Abstract
The production of ethanol from xylose by ethanologenic Escherichia coli strain KO11 was improved by adding various medium supplements (acetate, pyruvate, and acetaldehyde) that prolonged the growth phase by increasing cell yield and volumetric productivity (approximately twofold). Although added pyruvate and acetaldehyde were rapidly metabolized, the benefit of these additives continued throughout fermentation. Both additives increased the levels of extracellular acetate through different mechanisms. Since acetate can be reversibly converted to acetyl coenzyme A (acetyl-CoA) by acetate kinase and phosphotransacetylase, the increase in cell yield caused by each of the three supplements is proposed to result from an increase in the pool of acetyl-CoA. A similar benefit was obtained by inactivation of acetate kinase (ackA), reducing the production of acetate (and ATP) and sparing acetyl-CoA for biosynthetic needs. Inactivation of native E. coli alcohol-aldehyde dehydrogenase (adhE), which uses acetyl-CoA as an electron acceptor, had no beneficial effect on growth, which was consistent with a minor role for this enzyme during ethanol production. Growth of KO11 on xylose appears to be limited by the partitioning of carbon skeletons into biosynthesis rather than the level of ATP. Changes in acetyl-CoA production and consumption provide a useful approach to modulate carbon partitioning. Together, these results demonstrate that xylose fermentation to ethanol can be improved in KO11 by redirecting small amounts of pyruvate away from fermentation products and into biosynthesis. Though negligible with respect to ethanol yield, these small changes in carbon partitioning reduced the time required to complete the fermentation of 9.1% xylose in 1% corn steep liquor medium from over 96 h to less than 72 h.
Genetic engineering of metabolic pathways for the production of ethanol and plastics from carbohydrates (cellulose, hemicellulose, starch, and soluble sugars) offers the potential to replace a significant portion of imported petroleum (3, 14, 23). Glucose (cellulose and starch) represents the most abundant renewable feedstock, followed by xylose and other sugars derived from the hydrolysis of hemicellulose (3). Approximately 2 billion gallons of ethanol will have been produced by the United States in 2002, primarily from cornstarch. Although this represents less than 2% of U.S. automotive fuel, sufficient renewable lignocellulose is available from crop and timber residues to increase fuel ethanol production by at least 10-fold (3).
First-generation metabolic engineering for renewable chemicals such as ethanol (23, 39), lactate (5, 10, 19, 27), 1,3-propanediol (18, 36, 46), adipic acid (38), and succinate (20, 48) has focused primarily on product yields. The metabolic engineering of these new products often resulted in unexpected changes which increased the need for complex nutrients and decreased potential utility (9, 10, 12, 13, 31). For example, replacement of native fermentation pathways in Escherichia coli (23, 39) with the ethanol pathway from Zymomonas mobilis (Fig. 1) provided high ethanol yields from 10% xylose (or glucose) due to the low Km for pyruvate decarboxylase (PDC; 0.4 mM). However, the high efficiency of Z. mobilis PDC in this strain (KO11) also restricted carbon flow into the oxidizing arm of the tricarboxylic acid pathway, limiting cell growth and the rate of ethanol production (47).
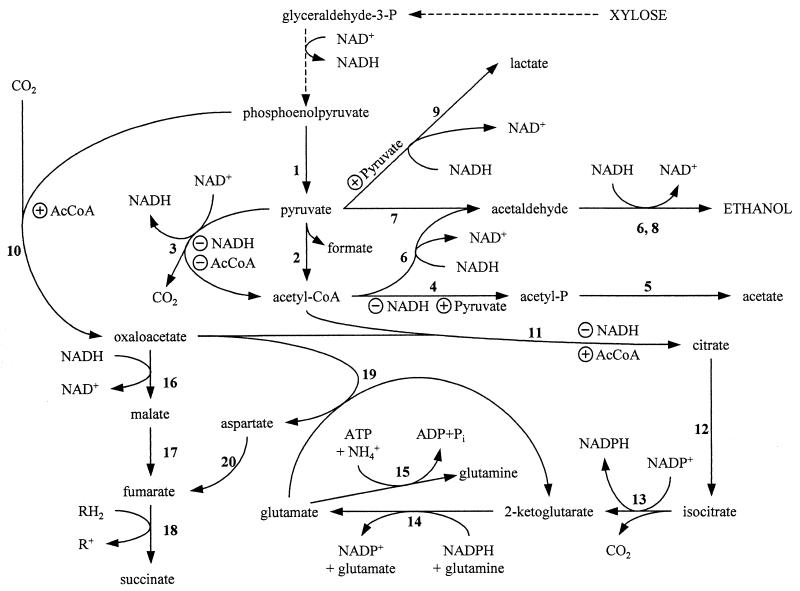
FIG. 1.
Carbon flow through central metabolism in KO11. Unless noted otherwise, enzymes listed are native to E. coli. Enzymes: 1, pyruvate kinase (pykA, pykF); 2, PFL (pflB); 3, PDH (aceEF, lpd); 4, phosphotransacetylase (pta); 5, acetate kinase (ackA); 6, alcohol-aldehyde dehydrogenase (adhE); 7, Z. mobilis PDC (pdc); 8, Z. mobilis alcohol dehydrogenase II (adhB); 9, LDH (ldhA); 10, PPC (ppc); 11, citrate synthase (gltA); 12, aconitase (acn); 13, isocitrate dehydrogenase (icd); 14, glutamate synthase (gltB, gltD); 15, glutamine synthetase (glnA); 16, malate dehydrogenase (mdh); 17, fumarase (fumB); 18, fumarate reductase (frdABCD); 19, aspartate transaminase (aspA); 20, aspartase (aspC). ⊕ indicates allosteric activation; ⊖ indicates allosteric inhibition.
Citrate synthase, a key enzyme in the partitioning of carbon into biosynthesis in native E. coli strains (50), was shown to be growth limiting for ethanologenic KO11 (47). Native citrate synthase is allosterically inhibited by high levels of NADH, which are typical of fermentation (17, 51). Growth and ethanol production were substantially improved in KO11 by the expression of an NADH-insensitive citrate synthase (citZ) from Bacillus subtilis (47). A similar stimulation of growth and ethanol production was observed during low aeration (oxidation of NADH) and upon addition of pyruvate, 2-ketoglutarate, and acetaldehyde (individually) to fermentation broth.
An alternative approach to enhance citrate synthase activity in KO11 is to increase available substrate pools (oxaloacetate and acetyl coenzyme A [acetyl-CoA]). In vitro, acetyl-CoA has been shown to serve as an allosteric activator of phosphoenolpyruvate carboxylase (PPC) (24) for the production of oxaloacetic acid and to relieve the allosteric inhibition of citrate synthase by NADH (51). In this paper, we show that physiological and genetic approaches which increase the availability of acetyl-CoA for biosynthesis increase cell yield and ethanol production. These results were used to engineer a second-generation biocatalyst, strain SU102, in which a small additional portion of substrate carbon was redirected from fermentation products to cellular biosynthesis.
MATERIALS AND METHODS
Microorganisms and media.
Strains and plasmids used in this study are listed in Table 1. KO11 and its derivatives (SU102 and SU104) are prototrophic. Working cultures of ethanologenic strains were transferred daily on solid medium (1.5% agar) containing mineral salts, 2% xylose, and 1% corn steep liquor (47). Stock cultures were stored frozen at −70°C. Luria-agar plates were used for the maintenance of other strains. Ampicillin (50 μg/ml), kanamycin (50 μg/ml), and tetracycline (5 or 10 μg/ml) were added as appropriate.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| Strains | ||
| KO11 | Δfrd cat pfl+pfl:: (Z. mobilis pdc+adhB+) | 39 |
| SU102 | KO11 ackA::FRT | This work |
| SU104 | KO11 ΔadhE::FRT | This work |
| W3110 | Wild type | ATCC 27325 |
| WA837 | rB− mB+gal met | 52 |
| Plasmids | ||
| pKD46 | γ β exo repA101 pSC101 repliconts (red recombinase) | 16 |
| pFT-A | bla flp pSC101 repliconts (FLP recombinase) | 40 |
| pCR2.1-TOPO | bla kan colE1 | Invitrogen |
| pKNOCK-Tc | tet R6K (pir-dependent replicon) | 1 |
| pSG76-K | kan FRT R6K (pir-dependent replicon) | 40 |
| pSG76-A | bla FRT R6K (pir-dependent replicon) | 40 |
| pLOI2065 | bla FRT-tet-FRT colE1 | This work |
| pLOI2224 | kan R6K (pir-dependent replicon) | 32 |
| pLOI2302 | bla colE1 (EcoRI flanked by AscI sites) | 55 |
| pLOI2375 | ackA::FRT-tet-FRT kan R6K (pir-dependent replicon) | This work |
| pLOI2803 | ΔadhE::FRT-tet-FRT kan colE1 | This work |
Fermentation.
Seed cultures and fermentations (35°C, 150 rpm) were grown in mineral salts medium containing 1% corn steep liquor and 9.1% xylose (47). Fermentations were maintained at pH 6.5 by the automatic addition of 2 N KOH (35). Fermentations were carried out in custom-designed fermentors with a 350-ml working volume, 500-ml total volume. With these units, temperature control was provided by immersion in a thermoregulated water bath. Vessels were continuously mixed at 100 rpm with 2.5-cm-long magnetic bars by a multiposition magnetic stirrer beneath the bath. Media supplements were filter sterilized as concentrates and added directly to fermentation broth. The pH of each vessel was independently regulated. Selenoid valves permitted base addition from graduated cylinders mounted above vessels. Samples were removed during fermentation for the measurement of cell mass, ethanol, organic acids, and sugars.
Analytical methods.
Cell mass was estimated from the optical density at 550 nm (0.33 mg [cell dry weight] ml−1) with a Bausch & Lomb Spectronic 70 spectrophotometer. Ethanol and acetaldehyde were measured by gas chromatography (Varian 3400CX) (35). Organic acids and sugars were analyzed by high-performance liquid chromatography (Hewlett Packard 1090 series II chromatograph equipped with refractive index and UV210 detectors) with a Bio-Rad Aminex HPX-87H ion exclusion column. Maximum volumetric productivity in millimoles/liter/hour was estimated as the first derivative of ethanol production by using PSI-Plot software (Poly Software International, Salt Lake City, Utah). Specific productivity was estimated by dividing volumetric productivity by cell mass; units are millimoles/gram (cell dry weight)/hour.
Genetic methods.
Standard methods were used for plasmid construction, DNA amplification by PCR, transformation, electroporation, and P1 phage transduction (34, 41). Primers (ORFmers) for the amplification of the E. coli ackA and adhE coding regions were purchased from the Sigma Scientific Company (St. Louis, Mo.). Chromosomal DNA from E. coli strain W3110 (ATCC 27325) served as the template for amplification. This strain was also used as an intermediate during the construction of ΔadhE in KO11.
Chromosomal insertion of deleted genes (adhE and ackA) was facilitated by inserting a tet gene flanked by the FRT sites for removal of the antibiotic marker by the chlortetracycline-inducible FLP recombinase (pFT-A) in the final construct (32, 40). Integration of linearized DNA was facilitated by using pKD46 (temperature conditional), containing an arabinose-inducible red recombinase (16). Putative deletion mutants were selected for tetracycline resistance (5 mg/liter) and screened for appropriate antibiotic resistance markers. At each step, mutants were verified by analyses of PCR and fermentation products.
Construction of pLOI2065 containing a removable tetracycline resistance cassette.
To facilitate antibiotic removal after chromosomal integration, a reusable cassette was constructed from the tet gene in pKNOCK-Tc (1) and the FRT sites in pSG76-A and pSG76-K (40). Both FRT sites were oriented in the same direction to allow efficient in vivo excision of the tet gene by the flp-encoded recombinase (32). This cassette was inserted into a modified plasmid, pUC18, to produce pLOI2065. Plasmid pLOI2065 contains two EcoRI sites and two SmaI sites oriented to allow the isolation of the FRT-tet-FRT cassette as a SmaI-to-EcoRI fragment for directional insertion, as a blunt fragment (SmaI), and as a sticky-ended fragment (EcoRI).
Construction of SU102 containing an insertion mutation in ackA.
The PCR-amplified coding region of ackA was cloned into pCR2.1-TOPO. After digestion with EcoRI, the 1.2-kbp fragment containing the ackA coding region was ligated into the unique EcoRI site of pLOI2302. A recombinant plasmid was selected in which the directions of transcription of the lac and ackA genes were opposite. The ackA gene was disrupted by digestion with EcoRV (one site) and the insertion of a 1.7-kbp SmaI fragment from pLOI2065 containing a tet gene flanked by two FRT sites for FLP recombinase. A 2.8-kbp AscI fragment containing ackA′-FRT-tet-FRT-′ackA was isolated from this plasmid and ligated into the AscI site of pLOI2224 containing a conditional R6K replicon. The resulting plasmid, pLOI2375, was used as a template for PCR amplification of the 2.8-kbp AscI fragment with ackA primers. After purification by phenol extraction, amplified DNA was used for the electroporation of E. coli KO11(pKD46) expressing phage lambda red recombinase (16). Recombinants were selected for tetracycline resistance. Plasmid pKD46 was eliminated by growth at 40°C. The integrated tet gene was deleted by using pFT-A expressing the FLP recombinase (32, 40). After removal of this plasmid by growth at 40°C, the resulting strain containing a mutation in ackA (insertion of 98 bases including stop codons in all three reading frames) was designated SU102.
Construction of SU104 containing a deletion in adhE.
The PCR-amplified coding region of adhE (2.7 kbp) was cloned into pCR2.1-TOPO. A recombinant plasmid was selected in which the transcription of lac and adhE was oriented in the same direction. The central region of the adhE gene (1.1 kbp) was deleted by digestion with HincII (two sites) and replaced with a 1.6-kbp SmaI fragment from pLOI2065 containing the FRT-tet-FRT cassette (1.7 kbp) to produce pLOI2803. After digestion of pLOI2803 with both PvuI and ScaI, this plasmid served as a template to amplify the 3.2-kbp region containing adhE′-FRT-tet-FRT-′adhE by using the adhE primers. This amplified DNA was used for the electroporation of W3110. Recombinants were selected for tetracycline resistance and analyzed to identify a clone containing the ΔadhE::FRT-tet-FRT mutation. Plasmid pKD46 was eliminated by growth at 42°C.
P1 transduction was used to transfer the adhE deletion in W3110 to KO11. To circumvent differences in restriction systems, the ΔadhE::FRT-tet-FRT mutation was transduced into a restriction-negative (modification-positive) derivative of E. coli B (strain WA837) prior to transduction into KO11. The tetracycline resistance gene was deleted from the KO11 derivative using pFT-A expressing the flp recombinase (32, 40). After removal of this plasmid by growth at 40°C, the resulting strain containing an internal deletion in adhE was designated SU104.
Nucleotide sequence accession number.The sequence for plasmid pLOI2065 has been deposited in GenBank under acquisition number AF521666.
RESULTS AND DISCUSSION
Acetate addition stimulates growth and ethanol production by reducing net acetate production during sugar metabolism.
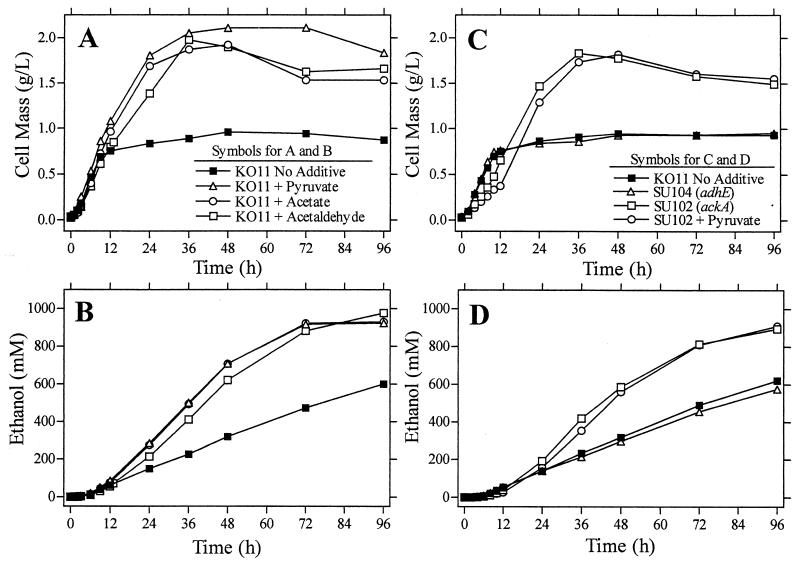
During the aerobic metabolism of sugars by E. coli, acetate production has been associated with a decrease in growth rate. Considerable effort has been made to minimize acetate production as a means of increasing cell density and the production of recombinant proteins (2, 4, 11, 15, 53, 54). The addition of as little as 2 g of sodium acetate (24 mM)/liter has been shown to decrease growth rate during oxidative sugar metabolism (29). During xylose fermentation by KO11, however, the addition of acetate stimulated growth and ethanol production (Fig. 2A and B; Table 2). A portion of the added acetate was initially consumed, in contrast to control fermentations where acetate was continuously produced (Fig. 3A). Rates of acetate production declined during subsequent incubation in both control and acetate-supplemented fermentations. Although almost twice as much sugar was metabolized by acetate-supplemented fermentations as by control fermentations (no additions), net acetate production in the acetate-supplemented culture (7.0 mmol/liter) was less than half that of the control (18.6 mmol/liter) after 72 h.
FIG. 2.
Effect of media additions and mutations on growth (A and C) and ethanol production (B and D).
TABLE 2.
Effects of mutations and additives on cell yield and ethanol productivity
| Strain and additive | Concn (mM) | n | Cell mass
|
Ethanol
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| Maximum concn (g/liter) | Time (h) | μa (h−1) | Maximum concn (mM) | Maximum VPb (mmol/liter/h) | SPc (mmol/g/h) | Theoretical yieldd (%) | |||
| KO11 | None | 44 | 0.95 ± 0.13 | 72 | 0.63 | 641 ± 93 | 8.3 | 10.1 | 63 |
| Pyruvate | 21 | 18 | 2.00 ± 0.18 | 72 | 0.76 | 955 ± 55 | 18.3 | 11.5 | 94 |
| Acetate | 24 | 2 | 1.92 ± 0.01 | 48 | 0.75 | 930 ± 11 | 18.7 | 12.0 | 92 |
| Acetate | 19 | 6 | 1.61 ± 0.30 | 72 | NDf | 901 ± 118 | ND | ND | 89 |
| Acetaldehydee | 11 | 6 | 1.51 ± 0.15 | 96 | 0.47 | 909 ± 98 | 18.2 | 11.3 | 90 |
| 2-Ketoglutarate | 12 | 6 | 1.84 ± 0.20 | 72 | ND | 907 | ND | ND | 90 |
| SU102 | None | 8 | 1.94 ± 0.14 | 48 | 0.66 | 946 ± 20 | 17 | 13.7 | 93 |
| Pyruvate | 21 | 4 | 1.93 ± 0.13 | 48 | 0.44 | 926 ± 19 | 17.4 | 10.0 | 92 |
| Acetate | 19 | 2 | 2.24 ± 0.17 | 48 | ND | 933 ± 26 | ND | ND | 92 |
| Acetaldehyde | 11 | 2 | 2.02 ± 0.17 | 48 | ND | 952 ± 6 | ND | ND | 94 |
| 2-Ketoglutarate | 12 | 2 | 1.83 ± 0.01 | 96 | ND | 901 ± 1 | ND | ND | 89 |
| SU104 | None | 8 | 1.02 ± 0.08 | 96 | 0.71 | 550 ± 89 | 8.9 | 13.7 | 55 |
| Pyruvate | 21 | 2 | 1.96 ± 0.06 | 48 | ND | 885 ± 24 | ND | ND | 87 |
| Acetate | 19 | 2 | 1.68 ± 0.02 | 48 | ND | 889 ± 10 | ND | ND | 88 |
| Acetaldehyde | 11 | 2 | 1.57 ± 0.31 | 48 | ND | 856 ± 100 | ND | ND | 84 |
| 2-Ketoglutarate | 12 | 2 | 1.97 ± 0.15 | 48 | ND | 971 ± 20 | ND | ND | 96 |
Specific growth rate at 2 h.
VP, volumetric productivity.
SP, specific productivity at 12 h.
Observed ethanol yield expressed as a percentage of the maximum theoretical yield from 91 g of xylose liter−1 (1.667 mmol of ethanol/mmol of xylose).
Half added initially, half added after 12 h.
ND, not determined. Estimates of specific and volumetric productivity were not calculated due to the limited number of sampling times.
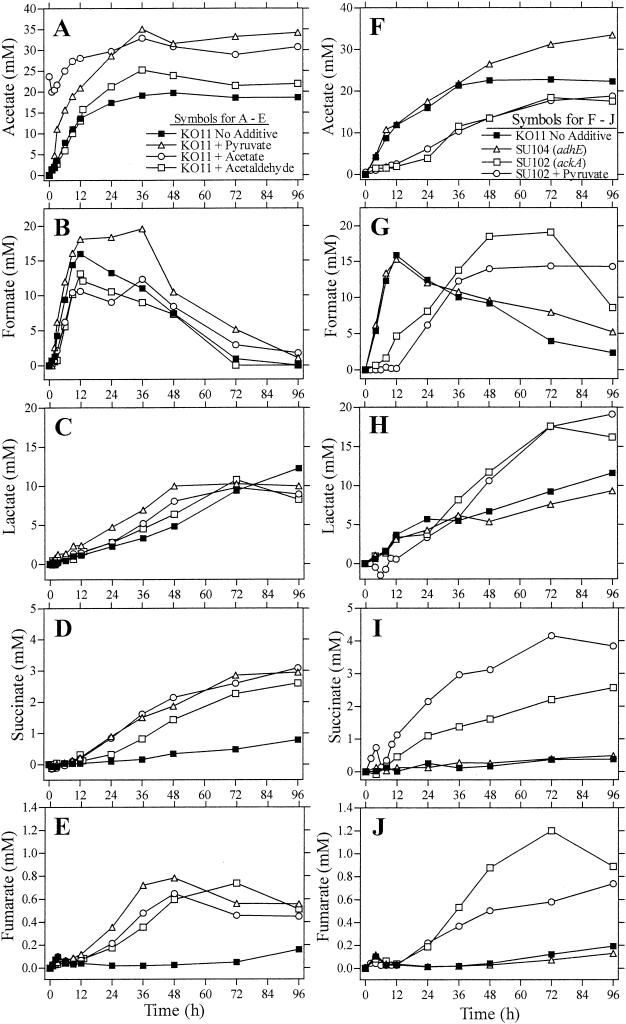
FIG. 3.
Effects of media additions and mutations on organic acid production: acetate (A and F), formate (B and G), lactate (C and H), fumarate (D and I), and succinate (E and J).
Previous studies have shown that the reversible phosphotransacetylase-acetate kinase pathway can serve as a route for entry of added acetate into the intracellular pool of acetyl-CoA (8, 22). Additional acetate uptake activity may be provided by the inducible acetyl-CoA synthetase, although this gene is typically repressed under fermentative conditions (26). Thus, the stimulation of growth and ethanol production by added acetate is presumed to result from the increased availability of acetyl-CoA. Under anaerobic conditions, the primary role of the tricarboxylic acid (TCA) pathway is to supply carbon skeletons for biosynthesis. Increasing the availability of acetyl-CoA would promote biosynthesis by relieving the NADH-mediated allosteric inhibition of citrate synthase (51) and by serving as an allosteric activator of PPC (24).
Stimulation of growth and ethanol production by added pyruvate can be primarily attributed to the increased production of acetate.
The stimulation of growth and ethanol production by pyruvate reported previously (47) appeared quite similar to the effects of added acetate (Fig. 2A and B). Analysis of products during fermentation provided further evidence of a related mechanism of action for acetate and pyruvate (Fig. 3A through E). With the exception of formate (Fig. 3B), profiles of organic acids were similar for acetate- and pyruvate-supplemented cultures. Both were distinctive from the control lacking supplements. Control fermentations produced lower levels of lactate than pyruvate-supplemented and acetate-supplemented fermentations during the initial 72 h (Fig. 3C). Addition of pyruvate stimulated the production of acetate to levels equivalent to those of acetate-supplemented fermentations (Fig. 3A). In both pyruvate- and acetate-supplemented fermentations, acetate concentrations were approximately twofold higher than those in the control after 36 h. Acetate concentrations in all fermentations remained relatively constant during further incubation.
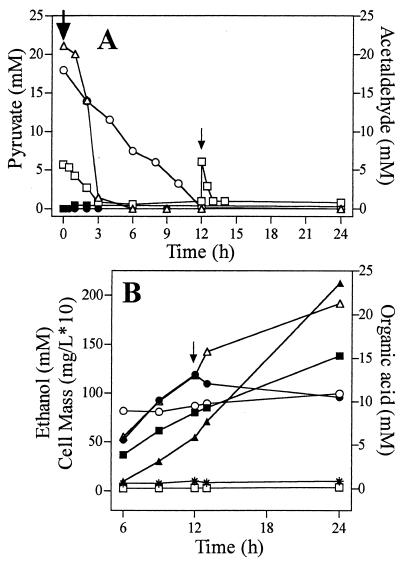
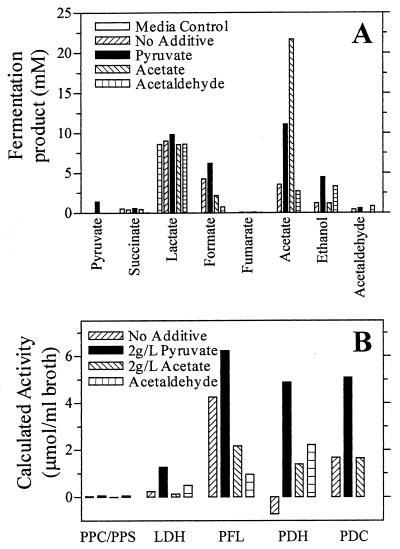
Most of the supplemental pyruvate (22 mM) was metabolized during the initial 3 h of incubation (Fig. 4), although the benefits for growth and ethanol production persisted throughout fermentation. For the initial 3 h following pyruvate addition, an increase in acetate was the largest change in the fermentation products (Fig. 5A). Smaller pyruvate-dependent increases were observed for ethanol, formate, lactate, and acetaldehyde. Biosynthetic needs were estimated to be small (increase of approximately 0.06 mg [cell dry weight]/liter) and did not represent a significant sink for the added pyruvate (2 g/liter). The partitioning of pyruvate between these different fermentation products (and biosynthesis) in KO11 is generally regarded as the result of five competing reactions: those of PDC, pyruvate formate lyase (PFL), pyruvate dehydrogenase (PDH), lactate dehydrogenase (LDH), and PPC. On a triose basis, relative activities can be estimated from the distribution of fermentation products (Fig. 1) (17). The large pyruvate-dependent increase in acetate after 3 h (Fig. 5A) reflects an increase in acetyl-CoA production (PFL and PDH activities). In the absence of formate hydrogen lyase induction (6), formate production provides an independent measure of PFL activity and exhibited a modest increase compared to that for acetate. These results indicate that PDH activity (estimated as acetate concentration minus the activity of formate concentration) serves as the primary source of additional acetyl-CoA during the metabolism of added pyruvate (Fig. 5B). Production of ethanol also increased immediately after the addition of pyruvate due to an increase in the production of acetaldehyde by PDC. Increased pyruvate oxidation by PDH is presumed to provide the additional NADH required to reduce acetaldehyde produced from added pyruvate. The increase in LDH activity (estimated as lactate production) can be attributed to substrate activation (45). Elevated extracellular levels of acetate in pyruvate-supplemented fermentations may serve to increase intracellular acetyl-CoA pools, extending the period of growth and thereby increasing the volumetric rate of ethanol production.
FIG. 4.
Metabolism of added acetaldehyde and pyruvate during fermentation. Pyruvate addition is indicated by the large arrow. Acetaldehyde additions (5.6 mM each) are indicated by the large arrow (initial addition) and the small arrow (second addition at 12 h). (A) Utilization of added pyruvate and acetaldehyde. Symbols: ▵, pyruvate utilization by KO11; ○, pyruvate utilization by SU102; □, acetaldehyde utilization by KO11; ▪, acetaldehyde in KO11 broth with no additions; •, pyruvate in KO11 broth with no additions. (B) Effect of the second acetaldehyde addition on the production of fermentation products by KO11. Symbols: ▪, cell mass; ▴, ethanol; •, formate; ○, lactate; ▵, acetate; *, succinate; □, fumarate.
FIG. 5.
Partitioning of carbon among competing pathways during the initial 3 h of fermentation. (A) Fermentation products after 3 h are illustrated. (B) Relative activity of primary enzymes that partition 3-carbon intermediates carbon (pyruvate and phosphoenolpyruvate) through competing pathways. Relative activities were estimated by using fermentation products and expressed as micromoles of product per milliliter during the initial 3 h of incubation. Endogenous production of acetate in acetate-supplemented fermentations was assumed to be equal to that for the control fermentation without additives. PDC activity was assumed to be equal to ethanol production, except for the acetaldehyde-supplemented fermentations where it could not be calculated. PDH was calculated as the difference between acetate and formate production. LDH, PFL, and PPC activities were assumed to be equal to the production of lactate, formate, and succinate, respectively.
The channeling of pyruvate to acetyl-CoA and acetate by the addition of pyruvate can be readily explained based on known allosteric controls (Fig. 1). Pyruvate is both a substrate for acetyl-CoA production and a strong allosteric activator of phosphotransacetylase (43). Addition of pyruvate has also been shown to increase acetaldehyde and decrease the level of NADH (47), an allosteric inhibitor of phosphotransacetylase (43), and PDH (17, 21). These actions would also tend to increase the partitioning of carbon into acetate.
Higher levels of succinate and fumarate (3-fold to over 10-fold, respectively) were produced by acetate- and pyruvate-supplemented fermentations (Fig. 3D and E). PPC (24) and citrate synthase (51) are both activated by acetyl-CoA and link the supply of this important intermediate to fermentation and biosynthesis. Under anaerobic conditions, the reductive portion of the TCA pathway is used to produce succinate. Due to the deletion of fumarate reductase (frd) in KO11, little succinate was produced and a small amount of fumarate accumulated. The increases in succinate and fumarate levels in acetate- and pyruvate-supplemented fermentations may result from an excess of citrate. Excess citrate can be cleaved into acetate and oxaloacetate by an inducible citrate lyase (30). Additional succinate can be produced from isocitrate by isocitrate lyase (51).
Pyruvate and free CoA are cosubstrates for formate production by PFL (Fig. 1). Formate levels increased during the initial 12 h of incubation in all fermentations and declined thereafter (Fig. 3B). The decline in formate can be attributed to the formate-inducible formate hydrogen lyase (6). Supplementing with acetate and pyruvate had opposite effects on formate production (Fig. 3B): higher concentrations in pyruvate-supplemented fermentations and lower concentrations in acetate-supplemented fermentations in comparison to those in the control. Both differences are in general agreement with the central role of acetyl-CoA and free CoA in metabolism (11, 15, 25). A lack of free CoA in acetate-supplemented fermentations may limit formate production by PFL. Conversely, higher levels of formate in pyruvate-supplemented fermentations may result from an increase in free CoA due to the allosteric activation of phosphotransacetylase by pyruvate (43).
Stimulation of growth and ethanol production by acetaldehyde can be attributed to increased acetyl-CoA.
Growth and ethanol production were also stimulated by acetaldehyde (47) (Fig. 2A and B). At concentrations above 5.6 mM, acetaldehyde strongly inhibited growth. It was empirically determined that stimulation equivalent to that of pyruvate could be achieved by the addition of 11.2 mM acetaldehyde, 5.6 mM initially and 5.6 mM after 12 h of fermentation (47). Previous studies also demonstrated that the addition of acetaldehyde caused a rapid decrease in the intracellular concentration of NADH (47).
The initial addition of acetaldehyde was metabolized within 3 h (Fig. 4A). During this time, ethanol increased by an amount equal to 70% of the added acetaldehyde (Fig. 5A). Increased pyruvate flux through PDH appears to provide the additional NADH required for acetaldehyde reduction (Fig. 5B). The second acetaldehyde addition was metabolized within 1 h (Fig. 4A), although benefits persisted throughout fermentation (Fig. 2A and B). Following the second addition, production of acetate and ethanol was increased while formate production was reduced. The persisting benefit of acetaldehyde additions for growth and ethanol production are presumed to result from an increase in the intracellular acetyl-CoA pool as a consequence of higher extracellular levels of acetate. High levels of NADH and global regulation by ArcA and FNR (17) may also limit PDH function in the absence of supplements. Increased production of acetyl-CoA by PDH (and perhaps increased synthesis of PDH) would be expected in response to NADH oxidation (50).
The production of formate by PFL may be limited by competition with PDH for free CoA. Patterns of organic acid production in acetaldehyde-supplemented cultures provide further support for a mechanism of action similar to that for pyruvate and acetate (Fig. 3A through E). Acetate levels were higher in all three supplemented cultures than in the unsupplemented control. Each supplemented fermentation also produced higher levels of succinate, lactate, and fumarate than the control.
Stimulation of growth and ethanol production by the inactivation of nonbiosynthetic pathways which consume acetyl-CoA.
Acetyl-CoA serves as the single most important intermediate for cellular biosynthesis, providing over half of the cellular carbon during sugar metabolism (37). Previous studies have shown that cell growth is limited by the availability of carbon skeletons during the fermentation of xylose (47), a limitation which was relieved (Fig. 2A and B) by supplements that increase the extracellular levels of acetate (acetate, pyruvate, acetaldehyde). During fermentation (Fig. 1), two pathways drain acetyl-CoA from the intracellular pool but provide limited benefit for biosynthesis. Acetyl-CoA can be reduced to acetaldehyde and ethanol by alcohol-aldhehyde dehydrogenase E (adhE) as an alternative route for NADH oxidation in KO11 (Fig. 1). Acetyl-CoA can also be converted to acetate by phosphotransacetylase (pta) and acetate kinase (ackA), increasing the production of ATP. Mutations in these pathways were investigated as a means of reducing acetate production and sparing acetyl-CoA for biosynthetic needs.
Inactivation of ackA rather than pta was chosen to minimize the potential problems associated with global regulation. Acetyl-P is proposed to serve as an important global regulator in E. coli (7, 25, 33), affecting gene expression and fundamental processes such as the turnover of RpoS. During oxidative metabolism, inactivation of the acetate pathway is detrimental to growth (11, 15, 25). Although not fully understood, this detrimental effect has been attributed to the depletion of free CoA due to low rates of acetyl-CoA turnover (11). In contrast to that found in previous studies concerning oxidative metabolism, inactivation of ackA (SU102) stimulated growth and ethanol production during the fermentation of xylose (Fig. 2C and D). An adhE mutation in strain KO11 (strain SU104) was of no benefit during xylose fermentation. Together, these results suggest that ADH contributes little to metabolism in KO11. The beneficial effect of inactivating ackA is presumed to result from a decrease in the production of acetate, increasing the availability of acetyl-CoA for biosynthesis. With regard to biosynthesis, the ackA mutation is a genetic equivalent of adding acetate, pyruvate, or acetaldehyde.
Strains SU104 (adhE mutant) and SU102 (ackA mutant) were also tested in fermentations with supplements that had been shown to increase the growth and ethanol production in KO11 (Table 2). The addition of acetate, pyruvate, and acetaldehyde to SU104 increased growth and ethanol production, indicating that the native alcohol dehydrogenase (adhE) was not essential for this response. Growth and ethanol production by SU102 (ackA) without supplements were equivalent to those by KO11 with supplements. The addition of pyruvate, acetate, 2-ketoglutarate, or acetaldehyde to SU102 provided little further improvement in growth or ethanol production.
High-performance liquid chromatography analysis of organic acids revealed similarities in the patterns of fumarate (Fig. 3J) and succinate (Fig. 3I) production between SU102 (ackA mutant) and KO11 supplemented with acetate, pyruvate, or acetaldehyde (Fig. 3E). The ackA mutation in SU102 also increased lactate production (Fig. 3I) and delayed the production of formate (Fig. 3G) and acetate (Fig. 3F). The delay in formate production in SU102 could result from increased acetyl-CoA, reducing the pool of free CoA (cosubstrate for PFL) analogous to that in acetate-supplemented KO11. Both acetate addition and mutations in the acetate pathway have been shown to cause a similar repression of 37 genes (25), attributed to an increase in the acetyl-CoA pool.
Inactivation of acetate kinase (SU102) caused an initial delay in acetate production but did not block later synthesis. The pathway responsible for acetate production during the latter stages of fermentation remains unknown but may be the result of the spontaneous dephosphorylation of acetyl-P as previously proposed (8) or from the induction of a cryptic enzyme(s). Despite the potential benefit of increased ATP production by acetate kinase, the increased drain of acetyl-CoA to acetate through this pathway appears to be more detrimental for growth and ethanol production than the reduction in ATP. With the exception of acetate (Fig. 3F), the production of fermentation products by the adhE mutant (strain SU104) was essentially the same as that for the parent strain, KO11 (Fig. 3A). Acetate production by SU104 continued throughout fermentation and reached higher final concentrations than that by KO11.
Conclusions.
Increasing the availability of acetyl-CoA stimulated growth and ethanol production from xylose by prolonging the growth phase of ethanologenic E. coli. The resulting increase in biocatalyst rather than an increase in cellular activity was responsible for the increased rate of ethanol production (Table 2). Similar benefits were obtained by minimizing the loss of acetyl-CoA as acetate (ackA mutation) and by increasing intracellular levels of acetate (supplementing with acetate, pyruvate, or acetaldehyde). Inactivation of the native E. coli alcohol-aldehyde dehydrogenase gene (adhE) had little effect, indicating that this pathway has limited function in ethanologenic KO11.
ATP production during xylose fermentation does not appear to limit cell yield in KO11. Including the energy required for xylose uptake and activation, less than 1 ATP (net) is produced from the metabolism of each xylose converted to ethanol (44, 47). During the initial 12 h of growth, up to 31% of the ATP (net) produced by KO11 is provided by the acetate pathway (calculated by assuming 1 ATP per acetate from acetate kinase and 0.4 ATP per pyruvate from glycolysis [47]). Disruption of this pathway (ackA) in SU102 increased cell yield twofold (Table 2). Thus, the partitioning of carbon skeletons rather than the production of ATP appears to limit the growth of ethanologenic E. coli during xylose fermentation. It is interesting to note that a similar conclusion has been reached for native strains of Saccharomyces cerevisiae (42) and Candida utilis (49). With both yeasts, cell yield appears to be limited by the partitioning of carbon into biosynthetic pathways rather than available ATP.
The mechanism for the stimulation of growth in ethanologenic E. coli KO11 is consistent with established patterns of allosteric regulation, although further controls of gene expression (FNR, ArcA) may also contribute to the observed effects. More than half the amino acids produced in the cell are derived from the TCA pathway. Flux through this pathway is controlled by PPC and citrate synthase (28, 50), activities which can be stimulated by acetyl-CoA (51). The individual addition of pyruvate, acetate, and acetaldehyde increased the extracellular levels of acetate, which can in turn serve to elevate intracellular pools of acetyl-CoA by reversible reactions. Additional benefits of supplements include a reduction in the level of NADH (added pyruvate and acetaldehyde), an allosteric inhibitor of citrate synthase that is antagonized by high levels of acetyl-CoA. Based on these results with mutants and with supplements, we conclude that the regulation of acetyl-CoA production and consumption can be used to make small changes in the partitioning of carbon between biosynthesis and fermentation during ethanol production by E. coli KO11. Though negligible with respect to ethanol yield, these small changes in carbon partitioning reduced in half the time required to complete the fermentation of 9.1% xylose.
Acknowledgments
This research was supported by the Florida Agricultural Experiment and grants from the U.S. Department of Agriculture (01-35504-10669 and 00-52104-9704) and the U.S. Department of Energy (FG02-96ER20222).
Footnotes
Florida Agricultural Experiment Station Publication no. R08879.
REFERENCES
- 1.Alexeyev, M. F. 1999. The pKNOCK series of broad-host-range mobilizable suicide vectors for gene knockout and targeted DNA insertion into the chromosome of gram-negative bacteria. BioTechniques 26:824-828. [DOI] [PubMed] [Google Scholar]
- 2.Aristidou, A. A., K. San, and G. N. Bennett. 1995. Metabolic engineering of Escherichia coli to enhance recombinant protein production through acetate reduction. Biotechnol. Prog. 11:475-478. [DOI] [PubMed] [Google Scholar]
- 3.Arntzen, C. E., and B. E. Dale. 1999. Biobased industrial products. Priorities for research and commercialization. National Academy Press, Washington, D.C. [PubMed]
- 4.Bauer, K. A., A. Ben-Bassat, M. Dawson, V. T. de la Puente, and J. O. Neway. 1990. Improved expression of human interleukin-2 in high-cell-density fermentor cultures of Escherichia coli K-12 by a phosphotransacetylase mutant. Appl. Environ. Microbiol. 56:1296-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bianchi, M. M., L Brambilla, F. Protani, C.-L. Liu, J. Lievense, and D. Porro. 2001. Efficient homolactic fermentation by Kluyveromyces lactis strains defective in pyruvate utilization and transformed with the heterologous LDH gene. Appl. Environ. Microbiol. 67:5621-5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bock, A., and G. Sawers. 1996. Fermentation, p. 262-282. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. American Society for Microbiology, Washington, D.C.
- 7.Bouche, S., E. Klauck, D. Fischer, M. Lucassen, K. Jung, and R. Hengge-Aronis. 1998. Regulation of RssB-dependent proteolysis in Escherichia coli: a role for acetyl phosphate in a response regulator-controlled process. Mol. Microbiol. 27:787-795. [DOI] [PubMed] [Google Scholar]
- 8.Brown, T. D. K., M. C. Jones-Mortimer, and H. L. Kornberg. 1977. The enzymic interconversion of acetate and acetyl-coenzyme A in Escherichia coli. J. Gen. Microbiol. 102:327-336. [DOI] [PubMed] [Google Scholar]
- 9.Bunch, P. K., F. Mat-Jan, N. Lee, and D. P. Clark. 1997. The ldhA gene encoding the fermentative lactate dehydrogenase of Escherichia coli. Microbiology 143:187-195. [DOI] [PubMed] [Google Scholar]
- 10.Chang, D.-E., H.-C. Jung, J.-S. Rhee, and J.-G. Pan. 1999. Homofermentative production of d- or l-lactate in metabolically engineered Escherichia coli RR1. Appl. Environ. Microbiol. 65:1384-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang, D.-E., S. Shin, J.-S. Rhee, and J.-G. Pan. 1999. Acetate metabolism in a pta mutant of Escherichia coli W3110: importance of maintaining acetyl coenzyme A flux for growth and survival. J. Bacteriol. 181:6656-6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chao, Y.-P., and J. C. Liao. 1994. Metabolic responses to substrate futile cycling in Escherichia coli. J. Biol. Chem. 269:5122-5126. [PubMed] [Google Scholar]
- 13.Chao, Y.-P., R. Patnaik, W. D. Roof, R. F. Young, and J. C. Liao. 1993. Control of gluconeogenic growth by pps and pck in Escherichia coli. J. Bacteriol. 175:6939-6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chotani, G., T. Dodge, A. Hsu, M. Kumar, R. LaDuca, D. Trimbur, W. Weyler, and K. Sanford. 2000. The commercial production of chemicals using pathway engineering. Biochim. Biophys. Acta 1543:434-455. [DOI] [PubMed] [Google Scholar]
- 15.Contiero, J., C. Beatty, S. Kumar, C. L. DeSanti, W. R. Strohl, and A. Wolfe. 2000. Effects of mutations in acetate metabolism on high-cell-density growth of Escherichia coli. J. Ind. Microbiol. 24:421-430. [Google Scholar]
- 16.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Graef, M. R., S. Alexeeva, J. L. Snoep, and M. J. Teixeira de Mattos. 1999. The steady-state internal redox state (NADH/NAD) reflects the external redox state and is correlated with catabolic adaptation in Escherichia coli. J. Bacteriol. 181:2351-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diaz-Torres, M., N. S. Dunn-Coleman, M. W. Chase, and D. Trimbur. August2000. Method for the recombinant production of 1,3-propanediol. U.S. patent 6,136,576.
- 19.Dien, B. S., N. N. Nichols, and R. J. Bothast. 2001. Recombinant Escherichia coli engineered for the production of l-lactic acid from hexose and pentose sugars. J. Ind. Microbiol. Biotechnol. 27:259-264. [DOI] [PubMed] [Google Scholar]
- 20.Donnelly, M. I., C. Sanville-Millard, and R. Chatterjee. December1998. Method for construction of bacterial strains with increased succinic acid production. U.S. patent 6,159,738.
- 21.Hansen, R. G., and U. Henning. 1966. Regulation of the pyruvate dehydrogenase activity in Escherichia coli K-12. Biochim. Biophys. Acta 122:355-358. [DOI] [PubMed] [Google Scholar]
- 22.Higgins, T. E., and M. J. Johnson. 1970. Pathways of anaerobic acetate utilization in Escherichia coli and Aerobacter cloacae. J. Bacteriol. 101:885-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ingram, L. O., H. C. Aldrich, A. C. C. Borges, T. B. Causey, A. Martinez, F. Morales, A. Saleh, S. A. Underwood, L. P. Yomano, S. W. York, J. Zaldivar, and S. Zhou. 1999. Enteric bacterial catalyst for fuel ethanol production. Biotechnol. Prog. 15:855-866. [DOI] [PubMed] [Google Scholar]
- 24.Izui, K., M. Taguchi, M. Morikawa, and H. Katsuki. 1981. Regulation of Escherichia coli phosphoenolpyruvate carboxylase by multiple effectors in vivo. II. Kinetic studies with a reaction system containing physiological concentrations of ligands. J. Biochem. 90:1321-1331. [DOI] [PubMed] [Google Scholar]
- 25.Kirkpatrick, C., L. M. Maurer, N. E. Oyelakin, Y. N. Yoncheva, R. Maurer, and J. L. Slonczewski. 2001. Acetate and formate stress: opposite responses in the proteomes of Escherichia coli. J. Bacteriol. 183:6466-6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumari, S., R. Tishel, M. Eisenbach, and A. J. Wolfe. 1995. Cloning, characterization, and functional expression of acs, the gene which encodes acetyl coenzyme A synthetase in Escherichia coli. J. Bacteriol. 177:2878-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kylä-Nikkilä, K., M. Hujanen, M. Leisola, and A. Palva. 2000. Metabolic engineering of Lactobacillus helveticus CNRZ32 for production of pure l-(+)-lactic acid. Appl. Environ. Microbiol. 66:3835-3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee, J., A. Goel, M. M. Ataai, and M. M. Domach. 1994. Flux analysis of citrate synthase-deficient Escherichia coli. Ann. N. Y. Acad. Sci. 745:35-50. [DOI] [PubMed] [Google Scholar]
- 29.Luli, G. W., and W. R. Strohl. 1990. Comparison of growth, acetate production, and acetate inhibition of Escherichia coli strains in batch and fed-batch fermentations. Appl. Environ. Microbiol. 56:1004-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lutgens, M., and G. Gottschalk. 1980. Why a co-substrate is required for the anaerobic growth of Escherichia coli on citrate. J. Gen. Microbiol. 119:63-70. [DOI] [PubMed] [Google Scholar]
- 31.Martinez, A., S. W. York, L. P. Yomano, V. L. Pineda, F. C. Davis, J. C. Shelton, and L. O. Ingram. 1999. Biosynthetic burden and plasmid burden limit expression of chromosomally integrated heterologous genes (pdc, adhB) in Escherichia coli. Biotechnol. Prog. 15:891-897. [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Morales, F., A. G. Borges, A. Martinez, K. T. Shanmugam, and L. O. Ingram. 1999. Chromosomal integration of heterologous DNA in Escherichia coli with precise removal of markers and replicons during construction. J. Bacteriol. 181:7143-7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCleary, W. R., J. B. Stock, and A. J. Ninfa. 1993. Is acetyl phosphate a global signal in Escherichia coli? J. Bacteriol. 175:2793-2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller, J. H. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 35.Moniruzzaman, M., and L. O. Ingram. 1998. Ethanol production from dilute acid hydrolysate of rice hulls using genetically engineered Escherichia coli. J. Ind. Microbiol. Biotechnol. 20:943-947. [Google Scholar]
- 36.Nakamura, C. E., A. A. Gatenby, A. K.-H. Hsu, R. D. La Reau, S. L. Haynie, M. Diaz-Torres, D. E. Trimbur, G. M. Whited, V. Nagarajan, M. S. Payne, S. K. Picataggio, and R. V. Nair. January2000. Method for the production of 1,3-propanediol by recombinant microorganisms. U.S. patent 6,013,494.
- 37.Neidhardt, F. C., J. L. Ingraham, and M. Schaechter. 1990. Physiology of the bacterial cell: a molecular approach. Sinauer Associates, Inc., Sunderland, Mass.
- 38.Niu, W., K. M. Draths, and J. W. Frost. 2002. Benzene-free synthesis of adipic acid. Biotechnol. Prog. 18:201-211. [DOI] [PubMed] [Google Scholar]
- 39.Ohta, K., D. S. Beall, J. P. Mejia, K. T. Shanmugam, and L. O. Ingram. 1991. Genetic improvement of Escherichia coli for ethanol production: chromosomal integration of Zymomonas mobilis genes encoding pyruvate decarboxylase and alcohol dehydrogenase II. Appl. Environ. Microbiol. 57:893-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Posfai, G., M. D. Koob, H. A. Kirkpatrick, and F. C. Blattner. 1997. Versatile insertion plasmids for targeted genome manipulations in bacteria: isolation, deletion, and rescue of the pathogenicity island LEE of the Escherichia coli O157:H7 genome. J. Bacteriol. 179:4426-4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 42.Somsen, O. J. G., M. A. Hoeben, E. Esgalhado, J. L. Snoep, D. Visser, R. T. van der Heijden, J. J. Heijnen, and H. V. Westerhoff. 2000. Glucose and the ATP paradox in yeast. Biochem. J. 352:593-599. [PMC free article] [PubMed] [Google Scholar]
- 43.Suzuki, T. 1969. Phosphotransacetylase of Escherichia coli B, activation by pyruvate and inhibition by NADH and certain nucleotides. Biochim. Biophys. Acta 191:559-569. [DOI] [PubMed] [Google Scholar]
- 44.Tao, H., R. Gonzales, A. Martinez, M. Rodriguez, L. O. Ingram, J. F. Preston, and K. T. Shanmugam. 2001. Use of expression arrays to investigate the basis for increased glycolytic flux (xylose) in ethanologenic Escherichia coli KO11. J. Bacteriol. 183:2979-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tarmy, E. M., and K. O. Kaplan. 1968. Chemical characterization of the d-lactate dehydrogenase from Escherichia coli B. J. Biol. Chem. 243:2579-2586. [PubMed] [Google Scholar]
- 46.Tong, I.-T., H. H. Liao, and D. C. Cameron. 1991. 1,3-Propanediol production by Escherichia coli expressing genes from the Klebsiella pneumoniae dha regulon. Appl. Environ. Microbiol. 57:3541-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Underwood, S. A., M. L. Buszko, K. T. Shanmugam, and L. O. Ingram. 2002. Flux through citrate synthase limits the growth of ethanologenic Escherichia coli KO11 during xylose fermentation. Appl. Environ. Microbiol. 68:1071-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vemuri, G. N., M. A. Altman, and E. Altman. 2002. Effects of growth mode and pyruvate carboxylase on succinic acid production by metabolically engineered strains of Escherichia coli. J. Bacteriol. 68:1715-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verduyn, C. 1991. Physiology of yeasts in relation to biomass yields. Antonie Leeuwenhoek 60:325-353. [DOI] [PubMed] [Google Scholar]
- 50.Walsh, K., and D. E. Koshland, Jr. 1985. Characterization of rate-controlling steps in vivo by use of an adjustable expression vector. Proc. Natl. Acad. Sci. USA 82:3577-3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weitzman, P. D. J. 1966. Regulation of citrate synthase activity in Escherichia coli. Biochim. Biophys. Acta 128:213-215. [DOI] [PubMed] [Google Scholar]
- 52.Wood, W. B. 1966. Host specificity of DNA produced by Escherichia coli: bacterial mutations affecting the restriction and modification of DNA. J. Mol. Biol. 16:118-133. [DOI] [PubMed] [Google Scholar]
- 53.Yang, Y., A. A. Aristidou, K. San, and G. N. Bennett. 1999. Metabolic flux analysis of Escherichia coli deficient in the acetate production pathway and expressing the Bacillus subtilis acetolactate synthase. Metab. Eng. 1:26-34. [DOI] [PubMed] [Google Scholar]
- 54.Yang, Y., K. San, and G. N. Bennett. 1999. Redistribution of metabolic fluxes in Escherichia coli with fermentative lactate dehydrogenase overexpression and deletion. Metab. Eng. 1:141-152. [DOI] [PubMed] [Google Scholar]
- 55.Zhou, S., and L. O. Ingram. 1999. Engineering endoglucanase-secreting strains of ethanologenic Klebsiella oxytoca P2. J. Ind. Microbiol. Biotechnol. 22:600-607. [DOI] [PubMed] [Google Scholar]