Abstract
So far, the inability to establish viable Lactobacillus surface layer (S-layer) null mutants has hampered the biotechnological applications of Lactobacillus S-layers. In this study, we demonstrate the utilization of Lactobacillus brevis S-layer subunits (SlpA) for the surface display of foreign antigenic epitopes. With an inducible expression system, L. brevis strains producing chimeric S-layers were obtained after testing of four insertion sites in the slpA gene for poliovirus epitope VP1, that comprises 10 amino acids. The epitope insertion site allowing the best surface expression was used for the construction of an integration vector carrying the gene region encoding the c-Myc epitopes from the human c-myc proto-oncogene, which is composed of 11 amino acids. A gene replacement system was optimized for L. brevis and used for the replacement of the wild-type slpA gene with the slpA-c-myc construct. A uniform S-layer, displaying on its surface the desired antigen in all of the S-layer protein subunits, was obtained. The success of the gene replacement and expression of the uniform SlpA-c-Myc recombinant S-layer was confirmed by PCR, Southern blotting MALDI-TOF mass spectrometry, whole-cell enzyme-linked immunosorbent assay, and immunofluorescence microscopy. Furthermore, the integrity of the recombinant S-layer was studied by electron microscopy, which indicated that the S-layer lattice structure was not affected by the presence of c-Myc epitopes. To our knowledge, this is the first successful expression of foreign epitopes in every S-layer subunit of a Lactobacillus S-layer while still maintaining the S-layer lattice structure.
Many organisms from the domains Bacteria and Archaea possess a surface layer (S-layer) as the outermost structure of the cell envelope. S-layers are composed of regularly arranged proteinaceous subunits of a single protein or glycoprotein species with molecular masses ranging from 40 to 170 kDa (43, 48). S-layer proteins represent 10 to 15% of the total protein of the bacterial cell, and S-layer lattices cover the cell surface during all stages of growth, which indicates that efficient gene expression, S-layer protein synthesis, and secretion take place (4). A high content of hydrophobic and acidic amino acids and a low theoretical isoelectric point (pI) are typical features of S-layer proteins (48). In contrast, very high pI values have been described for the S-layer proteins from various lactobacilli (4, 54) and Methanothermus fervidus (6). In general, S-layers have been considered to function as cell shape determinants, protective coats, promoters for cell adhesion and surface recognition, and molecular and ion traps; however, no general function found in all S-layers has been recognized (47, 48).
S-layers have been found from many species of the genus Lactobacillus (31, 56). However, the S-layer protein genes have been cloned and sequenced only from Lactobacillus brevis ATCC 8287 (54) and from the closely related species L. acidophilus (3), L. helveticus (8), and L. crispatus (46). The molecular masses of Lactobacillus S-layer proteins are among the smallest (43 to 46 kDa) known for the S-layer proteins, and in the L. acidophilus group they have sequence similarity mainly in the C-terminal part of the proteins. A highly conserved domain was recently shown to mediate the binding of the S-layer protein subunits to the cell wall in the C-terminal part of the L. acidophilus group S-layer proteins (49). However, in L. brevis strains, the C-terminal part of the S-layer protein is highly heterogenous and lacks a common cell wall anchoring domain (A. Palva et al., unpublished results). The S-layer protein gene (slpA) of L. brevis ATCC 8287 (54) is expressed by two active adjacent slpA promoters (17), and its expression and secretion signals direct high-level heterologous protein secretion and intracellular protein production in various Lactococcus and Lactobacillus hosts (18, 44). This L. brevis strain also strongly adheres to several human epithelial cell types and fibronectin via a receptor-binding site that is located at the N- terminal region of the slpA gene product (16). Adherence of L. brevis ATCC 8287 has also been demonstrated to pig intestinal epithelial cells (M. Jakava-Viljanen et al., unpublished results).
Lactic acid bacteria (LAB), especially lactococci and lactobacilli, are currently being developed as antigen delivery vehicles (36, 37, 45). Delivery of antigens to mucosal surfaces by LAB has been considered to offer a safer alternative to live attenuated pathogens such as Salmonella, Mycobacterium, Bordetella, and Vibrio, which have been widely studied as putative vaccine vectors (7, 32, 40, 51). Lactobacilli have several properties that make them attractive candidates for mucosal vaccine delivery vehicles. Due to their long history of use in dairy and other food fermentations, lactobacilli can be generally recognized as safe. Furthermore, many Lactobacillus strains possess intrinsic adjuvanticity (28, 33) and health-promoting, i.e., probiotic properties (53). The main advantages of lactococci as vaccine vectors include their food grade status and their use as well-developed genetic tools. With lactococci and lactobacilli, different cellular locations have been tested for antigen production (34, 37). A very large number of surface layer protein subunits present in an S-layer and the possibility to detach the S-layer from the cell to a cell-free self-assembling S-layer protomer would make the use of a Lactobacillus S-layer a very interesting alternative to surface display antigens in LAB. In addition to the well-characterized S-layer protein of L. brevis ATCC 8287, this strain is a promising host candidate for use as a live mucosal antigen delivery vehicle because it possesses several properties required from a probiotic bacterium. These L. brevis traits include tolerance to low pH, bile acids, and pancreatic fluid in vitro and survival in the gastrointestinal tract in vivo (38), antimicrobial activity against some pathogens (20, 38) and ability to adhere to several types of human intestinal epithelial cells (16).
In this study, we describe the successful expression of two different model epitopes from two insertion sites of the L. brevis slpA gene. With a nisin-inducible plasmid system, a chimeric S-layer was produced in which only a minor portion of the S-layer protein subunits carried the epitope. By using the thermosensitive (ts) plasmids pVE6007 and pKTH5115, a pORI280 derivative, a stable integrant strain was formed which produced a uniform S-layer with all the S-layer protein subunits expressing the c-Myc epitope on their surface.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The strains and plasmids used in this study are listed in Table 1. Lactobacillus strains were grown at 37°C in MRS broth (Difco, Detroit, Mich), and Lactococcus lactis strains were grown at 30°C in M17 (Difco) containing 0.5% (wt/vol) glucose. Escherichia coli strains were cultivated in Luria- Bertani medium at 37°C under aeration. When appropriate, media were supplemented with the following antibiotics: chloramphenicol, 10 μg/ml (for L. lactis) or 7.5 μg/ml (for L. brevis); erythromycin, 7.5 μg/ml (for L. brevis); ampicillin, 100 μg/ml (for E. coli). Plasmid pNZ9530 is a low-copy-number expression vector carrying the regulatory genes nisR and nisK required for nisin-induced transcription from PnisA (19). The optimal nisin concentration for pNZ9530 was determined to be 10 ng/ml.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Source or referencea |
|---|---|---|
| Strains | ||
| L. brevis ATCC 8287 (GRL1) | ATCC | |
| L. lactis MG1363 | Plasmid-free derivative of NCDO 712, Lac− | 12 |
| L. lactis NZ9000 | pepN::nisR nisK | 9 |
| L. lactis LL108 | RepA+ MG1363, Cmr, carrying multiple copies of the pWV01 repA gene in the random chromosomal fragment A | 25 |
| L. brevis GRL1045 | Emr Cms, derivative of GRL1 with the c-Myc epitope insert in the genomic slpA and unintegrated pKTH5115 | This study |
| L. brevis GRL1046 | Emr Cms, derivative of GRL1 with the c-Myc epitope insert in the genomic slpA | This study |
| Plasmids | ||
| pLET1 | Cmr Kmr, lactococcal expression vector with T7 RNA polymerase promoter | 55 |
| pKTH 2151 | Cmr Kmr, pLET1 derivative carrying the L. brevis slpA gene | This study |
| pKTH2152 | Cmr Kmr, pKTH 2151 derivative; slpA fused with a poliovirus VP1 epitope insert in insertion site I | This study |
| pNZ8032 | Cmr, pNZ8008 derivative carrying the gusA gene translationally fused to the nisA promoter (PnisA) | 10 |
| pNZ9530 | Emr, nisRK cloned in pIL252, expression of nisRK driven by rep readthrough | 19 |
| pKTH5006 | Cmr, pNZ8032 derivative carrying the slpA gene under PnisA; the VP1 epitope encoding nucleotides in slpA insertion site I | This study |
| pKTH5007 | Cmr, as pKTH5006, the VP1 insert in slpA insertion site II | This study |
| pKTH5008 | Cmr, as pKTH5006, the VP1 insert in slpA insertion site III | This study |
| pKTH5063 | Cmr, as pKTH5006, the VP1 insert in slpA insertion site IV | This study |
| pKTH5074 | Cmr, as pNZ8032 derivative carrying the slpA gene under PnisA; slpA fused with c-Myc epitope encoding nucleotides in slpA insertion site II | This study |
| pVE6007 | Cmr, ts derivative of pWV01 | 29 |
| pORI280 | Emr, lacZ+ ori+ derivative of pWV01, replicates only in strains providing RepA in trans | 25 |
| pKTH5115 | Emr, pORI280 derivative with slpA lacking its promoters, RBS, and transcription terminator and carrying the c-Myc | This study |
Cmr, resistance to chloramphenicol; Kmr, resistance to kanamycin; Emr, resistance to erythromycin; ATCC, American Type Culture Collection.
DNA modifications and transformation.
Routine molecular biology techniques were performed essentially as described previously (41). Plasmid DNA was isolated from L. lactis and L. brevis by using the QIAfilter Plasmid Midi Kit (Qiagen GmbH, Hilden, Germany) and 8 mg of lysozyme per ml and from E. coli by using the Wizard Minipreps kit (Promega, Madison, Wis.). DNA restriction enzymes and modification enzymes were used as recommended by the manufacturers (Promega; New England Biolabs Inc., Beverly, Mass.). Transformation of L. lactis was done by the method of Holo and Nes (14), and transformation of L. brevis was done essentially as described by Bhowmik and Steele (1).
Oligonucleotides and DNA sequencing.
The oligonucleotides used in this study are listed in Table 2. They were purchased from the oligonucleotide synthesis service of MedProbe (Norway) or from MWG-Biotech AG (Germany). Nucleotide sequencing was performed by the dideoxy chain termination method of Sanger et al. (42) by using an ABI Prism 310 Genetic Analyzer (Applied Biosystems, Foster City, Calif.) with the ABI Prism BigDye Terminators v3.0 cycle-sequencing kit (Applied Biosystems).
TABLE 2.
Oligonucleotides used in the study
| Oligonucleotide | Nucleotide sequence (5′→3′)a | Useb |
|---|---|---|
| p1 | CGATCCTGCTTTAACTGCTGTTGAAACTGGTGCTACTAT | VP1 |
| p2 | CGATAGTAGCACCAGTTTCAACAGCAGTTAAAGCAGGAT | VP1 |
| p3 | CTGAGAATTCAGTTACAGCAACCAACG | PCR |
| p4 | TTTAAAGCTTGTTTTTCCTAACAAAGGCC | PCR |
| p5 | CCATGGTACAATCAAGTTTAAAGAAATCTC | PCR |
| p6 | CCTGCTTTAACTGCTGTTGAAACTGGTGCTACTGCCGCCGATCAAACTGCTC | R-PCR, site II/VP1 |
| p7 | AGTAGCACCAGTTTCAACAGCAGTTAAAGCAGGCTTATCGCTTACCTTAGAACCTG | R-PCR, site II/VP1 |
| p8 | CCTGCTTTAACTGCTGTTGAAACTGGTGCTACTAATGATAAGGTTGCAGCTAACG | R-PCR, site III/VP1 |
| p9 | AGTAGCACCAGTTTCAACAGCAGTTAAAGCAGGAGCATCAGCTGTAGTCAATGC | R-PCR, site III/VP1 |
| p10 | TACCGAATTCGGGACAGGTGCTAGAGAC | PCR |
| p11 | AGTAGCACCAGTTTCAACAGCAGTTAAAGCAGGAGCCATAGTAGCCTTAGAAG | R-PCR, site IV/VP1 |
| p12 | CCTGCTTTAACTGCTGTTGAAACTGGTGCTACTAAGTTAGCTTCTTCAAAGAGTC | R-PCR, site IV/VP1 |
| p13 | GTATGAATTCGAAATGACTTCAGAAAAGG | PCR |
| p14 | GACAGGATCCATATAGAAGAAAAGGGC | PCR |
| p15 | CACAAAGCTTTGAAGAAGCAGCACTGTCC | PCR |
| p16 | CCAAGATCTAGTCTTATAACTATACTG | PCR |
| p17 | CAGATCCTCTTCTGAGATGAGTTTTTGTTCCTTATCGCTTACCTTAGAACCTG | R-PCR, site II/c-Myc |
| p18 | GAACAAAAACTCATCTCAGAAGAGGATCTGGCCGCCGATCAAACTGCTC | R-PCR, site II/c-Myc |
| p19 | GTTCGGTACCATCAACAAAGCTCACCTA | PCR |
| p20 | CTGAGGTACCTCTCTTTACTTGGGCCTTGC | PCR |
| p21 | CAGATCCTCTTCTGAGATGAG | PCR |
| p22 | TCATCTCAGAAGAGGATCTG | PCR |
| p23 | ACTATCCCGACCGCCTTAC | PCR |
| p24 | CGTAACTGCCATTGAAATAGACC | PCR |
| p25 | GAGATAAGAATTGTTCAAAGCTA | PCR |
| p26 | TGCAGGTACCGATTACAAAGGCTTTAAGC | PCR |
| P27 | CATGGGATCCCTGGTACACAAGTACTTGG | PCR |
| p28 | ATTTGAATTCAGCAGCATAGACTGTTGAG | PCR |
The epitope-encoding nucleotides are underlined.
R-PCR, recombinant PCR; VP1, a poliovirus type 3 VP1 epitope; c-Myc, an epitope from the human c-myc proto-oncogene; sites II to IV refer to the insertion sites of the epitope-encoding nucleotides in the slpA gene.
Plasmid constructions.
Four putative epitope insertion sites in the slpA gene of L. brevis ATCC 8287 (designated L. brevis GRL1 below) were chosen on the basis of the hydrophilicity profile of the SlpA protein analyzed by the PC-Gene program Antigen (Genofit, Geneva, Switzerland). A DNA fragment encoding the poliovirus type 3 VP1 epitope (amino acid sequence PALTAVETGAT), which forms part of an immunodominant region of the VP1 capsid protein of enteroviruses (15), was inserted into four different insertion sites (I to IV) in the L. brevis slpA gene. A DNA region derived from the human c-myc proto-oncogene (11) and encoding was inserted into insertion site II.
Insertion site I consisted of a native ClaI restriction site between nucleotides 1447 and 1452 in slpA (GenBank accession number Z14250). A double-stranded hybrid of oligonucleotides p1 and p2 (Table 2), containing the nucleotides encoding the VP1 epitope and ClaI cohesive ends, was cloned as the ClaI fragment into pKTH2151, resulting in pKTH2152. An EcoRI-BglII fragment from pKTH2152 was ligated with a BglII-digested PCR product amplified with primers p3 and p4 (Table 2) from chromosomal DNA of L. brevis. The PCR product amplified with primers p3 and p4 contained the translation stop codon and the transcription terminator of the slpA gene, missing from the slpA region cloned in pKTH2152. The ligation mixture was used as the template in PCR with the primer pair p5 and p4 (Table 2), resulting in a PCR product carrying new NcoI and HindIII sites at its 5′ and 3′ ends, respectively, and an extra codon for a valine (V) residue after the translation initiation codon. The resulting PCR product was transferred as the NcoI-HindIII fragment into pNZ8032, resulting in pKTH5006.
Cloning of the VP1 epitope-encoding nucleotides into insertion sites II, III, and IV and c-myc into insertion site II was carried out by applying the recombinant PCR technique essentially as described previously (13, 21). Briefly, to insert the VP1 epitope-encoding DNA between nucleotides 1110 and 1111 (insertion site II) of slpA, two PCR products, which were amplified with the primer pairs p6 plus p4 and p5 plus p7 (Table 2) from GRL1 DNA, were hybridized and the hybrid formed was PCR amplified with primers p5 and p4. The resulting PCR product had an extra codon for V after the slpA translation initiation codon generated by primer p5 and added NcoI and HindIII sites at its 5′ and 3′ ends, respectively. The PCR product was cloned as the NcoI-HindIII fragment into pNZ8032, resulting in pKTH5007. Insertion of the VP1 epitope-encoding region between slpA nucleotides 1305 and 1306 (insertion site III) was done essentially as described for the insertion site II, except that slpA fragments PCR amplified with the primer pair p8 and p9 (Table 2) were used and the primer pair p4 and p5 was used in the formation of the double-stranded recombinant molecule. The plasmid formed after the cloning of the NcoI-HindIII PCR fragment into pNZ8032 was designated pKTH5008. The insertion of the VP1 epitope-encoding region between slpA nucleotides 504 and 505 (insertion site IV) was carried out similarly using primer pairs p10 plus p11 and p12 plus p13 to generate the PCR products for hybridization, and the further PCR amplification of the hybrid formed was done with primers p14 and p15. The resulting PCR product was then transferred as a PstI fragment into pKTH5007, giving pKTH5063.
Cloning of the c-Myc epitope encoding nucleotides into the insertion site II was carried out by first generating PCR products for hybridization with the primer pairs p16 plus p17 and p18 plus p4 (Table 2) and pKTH5007 as the template. The p16- plus p17-amplified fragment contained the nisA promoter and nucleotides 1 to 1110 from the slpA gene, and the p18- plus p4-amplified fragment carried the 3′ end of the slpA gene starting from nucleotide 1111 and spanning the slpA transcription terminator region. The p17 and p18 R-PCR primers also contained the c-myc region. The hybridized PCR fragments were PCR amplified with primers p16 and p4, and the resulting PCR product was cloned as an NcoI-HindIII fragment into pKTH5007, giving plasmid pKTH5074. DNA sequencing of pKTH5074 revealed a point mutation changing nucleotide 1111 from G to A, thus changing the first amino acid after the c-Myc epitope insertion site from alanine to threonine.
The slpA gene without its promoters, ribosome binding site, (RBS) and transcription terminator, but containing the c-Myc epitope-encoding nucleotides in insertion site II, was amplified from pKTH5074 by using primers p19 and p20, resulting in an approximately 1.4-kb PCR fragment with added KpnI sites at its 5′ and 3′ ends. The amplified PCR product was transferred as a KpnI fragment into pORI280, resulting in pKTH5115. Plasmid pKTH5115 can replicate only when RepA is provided in trans; therefore, pKTH5115 was first constructed in Lactococcus lactis LL108, providing chromosomally encoded RepA. All the plasmid constructs were verified by DNA sequencing.
Integration of the c-myc region on the GRL1 chromosome.
The ts plasmids pVE6007, which encodes RepA protein at the permissive temperature, and pKTH5115 were subsequently used to transform L. brevis GRL1. To cause the loss of pVE6007 and concomitant chromosomal integration of pKTH5115 via its homology regions with the genomic slpA gene at the restrictive temperature, GRL1 cells harboring pVE6007 and pKTH5115 were first grown overnight at +28°C in MRS broth supplemented with 7.5 μg each of erythromycin and chloramphenicol per ml. From overnight culture, a 1% inoculation was made into a MRS broth supplemented with erythromycin (5 μg/ml) and the bacteria were grown at 28°C to an optical density at 600 nm (OD600) of 0.2. Thereafter the culture was transferred to 39°C and incubated to an OD600 of 1.0. A 0.2% dilution of the culture was made into MRS broth supplemented with 5 μg of erythromycin per ml, and the culture was incubated at 39°C overnight. Thereafter, the culture was again diluted and plated on MRS plates supplemented with 5 μg of erythromycin per ml. After overnight incubation at 39°C, colonies were picked and streaked on MRS plates supplemented either with erythromycin at 5 μg/ml or with chloramphenicol at 7.5 μg/ml. Subsequent confirmation of Cms Emr colonies was carried out by PCR. The L. brevis strain carrying the c-myc in the genomic slpA was designated GRL1046.
Enzymatic assay for the detection of the S-layer-displayed epitope.
Cells, harvested after overnight incubation with or without overnight induction by nisin (10 ng/ml), were resuspended in phosphate-buffered saline (PBS) to an OD600 of 1.0. Aliquots of 1 ml from these solutions were centrifuged at 3,000 × g and washed twice with phosphate-buffered saline PBS before being resuspended in 200 μl of PBS. Resuspended cells were mixed with 200 μl of horseradish peroxidase-conjugated anti-Myc antibody (InVitrogen, Leek, The Netherlands) or with polyclonal antibodies obtained by immunization with a synthetic peptide designed on the basis of the VP1 sequence of type 3 poliovirus strain Sabin (anti-VP1 antibody [13]), diluted 1:50 in PBS, and incubated for 1 h. After incubation with antisera, cells were washed twice with PBS and anti-VP1 antibody-treated cells were incubated for 1 h with HRP-conjugated goat anti-rabbit immunoglobulin G (IgG) (Bio-Rad Laboratories, Richmond, Calif.), diluted 1:100, and washed twice with PBS. The cells were then washed once with the substrate buffer (40 mM sodium acetate, 40 mM sodium citrate [pH 4.4]) and resuspended in 1 ml of the same buffer. They were further diluted 1:5 to 1:50 into substrate buffer, resulting in cell suspensions with OD600 values of 0.2 to 0.02. Microtiter plates were loaded with 100 μl of each cell suspension, and plates were developed essentially as described earlier (22).
Detection of L. brevis S-layer proteins.
GRL1 and GRL1046 cells were grown to an OD600 of 0.8 in MRS broth, harvested by centrifugation (2 min at 18,000 × g), and washed once with PBS. The pellet, equivalent to 1.5 ml of culture, was dissolved directly in 50 μl of Laemmli buffer. The polypeptides were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) by the method of Laemmli (23) and visualized by Coomassie brilliant blue staining. The growth supernatants, concentrated by ultrafiltration, were analyzed for S-layer protein content by SDS-PAGE.
Isolation of S-layer proteins.
The S-layer proteins were extracted from overnight L. brevis cells by using 6 M LiCl. Cells from 100 ml of culture were harvested and washed once with PBS. The pellet was resuspended in 10 ml of 6 M LiCl and incubated for 30 min at room temperature followed by centrifugation (15,000 × g for 15 min). The supernatant was dialyzed against distilled H2O overnight at 4°C and centrifuged (10,000 × g for 30 min). The pellet containing the S-layer self-assembly products was resuspended in water and kept at −20°C until further use. For matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry, the protein samples were dissolved in 0.1% trifluoroacetic acid and analyzed on a Biflex time-of-flight instrument (Bruker-Daltonics, Bremen, Germany) equipped with a nitrogen laser operating at 337 nm essentially as described previously (35). Briefly, analyses were carried out in the linear positive-ion delayed-extraction mode using saturated sinapic acid in a mixture of 0.1% trifluoroacetic acid and 50% acetonitrile (1:2) as a matrix. Samples were prepared by mixing 1 μl of protein with 1 μl of sinapic acid matrix on the target plate and dried under a gentle stream of warm air. All mass spectra were calibrated externally with bovine serum albumin as a standard. For the prediction of the molecular masses of S-layer proteins, the ProtParam tool (available at http://www.expasy.ch/tools/protparam.html) was used.
Immunofluorescence.
Bacteria were harvested from overnight cultures or after nisin induction, washed with PBS, applied to microscope slides, and fixed with 2% (vol/vol) formaldehyde. The slides were incubated for 1 h with diluted (1:20 to 1:160 in PBS) anti-VP1 antibody or with diluted (1:10 to 1:40) anti-Myc antibody (Invitrogen) in a moist chamber at 37°C, washed twice with PBS, and finally incubated for 1 h in a moist chamber at 37°C either with fluorescein isothiocyanate (FITC)-conjugated swine anti-rabbit IgG antiserum (dilution 1:20; Dako A/S, Copenhagen, Denmark,) or with FITC-conjugated rabbit anti-mouse IgG antiserum (dilution 1:100; Jackson ImmunoResearch Laboratories Inc., West Grove, Pa.). After two washes with PBS, the slides were mounted by using Fluoprep (bioMérieux sa, Marcy l'Etoile, France) and examined under a UV microscope.
Southern blot hybridization.
Restriction enzyme-digested chromosomal DNA was separated on a 0.8% agarose gel and transferred to a nylon membrane essentially as described by Southern (50). The PCR probe specific for the slpA gene was amplified from GRL1 chromosomal DNA using primers p19 and p20, purified by using the QIAquick PCR purification kit (Qiagen), and labeled with digoxigenin-dUTP using the DIG high-prime kit (Roche Diagnostics, Basel, Switzerland). Hybridization and probe detection were performed as specified in the instructions provided with the DIG detection system (Roche Diagnostics).
EM.
GRL1 and GRL1046 cells from prolonged cultivation on MRS agar plates were studied by the negative-stain technique. A drop of suspension was put on a grid coated with Formvar film and carbon. After 30 s, the drop was dried with a piece of filter paper and a drop of 1% phosphotungstic acid (pH 7.0) was added on the grid. After 1 min, the stain was dried as described above and the specimen was studied by JEOL 1200-EX transmission electron microscopy (EM) at 60 kV (26).
RESULTS
Epitope insertion sites in the slpA gene.
To study the surface expression of foreign epitopes on the L. brevis S-layer, we chose insertion sites in the slpA gene on the basis of the hydrophilicity profile of the SlpA protein. The four most hydrophilic parts of the SlpA protein were chosen for testing, since it was expected that part of them were likely to be sites where epitopes would be accessible to the cell surface. The four epitope insertion sites I to IV in the slpA gene product tested were between amino acid residues Asp362 and Thr363, Lys249 and Ala250, Ala313 and Asn314 and Ala49 and Lys50 of the mature SlpA, respectively. An 11-amino-acid immunodominant region of the VP1 capsid protein of enteroviruses (15) and a 10-amino-acid c-Myc epitope (11) were used as model epitopes. The nucleotides encoding the VP1 epitope were inserted in all four insertion sites, whereas the nucleotides encoding the c-Myc epitope were inserted only in site II.
Construction of epitope-expressing L. brevis strains producing chimeric S-layers.
Plasmids pKTH5006, pKTH5007, pKTH5008, pKTH5063, and pKTH5074, which carried under the nisA promoter (PnisA) (10) the L. brevis slpA gene and the VP1 epitope DNA in slpA insertion sites I, II, III, and IV and c-myc in insertion site II, respectively (Table 1 and Materials and Methods), were first constructed in L. lactis, and their DNA was sequenced to verify their correctness. All five plasmids were used to transform L. brevis GRL1, harboring pNZ9530 for nisin induction. All the double transformants were stable, and the optimal nisin concentration was determined.
To study the surface accessibility of the VP1 epitopes expressed from pKTH5006, pKTH5007, pKTH5008, or pKTH5063 and the c-Myc epitope expressed from pKTH5074, whole-cell enzyme-linked immunosorbent assays (ELISA) using anti-VP1 or anti-c-Myc antibodies were performed. The strongest color response in the enterovirus epitope constructs was obtained with the recombinant strain harboring pKTH5007, compared with the OD values obtained with wild-type GRL1 cells (data not shown), demonstrating that the VP1 epitope in site II was accessible on the surface of the recombinant GRL1 cells. Recombinant GRL1 carrying pKTH5006 also gave a positive color response, whereas with the other constructs, the OD values remained at the same level as that with the wild-type GRL1 strain. Accordingly, recombinant GRL1 strain harboring pKTH5074, which carried the c-Myc epitope-encoding nucleotides in the insertion site II, also gave a positive signal in the whole-cell ELISA (data not shown).
The surface accessibility of the test epitopes in the recombinant GRL1 strains with pKTH5007, pKTH5006, or pKTH5074, in addition to the control GRL1 strain with pNZ9530, was also assayed by immunofluorescence microscopy using either anti-VP1 or anti-c-Myc antibodies and a FITC-conjugated secondary antibody. In this assay, however, the epitopes could not be detected (data not shown).
Gene replacement of the native L. brevis slpA gene with the slpA-c-myc-fusion with the integration plasmid pKTH5115.
To increase the expression level of the epitope-SlpA protein from that obtained with the nisin-inducible double-plasmid GRL1 strains, different gene replacement systems to integrate the c-Myc epitope-encoding nucleotides into the chromosomal slpA gene were tested (data not shown). The successful gene replacement strategy involved the use of two pWV01-derived plasmids, i.e., pKTH5115, which is a pORI280 (25) derivative constructed in this study to contain c-myc in slpA insertion site II, and pVE6007 (29), which is a ts derivative of pWV01. To allow the integration event to occur, the growth temperature of a transformant, harboring both plasmids, was shifted from 28 to 39°C under erythromycin selection. After this enrichment, 50 colonies were selected for further testing, and 8 of them were found to be Cms, indicating the loss of pVE6007. PCR and DNA-sequencing analyses (data not shown) showed that in one of these transformants, a direct double-crossover integration of c-myc into the GRL1 chromosome had occurred. However, this transformant, designated GRL1045, still harbored unintegrated plasmid pKTH5115. To cure GRL1045 from the unintegrated pKTH5115, it was cultivated for approximately 50 generations in MRS broth without antibiotic selection, resulting in 1 Ems colony from approximately 400 colonies tested. This strain, designated L. brevis GRL1046, was chosen for further analyses.
To confirm the presence of the genomic slpA-c-myc fusion and the absence of the intact slpA and pKTH5115 in GRL1046, PCR analyses and DNA sequencing were carried out. When PCR primers specific for the slpA gene region outside of that cloned in pKTH5115 and for c-myc were used, PCR products of the expected sizes were amplified from GRL1046 (Fig. 1A, lanes 2 and 4) whereas no PCR products were formed from the GRL1 control DNA (lanes 1 and 3). When a primer pair specific for c-myc and pORI280 was used (lanes 5 and 6), no products were amplified from GRL1046 or from GRL1. A PCR product of the expected size was amplified from pORI280 but not from GRL1046 when primers specific for pORI280 were used (lanes 7 and 8). With PCR primers annealing at close proximity on both sides of the c-myc insertion site in slpA, a clear size difference between the amplified PCR products of GRL1 and GRL1046 was detected (Fig. 1B). By PCR analysis, it was thus verified that the DNA region encoding the c-Myc epitope was located in insertion site II in the slpA gene of GRL1046 and that this strain did not harbor unintegrated or integrated plasmid pKTH5115 or the wild-type slpA gene. DNA sequencing (data not shown) showed that no unexpected mutations had taken place in the slpA gene region of GRL1046.
FIG. 1.
PCR analysis of the slpA-c-myc gene replacement in L. brevis GRL1046. (A) Total chromosomal DNA of the GRL1046 (lanes 2, 4, 6, 7, and 10) and GRL1 wild-type (lanes 1, 3, 5, and 9) strains and pORI280 plasmid DNA (lane 8) were used as templates in PCR with the primer pairs p14 and p21 (lanes 1 and 2), p22 and p4 (lanes 3 and 4), p22 and p23 (lanes 5 and 6), p24 and p25 (lanes 7 and 8) and p26 and p13 (lanes 9 and 10). (B) Total DNA of GRL1 (lane 1) and GRL1046 (lane 2) amplified with primers p27 and p28. PCR products were separated by electrophoresis on a 0.8% (A) or 2% (B) agarose gel and stained with ethidium bromide. Values of the molecular size markers (SmartLadder; Eurogentec) are indicated on the left (A) or on the right (B).
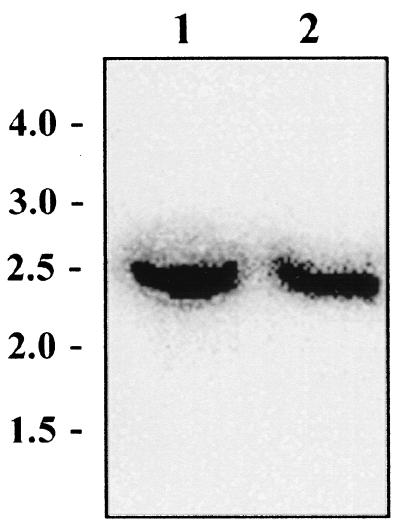
Southern hybridization was performed on EcoRI-digested chromosomal DNA of strains GRL1046 and GRL1 to verify that no chromosomal rearrangements had occurred in strain GRL1046. A PCR-amplified slpA specific probe hybridized to an approximately 2.5-kb fragment in both GRL1046 and GRL1 (Fig. 2), indicating that, excluding the c-myc insert in GRL1046, no major changes had occurred on the slpA gene region in these strains.
FIG. 2.
Southern blot analysis of L. brevis strains GRL1046 and GRL1. Total DNA extracted from strains GRL1046 (lane1) and GRL1 (lane 2) was digested with EcoRI and hybridized with a 1,406-bp internal slpA fragment probe amplified with the primer pair p19 and p20. Values (in kilobase pairs) of the molecular size markers (SmartLadder) are indicated on the left.
Characterization of the S-layer protein produced by the integrant strain GRL1046.
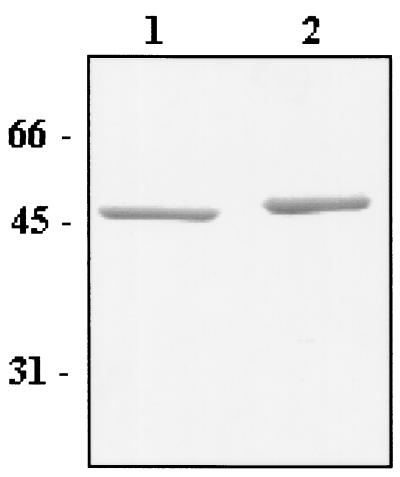
SDS-PAGE analysis of whole cells of L. brevis GRL1046 strain revealed only one protein band with an apparent molecular mass of 46 kDa, representing the putative recombinant S-layer protein (Fig 3, lane 2). The electrophoretic mobility of the S-layer protein of strain GRL1046 was slightly lower than that of strain GRL1 (lane 1). There were no significant differences in the amount of the S-layer proteins in GRL1 and GRL1046, indicating equally strong expression of SlpA in these strains (Fig. 3). Concentrated growth supernatants of both GRL1 and GRL1046 were analyzed by SDS-PAGE, but no detectable release of SlpA into the culture medium could be found from either of the strains studied (data not shown).
FIG. 3.
SDS-PAGE analysis of L. brevis GRL1 and GRL1046 cells. Equal amounts of GRL1 (lane 1) and GRL1046 (lane 2) cells from an OD600 of 0.8 were boiled in Laemmli sample buffer before being subjected to protein gel analysis. Values of the molecular mass marker proteins are indicated on the left.
For an exact determination of the molecular masses of the S-layer protein subunits produced by GRL1046 and GRL1, S-layer proteins were isolated by LiCl extraction. By MALDI-TOF mass spectrometry analysis, the molecular masses of the isolated SlpA proteins of GRL1046 and GRL1 were determined to be 46,399 and 45,143 Da, respectively. These values were in accordance with the molecular masses of 46,448 and 45,232 Da, from the amino acid sequences of the S-layer proteins of GRL1046 and GRL1, respectively, predicted by the ProtParam tool. The MALDI-TOF analysis thus confirmed that strain GRL1046 produced only the SlpA-c-Myc epitope fusion protein, leading to the observed 1,256-Da size difference between the intact and recombinant S-layer protein subunits.
Surface display of the c-Myc epitope from the integrant strain GRL1046.
The surface accessibility of the c-Myc epitope on the recombinant S-layer protein subunits produced by strain GRL1046 was verified by whole-cell ELISA and immunofluorescence microscopy. For the whole-cell ELISA, equal amounts of GRL1 and GRL1046 cells were harvested and subjected to enzymatic detection with anti-Myc antibodies. The ELISA color shift increased with increasing cell density of the GRL1046 strain, whereas the color shift for GRL1 remained at a very low level (Fig 4). This demonstrated that the c-Myc epitope incorporated into the S-layer protein subunits of GRL1046 was accessible on the cell surface.
FIG. 4.
Whole-cell ELISA for detection of surface-exposed c-Myc epitope in L. brevis GRL1046. Anti-Myc antibody was allowed to bind to lactobacillar cells, after the addition of a horseradish peroxidase conjugate, different lactobacillar cell densities were incubated with a chromogenic substrate. Symbols: •, GRL1046; ▴, GRL1 wild-type strain.
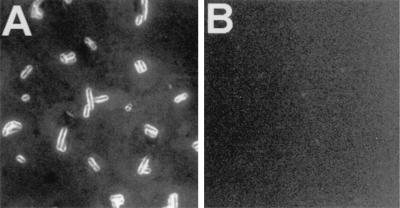
Immunofluorescence microscopy verified the results obtained by whole-cell ELISA. When reacted with anti-Myc antibodies, L. brevis GRL1046 cells exhibited a strong fluorescence at the cell surface (Fig. 5A), confirming that the c-Myc epitope was indeed surface exposed in this strain. Instead, no fluorescence was observed with wild-type GRL1 cells reacted with anti-Myc antibodies (Fig. 5B).
FIG. 5.
Immunofluorescence microscopy of L. brevis GRL1046 (A) and wild-type L. brevis GRL1 (B). Bacteria harvested from overnight cultures were treated with anti- Myc antibodies (diluted 1:10 in PBS) and FITC-conjugated secondary antibody. Both pictures were taken after 5-s exposures. Magnification, ×4,000.
S-layer formation in L. brevis GRL1046.
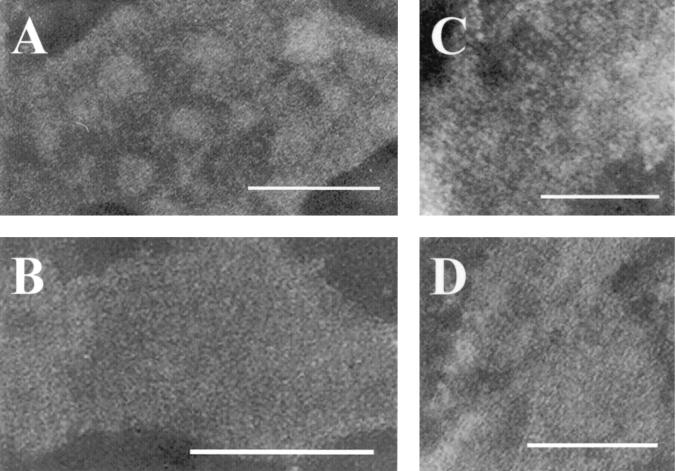
To study the effect of the c-Myc epitope on the S-layer protein lattice structure of GRL1046, negative staining of L. brevis GRL1046 and GRL1 cells from aged colonies and of LiCl-isolated S-layer protein preparations was analyzed by transmission electron microscopy. In the electron micrographs, similar structures with an oblique arrangement of the subunits could be clearly observed from both strains (Fig. 6A and B). The oblique periodicity was also very clearly seen in the S-layer protein preparations isolated with LiCl from both strains GRL1046 (Fig. 6C) and GRL1 (Fig. 6D). Thus, the c-Myc epitope expressed in the slpA insertion site II did not appear to affect either the ability of GRL1046 to form a native-like S-layer lattice in vivo or the self-assembly of the S-layer in vitro.
FIG. 6.
Transmission electron microscopy of L. brevis GRL1046 and GRL1 S-layers. Negative staining was performed with cells of GRL1046 (A) and GRL1 (B) derived from aged colonies and with S-layer protein preparations from LiCl isolations of GRL1046 (C) and GRL1 (D). Bars, 0.1 μm.
DISCUSSION
In this paper, we describe constructions of L. brevis strains displaying heterologous model epitopes, a VP1 enterovirus epitope of 11 amino acids and a c-Myc epitope of 10 amino acids, as part of the outermost proteinacous S-layer of the cell. Four putative insertion sites in the slpA gene, based on a hydrophilicity profile of the SlpA protein, were tested to reveal the region allowing the most efficient surface expression of the epitopes. The number of insertion sites to be tested was restricted because the L. brevis slpA gene contains a large number of inverted and direct repeats (54), and it is very difficult to clone due to its instability and toxicity in heterologous hosts. Furthermore, genetic modifications of the L. brevis slpA gene have been hampered by the inability to establish a viable slpA-null mutant and the low transformation frequency of L. brevis (Palva et al., unpublished). It has become common knowledge in Lactobacillus S-layer research that the S-layer protein genes of other Lactobacillus species, even though some of them are easier to clone and modify in heterologous hosts than that of L. brevis, have also been recalcitrant for inactivation.
In this study, we were, however, able to establish chimeric S-layer by expressing the epitope carrying the slpA gene in a plasmid under the inducible nisin promoter system in L. brevis that was still producing chromosomally encoded wild-type S-layer proteins. This allowed us to identify two slpA insertion sites resulting in surface display of the antigen of interest. The expression level of the epitopes in this system was, however, insufficient. This was probably due to the low expression level of the epitope-SlpA constructs under the nisA promoter compared to the high-level expression of the chromosomal slpA gene, which was still present in these double-plasmid strains.
For expression of the desired epitope at a very high level in a uniform recombinant S-layer, several ts integration vectors were tested to obtain gene replacement by two subsequent single-crossover events (data not shown). With these vectors, a primary integration of the model epitopes to slpA could be demonstrated but the transformants obtained turned out to be highly unstable (data not shown). Development of a gene replacement system was also hampered by the lack of certainty that the epitopes in the chosen insertion sites would not be toxic or retard cell growth when produced as part of a uniform S-layer. Finally, by using a gene replacement method based on the two-plasmid (pORI280 and pVE6007) integration system, a pure double- crossover integrant, producing a uniform modified S-layer, could be obtained. This system was chosen to minimize the concomitant slpA transcription and plasmid replication functions in the L. brevis chromosome, which are likely to result in DNA rearrangements. By supplying the RepA protein in another plasmid, a tighter replication control was achieved compared to the direct ts integration vectors, where the restrictive temperature in L. brevis did not completely shut down the replication functions. This integration strategy has been used previously in Lactococcus for the generation of chromosomal mutations (24), and a similar two-plasmid system was recently modified for L. gasseri and L. acidophilus to increase the range of applicable gene inactivation temperatures (39).
Because the integration of the c-Myc epitope-encoding region into the L. brevis chromosome apparently occurred by a direct double-crossover event, no plasmid DNA remained in the genome. By PCR, however, we could detect unintegrated pKTH5115 DNA in the integrant strain, GRL1045. A possible explanation was that the resident plasmid of unknown functions, harbored by L. brevis GRL1 (38), could provide some residual replication function to pKTH5115. The final integrant strain, GRL1046, harboring no unintegrated pKTH5115, was obtained by culturing GRL1045 for approximately 50 generations without antibiotics.
Production of c-Myc epitope in the C-terminal half of L. brevis SlpA affected neither the anchoring of SlpA subunits to the cell wall, since no fusion protein was released into the culture medium, nor the formation of the S-layer lattice, as revealed by negative-staining results from EM. The region responsible for the binding of the SlpA protein of L. brevis to the cell wall has not yet been determined. The only cell wall binding domain of a Lactobacillus S-layer protein characterized so far is that of the SA protein of L. acidophilus ATCC 4356 (49). The lack of amino acid sequence similarity and the functional differences (16, 52) of the S-layer proteins from L. brevis GRL1 and L. acidophilus ATCC 4356, however, suggest different locations of the functional domains in these proteins. The receptor binding domain of SlpA, which is responsible for the affinity of L. brevis cells for human epithelial cells and fibronectin, is located within a fragment of 81 amino acids in the N-terminal part of the protein (16). The adhesive properties of the integrant strain, GRL1046, are thus expected to be unchanged from that of the wild-type strain, GRL1, since the c-Myc epitope resides within the C-terminal region of the mature fusion protein, not involved in adhesion.
To our knowledge, only the cell wall-targeting domains of S-layer proteins have so far been used to produce chromosomally encoded S-layer fusion proteins. A chromosomal integration, utilizing the S-layer homologous motifs of the Bacillus anthracis S-layer proteins EA1 or Sap fused with levansucrase of B. subtilis, resulted in anchoring of the fusion protein to the bacterial cell surface (30). There are also reports on several non-Lactobacillus S-layer fusion proteins for which the protein production is plasmid derived either in a heterologous host (5, 49) or in a null mutant lacking the wild-type S-layer protein gene (2). Our work is thus the first to describe the construction of a chromosomally encoded S-layer fusion protein, utilizing the entire S-layer protein gene.
As shown by the EM results, the epitope insertion into the slpA gene could be done without affecting the S-layer protein lattice symmetry or the self-assembly of isolated S-layer protein subunits. Due to a very delicate nature of the L. brevis S-layer, negative staining was the technique of choice for EM. The EM data showed that the S-layers from both GRL1 and the epitope strain GRL1046 formed an oblique structure. The negative-staining technique allowed visualization only of disrupted cells and isolated self-assembled S-layers because of a high staining background of intact cells. To visualize the cell surface of intact L. brevis cells, the freeze-etching technique and certain stainings in the thin-sectioning technique could be applied. However, these techniques could result in resolution problems due to the nature of this S-layer and because it is superimposed by the irregular structures (e.g., the polymers) of the cell wall.
Various recombinant Lactobacillus strains producing surface-exposed antigens have been constructed and used in immunization experiments (27, 37, 45). On the surface of L. casei, approximately 1.4 × 103 to 3.9 × 103 tetanus toxin fragment C molecules have been produced with vectors driving the tetanus toxin fragment C expression under the control of an α-amylase or l-(+)-lactate dehydrogenase promoter (27). It has been theoretically calculated that the encompassing S-layer on an average-size cell consists of approximately 5 × 105 monomers (47). Thus, with the surface display system developed in this study, it is possible to present at least such a large number of antigen epitope molecules on the cell surface of each L. brevis cell. Surface displaying of vaccines as part of an S-layer would thus be a very efficient way to present antigens to the mucosa-associated lymphoreticular tissue. Expression of vaccine antigens of different sizes in the L. brevis S-layer is now in progress in our laboratory.
Acknowledgments
We thank Ilkka Palva for valuable discussions and critical reading of the manuscript. We also thank Merja Roivanen and Tapani Hovi for providing the anti-VP1 antibodies, Kari Lounatmaa for EM work, and Esa Pohjolainen for technical assistance.
This work was supported by the Academy of Finland (grants 40836 and 44602).
REFERENCES
- 1.Bhowmik, T., and J. L. Steele. 1993. Development of an electroporation procedure for gene disruption in Lactobacillus helveticus CNRZ32. J. Gen. Microbiol. 139:1-7.8450303 [Google Scholar]
- 2.Bingle, W. H., J. F. Nomellini, and J. Smit. 1997. Cell-surface display of a Pseudomonas aeruginosa strain K pilin peptide within the paracrystalline S-layer of Caulobacter crescentus. Mol. Microbiol. 26:277-288. [DOI] [PubMed] [Google Scholar]
- 3.Boot, H. J., C. P. A. M. Kolen, J. M. van Noort, and P. H. Pouwels. 1993. S-layer protein of Lactobacillus acidophilus ATCC 4356: purification, expression in Escherichia coli and nucleotide sequence of the corresponding gene. J. Bacteriol. 175:6089-6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boot, H. J., and P. H. Pouwels. 1996. Expression, secretion and antigenic variation of bacterial S-layer proteins. Mol. Microbiol. 21:1117-1123. [DOI] [PubMed] [Google Scholar]
- 5.Breitwieser, A., E. M. Egelseer, D. Moll, N. Ilk, C. Hotzy, B. Bohle, C. Ebner, U. B. Sleytr, and M. Sára. 2002. A recombinant bacterial cell surface (c-layer)-major birch pollen allergen-fusion protein (rSbsC/Bet v1) maintains the ability to self- assemble into regularly structured monomolecular lattices and the functionality of the allergen. Protein Eng. 15:243-249. [DOI] [PubMed] [Google Scholar]
- 6.Bröckel, G., M. Behr, S. Fabry, R. Hensel, H. Kaudewitz, E. Biendl, and H. König. 1991. Analysis and nucleotide sequence of the genes encoding the surface- layer glycoprotein of the hyperthermophilic methanogens Methanothermus fervidus and Methanothermus sociabilis. Eur. J. Biochem. 199:147-152. [DOI] [PubMed] [Google Scholar]
- 7.Bumann, D., C. Hueck, T. Aebischer, and T. F. Meyer. 2000. Recombinant live Salmonella spp. for human vaccination against heterologous pathogens. FEMS Immunol. Med. Microbiol. 27:357-364. [DOI] [PubMed] [Google Scholar]
- 8.Callegari, M. L., B. Riboli, J. W. Sanders, P. S. Cocconcelli, J. Kok, G. Venema, and L. Morelli. 1998. The S-layer gene of Lactobacillus helveticus CNRZ 892: cloning, sequence and heterologous expression. Microbiology 144:719-726. [DOI] [PubMed] [Google Scholar]
- 9.de Ruyter, P. G. G. A., O. P. Kuipers, M. M. Beerthuyzen, I. van Alen-Boerrigter, and W. M. de Vos. 1996. Functional analysis of promoters in the nisin gene cluster of Lactococcus lactis. J. Bacteriol. 178:3434-3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Ruyter P. G. G. A., O. P. Kuipers, and W. M. de Vos. 1996. Controlled gene expression systems for Lactococcus lactis with food-grade inducer nisin. Appl. Environ. Microbiol. 62:3662-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evan G. I., G. K. Lewis, G. Ramsay, and J. M. Bishop. 1985. Isolation of monoclonal antibodies spesific for human c-myc proto-oncogene product. Mol. Cell. Biol. 5:3610-3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gasson, M. J. 1983. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 154:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higuchi, R. 1990. Recombinant PCR, p.177-183. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (ed.), PCR protocols. Academic Press, San Diego, Calif.
- 14.Holo, H., and I. F. Nes. 1989. High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osomotically stabilized media. Appl. Environ. Microbiol. 55:3119-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hovi, T., and M. Roivainen. 1993. Peptide antisera targeted to a conserved sequence in poliovirus capsid protein VP1 cross-react widely with members of the genus Enterovirus. J. Clin. Microbiol. 31:1083-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hynönen, U., B. Westerlund-Wikström, A. Palva, and T. K. Korhonen. 2002. Fibronectin-binding function in the SlpA surface protein of Lactobacillus brevis. J. Bacteriol. 184:3360-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahala, M., and A. Palva. 1997. In vivo expression of the Lactobacillus brevis s-layer gene. J. Bacteriol. 179:284-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahala, M., and A. Palva. 1999. The expression signals of the Lactobacillus brevis slpA gene direct efficient heterologous protein production in lactic acid bacteria. Appl. Microbiol. Biotechnol. 51:71-78. [DOI] [PubMed] [Google Scholar]
- 19.Kleerebezem M., M. M. Beerthuyzen, E. E. Vaughan, W. M. de Vos, and O. P. Kuipers. 1997. Controlled gene expression systems for lactic acid bacteria: transferable nisin-inducible expression cassettes for Lactococcus, Leuconostoc, and Lactobacillus spp. Appl. Environ. Microbiol. 63:4581-4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koga, T., T. Mizobe, and K. Takumi. 1998. Antibacterial activity of Lactobacillus species against Vibrio species. Microbiol. Res. 153:271-275. [DOI] [PubMed] [Google Scholar]
- 21.Kylä-Nikkilä, K., M. Hujanen, M. Leisola, and A. Palva. 2000. Metabolic engineering of Lactobacillus helveticus CNRZ32 for production of pure l-(+)- lactic acid. Appl. Environ. Microbiol. 66:3835-3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laitinen, R., E. Malinen, and A. Palva. 2002. PCR-ELISA. I. Application to simultaneous analysis of mixed bacterial samples composed of intestinal species. Syst. Appl. Microbiol. 25:241-248. [DOI] [PubMed] [Google Scholar]
- 23.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 227:680-685. [DOI] [PubMed] [Google Scholar]
- 24.Law, J., G. Buist, A. Haandrikman, J. Kok, G. Venema, and K. Leenhouts. 1995. A system to generate chromosomal mutations in Lactococcus lactis which allows fast analysis of targeted genes. J. Bacteriol. 177:7011-7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leenhouts, K., G. Buist, A. Bolhuis, A. ten Berge, J. Kiel, I. Mierau, M. Dabrowska, G. Venema, and J. Kok. 1996. A general system for generating unlabelled gene replacements in bacterial chromosomes. Mol. Gen. Genet. 253:217-224. [DOI] [PubMed] [Google Scholar]
- 26.Lounatmaa, K. 1985. Electron microscopic methods for the study of bacterial surface structures, p. 243-261. In T. K. Korhonen, E. A. Dawes and P. H. Mäkelä (ed.), Enterobacterial surface antigens: methods for molecular characterization. Elsevier Science Publishers (Biomedical Division), Amsterdam, The Netherlands.
- 27.Maassen, C. B. M., J. D. Laman, M.-J. Heijne den Bak-Glashouwer, F. J. Tielen, J. C. P. A. van Holten-Neelen, L. Hoogteijling, C. Antonissen, R. J. Leer, P. H. Pouwels, W. J. A. Boersma, and D. M. Shaw. 1999. Instruments for oral disease- intervention strategies: recombinant Lactobacillus casei expressing tetanus toxin fragment C for vaccination or myelin proteins for oral tolerance induction in multiple sclerosis. Vaccine 17:2117-2128. [DOI] [PubMed] [Google Scholar]
- 28.Maassen, C. B. M., C. van Holten-Neelen, F. Balk, M-J. Heijne den Bak- Glashouwer, R. J. Leer, J. D. Laman, W. J. A. Boersma, and E. Claassen. 2000. Strain dependent induction of cytokine profiles in the gut by orally administered Lactobacillus strains. Vaccine 18:2613-2623. [DOI] [PubMed] [Google Scholar]
- 29.Maguin, E., P. Duwat, T. Hege, D. Ehrlich, and A. Gruss. 1992. New thermosensitive plasmid for gram-positive bacteria. J. Bacteriol. 174:5633-5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mesnage, S., E. Tosi-Couture, and A. Fouet. 1999. Production and cell surface anchoring of functional fusions between the SLH motifs of the Bacillus anthracis S-layer proteins and the Bacillus subtilis levansucrase. Mol. Microbiol. 31:927-936. [DOI] [PubMed] [Google Scholar]
- 31.Masuda, K., and T. Kawata. 1983. Distribution and chemical characterization of regular arrays in the cell walls of strains of the genus Lactobacillus. FEMS Microbiol. Lett. 20:145-150. [Google Scholar]
- 32.Mielcarek, N., J. Cornette, A. M. Schacht, R. J. Pierce, C. Locht, A. Capron, and G. Riveau. 1997. Intranasal priming with recombinant Bordetella pertussis for the induction of a systemic immune response against a heterologous antigen. Infect. Immun. 65:544-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miettinen, M., J. Vuopio-Varkila, and K. Varkila. 1996. Production of human tumor necrosis factor alpha, interleukin-6, and interleukin-10 is induced by lactic acid bacteria. Infect. Immun. 64:5403-5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norton, P. M., H. W. G. Brown, J. M. Wells, A. M. Macpherson, P. W. Wilson, and R. W. F. Le Page. 1996. Factors affecting the immunogenicity of tetanus toxin fragment C expressed in Lactococcus lactis. FEMS Immunol. Med. Microbiol. 14:167-177. [DOI] [PubMed] [Google Scholar]
- 35.Nyman, T. A., S. Matikainen, T. Saraneva, I. Julkunen, and N. Kalkkinen. 2000. Proteome analysis reveals ubiquitin-conjugating enzymes to be a new family of interferon-alpha-regulated genes. Eur. J. Biochem. 267:4011-4019. [DOI] [PubMed] [Google Scholar]
- 36.Pouwels, P. H., R. J. Leer, M. Shaw, M.-J. Heijne den Bak-Glashouwer, F. D. Tielen, E. Smit, B. Martinez, J. Jore, and P. L. Conway. 1998. Lactic acid bacteria as antigen delivery vehicles for oral immunization purposes. Int. J. Food Microbiol. 41:155-167. [DOI] [PubMed] [Google Scholar]
- 37.Reveneau, N., M.-C. Geoffroy, C. Locht, P. Chagnaud, and A. Mercenier. 2002. Comparison of the immune responses induced by local immunizations with recombinant Lactobacillus plantarum producing tetanus toxin fragment C in different cellular locations. Vaccine 20:1769-1777. [DOI] [PubMed] [Google Scholar]
- 38.Rönkä, E., E. Malinen, M. Saarela, M. Rinta-Koski, J. Aarnikunnas, and A. Palva. Probiotic and milk technological properties of Lactobacillus brevis. Int. J. Food Microbiol. in press. [DOI] [PubMed]
- 39.Russell, W. M., and T. R. Klaenhammer. 2001. Efficient system for directed integration into the Lactobacillus acidophilus and Lactobacillus gasseri chromosomes via homologous recombination. Appl. Environ. Microbiol. 67:4361-4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryan E. T., J. R. Butterton, T. Zhang, M. A. Baker, S. L. Stanley, and S. B. Calderwood. 1997. Oral immunization with attenuated vaccine strains of Vibrio cholerae expressing a dodecapeptide repeat of the serine-rich Entamoeba histolytica protein fused to the cholera toxin B subunit induces systemic and mucosal antiamebic and anti-V. cholerae antibody responses in mice. Infect. Immun. 65:3118-3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 42.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain- terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sára, M., and U. B. Sleytr. 2000. S-layer proteins. J. Bacteriol. 182:859-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Savijoki, K., M. Kahala, and A. Palva. 1997. High level heterologous protein production in Lactococcus and Lactobacillus using a new secretion system based on the Lactobacillus brevis S-layer signals. Gene 186:255-262. [DOI] [PubMed] [Google Scholar]
- 45.Shaw, D. M., B. Gaerthé, R. J. Leer, J. G. M. M. van der Stap, C. Smittenaar, M.-J. J. Heijne den Bak-Glashouwer, J. E. R. Thole, F. J. Tielen, P. H. Pouwels, and C. E. G. Havenith. 2000. Engineering the microflora to vaccinate the mucosa: serum immunoglobulin G responses and activated draining cervical lymph nodes following mucosal application of tetanus toxin fragment C-expressing lactobacilli. Immunology 100:510-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sillanpää, J., B. Martinez, J. Antikainen, T. Toba, N. Kalkkinen, S. Tankka, K. Lounatmaa, J. Keränen, M. Höök, B. Westerlund-Wikström, P. H. Pouwels, and T. K. Korhonen. 2000. Characterization of the collagen-binding S-layer protein CbsA of Lactobacillus crispatus. J. Bacteriol. 182:6440-6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sleytr, U. B., and P. Messner. 1988. Crystalline surface layers in procaryotes. J. Bacteriol. 170:2891-2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sleytr, U. B., and T. J. Beveridge. 1999. Bacterial S-layers. Trends Microbiol. 7:253-260. [DOI] [PubMed] [Google Scholar]
- 49.Smit, E., F. Oling, R. Demel, B. Martinez, and P. H. Pouwels. 2001. The S-layer protein of Lactobacillus acidophilus ATCC 4356: identification and characterisation of domains responsible for S-layer protein assembly and cell wall binding. J. Mol. Biol. 305:245-257. [DOI] [PubMed] [Google Scholar]
- 50.Southern, E. M. 1975. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J. Mol. Biol. 98:503-517. [DOI] [PubMed] [Google Scholar]
- 51.Stover, C. K., V. F. de la Cruz, G. P. Bansal, M. S. Hanson, T. R. Fuerst, W. R. Jacobs, and B. R. Bloom. 1992. Use of recombinant BCG as a vaccine delivery vehicle. Adv. Exp. Med. Biol. 327:175-182. [DOI] [PubMed] [Google Scholar]
- 52.Toba, T., R. Virkola, B. Westerlund, Y. Björkman, J. Sillanpää, T. Vartio, N. Kalkkinen, and T. K. Korhonen. 1995. A collagen binding S-layer protein in Lactobacillus crispatus. Appl. Environ. Microbiol. 61:2467-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vaughan, E. E., B. Mollet, and W. M. de Vos. 1999. Functionality of probiotics and intestinal lactobacilli: light in the intestinal tract tunnel. Curr. Opin. Biotechnol. 10:505-510. [DOI] [PubMed] [Google Scholar]
- 54.Vidgrén, G., I. Palva, R. Pakkanen, K. Lounatmaa, and A. Palva. 1992. S-layer protein gene of Lactobacillus brevis: cloning by polymerase chain reaction and determination of the nucleotide sequence. J. Bacteriol. 174:7419-7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wells, J. M., P. W. Wilson, P. M. Norton, M. J. Gasson, and R. W. F. Le Page. 1993. Lactococcus lactis: high-level expression of tetanus toxin fragment C and protection against lethal challenge. Mol. Microbiol. 8:1155-1162. [DOI] [PubMed] [Google Scholar]
- 56.Yasui T., K. Yoda, and T. Kamiya. 1995. Analysis of S-layer proteins of Lactobacillus brevis. FEMS Microbiol. Lett. 133:181-186. [DOI] [PubMed] [Google Scholar]