Abstract
Aerobic bacteria that grow on vinyl chloride (VC) have been isolated previously, but their diversity and distribution are largely unknown. It is also unclear whether such bacteria contribute to the natural attenuation of VC at chlorinated-ethene-contaminated sites. We detected aerobic VC biodegradation in 23 of 37 microcosms and enrichments inoculated with samples from various sites. Twelve different bacteria (11 Mycobacterium strains and 1 Nocardioides strain) capable of growth on VC as the sole carbon source were isolated, and 5 representative strains were examined further. All the isolates grew on ethene in addition to VC and contained VC-inducible ethene monooxygenase activity. The Mycobacterium strains (JS60, JS61, JS616, and JS617) all had similar growth yields (5.4 to 6.6 g of protein/mol), maximum specific growth rates (0.17 to 0.23 day−1), and maximum specific substrate utilization rates (9 to 16 nmol/min/mg of protein) with VC. The Nocardioides strain (JS614) had a higher growth yield (10.3 g of protein/mol), growth rate (0.71 day−1), and substrate utilization rate (43 nmol/min/mg of protein) with VC but was much more sensitive to VC starvation. Half-velocity constant (Ks) values for VC were between 0.5 and 3.2 μM, while Ks values for oxygen ranged from 0.03 to 0.3 mg/liter. Our results indicate that aerobic VC-degrading microorganisms (predominantly Mycobacterium strains) are widely distributed at sites contaminated with chlorinated solvents and are likely to be responsible for the natural attenuation of VC.
Vinyl chloride (VC) is a common groundwater contaminant (49) which is of concern due to its carcinogenicity (7). Although VC can be produced naturally at very low levels in some soils (32), the industrial synthesis of polyvinyl chloride plastics (27 million tons per year globally [33]) and the bacterial metabolism of chlorinated solvents (36, 41) are the most problematic sources of VC contamination. Many anaerobic bacteria can reductively dechlorinate the widely used solvents tetrachloroethene (PCE) and trichloroethene (TCE), producing cis-dichloroethene (cDCE), VC, or ethene (ETH) (19, 30, 35, 40, 59). However, microbes capable of reducing VC to ETH are often absent or inactive in subsurface ecosystems, and thus, VC commonly accumulates as an end product of anaerobic dechlorination (17, 37, 39).
VC can be oxidized to CO2 under anaerobic conditions in the presence of Fe(III) or humic acids (4, 5), but the microbiology and biochemistry of such anaerobic oxidations have not been investigated. Aerobic bacteria can catalyze the cometabolic oxidation of VC in the presence of monooxygenase inducers such as methane (18), ethane (20), ETH (34), propane (38), propene (15), isoprene (16), toluene (48), and ammonia (56). Bioremediation strategies based on aerobic cometabolism have been examined for VC and other chlorinated ethenes (36, 47), but there are several problems with cometabolic systems—electron donors are required (2), the growth-supporting substrate and the pollutant compete for the same enzymes (15), and reactive toxic metabolites may accumulate and inhibit biodegradation (43, 61).
A few strains of Mycobacterium (25, 26) and Pseudomonas (57, 58) that can grow aerobically on VC as the sole carbon and energy source have been isolated from soil, river water, and activated sludge. Such bacteria are not subject to cometabolic limitations and may play a role in the natural attenuation of VC if they occur in the subsurface at contaminated sites. The origin of VC-assimilating bacteria is unclear, although evolution from strains that grow on ETH seems likely (58). ETH is produced via biosynthesis in plants and soil microorganisms, and aerobic ETH-assimilating bacteria appear to be fairly widespread and easily isolated (10, 23). The VC- and ETH-assimilation pathways in bacteria are not well understood, but there is evidence for an initial monooxygenase attack and the production of VC epoxide (chlorooxirane) (25) and epoxyethane (ethylene oxide) (11), respectively.
While several recent studies (17, 28, 29) have examined the distribution and diversity of anaerobic PCE-TCE-dechlorinating bacteria, similar studies on aerobic VC-assimilating bacteria are lacking, and it is not known whether such bacteria might contribute to the natural attenuation of VC. Evidence from the field (14) suggests that VC can disappear in the aerobic downgradient portions of chlorinated ethene plumes, but whether VC-assimilating bacteria are responsible for the effect is unknown. There is great variation in the kinetic parameters of the few aerobic VC degraders examined thus far, making it difficult to predict the rate and extent of in situ VC oxidation. To address the above issues, we investigated aerobic VC biodegradation in samples from chlorinated-ethene-contaminated sites and examined the phylogeny and kinetic parameters of VC-assimilating strains isolated from the site samples.
MATERIALS AND METHODS
Chemicals, media, and incubation conditions.
The VC (99.5%) was from Fluka, and the ETH (99.5%) was from Scott. All other chemicals were reagent grade. A minimal salts medium (MSM) (24), modified as described previously (9), was used for both enrichments and pure cultures. One-tenth-strength Trypticase soy agar (1/10-TSA; pH 7.0) and 1/10-strength Trypticase soy broth (1/10-TSB; pH 7.0) were used as nonselective media and contained TSB (3 g/liter; Difco) and Bacto agar (20 g/liter; Difco) where required. In some cases, glucose (10 g/liter) and/or bovine serum albumin (Fraction V; 5 g/liter; Sigma) was added to 1/10-TSA and 1/10-TSB. All cultures were incubated aerobically. Broths were shaken at 150 to 165 rpm. Incubation temperatures ranged from 20 to 30°C, as listed specifically below.
Analytical methods.
VC, ETH, and epoxyethane were analyzed in headspace samples by gas chromatography with flame-ionization detection as described previously (9). For analysis of kinetic data, aqueous concentrations of VC were calculated from total amounts, using a dimensionless Henry's constant of 0.9079 at 20°C (22). For ETH, a Henry's constant of 7.24 at 20°C was estimated by using the technique of Gossett (22). A Hewlett Packard HP 5890 Series II gas chromatograph equipped with a thermal conductivity detector and a 1/8-in.-diameter stainless steel column packed with a 60/80-mesh 5A molecular sieve (Supelco) was used to analyze oxygen in headspace samples (0.5 ml from 160-ml serum bottles). Protein concentrations were routinely measured by a UV absorbance assay based on previously described methods (31, 42). Samples of culture fluid (0.45 ml) were mixed with NaOH (0.15 ml, 10 M) and heated (90°C, 10 min). The samples were cooled, an HCl solution was added (400 μl; 10 M HCl and MSM in a 3:5 ratio), and the tubes were centrifuged (16,000 × g) for 5 min. The absorbance of the supernatants was measured at 230 and 260 nm, and the protein content was calculated in micrograms per milliliter as (183 × A230) − (75.8 × A260). Protein in neutralized lysates was also determined on occasion by using the MicroBCA assay kit (Pierce) as described previously (9). Dry-weight growth yields were determined with 700-ml cultures grown on 100 ml (4,167 μmol) of VC or ETH. Cells were washed in deionized water and then dried at 105°C to a constant weight. The optical density at 600 nm (OD600) was also used to monitor growth in some cases.
Microcosms and enrichment cultures.
Samples of groundwater (50% [vol/vol]), soil (5% [wt/vol]), sediment (5% [wt/vol]), or activated carbon (5% [wt/vol]) were mixed with MSM in 160-ml serum bottles (50-ml or 72-ml [liquid phase]) sealed with Teflon-faced rubber stoppers and aluminum crimp caps. In cases where samples contained solids and groundwater, initial microcosms were set up without the addition of medium. VC was added at 20 to 40 μmol/bottle (0.13 to 0.26 mM aqueous concentration) in the initial microcosms and at 100 to 400 μmol/bottle in later transfers (0.66 to 2.64 mM). The microcosms and enrichments were incubated with the bottles in an inverted position and with shaking at the ambient temperature (20 to 24°C), and active cultures (5% [vol/vol]) were transferred to fresh MSM at intervals. Sterile controls (autoclaved samples in MSM) were set up in accompaniment to the initial microcosms for the monitoring of abiotic losses.
Isolation and identification of VC degraders.
Pure cultures were isolated from the enrichments by spreading dilutions on MSM plates and incubating in desiccators in a 1% (vol/vol) VC-air atmosphere. After incubation at ambient temperature for 1 to 3 months, representative colonies were restreaked on two MSM plates, one of which was reincubated with 1% VC and the other of which was reincubated in air alone. Isolates showing significantly more growth in the presence of VC were investigated further. In some cases, isolations were also done on 1/10-TSA plates both with and without added bovine serum albumin. Utilization of VC was confirmed by reinoculation of isolates into MSM-VC broths and the monitoring of VC consumption (gas chromatography [GC] analysis) and cell growth (OD600 or protein assay). Identification of strains was done by partial sequencing of amplified 16S rDNA (MIDI Labs, Newark, Del.). Phylogenetic analysis was done with ClustalX and TreeView software (see Fig. 1; see legend for details).
FIG. 1.
Phylogeny of VC-assimilating isolates based on partial 16S rRNA gene sequences. Names of VC-degrading isolates from the present study are in boxes. A total of 400 bases were used for analysis after removal of positions containing gaps or ambiguous nucleotides. Bootstrap values from 100 neighbor-joining trees are indicated to the left of the nodes. Branch points with less than 50% bootstrap support were collapsed. The bar represents 3 nucleotide changes per 100 nucleotides. The consensus tree was rooted by using Escherichia coli as the outgroup. GenBank accession numbers are given below each strain.
DNA extraction and repetitive extragenic palindromic (REP)-PCR analysis.
Cultures were inoculated into 50 ml of 1/10-TSB-glucose (1%)-glycine (0.6%) at an OD600 of approximately 0.5 and grown for 16 to 24 h at 30°C to an OD600 of approximately 1.0 to 1.5. Cells were washed in STE buffer (as described previously (45), but with EDTA at 50 mM), treated with acetone (27), and incubated at 37°C overnight in lysozyme (5 mg in 1 ml of STE buffer). Cells were lysed with proteinase K (0.5 mg/ml), sodium dodecyl sulfate (1%), and heat (55°C, 2 to 16 h), and the DNA was purified via phenol-chloroform extraction, isopropanol precipitation, RNase treatment, and ethanol precipitation (45). Typical yields were 0.5 to 1.5 μg of genomic DNA/ml of culture. DNA was diluted to 50 ng/μl in water and used as the template for PCR with the REP1R-I and REP2-I primers as described by de Bruijn (13), except dNTPs were added at 0.2 mM.
Determination of growth rates and growth yields.
VC-grown cells were washed with MSM and inoculated into 50 ml of MSM (OD600 = 0.05, protein = 7 to 11 μg/ml). VC was added at 150 μmol/bottle (1 mM), and the cultures were incubated at 20°C with sampling at intervals for determination of VC and protein concentrations. VC (150 μmol) was added as required. Another set of cultures inoculated with ETH-grown cells was similarly treated, except ETH was provided as the substrate (one addition of 600 μmol/bottle = 0.7 mM). Growth yields were calculated from the linear regression (milligrams of protein produced/mmol of substrate consumed). Maximum specific growth rates were subsequently calculated by fitting an exponential curve to a plot showing protein yield versus time. In this case, protein yield was calculated from substrate depletion data as X0 + Y ΔS, where X0 is the initial biomass (milligrams of protein), Y is the growth yield (milligrams of protein/millimole of substrate), and ΔS is the cumulative substrate consumed (millimoles). This method of calculating the growth rate was used due to the availability of more substrate depletion data than protein data and because of the relatively large scatter in the protein measurements for some cultures. The growth yield of strain JS616 was determined by using 3-ml cultures in 10-ml serum bottles. The whole culture was lysed by injecting NaOH (1 ml, 10 M) into the bottle and heating (90°C, 15 min), and then the lysate was neutralized and assayed for protein by measurement of UV absorbance.
VC-ETH utilization kinetics.
VC- or ETH-grown cells were washed in MSM and divided among three 160-ml serum bottles containing 72 ml of MSM each. The cultures were incubated with the bottles inverted at an angle at 20°C and with rotary shaking at 165 rpm, and the headspace gases were sampled at intervals for GC analysis. Estimates of the maximum specific substrate utilization rate (k) and the half-velocity constant (Ks) were obtained from VC or ETH depletion curves. The data were fitted to the Michaelis-Menten model by using the AQUASIM software program, as described previously (9). VC kinetics was determined with cultures pregrown on VC, while ETH kinetics was determined with cultures pregrown on ETH. Protein concentrations were measured at the end of each depletion experiment by lysing the entire contents of each culture bottle, as described previously. Experiments were designed such that biomass increases over the observed depletion curves were negligible (typically < 0.5%).
Oxygen utilization kinetics.
The half-velocity constant with respect to oxygen (Ks[O2]) was determined with 72-ml cultures in triplicate as described above for the VC-ETH kinetics study. After inoculation, the headspace was purged briefly with nitrogen to remove excess oxygen and then 300 μmol of VC or 800 μmol of ETH was added to the culture. Ks(O2) values were estimated using AQUASIM as described above. A zero-oxygen standard was prepared by adding excess sodium sulfite to a 160-ml serum bottle containing 72 ml of distilled water in the presence of cobalt catalyst. The oxygen peak area for the mixture from this bottle, as measured by GC, was defined as corresponding to 0 mg of dissolved oxygen/liter. Oxygen threshold levels (i.e., oxygen uptake levels too low to measure at oxygen concentrations greater than zero) were observed during the study, which required a modification of the Michaelis-Menten model for the AQUASIM program as follows:
 |
where V is the rate of oxygen depletion, Vmax is the maximum rate of oxygen depletion (−dS/dt), S is the oxygen concentration (in milligrams per liter), and St is the threshold oxygen concentration (in milligrams per liter). The model-fitting parameters were thus Vmax, Ks(O2), and St.
Epoxyethane production assay.
Cells from 50-ml MSM cultures grown to mid-exponential phase on either VC or sodium acetate (20 mM) were washed in KP buffer (K2HPO4, 20 mM [pH 7.0]) and suspended to 0.2 ml with KP buffer in 10-ml serum bottles. The bottles were capped and an epoxypropane solution was added (2.5 mM in 0.8 ml of KP buffer), yielding 1-ml suspensions at an OD600 between 5 and 10 (protein = 0.7 to 2.3 mg/ml). ETH (3 μmol) was added, and the bottles were incubated with shaking (300 rpm) at 20°C. After 10 min of equilibration, 100-μl headspace samples were taken at intervals and analyzed by GC to determine the epoxyethane accumulation rate. At the end of the experiment, protein was assayed and monooxygenase activity was calculated as nanomoles of epoxyethane produced per minute per milligram of protein. In the case of strain JS616, Tween 80 (0.1% [vol/vol]) was added to cultures just before harvesting and was included in the washing buffer to facilitate centrifugation and reduce clumping during the assay.
Bacterial strain accession numbers.
Several of the VC-degrading strains have been deposited with the American Type Culture Collection, with accession numbers as follows: JS60, BAA-494; JS61, BAA-495; JS616, BAA-496; JS617, BAA-497; JS621, BAA-498; and JS614, BAA-499.
RESULTS AND DISCUSSION
Distribution of VC assimilation activity.
Aerobic biodegradation of VC occurred in 23 of 37 samples from 22 sites (Table 1). Twenty of 31 chlorinated-ethene-contaminated samples yielded positive enrichments, while 3 of 6 uncontaminated samples were positive. The lag time before VC degradation began was highly variable, ranging from 20 to 110 days. In most cases, VC-degrading activity in the initial microcosms was readily transferable in enrichment cultures with VC as the sole carbon and energy source. It is unclear from our data whether the presence of VC degraders correlated with site contamination. This is due to the small number of uncontaminated samples investigated and the lack of rigorous site characterization in many cases.
TABLE 1.
VC biodegradation in aerobic microcosms and enrichments
| Sample (location) | Contaminants present at sitea | VC oxidation | Lag time (days) | Strain isolated |
|---|---|---|---|---|
| Groundwater (industrial site; Plaquemine, La.) | VC, cDCE, TCE, PCE | + | 70 | Mycobacterium sp. strain JS60 |
| Activated sludge (Ithaca, N.Y.). | None identified | + | 50 | Mycobacterium sp. strain JS61 |
| Soil (industrial site; Midland, Mich.) | None identified | − | None (no activity after 4 mo) | |
| Pond water (Tyndall AFB, Fla.) | None identified | − | None (no activity after 4 mo) | |
| Wastewater (Tyndall AFB, Fla.) | None identified | − | None (no activity after 4 mo) | |
| Soil (industrial site; Boeblingen, Germany) | cDCE, TCE, PCE | + | 50 | None (isolation attempts failed) |
| Soil (industrial site; Dusseldorf, Germany) | VC, cDCE | + | 40 | Mycobacterium sp. strain JS618 |
| Activated carbon (pump and treatment plant; Dortmund, Germany) | VC, cDCE, TCE, PCE | + | 30 | Mycobacterium sp. strain JS617 |
| Sediment (industrial site; Carlyss, La.)b | VC, cDCE, 1, 1-DCE, TCE | + | 20 | Mycobacterium sp. strain JS616 |
| Soil (Dover AFB, Del.)b | cDCE, TCE, TCA | + | 110 | None (no activity after 2nd transfer) |
| Soil (Hill AFB, Utah)b | TCE, TCA | + | 90 | None (low activity) |
| Groundwater (Phoenix, Ariz.) | VC, cDCE, TCE, TCA, benzene | − | None (no activity after 4 mo) | |
| Soil (industrial site; Carson, Calif.) | VC, DCA | + | 20 | Nocardioides sp. strain JS614 |
| Aquifer solids (Travis AFB, Calif.)b | VC, cDCE, TCE | + | 50 | Mycobacterium sp. strain JS619 |
| Groundwater MW16-10S (Cecil Field NASc, Fla.) | VC, cDCE, TCE, DCA | + | 60 | Mycobacterium sp. strain JS615 |
| Groundwater (Cecil Field NAS, Fla.) MW16-13S | None identified | + | 90 | None (enrichment frozen) |
| Groundwater (Cecil Field NAS, Fla.) MW3-13S | cDCE, TCE, DCA, TCA | − | None (no activity after 4 mo) | |
| Sediment (Cecil Field NAS, Fla.) Rowell Ck. | cDCE, DCA, chloroform, benzene | − | None (no activity after 4 mo) | |
| Groundwater (Cape Canaveral, Fla.) MW S-14 | VC, cDCE | + | 30 | Mycobacterium sp. strain JS620 |
| Groundwater (Cape Canaveral, Fla.) MW S-19 | VC, cDCE | + | 110 | None (enrichment frozen) |
| Groundwater (Cape Canaveral, Fla.) MW I-11 | VC, cDCE | − | None (no activity after 4 mo) | |
| Groundwater (Moody AFB, Ga.) MW-14 | None identified | + | 90 | Mycobacterium sp. strain JS621 |
| Groundwater (Moody AFB, Ga.) MW-9 | VC, cDCE, TCE | + | 40 | Mycobacterium sp. (JS621 like) |
| Groundwater (Moody AFB, Ga.) MW-12 | VC, cDCE, TCE | + | 70 | Mycobacterium sp. (JS621 like) |
| Groundwater (Moody AFB, Ga.) MW-19 | VC, cDCE, TCE | + | 70 | None (isolations in progress) |
| Groundwater (Moody AFB, Ga.) MW-27 | VC, cDCE, TCE | + | 50 | Mycobacterium sp. (JS621 like) |
| Soil-groundwater (Cape Canaveral, Fla., site 1381) | VC, cDCE | − | None (no activity after 4 mo) | |
| Soil-groundwater (Alameda NAS, Calif., site 4) | VC, cDCE, TCE | − | None (no activity after 4 mo) | |
| Soil-groundwater Fort Lewis (Wash.) | VC, cDCE, TCE | + | 50 | Mycobacterium sp. strain TM2 |
| Groundwater-fines (industrial site, Sydney, Australia) | Chlorinated methanes, ethanes, and ethenes | − | None (no activity after 4 mo) | |
| Groundwater-fines (landfill, Voorhees, N.J.) | cDCE | + | 70 | Mycobacterium sp. strain TM1 |
| Soil (Savannah River, Ga.) | TCE | − | None (no activity after 4 mo) | |
| Groundwater (Camp LeJeune, N.C., site 73) MW-13 | VC, cDCE, TCE | − | None (no activity after 4 mo) | |
| Soil-groundwater (Camp LeJeune, N.C., site 73) MW-15 | VC, cDCE, TCE | − | None (no activity after 4 mo) | |
| Soil-groundwater (Camp LeJeune, N.C., site 73) MW-29 | VC, cDCE, TCE | − | None (no activity after 4 mo) | |
| Soil-groundwater (Camp LeJeune, N.C., site 73) A47/3-11 | VC, cDCE, TCE | + | 60 | None (isolations in progress) |
| Soil-groundwater (Camp LeJeune, N.C., site 73) A47/3-8 | VC, cDCE, TCE | + | 80 | None (isolations in progress) |
Information obtained from site managers and our own GC analyses. DCA, 1,2-dichloroethane; TCA, 1,1,2-trichloroethane.
Sample was an experimental microcosm received from another laboratory.
NAS, Naval Air Station.
One interesting trend noted with the samples from contaminated sites was that VC degraders were not detected in the two samples (Phoenix groundwater and Cecil Field sediment) that contained benzene in addition to chlorinated ethenes. It is possible that at such sampling locations, hydrocarbons interfered with the development of VC-degrading populations by exerting toxic effects or by acting as alternative carbon sources. Another unexpected result with the samples from Cecil Field was that a positive enrichment was obtained from groundwater outside the contaminant plume (sample MW16-13S), while no activity was seen in groundwater from a highly contaminated area of the site (MW3-13S). Active enrichments were also obtained from uncontaminated samples in two other cases (Moody Air Force Base [AFB] sample MW-14 groundwater and Ithaca activated sludge).
From our survey of sites, it appears that VC-degrading microorganisms are fairly widespread and arise wherever conditions are appropriate for their growth. The presence of chlorinated ethenes is apparently not the sole factor that determines whether VC degraders are present at a site. Other site conditions, such as redox potential, pH, and the presence of other contaminants and nutrients, would be expected to influence the development of VC-degrading microbial populations. The most favorable niches for the growth of VC degraders may be in aerobic areas downgradient of chlorinated ETH plumes, where anaerobic electron donors are depleted and the end products of anaerobic chloroethene metabolism (i.e., VC, ETH, and ethane) accumulate.
Isolation and characterization of bacteria.
Pure cultures of VC-assimilating bacteria were isolated from 15 of the enrichments. All of the isolated strains grew on both VC and ETH as the sole carbon source and were relatively slow growing, requiring 1 to 3 weeks to form colonies on 1/10-TSA plates and 1 to 3 months to form colonies on MSM-VC plates. The optimum temperature and pH for the isolates was determined in two cases (i.e., those of strains JS60 and JS61) to be 30°C and pH 6.0 to 6.5. For the sake of consistency, however, further growth and kinetic experiments with the isolates were performed at 20°C with a pH of 7.0.
On 1/10-TSA plates, colonies of the VC-degrading strains were white, cream, yellow, or orange in color and ranged in form from smooth and circular to raised and irregular. Large- and small-colony variants were produced by pure cultures in several cases, a trait that was particularly noticeable with strain JS614. Strains JS61, JS617, and JS621 did not readily form single colonies on 1/10-TSA or MSM plates, despite growing well in broth medium. The addition of glucose and particularly albumin (as used in mycobacterial media such as Middlebrook 7H9) greatly facilitated colony development with such strains. In liquid culture, all of the isolates except strain JS614 showed some degree of hydrophobic behavior-either clumping or adhering to the walls of serum bottles.
In groundwater enrichments, there was a tendency for less hydrophobic strains (e.g., JS60, JS615, and JS620) related to Mycobacterium rhodesiae (see below) to dominate, while with solid sample types a diverse range of more hydrophobic strains (e.g., JS616, JS617, and JS619) tended to be isolated. The use of groundwater samples in many cases may have biased our survey against the more hydrophobic, possibly surface-associated bacteria at the sampling site. Future sampling efforts should therefore focus on samples of soil or aquifer solids to more fully investigate the diversity of VC-assimilating bacteria. The fact that we could not isolate pure cultures from some enrichments despite repeated attempts also indicates that the biodiversity of aerobic VC degraders is not fully represented by the isolates obtained thus far.
Phylogenetic analysis.
Comparison of partial 16S rDNA sequences from the VC-degrading isolates with sequences from GenBank indicated that with one exception (strain JS614), all of the isolates were Mycobacterium species (Fig. 1). Many of the strains (i.e., TM1, JS60, JS61, JS615, JS618, JS620, and JS621) clustered in a loose group which includes M. rhodesiae, M. sphagni, M. aichiense, M. fortuitum, and M. mucogenicum (44, 52). This group also includes the ETH-degrading strain K1 (34), the trichloroethane-degrading strain TA27 (60), and several polyaromatic hydrocarbon-degrading bacteria (e.g., strains RJGII-135 [46] and SM7.6.1 [21]). Based on analysis of some near-full-length 16S rDNA sequences (1,420 bases), we have tentatively assigned strains TM1, JS60, JS615, and JS620 to M. rhodesiae and strains JS61 and JS618 to M. aichiense.
The strain JS621 partial 16S rDNA sequence (400 bp) was most similar to that of M. mucogenicum (Fig. 1), but comparison of near-full-length sequences (data not shown) revealed a closer relationship to M. rhodesiae (98.2% sequence identity). However, JS621 was slow growing and nonpigmented on plates, in contrast to M. rhodesiae (52), and the other M. rhodesiae-like VC degraders, which grew more rapidly and featured yellow pigmentation. Thus, strain JS621 may represent a novel species of mycobacterium. Based on 16S rDNA analysis, the mycobacterial isolates JS617 and TM2 are most likely to be strains of M. tusciae (51), while JS616 and JS619 are probably strains of M. gadium (8), and M. moriokaense (53), respectively. The 16S rDNA sequence from strain JS614 was most similar to sequences from Nocardioides strains. Analysis of near-full-length 16S rDNA sequences (1,469 bases) indicated that the JS614 gene was 97.4% identical to that of Nocardioides pyridinolyticus, the closest-matching well-established species. Based on these results, it is possible that strain JS614 represents a new Nocardioides species (50).
In two cases, VC-degrading isolates from geographically distant sites had identical partial 16S rDNA sequences (isolates JS60 and JS620 and isolates JS61 and JS618). On the basis of phenotypic differences such as pigmentation and the results of REP-PCR amplification (Fig. 2), which showed all four strains to be distinct, we believe these strains to be independent isolates. The four isolates derived from Moody AFB groundwater (JS621 and three JS621-like strains) had identical partial 16S rDNA sequences and the same colony morphology. Further tests (e.g., REP-PCR assays) are required to determine the relationship between these isolates. The JS621-like strains were isolated from monitoring wells covering the entire length of the VC plume, suggesting that a single VC-degrading strain dominates this site. A similar phenomenon has been observed at Kelly AFB (Texas), where chlorobenzene-degrading bacteria isolated from different groundwater samples were all found to carry identical 16S rDNA genes and degradation pathways (54).
FIG. 2.
Discrimination of closely related VC-degrading mycobacteria by REP-PCR.
The fact that all our VC-degrading isolates were gram-positive bacteria (order Actinomycetales) seems unusual in light of the recent isolations of VC-degrading Pseudomonas strains (57, 58). As the media and temperatures used in our study were similar to those of Verce et al. (57, 58), the differences in phylogeny of isolates may be due to the sole use of VC for selection in our work rather than of ethane (57) or ETH (58) in initial enrichments. Differences in sample types (i.e., groundwater and soil versus sewage sludge) may also have influenced the types of isolates recovered, although it should be noted that we isolated Mycobacterium JS61 from activated sludge. Based on the results of our isolations, it appears that mycobacteria are more likely candidates than pseudomonads as the agents responsible for the natural attenuation of VC in the subsurface at contaminated sites.
Growth and kinetic parameters.
Five representative strains were selected for more detailed analysis of growth and kinetic parameters with ETH and VC (Table 2). The growth yields and growth rates of the four Mycobacterium strains examined (JS60, JS61, JS616, and JS617) were similar, and all were lower than the values obtained for the Nocardioides strain (JS614). We considered the possibility that the apparent high yields of strain JS614 were due to biases in the lysis or protein quantitation methods used, but alternative protein assays (Micro-BCA) and measurement of dry-weight yields confirmed the initial data. The dry-weight growth yields with JS614 were 20.1 g/mol for VC and 29.4 g/mol for ETH compared to yields with JS60 of 10.8 g/mol for VC and 22.0 g/mol for ETH (averages of two experiments).
TABLE 2.
Growth and kinetic parameters of VC-assimilating bacteria
| Strain | Growth yielda (g of protein/mol of substrate)
|
Maximum specific growth rate (day−1)
|
k (nmol/min/mg of protein)
|
Ks (μM)
|
Epoxyethane production from ETH (nmol/min/mg of protein)
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| VC | ETH | VC | ETH | VC | ETH | VC | ETH | VC-grown | Acetate-grown | |
| Mycobacterium | ||||||||||
| JS60 | 6.6 ± 0.7 | 11.2 ± 0.6 | 0.22 ± 0.02 | 0.58 ± 0.05 | 9.7 ± 0.2 | 25.4 ± 0.8 | 0.5 ± 0.1 | 0.9 ± 0.1 | 12.1 ± 3.7 | 0 |
| JS61 | 6.2 ± 1.5 | 12.1 ± 1.6 | 0.21 ± 0.02 | 0.76 ± 0.07 | 9.0 ± 0.2 | 43.5 ± 1.4 | 0.8 ± 0.2 | 4.0 ± 0.2 | 8.0 ± 2.8 | 0 |
| JS616 | 5.4 ± 1.1 | 12.8 ± 1.1 | 0.17 ± 0.02 | 0.39 ± 0.02 | 15.4 ± 0.6 | 20.4 ± 1.0 | 3.2 ± 0.3 | 4.0 ± 0.5 | 13.9 ± 9.3 | 0 |
| JS617 | 6.0 ± 0.6 | 11.1 ± 0.5 | 0.23 ± 0.02 | 0.64 ± 0.05 | 16.0 ± 0.4 | 30.6 ± 1.4 | 0.8 ± 0.1 | 1.5 ± 0.1 | 6.7 ± 0.7 | 0 |
| Nocardioides sp. strain JS614 | 10.3 ± 0.8 | 21.2 ± 1.8 | 0.71 ± 0.04 | 0.91 ± 0.08 | 43.1 ± 4.2 | 21.5 ± 1.6 | 1.2 ± 0.2 | 1.0 ± 0.2 | 22.0 ± 4.1 | 0 |
| Mycobacterium sp. strain L1b | 7.6 | 11.9 | 0.96 | NRc | 100 | NR | 3.2 | NR | NR | NR |
| Pseudomonas sp. strain DL1b | 7.2 ± 0.3 | 13.1 ± 1.7 | 0.046 | 0.071 | 4.6 ± 0.2 | 3.8 ± 0.1 | 1.2 ± 0.2 | 18.8 ± 1.2 | NR | NR |
The growth yields of the Mycobacterium strains agreed fairly well with the yields of previously isolated Mycobacterium and Pseudomonas strains (57), assuming a value of 55% protein in dry weight of bacteria (6) (a ratio of 56% was calculated from the growth yields of strain JS60 in the present study). The higher growth yields of strain JS614 may reflect a different pathway of VC assimilation in this bacterium. For example, carboxylation of the epoxides resulting from VC and ETH oxidation (1) would allow the net incorporation of more carbon per mole of VC utilized than alternatives such as conjugation to coenzyme A (11) or glutathione (55). Further investigation into the biochemistry of VC assimilation in strain JS614 and the other isolates is required to test this hypothesis.
The maximum specific growth rates on VC of our mycobacteria were about 5-fold lower than that for Mycobacterium sp. strain L1 (25), 5-fold higher than that for Pseudomonas sp. strain DL1, and 50-fold higher than that for Pseudomonas sp. strain MF1 (58). The comparison with strain L1 may not be entirely appropriate given the higher growth temperature used in that study (30°C), but the comparison with the Pseudomonas strains is appropriate. The more rapid growth of the Mycobacterium strains adds weight to our argument that this group is more likely to be influential in the natural attenuation of VC. The maximum specific growth rate of Nocardioides sp. strain JS614 at 20°C was 15 times higher than that of strain DL1 and 150 times higher than that of strain MF1. Although Nocardioides-type VC degraders appear to be rare based on the results of our site-screening study, they are likely to have a large impact on the natural attenuation of VC where they occur due to their rapid growth and high biomass yields.
In general, the substrate utilization kinetics of the four Mycobacterium strains were more similar to each other than they were to the Nocardioides strain (Table 2). The k values for VC utilization ranged from 9 to 16 nmol/min/mg of protein among the four Mycobacterium strains; however, the corresponding k value for the Nocardioides strain was 43 nmol/min/mg of protein. The k values for ETH were greater than those for VC with the Mycobacterium strains, but the reverse was true with the Nocardioides strain (JS614).
The Ks values of the VC-assimilating strains showed no apparent grouping by phylogeny and were generally similar (ca. 1 μM). The Ks values agreed fairly well with data from other bacteria that grow on VC (58) and were much lower than the Ks values reported for aerobic cometabolic chlorinated-ethene-degrading cultures (3). Ks values for ETH among the Mycobacterium strains were higher than Ks values for VC, suggesting that the enzymes involved have a somewhat greater affinity for VC than for ETH. No such difference in affinity between VC and ETH was apparent for the Nocardioides strain JS614. The low Ks values for VC (and the absence of a measurable VC threshold) suggests that these bacteria are capable of degrading VC to very low levels under appropriate field conditions and are therefore environmentally relevant strains. In terms of kinetic parameters, it appears that it matters very little which species of VC-degrading Mycobacterium resides at a contaminated site but the presence of an organism like Nocardioides JS614 would represent a significantly greater VC oxidation potential.
All five strains examined were able to oxidize ETH to epoxyethane after growth on VC but not after growth on acetate, indicating the presence of an inducible alkene monooxygenase (Table 2). The specific activities measured for epoxyethane production ranged from 7 to 22 nmol/min/mg of protein. The measured activities are lower than the true activity (k value for ETH oxidation; Table 2), probably due to the inhibitory presence of epoxypropane in the cell suspensions. The addition of epoxypropane was required to stop the metabolism of epoxyethane (12) to allow an accumulation rate to be measured, but epoxypropane also partially inhibited ETH oxidation in experiments with strain JS60 (data not shown). In further experiments with JS60 (data not shown), ETH-grown cells also readily metabolized VC, suggesting that the same monooxygenase enzyme is active on VC and ETH and is inducible by both substrates. Alkene monooxygenase activity has also been detected in Mycobacterium sp. strain L1, Pseudomonas sp. strain MF1, and Pseudomonas sp. strain DL1 (25, 57, 58), indicating that monooxygenase-catalyzed epoxidation is common to all VC-assimilating bacteria. The monooxygenase activities calculated in the present study were significantly lower than the corresponding activities reported for Mycobacterium sp. strain L1 (25) and ETH-assimilating mycobacteria (23). This difference is at least partly due to the use of lower incubation temperatures in our experiments (20°C) and the inhibitory effects of epoxypropane.
Oxygen utilization kinetics.
Ks(O2) values were determined for five of the VC-assimilating strains (Table 3). For each strain, the Ks(O2) value for VC was lower than the Ks(O2) value for ETH, similar to the pattern seen with the Ks values for substrate utilization (Table 2). The very low Ks(O2) values measured (0.03 to 0.30 mg/liter) during growth on VC indicate that these five strains can effectively biodegrade VC under conditions of low-oxygen tension similar to those commonly encountered in the subsurface. Although the Ks(O2) values were low, all the strains exhibited oxygen threshold behavior during growth on VC, in which O2 uptake ceased when the O2 concentration dropped below 0.02 to 0.1 mg/liter. The Nocardioides strain (JS614) was the only strain to exhibit any significant oxygen threshold behavior during growth on ETH. It is unlikely that the O2 thresholds observed were analytical artifacts, as in every case elevated oxygen levels in experimental bottles were confirmed by comparison to an identically treated zero-oxygen standard. In addition, the cultures were not limited by lack of VC or ETH at the oxygen threshold point and respiking of oxygen (tested with strain JS616 on VC and strain JS614 on ETH) resulted in further O2 uptake until a threshold was again reached. Thus, cessation of oxygen uptake at the measured threshold value was not a result of substrate depletion or cell death. We cannot explain these observations at present, but they could reflect some properties of the initial monooxygenase in the VC-ETH pathway.
TABLE 3.
Oxygen half-velocity constants of VC-assimilating bacteria
| Strain |
Ks(O2) (mg/liter)a
|
O2 threshold (mg/liter)
|
||
|---|---|---|---|---|
| VC | ETH | VC | ETH | |
| Mycobacterium | ||||
| JS60 | 0.17 ± 0.06 | 0.18 ± 0.1 | 0.02 ± 0.01 | 0.00 |
| JS61 | 0.03 ± 0.04 | 0.25 ± 0.1 | 0.07 ± 0.01 | 0.00 |
| JS616 | 0.30 ± 0.24 | 1.1 ± 1.0 | 0.10 ± 0.02 | 0.00 |
| JS617 | 0.07 ± 0.06 | 0.2 ± 0.1 | 0.06 ± 0.02 | 0.02 ± 0.01 |
| Nocardioides sp. strain JS614 | 0.11 ± 0.04 | 0.29 ± 0.1 | 0.06 ± 0.01 | 0.12 ± 0.02 |
Data are averages of three replicates; error values represent 95% confidence intervals.
Effect of starvation on VC degradation.
To determine the response of the VC-assimilating isolates to periods of VC depletion, two representative strains were examined—Mycobacterium sp. strain JS60 and Nocardioides sp. strain JS614. VC starvation affected these two strains very differently (Fig. 3). Strain JS60 readily recovered from periods of VC starvation of at least 1 week of duration, and the time required for degradation of 150 μmol of VC increased in proportion to the length of the preceding starvation period. In its tolerance of VC starvation, strain JS60 resembled the pseudomonads of Verce et al. (57, 58) more than the Mycobacterium sp. strain L1 of Hartmans and de Bont (25), although it is difficult to directly compare these starvation experiments due to differences in the experimental conditions (e.g., cell density, temperature, and batch versus continuous culture).
FIG. 3.
Effect of VC starvation on VC degradation. Cultures of JS60 and JS614 were grown on VC in 50-ml MSM broths to mid-exponential phase (OD600 = 0.2 to 0.3; protein = 30 to 70 μg/ml) until the VC (additions of 2 × 150 μmol) was depleted. The cultures were then incubated for 0, 0.5, 1, or 7 days before readdition of VC (150 μmol = 1.0 mM initial aqueous concentration) and monitoring by GC analysis. ○, no starvation; ⋄, 12 h of starvation; □, 1 day of starvation; ▵, 1 week of starvation. Data are averages of three replicate cultures; error bars represent standard deviations.
In contrast to JS60, strain JS614 did not recover from periods of VC starvation of 1 day or longer. The sensitivity of JS614 to starvation is also illustrated by the fact that during kinetics experiments, its maximum VC utilization rate was reduced by approximately 50% after less than 5 min of VC starvation (data not shown). The physiological basis of the different responses of strains JS60 and JS614 to VC starvation is unknown. The sensitivity of JS614 could be due to activity of alkene monooxygenase combined with a lack of activity of the epoxide-transforming enzymes, resulting in the accumulation of toxic VC epoxide (25). If these experiments were to be considered representative of the behavior of VC-assimilating strains in the environment, Nocardioides VC degraders would be expected to be ineffective in locations where VC is not constantly present while Mycobacterium strains would be more robust. It is possible that the sensitivity to VC starvation of Nocardioides VC degraders explains the paradox of their apparently limited distribution in the environment (Table 1) despite their impressive growth and kinetic parameters (Table 2).
Testing of ETH degraders for growth on VC.
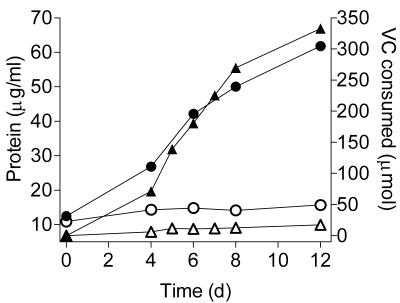
In light of the recent report describing an ETH-assimilating Pseudomonas strain that could adapt to growth on VC (58), we decided to investigate whether a similar adaptation can occur in gram-positive bacteria. Mycobacterium sp. strain K1 and Corynebacterium sp. strain K3, previously isolated from a consortium grown on ETH (34), were tested for their ability to grow on VC (Fig. 4). Strain K3 did not grow on VC, although cells from the same inoculum grew when ETH was used as the carbon source (data not shown). In contrast, strain K1 readily adapted to growth on VC as the sole carbon source, even after growth on nonselective medium. This finding was unexpected, as the consortium that was the source of strain K1 did not grow on VC (34). It is possible that in the present study, the use of a pure culture or the different experimental conditions permitted the growth of strain K1 on VC. Interestingly, the partial 16S rDNA sequence of strain K1 is similar to those of the M. rhodesiae-like strains we isolated on VC as the sole carbon source (Fig. 1).
FIG. 4.
Testing of ETH-assimilating bacteria for growth on VC as the carbon source. Mycobacterium sp. strain K1 and Corynebacterium sp. strain K3 (34) were grown on 1/10-TSA-1% glucose plates and inoculated into duplicate 50-ml MSM broth volumes at an initial OD600 of 0.10 (protein = 10 to 15 μg/ml). VC was added (150 μmol = 1.0 mM initial aqueous concentration), and the cultures were incubated at 20°C and sampled at intervals to quantify VC and protein. In the case of strain K1, VC was added again (2 × 150 μmol) after the initial amount had been degraded. ▴, VC-strain K1; •, protein-strain K1; ▵, VC-strain K3; ○, protein-strain K3. Data are averages of two replicate cultures.
The rapid growth of Mycobacterium strain K1 on VC is in contrast to the approximately 40-day lag period seen before an ETH-degrading Pseudomonas adapted to growth on VC (58), suggesting that in the case of strain K1, the ETH assimilation pathway functioned equally well for VC assimilation. This finding has significant implications for the natural attenuation of VC, because ETH-assimilating bacteria are likely to be present at chlorinated-ethene-contaminated sites, particularly when some ETH is produced as an end product of the anaerobic respiration of more highly chlorinated ethenes. It remains to be determined what proportion of ETH-assimilating bacteria can also grow on VC—our results with strain K3 and those of a previous study (25) suggest that the ability is not ubiquitous.
Conclusion.
Our findings indicate that the bacterial assimilation of VC is an ecologically significant phenomenon of an importance equal to or greater than that of cometabolic VC degradation. Members of a diverse group of Mycobacterium strains are capable of growth on VC, and these bacteria are indigenous and widely distributed at chlorinated-ethene-contaminated sites. Based on their distribution, growth rates, and kinetic parameters, we believe that Mycobacterium strains are most likely to be responsible for the aerobic natural attenuation of VC that has been observed at many sites. Further characterization of the strains we have isolated will provide a basis for developing molecular methods for detecting VC degraders in the environment and assessing the natural attenuation potential of individual sites. Our VC-assimilating strains may also be useful for enhanced bioremediation by complementing the activities of anaerobic bacteria and providing a mechanism for the complete mineralization of chlorinated ETHs.
Acknowledgments
We thank Shirley Nishino for help with sample collection and setup of enrichments and Juli Rubin and Sue Broxson for technical assistance. We are also grateful to the many people who provided information or material from chloroethene-contaminated sites: Petra Koziollek (who also provided strains K1 and K3), Melinda Chambless, Mike Witt, Sandra Dworatzek, Evan Cox, Elizabeth Edwards, Lori Moser, Bella Chu, Jim Clarke, Todd Wiedemeier, Bob Davis, Merv Dale, Scott Glass, Rob Simcik, John Nash, Kandi Brown, and Mike Miracle. This work was funded by the U.S. Strategic Environmental Research and Development Program.
N.V.C. was supported by a postdoctoral fellowship from the Oak Ridge Institute for Science and Education (U.S. Department of Energy).
REFERENCES
- 1.Allen, J. R., and S. A. Ensign. 1997. Purification to homogeneity and reconstitution of the individual components of the epoxide carboxylase multiprotein enzyme complex from Xanthobacter strain Py2. J. Biol. Chem. 272:32121-32128. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Cohen, L., and P. L. McCarty. 1991. Effects of toxicity, aeration, and reductant supply on trichloroethylene transformation by a mixed methanotrophic culture. Appl. Environ. Microbiol. 57:228-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez-Cohen, L., and G. E. Speitel, Jr. 2001. Kinetics of aerobic cometabolism of chlorinated solvents. Biodegradation 12:105-126. [DOI] [PubMed] [Google Scholar]
- 4.Bradley, P. M., and F. H. Chapelle. 1996. Anaerobic mineralization of vinyl chloride in Fe(III)-reducing aquifer sediments. Environ. Sci. Technol. 30:2084-2086. [Google Scholar]
- 5.Bradley, P. M., F. H. Chapelle, and D. R. Lovley. 1998. Humic acids as electron acceptors for anaerobic microbial oxidation of vinyl chloride and dichloroethene. Appl. Environ. Microbiol. 64:3102-3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brock, T. D., and M. T. Madigan. 1991. Biology of microorganisms, 6th ed. p. 121. Prentice-Hall, Englewood Cliffs, N.J.
- 7.Bucher, J. R., G. Cooper, J. K. Haseman, C. W. Jameson, M. Longnecker, F. Kamel, R. Maronpot, H. B. Matthews, R. Melnick, R. Newbold, R. W. Tennant, C. Thompson, and M. Waalkes. 2001. Ninth report on carcinogens. National Toxicology Program, U.S. Dept. of Health and Human Services, Washington, D.C. [Online.] http://ehis.niehs.nih.gov/roc/ninth/known/vinylchloride.pdf.
- 8.Casal, M., and J. R. Calero. 1974. Mycobacterium gadium sp. nov., a new species of rapid-growing scotochromogenic mycobacteria. Tubercle 55:299-308. [DOI] [PubMed] [Google Scholar]
- 9.Coleman, N. V., T. E. Mattes, J. M. Gossett, and J. C. Spain. 2002. Biodegradation of cis-dichloroethene as the sole carbon source by a β-proteobacterium. Appl. Environ. Microbiol. 68:2726-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Bont, J. A. M. 1976. Oxidation of ethylene by soil bacteria. Antonie Leeuwenhoek 42:59-71. [DOI] [PubMed] [Google Scholar]
- 11.de Bont, J. A. M., and W. Harder. 1978. Metabolism of ethylene by Mycobacterium E 20. FEMS Microbiol. Lett. 3:89-93. [Google Scholar]
- 12.de Bont, J. A. M., M. M. Atwood, S. B. Primrose, and W. Harder. 1979. Epoxidation of short-chain alkenes in Mycobacterium E20: the involvement of a specific monooxygenase. FEMS Microbiol. Lett. 6:183-188. [Google Scholar]
- 13.de Bruijn, F. J. 1992. Use of repetitive (repetitive extragenic palindromic and enterobacterial repetitive intergeneric consensus) sequences and the polymerase chain reaction to fingerprint the genomes of Rhizobium meliloti isolates and other soil bacteria. Appl. Environ. Microbiol. 58:2180-2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards, E. A., and E. E. Cox. 1997. Field and laboratory studies of sequential anaerobic-aerobic chlorinated solvent biodegradation, p. 261-265. In B. C. Alleman, and A. Leeman, (ed.), In situ and on-site bioremediation, vol. 3. Battelle Press, Columbus, Ohio.
- 15.Ensign, S. A., M. R. Hyman, and D. J. Arp. 1992. Cometabolic degradation of chlorinated alkenes by alkene monooxygenase in a propylene-grown Xanthobacter strain. Appl. Environ. Microbiol. 58:3038-3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ewers, J., D. Freier-Schröder, and H.-J. Knackmuss. 1990. Selection of trichloroethene (TCE) degrading bacteria that resist inactivation by TCE. Arch. Microbiol. 154:410-413. [DOI] [PubMed] [Google Scholar]
- 17.Fennell, D. E., A. B. Carroll, J. M. Gossett, and S. H. Zinder. 2001. Assessment of indigenous reductive dechlorinating potential at a TCE-contaminated site using microcosms, polymerase chain reaction analysis, and site data. Environ. Sci. Technol. 35:1830-1839. [DOI] [PubMed] [Google Scholar]
- 18.Fox, B. G., J. G. Borneman, L. P. Wackett, and J. D. Lipscomb. 1990. Haloalkene oxidation by the soluble methane monooxygenase from Methylosinus trichosporium OB3b: mechanistic and environmental implications. Biochemistry 29:6419-6427. [DOI] [PubMed] [Google Scholar]
- 19.Freedman, D. L., and J. M. Gossett. 1989. Biological reductive dechlorination of tetrachloroethylene and trichloroethylene to ethylene under methanogenic conditions. Appl. Environ. Microbiol. 55:2144-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freedman, D. L., and S. D. Herz. 1996. Use of ethylene and ethane as primary substrates for aerobic cometabolism of vinyl chloride. Water Environ. Res. 68:320-328. [Google Scholar]
- 21.Friedrich, M., R. J. Grosser, E. A. Kern, W. P. Inskeep, and D. M. Ward. 2000. Effect of model sorptive phases on phenanthrene biodegradation: molecular analysis of enrichments and isolates suggests selection based on bioavailability. Appl. Environ. Microbiol. 66:2703-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gossett, J. M. 1987. Measurement of Henry's Law constants for C1 and C2 chlorinated hydrocarbons. Environ. Sci. Technol. 21:202-208. [Google Scholar]
- 23.Habets-Crützen, A. Q. H., L. E. S. Brink, C. G. van Ginkel, J. A. M. de Bont, and J. Tramper. 1984. Production of epoxides from gaseous alkenes by resting-cell suspensions and immobilized cells of alkene-utilizing bacteria. Appl. Microbiol. Biotechnol. 20:245-250. [Google Scholar]
- 24.Hartmans, S., A. Kaptein, J. Tramper, and J. A. M. de Bont. 1992. Characterization of a Mycobacterium sp. and a Xanthobacter sp. for the removal of vinyl chloride and 1,2-dichloroethane from waste gases. Appl. Microbiol. Biotechnol. 37:796-801. [Google Scholar]
- 25.Hartmans, S., and J. A. M. de Bont. 1992. Aerobic vinyl chloride metabolism in Mycobacterium aurum L1. Appl. Environ. Microbiol. 58:1220-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartmans, S., J. A. M. de Bont, J. Tramper, and K. C. A. M. Luyben. 1985. Bacterial degradation of vinyl chloride. Biotechnol. Lett. 7:383-388. [Google Scholar]
- 27.Heath, L. S., G. L. Sloan, and H. E. Heath. 1986. A simple and generally applicable procedure for releasing DNA from bacterial cells. Appl. Environ. Microbiol. 51:1138-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hendrickson, E. R., J. A. Payne, R. M. Young, M. G. Starr, M. P. Perry, S. Fahnestock, D. E. Ellis, and R. C. Ebersole. 2002. Molecular analysis of Dehalococcoides 16S ribosomal DNA from chloroethene-contaminated sites throughout North America and Europe. Appl. Environ. Microbiol. 68:485-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hohnstock-Ashe, A. M., S. M. Plummer, R. M. Yager, P. Baveye, and E. L. Madsen. 2001. Further biogeochemical characterization of a trichloroethene-contaminated fractured dolomite aquifer: electron source and microbial communities involved in reductive dechlorination. Environ. Sci. Technol. 35:4449-4456. [DOI] [PubMed] [Google Scholar]
- 30.Holliger, C., D. Hahn, H. Harmsen, W. Ludwig, W. Schumacher, B. Tindall, F. Vasquez, N. Weiss, and A. J. Zehnder. 1998. Dehalobacter restrictus gen. nov. and sp. nov., a strictly anaerobic bacterium that reductively dechlorinates tetra- and trichloroethene in an anaerobic respiration. Arch. Microbiol. 169:313-321. [DOI] [PubMed] [Google Scholar]
- 31.Kalb, V. F., and R. W. Bernlohr. 1977. A new spectrophotometric assay for protein in cell extracts. Anal. Biochem. 82:362-371. [DOI] [PubMed] [Google Scholar]
- 32.Keppler, F., R. Borchars, J. Pracht, S. Rheinberger, and H. Scholer. 2002. Natural formation of vinyl chloride in the terrestrial environment. Environ. Sci. Technol. 36:2479-2483. [DOI] [PubMed] [Google Scholar]
- 33.Kielhorn, J., C. Melber, U. Wahnschaffe, A. Aitio, and I. Mangelsdorf. 2000. Vinyl chloride: still a cause for concern. Environ. Health Perspect. 108:579-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koziollek, P., D. Bryniok, and H.-J. Knackmuss. 1999. Ethene as an auxiliary substrate for the cooxidation of cis-dichloroethene and vinyl chloride. Arch. Microbiol. 172:240-246. [DOI] [PubMed] [Google Scholar]
- 35.Krumholz, L. R., R. Sharp, and S. S. Fishbain. 1996. A freshwater anaerobe coupling acetate oxidation to tetrachloroethylene dehalogenation. Appl. Environ. Microbiol. 62:4108-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee, M. D., J. M. Odom, and R. J. Buchanan, Jr. 1998. New perspectives on microbial dehalogenation of chlorinated solvents: insights from the field. Annu. Rev. Microbiol. 52:423-452. [DOI] [PubMed] [Google Scholar]
- 37.Lorah, M. M., and L. D. Olsen. 1999. Degradation of 1,1,2,2-tetrachloroethane in a freshwater tidal wetland: field and laboratory evidence. Environ. Sci. Technol. 33:227-234. [Google Scholar]
- 38.Malachowsky, K. J., T. J. Phelps, A. B. Teboli, D. E. Minnikin, and D. C. White. 1994. Aerobic mineralization of trichloroethylene, vinyl chloride, and aromatic compounds by Rhodococcus species. Appl. Environ. Microbiol. 60:542-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maymó-Gatell, X., T. Anguish, and S. H. Zinder. 1999. Reductive dechlorination of chlorinated ethenes and 1,2-dichloroethane by “Dehalococcoides ethenogenes” 195. Appl. Environ. Microbiol. 65:3108-3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maymó-Gatell, X., Y.-T. Chien, J. M. Gossett, and S. H. Zinder. 1997. Isolation of a bacterium that reductively dechlorinates tetrachloroethene to ethene. Science 276:1568-1571. [DOI] [PubMed] [Google Scholar]
- 41.McCarty, P. L. 1997. Breathing with chlorinated solvents. Science 276:1521-1522. [DOI] [PubMed] [Google Scholar]
- 42.Meyers, P. R., W. R. Bourn, L. M. Steyn, P. D. van Helden, A. D. Beyers, and G. D. Brown. 1998. Novel method for rapid measurement of growth of mycobacteria in detergent-free media. J. Clin. Microbiol. 36:2752-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newman, L. M., and L. P. Wackett. 1997. Trichloroethylene oxidation by purified toluene 2-monooxygenase: products, kinetics, and turnover-dependent inactivation. J. Bacteriol. 179:90-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pitulle, C., M. Dorsch, J. Kazda, J. Wolters, and E. Stackebrandt. 1992. Phylogeny of rapidly growing members of the genus Mycobacterium. Int. J. Syst. Bacteriol. 42:337-343. [DOI] [PubMed] [Google Scholar]
- 45.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, New York, N.Y.
- 46.Schneider, J., R. Grosser, K. Jayasimhulu, W. Xue, and D. Warshawsky. 1996. Degradation of pyrene, benz[a]anthracene, and benzo[a]pyrene by Mycobacterium sp. strain RJGII-135, isolated from a former coal gasification site. Appl. Environ. Microbiol. 62: 13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Semprini, L. 1997. Strategies for the aerobic co-metabolism of chlorinated solvents. Curr. Opin. Biotechnol. 8:296-308. [DOI] [PubMed] [Google Scholar]
- 48.Shim, H., D. Ryoo, P. Barbieri, and T. K. Wood. 2001. Aerobic degradation of mixtures of tetrachloroethylene, trichloroethylene, dichloroethylenes, and vinyl chloride by toluene-o-xylene monooxygenase of Pseudomonas stutzeri OX1. Appl. Microbiol. Biotechnol. 56:265-269. [DOI] [PubMed] [Google Scholar]
- 49.Squillace, P. J., M. J. Moran, W. W. Lapham, C. V. Price, R. M. Clawges, and J. S. Zogorski. 1999. Volatile organic compounds in untreated ambient groundwater of the United States, 1985-1995. Environ. Sci. Technol. 33:4176-4187. [Google Scholar]
- 50.Stackebrandt, E., and B. M. Goebel. 1994. A place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Bacteriol. 44:846-849. [Google Scholar]
- 51.Tortoli, E., R. M. Kroppenstedt, A. Bartoloni, G. Caroli, I. Jan, J. Pawlowski, and S. Emler. 1999. Mycobacterium tusciae sp. nov. Int. J. Syst. Bacteriol. 49:1839-1844. [DOI] [PubMed] [Google Scholar]
- 52.Tsukamura, M., and S. Mizuno. 1977. Numerical analysis of relationships among rapidly growing, scotochromogenic mycobacteria. J. Gen. Microbiol. 98:511-517. [DOI] [PubMed] [Google Scholar]
- 53.Tsukamura, M., and S. Ichiyama. 1986. Numerical classification of rapidly growing nonphotochromogenic mycobacteria. Microbiol. Immunol. 30:863-882. [DOI] [PubMed] [Google Scholar]
- 54.van der Meer, J. R., C. Werlen, S. F. Nishino, and J. C. Spain. 1998. Evolution of a pathway for chlorobenzene metabolism leads to natural attenuation in contaminated groundwater. Appl. Environ. Microbiol. 64:4185-4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Hylckama Vlieg, J. E. T., J. Kingma, A. J. van den Wijngaard, and D. B. Janssen. 1998. A glutathione S-transferase with activity towards cis-dichloroepoxyethane is involved in isoprene utilization by Rhodococcus sp. strain AD45. Appl. Environ. Microbiol. 64:2800-2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vannelli, T., M. Logan, D. M. Arciero, and A. B. Hooper. 1990. Degradation of halogenated aliphatic compounds by the ammonia-oxidizing bacterium Nitrosomonas europaea. Appl. Environ. Microbiol. 56:1169-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Verce, M. F., R. L. Ulrich, and D. L. Freedman. 2000. Characterization of an isolate that uses vinyl chloride as a growth substrate under aerobic conditions. Appl. Environ. Microbiol. 66:3535-3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verce, M. F., R. L. Ulrich, and D. L. Freedman. 2001. Transition from cometabolic to growth-linked biodegradation of vinyl chloride by a Pseudomonas sp. isolated on ethene. Environ. Sci. Technol. 35:4242-4251. [DOI] [PubMed] [Google Scholar]
- 59.Vogel, T. M., and P. L. McCarty. 1985. Biotransformation of tetrachloroethylene to trichloroethylene, dichloroethylene, vinyl chloride, and carbon dioxide under methanogenic conditions. Appl. Environ. Microbiol. 49:1080-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yagi, O., A. Hashimoto, K. Iwasaki, and M. Nakajima. 1999. Aerobic degradation of 1,1,1-trichloroethane by Mycobacterium spp. isolated from soil. Appl. Environ. Microbiol. 65:4693-4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yeager, C. M., P. J. Bottomley, and D. J. Arp. 2001. Cytotoxicity associated with trichloroethylene oxidation in Burkholderia cepacia G4. Appl. Environ. Microbiol. 67:2107-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]