Abstract
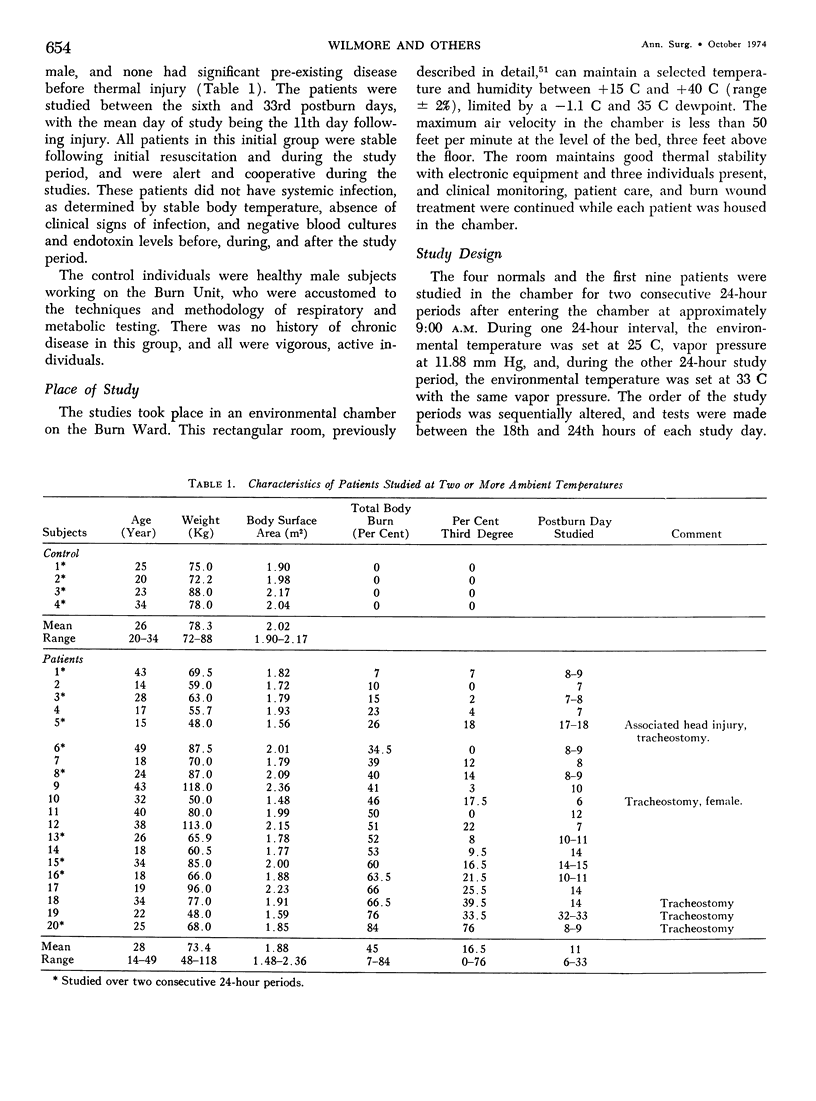
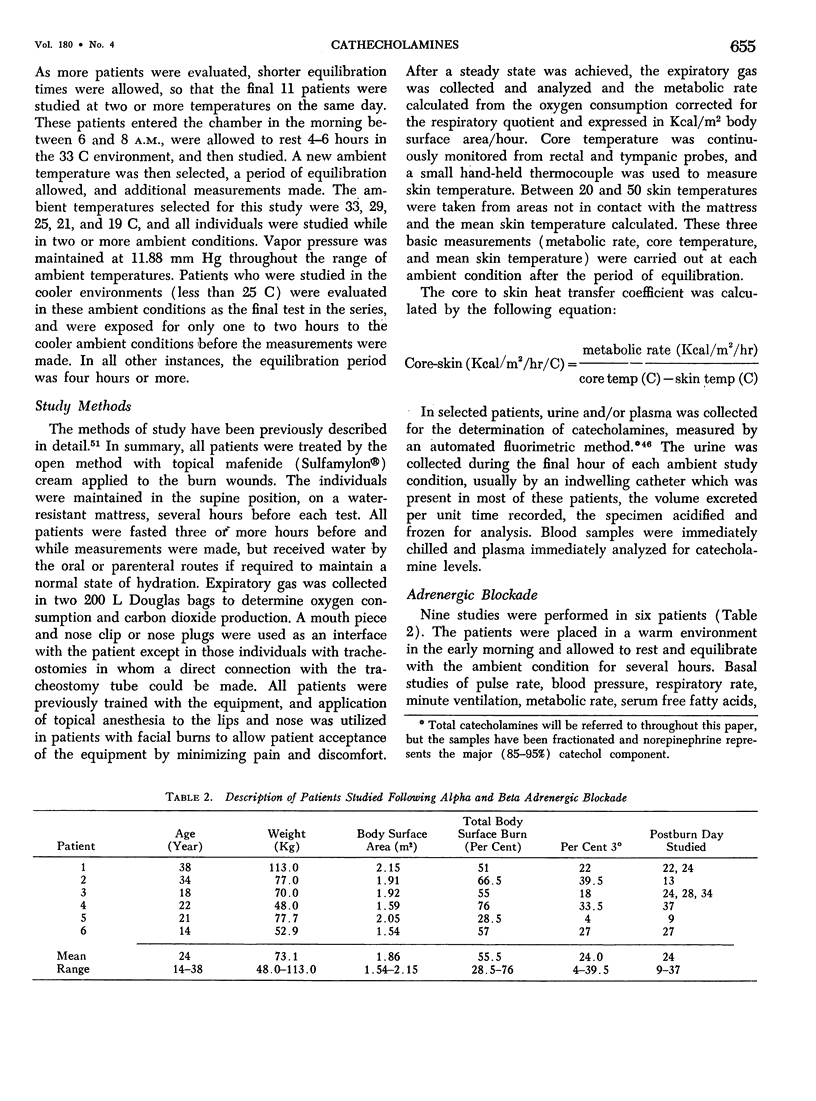
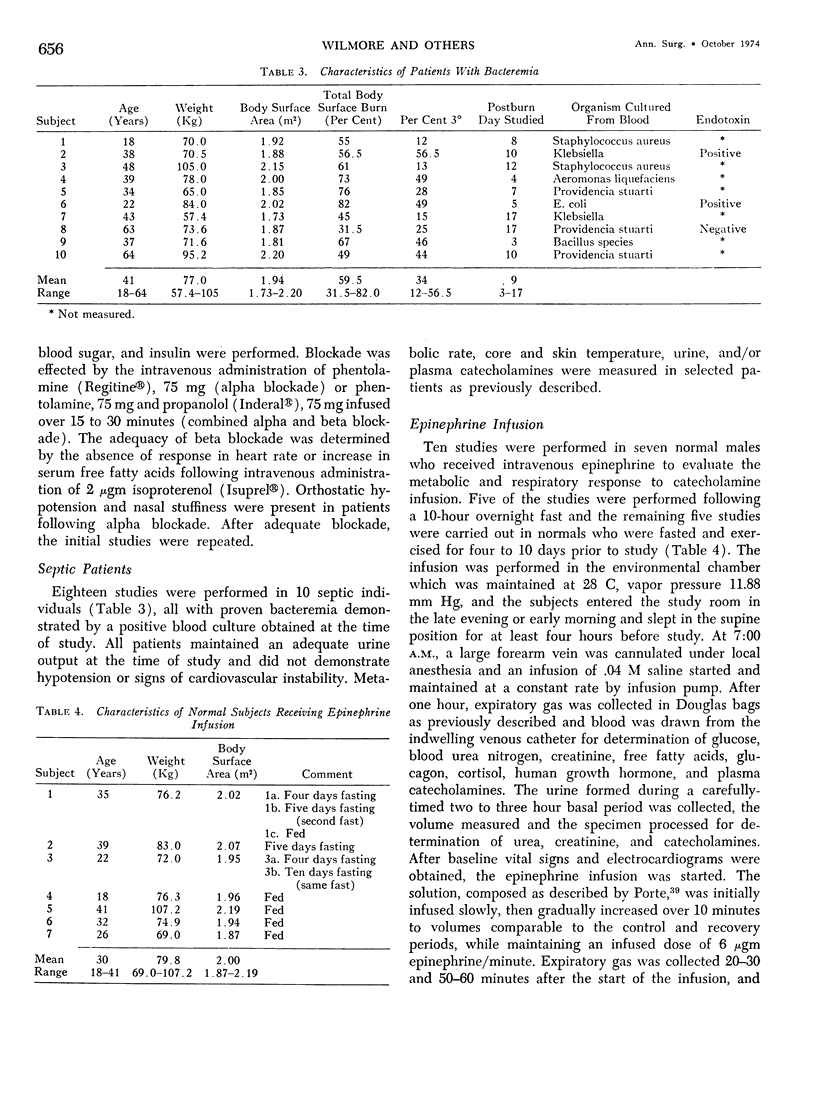
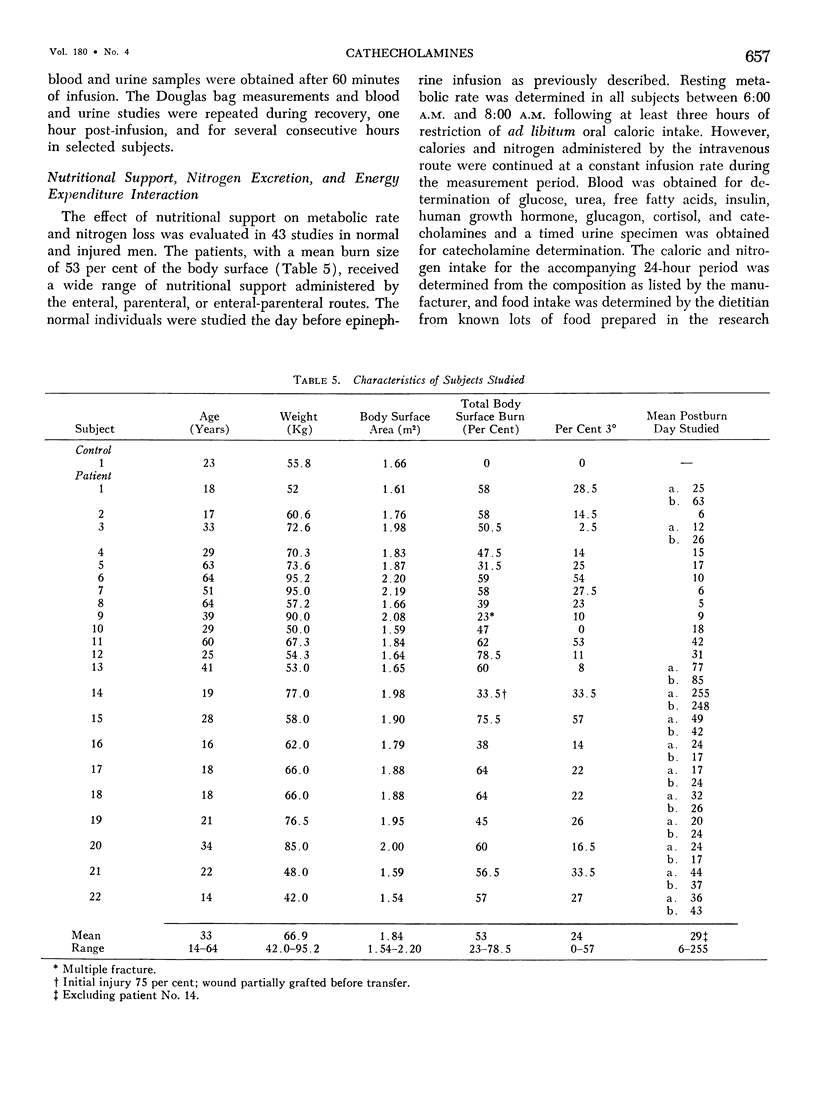
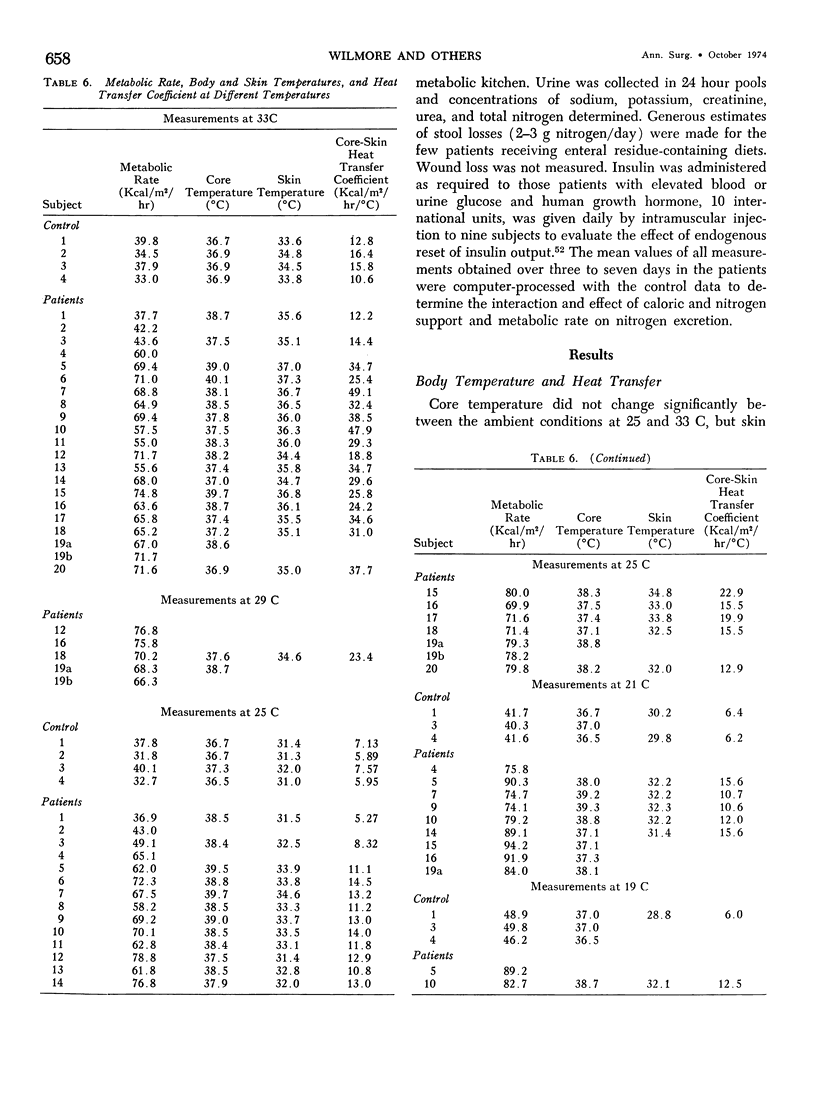
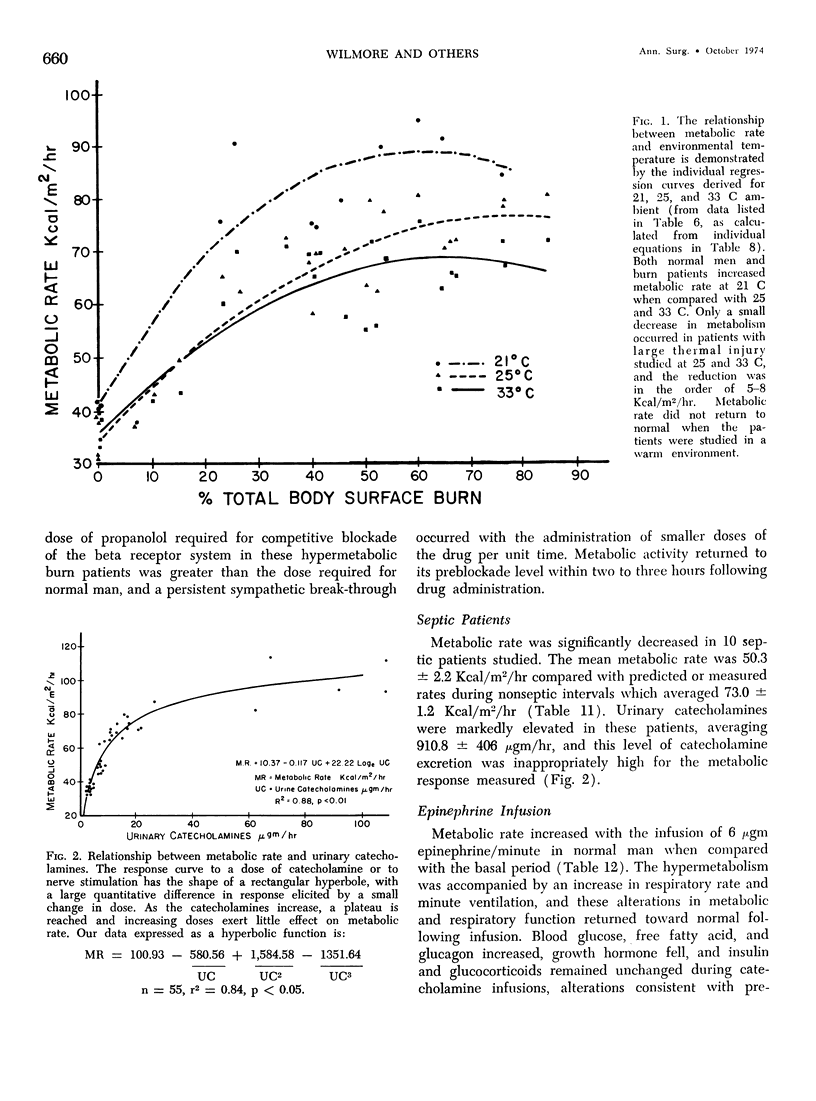
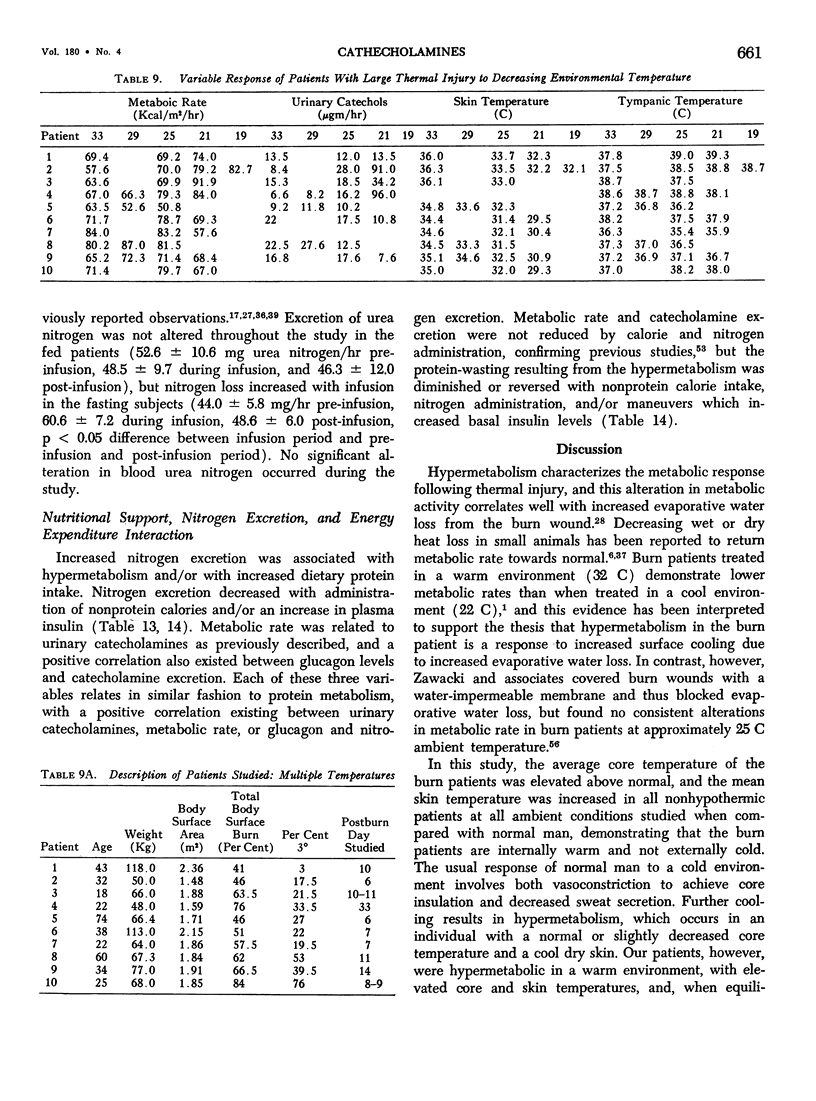
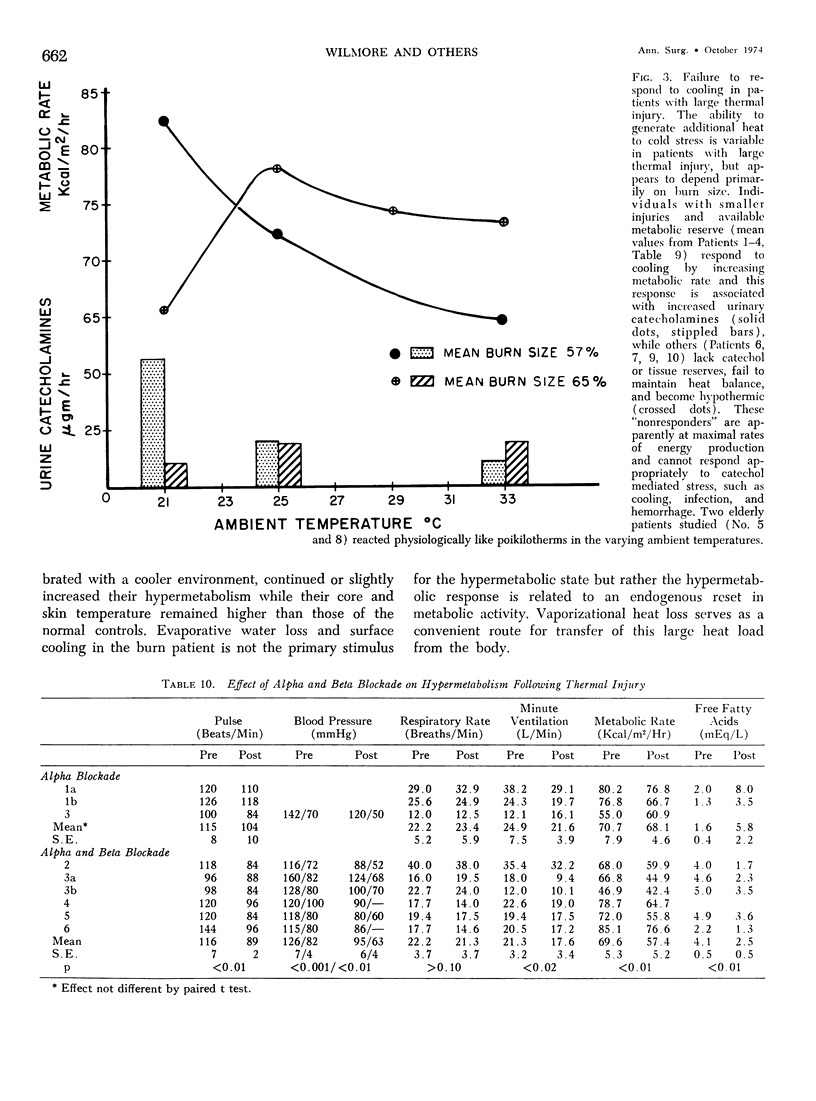
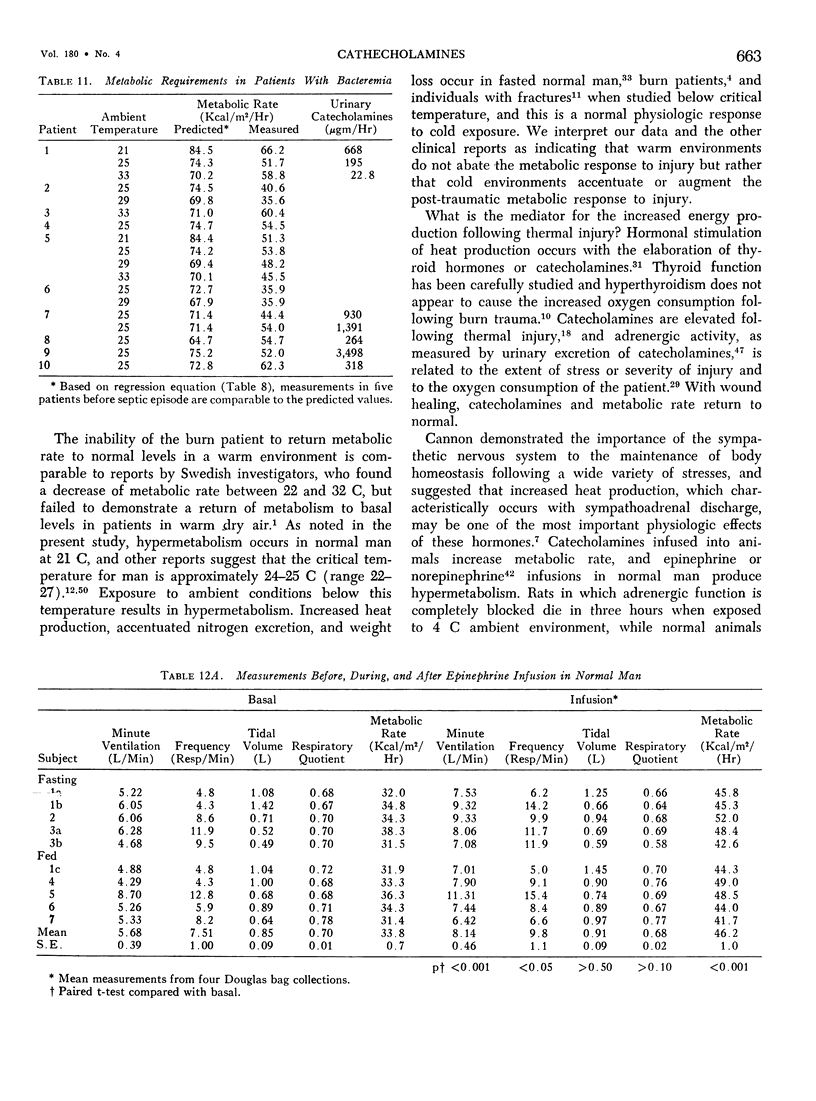
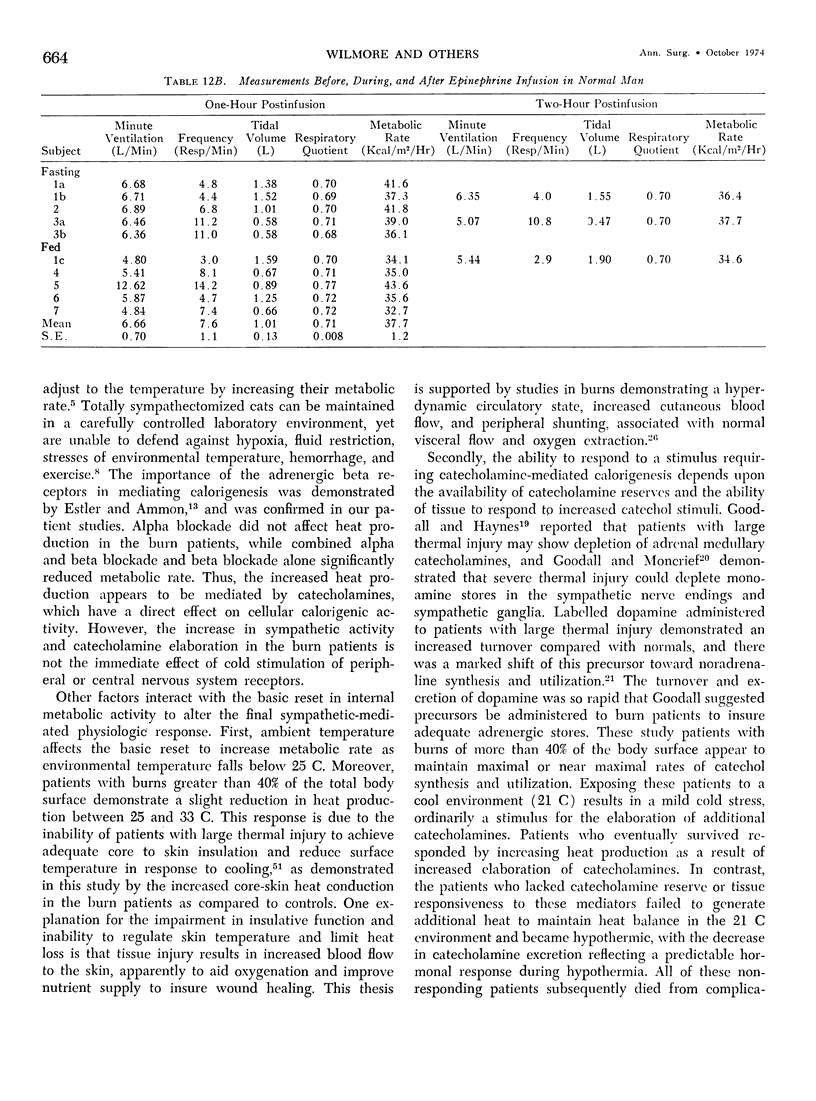
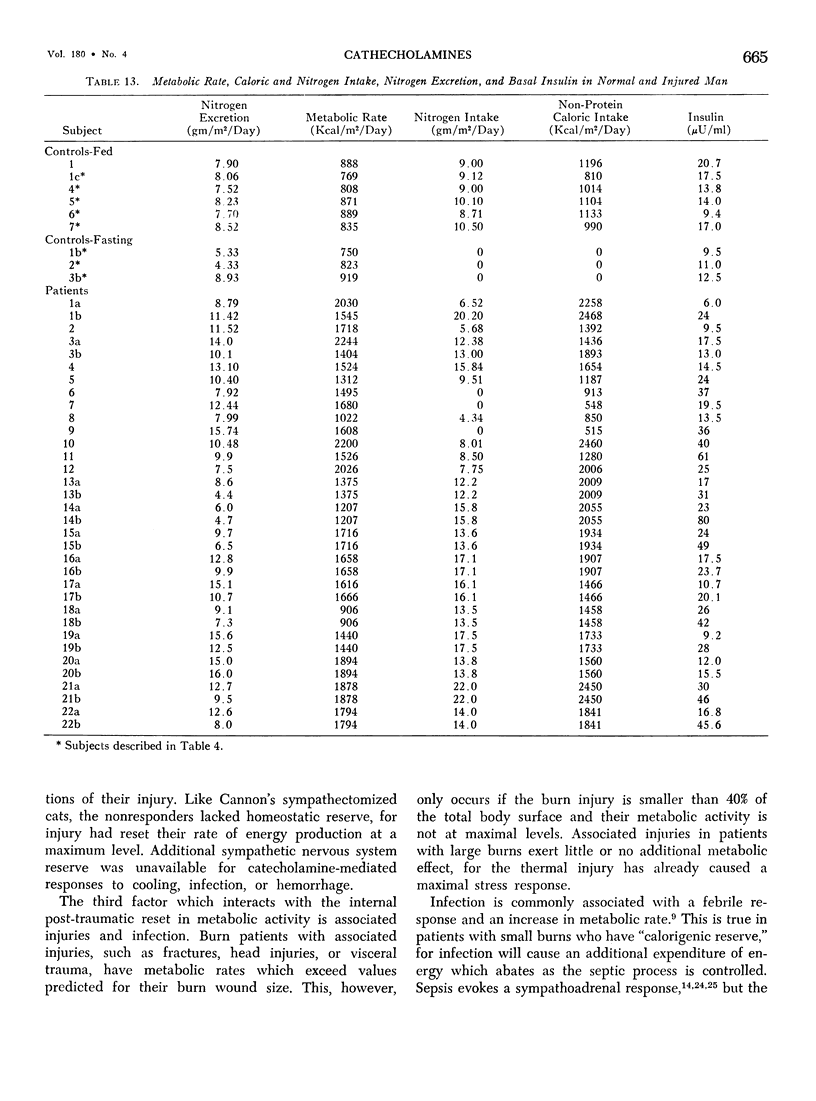
Hypermetabolism characterizes the metabolic response to thermal injury and the extent of energy production is positively related to the rate of urinary catecholamine excretion. Alpha and beta adrenergic blockade decreased metabolism from 69.6 ± 5.3 Kcal/m2/hr to 57.4 ± 5.2 (p < 0.01), and infusion of 6 µgm epinephrine/minute in normal man significantly increased metabolic rate. Twenty noninfected burned adults with a mean burn size of 45% total body surface (range 7-84%) and four normal controls were studied in an environmental chamber at two or more temperatures between 19 and 33 C with vapor pressure constant at 11.88 mm Hg. All burn patients were hypermetabolic at all temperatures studied and their core and mean skin temperatures were significantly elevated above control values. Between 25 and 33 C ambient, metabolism was unchanged in controls and burns of less than 40% total body surface (48.9 ± 4.6 Kcal/m2/hr vs. 48.9 ± 4.5), but metabolic rate decreased in larger burns in the warmer environment (72.0 ± 1.9 vs. 65.8 ± 1.7, p < 0.001). At 21 C, metabolism and catecholamines increased, except in four nonsurvivors who became hypothermic with decreased catechol elaboration. Metabolic rate in ten patients with bacteremia was below predicted levels while catecholamines were markedly elevated suggesting interference with tissue uptake of the neurohormonal transmitters. Feeding burn patients or administering glucose and insulin improved nitrogen retention and altered substrate flow but did not significantly reduce urinary catecholamines or metabolic rate. Burned patients are internally warm, not externally cold, and catecholamines appear to mediate their increased heat production. Hypermetabolism may be modified by ambient temperature, infection, and pharmacologic means. Alterations in hypothalamic function due to injury, resulting in increased catecholamine elaboration, would explain the metabolic response to thermal injury.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birke G., Carlson L. A., von Euler U. S., Liljedahl S. O., Plantin L. O. Studies on burns. XII. Lipid metabolism, catecholamine excretion, basal metabolic rate, and water loss during treatment of burns with warm dry air. Acta Chir Scand. 1972;138(4):321–333. [PubMed] [Google Scholar]
- Brodie B. B., Davies J. I., Hynie S., Krishna G., Weiss B. Interrelationships of catecholamines with other endocrine systems. Pharmacol Rev. 1966 Mar;18(1):273–289. [PubMed] [Google Scholar]
- COPE O., NARDI G. L., QUIJANO M., ROVIT R. L., STANBURY J. B., WIGHT A. Metabolic rate and thyroid function following acute thermal trauma in man. Ann Surg. 1953 Feb;137(2):165–174. doi: 10.1097/00000658-195302000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell F. T. Metabolic Response to Thermal Trauma: II. Nutritional Studies with Rats at Two Environmental Temperatures. Ann Surg. 1962 Jan;155(1):119–126. doi: 10.1097/00000658-196201000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERIKSON H., KROG J., ANDERSEN K. L., SCHOLANDER P. F. The critical temperature in naked man. Acta Physiol Scand. 1956 Jul 17;37(1):35–39. doi: 10.1111/j.1748-1716.1956.tb01339.x. [DOI] [PubMed] [Google Scholar]
- Estler C. J., Ammon H. P. The importance of the adrenergic beta-receptors for thermogenesis and survival of acutely cold-exposed mice. Can J Physiol Pharmacol. 1969 May;47(5):427–434. doi: 10.1139/y69-077. [DOI] [PubMed] [Google Scholar]
- FRANKSSON C., GEMZELL C. A., VON EULER U. S. Cortical and medullary adrenal activity in surgical and allied conditions. J Clin Endocrinol Metab. 1954 Jun;14(6):608–621. doi: 10.1210/jcem-14-6-608. [DOI] [PubMed] [Google Scholar]
- GOODALL M., HAYNES B. W., Jr Adrenal medullary insufficiency in severe thermal burn. J Clin Invest. 1960 Dec;39:1927–1932. doi: 10.1172/JCI104217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODALL M., STONE C., HAYNES B. W., Jr Urinary output of adrenaline and noradrenaline in severe thermal burns. Ann Surg. 1957 Apr;145(4):479–487. doi: 10.1097/00000658-195704000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON M. L. An evaluation of afferent nervous impulses in the adrenal cortical response to trauma. Endocrinology. 1950 Nov;47(5):347–350. doi: 10.1210/endo-47-5-347. [DOI] [PubMed] [Google Scholar]
- GRIFFITH F. R., Jr Fact and theory regarding the calorigenic action of adrenaline. Physiol Rev. 1951 Apr;31(2):151–187. doi: 10.1152/physrev.1951.31.2.151. [DOI] [PubMed] [Google Scholar]
- Goodall M. C., Alton H. Dopamine (3-hydroxytyramine) replacement and metabolism in sympathetic nerve and adrenal medullary depletions after prolonged thermal injury. J Clin Invest. 1969 Sep;48(9):1761–1767. doi: 10.1172/JCI106142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall M., Moncrief J. A. Sympathetic Nerve Depletion in Severe Thermal Injury. Ann Surg. 1965 Nov;162(5):893–900. doi: 10.1097/00000658-196511000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths J., Groves A. C., Leung F. Y. Hypertriglyceridemia and hypoglycemia in gram-negative sepsis in the dog. Surg Gynecol Obstet. 1973 Jun;136(6):897–903. [PubMed] [Google Scholar]
- Groves A. C., Griffiths J., Leung F., Meek R. N. Plasma catecholamines in patients with serious postoperative infection. Ann Surg. 1973 Jul;178(1):102–107. doi: 10.1097/00000658-197307000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gump F. E., Price J. B., Jr, Kinney J. M. Blood flow and oxygen consumption in patients with severe burns. Surg Gynecol Obstet. 1970 Jan;130(1):23–28. [PubMed] [Google Scholar]
- HARRISON H. N., MONCRIEF J. A., DUCKETT J. W., Jr, MASON A. D., Jr THE RELATIONSHIP BETWEEN ENERGY METABOLISM AND WATER LOSS FROM VAPORIZATION IN SEVERELY BURNED PATIENTS. Surgery. 1964 Jul;56:203–211. [PubMed] [Google Scholar]
- HSIEH A. C., CARLSON L. D. Role of adrenaline and noradrenaline in chemical regulation of heat production. Am J Physiol. 1957 Aug;190(2):243–246. doi: 10.1152/ajplegacy.1957.190.2.243. [DOI] [PubMed] [Google Scholar]
- HUME D. M., EGDAHL R. H. The importance of the brain in the endocrine response to injury. Ann Surg. 1959 Oct;150:697–712. doi: 10.1097/00000658-195910000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson E. L., Brennan M. F., O'Connell R. C., Moore F. D. Response of glucose, insulin, free fatty acid, and human growth hormone to norepinephrine and hemorrhage in normal man. Ann Surg. 1973 Apr;177(4):453–457. doi: 10.1097/00000658-197304000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison T. S., Seaton J. F., Feller I. Relationship of increased oxygen consumption to catecholamine excretion in thermal burns. Ann Surg. 1967 Feb;165(2):169–172. doi: 10.1097/00000658-196702000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himms-Hagen J. Sympathetic regulation of metabolism. Pharmacol Rev. 1967 Sep;19(3):367–461. [PubMed] [Google Scholar]
- Kris A. O., Miller R. E., Wherry F. E., Mason J. W. Inhibition of insulin secretion by infused epinephrine in rhesus monkeys. Endocrinology. 1966 Jan;78(1):87–97. doi: 10.1210/endo-78-1-87. [DOI] [PubMed] [Google Scholar]
- LIEBERMAN Z. H., LANSCHE J. M. Effects of thermal injury on metabolic rate and insensible water loss in the rat. Surg Forum. 1957;7:83–88. [PubMed] [Google Scholar]
- Porte D., Jr, Graber A. L., Kuzuya T., Williams R. H. The effect of epinephrine on immunoreactive insulin levels in man. J Clin Invest. 1966 Feb;45(2):228–236. doi: 10.1172/JCI105335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porte D., Jr, Robertson R. P. Control of insulin secretion by catecholamines, stress, and the sympathetic nervous system. Fed Proc. 1973 Jul;32(7):1792–1796. [PubMed] [Google Scholar]
- Stone D. J., Keltz H., Sarkar T. K., Singzon J. Ventilatory response to alpha-adrenergic stimulation and inhibition. J Appl Physiol. 1973 May;34(5):619–623. doi: 10.1152/jappl.1973.34.5.619. [DOI] [PubMed] [Google Scholar]
- Taggart P., Carruthers M., Somerville W. Electrocardiogram, plasma catecholamines and lipids, and their modification by oxyprenolol when speaking before an audience. Lancet. 1973 Aug 18;2(7825):341–346. doi: 10.1016/s0140-6736(73)93190-5. [DOI] [PubMed] [Google Scholar]
- Unger R. H. Glucagon and the insulin: glucagon ratio in diabetes and other catabolic illnesses. Diabetes. 1971 Dec;20(12):834–838. doi: 10.2337/diab.20.12.834. [DOI] [PubMed] [Google Scholar]
- VON EULER U. S., HELLNER S. Excretion of noradrenaline and adrenaline in muscular work. Acta Physiol Scand. 1952 Sep 10;26(2-3):183–191. doi: 10.1111/j.1748-1716.1952.tb00900.x. [DOI] [PubMed] [Google Scholar]
- VON EULERU QUANTITATION OF STRESS BY CATECHOLAMINE ANALYSIS. Clin Pharmacol Ther. 1964 Jul-Aug;5:398–404. doi: 10.1002/cpt196454398. [DOI] [PubMed] [Google Scholar]
- Vetter N. J., Strange R. C., Adams W., Oliver M. F. Initial metabolic and hormonal response to acute myocardial infarction. Lancet. 1974 Feb 23;1(7852):284–288. doi: 10.1016/s0140-6736(74)92595-1. [DOI] [PubMed] [Google Scholar]
- Viktora J. K., Baukal A., Wolff F. W. New automated fluorometric methods for estimation of small amounts of adrenaline and noradrenaline. Anal Biochem. 1968 Jun;23(3):513–528. doi: 10.1016/0003-2697(68)90243-1. [DOI] [PubMed] [Google Scholar]
- Wilkerson J. E., Raven P. B., Horvath S. M. Critical temperature of unacclimatized male Caucasians. J Appl Physiol. 1972 Oct;33(4):451–455. doi: 10.1152/jappl.1972.33.4.451. [DOI] [PubMed] [Google Scholar]
- Wilmore D. W., Curreri P. W., Spitzer K. W., Spitzer M. E., Pruitt B. A., Jr Supranormal dietary intake in thermally injured hypermetabolic patients. Surg Gynecol Obstet. 1971 May;132(5):881–886. [PubMed] [Google Scholar]
- Wilmore D. W., Lindsey C. A., Moyland J. A., Faloona G. R., Pruitt B. A., Unger R. H. Hyperglucagonaemia after burns. Lancet. 1974 Jan 19;1(7847):73–75. doi: 10.1016/s0140-6736(74)92290-9. [DOI] [PubMed] [Google Scholar]
- Wilmore D. W., Moylan J. A., Jr, Bristow B. F., Mason A. D., Jr, Pruitt B. A., Jr Anabolic effects of human growth hormone and high caloric feedings following thermal injury. Surg Gynecol Obstet. 1974 Jun;138(6):875–884. [PubMed] [Google Scholar]
- Zawacki B. E., Spitzer K. W., Mason A. D., Jr, Johns L. A. Does increased evaporative water loss cause hypermetabolism in burned patients? Ann Surg. 1970 Feb;171(2):236–240. doi: 10.1097/00000658-197002000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]


