The massive quantities of phytoplankton in the North Atlantic and Antarctic oceans producing dimethylsulfoniopropionate (DMSP) as an osmoprotectant, much of which is degraded by marine bacteria to dimethylsulfide (DMS), ensures an important role for both compounds in the global sulfur cycle. The closest to a comprehensive review on this topic is a book of symposium proceedings edited by Kiene et al. (75); the more recent developments related specifically to DMSP degradation by microbial communities are found elsewhere (68). This article is more comprehensive, as it includes some of the earlier literature in describing the sources of DMSP, its release and linkage to the marine (primarily microbial) food web and subsequent degradation via cleavage to DMS and acrylic acid or demethylation and demethiolation to methanethiol.
DMS production from DMSP has long been associated with marine algae according to the following reaction (20, 22):
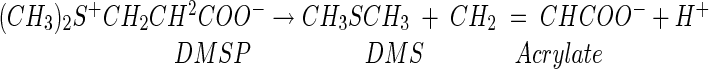 |
(1) |
DMSP is a tertiary sulfonium compound produced in high concentration by certain species of marine algae and plant halophytes for the regulation of their internal osmotic environment (1, 41, 47, 120), although its role in plants remains unclear. This alga-associated, i.e., particulate DMSP (DMSPp), when released into the marine environment as dissolved DMSP (DMSPd), can serve as a link between primary production and the microbial population, as it is readily degraded by chemoheterotrophic bacteria (59). DMSP turnover usually exceeds DMS production in natural waters (60) because DMSP is also demethylated to 3-methiolpropionate, which can be further demethylated to 3-mercaptopropionate or demethiolated, releasing methanethiol (72, 118). These reactions will be discussed in more detail below.
The biogeochemical significance of DMSP cleavage was first suggested in 1972, when DMS was found to be universally present in seawater and emitted at a significant rate to the atmosphere (87). It was proposed that DMS, rather than H2S from coastal waters and mud flats, was the missing gaseous sulfur compound needed to enable the steady-state flow of sulfur between marine and terrestrial environments, making DMS emissions a key step in the global sulfur cycle (87). Atmospheric H2S, which arises primarily from dissimilatory sulfate reduction in organic matter-rich environments, could never be measured in sufficient quantity to be the vehicle for transferring large quantities of sulfur from sea to air to land. The total annual flux of biogenic DMS released to the atmosphere ranges from 28 to 45 Tg of S year−1, at least 10-fold higher than from all other sources (Table 1). Recent, more comprehensive calculations of global annual DMS flux from the oceans gave values that ranged from 13 to 37 Tg of S year−1 (57). This sea-to-air flux represents about 50% of the global biogenic sulfur flux to the atmosphere (3). However, anthropogenic sulfur emissions dominate the sulfur flux, representing 80 to 90% of the input to the global sulfur cycle (12, 23, 88).
TABLE 1.
Estimates of natural emissions of organosulfur compoundsa
| Source | Sulfur compounds releasedb (Tg of S yr−1)
|
|||
|---|---|---|---|---|
| DMS | COS | DMDS | CS2 | |
| Ocean | 27-40 (13-37)c | 0.4 | 0-1 | 0.3 |
| Salt marsh | 0.6 | 0.12 | 0.1 | 0.1 |
| Freshwater swamp | 0.8 | 1.85 | 0.2 | 2.8 |
| Soils and plants | 0.2-0.4 | 0.2-1.0 | 1 | 0.6-1.5 |
| Total | 28.6-45.5 | 2.57-3.37 | 0-2.3 | 0.1-4.7 |
Modified from Kelly and Smith (56), who used data from Andreae (3, 4), and Steudler and Peterson (112) with permission. The factor of uncertainty with these types of data is believed to be about 2.
COS, carbonyl sulfide; DMDS, dimethyl disulfide; CS2, carbon disulfide.
Data from recent updated data sets and flux models of Kettle and Andreae (57) are shown in parentheses.
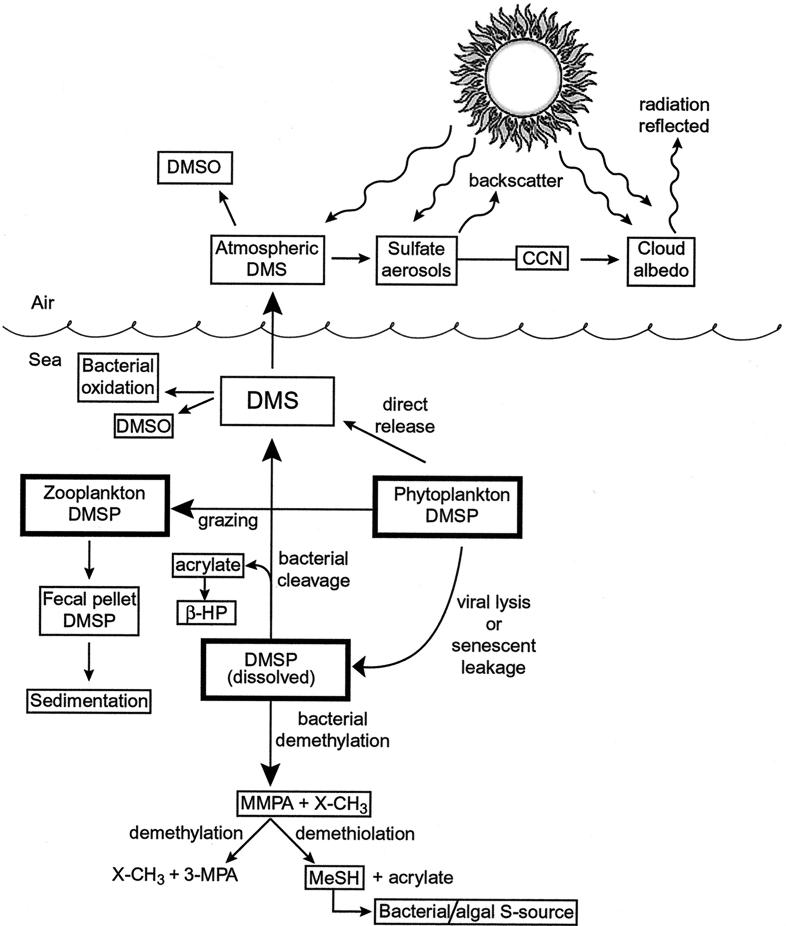
The magnitude of the marine DMS emissions is all the more remarkable considering that over half of the DMSP released is demethylated (68) and that a significant fraction of the DMS is oxidized by bacteria in the water column before it can be released to the atmosphere (13, 64). While most of the biogenic sulfur emissions (primarily DMS) come from the oceans, those coming from salt marshes and coastal wetlands are many times higher on a unit area basis (112). DMS flux per unit area from these marine wetlands is also much higher than from any known terrestrial soil (2). The biogeochemical cycling of DMSP and its biological degradation products are shown in Fig. 1.
FIG. 1.
Scheme representing the mechanisms of DMSP and DMS cycling in the marine water column and atmosphere. DMSO, dimethyl sulfoxide; CCN, cloud-condensing nuclei; MMPA, 3-methiolpropionate; β-HP, β-hydroxypropionate; 3-MPA, 3-mercaptopropionate; MeSH, methanethiol; X-CH3, unidentified molecule with a terminal methyl group.
MARINE DMS EMISSIONS AND CLIMATE
Microbe- (and phytoplankton)-generated DMS resulting from DMSP cleavage not only serves to complete the global sulfur cycle; it may also have an impact on the earth's climate, at least in remote oceanic areas (3, 4). Atmospheric DMS is rapidly oxidized to acidic aerosol sulfates via methanesulfonic acid (CH3SO3H) and sulfur dioxide (SO2) (see review by Malin [88]). These sulfur-containing aerosols serve as “cloud-condensing nuclei” which can absorb and scatter incoming radiation, altering the global radiation budget (24), before falling to earth as acid rain. Based on model calculations, it has been postulated that the link between DMS generated by biological communities and sulfate aerosols acting as cloud-condensing nuclei reducing the amount of sunlight that reaches the earth's surface could (theoretically) decrease the global mean temperature (3, 23, 24). Such a phenomenon would have a negative feedback affect on the marine community that generates the DMS (24, 88, 89).
SOURCES OF DMSP
Micro- and macroalgae and halophytic plants are abundant sources of DMSP in the marine environment (22, 48, 81). The synthesis of DMSP originates from methionine in both plants and algae, but the pathways differ from there. The pathway in Spartina alterniflora is as follows: methionine → S-methylmethionine → DMSP-amine → DMSP-aldehyde → DMSP (76). Algae appear to synthesize DMSP as follows: methionine → 4-methylthio-2-oxobutyrate → 4-methylthio-2-hydroxybutyrate → 4-dimethylsulfonio-2-hydroxybutyrate → DMSP (42). Only a limited number of species of algae and halophytes are capable of DMSP biosynthesis (28, 29, 54, 55), and its concentration can vary considerably from one species to another (55).
In a recently studied Phaeocystis spring bloom, the DMSPp concentration in the water column averaged about 500 nM and spiked to 1,650 nM at its peak (129); in this case, algal biomass showed a positive overall correlation with DMS production. Blooms of this magnitude, however, are not typical in marine waters. In other locations and times, the correlation between algal chlorophyll a and DMSP/DMS concentrations in seawater were seen to be inconsistent (3, 83, 90, 119). Andreae (3) examined DMS and chlorophyll a data from many locations and reported “values of r2 near 0.3, which, because of the large number of data (over 1,000 measurements), are highly significant.” However, as he pointed out, this is not a simple relationship, as the correlation explained only about 30% of the variability and suggested “a complicated interplay of algal speciation and trophic interactions.” Although sophisticated methods of relating certain pigments to specific groups or classes of algae could theoretically be useful in identifying DMSP-rich phytoplankton taxa in situ, this proved unsuccessful in one study, leading to the suggestion that DMSP may be present in species that do not have common algal pigments (27).
Phytoplankton that contain the highest cytosolic levels of DMSP are shown in Table 2; they are the small dinoflagellates (<10 μm), prymnesiophytes (mainly coccolithophores), and chrysophytes. Only those hyperproducing species were chosen for Table 2 to emphasize that high concentrations of DMSP would probably exist in the seawater around these cells following their disruption by grazing or senescence, providing a substantial level of dissolved organic carbon for heterotrophic bacteria. In the gigantic coccolithophore blooms found in the North Sea, DMSP can reach 10% of the total carbon fixed (8). Similar amounts of carbon fixed were shown to go into DMSP in the <2-μm fraction from the North Atlantic (106). The chrysophytes and prymnesiophytes are in this size range, and since they often occur in high concentrations in various areas of the ocean, DMSP may also be an important source of carbon in the microbial food web in those localized areas of the ocean. Other marine sources of DMSP are shown in Table 3.
TABLE 2.
Intracellular concentration of DMSP in marine phytoplankton
| Organism | Cytosolic DMSPa (mM) | Reference |
|---|---|---|
| Hymenomonas carterae | 120 | Vairavamurthey et al. (120) |
| Gymnodinium nelsoni | 280 | Dacey and Wakeham (30) |
| Platymonas subcordiformis | 170 | Dickson and Kirst (40) |
| Phaeocystis sp. | 71-169 | Stefels and van Boekel (109) |
| Melosira numuloides (Diatoms) | 264 | Keller et al. (55) |
| Chrysameoba sp. (chyrosphyte) | 596 | |
| Ochromonas sp. (chrysophyte) | 529 | |
| Prorocentrum sp. strain IIB2b1 (dinoflagellate) | 1,082b | |
| Emiliania huxleyi BT6 (coccolithophore) | 166 | |
| Diatoms (from sea water) | 38 ± 18 | Turner et al. (119) |
| <10-μm flagellates (from sea water) | 125 | Belviso et al. (15) |
| 650 ± 429 | Turner et al. (119) | |
| Dinoflagellates (from sea water) | 640 | Belviso et al. (15) |
| 70 ± 30 | Turner et al. (119) |
Per liter of cell volume.
A reviewer of this article pointed out that “seawater osmolarity is about 0.7 M, and DMSP is never the only osmolyte, so its intracellular concentrations can't be >1 M.”
TABLE 3.
Other marine sources of DMSP
| Source | DMSP concn (mM) | Reference(s) |
|---|---|---|
| Spartina alterniflora | 20-70a | Dacey et al. (29) |
| Copepod fecal pellets | 0.17b | Tang (115) |
| 33c | Kwint et al. (77) | |
| Microbial matd | 0.85 | Jonkers et al. (53) |
| Sea water (soluble DMSP)e | 5-290 | Turner et al. (119) |
| Belviso et al. (15) |
Based on the assumption that wet weight was all due to cell cytoplasmic water.
Acartia tonsa pellets after 24 h.
Eurytemora affinis, fresh pellets.
Primarily surface diatoms. The value is based on mat volume.
From North Atlantic and Mediterranean surface waters. Values are micromolar.
Benthic diatoms are also DMSP producers and appeared to be the major source of DMSP in microbial mats found in salt marshes (53). In the summer months, these mat surfaces contained 550 to 850 μM DMSP (based on sediment volume), which was a source of DMS under anoxic-dark conditions and served as a sink for DMS under oxic-light conditions (53, 122). While marine microbial mats are rich in cyanobacteria, the cyanobacteria themselves produce negligible amounts of DMSP (53, 55). A few species of freshwater cyanobacteria produce low but measurable levels of DMS in culture (92) and on alkali treatment (21, 22); the latter process cleaves DMSP, releasing DMS that is easily measured by gas chromatography. These methods of DMSP analysis are indirect, and the occurrence of DMSP in cyanobacteria needs to be proved by more rigorous methods.
One of the most unusual sources of DMS is that emitted from a benthic flatworm found in intertidal mudflats, Convoluta roscoffensis, which contains an algal symbiont, Tetraselmis sp. (121). These worms aggregate in dense colonies in the surface sediments during receding tides while emitting large amounts of DMS. DMSP was present in high levels in sediments containing the worms. Pure cultures of the alga showed that it had high concentrations of DMSP but no DMSP lyase activity, leading to the deduction that worm tissue contained both DMSP and the DMSP lyase activity. Although the role of microbes could not be excluded, it appeared that they did not contribute significantly to the DMS production observed in these worm-populated sediments (121).
Cytosolic DMSP in algae is physically separated from the DMSP lyase(s), as suggested by the fact that healthy populations produce little DMS (109, 119, 136). DMSP lyases in phytoplankton that are membrane bound are presumably not in contact with cytosolic DMSP. These phytoplankton lyases were tabulated by Steinke et al. (111) and included species of Phaeocystis, Polysiphonia, and Ulva. Several Emiliania huxleyi isolates are claimed to have membrane-bound lyase activity, but the evidence presented was that only a fraction of the total activity (4 and 54% in two different isolates) appeared in the supernatant after high-speed centrifugation (111). The apparent assumption was that the remainder of the activity was in the pellet (i.e., membrane bound).
It appears that the DMSP lyase in all phytoplankton species, including E. huxleyi, is expressed constitutively, but its characteristics in isolates of the latter were quite different from one another, suggesting they were isoenzymes with “possibly different cellular locations and functions and varying DMS production under natural conditions” (111). DMSP lyases have been purified from two different species of macroalgae, the red alga Polysiphonia paniculata (113) and the green alga Ulva curvata (34). Both species yielded several lyase proteins of different molecular weights. The major enzyme components purified from these species differed considerably in their Kms for DMSP; the Kms of the Polysiphonia lyase was 73 μM, while that of the Ulva lyase was 520 μM. The specific activity of the Polysiphonia enzyme remains uncertain, as the values calculated from the figures differed from those in the text by 150-fold (100).
Although most, but not all (99), DMSP-producing species of phytoplankton have DMSP lyase activity, its physiological role remains unknown. Bacteria are generally thought to dominate DMS production (60), but recent data suggested that within aging E. huxleyi blooms at some North Atlantic sites where the photosynthetic dinoflagellates biomass was greater than that of the coccolithophores, 75 to 80% of the total DMSP lyase activity was associated with the dinoflagellates (110). Bacterium-sized particles at these sites had <5% of the total DSMP lyase activity. This is complicated, however, because of the possibility of particle-attached bacteria having DMSP lyase activity.
Higher plants that produce the highest levels of DMSP are generally marine, salt-tolerant species that include the salt marsh cordgrass Spartina alterniflora (29), a Pacific beach plant, Wollastonia biflora (113), and sugarcane (103), the only nonmarine exception. In the fall, dissolved DMSP (DMSPd) leaches from S. alterniflora leaves into the creek waters (102). While nothing is known about the microbial degradation of and growth on the DMSP associated with these plants, it can be surmised that both chemoheterotrophic parasites (endophytes and epiphytes) and saprophytes make use of this plentiful, energy-rich monomeric substrate.
While there is no information on DMSP-utilizing bacterial epiphytes, there is evidence that fungi involved in decay of Spartina cordgrass are capable of using the cytosolic DMSP, estimated to be 20 to 70 mM (Table 2). Of 11 species of ascomycete fungi, which are the predominant decomposers of DMSP-producing salt marsh grasses, all but 2 had DMSP lyase activity and could grow in vitro on DMSP. Conversely, only 3 of 21 fungal species that decompose non-DMSP-producing halophytes had DMSP lyase activity (11). These data suggested coevolution of the DMSP-utilizing fungi and the DMSP-producing halophyte. Finally, there is only speculation as to the function of DMSP in these marsh plants. While it may be presumed to be related to osmotic regulation (29), an experiment designed to test this hypothesis yielded negative results in S. alterniflora and related species (reference 101 and references therein).
LINKING PHYTOPLANKTON DMSP TO THE MICROBIAL FOOD WEB
The bulk of the DMS produced on this planet comes from DMSP produced and stored in marine phytoplankton (DMSPp), and therefore processes that release it are critical to understanding DMS production. DMSPd, which is a constituent of dissolved organic carbon, ranges from 5 to 50 nM, with concentrations of >100 nM in blooms of DMSP-producing algae. DMSPd leaches from phytoplankton during stress, senescence, viral lysis, microbial attack, and grazing by zooplankton (17, 123, 135); its flow to marine heterotrophs constitutes a part of the microbial loop (10, 104). Since DMSP comprises 1 to 10% of the carbon in some algal species (91), it is assumed to be an important part of the dissolved organic carbon available to bacteria. The strong emissions of DMS from the water column and the appearance of DMSP demethylation products in anoxic sediment pore waters (72, 94) indicate that DMSP is a readily metabolizable form of dissolved organic carbon, with all the products of its degradation used as microbial substrates (104).
With some assumptions relating to assimilation efficiency, it was estimated that DMSPd released from North Atlantic and North Sea phytoplankton (coccolithophore) blooms was of sufficient quantity to support over 5% (range, 1 to 15%) of the microbial carbon demand (106, 140). DMSPd turnover in the Gulf of Mexico supported a slightly lower percentage of microbial growth (67). In addition, DMSP contributed nearly all the sulfur requirement to the microbes at these locations. No other single compound is known to contribute so much carbon and sulfur to the microbial food web. This is perhaps not surprising, because the concentration of cytosolic DMSP in some algal species is very high (ca. 0.5 M), with many in the range of 100 to 200 mM (Table 2), and DMSP is a readily metabolizable monomer requiring no previous hydrolytic enzyme activity to release it. The importance of DMSP-sulfur in the microbial food web (69) is discussed below. The conclusion is that DMSP is intimately linked to the pelagic food web and the microbial loop and could be a key to culturing some of the currently unculturable marine microbes.
The coupling between phytoplankton bloom formation and the resulting increase in bacterial biomass in numerous studies usually showed a distinct delay of several days (references 16 and 123 and references therein). Similarly, DMS emissions lagged behind the collapse of diatom blooms (by 2 to 10 days) as seen in large-scale mesocosm experiments (78, 91), but could also occur at the peak of the algal bloom (63, 79, 129). As the mesocosm experiments were carried out in the absence of grazing by zooplankton predators, these workers concluded that senescence-related cell lysis was responsible for release of the DMS. Senescence was also attributed to the release of DMSPd and DMS in natural assemblages of phytoplankton (90) and in experiments with axenic Phaeocystis cultures (109). The latter was taken to suggest that algal DMSP lyase “may contribute significantly to DMS production from DMSPp during bloom situations in the field.” This and other studies (19) indicate that bacteria accompanying phytoplankton blooms, such as these high DMSPp-containing colonial forms, are not responsible for all the DMS produced during senescence. Finally, evidence indicates that phytoplankton senescence and the concomitant DMS production are linked to nutrient deficiency (97), which is believed to be responsible for the “crash” of most blooms.
DMSP released by zooplankton grazing.
Zooplankton (dinoflagellates, ciliates, copepods, and krill) are known to process a large fraction (10 to 25%, or higher) of the daily oceanic primary production (80, 114), but their role in the DMSP recycling processes is still not fully understood. Laboratory studies by Dacey and Wakeham (30) were the first to show that marine copepods grazing on Gymnodinium nelsoni greatly stimulated DMS production, and other workers have made similar observations (15, 25, 31, 82). In surface waters of the North Sea, Archer et al (8) found that 16 to 43% of the DMSPp pool turned over daily due to zooplankton grazing of coccolithophore blooms. However, the importance of grazing is debated, as others found that the distribution of DMS was not statistically related to the abundance of zooplankton (copepods and ciliates) in the water column and that it was probably not important to the biogeochemistry of DMS (18, 25, 77, 82, 116).
In one study, grazing of DMSP-rich Phaeocystis spp. removed <1% of the standing stock in a spring bloom day−1 (123) because, it appears, its colonies reach 2 mm in size and are too large for predation by most zooplankton. Copepods do, however, repackage some unknown quantity of phytoplankton biomass, including DMSP, in the gut as fecal pellets (14, 25, 30, 77, 82, 116), making it readily available to bacteria. Copepod bodies contain micro- to millimolar concentrations of DMSP (116), presumably much of it in fecal pellets; 33 mM was measured in fresh pellets in one experiment (77). Tang (115) believes the rapid diffusion of DMSPd from zooplankton fecal pellets has been incorrectly described as “sloppy grazing.” Although the gut contents of grazing zooplankton are rich in DMSP, relatively little DMS is produced (8, 18, 77, 131). This could result if (i) the DMS produced was rapidly consumed as an energy source by other gut bacteria (64, 33), (ii) the DMSP was demethylated or demethiolated (62, 118), or (iii) other soluble, nonvolatile DMSP degradation products were produced, as suspected (67).
Some DMSPp is used by microzooplankton as a carbon source, but it appears to be used primarily as their sulfur source (8). Taken together, the DMSP/DMS sinks are many and varied, which appears to explain why so little of the total DMSP produced by grazed phytoplankton ends up as atmospheric DMS. The rapidity with which DMSP in the fecal pellets is metabolized is inferred from the fact that most pellets sink rapidly (50 to 1,000 m day−1) (52), yet little or no DMS(P) was measured beneath the euphotic zone (13, 90). Both DMSP and DMS were also closely associated with the algal biomass in a shallow coastal salt pond (133). All indications point to little fecal pellet DMSPp escaping the phytoplankton-rich zone in the water column. Finally, DMSP, DMS, and acrylate also get into the microbial food web via the gut contents of some fish, turtles, and birds that feed on either DMSP-rich phytoplankton, epiphytized reef plants, or krill (reference 28 and references therein).
BACTERIAL DEGRADATION OF DMSP
Since most DMS produced on earth originates from marine sources, it is somewhat ironic that the first bacterial isolate capable of producing DMS from DMSP came from a freshwater river sediment. That isolate, an anaerobe resembling Clostridium propionicum, grew by fermenting DMSP to DMS, propionate, acetate, CO2, and a proton (132). Anaerobic bacteria in marine sediments also produced these products when amended with acrylate (73). Alternatively, a newly discovered marine sulfate reducer, Desulfovibrio acrylicus, cleaved DMSP to DMS and acrylate and used the acrylate as a terminal electron acceptor (127). Anaerobic bacteria in Spartina salt marsh sediments can use DMSP and acrylate as a source of energy for growth and N2 fixation (36). However, microbes with this capability appeared to be unique to this environment (salinity of 17 to 24 ppt and 10 to 15 nM DMSP in the sediment porewater); they could not be detected in marsh sediments along brackish waters (salinity below 8 ppt and no DMSP).
The first (59, 118) and all subsequent aerobic DMS-producing isolates have been assumed to cleave DMSP similar to algae (see equation 1 above), but acrylate has apparently only been demonstrated to be a product of this reaction in anoxic sediments (72) and the anaerobe D. acrylicus (124). It may be that no one has looked for acrylate production concomitant with DMSP cleavage by aerobes, but its production remains to be assumed. In this regard, 13C-labeled DMSP uptake could be demonstrated in a cultured DMS producer, but labeled acrylate could not be detected (6, 7). From the results of a more extensive study (discussed below), it was surmised that acrylate turned over rapidly, resulting in low, undetectable pool sizes. DMS-producing aerobes do, however, grow equally well on DMSP and acrylate as the only source of carbon (38, 59, 86, 118), and both molecules are equivalent as inducers of DMSP lyase activity (35, 83), all consistent with acrylate's being produced along with DMS.
Microbial production of DMS from either naturally occurring or added DMSPd has been well documented in both oceanic and estuarine waters (59, 71, 118) and salt marsh sediments (65, 74). Marine sediments produce approximately 1,000-fold more DMSP and DMS than the overlying water (96), with rates of gaseous sulfur emissions being 10- to 100-fold higher (112). Consistent with these findings, most-probable-number analyses of aerobic DMS producers in sediments from a Spartina salt marsh and sea grass-dominated intertidal zone were 106 to 107 (ml of sediment)−1 (53, 138) compared to 103 to 105 ml−1 in seawater (98, 119). Nevertheless, the relatively small area of salt marshes and near-shore sediments compared to the oceans greatly reduces the relevance of these sediments in the global biogeochemistry of DMS and the sulfur cycle.
As phytoplankton blooms decline, bacteria attached to these cells were seen to increase relative to the number of free-living bacteria present in the samples (10, 16, 93). For example, Phaeocystis epiphytes were eightfold more numerous than those surrounding the algal cells (99). The number of these epiphytes that were DMSP utilizers is unknown. In another study, phytoplankton particles >20 μm in size had high rates of DMS production (from added DMSP) compared to 0.7- to 2-μm-sized particles (free-living bacteria), which were negligible (19). The higher rates of DMS production by the larger particles were due either to attached bacteria or to the size of the phytoplankton cells; the researchers speculated that it might be due to attached bacteria. Until an inhibitor that can distinguish between the DMSP lyases of bacteria and phytoplankton is identified, there is no apparent way to assess the relative contribution of each, which is one of the important unanswered questions concerning the biogeochemistry of DMS.
DMSP-consuming bacteria are also found attached to or associated with copepods. Microbial most-probable-number analysis of Acartia tonsa that had been homogenized showed >104 DMSP consumers per animal after feeding on DMSP-containing algae (117). This number was estimated to be about 1% of the total bacteria associated with this copepod. These workers also estimated that 1 cm3 of copepod fecal material contained about 1010 to 1011 DMSP-consuming bacteria. The breakdown between the bacteria attached to particulate algal debris and those living free in the feces is unknown and probably irrelevant.
Free-living assemblages of aerobic DMSP-degrading bacteria are commonly found in oceans and estuaries, where they either produce DMS concurrent with growth (70, 83) or demethylate it and grow on the product(s) (118). In coastal waters and the open waters of the Gulf of Mexico, free-living bacteria (the <1-μm filtrate) were shown to be responsible for most of the DMSPd degradation and are believed to dominate DMSPd utilization (63, 67). A number of studies have focused on obtaining the kinetic parameters of DMSP loss, DMS production rates, and Kms in natural waters. DMSP turnover rates in coastal waters, although about 10-fold higher than in oceanic waters (67), were in the range of <1 to ca. 100 nM day−1 (60, 63, 64, 71, 84, 85).
Deductions about DMSP degradation based on measurements of Kms, rates of DMSP loss, and appearance of products would seem to be confounded by some or all of the following factors: (i) the presence of organisms ranging from small protists to a heterogeneous bacterial assemblage to the presence of free enzymes in the water column, all capable of carrying out these reactions (59, 62), (ii) because of the multiplicity of species acting on DMSP, both cleavage and demethylation may occur at the same time, and even by the same organism (43, 69), (iii) some products of DMSP degradation may in turn be degraded by the same or other bacteria, thus underestimating their production, (iv) some DMS producers have extracellular DMSP lyases, while others have a combination of uptake protein and a cytosolic DMSP lyase, meaning that the lyases of the bacterial assemblage are responding to a combination of environmental and physiological DMSP concentrations, (v) interactions occur between uptake, accumulation, and lyase induction, and finally (vi) some microbes concentrate DMSP for possible use as an osmotic regulator (39, 134). As these factors would all vary from one site to another, kinetic data from natural samples would certainly be prone to some misinterpretation. Nonetheless, these types of data contributed to establishing a consensus over the past 15 years of the biogeochemistry of DMSP and DMS (Fig. 1).
DMSP demethylation.
One of the more significant findings in studying DMSP degradation was that microbes that could degrade DMSP without producing DMS existed in anoxic sediments and in seawater (62, 72, 74, 118). The explanation was that DMSP could also be demethylated in a series of reactions (equations 2 and 3) to produce 3-mercaptopropionate (3-MPA) or the intermediate, 3-methiolpropionate (MMPA), can be demethiolated to yield methanethiol (MeSH) and acrylate (equation 4), as indicated below.
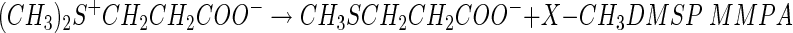 |
(2) |
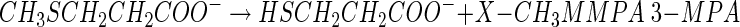 |
(3) |
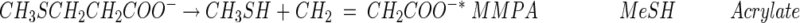 |
(4) |
A product that is suspected but not proven to be the other product of this demethiolation reaction is marked with an asterisk.
This process appears to explain numerous observations in natural samples where the rate of loss of DMSPd exceeded the rate of DMS production (62, 67, 71, 79, 84-86, 135). In most cases, the extent of demethylation in natural water and sediments was reported as the difference between the DMSP consumed and DMS produced after taking into account DMS uptake by bacteria. These DMS utilizers, as measured by most-probable-number analyses, are ubiquitous in the marine environment, being present at ca. 103 (ml of estuarine water)−1 (70) and 105 (ml of anoxic intertidal sediment)−1 (53). In sediments, DMS utilization would be due, at least in part, to methanogenic archaea (70, 74, 126) and methylotrophic bacteria (131).
Even though marine prokaryotes are a major sink for DMS, methanethiol resulting from demethylation is metabolized much more rapidly (62, 140), presumably because it is a major source of sulfur for the biosynthesis of methionine by some marine bacterioplankton (67, 69). Methanethiol derived from DMSP appears to be the major source of sulfur for marine bacteria in general and supplies Roseobacter species with ≥95% of their sulfur needs (69, 140). This is significant because α-proteobacterial DMS producers, the “Roseobacter lineage” (43), have been reported to be abundant in marine waters (32, 46, 95).
In anoxic marine sediments, DMSP is both cleaved to DMS and demethylated, yielding 3-mercaptopropionate and methanethiol (72), with most of the methanethiol being converted to methane, a sink for these types of sulfur molecules (74). A methanogen isolated from anoxic sediments was capable of demethylating 3-methiolpropionate to 3-mercaptopropionate (71, 128), and a sulfate reducer that cleaved DMSP to DMS and acrylate was isolated (127). Another sulfate-reducing isolate demethylated DMSP to yield 3-methiolpropionate (126) by using a unique DMSP-tetrahydrofolate methyltransferase (50).
Factors controlling the pathways of bacterial degradation of DMSP to DMS (cleavage) versus methanethiol (demethylation/demethiolation) are probably numerous and not well understood (53, 129, 140). Different marine environments would be expected to produce different ratios of DMS to methanethiol depending on the ambient levels of DMSPd. This would be influenced by the local phytoplankton bloom, whose stage of development would be influenced by the level of nutrients in the water column, and the season. For example, the microbial assemblage within a DMSPp-rich Phaeocystis bloom varied in its production of DMS and demethylation products depending on the stage of the bloom and the concentration of DMSPd that it produced (129). The microbial species found in particular blooms also have a great influence on DMS emissions. For example, the dominant bacterial species in the large Emiliania huxleyi blooms of the North Atlantic were related to the Roseobacter group (140), many of which are known to be methanethiol producers. DMSPd turnover in these Roseobacter-rich waters was 10-fold higher than the DMS produced, with the difference presumed to be shunted toward methanethiol production to be used for the assemblages' sulfur and carbon growth needs (69). This work has led to the suggestion that the composition of the DMSP-degrading assemblage in special environments, such as around the coccolithophore blooms of the North Sea, can in fact regulate the amount of DMS produced and thus emitted to the atmosphere (85, 129, 140).
Phylogeny of DMSP degraders.
The first DMSP-degrading microbial isolates shown to be capable of producing DMS, 3-methiolpropionate, or methanethiol (59, 118) or accumulate DMSP when placed under osmotic stress (39, 134) were all of undetermined phylogeny. The first indication that certain phylogenetic groups might be rich in DMSP degraders came indirectly from studies of culturable lignin-utilizing bacteria and indicated that many of the marine α-subclass of the Proteobacteria species had this capability (46). Nearshore and estuarine lignin enrichment cultures, when screened against community DNA, resulted in the identification of numerous isolates, many of which were closely related to the genus Roseobacter (44, 46). Probes of 16S ribosomal DNA designed to quantify this group showed them to be an important community in coastal seawater.
Among the microbes having homology with theses probes (MALF-1 and MALF-2) was the DMSP degrader strain LFR. Subsequent analysis showed that many of the lignin and DMSP enrichment isolates were DMSP-degrading α-proteobacteria (43). Members of the Roseobacter clade were prominent in the algal booms of the North Atlantic, and their abundance correlated positively with DMSP concentration and phytoplankton biomass (45). DMSP enrichments of estuarine creek water and surface sediments from a Spartina salt marsh yielded isolates predominantly (ca. 80%) of the γ-subclass of Proteobacteria, with many showing a phenotypic relationship to pseudomonads (5). The remainder were α-Proteobacteria, with one exception, Alcaligenes faecalis, the only β-subclass member of the Proteobacteria isolated, but it was a phylotype isolated numerous times.
Terrestrial and freshwater sediments contain only minute amounts of DMSP compared to marine sources (R. P. Kiene, unpublished data quoted in reference 61); nonetheless, DMSP amendments to river sediments showed DMS production rates and contained numbers of DMS producers of about 1% of those measured in marine sediments (138). Furthermore, the isolates obtained were aerobic DMS producers. Tellurite followed by DMSP enrichment provided an environment to find heretofore unrecognized genera of DMS producers. The most numerous of these tellurite-resistant isolates were gram-positive, Nocardia-like organisms which phylogenetic analysis (16S rRNA gene sequences) showed to be Rhodococcus equi and Rhodococcus rhodochrous. Also isolated by this enrichment regime were gram-negative, tellurite-resistant DMS producers having phylotype similarities to Sinorhizobium and Paracoccus and an isolate having 100% 16S rRNA sequence similarity to Pseudomonas aeruginosa. Parallel enrichments of marine sediments yielded only gram-negative DMS producers (138). The usefulness of the DMSP-cleaving phenotype to microbes living in a freshwater DMSP-free environment as far inland as the Missouri River in the middle of North America (N. Kulkarni and D. C. Yoch, unpublished data) remains a mystery, since this area has not had a marine environment, and therefore presumably no exposure to DMSP, for the past 70 million years.
DMSP uptake and cleavage.
Detailed studies of the physiology of DMSP-cleaving aerobic bacteria began with Roseobacter-related isolates from the Sagassso Sea, strain LFR (83, 86), and Sagittula stellata, a coastal isolate (43); Alcaligenes faecalis M3A, a β-proteobacterium salt marsh surface isolate (35, 38); and Pseudomonas doudoroffii, a γ-proteobacterial oceanic isolated (35, 37). Some of the Roseobacter-related coastal/salt marsh isolates appeared to be capable only of DMS production, with none apparently being strict DMSP demethylators or demethylators/demethiolators (43). However, since these analyses were carried out with 2 to 10 mM DMSP, it is possible that the lack of methanethiol production, indicative of demethylation/demethiolation, was inhibited by these high substrate concentrations.
One group of these isolates, strains DSS-3, DSS-8, ISM, GAI-5, and GIA-8, stood out as highly versatile in their metabolism of DMSP in that all had DMSP lyase, DMSP demethylase, and 3-methiolpropionate demethiolase activities (43). Their DMSP-degrading phenotypes were not identical, as only two could grow on both DMSP and acrylate, suggesting that they also had acrylase activity. Strains ISM, GAI-21, and GAI-109 were similar to a phototroph, Thiocapsa roseopersicina M11, isolated from a marine microbial mat (53) in that they had DMSP lyase activity but could not grow on either DMSP or acrylate. Furthermore, strain GAI-21 could not extract methanethiol from DMSP for use as a sulfur source (43), leaving one to wonder what advantage this microbe gains from degrading DMSP. Another group, composed of α-and γ-proteobacteria isolated from DMSP enrichments of salt marsh sediments and estuarine water were all DMS producers, with none capable of DMSP demethylation/demethiolation (5).
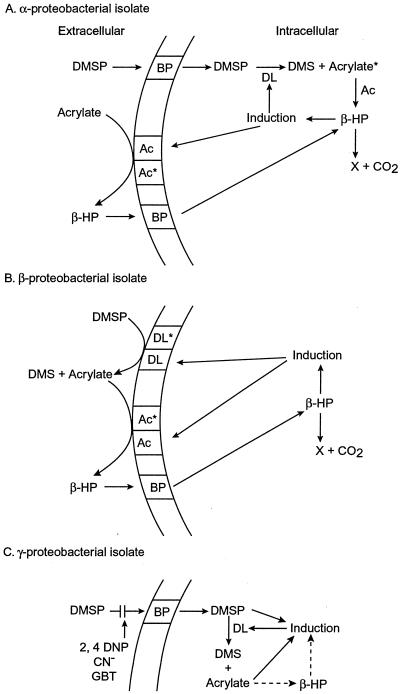
DMSP uptake and cleavage have been studied in some detail in an isolate from each of the α-, β-, and γ-subclasses of the Proteobacteria (6, 7, 124, 137). These cultured isolates may serve as models for additional biochemical studies even though they may not be representative of the dominant DMS-producing phylotypes in natural waters. In strain LFR, the most thoroughly studied isolate of the α-subclass, DMSP uptake preceded production of DMS, indicating an intracellular location of DMSP lyase (83). Accumulation of [1-13C]DMSP in the cytosol confirmed the intracellular location of the lyase (Fig. 2A) (7). Its disappearance coincided with the appearance of an unsuspected degradation product, [1-13C]β-hydroxypropionate, which in turn was decarboxylated in the cytosol. Acrylate, the putative intermediate, was not detected, suggesting that it may have turned over so rapidly that the pool size remained below the nuclear magnetic resonance detection limit. [1-13C]acrylate additions to cell suspensions of strain LFR resulted in [13C]β-hydroxypropionate first accumulating outside the cell and then appearing the cytosol, where it was subsequently metabolized. The appearance of β-hydroxypropionate both outside the cell and in the cytosol suggested that acrylase, the enzyme responsible for converting acrylate to β-hydroxypropionate, operated (7) both outside (in either the membrane or periplasm) and inside the cell.
FIG. 2.
Models comparing the uptake and metabolism of DMSP and acrylate in (A) strain LFR, (B) Alcaligenes faecalis, and (C) Pseudomonas doudoroffii. Abbreviations: β-HP, beta-hydroxypropionate; DL and DL*, inducible and constitutive DMSP lyase, respectively; Ac and Ac*, inducible and constitutive acrylase, respectively; acrylate∗, assumed to be present but not detectable; 2,4 DNP, the energy uncoupler 2,4-dinitrophenol; BP, binding protein; GBT, glycine betaine. Dashed lines in C represent the assumed mechanism of acrylate (via β-hydroxypropionate) induction of DMSP lyase in this organism.
The β-subclass isolate Alcaligenes faecalis M3A was shown to possess an extracellular DMSP lyase and acrylase which converted DMSP and acrylate, respectively, to β-hydroxypropionate, which was detected externally prior to its disappearance and accumulated in the cytosol, where it was metabolized (Fig. 2B) (6, 35). In both the α- and β-subclass isolates, DMSP and acrylate induced both DMSP lyase and acrylase, but these were only effective because they are the precursors of β-hydroxypropionate, the actual inducer. In both isolates, low endogenous (preinduced) levels of DMSP lyase and acrylase (designated DL* and Ac*, respectively, in Fig. 2A and B) provided sufficient β-hydroxypropionate to induce higher levels of these enzymes in response to higher levels of substrate.
The γ-proteobacterial isolate Pseudomonas doudoroffii took up DMSP before cleaving it to DMS and acrylate, action indicative of a cytosolic DMSP lyase similar to that of strain LFR (Fig. 2C) (35, 137). DMSP was taken up by an energy-dependent (cyanide-sensitive) transport system which saturated at several levels, the lowest having a half saturation constant (Kt transport) of 3.4 μM, substantially higher than the DMSPd found in situ, suggesting that it may have binding sites of higher affinity. 3-Methiolpropionate and glycine betaine inhibited DMSP uptake by P. doudoroffii suspensions, but glycine betaine was far less effective than it was in natural assemblages of DMS producers (66). The details of how these or related organisms in natural waters take up DMSP and acrylate, induce the appropriate enzymes, and metabolize these molecules in response to their in situ concentrations remain unknown.
DMSP lyases have been purified from three species of bacteria; all are composed of a single 48-kDa polypeptide having a Km for DMSP in the range of 0.5 to 2.0 mM (36, 38, 125). In a cell extract of the Phaeocystis sp., the Km of the lyase was 2.25 mM (101). These values are all many orders of magnitude higher than the levels of DMSPd found in oceanic waters and sediments. Those values range from 2 to 20 nM (with most values being >5 nM) in oceanic and nearshore waters (15, 49, 119, 133). DMSP measured as base hydrolyzable DMS in salt marsh sediments was ca. 200 μM (58), suggesting that the sediment pore water may in fact be considerably higher than the 5 nM measured in oceans. Km values for various assemblages in natural waters ranged from high-affinity systems in the low nanomolar range (66, 84, 85, 105) to low-affinity turnover in the micromolar range (53, 84, 85, 124). In these types of studies, it might be useful to test higher levels of DMSP (which has never been done) to ensure that uptake systems and lyase activities are truly saturated and that any low-affinity activity is not missed.
Isolates grown on substrate levels of DMSP show even greater variability in their Kms for DMSP cleavage, ranging from millimolar (37, 125, 127) to high nanomolar in range (83). Cantin et al. (19) suggested that microbes with these low-affinity DMSP-cleaving systems might solve this apparent dilemma by attaching or closely associating, probably via chemotactic attraction (139), to phytoplankton cells, as DMS production was correlated with filter fractions (>20 μm) having high DMSPp levels. Such attachment was particularly high during senescence (100), when the high millimolar levels of DMSP are leaking from high DMSP-containing species of phytoplankton (Table 2).
Such a broad range of Kms has been documented previously for glucose utilization by marine populations and ascribed simply to the complexity of the microbial environment (9), which may provide a rationalization for the findings cited here. Alternatively, high Km values, it was suggested, result from bacteria being acclimated to (or perhaps evolving in) environments having a high substrate concentration (105), such as DMSP found around senescent or virally lysed phytoplankton or salt marsh sediments, where DMSP levels are high (58). The fact that phytoplankton also have DMSP lyases with Kms varying from micromolar (100) to millimolar (108) further complicates the study and interpretation of DMS production in situ. Presumably these low-affinity algal lyases would be competitive with the putative low-affinity enzymes from bacteria, since the algal enzyme would be in the immediate proximity of the DMSP leaking from the old or damaged cell.
Conclusions.
DMSP cleavage to DMS plays an important biogeochemical role in the global sea-to-land transfer of sulfur, and it has a potential effect on remote oceanic weather patterns due to its oxidation in the atmosphere, which generates cloud-condensing nuclei leading to reflection of solar radiation (Fig. 1). The amount of DMS released to the atmosphere is all the more amazing now that we understand that a sizable fraction of the marine DMSPd is not cleaved but demethylated to yield methanethiol. Methanethiol appears to be particularly important as a source of sulfur for marine bacteria and perhaps some eukaryotic organisms. An emerging hypothesis suggests that as the DMSP concentration increases, DMS production increases until the demethylation/demethiolation pathway is saturated, at which point all the DMSP is cleaved to yield DMS. If microbial sulfur demand is low, a higher proportion of DMSP is degraded via the demethylation pathway, whereas at higher DMSP concentrations, more DMS is produced (68). The importance of DMS production justifies the intense study of this process by bacteria and algae during the past 15 years, and this review was an attempt to document that progress.
REFERENCES
- 1.Ackman, R. G., C. S. Tocher, and J. McLachan. 1966. Occurrence of dimethyl-β-propiothetin in marine phytoplankton. J. Fish. Res. Board Can. 23:357-364. [Google Scholar]
- 2.Adams, D. F., S. O. Farwell, D. Robinson, M. R. Merrill, and W. L. Bamesberger. 1981. Biogenic sulfur source strengths. Environ. Sci. Technol. 15:1493-1498. [Google Scholar]
- 2a.Names?. Yr? bacteria inhabiting macroscopic organic aggregates (marine snow) from surface waters. Limnol. Oceanogr. 31:68-78.
- 3.Andreae, M. O. 1990. Ocean-atmosphere interactions in the global biogeochemical sulfur cycle. Mar. Chem. 30:1-29. [Google Scholar]
- 4.Andreae, M. O. 1985. The emission of sulfur to the remote atmosphere, p. 5-25. In J. N. Galoway, R. J. Charlson, M. O. Andreae, and H. Rode (ed.). The biogeochemical cycling of sulfur and nitrogen in the remote atmosphere. D. Reidel Publishing Co., Boston, Mass.
- 5.Ansede, J. H., R. F. Friedman, and D. C. Yoch. 2001. Phylogenetic analysis of culturable dimethyl sulfide-producing bacteria from a Spartina-dominated salt marsh and estuarine water. Appl. Environ. Microbiol. 67:1210-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ansede, J. H., P. J. Pellechia, and D. C. Yoch. 1999. Metabolism of acrylate to β-hydroxypropionate and its role in dimethylsulfoniopropionate lyase induction by a salt marsh sediment bacterium. Alcaligenes faecalis. Appl. Environ. Microbiol. 65:5075-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ansede, J. H., P. J. Pellechia, and D. C. Yoch. 2001. Nuclear magnetic resonance of [1-13C]dimethylsulfoniopropionate (DMSP) and [1-13C]acrylate metabolism by a DMSP lyase-producing marine isolate of the α-subclass of Proteobacteria. Appl. Environ. Microbiol. 67:3134-3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Archer, S. D., C. E. Widdicombe, G. A. Tarran, A. P. Rees, and P. H. Burkill. 2001. Production and turnover of particulate dimethylsulfoniopropionate during coccolithophore bloom in the northern North Sea. Aquat. Microb. Ecol. 24:225-241. [Google Scholar]
- 9.Azam, F., and R. E. Hodson. 1981. Multiphasic kinetics for d-glucose uptake by assemblages of natural marine bacteria. Mar. Ecol. Prog. Ser. 6:213-222. [Google Scholar]
- 10.Azam, F., D. C. Smith, G. F. Steward, and Å. Hagström. 1993. Bacteria-organic matter coupling and its significance for carbon cycling. Microb. Ecol. 28:167-179. [DOI] [PubMed] [Google Scholar]
- 11.Bacic, M. K., S. Y. Newell, and D. C. Yoch. 1998. Release of dimethylsulfide from dimethylsulfoniopropionate by plant-associated salt marsh fungi. Appl. Environ. Microbiol. 64:1484-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bates, T. S., B. K. Lamb, A. Guenther, J. Dignon, and R. E. Stoiber. 1992. Sulfur emissions to the atmosphere from natural sources. J. Atmos. Chem. 14:315-337. [Google Scholar]
- 13.Bates, T. S., Kiene, R. P., G. V. Wolfe, P. A. Matrai, F. P. Chavez, K. R. Buck, B. W. Bloquist, and R. L. Cuhel. 1994. The cycling of sulfur in surface seawater of the northeast Pacific. J. Geophys. Res. 99:7835-7843. [Google Scholar]
- 14.Belviso, S., P. Buat-Menard, P. Putaud, B. C. Nguyen, H. Claustre, and J. Neveux. 1993. Size distribution of dimethylsulfoniopropionate (DMSP) in areas of the tropical northeastern Atlantic Ocean and the Mediterranean Sea. Mar. Chem. 44:55-71. [Google Scholar]
- 15.Belviso, S., S.-K. Kim, F. Rassoulzadegan, B. Krajka, B. C. Nguyen, N. Mihalopoulos, and P. Buat-Menard. 1990. Production of dimethylsulfonium propionate (DMSP) and dimethylsulfide (DMS) by a microbial food web. Limnol. Oceanogr. 35:1810-1821. [Google Scholar]
- 16.Billen, G., C. Joiris, L. Meyer-Reil, and H. Lindeboom. 1990. Role of bacteria in the north sea ecosystem. Nether. J. Sea Res. 26:265-293. [Google Scholar]
- 17.Bratbak. G., M. Levasseur S. Michaud, G. Cantin, et al. 1995. Viral activity in relation to Emiliania huxleyi blooms: a mechanism of DMSP release? Mar. Ecol. Prog. Ser. 128:133-142. [Google Scholar]
- 18.Cantin, G., M. Levasseur, M. Gosselin, and S. Michaud. 1996. Role of zooplankton on the mesoscale distribution of dimethylsulfide concentrations in the Gulf of St. Lawrence, Canada. Mar. Ecol. Prog. Ser. 141:103-117. [Google Scholar]
- 19.Cantin, G., M. Levasseur, S. Schultes, and S. Michaud. 1999. Dimethylsulfide (DMS) production by size-fractionated particles in the Labrador Sea. Aquat. Microb. Ecol. 19:307-312. [Google Scholar]
- 20.Cantoni, G. L., and D. G. Anderson. 1956. Enzymatic cleavage of dimethylpropiothetin by Polysiphonia lanosa. J. Biol. Chem. 222:171-177. [PubMed] [Google Scholar]
- 21.Challenger, F., R. Bywood, P. Thomas, and B. Haywood. 1957. Studies on biological methylation. XVII. The natural occurrence and chemical reactions of some thetins. Arch. Biochem. Biophys. 69:514-523. [DOI] [PubMed] [Google Scholar]
- 22.Challenger, F., and M. I. Simpson. 1948. Studies on biological methylation. XII. A precursor of the dimethyl sulfide evolved by Polysiphonia fastigiata, dimethyl-2-carboxyethyl sulfonium hydroxide and its salts. J. Chem. Soc. 3:1591-1597. [DOI] [PubMed] [Google Scholar]
- 23.Charlson, R. J. 1993. Gas-to-particle conversion and CNN production, p. 275-286. In G. Restelli and G. Angeleitti (ed.), Dimethylsulfide: oceans, atmosphere and climate. Kluwer, Dordrecht, The Netherlands.
- 24.Charlson, R. J., J. E. Lovelock, M. O. Andreae, and S. G. Warren. 1987. Oceanic phytoplankton, atmospheric sulfur, cloud albedo and climate. Nature 326:655-661. [Google Scholar]
- 25.Christaki, U., S. Belviso, J. R. Dolan, and M. Corn. 1996. Assessment of the role of copepods and ciliates in the release to solution of particulate DMSP. Mar. Ecol. Prog. Ser. 141:119-127. [Google Scholar]
- 26.Dacey, J. W. H., and N. V. Blough. 1987. Hydroxide decomposition of dimethylsulfoniopropionate to form dimethylsulfide. Geophys. Res. Lett. 14:1246-1249. [Google Scholar]
- 27.Dacey, J. W. H., F. A. Howse, A. F. Michaels, and S. G. Wakeham. 1998. Temporal variability of dimethylsulfide and dimethylsulfoniopropionate in the Sargasso Sea. Deep-Sea Res. I 45:2085-2104. [Google Scholar]
- 28.Dacey, J. W. H., G. M. King, and P. S. Lobel. 1994. Herbivory by reef fishes and the production of dimethylsulfide and acrylic acid. Mar. Ecol. Prog. Ser. 112:67-74. [Google Scholar]
- 29.Dacey. J. W. H., G. M. King, and S. G. Wakeham. 1987. Factors controlling emission of dimethylsulfide from salt marshes. Nature 330:643-645. [Google Scholar]
- 30.Dacey, J. W. H., and S. G. Wakeham. 1986. Oceanic dimethyl sulfide: production during zooplankton grazing on phytoplankton. Science 233:13141.316.. [DOI] [PubMed] [Google Scholar]
- 31.Daly, K. L., and G. R. DiTullio. 1996. Particulate dimethylsulfoniopropionate removal and dimethylsulfide production by zooplankton in the southern ocean, p. 238-243. In R. P. Kiene, P. T. Visscher, M. D. Keller, and G. O. Kirst (ed.), Biological and environmental chemistry of DMSP and related sulfonium compounds. Plenum Press, New York, N.Y.
- 32.Dang, H., and C. R. Lovell. 2002. Numerical dominance and phylotype of marine Roseobacter species during early colonization of submerged surface in coastal marine waters as determined by 16S ribosomal DNA sequence analysis and fluorescence in situ hybridization. Appl. Environ. Microbiol. 69:496-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delille, D., and S. Razouls. 1996. Community structures of heterotrophic bacteria of copepod fecal pellets. J. Plank. Res. 16:603-615. [Google Scholar]
- 34.de Souza, M. P., Y. P. Chen, and D. C. Yoch. 1996. Dimethylsulfoniopropionate lyase from the marine macrolaga Ulva curvata: purification and characterization of the enzyme. Planta 199:433-438. [Google Scholar]
- 35.de Souza, M. P., and D. C. Yoch. 1995. Comparative physiology of dimethyl sulfide production by dimethylsulfoniopropionate lyase in Pseudomonas doudoroffii and Alcaligenes sp. strain M3A. Appl. Environ. Microbiol. 61:3986-3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Souza, M. P., and D. C. Yoch. 1996. Differential metabolism of dimethylsulfoniopropionate and acrylate in saline and brackish intertidal sediments. Microb. Ecol. 31:319-330. [DOI] [PubMed] [Google Scholar]
- 37.de Souza, M. P., and D. C. Yoch. 1996. N-terminal amino acid sequences and comparison of DMSP lyases from Pseudomonas doudoroffii and Alcaligenes strain M3A, p. 293-304. In R. P. Kiene, P. T. Visscher, M. D. Keller, and G. O. Kirst (ed.), Biological and environmental chemistry of DMSP and related sulfonium compounds. Plenum Press, New York, N.Y.
- 38.de Souza, M. P., and D. C. Yoch. 1995. Purification and characterization of dimethylsulfoniopropionate lyase from an Alcaligenes-like dimethyl sulfide-producing marine isolate. Appl. Environ. Microbiol. 61:21-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diaz, M. R., P. T. Visscher, and B. F. Taylor. 1992. Metabolism of dimethylsulfoniopropionate and glycine betaine by a marine bacterium. FEMS Microbiol. Lett. 96:61-66. [Google Scholar]
- 40.Dickson, D. M. J., and G. O. Kirst. 1986. The role of dimethylsulfoniopropionate, glycine betaine and homarine in the osmoacclimation of Platymonas subcordiformis. Planta 167:536-543. [DOI] [PubMed] [Google Scholar]
- 41.Dickson, D. M., R. G. Wyn Jones, and J. Davenport. 1980. Steady state osmotic adaptation in Ulva lactuca. Planta 150:158-165. [DOI] [PubMed] [Google Scholar]
- 42.Gage, D. A., D. Rhodes, K. D. Nolte, W. A. Hicks, T. Leustek, A. J. L. Cooper, and A. D. Hanson. 1997. A new route for synthesis of dimethylsulphoniopropionate in marine algae. Nature 387:891-894. [DOI] [PubMed] [Google Scholar]
- 43.González, J. M., R. P. Kiene, and M. A. Moran. 1999. Transformation of sulfur compounds by an abundant lineage of marine bacteria in the α-subclass of the class Proteobacteria. Appl. Environ. Microbiol. 65:3810-3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.González, J. M., and M. A. Moran. 1997. Numerical dominance of a group of marine bacteria in the α-subclass of the class Proteobacteria in coastal waters. Appl. Environ. Microbiol. 63:4237-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.González, J. M., and R. Simó, R. Massana, J. S. Covert, E. O. Casamayor, C. Pedrós-Alio, and M. A. Moran. 2000. Bacterial community structure associated with a dimethylsulfoniopropionate-producing North Atlantic algal bloom. Appl. Environ. Microbiol. 66:4237-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.González, J. M., W. B. Whitman, R. E. Hudson, and M. A. Moran. 1996. Identifying numerically culturable bacteria from complex communities: an example from a lignin enrichment culture. Appl. Environ. Microbiol. 62:4433-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ishida, Y. 1996. 30 years of research on dimethylsulfoniopropionate, p. 1-12. In R. P. Kiene, P. T. Visscher, M. D. Keller, and G. O. Kirst (ed.), Biological and environmental chemistry of DMSP and related sulfonium compounds. Plenum Press, New York, N.Y.
- 48.Ishida, Y., and H. Kodota. 1967. Production of dimethyl sulfide from unicellular algae. Bull. Jpn. Soc. Sci. Fish. 33:782-787. [Google Scholar]
- 49.Iverson, R. L., F. L. Nearhoof, and M. O. Andreae. 1989. Production of DMSP and DMS by phytoplankton in estuarine and coastal waters. Limnol. Oceanogr. 34:53-67. [Google Scholar]
- 50.Jansen, M., and T. A. Hansen. 1998. Tetrahydrofolate serves as a methyl acceptor in the demethylation of dimethylsulfoniopropionate in cell extracts of sulfate-reducing bacteria. Arch. Microbiol. 169:84-87. [DOI] [PubMed] [Google Scholar]
- 51.Jansen, M., and T. A. Hansen. 2000. DMSP:tetrahydrofolate methyltransferase from the marine sulfate-reducing strain WN. J. Sea Res. 43:225-231. [Google Scholar]
- 52.Jacobsen, T. R., and F. Azam. 1984. Role of bacteria in copepod fecal pellet decomposition: colonization, growth rates and mineralization. Bull. Mar. Sci. 35:495-502. [Google Scholar]
- 53.Jonkers, H. M., G. F. Koopmans, and H. van Gererden. 1998. Dynamics of dimethyl sulfide (DMS) in a marine microbial. Microb. Ecol. 36:93-100. [DOI] [PubMed] [Google Scholar]
- 54.Karsten, U., C. Wiencke, and G. O. Kirst. 1990. The β-dimethylsulphoniopropionate (DMSP) content of macroalgae from Antarctica and southern Chile. Bot. Mar. 33:143-146. [Google Scholar]
- 55.Keller, M. D., W. K. Bellows, and R. R. L. Guillard. 1989. Dimethyl sulfide production in marine phytoplankton, p.167-182. In E. S. Saltzman and W. J. Cooper (ed.), Biogenic sulfur in the environment. American Chemical Society, Washington, D.C.
- 56.Kelly, D. P., and N. A. Smith. 1990. Organic sulfur compounds in the environment. Biogeochemistry, microbiology and ecological aspects. Adv. Microb. Ecol. 11:345-385. [Google Scholar]
- 57.Kettle, A. J., and M. O. Andreae. 2000. Flux of dimethylsulfide from the oceans: a comparison of updated data sets and flux models. J. Geophys. Res. 26:26793-26808. [Google Scholar]
- 58.Kiene, R. P. 1988. Dimethyl sulfide metabolism in salt marsh sediments. FEMS Microbiol. Ecol. 53:71-78. [Google Scholar]
- 59.Kiene, R. P. 1990. Dimethyl sulfide production from dimethylsulfoniopropionate in coastal seawater samples and bacterial cultures. Appl. Environ. Microbiol. 56:3292-3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kiene, R. P. 1992. Dynamics of dimethyl sulfide and dimethylsulfoniopropionate in oceanic waters. Mar. Chem. 37:29-52. [Google Scholar]
- 61.Kiene, R. P. 1996. Microbial cycling of organosulfur gases in marine and freshwater environments, p. 137-151. In D. Adams, S. Seitzinger, and P. Crill (ed.), Cycling of reduced gases in the hydrosphere, vol. 23E. Schweitzerbart'sche Verlagsbuchhandlung, Nagele U. Obermiller, Stuttgart, Germany.
- 62.Kiene, R. P. 1996. Production of methanethiol from dimethylsulfoniopropionate in marine surface waters. Mar. Chem. 54:69-83. [Google Scholar]
- 63.Kiene, R. P. 1996. Turnover of dissolved DMSP in estuarine and shelf waters of the northern Gulf of Mexico, p. 337-349. In R. P. Kiene, P. T. Visscher, M. D. Keller, and G. O. Kirst (ed.), Biological and environmental chemistry of DMSP and related sulfonium compounds. Plenum Press, New York, N.Y.
- 64.Kiene, R. P., and T. S. Bates. 1990. Biological removal of dimethyl sulfide from seawater. Nature 345:702-705. [Google Scholar]
- 65.Kiene, R. P., and D. G. Capone. 1988. Microbial transformations of methylated sulfur compounds in anoxic salt marsh sediments. Microb. Ecol. 15:275-291. [DOI] [PubMed] [Google Scholar]
- 66.Kiene, R. P., L. P. Hoffman Williams, and J. E. Walker. 1998. Seawater microorganisms have a high affinity glycine betaine uptake system which also recognizes dimethylsulfoniopropionate. Aquat. Microb. Ecol. 15:39-51. [Google Scholar]
- 67.Kiene, R. P., and L. J. Linn. 2000. Distribution and turnover of dissolved DMSP and its relationship with bacterial production and dimethylsulfide in the Gulf of Mexico. Limnol. Oceanogr. 45:848-861. [Google Scholar]
- 68.Kiene, R. P., L. J. Linn, and J. A. Bruton. 2000. New and important roles for DMSP in marine microbial communities. J. Sea Res. 43:209-224. [Google Scholar]
- 69.Kiene, R. P., L. J. Linn, J. González, M. A. Moran, and J. A. Burton. 1999. Dimethylsulfoniopropionate and methanethiol are important precursors of methionine and protein-sulfur in marine bacterioplankton. Appl. Environ. Microbiol. 65:45-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kiene, R. P., R. S. Oremland, A. Catena, L. G. Miller, and D. G. Capone. 1986. Metabolism of reduced methylated sulfur compounds in anaerobic sediments and by a pure culture of an estuarine methanogen. Appl. Environ. Microbiol. 52:1037-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kiene, R. P., and S. K. Service. 1991. Decomposition of dissolved DMSP and DMS estuarine waters: dependence on temperature and substrate concentration. Mar. Ecol. Prog. Ser. 76:1-11. [Google Scholar]
- 72.Kiene, R. P., and B. F. Taylor. 1988. Demethylation of dimethylsulfoniopropionate and production of thiols in anoxic marine sediments. Appl. Environ. Microbiol. 54:2208-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kiene, R. P., and B. F. Taylor. 1989. Metabolism of acrylate and 3-mercaptopropionate: decomposition products of (dimethylsulfonio)propionate in anoxic marine sediments, p. 222-230. In E. S. Saltzman and W. J. Cooper (ed.), Biogenic sulfur in the environment. American Chemical Society, Washington, D.C.
- 74.Kiene, R. P., and P. T. Visscher. 1987. Production and fate of methylated sulfur compounds from methionine and dimethylsulfoniopropionate in anoxic salt marsh sediments. Appl. Environ. Microbiol. 53:2426-2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kiene, R. P., P. T. Visscher, M. D. Keller, and G. O. Kirst (ed.). 1996. Biological and environmental chemistry of DMSP and related sulfonium compounds. Plenum Press, New York, N.Y.
- 76.Kocsis, M. G., K. D. Nolte, D. Rhodes, T. L. Shen, D. A. Gage, and A. D. Hanson. 1998. Dimethylsulfoniopropionate biosynthesis in Spartina alterniflora—evidence that S-methylmethionine and dimethylsulfoniopropylamine are intermediates. Plant Physiol. 117:273-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kwint, R. L., J., X. Irigoien, and K. J. M. Kramer. 1996. Copepods and DMSP, p. 239-252. In R. P. Kiene, P. T. Visscher, M. D. Keller, and G. O. Kirst (ed.), Biological and environmental chemistry of DMSP and related sulfonium compounds. Plenum Press, New York, N.Y.
- 78.Kwint, R. L. J., and K. J. M. Kramer. 1995. Dimethylsulfide production by plankton communities. Mar. Ecol. Prog. Ser. 121:227-237. [Google Scholar]
- 79.Kwint, R. L. J., P. Quist, T. A. Hansen, L. Dijkhuizen, and K. J. M. Kramer. 1996. Turnover of dimethylsulfoniopropionate and dimethylsulfide in the marine environment: a mesocosm experiment. Mar. Ecol. Prog. Ser. 145:223-232. [Google Scholar]
- 80.Lancelot, C., and G. Billen. 1985. Carbon-nitrogen relationships in nutrient metabolism of coastal marine ecosystems. Adv. Aquat. Microbiol. 3:263-321. [Google Scholar]
- 81.Larher, F., J. Hamelin, and G. R. Stewart. 1977. L'acide diméthylsulfonium-5 propanoïque de Spartina anglica. Phytochemistry (Oxford) 16:2019-2020. [Google Scholar]
- 82.Leck, C., U. Larsson, L. E. Bågander, S. Johansson, and S. Hajdu. 1990. Dimethyl sulfide in the Baltic Sea: annual variability in relation to biological activity. J. Geophys. Res. 95:3353-3363. [Google Scholar]
- 83.Ledyard, K. M., and J. W. H. Dacey. 1994. Dimethylsulfide production from dimethylsulfoniopropionate by a marine bacterium. Mar. Ecol. Prog. Ser. 110:95-103. [Google Scholar]
- 84.Ledyard, K. M., and J. W. H. Dacey. 1996. Kinetics of DMSP-lyase activity in coastal waters, p. 325-335. In R. P. Kiene, P. T. Visscher, M. D. Keller, and G. O. Kirst (ed.), Biological and environmental chemistry of DMSP and related sulfonium compounds. Plenum Press, New York, N.Y.
- 85.Ledyard, K. M., and J. W. H. Dacey. 1996. Microbial cycling of DMSP and DMS in coastal and oligotrophic seawater. Limnol. Oceanogr. 41:33-40. [Google Scholar]
- 86.Ledyard, K. M., E. F. DeLong, and J. W. H. Dacey. 1993. Characterization of a DMSP-degrading bacterial isolate from the Sargasso Sea. Arch. Microbiol. 160:312-318. [Google Scholar]
- 87.Lovelock, J. E., R. J. Maggs, and R. A. Rasmussen. 1972. Atmospheric dimethyl sulfide and the natural sulfur cycle. Nature 237:452-453. [Google Scholar]
- 88.Malin, G. 1996. The role of DMSP and DMS in the global sulfur cycle and climate regulation, p. 177-189. In R. P. Kiene, P. T. Visscher, M. D. Keller, and G. O. Kirst (ed.), Biological and environmental chemistry of DMSP and related sulfonium compounds. Plenum Press, New York, N.Y.
- 89.Malin, G., P. S. Liss, and S. M. Turner. 1994. Dimethyl sulfide: production and atmospheric consequences, p. 303-320. In J. C. Green and B. S. C. Leadbeater (ed.), The haptophyte algae. Clarendon, London, UK.
- 90.Matrai, P., and M. D. Keller. 1993. DMS in a large scale coccolithophore bloom in the Gulf of Maine. Cont. Shelf Res. 13:831-843. [Google Scholar]
- 91.Matrai, P. A., and M. D. Keller. 1994. Total organic sulfur and dimethylsulfoniopropionate in marine phytoplankton: intracellular variations. Mar. Biol. 119:61-68. [Google Scholar]
- 92.Mechard, M. J., and W. R. Rayburn. 1979. Volatile organic sulfides from freshwater algae. J. Phycol. 15:379-383. [Google Scholar]
- 93.Middelboe, M. M. Søndergaard, Y. Letarte, and N. H. Borch. 1995. Attached and free-living bacteria: production and polymer hydrolysis during a diatom bloom. Microb. Ecol. 29:231-248. [DOI] [PubMed] [Google Scholar]
- 94.Mopper, K., and B. F. Taylor. 1986. Biogeochemical cycling of sulfur-thiols in coastal marine sediments. p. 324-339. In M. Sohn (ed.), Organic marine geochemistry. American Chemical Society, Washington, D.C.
- 95.Mullins, T. D. T. B. Britschgi, R. L. Krest, and S. J. Giovannoni. 1995. Genetic comparisons reveal the same unknown bacterial lineages in Atlantic and Pacific bacterioplankton communities. Limnol. Oceanogr. 41:148-158. [Google Scholar]
- 96.Nedwell, D. B., M. T. Shabbeer, and R. M. Harrison. 1994. Dimethyl sulfide in North Sea waters and sediments. Est. Coast. Shelf Sci. 39:209-217. [Google Scholar]
- 97.Nguyen, B. C., S. Belviso, N. Mihalopoulos, J. Gostan, and P. Nival. 1988. Dimethyl sulfide production during natural phytoplanktonic blooms. Mar. Chem. 24:133-142. [Google Scholar]
- 98.Niki, T., M. Kunugi, K. Kohata, and A. Otsuki. 1997. Annual monitoring of DMS-producing bacteria in Tokyo Bay, Japan, in relation to DMSP. Mar. Ecol. Prog. Ser. 156:17-24. [Google Scholar]
- 99.Niki, T., M. Kunugi, and A. Otsuki. 2000. DMSP-lyase activity in five marine phytoplankton species: its potential importance in DMS production. Mar. Biol. 136:759-764. [Google Scholar]
- 100.Nishiguchi, M. K., and L. J. Goff. 1995. Isolation, purification, and characterization DMSP lyase (dimethylpropiothetin dethiomethylase (4.4.1.3) from the red alga Polysiphonia paniculata. J. Phycol. 31:567-574. [Google Scholar]
- 101.Otte, M. L., and J. T. Morris. 1994. Dimethylsulfoniopropionate (DMSP) in Spartina alterniflora Loisel. Aquat. Bot. 48:239-259. [Google Scholar]
- 102.Pakulski, J. D., and R. P. Kiene. 1992. Foliar release of dimethylsulfoniopropionate from Spartina alterniflora. Mar. Ecol. Prog. Ser. 81:277-287. [Google Scholar]
- 103.Paquet, L., B. Rathinasabapathi, H. Saini, L., Zamir, D. A. Gage, Z.-H. Huang, and A. D. Hanson. 1994. Accumulation of the compatible solute 3-dimethylsulfoniopropionate in sugarcane and its relatives, but not other gramineous crops. Aust. J. Plant Physiol. 21:37-48. [Google Scholar]
- 104.Pomeroy, L. R., and W. J. Wiebe. 1993. Energy sources from microbial food webs. Mar. Microb. Food Webs 7:101-118. [Google Scholar]
- 105.Scarratt, M., G. Cantin, M. Levasseur, and S. Michaud. 2000. Particle size-fractioned kinetics of DMS production: where does DMSP cleavage occur in the microscale. J. Sea Res. 43:245-252. [Google Scholar]
- 106.Simó, R., S. D. Archer, C. Pedró-Alió, L. Gilpin, and C. E. Stelfox-Widdicombe. 2002. Coupled dynamics of dimethylsulfoniopropionate and dimethylsulfide cycling and the microbial food web in surface waters of the North Atlantic. Limnol. Oceanogr. 47:53-61. [Google Scholar]
- 107.Smith, D. C., G. F. Steward, R. A. Long, and F. Azam. 1995. Bacterial mediation of carbon fluxes during a diatom bloom in a mesocosm. Deep Sea Res. 42:75-97. [Google Scholar]
- 108.Stefels, J., and L. Dijkhuizen. 1996. Characteristics of DMSP-lyase in Phaeocystis sp. (Prymnesiophyceae). Mar. Ecol. Prog. Ser. 131:307-313. [Google Scholar]
- 109.Stefels, J., and W. H. M. van Boekel. 1993. Production of DMS from dissolved DMSP in axenic cultures of the marine phytoplankton species Phaeocystis sp. Mar. Ecol. Prog. Ser. 97:11-18. [Google Scholar]
- 110.Steinke, M., G. Malin, S. D. Archer, P. H. Burkill, and P. S. Liss. 2002. DMS production in a cocclithophorid bloom: evidence for the importance of dinoflagellate DMSP lyases. Aquat. Microb. Ecol. 26:259-270. [Google Scholar]
- 111.Steinke, M., G. V. Wolfe, and G. O. Kirst. 1998. Partial characterization of dimethylsulfoniopropionate (DMSP) lyase isozymes in 6 strains of Emiliania huxleyi. Mar. Ecol. Prog. Ser. 175:215-225. [Google Scholar]
- 112.Steudler, P. A., and B. J. Peterson. 1984. Contribution of gaseous sulphur from salt marshes to the global sulphur cycle. Nature (London) 311:455-457. [Google Scholar]
- 113.Storey, R., J. Gorham, M. G. Pitman, A. D. Hanson, and D. Gage. 1993. Response of Melanthera biflora to salinity and water stress. J. Exp. Bot. 44:1551-1560. [Google Scholar]
- 114.Tackx, M. L. M., C. Bakker, and P. Van Rijswijk. 1990. Zooplankton grazing pressure in the Oosterschelde (the Netherlands). Neth. J. Sea Res. 25:405-415. [Google Scholar]
- 115.Tang, K. W. 2001. Defecation of dimethylsulfoniopropionate (DMSP) by the copepod Acartia tonsa as functions of ambient food concentration and body DMSP content. J. Plankton Res. 23:549-553. [Google Scholar]
- 116.Tang, K. W., D. R. Rogers, H. G. Dam, and P. T. Visscher. 2000. Seasonal distribution of DMSP among seston, dissolved matter and zooplankton along a transect in the Long Island Sound estuary. Mar. Ecol. Prog. Ser. 206:1-11. [Google Scholar]
- 117.Tang, W., P. T. Visscher, and H. G. Dam. 2001. DMSP-consuming bacteria associated with the calanoid copepod Acartia tonsa (Dana). J. Exp. Mar. Biol. Ecol. 256:185-198. [DOI] [PubMed] [Google Scholar]
- 118.Taylor, B. F., and D. C. Gilchrist. 1991. New routes of aerobic biodegradation of dimethylsulfoniopropionate. Appl. Environ. Microbiol. 57:3581-3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Turner, S. M., G. Malin, P. S. Liss, D. S. Harbour, and P. M. Holligan. 1998. The seasonal variation of dimethyl sulfide and dimethylsulfoniopropionate concentrations in nearshore waters. Limnol. Oceanogr. 33:364-375. [Google Scholar]
- 120.Vairavamurthy, A., M. O. Andreae, and R. L. Iverson. 1985. Biosynthesis of dimethyl sulfide and dimethylpropiothetin by Hymenomonas carterae in relation to sulfur sources and salinity variations. Limnol. Oceanogr. 30:59-70. [Google Scholar]
- 121.Van Bergeijk, S. A., and L. J. Stal. 2001. Dimethylsulfoniopropionate and dimethylsulfide in the marine flatworm Convoluta roscoffensis and its algal symbiont. Mar. Biol. 138:209-216. [Google Scholar]
- 122.Van Bergeijk, S. A., and L. J. Stal. 1996. The role of oxygenic phototrophic microorganisms in production and conversion of dimethylsulfoniopropionate and dimethylsulfide in microbial mats, p. 369-379. In R. P. Kiene, P. T. Visscher, M. D. Keller, and G. O. Kirst (ed.), Biological and environmental chemistry of DMSP and related sulfonium compounds. Plenum Press, New York, N.Y.
- 123.van Boekel, W. H. M., F. C. Hansen, R. Riegman, and R. P. M. Bak. 1992. Lysis-induced decline of a Phaeocystis spring bloom and coupling with the microbial foodweb. Mar. Ecol. Prog. Ser. 81:269-276. [Google Scholar]
- 124.van der Maarel, M. J. E. C., W. Aukema, and T. A. Hansen. 1996. Purification and characterization of a dimethylsulfoniopropionate cleavage enzyme from Desulfovibrio acrylicus. FEMS Microbiol. Lett. 143:241-245. [Google Scholar]
- 125.van der Maarel, M. J. E. C., and T. A. Hansen. 1996. Anaerobic microorganisms involved in the degradation of DMS(P), p. 351-360. In R. P. Kiene, P. T. Visscher, M. D. Keller, and G. O. Kirst (ed.), Biological and environmental chemistry of DMSP and related sulfonium compounds. Plenum Press, New York, N.Y.
- 126.van der Maarel, M. J. E. C., P. Quist, L. Dijkhuizen, and T. A. Hansen. 1993. Anaerobic degradation of dimethylsulfoniopropionate to 3-S-methylmercaptopropionate by a marine Desulfobacterium strain. Arch. Microbiol. 160:411-412. [Google Scholar]
- 127.van der Maarel, M. J. E. C., S. van Bergeijk, A. F. van werkhoven, A. M. Laverman, W. G. Meijer, W. T. Stam, and T. A. Han. 1996. Cleavage of dimethylsulfoniopropionate and reduction of acrylate by Desulfovibrio acrylicus sp. nov. Arch. Microbiol. 166:109-115. [Google Scholar]
- 128.van der Maarel, M. J. E. C., M. Jansen, and T. A. Hansen. 1995. Methanogenic conversion of 3-S-methylmercaptopropionate to 3-mercaptopropionate. Appl. Environ. Microbiol. 61:48-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.van Duyl, F. C., W. W. C. Gieskes, A. J. Kop, and W. E. Lewis. 1998. Biological control of short-term variations in the concentration of DMSP and DMS during a Phaecystis spring bloom. J. Sea Res. 40:221-231. [Google Scholar]
- 130.Visscher, P. T., M. R. Diaz, and B. F. Taylor. 1992. Enumeration of bacteria which cleave or demethylate dimethylsulfoniopropionate in the Caribbean Sea. Mar. Ecol. Prog. Ser. 89:293-296. [Google Scholar]
- 131.Visscher, P. T., R. P. Kiene, and B. F. Taylor. 1994. Demethylation and cleavage of demethylsulfoniopropionate in marine intertidal sediments. FEMS Microbiol. Ecol. 14:179-190. [Google Scholar]
- 132.Wagner, C., and E. R. Stadtman. 1962. Bacterial fermentation of dimethyl-β-propiothetin. Arch. Biochem. Biophys. 98:331-336. [DOI] [PubMed] [Google Scholar]
- 133.Wakeham, S. G., B. L. Howes, J. W. H. Dacey, R. P. Schwarzenbach, and J. Zeyer. 1987. Biogeochemistry of dimethylsulfide in a seasonally stratified coastal salt pond. Geochim. Cosmochim. Acta 51:1675-1684. [Google Scholar]
- 134.Wolfe, G. V. 1996. Accumulation of dissolved DMSP by marine bacteria and its degradation via bacterivory, p. 277-291. In R. P. Kiene, P. T. Visscher, M. D. Keller, and G. O. Kirst (ed.), Biological and environmental chemistry of DMSP and related sulfonium compounds. Plenum Press, New York, N.Y.
- 135.Wolfe, G. V., E. B. Sherr, and B. F. Sherr. 1994. Release and consumption of DMSP from Emiliania huxleyi during grazing by Oxyrrhis marina. Mar. Ecol. Prog. Ser. 111:111-119. [Google Scholar]
- 136.Wolfe, G. V., and M. Steinke. 1996. Grazing-activated production of dimethyl sulfide (DMS) by two clones of Emiliania huxleyi. Limnol. Oceanogr. 41:1151-1160. [Google Scholar]
- 137.Yoch, D. C., J. H. Ansede, and K. S. Rabinowitz. 1997. Evidence for intracellular and extracellular dimethylsulfoniopropionate (DMSP) lyases and DMSP uptake sites on two species of marine bacteria. Appl. Environ. Microbiol. 63:3182-3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yoch, D. C., R. H. Carraway, R. Friedman, and N. Kulkarni. 2001. Dimethylsulfide (DMS) production from dimethylsulfoniopropionate by freshwater river sediments: phylogeny of gram-positive DMS-producing isolates. FEMS Microbiol. Ecol. 37:31-37. [Google Scholar]
- 139.Zimmer-Faust, R., M. P. de Souza, and D. C. Yoch. 1996. Bacterial chemotaxis and its potential in marine dimethylsulfide production and biogeochemical sulfur cycling. Limnol. Oceanogr. 41:1330-1334. [Google Scholar]
- 140.Zubkov, M. V., B. M. Fuchs, S. D. Archer, R. P. Kiene, R. Amann, and P. H. Burkill. 2001. Linking the composition of bacterioplankton to rapid turnover of dissolved dimethylsulfoniopropionate in an algal bloom in the North Sea. Environ. Microbiol. 3:304-311. [DOI] [PubMed] [Google Scholar]