Abstract
Microorganisms that use nitrate as an alternative terminal electron acceptor play an important role in the global nitrogen cycle. The diversity of the nitrate-reducing community in soil and the influence of the maize roots on the structure of this community were studied. The narG gene encoding the membrane bound nitrate reductase was selected as a functional marker for the nitrate-reducing community. The use of narG is of special interest because the phylogeny of the narG gene closely reflects the 16S ribosomal DNA phylogeny. Therefore, targeting the narG gene provided for the first time a unique insight into the taxonomic composition of the nitrate-reducing community in planted and unplanted soils. The PCR-amplified narG fragments were cloned and analyzed by restriction fragment length polymorphism (RFLP). In all, 60 RFLP types represented by two or more clones were identified in addition to the 58 RFLP types represented by only one clone. At least one clone belonging to each RFLP type was then sequenced. Several of the obtained sequences were not related to the narG genes from cultivated bacteria, suggesting the existence of unidentified nitrate-reducing bacteria in the studied soil. However, environmental sequences were also related to NarG from many bacterial divisions, i.e., Actinobacteria and α, β, and γ Proteobacteria. The presence of the plant roots resulted in a shift in the structure of the nitrate-reducing community between the unplanted and planted soils. Sequencing of RFLP types dominant in the rhizosphere or present only in the rhizosphere revealed that they are related to NarG from the Actinobacteria in an astonishingly high proportion.
Nitrate-reducing prokaryotes constitute a wide group with members among the α, β, and γ Proteobacteria, gram-positive Bacteria, and even Archea, sharing the ability to obtain energy from dissimilatory reduction of nitrate into nitrite. The nitrite can then be reduced into gaseous nitrogen compounds by denitrification or into NH4 by dissimilatory nitrate reduction into ammonia. Denitrification is the dominant process in soils and lake sediment, whereas dissimilatory nitrate reduction into ammonia occurs mainly in the rumen and in digested sludge (30). Due to nitrogen losses in agricultural soils and production of the greenhouse gases NO and N2O, denitrification has received a lot of attention over the last 20 years (7). There are two distinct forms of dissimilatory nitrate reductase; a periplasmic nitrate reductase and a membrane-bound nitrate reductase (reviewed in references 16 and 22). Only the nitrate reductase is found universally in prokaryotes and can therefore be used as a functional marker for these microorganisms. In addition, many microorganisms can contain both nitrate reductases (22). The membrane-bound nitrate reductase is composed of a soluble β subunit containing four [Fe-S] clusters and the γ subunit containing two b-type hemes. The last subunit, encoded by the narG gene, contains a [4Fe-4S] cluster and a molybdopterin guanine dinucleotide cofactor. This α subunit being the catalytic site of nitrate reduction, it was selected as a functional marker for the dissimilatory nitrate-reducing community and a highly specific set of primers was designed to amplify a fragment of the narG gene from a variety of nitrate reducing prokaryotes.
It is well established that roots of growing plants can not only release carbon compounds via the exudation but also modify the soil oxygen partial pressure and the nitrate concentration in the rhizosphere. The impact of the rhizosphere on microbial activities such as denitrification has been previously reported (14, 23, 28, 31). However, little is known about the influence of the rhizosphere on the structure of the nitrate-reducing community and previous studies on this community were performed mainly in the rhizosphere of aerenchymatous plant by using cultivation-based approaches (4, 17). The application of molecular biological techniques targeting the 16S ribosomal DNA gene or, more recently, functional genes has provided a pertinent alternative to these culture-based methods, providing unique insight into the composition, richness, and structure of microbial communities (3, 8, 13, 18, 32). This study has two aims. First we wish to assess the natural diversity of the narG gene in soil by direct PCR amplification, cloning, and restriction fragment and sequence analysis. The second aim is to examine the influence of the rhizosphere of maize (Zea mays cv. Dea) on the composition of the nitrate-reducing community. The initial analysis revealed a very high diversity and the presence of a large proportion of uncharacterized dissimilatory nitrate reducers in the studied soil. Our analysis also demonstrated specific changes in the structure of the nitrate-reducing community in the planted soil without modification of the diversity indices.
MATERIALS AND METHODS
Soil and treatment.
The soil used in this study was collected from the 20-cm-deep top layer of an experimental field of the ENSAIA domain of La Bouzule (Nancy, France). It is a redoxic neoluvisol with 33.3% clay, 51.3% silt, 15.4% sand, and a pH in water of 5.8. Just after collection, the soil was sieved (5-mm mesh size), moistened to 80% of the water holding capacity, and distributed into plastic pots (amounts equivalent to 1.5 kg of dry soil per pot). Some plastic pots were kept uncultivated, and others were cultivated with maize (Zea mays cv. Dea). Maize seeds were sterilized with a solution of sodium hypochlorite (33 mg · liter−1) under agitation for 5 min. They were then thoroughly rinsed with sterile distilled water and sowed (four seeds per pot). The experiment was conducted in a greenhouse under controlled conditions as follows: day temperature, 17°C; night temperature, 15°C; daylight period, 11 h; relative humidity, 60%; and cooling above 20°C. The pots were weighed every day in order to maintain soil water content. Eight weeks after planting, soil from planted and nonplanted plastic pots were recovered.
Design of the narG-specific primers.
The narG sequences of bacteria and archea currently available from the GenBank database and from genome projects were aligned and then scanned for conserved regions that could provide suitable primer target sites. From this analysis, two degenerated primers, narG1960f (5′ TAYGTSGGSCARGARAA 3′) and narG2650r (5′ TTYTCRTACCABGTBGC 3′), were designed to amplify a 650-bp internal section of the narG gene.
DNA extraction.
Soil samples from all pots of the same treatment were mixed and then subsampled for DNA extraction. DNA was extracted from three 250-mg aliquots of soil from the unplanted treatment (samples BSA, BSB, and BSC) and the planted treatment (samples PSD, PSE, and PSF) according to the method of Martin-Laurent et al. (15). Briefly, samples were homogenized in 1 ml of extraction buffer for 30 s at 1,600 rpm in a mini-bead beater cell disruptor (Mikro-Dismembrator S; B. Braun Biotech International). Soil and cell debris were removed by centrifugation. After sodium acetate precipitation, proteins were removed. Nucleic acids were then precipitated with cold isopropanol, washed with 70% ethanol, and purified by using a Sepharose 4B spin column. The quality and the size of the soil DNAs were checked by electrophoresis on 1% agarose. DNA was quantified by use of a BioPhotometer (Eppendorff).
RISA and narG amplification.
The intergenic spacer region between the small and large subunit of the ribosomal genes was amplified (ribosomal intergenic spacer region amplification [RISA]) from 25 ng of purified soil DNA with a total volume of 50 μl by using the universal primers 38r (5′-CCG GGT TTC CCC ATT CGG-3′) and 72f (5′-TGC GGC TGG ATC TCC TT-3′) under the following conditions: 5 min at 94°C, 35 cycles of 1 min at 94°C, 1 min at 55°C, and 2 min at 72°C, plus an additional 15-min cycle at 72°C. Eight-microliter aliquots were then separated on a native 6% acrylamide gel run for 17 h at 4 mA. The gel was stained with SYBR green II (Molecular Probes, Leiden, Netherlands). Amplification of purified soil DNA was also carried out by using the primers narG1960f and narG2650r. Three independent PCR amplifications were performed for each replicate in a total of 50 μl containing 5 μl of 10× PCR buffer, 200 μM concentrations of each deoxyribonucleoside triphosphate, 1 U of Taq polymerase, 25 ng of purified soil DNA, and 500 pM concentrations of each primer. The enzyme was added after the first denaturation step. Thermal cycling was carried out by an initial denaturation step of 5 min at 95°C, followed by a touch down PCR (Thermocyclor; M.J. Research). This consisted of a denaturation step of 30 s at 94°C, a primer annealing step of 30s at 60°C, and an elongation step of 45 s at 72°C; cycling was completed by a final elongation step of 72°C for 6 min. During the first eight cycles, the temperature was decreased by 0.5°C for each cycle, starting at 59°C, until it reached 55°C. The additional 30 cycles were performed at an annealing temperature of 55°C. The presence and size of the amplification products were determined by agarose (1%) gel electrophoresis of the reaction product and ethidium bromide staining.
Restriction fragment length polymorphism (RFLP) fingerprinting analysis and clone library construction.
Gel slices containing the narG PCR products were excised, and DNA was purified by using the Qiaex II kit (Qiagen) as specified by the manufacturer. PCR products belonging to the same replicate were then pooled and concentrated by using the QIAquick columns (Qiagen). Aliquots of the excised and purified PCR products were digested with AluI restriction enzyme at 37°C for 12 h and separated by electrophoresis on a native 6% acrylamide gel for 15 h at 4 mA. In addition, aliquots of the excised and purified PCR products from each soil replicate were cloned by using the pGEM-T Easy Vector System (Promega). Ligation and transformation reactions were performed according to the manufacturer's instructions. Approximately 90 recombinants from each of the six libraries were screened for full-size inserts (approximately 650 bp) by transferring small aliquots of cells to PCR mixtures containing the vector primers T-7 and SP-6 and thermal cycling. Colonies that did not produce amplifications were eliminated from the libraries. The PCR products were digested with the restriction endonuclease AluI. The restriction fragments were resolved by agarose gel electrophoresis with 3% small fragment agarose (FMC, Rockland, Maine). Clones with identical restriction patterns were grouped together into clone families.
Sequencing of the cloned narG products.
The nucleotide sequences of the cloned PCR products (650 bp) were determined by using the DTCS-1 kit (Beckman Coulter) and a Ceq 2000 XL sequencer (Beckman Coulter) according to the manufacturer's instructions. Vector primers T7 and SP6 were used for sequencing reactions.
Phylogenetic analysis, rarefaction, and diversity analysis.
An alignment of the deduced protein sequences of the narG genes was made by using the CLUSTALX software version V. 1.0.1 (29). To construct the phylogenic tree based on amino acids alignments, protein distances were inferred by the neighbor-joining method. Bootstrap analysis for protein level (NarG) phylogenetic analyses were performed by using the CLUSTALX software version V. 1.0.1. For each calculation, 1,000 bootstrap resamplings were performed. The diversity of the phylotypes in different samples was compared by rarefaction analysis. Rarefaction calculations were done by using the software Analytic Rarefaction (version 2.1; Stratigraphy laboratory, University of Georgia). The Shannon-Weaver index of general diversity (H′) was calculated with the following equation: H′ = −ΣPi*log2Pi, where Pi was calculated as follows: Pi= ni/N, where ni is the number of clones in an RFLP type and N is the total number of clones (24). Using the same data, the Simpson index of dominance concentration (D) was calculated by using the following function: D = ΣPi2 (26). The distributions of narG RFLP types between unplanted and planted soil populations were statistically compared by analysis of molecular variance (AMOVA version 1.55, 1995 [9]).
Nucleotide accession number.
The environmental narG gene sequences have been deposited in the GenBank sequence database under the accession numbers AY113707 to AY113839.
RESULTS
Specificity of the narG assay.
Based on the alignment of publicly available narG sequences from GenBank but also from finished and unfinished microbial genome projects, a couple of primers were designed to amplify a partial stretch of the narG gene from a wide range of microorganisms belonging to Proteobacteria, gram-positive bacteria, and also Archea (data not shown). These primers were used to amplify DNA extracted from each sample of the unplanted and planted soils. PCR products of the predicted size were recovered from each sample. The BLASTP program (1) produced statistically significant alignments for 132 of 144 sequences with dissimilatory nitrate reductase. The 12 sequences that did not show significant identity with the narG gene were dismissed.
Fingerprinting analysis of amplified intergenic spacer region between the small and large subunits of the ribosomal genes (RISA) and of narG gene.
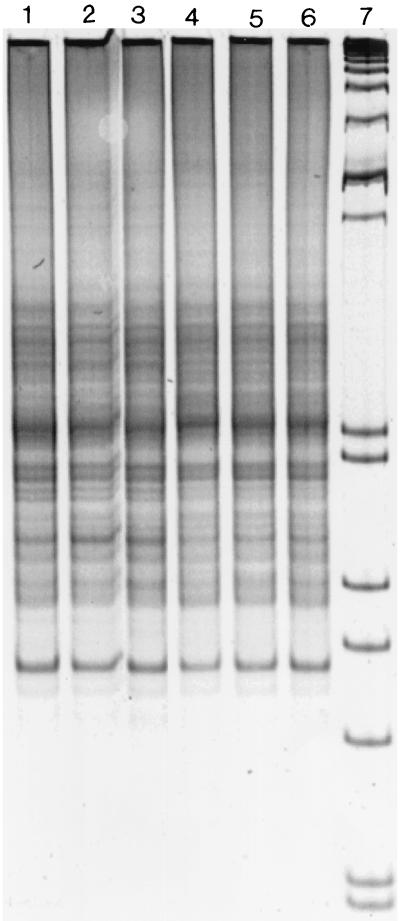
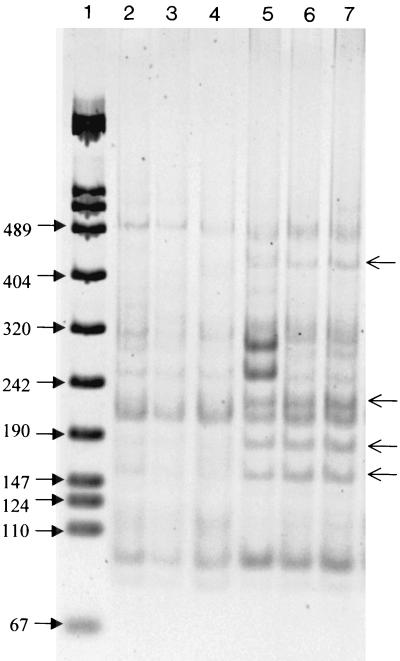
Similar RISA patterns were obtained for the unplanted and planted soil samples (Fig. 1). Evaluation of the impact of the rhizosphere was further conducted on the nitrate-reducing community. In contrast to the RISA patterns, RFLP analysis of the amplified narG gene indicated differences in the composition of the membrane bound nitrate-reducing community between the planted and unplanted soil. Thus, a higher number of dominant bands at 150, 180, 230, and 450 bp was observed in the planted soil than in the unplanted soil (Fig. 2). Differences between RFLP patterns from the planted soil have been observed with the presence of the two bands of higher intensity at 280 and 250 bp in the PSD sample (Fig. 2), while RFLP patterns of the unplanted soil were very similar.
FIG. 1.
Comparison of the RISA patterns obtained from the unplanted soil (lane 1, replicate BSA; lane 2, replicate BSB; lane 3, replicate BSC) and from the maize-planted soil (lane 4, replicate PSD; lane 5, replicate PSE; lane 6, replicate PSF) with 16S ribosomal DNA intergenic spacer universal primers. Migration was performed with a 6% polyacrylamide gel, and the molecular size marker was the VIII from Boehringer Mannheim (lane 7).
FIG. 2.
Comparison of the AluI RFLPs of the narG PCR products obtained from the unplanted soil (lane 2, replicate BSA; lane 3, replicate BSB; lane 4, replicate BSC) and from the maize-planted soil (lane 5, replicate PSD; lane 6, replicate PSE; lane 7, replicate PSF). Migration was performed on a 6% polyacrylamide gel, and the molecular size marker was the VIII from Boehringer Mannheim (lane 1).
Phylotype richness and distribution.
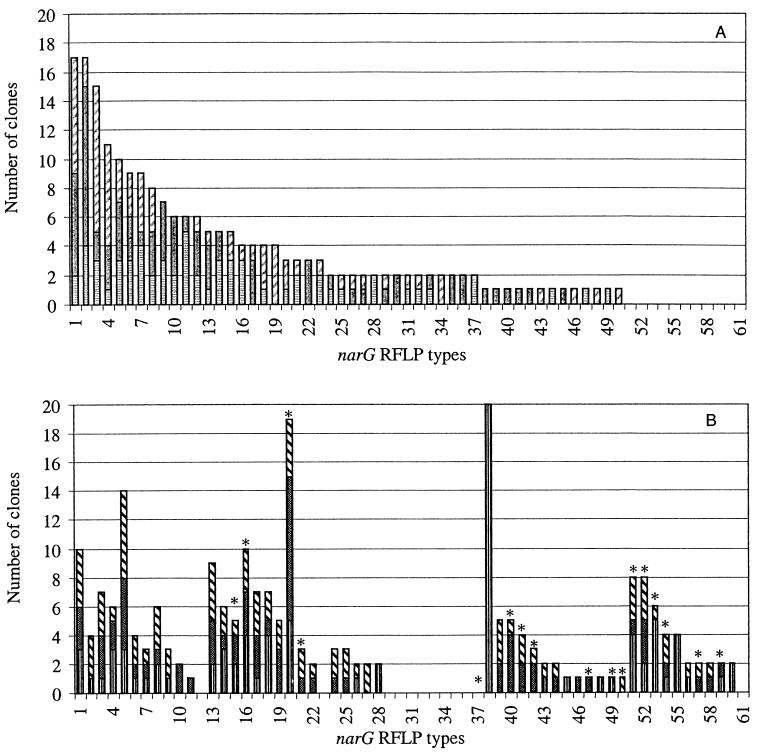
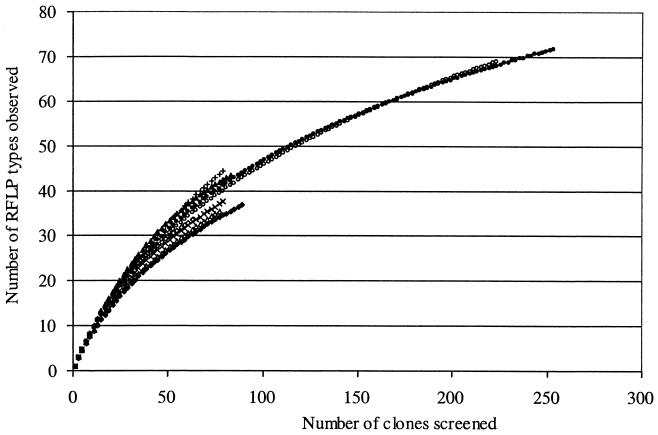
Libraries of approximately 90 randomly selected clones were constructed for each sample of the unplanted and planted soil (six libraries in total). The clones were subjected to RFLP analysis and grouped based on their representative RFLP patterns. A total of 472 clones were then screened by RFLP, resulting in the identification of more than 100 AluI restriction fragment banding patterns. Sixty of these patterns represented two or more clones, and they accounted for more than 90% of the clones examined. Comparison of the distribution of phylotypes from the planted and unplanted soils clearly exhibited significant differences (P < 0.05). The unplanted soil libraries were dominated by the phylotype numbers 1, 2, and 3, which together accounted for 26% of these clone libraries (Fig. 3), while the libraries from the planted soil were dominated by RFLP type numbers 38, 20, and 5, which together accounted for 24%. Interestingly, a similar number of phylotypes (about 10) was detected only in the unplanted or in the planted soil. The phylotype number 38 contained only clones from one sample (PSD). This result is in accordance with Fig. 2, which shows, only in sample PSD, two bands at 280 and 250 bp of higher intensity corresponding to this phylotype. However, excepting the phylotype number 38, phylotypes containing at least seven clones were identified in each of three samples, confirming the good reproducibility. To estimate how well the libraries were sampled, rarefaction curves were plotted. These curves indicated a high degree of diversity illustrated by the number of clones necessary to estimate the total number of phylotypes in the studied soil (Fig. 4). As a result, the rarefaction curves from the unplanted soil narG clone libraries are similar to those from the planted soil narG clone libraries. Calculation of the Shannon-Weiner index (H = 3) or Simpson's index (D = 0.04) did not reveal significant differences between unplanted and planted soil clone libraries, indicating that the unplanted soil clone libraries are as diverse as the planted soil libraries.
FIG. 3.
Distribution of the narG RFLP types from the unplanted soil (A) samples BSA (▤), BSB (gray with white dots) and BSC (▨) and distribution of the same RFLP types from the planted soil (B) samples PSD (▥), PSE (▩), and PSF (▧). Only RFLP types containing more than one clone are represented. The asterisks indicate RFLP types belonging to cluster 2.
FIG. 4.
Rarefaction curves of observed diversity of narG RFLP types in the unplanted soil (○) samples BSA (⋄), BSB (x), and BSC (▵) and in the planted soil (•) samples PSD (♦), PSE (+), and PSF (▴).
Phylogenetic diversity among RFLP phylotypes.
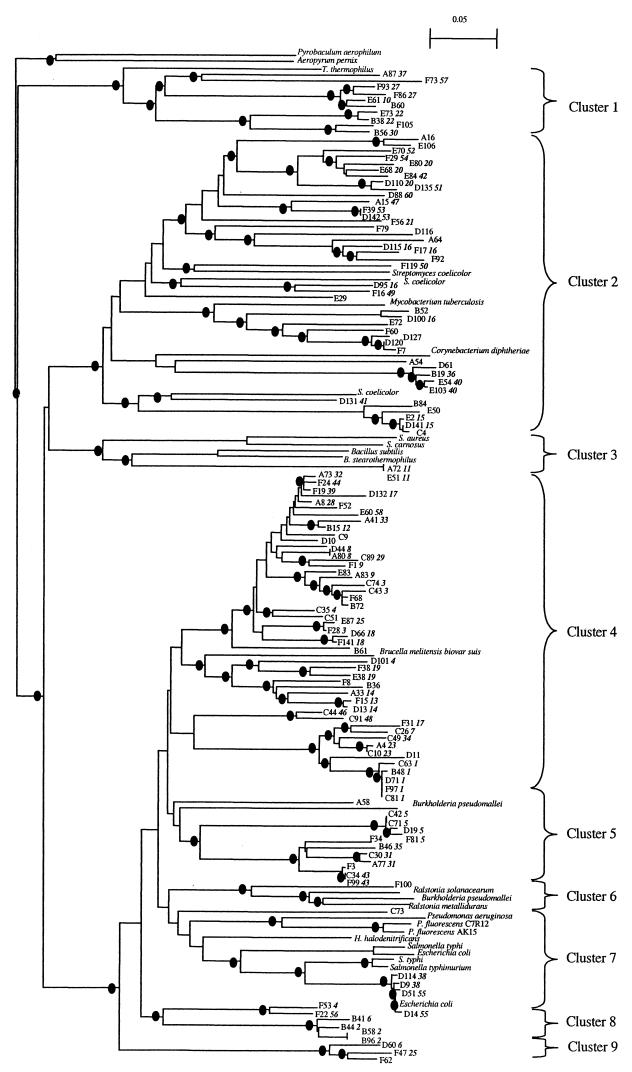
To evaluate the phylogenetic diversity represented by the 118 RFLP patterns identified in the narG gene clone libraries, 132 sequences from all the RFLP types were obtained and used for phylogenetic analysis; sequences of 30 narG genes previously described were also included. Of the 144 sequences obtained, 12 non-nitrate reductase sequences were dismissed. Levels of translated amino acid identity of the narG clones ranged from 62 to 99.5% with the nearest known NarG. The obtained tree can be divided into nine clusters based on the location of NarG from cultivated microorganisms (Fig. 5). The first cluster contained clones representing nine phylotypes and the NarG sequence of the extremophile bacteria, Thermus thermophilus. Cluster 2 contained the NarG of the Actinobacteria genera Streptomyces, Corynebacterium, and Mycobacterium and several phylotypes representing approximately 37 and 12% of the planted and unplanted soil clone libraries, respectively. Interestingly, most of the phylotypes dominant in the planted soil or detected only in the planted soil belong to this cluster (Fig. 3 and 5). Only two clones belonging to the same phylotype and related to NarG of other gram-positive bacteria, Bacillus spp. and Staphylococcus spp., are present in cluster 3. Cluster 4 contained only one NarG sequence from cultivated bacteria, a member of alpha Proteobacteria, Brucella melitensis biovar suis. This cluster is divided into two subclusters, and in the first subcluster clones were more closely related to each other than in other clusters. Analysis of the distribution of the clones in cluster 4 revealed that this cluster represented 56 and 36% of the unplanted and planted soil clones libraries, respectively. Cluster 6 consisted of cultivated bacteria belonging to the beta Proteobacteria, which are related to only one environmental clone (75% amino acid identity with Ralstonia solanacearum). In contrast, only environmental NarG sequences were present in the last two clusters. Clones belonging to RFLP types 4 and 26 are grouped in different clusters. However, only one restriction site was present in RFLP type 4; and concerning RFLP type 26, in silico restriction profiles of the corresponding clones were very similar and could not be distinguished on an agarose gel. Except for these clones, all clones belonging to the same RFLP type were grouped in the same cluster. Altogether, these data confirmed that the clone libraries represented a phylogenetically broad spectrum of microorganisms.
FIG.5.
Phylogenetic relationship of translated narG gene product. Phylogenetic distances were determined by neighbor-joining analysis. The corresponding RFLP types are indicated in bold and italics after the clone number. Nodes with more than 750 (of 1,000) bootstrap iterations are highlighted by a black circle. The accession numbers or website resources for the narG genes are as follows: Pseudomonas fluorescens C7R12, AF197465; P. fluorescens AK15 U71398; Pseudomonas aeruginosa, Y15252; E. coli, X16181 and X17110; Halobacterium denitrificans, AB076402; Thermus thermophilus, Y10124; Mycobacterium tuberculosis, NC_000962; Ralstonia solanacearum, NC_003296; Bacillus subtilis, X91819; Bacillus stearothermophilus, http://www.genome.ou.edu/bstearo.html; Streptomyces coelicolor, AL512667, AL109989, and AL031515; Staphylococcus carnosus, AF029224; Staphylococcus aureus, NC_002745, Brucella melitensis biovar suis, http://www.tigr.org; B. pseudomallei, http://www.sanger.ac.uk/Projects/; Mycobacterium tuberculosis, Z81360; Corynebacterium diphtheriae, http://www.sanger.ac.uk/Projects/C_diphtheriae/; Ralstonia metallidurans, http://www.jgi.doe.gov/JGI_microbial; R. solanacearum, AL646082; Pyrobaculum aerophilum, NC_003364; Aeropyrum pernix, http://www.bio.nite.go.jp; S. enterica serovar Typhi, NC_003198; S. enterica serovar Typhimurium, NC_003197.
DISCUSSION
Both DNA extraction and amplification suffer from biases that can distort community composition, richness, and structure. We assumed that the biases operated uniformly for the unplanted and planted soil and therefore the soil samples could be compared as was done in previous studies (5, 8, 19).
Designing a primer specific to all narG sequences was problematic due to the high taxonomic diversity of nitrate-reducing prokaryotes and to the low degree of conservation of this gene between Proteobacteria and gram-positive bacteria (22). In a recent study, another set of primers for the narG gene has been described (11). However a nested PCR was required for successful amplification and only six different clones were retrieved, all of them related to the narG of Escherichia coli. The primers described in this study and designed to amplify the narG gene from Proteobacteria, gram-positive Bacteria, and even Archea have allowed the amplification of the target gene in a direct PCR. The amplified fragment was chosen in a conserved region of molybdopterin guanine dinucleotide dependent enzymes (2). A nonspecific amplification has been observed in our study with 12 of 132 sequences showing no significant identity with NarG. However, each of these sequences was present only in one or two clones; thus, only 14 of the 472 clones of our gene libraries did not match with NarG. Nitrate-reducing prokaryotes possessing at least the membrane-bound nitrate reductase were targeted in this analysis, excluding nitrate-reducing prokaryotes possessing only the periplasmic nitrate reductase or an unknown nitrate reductase. However, the membrane bound nitrate reductase is more widely spread among microorganisms, the periplasmic one being restricted to the gram-negative bacteria. Moreover, the use of narG is of special interest, because the phylogeny of the narG functional genes reflects the 16S rRNA phylogeny of the organisms from which the gene sequences were retrieved (20). Therefore, retrieval of narG gene sequences can provide valuable information on the phylogenetic affiliation of these organisms in the environment. By targeting the narG gene, this study provides for the first time a unique insight into the structure of the nitrate-reducing community in planted and unplanted soils by use of a molecular approach.
In a culture-based study on the composition of the nitrate-reducing community, Nijburg et al. (17) suggested the presence of a specific nitrate-reducing community for each environment. In the sediment samples from the rhizosphere of the aerenchymatous plant Glyceria maxima, Bacillus, Moraxella, and Pseudomonas species were isolated (17). Enterobacteriacea, Vibrio, and Aeromonas were identified in another sediment (4). A larger diversity was observed by Shirey and Sextone (25) in abandoned and reclaimed mine soils with nitrate-reducing isolates identified as Streptomyces, Bacillus, and Enterobacteriacea strains. Finally Cheneby et al. (6) reported the isolation of Bacillus, Streptomyces, Pseudomonas, Burkholderia, and Xanthomonas species from three different soils. In the nested-PCR-based study of Gregory et al. (11), the six narG sequences detected in a clone library established from freshwater surface sediment were tightly clustered and related to the E. coli sequence. Our narG clone libraries provided a much higher phylogenetic diversity of the nitrate-reducing community than the previous studies provided. Only few clones showed high levels of amino acids identity (>90%) to sequences of known NarG from cultivated bacteria. However, it should be stressed that two copies of the narG gene have been identified in some bacterial genomes such as E. coli, Salmonella enterica serovar Typhi, and Burkholderia pseudomallei. Sequencing of the genome of Streptomyces coelicolor has even revealed the presence of three copies of this gene. Phylogenetic analysis showed that these narG copies are not always closely related (Fig. 5) (20). Consequently, diversity data of the narG gene have to be interpreted with caution. The tree obtained from sequence analysis can be divided into nine major clusters. Clones from many bacterial divisions, i.e., the Bacillus-Staphylococcus group, Actinobacteria, α, β, and γ Proteobacteria, were found to be related to NarG. Some clones are even related to NarG of the extremophile bacterium Thermus thermophilus. In contrast, clusters 4, 8, and 9 are related to only one known narG gene, indicating that an important proportion of uncharacterized dissimilatory nitrate reducers are present in the studied soil. In contrast to culture-based studies and despite the high number of clones analyzed, few sequences related to NarG of gamma Proteobacteria were recovered. This suggests either that the soil used in this study contained few gamma Proteobacteria or that the previous culture-based experiments selected fast-growing, cultivable heterotrophs belonging to the Proteobacteria divisions, resulting in overestimation of this group.
While no differences in the genetic structure of the total bacterial community were observed by RISA analysis, focusing on the nitrate-reducing community revealed a shift in the structure of this community between the unplanted and planted soils (Fig. 2). Therefore, it appears that under some functional or ecological aspects, genes encoding key enzymes such as the membrane bound nitrate reductase could be better targets for fine-scale resolution of microbial diversity than the intergenic spacer region between the small and large subunit of the ribosomal genes. Analysis of the clone libraries by RFLP confirmed that the diversity of the planted soil was different from that of the unplanted soil (Fig. 3). Interestingly, sequence analysis of the narG clone libraries obtained from the unplanted and planted soils showed that most of the RFLP types dominant in the rhizosphere or present only in the rhizosphere are related to NarG of Actinobacteria (Fig. 3 and 5). In silico restriction analysis of the narG sequences from Actinobacteria resulted in fragments having sizes identical to the dominant bands observed only in the planted soil (Fig. 2). Thus, comparison of the narG restriction profiles of the PCR products obtained from DNA extracted from soil and from the clone libraries indicated that no important bias occurred during the cloning step. These data are in accordance with the results of Miller et al. (15), who found 10 times more actinomycetes in wheat rhizosphere than in unplanted soil. More recently, after finding that a high proportion of dominant populations in the rhizosphere belonging to high-G+C gram-positive bacteria, Smalla et al. underlined that high G+C gram-positive bacteria might be more dominant in the rhizosphere than previously suggested (27). Our data provide evidence for the assumption that plants could select bacterial groups within a functional community in the proximity of their roots.
Measuring Simpson and Shannon-Weaver indices among the clone libraries did not show differences between unplanted and planted soil samples, indicating that the diversity was in fact similar in the planted and unplanted soils. Thus, our results suggest that the structure of the nitrate-reducing community has been modified by the maize roots but without significant impact on the diversity.
These differences in the nitrate-reducing community composition could be ascribed to a selective advantage of some strains over the others in the rhizosphere. The ability to dissimilate NO3− could contribute to explain the differences in the composition of this community between unplanted and planted soils. It has been demonstrated in previous studies that strains able to dissimilate nitrogenous oxide exhibited a higher rhizosphere competence (10, 21). However, other bacterial traits such as the ability to use specific carbon compounds present in the root exudates could also contribute to explain the observed differences. Further studies are also required to determine whether the nitrate concentration, or the oxygen partial pressure, or the exudation of carbon substrate, or a combination of these parameters is important for the composition of the nitrate-reducing community.
In modeling the denitrifying activity and N2O emission, it is often assumed that the underlying denitrifying microorganisms in soils are identical in terms of electron donor, induction of the enzyme synthesis or of the specific activity of the enzymes catalyzing the different denitrifying steps. It has been recently demonstrated that global trace gas emissions can be affected by changes in the community composition, indicating that studies on the structure of functional communities are required to understand and to model fluxes (12). Using a molecular approach based on the narG gene, this study provided insight into the structure of the nitrate-reducing community, which performs the first step of the denitrification pathway. However, a gene sequence indicates the potential presence of the corresponding function, but there is evidence that this potential is not always converted to an actual metabolic role. Therefore, information is needed not only on the structure of communities genetically able to denitrify but also on how and when these denitrifying genes are expressed within these communities. The development of protocols to extract mRNA in the environment can provide a starting point for novel approaches to the study of gene expression within a functional community.
Acknowledgments
We thank G. Laguerre for her helpful comments on diversity and statistical analysis.
This work was supported by the PNSE program of the Institut National des Sciences de l'Univers and the Burgundy region program B03725.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Berks, B. C., S. J. Ferguson, J. W. B. Moir, and D. J. D. Richardson. 1995. Enzymes and associated electron transports systems that catalyse the respiratory reduction of nitrogen oxides and oxyanions. Biochim. Biophys. Acta 1232:97-173. [DOI] [PubMed] [Google Scholar]
- 3.Braker, G., H. L. Ayala-del-Rio, A. H. Devol, A. Fesefeldt, and J. M. Tiedje. 2001. Community structure of denitrifiers, bacteria, and archaea along redox gradients in Pacific Northwest marine sediments by terminal restriction fragment length polymorphism analysis of amplified nitrite reductase (nirS) and 16S rRNA genes. Appl. Environ. Microbiol. 67:1893-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunel, B., J. D. Janse, H. J. Laanbroek, and J. D. Woldendorp. 1992. Effect of transient oxic conditions on the composition of the nitrate-reducing community from the rhizosphere of Typha angustipholia. Microbiol. Ecol. 24:51-61. [DOI] [PubMed] [Google Scholar]
- 5.Bruns, M. A., J. R. Stephen, G. A. Kowalchuk, J. I. Prosser, and E. A. Paul. 1999. Comparative diversity of ammonia oxidizer 16S rRNA gene sequences in native, tilled, and successional soils. Appl. Environ. Microbiol. 65:2994-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheneby, D., L. Philippot, A. Hartmann, C. Henault, and J. C. Germon. 2000. 16S rDNA analysis for characterization of denitrifying bacteria isolated from three agricultural soils. FEMS Microbiol. Ecol. 34:121-128. [DOI] [PubMed] [Google Scholar]
- 7.Conrad, R. 1996. Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO). Microbiol. Rev. 60:609-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duineveld, B. M., A. S. Rosado, J. D. van Elsas, and J. A. van Veen. 1998. Analysis of the dynamics of bacterial communities in the rhizosphere of the chrysanthemum via denaturing gradient gel electrophoresis and substrate utilization patterns. Appl. Environ. Microbiol. 64:4950-4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Excoffier, L., P. E. Smouse, and J. M. Quattro. 1992. Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 134:479-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghiglione, J. F., F. Gourbiere, P. Potier, L. Philippot, and R. Lensi. 2000. Role of respiratory nitrate reductase in ability of Pseudomonas fluorescens YT101 to colonize the rhizosphere of maize. Appl. Environ. Microbiol. 66:4012-4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gregory, L. G., A. Karakas-Sen, D. J. Richardson, and S. Spiro. 2000. Detection of genes for membrane-bound nitrate reductase in nitrate-respiring bacteria and in community DNA. FEMS Microbiol. Lett. 183:275-279. [DOI] [PubMed] [Google Scholar]
- 12.Holtan-Hartwig, L., P. Dörsch, and L. R. Bakken. 2000. Comparison of denitrifying communities in organic soils: kinetics of NO3− and N2O reduction. Soil Biol. Biochem. 32:833-843. [Google Scholar]
- 13.Kowalchuk, G. A., A. W. Stienstra, G. H. Heilig, J. R. Stephen, and J. W. Woldendorp. 2000. Molecular analysis of ammonia-oxidising bacteria in soil of successional grasslands of the Drentsche A (The Netherlands). FEMS Microbiol. Ecol. 31:207-215. [DOI] [PubMed] [Google Scholar]
- 14.Mahmood, T., R. Ali, K. A. Malik, and S. R. A. Shamsi. 1997. Denitrification with and without maize plant (Zea mays L.) under irrigated field conditions. Biol. Fertil. Soils 24:323-328. [Google Scholar]
- 15.Miller, H. J., E. Liljeroth, G. Henken, and J. A. van Veen. 1990. Fluctuations in the fluorescent pseudomonad and actinomycete populations of rhizosphere and rhizoplane during the growth of spring wheat. Can. J. Microbiol. 36:254-258. [Google Scholar]
- 16.Moreno-Vivian, C., P. Cabello, M. Martinez-Luque, R. Blasco, and F. Castillo. 1999. Prokaryotic nitrate reduction: molecular properties and functional distinction among bacterial nitrate reductases. J. Bacteriol. 181:6573-6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nijburg, J., M. J. L. Coolen, S. Gerards, P. J. A. K. Gunnewiek, and H. J. Lannbroek. 1997. Effects of nitrate availability and the presence of Glyceria maxima on the composition and activity of the dissimilatory nitrate-reducing bacteria community. Appl. Environ. Microbiol. 63:931-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nusslein, K., and J. M. Tiedje. 1998. Characterization of the dominant and rare members of a young Hawaiian soil bacterial community with small-subunit ribosomal DNA amplified from DNA fractionated on the basis of its guanine and cytosine composition. Appl. Environ. Microbiol. 64:1283-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nusslein, K., and J. M. Tiedje. 1999. Soil bacterial community shift correlated with change from forest to pasture vegetation in a tropical soil. Appl. Environ. Microbiol. 65:3622-3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Philippot, L. 2002. Denitrifying genes in bacterial and archeal genomes. Biochim. Biophys. Acta 1 577:355.. [DOI] [PubMed] [Google Scholar]
- 21.Philippot, L., C. Clays-Josserand, and R. Lensi. 1995. Use of Tn5 mutants to assess the role of the dissimilatory nitrite reductase in the competitive abilities of two Pseudomonas strains in soil. Appl. Environ. Microbiol. 61:1426-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Philippot, L., and O. Hojberg. 1999. Dissimilatory nitrate reductases in bacteria. Biochim. Biophys. Acta 1446:1-23. [DOI] [PubMed] [Google Scholar]
- 23.Prade, K., and G. Trolldenier. 1988. Effect of wheat roots on denitrification at varying soil air-filled porosity and organic-carbon content. Biol. Fertil. Soils 7:1-6. [Google Scholar]
- 24.Shannon, C., and W. Weaver. 1963. The mathematical theory of communication, 5th ed. Urbana University of Illinois Press, Chicago, Ill.
- 25.Shirey, J. J., and A. J. Sextone. 1989. Denitrification and nitrate-reducing bacterial populations. FEMS Microbiol. Ecol. 62:59-70. [Google Scholar]
- 26.Simpson, E. H. 1949. Measurement of diversity. Nature 163:688. [Google Scholar]
- 27.Smalla, K., G. Wieland, A. Buchner, A. Zock, J. Parzy, S. Kaiser, N. Roskot, H. Heuer, and G. Berg. 2001. Bulk and rhizosphere soil bacterial communities studied by denaturing gradient gel electrophoresis: plant-dependent enrichment and seasonal shifts revealed. Appl. Environ. Microbiol. 67:4742-4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith, S., and J. M. Tiedje. 1979. The effect of roots on soil denitrification. Soil Sci. Soc. Am. J. 43:951-955. [Google Scholar]
- 29.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 24:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiedje, J. M. 1988. Ecology of denitrification and dissimilatory nitrate reduction to ammonium, p. 179-244. In A. J. Zehnder (ed.), Biology of anaerobic microorganisms. John Wiley & Sons, Inc., New York, N.Y.
- 31.Trolldenier, G. 1988. Plant nutritional and soil factors in relation to microbial activity in the rhizosphere, with particular emphasis on denitrification. Z. Pfanzenernähr. Boden. 152:223-230. [Google Scholar]
- 32.Zhou, J., M. E. Davey, J. B. Figueras, E. Rivkina, D. Gilichinsky, and J. M. Tiedje. 1997. Phylogenetic diversity of a bacterial community determined from Siberian tundra soil DNA. Microbiology 143:3913-3919. [DOI] [PubMed] [Google Scholar]