Abstract
The production of an ant-deterrent factor(s) (ADF) by Xenorhabdus nematophila and Photorhabdus luminescens, the symbiotic bacteria of the nematodes Steinernema carpocapsae and Heterorhabditis bacteriophora, respectively, was examined. In addition to an in vivo assay in which bacteria were tested for their ability to produce ADF within insect cadavers (M.E. Baur, H. K. Kaya, and D. R. Strong, Biol. Control 12:231-236, 1998), an in vitro microtiter dish assay was developed to monitor ADF activity produced by bacteria grown in cultures. Using these methods, we show that ADF activity is present in the supernatants of bacterial cultures, is filterable, heat stable, and acid sensitive, and passes through a 10-kDa-pore-size membrane. Thus, ADF appears to be comprised of a small, extracellular, and possibly nonproteinaceous compound(s). The amount of ADF repellency detected depends on the ant species being tested, the sucrose concentration (in vitro assays), and the strain, form, and age of the ADF-producing bacteria. These findings demonstrate that the symbiotic bacteria of some species of entomopathogenic nematodes produce a compound(s) that deters scavengers such as ants and thus could protect nematodes from being eaten during reproduction within insect cadavers.
Xenorhabdus spp. and Photorhabdus spp. are motile, gram-negative bacteria belonging to the family Enterobacteriaceae (5, 8) and are mutualistically associated with specific entomopathogenic nematodes in the families Steinernematidae and Heterorhabditidae, respectively. The bacteria are carried as symbionts in the intestinal tract of the only free-living stage of the nematode, the infective juvenile (IJ). The nematode-bacterium complex invades and kills the immature and adult stages of numerous insects (15). IJs enter insect species through natural orifices and subsequently penetrate into the hemocoel where bacteria are released, resulting in insect death within 48 h. In the insect cadaver, the bacteria serve as a food source and are required for nematode growth and reproduction. Once nutrients have been exhausted in the insect cadaver, progeny nematodes develop into IJs carrying the bacterial symbiont and exit the cadaver to search for a new host (7).
The symbiotic association with nematodes is thought to enable the bacteria to survive outside of and be effectively transmitted between insect hosts. The bacteria, in turn, are essential for killing most insect hosts and are required for the nematode to complete its life cycle. Xenorhabdus and Photorhabdus bacteria can occur as one of two phenotypic variants (previously referred to as phase variants) known as primary form (1° form) or secondary form (2° form) (7). The 1°-form bacteria are always found associated with field-collected nematodes and produce antibiotics, adsorb certain dyes, and develop large intracellular inclusions composed of crystal proteins (3, 6, 7). The 2° form can arise spontaneously in aged laboratory cultures of bacteria. It does not bind to dye products and is defective in the ability to produce many of the substances associated with the 1°-form variant.
Because of their wide host range, ease of production, and the need to find an alternative approach to chemical pesticides in the soil, entomopathogenic nematodes have been used extensively as bioinsecticides against soil pests (15), and levels of control equivalent to those of chemical pesticides have often been achieved (9). One limitation to their effective use is that the abundance of nematodes is greatly reduced after application (22). The causes of this rapid decline in the applied population have been attributed to the presence of antagonists in the soil (13) and the lack of appropriate insect hosts, as well as extremes of temperature, UV light, and desiccation (14). Furthermore, nematode recycling and infection of new insect hosts may be adversely affected when scavengers consume nematode-killed insects before IJs exit the insects and enter soil (2).
The bacterial symbionts of entomopathogenic nematodes produce many chemical compounds within the insect cadaver (26), some of which appear to protect against invasion by opportunistic organisms and thereby enhance the development and survival of the nematodes. For example, arthropod scavengers, such as the Argentine ant Linepithema humile (Mayr), are deterred from feeding on Photorhabdus- or Xenorhabdus-killed insects (2). While the capability of the bacteria to produce compounds that deter scavengers remains poorly understood, their activities apparently enhance the development and survival of their nematode hosts. We report here the responses of L. humile, Lasius alienus (Foerster), and Formica subsericea (Say) ants to Photorhabdus spp. and Xenorhabdus nematophila (Poinar and Thomas) bacteria. The activity, production, and some biochemical characteristics of an ant-deterrent factor(s) (ADF) produced by entomopathogenic bacteria are described.
MATERIALS AND METHODS
Strains, propagation, and storage conditions.
Nematode strains used were Steinernema carpocapsae (Weiser) All and Heterorhabditis bacteriophora Poinar NC1. All nematodes were reared in wax moth larvae as previously described (17) and stored in water at 15°C for no more than 3 weeks before they were used. S. carpocapsae nematodes carrying HGB007 bacteria were generated by cultivating sterile nematode eggs on lawns of HGB007 and harvesting the progeny IJs in water traps as previously described (11, 23, 24). Subsequent rearing was conducted in wax moth larvae as previously described (17). Xenorhabdus and Photorhabdus bacterial strains used in this study are listed in Table 1. The bacteria were streaked on NBTA (10) as needed to detect possible phenotypic variation before using them in experiments. 1°-form bacteria were used in all experiments except where specified. Bacteria taken from −70°C stocks were either transferred directly into liquid culture or were first transferred to NBTA medium, grown for 48 h, and then transferred to liquid culture. For liquid culture, bacteria were grown at 28 to 30°C aerated in nutrient broth (NB) (Difco, Detroit, Mich.) or Luria-Bertani broth (LB) (18) for the lengths of time indicated for each experiment.
TABLE 1.
Bacterial strains and sources of X. nematophila and P. luminescens used in ADF experiments
| Isolate | Strain | Forma | Source |
|---|---|---|---|
| HGB007 | X. nematophila ATCC 19061 | 1° | ATCCb |
| HGB030 | X. nematophila ATCC 19061 | 2° | HGBc |
| HGB001 | X. nematophila | 1° | HGBd |
| HGB008 | P. luminescens ATCC 29999 | 1° | ATCC |
| HGB159 | P. luminescens ATCC 29999 | 2° | HGBc |
| HGB325 | P. luminescens K122 | 1° | JCEe |
| HGB326 | P. luminescens NC1 | 1° | JCE |
| HGB327 | P. luminescens Hm | 1° | JCE |
Form variants were verified by observing colony color and zone of clearing on NBTA medium (10).
ATCC, American Type Culture Collection (Rockville, Md.).
In the lab of H. Goodrich-Blair, HGB030 was isolated from an aged culture of HGB007 and HGB159 was isolated from an aged culture of HGB008.
Isolated by H. Goodrich-Blair from surface-sterilized infective-stage S. carpocapsae All strain nematodes by homogenization (27).
Gift of J. C. Ensign, UW-Madison.
Ant test sites.
Three field sites-the University of California, Davis (UC-Davis) campus (Yolo County), a marina in Berkeley, California (Alameda County), and the University of Wisconsin, Madison (UW-Madison) campus (Dane County)-were chosen for experiments in the natural habitats of the ants. The Argentine ant, L. humile, which is common in disturbed areas such as urban and agricultural habitats throughout much of California (12, 25), was tested for its response to bacterial secondary metabolites at the first two locations. L. alienus and F. subsericea, identified by Philip Ward (UC-Davis), were studied at the Wisconsin site.
To establish laboratory ant colonies, L. humile workers, queens, and broods were collected in the field by digging up the nests with soil and leaf litter in Davis, California. The ants were allowed to form nests as described by Baker et al. (1). Briefly, plastic petri dishes (100 by 15 mm) were partially filled with plaster of Paris which, once hardened, was kept moist by a wick submerged in water. Ants established nests in the dishes after the infested material (soil or litter) was placed in a bucket, and water was added. As the water level rose, the ants took refuge in the petri dish, which was covered with red cellophane to create a dark environment. Eleven colonies, ranging from about 2,000 to 5,000 individuals/colony, were established.
In vivo ADF activity assays (California test sites only).
Bacterium-infected insects used in ADF assays were obtained by injecting last-instar Galleria mellonella (L.) larvae (Rainbow Mealworms, Inc., Compton, Calif.) with bacteria that had been grown on NBTA for 48 h and then suspended in sterile Ringer's solution (17). Using a hemocytometer, a bacterial count was made for each culture. On the basis of this count, each culture was diluted so that there were approximately 100 bacterial cells/μl. Ten microliters of the bacterial suspension was injected into the second proleg of a chilled larva (held at 4°C for 10 min) with a 27-gauge needle on a 2-ml syringe. Sterile Ringer's solution was used as a control. The injected larvae were placed in petri dishes and incubated at room temperature for 4 days. The 4-day-old bacterium-killed larvae were used for field and laboratory experiments. Control larvae injected with Ringer's solution were frozen for at least 2 h and used as freeze-killed controls.
To obtain nematode- or bacterium-infected insects for testing ADF activity, 10 wax moth larvae were placed in a 10-cm-diameter petri dish lined with a filter paper onto which 200 IJs had been applied. The petri dishes with infected wax moth larvae were kept at ambient laboratory conditions (23 ± 2°C) for 4 days (hereafter referred to as 4-day-old, postinfected insects). The weight of wax moth larvae used in all experiments ranged from 240 to 260 mg/larva.
For laboratory ant colony experiments, one cadaver from each treatment was placed on a filter paper and introduced into an ant colony. A minimum of 10 colonies was used for each experiment. The colonies were deprived of food but not water for 48 h prior to the experiment.
In field ant colony experiments, nematode-killed or bacterium-killed insects were placed on a filter paper (90-mm diameter) near ant foraging trails. To protect the insect cadavers from birds, filter papers containing the cadavers were covered by the bottom side of plastic petri dishes (100-mm diameter) with 8 holes (15-mm diameter) to allow ants to forage on the cadavers. Cadavers from each treatment were arranged randomly in a circle. Experiments were conducted under natural shade conditions.
Observations on the condition of the cadavers were made 24 h after placement by using a 10× hand lens in the field and a dissecting microscope in the laboratory. Cadavers were classified as “intact” if no breaks in the integument were observed or as “not intact” if one or more puncture wounds were observed. Ant counts were initiated 10 min after the cadavers were placed in the experiment site and recorded at 10-min intervals thereafter for 60 min.
In vitro assays for ADF activity.
Due to different response levels of California and Wisconsin ants to ADF and sucrose attractant, different protocols were developed for use at each location. However, unless noted otherwise, minor differences in protocols (e.g., bacterial growth medium, test suspension volume, monitoring method) did not cause qualitative differences in the results obtained for the California and Wisconsin field sites. Regardless of the protocol, 96-well polyvinyl chloride microtiter plates (from Becton Dickinson [Oxnard, Calif.] or Corning Laboratory Science Company [Corning, N.Y.]) were used to hold the test suspensions. In all experiments, the arrangement of bacterial suspension in the wells of each plate was completely randomized, and samples were compared with water controls to determine when the difference between the pre- and postexperimental amounts was due to evaporation. Each well contained 0.2 to 0.21 ml of test suspension, and the substrate remaining after testing was measured by volume or weight. We observed that L. humile ants congregated on the rows or columns near the edges of the plates and did not proceed inward until the sucrose suspension in the well near an edge was consumed. To avoid this “edge effect,” only the wells along one side were used for all experiments with L. humile ants, and one side of the plate was coated with a sucrose solution to attract ants to the plates. The Wisconsin ant species studied, L. alienus and F. subsericea, did not show a preference for edge wells. For these ants, flexible polyvinyl chloride microtiter plates were cut to size such that all wells in the block were used (e.g., 3 × 8 for an experiment with eight treatments). Each block was placed in a standard 10-cm-diameter plastic petri dish in which four holes were made at roughly equal distances in the side walls using a heated no. 1 cork borer to give ants access to the wells and placed near a different ant trail or nest.
California experiments were performed all year at temperatures ranging from 15 to 30°C for field experiments compared to 23 ± 2°C for laboratory experiments. Wisconsin experiments were limited to field trials between July and September, with temperatures ranging from 8 to 34°C.
In California, after placement, ant counts were conducted at the same intervals as for the field experiments, and the experiment was terminated in less than 60 min if any one suspension was consumed prior to this time. Two wells for each treatment were used in a plate (a replicate). Except where noted otherwise, eight replicates (plates) were included in each experiment. In Wisconsin, there were three replicate wells for each treatment in each well block, and there was a minimum of two blocks per experiment. L. alienus and F. subsericea were allowed to feed on the substances for a minimum of 6 h (visitation by ants was not monitored during this time).
Ant behavior.
L. humile was the only ant species observed in and around the field sites in Davis, California. Generally, it took 5 to 10 min and 1 to 2 min for worker ants to locate the insect cadavers or bacterial suspensions in the field and laboratory experiments, respectively. There were more ants on the cadavers during the first 1 to 2 h after placement than 2 h after placement in laboratory trials, but ants fed on freeze-killed insects at the same intensity during the duration of the field trials. Approximately 100 to 150 ants were required to completely consume the sucrose control solution in one well. A well can host ca. 45 ants at one time during the feeding period. An individual ant spent 1 to 2 min at a control well containing a sucrose suspension before leaving.
Similar observations were made at the Wisconsin site. Test suspensions were located by worker ants within 10 min and visited at high intensity within 30 min. ADF activity was evaluated by consumption only. Time for complete consumption of the sucrose control treatments depended on ant size, colony size, weather conditions, and frequency of testing at the specific site (i.e., starvation level of the ants) but usually varied between 2 and 6 h. Ants sampled all wells but concentrated their efforts on consuming control wells or those with least ADF activity. Once these wells were completely emptied, the process continued until the ants encountered the wells where the ADF activity completely prohibited consumption.
Biochemical characterization of ADF.
For experiments to test the effects of filtration on ADF activities from isolates HGB007 (cultured for 108 h), HGB008 (cultured for 132 h), and HGB325 (cultured for 132 h), cell-free supernatants were prepared by centrifuging bacterial suspensions at 2,000 × g for 20 min and passing each supernatant through a 0.45-μm-pore-size Millipore filter (Millipore, Bedford, Mass.). Nonfiltered cultures served as controls.
Heated samples for in vitro assays were obtained by autoclaving (121°C for 20 min) cell-free supernatants of HGB007, HGB008, and HGB325 isolates. Nonautoclaved cultures and autoclaved NB served as controls. For in vivo assays, autoclaved bacterium-killed insects served as the heated treatment, whereas nonautoclaved cadavers served as the nonheated treatment. Freeze-killed wax moth larvae that had been autoclaved or had not been autoclaved served as controls.
For experiments testing the effect of pH on ADF activity, a cell-free supernatant from a culture of the HGB007 isolate grown for 48 h (pH 8.4) was divided, put into six separate tubes, and adjusted to pH 2, 4, 6, 8, 10, or 12 with either 0.1 M HCl or 0.1 M NaOH, followed by the addition of sucrose to 25%. Control samples of 25% sucrose adjusted to the same range of pH were included in the experiment, which was conducted twice according to the Wisconsin trial conditions.
For molecular weight analyses, cell-free supernatants from HGB007 cultures that had been grown for 48 h were separated by size on a Centricon 10-kDa-cutoff filter (Millipore) into eluate (<10-kDa) and retentate (>10-kDa) fractions. Each fraction was normalized by the addition of water to the proportional weight/volume of the input solute. A reconstituted sample was prepared by mixing eluate with retentate in the same proportions as in the original sample. Sucrose and water samples served as a control. The experiment was performed twice, each time with a different supernatant preparation, according to the in vitro Wisconsin test site conditions.
Statistical analysis.
The number of worker ants visiting the insect cadavers or suspension treatments, the amount of suspension removed from wells, and the number of intact cadavers were transformed into proportional data. The proportion of ants visiting one treatment was calculated as follows. The number of ants visiting that treatment (total of two wells) at each 10-min interval was totaled and divided by the total number of ants visiting all the treatments in a given plate. The proportion of suspension removed in a treatment was obtained by dividing the suspension removed in one treatment (total of two wells) by the original volume of suspension present in the treatment at the start of the assay. Proportional data were arcsine-square root transformed before conducting a two-way analysis of variance and then Duncan's new multiple-range test (21). Untransformed data, such as percentage of suspension removed, are reported here, and mean values ± standard errors are provided.
RESULTS
Effect of sucrose concentration on ADF activity.
Sucrose was used to attract ants in in vitro assays, and its concentration was tested for its impact on L. humile ant visitation behavior and feeding during monitoring of bacterial deterrence. The bacterium used for this purpose was HGB007, an X. nematophila strain that had exhibited the ability to deter ants at the Wisconsin field site. It should be noted that Escherichia coli cultures are readily visited and consumed by ants (Table 2) and that ADF activity is not a general property of bacterial cultures. Sucrose was added to various concentrations to culture medium with and without HGB007 and tested against L. humile ants.
TABLE 2.
Effect of P. luminescens HGB008 phenotypic variation on ADF activity at California test sites
| Test conditiona | Strainb | Form | ADF activityc |
|---|---|---|---|
| Field in vitro | E. coli | NAg | 0.95 ± 0.11 Ad |
| HGB008 | 1° | 0.08 ± 0.01 Bd | |
| HGB159 | 2° | 0.08 ± 0.02 Bd | |
| Laboratory in vitro | E. coli | NA | 1 Ae |
| HGB008 | 1° | 0.05 ± 0.01 Be | |
| HGB159 | 2° | 0.05 ± 0.01 Be | |
| Field in vivo | Control | NA | 0.51 ± 0.08 Af |
| HGB008 | 1° | 0.019 ± 0.002 Bf | |
| HGB159 | 2° | 0.47 ± 0.05 Af |
See Materials and Methods for detailed explanation of test conditions.
See Table 1 for details on the strains. E. coli, E. coli (Migula). Control, freeze-killed insects used for in vivo experiments.
For in vitro assays, values are the proportion of volume (in microliters) removed from wells. For in vivo studies, values are the mean proportion of ants visiting. Different letters after values indicate significant difference within an experiment (P < 0.05 by Duncan's new multiple-range test).
There was a significant difference between the number of field ants visiting the E. coli control versus the bacterial treatments (F = 34.93, df = 2, 32; P < 0.05).
Significantly more suspension remained in the wells of 1°- or 2°-form bacterial treatments than that for the E. coli control treatment (F = 4697.98, df = 2, 32; P < 0.001).
There was a significant difference between the treatments of 1°- and 2°-form bacteria or freeze-killed control (F = 67.5, df = 2, 67; P < 0.0001).
NA, not applicable.
Ant visitation was measured in two independent trials of 10 treatments each (5 treatments for NB and 5 treatments for HGB007) (data not shown). The proportion of the ant population visiting wells containing sucrose concentrations of ≥10% was significantly higher than those visiting wells with no sucrose or with a sucrose concentration of 5% (trial 1, F = 117.83, df = 9, 140, P < 0.0001; trial 2, F = 51.76, df = 9, 140, P < 0.0001). In each of these trials, the proportion of ants visiting NB and HGB007 wells with no sucrose was low (< 0.02).
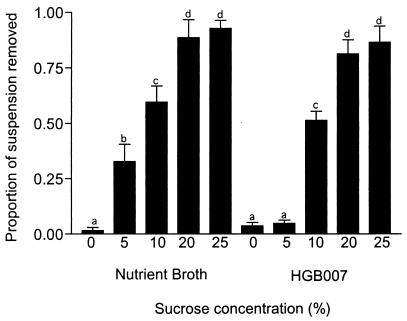
The extent to which ants consumed test solutions was proportional to the amount of sucrose that was present (Fig. 1). Ants do not consume solutions containing no added sucrose and visit such solutions less frequently. Interestingly, the amount of 5% sucrose solution consumed was decreased if a bacterial suspension was added to the sucrose solution (Fig. 1); however, this effect was masked at sucrose concentrations of ≥10%. We developed an assay using the 5% sucrose solution as a base, and we defined ADF activity as the ability of added bacterial suspensions or culture supernatants to deter normal levels of either ant visitation or solution consumption.
FIG. 1.
Effect of sucrose concentration on ADF activity toward L. humile feeding. The HGB007 isolate was grown in NB for 108 h, and powdered sucrose was added to the final sucrose concentrations indicated on the x axis. Sterile NB was used as a control. Eight plates (eight replicates) were used; each plate contained one well of each treatment. The experiment was conducted at four Davis field sites over the course of 4 days at temperatures ranging from 21 to 25°C. Experiments were terminated when the suspension in one of the wells on the plate was consumed. There was no significant difference (P > 0.05) between the values with the same lowercase letter above the bars.
These data are in contrast to the feeding behavior of L. alienus and F. subsericea ants. These ants appeared to be more sensitive to ADF activity and did not consume HGB007 cultures with 25% sucrose (Table 3). All subsequent experiments with L. humile were therefore performed with 5% sucrose concentrations, whereas for experiments with L. alienus or F. subsericea, 25% sucrose concentrations were used.
TABLE 3.
Effect of X. nematophila HGB007 phenotypic variation on ADF activity at Wisconsin test sitea
| Treatment | Bacterial form | Ant tested | Proportion of suspension removedb |
|---|---|---|---|
| Water only | NAc | L. alienus | 0.085 ± 0.002 |
| F. subsericea | 0.1 ± 0.01 | ||
| Water + sucrose | NA | L. alienus | 1 ± 0 |
| F. subsericea | 1 ± 0 | ||
| HGB007 + sucrose | 1° | L. alienus | 0.2 ± 0.15 |
| F. subsericea | 0.19 ± 0.02 | ||
| HGB030 + sucrose | 2° | L. alienus | 1 ± 0 |
| F. subsericea | 1 ± 0 |
Bacteria (see Table 1 for detailed description of strains) were grown aerated in LB for 48 h and tested at 25% sucrose.
Proportion of suspension removed from wells after 6 h of exposure to ant trails. Values are averages ± standard errors.
NA, not applicable.
Time course of ADF production in vitro.
At the Wisconsin test site, it was found that cultures of HGB007 (X. nematophila) grown for 48 h had more ADF activity, measured by a reduction in ant consumption of test solutions, than cultures grown for 24 h (data not shown). In fact, under Wisconsin in vitro assay conditions, the HGB007 cultures grown for 48 h usually had attained a maximal level of deterrence activity (i.e., very little suspension removed from the wells). Therefore, this growth time was used for all test cultures at the Wisconsin site.
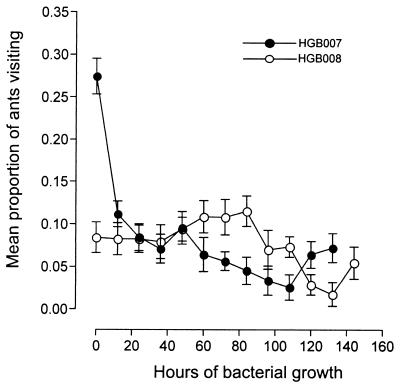
In contrast, maximal ADF activity was not observed by 48 h of bacterial growth at the California sites. Therefore, the timing of appearance of ADF activity at these sites was assessed. Samples from HGB007 (X. nematophila) and HGB008 (P. luminescens) cultures were taken at various times over a 132- and 144-h time period, respectively, and tested for deterrence of ant visitation (Fig. 2). There were significant differences in the mean proportions of ants visiting HGB008 with the different bacterial growth times (F = 89.94, df = 12, 436; P < 0.001). Ant visitation to samples taken after 84 h of bacterial growth decreased (i.e., ADF activity increased) and was lowest (P > 0.05) for samples taken at 132 h of bacterial growth.
FIG. 2.
Time course of ADF activity production by isolates HGB007 and HGB008. HGB007 and HGB008 were grown in NB, and samples were taken at 12-h intervals. Bacterial suspensions from the two species were placed into different plates and tested separately, and eight replicates per bacterium were used. Experiments were conducted at four Davis field sites, and experiments were conducted twice. The sample size ranged from 75 to 257 ants/plate for HGB008 and 51 to 141 ants/plate for HGB007.
The length of time of bacterial growth also affected the ants' response to HGB007 (F = 119.46, df = 11, 216; P < 0.0001) (Fig. 2). There was a sharp decrease in visitation to HGB007 samples taken from cultures grown for 0 versus 12 h and a steady decrease in visitation of ants to samples taken after 12 h of growth, with the lowest visitation to samples from cultures grown for 108 h. For all subsequent California in vitro ADF activity experiments, X. nematophila strains were grown for 108 h, and P. luminescens strains were grown for 132 h.
Production of ADF activity in vivo.
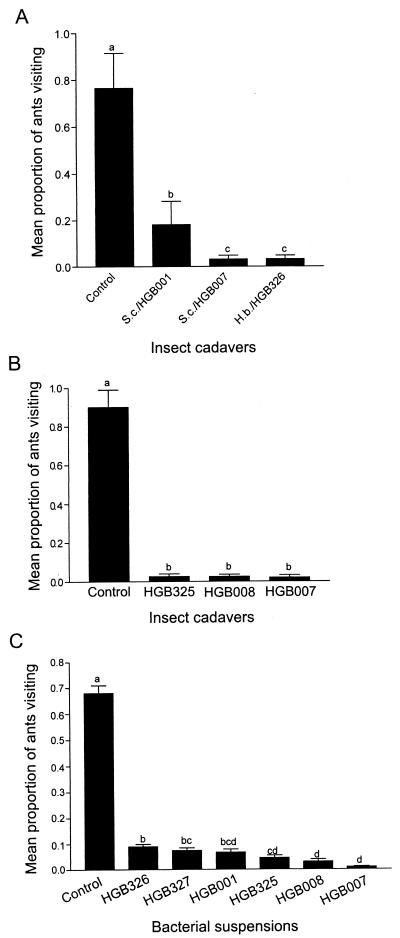
We tested the response of L. humile to insects killed by different nematode-bacterium pairs and found a significant difference in ant response depending on the bacterial strain harbored by the nematode (F = 412.38, df = 3, 203; P < 0.0001) (Fig. 3A). Ants approached and touched the insects killed by S. carpocapsae harboring HGB007 and H. bacteriophora harboring P. luminescens HGB326 and then left the cadavers. In contrast, the proportion of ants that fed on the insects killed by S. carpocapsae carrying HGB001 was significantly higher.
FIG. 3.
ADF activity produced by various Photorhabdus and Xenorhabdus strains. (A) The mean proportion of field ants visiting wax moth larvae killed by Heterorhabditis or Steinernema nematodes colonized by different bacterial isolates. S. carpocapsae All (S.c.) and H. bacteriophora NC1 (H.b.) nematodes with HGB001, HGB007, and HGB326 bacteria were studied. Freeze-killed insects were used as the control. There were eight replicates with two cadavers in each treatment, and the experiment was repeated twice. Means with the same letter above the bar are not significantly different (P > 0.05 by Duncan's new multiple range test). The sample size ranged from 47 to 196ants per plate. (B) The mean proportion of field ants visiting wax moth larvae killed by injection with the bacterial strains indicated below each bar or by freezing (Control). Twenty-five replicates were used, and the experiment was repeated six times. Each replicate consisted of two cadavers from each treatment (total of eight cadavers) in a plastic petri dish and placed at four field sites in Davis, Calif., over a 2-week period. There was no significant difference (P > 0.05 by Duncan's new multiple-range test) between the values with the same lowercase letter above the bars. The sample size ranged from 25 to 81 ants per plate. (C) Photorhabdus and Xenorhabdus strains were grown in NB for 132 and 108 h, respectively, and tested against field colonies of Argentine ants. Mean (± standard error) proportion of ants visiting the supernatants of six different bacterial strains (identified below each bar) or a medium-only control (NB) is given. The experiment was conducted once, with eight replicates, in the Davis field site. There was no significant difference (P > 0.05 by Duncan's new multiple-range test) between the values with the same lowercase letter above the bars. The sample size ranged from 50 to 136 ants per plate.
We then assessed ADF activity in larvae that had been killed by direct injection (i.e., without any nematodes) of strains of Xenorhabdus (HGB007) and Photorhabdus (HGB008 and HGB325) by monitoring L. humile ant visitation at the California test sites. As expected, the ants responded differently to bacterium-killed versus freeze-killed insect hosts in the field (Fig. 3B) and lab (data not shown). In field experiments, significantly more ants fed on the freeze-killed insects than on any of the bacterium-killed insects (F = 36.7, df = 3, 101; P < 0.001) (Fig. 3B). Ninety-six percent of the freeze-killed insects were no longer intact, and the other 4% were missing after the 24-h experiment. In contrast, only 6% of the bacterium-killed insects had their integument punctured, and the remaining cadavers were intact. Similar results were obtained when larvae killed by various bacterial strains were placed in 10 laboratory ant colonies (one larva for each treatment per colony).
Last, we compared ADF activity of various bacterial strains when grown in laboratory growth medium. Xenorhabdus (HGB001 and HGB007) and Photorhabdus (HGB008, HGB325, HGB326, and HGB327) strains were grown in NB and tested for visitation by L. humile in the California field sites (Fig. 3C). There were significant differences in the different treatments (F = 290.73, df = 6, 480; P < 0.0001), with the highest proportion of ants feeding on control wells (NB plus 5% sucrose), which was significantly higher than that for other treatments. All NB-cultured Xenorhabdus and Photorhabdus strains tested had some measurable ADF activity against Argentine ants, with HGB326 having the least activity and HGB007 having the most activity.
Effect of phenotypic variation on ADF activity.
The effect of phenotypic variation on ADF activity produced by HGB007 cultures grown for 48 h was assessed in field experiments in Wisconsin (Table 3). Cultures of 2°-form X. nematophila (HGB030) did not show ADF activity; ants consumed the entire mixture. The effect of HGB008 phenotypic variation was measured at field and laboratory sites at Davis, Calif. (Table 2). Surprisingly, phenotypic variation did not affect ant response to P. luminescens grown in laboratory culture but did affect ant response to P. luminescens in insect larvae. While ants did not consume either 1°- or 2°-form P. luminescens grown in NB, they did consume the sucrose-only control treatment. In contrast, while ants did not visit or attempt to eat larvae killed by 1°-form P. luminescens (HGB008), they did visit larvae killed by 2°-form P. luminescens (HGB159). In fact, there was no significant difference in the proportion of ants visiting 2°-form P. luminescens-killed insects versus freeze-killed insects (F = 0.14, df = 3, 127; P > 0.70). A similar response was observed with the cadavers in the 11 laboratory ant colonies (data not shown).
Biochemical characterization of ADF activity.
We conducted several experiments to begin characterizing the biochemical nature of ADF. First, the effect of filtration on ADF activity from supernatants of cultures of HGB007 (grown for 108 h), HGB008 (grown for 132 h), and HGB325 (grown for 132 h) was determined by monitoring L. humile visitation in laboratory and field Argentine ant colonies. It was found that laboratory ants were deterred by the filtered and nonfiltered bacterial cultures equally (data not shown). A similar response was detected in the field, where there was no significant difference between filtered and nonfiltered bacterial treatments, with 13% of ants visiting the wells for all the nonfiltered bacterial treatments and 12% of ants visiting for all the filtered treatments (F = 0.2, df = 1, 71; P = 0.655). The control treatments of filtered and nonfiltered NB showed a significantly higher (F = 318.78, df = 3, 71; P < 0.0001) proportion of ants visiting compared to the bacterial cultures, with a mean value of ca. 38% ants for both the filtered and nonfiltered control treatments.
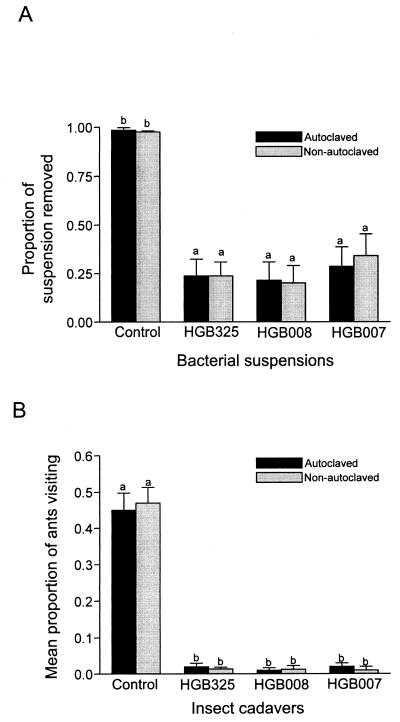
Cell-free supernatants of cultures of HGB007 (grown for 108 h), HGB008 (grown for 132 h), and HGB325 (grown for 132 h) were autoclaved to determine the effect of heat treatment on ADF activity as detected by consumption of solutions (Fig. 4A). There was no significant difference between autoclaved and nonautoclaved treatments (P > 0.9). Field experiments were consistent with lab experiments in that there was no significant difference between the amounts of suspension left in the heated and nonheated treatments (P > 0.79), with 0.12 ml (60%) for the nonautoclaved treatments and 0.11 ml (55%) for the autoclaved treatments (data not shown). Again, the difference among the bacterial treatments was not significant (P > 0.05) and a highly significant difference was observed between the bacterial and control treatments (F = 96.51, df = 7, 98; P < 0.0001) (data not shown).
FIG. 4.
Effect of autoclaving on ADF activity. (A) The mean proportion of bacterial suspension removed from wells for autoclaved and nonautoclaved treatments after exposure to laboratory ant colonies. Supernatants were taken from bacterial strains (indicated below each bar) grown for 132 h (HGB325 and HGB008) or 108 h (HGB007) before autoclaving and addition of sucrose to 5%. NB with 5% sucrose was used as a control. There was no significant difference (P > 0.05 by Duncan's new multiple-range test) between the values with the same lowercase letter above the bars. Ants consumed significantly more of the control treatment than the three bacterial treatments (F = 299.81; df = 7, 98; P < 0.0001). Between 18 and 97 ants visited each plate. (B) Mean proportion of Berkeley field ants visiting autoclaved and nonautoclaved treatments of 4-day-old, postinfected insects that had been killed with the bacterial strain indicated below each bar. Each replicate consisted of two cadavers from each treatment in a plastic petri dish. The experiment was conducted once with 25 replicates. There was no significant difference (P > 0.05 by Duncan's new multiple-range test) between the values with the same lowercase letter above the bars. A significantly higher proportion of ants visited the control larvae compared to that of ants visiting the bacterium-killed insects (F = 192, df = 7, 482; P < 0.0001). Sample size ranged from 21 to 124 ants/dish.
As a further test of the heat stability of ADF, insect larvae killed by HGB007, HGB008, or HGB325 were autoclaved and compared with nonautoclaved larvae for their effect on ant visitation. L. humile did not respond to the autoclaved and nonautoclaved bacterium-killed insects differently (Fig. 4B).
To assess the approximate molecular size of ADF, cell-free supernatants of HGB007 cultures (grown for 48 h) were fractionated on a 10-kDa-pore-size membrane and tested for ADF activity at the Wisconsin test site by measuring consumption of solutions. L. alienus and F. subsericea ants did not consume treatments of water (without attractant), eluate, or reconstituted samples. However, the sucrose-control treatment and most of the retentate treatment were consumed. The proportions of substrate (mean ± standard error) removed from wells (in ascending order) were as follows: water, 0.17 ± 0.04; eluate, 0.38 ± 0.03; supernatant, 0.45 ± 0.04; reconstituted eluate plus retentate, 0.47 ± 0.03; retentate, 0.89 ± 0.05; sucrose, 1 ± 0). There were significant differences (F = 26.7, df = 6, 10; P < 0.0001) among the treatments, demonstrating that ADF activity was present in the <10-kDa eluate and supernatant.
Cell-free supernatants of HGB007 cultures (grown for 48 h) were tested at the Wisconsin test site for the effect of pH on consumption. L. alienus and F. subsericea ants consumed sucrose controls of each pH tested (data not shown), but ADF activity in test strains was lost at low pH values. The precise pH at which activity was lost depended on the ant species. L. alienus ants consumed supernatants of pH 6.0 or less, while F. subsericea ants were still repelled by supernatants at pH 4.0 and pH 6.0 but not by supernatant at pH 2.0 (data not shown).
DISCUSSION
The results of our experiments show that the symbiotic bacteria of certain entomopathogenic nematodes produce a factor(s), ADF, which deters ants from visiting and/or feeding on otherwise attractive substrates of either sucrose or dead insect larvae. These results are consistent with the observations of Baur et al. (2), and differences in observed ADF activity between the two studies can be readily explained by differences in strains, forms, growth rate, and/or cell count.
Although Photorhabdus and Xenorhabdus species occupy the same pair of ecological niches (the intestines of symbiont nematodes and the hemocoel of insects) Heterorhabditis-Photorhabdus and Steinernema-Xenorhabdus entomopathogenic nematode-bacterium complexes are widely divergent and have separate origins (4, 20). In fact, there is less than 20% identity between the genomic DNA from species in the Xenorhabdus and Photorhabdus genera (5). Poinar (20) concluded that a similar pattern of infectivity, life cycle, and association with bacterial symbionts is the result of convergent evolution. In light of this hypothesis, it is possible that the ADF activities produced by Xenorhabdus and Photorhabdus are distinct compounds and that ADF production is also an example of a convergently evolved function.
There are major physiological differences between the 1° and 2° forms for both Xenorhabdus and Photorhabdus bacteria (3, 6, 8). For example, 1°-form variants produce more antibiotics and show differences in their ability to absorb certain dyes compared to 2°-form variants. The two variants produced different levels of ADF activity under some, but not all, test conditions. X. nematophila HGB030 (2° form derived from HGB007) did not produce ADF activity when tested in vitro at the Wisconsin test site (Table 3). In contrast, P. luminescens HGB159 (2° form) exhibited ADF activity under in vitro conditions, but not when injected into insects (in vivo) (Table 2). One possible explanation for these differences may be that growth of 2°-form P. luminescens within insects is not optimal for production of ADF. Alternatively, the mechanisms of phenotypic variation, and, in turn, the regulation of ADF production, may be distinct between Xenorhabdus and Photorhabdus genera.
Under in vitro conditions, higher sucrose concentrations in the suspensions resulted in more ants feeding, indicating that sucrose masked the effects of ADF. However, experimental conditions, such as test location or ant species used, yielded differences in the level of sucrose required for masking. These differences may have been due to different sensitivities of the ant species used in each location, differences in culture medium and time, or differences in some other variable parameter of the field sites.
The length of time postinfection or bacterial culture time can greatly impact the titer of ADF. Kaya et al. (16) observed that Pheidole megacephala (Fabricius) heavily scavenged insects killed by entomopathogenic nematodes that were less than 4 days postinfection of the nematode. The data indicated that the ADF titer was low, suggesting that the bacteria did not produce sufficient ADF during the early infection period. Other invertebrate scavengers such as earwigs, cockroaches, and slugs may also feed on nematode-killed insects in the early stages of nematode development and abort IJ production (16). California in vitro experiments (Fig. 2) showed that X. nematophila HGB007 ADF activity against L. humile was greatest when cells were grown for 108 h and P. luminescens HGB008 ADF activity was greatest in samples taken after 132 h of growth, consistent with the results of in vivo experiments. An extensive time course experiment was not conducted at the Wisconsin test site since, under these conditions, bacterial cultures grown for 48 h produced sufficient ADF activity to completely deter L. alienus and F. subsericea ants when resuspended in 25% sucrose.
Our data revealed a number of characteristics of ADF, although it should be stressed that more than one compound could have deterrent activity. ADF from X. nematophila HGB007 is present in the cell-free supernatant and can pass through a 0.45-μm-pore-size Millipore filter, indicating that ADF is extracellular. Autoclaving supernatants did not eliminate ADF activity; therefore, the compound(s) may be nonproteinaceous. Molecular size fractionation of filtered supernatants suggests that ADF from X. nematophila HGB007 is a small compound of less than 10 kDa, and it was found that ADF is sensitive to acidic pH. Interestingly, these attributes are similar to those of β-exotoxin of Bacillus thuringiensis Berliner, an insect feeding deterrent (19).
This work confirms and extends previous observations that the production of ADF activities by entomopathogenic bacteria plays a significant role in protecting nematode-killed insects from scavengers (2). Scavengers can be detrimental to the nematodes if the cadavers are damaged or consumed before the nematodes can complete their life cycle, and the production of ADF by symbiont bacteria may serve to protect the cadaver and the nematodes within. Thus, ADF production can be added to the list of compounds produced by symbiont bacteria (such as antibiotics) that increase the fitness of the nematodes (7).
Scavengers, such as ants, can adversely affect the persistence of entomopathogenic nematodes being used as biological control agents. We have shown that ADF produced by the nematodes' symbiotic bacteria can deter ants from feeding on nematode-killed insects. Future research is needed to elucidate the nature of ADF, isolate the gene(s) required for expression of ADF, and determine the range of its activity against insects and other arthropods or vertebrates. The results of such studies may lead to an enhancement of ADF activity and to ADF-fortified nematode application as a viable approach to agricultural and structural pest management.
Acknowledgments
Work in H. Goodrich-Blair's laboratory was supported in part by USDA/CREES grant CRHF-0-6055 and by National Institutes of Health R01 grant GM59776 (awarded to H.G.B. and used for K.H. support). Work in H. K. Kaya's laboratory was supported in part by the Genetics Resources Conservation Program.
We thank Lester E. Ehler, Eric Martens, and Samantha Orchard for helpful comments on the manuscript.
REFERENCES
- 1.Baker, T. C., S. E. Van Vorhis Key, and L. K. Gaston. 1985. Bait-preference tests for the Argentine ant (Hymenoptera: Formicidae). J. Econ. Entomol. 78:1083-1088. [Google Scholar]
- 2.Baur, M. E., H. K. Kaya, and D. R. Strong. 1998. Foraging ants as scavengers on entomopathogenic nematode-killed insects. Biol. Control 12:231-236. [Google Scholar]
- 3.Bintrim, S. B., and J. C. Ensign. 1998. Insertional inactivation of genes encoding the crystalline inclusion proteins of Photorhabdus luminescens results in mutants with pleiotropic phenotypes. J. Bacteriol. 180:1261-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boemare, N. E., and R. J. Akhurst. 1994. DNA homology between Xenorhabdus and Photorhabdus spp.: convergent evolution of two different genera, p. 59-69. In A. M. Burnell, R. U. Ehlers, and J. P. Masson (ed.), Genetics of entomopathogenic nematode-bacterium complexes. European Commission Publications, Brussels, Belgium.
- 5.Boemare, N. E., R. J. Akhurst, and R. G. Mourant. 1993. DNA relatedness between Xenorhabdus spp. (Enterobacteriaceae), symbiotic bacteria of entomopathogenic nematodes, and a proposal to transfer Xenorhabdus luminescens to a new genus, Photorhabdus gen. nov. Int. J. Syst. Bacteriol. 43:249-255. [Google Scholar]
- 6.Couche, G. A., and R. P. Gregson. 1987. Protein inclusions produced by the entomopathogenic bacterium Xenorhabdus nematophilus subsp. nematophilus. J. Bacteriol. 169:5279-5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forst, S., and D. Clarke. 2002. Bacteria-nematode symbioses, p. 57-77. In R. Gaugler (ed.), Entomopathogenic nematology. CABI Publishing, Wallingford, United Kingdom.
- 8.Forst, S., and K. Nealson. 1996. Molecular biology of the symbiotic-pathogenic bacteria Xenorhabdus spp. and Photorhabdus spp. Microbiol. Rev. 60:21-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Georgis, R., and R. Gaugler. 1991. Predictability in biological control using entomopathogenic nematodes. J. Econ. Entomol. 84:713-720. [Google Scholar]
- 10.Gerritsen, L. J. M., G. de Raay, and P. H. Smits. 1992. Characterization of form variants of Xenorhabdus luminescens. Appl. Environ. Microbiol. 58:1975-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han, R. C., and R. U. Ehlers. 1998. Cultivation of axenic Heterorhabditis spp. dauer juveniles and their response to non-specific Photorhabdus luminescens food signals. Nematologica 44:425-435. [Google Scholar]
- 12.Haney, P. B., R. F. Luck, and D. S. Moreno. 1987. Increases in densities of the citrus red mite Panonychus citri (Acarina: Tetranychidae) in association with the Argentine ant, Iridomyrmex humilis (Hymenoptera: Formicidae) in southern California citrus. Entomophaga 32:49-57. [Google Scholar]
- 13.Kaya, H. K. 2002. Natural enemies and other antagonists, p. 189-203. In R. Gaugler (ed.), Entomopathogenic nematology. CABI Publishing, Wallingford, United Kingdom.
- 14.Kaya, H. K. 1990. Soil ecology, p. 93-115. In R. Gaugler and H. K. Kaya (ed.), Entomopathogenic nematodes in biological control. CRC Press, Boca Raton, Fla.
- 15.Kaya, H. K., and R. Gaugler. 1993. Entomopathogenic nematodes. Annu. Rev. Entomol. 38:181-206. [Google Scholar]
- 16.Kaya, H. K., A. M. Koppenhöfer, and M. Johnson. 1998. Natural enemies of entomopathogenic nematodes. Jpn. J. Nematol. 28:13-21. [Google Scholar]
- 17.Kaya, H. K., and S. P. Stock. 1997. Techniques in insect nematology, p. 281-324. In L. A. Lacey (ed.), Manual of techniques in insect pathology. Academic Press, London, United Kingdom.
- 18.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 19.Mohd-Salleh, M. H., and L. C. Lewis. 1982. Feeding deterrent response of corn insects to β-exotoxin of Bacillus thuringiensis. J. Invertebr. Pathol. 39:323-328. [Google Scholar]
- 20.Poinar, G. O., Jr. 1993. Origins and phylogenetic relationships of the entomophilic rhabditids, Heterorhabditis and Steinernema. Fundam. Appl. Nematol. 16:333-338. [Google Scholar]
- 21.SAS Institute. 1988. SAS user's guide: statistics. SAS Institute, Cary, N.C.
- 22.Smits, P. H. 1996. Post-application persistence of entomopathogenic nematodes. Biocontrol Sci. Technol. 6:379-387. [Google Scholar]
- 23.Vivas, E. I., and H. Goodrich-Blair. 2001. Xenorhabdus nematophilus as a model for host-bacterium interactions: rpoS is necessary for mutualism with nematodes. J. Bacteriol. 183:4687-4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volgyi, A., A. Fodor, A. Szentirmai, and S. Forst. 1998. Phase variation in Xenorhabdus nematophilus. Appl. Environ. Microbiol. 64:1188-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ward, P. S. 1987. Distribution of the introduced Argentine ant (Iridomyrmex humilis) in natural habitats of the lower Sacramento Valley and its effects on the indigenous ant fauna. Hilgardia 55:1-16. [Google Scholar]
- 26.Webster, J. 2002. Bacterial metabolites, p. 99-114. In R. Gaugler (ed.), Entomopathogenic nematology. CABI Publishing, Wallingford, United Kingdom.
- 27.Woodring, J. L., and H. K. Kaya. 1988. Steinernematid and heterorhabditid nematodes: a handbook of biology and techniques. Southern Cooperative Series Bulletin 331. Arkansas Agricultural Experiment Station, Fayetteville.