Abstract
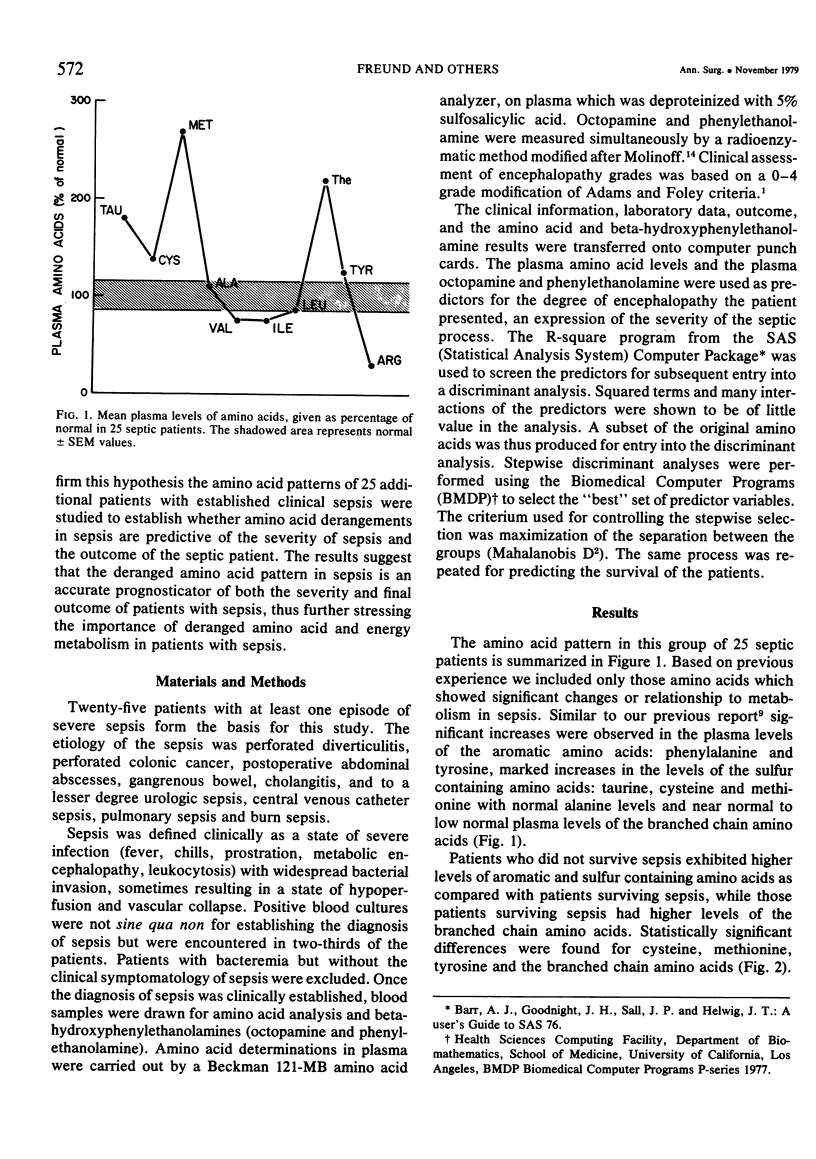
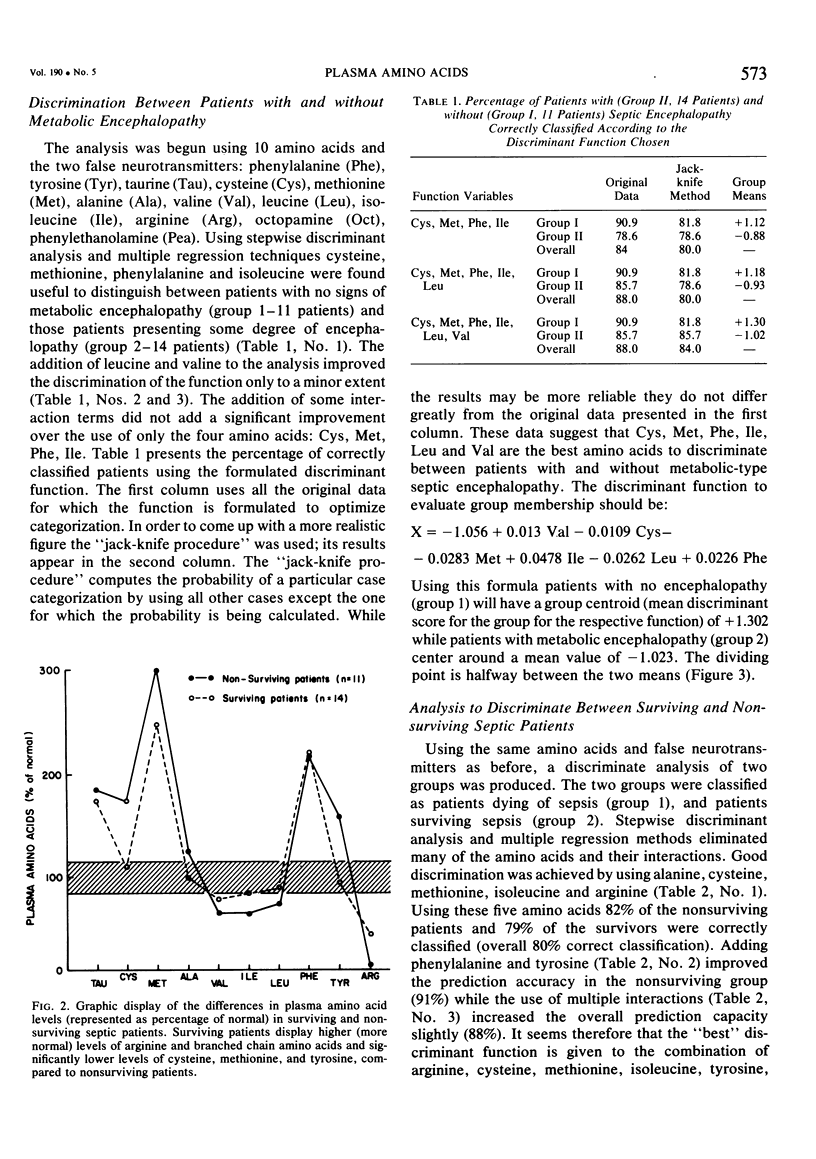
Sepsis is a major catabolic insult resulting in a peripheral energy deficit which is made up in part by increased breakdown of lean body mass and oxidation of amino acids, principally the branched chain amino acids. The prognosis in any given case of sepsis is difficult to predict, but should theoretically be related to the degree of disturbance in peripheral energy deficit, which may in turn, be related to plasma amino acid pattern. In order to study whether this hypothesis was correct, plasma amino acids and some of their metabolic byproducts, the beta-hydroxyphenylethanolamines, were studied in 25 septic patients, and were used as discriminant variables in a series of computer performed discriminant analyses and multiple regressions. The two functions tested were the degree of metabolic septic encephalopathy as a determinant of the severity of sepsis and the final outcome in the septic patient. Plasma amino acid patterns exhibited elevated levels of the aromatic and sulfur containing amino acids, phenylalanine, tryosine, tryptophan, methionine, cysteine, and taurine, normal concentrations of alanine, and low normal concentrations of the branched chain amino acids, valine, leucine and isoleucine. Arginine levels, as previously noted, were very low. Patients not surviving the septic episode exhibited higher concentrations of aromatic and sulfur containing amino acids, while patients surviving sepsis had higher concentrations of the branched chain amino acids and arginine. When the degree of encephalopathy as a determinant of the severity of sepsis and step wise discriminant analysis with multiple crescent techniques were used, the best discriminant function between patients with and without encephalopathy was found to result from the interaction of cysteine, methionine, phenylalanine, isoleucine, leucine, and valine. These amino acids gave a correct classification in 82% of patients with no encephalopathy, and 80% of patients with septic encephalopathy. When the same amino acids were used for the discriminant analysis for patients dying of sepsis and patients surviving, the best discriminant function was achieved by using plasma concentrations of alanine, cysteine, methionine, isoleucine, arginine, tyrosine and phenylalanine resulting in 91% of the nonsurvivors, and 79% of the survivors correctly classified. The results suggest a close and significant relationship between the deranged energy metabolism and muscle protein breakdown in sepsis, and the outcome. This further suggests a central role for certain amino acids in perhaps predicting the severity of sepsis and its outcome.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS R. D., FOLEY J. M. The neurological disorder associated with liver disease. Res Publ Assoc Res Nerv Ment Dis. 1953;32:198–237. [PubMed] [Google Scholar]
- Clowes G. H., Jr, O'Donnell T. F., Jr, Ryan N. T., Blackburn G. L. Energy metabolism in sepsis: treatment based on different patterns in shock and high output stage. Ann Surg. 1974 May;179(5):684–696. doi: 10.1097/00000658-197405000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbertson D. P. Post-traumatic metabolism: a multidisciplinary challenge. Surg Clin North Am. 1978 Oct;58(5):1045–1054. doi: 10.1016/s0039-6109(16)41643-9. [DOI] [PubMed] [Google Scholar]
- Duke J. H., Jr, Jørgensen S. B., Broell J. R., Long C. L., Kinney J. M. Contribution of protein to caloric expenditure following injury. Surgery. 1970 Jul;68(1):168–174. [PubMed] [Google Scholar]
- FLECK A., MUNRO H. N. PROTEIN METABOLISM AFTER INJURY. Metabolism. 1963 Sep;12:783–789. [PubMed] [Google Scholar]
- Fischer J. E., Baldessarini R. J. Pathogenesis and therapy of hepatic coma. Prog Liver Dis. 1976;5:363–397. [PubMed] [Google Scholar]
- Fischer J. E., Funovics J. M., Aguirre A., James J. H., Keane J. M., Wesdorp R. I., Yoshimura N., Westman T. The role of plasma amino acids in hepatic encephalopathy. Surgery. 1975 Sep;78(3):276–290. [PubMed] [Google Scholar]
- Fischer J. E., Rosen H. M., Ebeid A. M., James J. H., Keane J. M., Soeters P. B. The effect of normalization of plasma amino acids on hepatic encephalopathy in man. Surgery. 1976 Jul;80(1):77–91. [PubMed] [Google Scholar]
- Freund H. R., Ryan J. A., Jr, Fischer J. E. Amino acid derangements in patients with sepsis: treatment with branched chain amino acid rich infusions. Ann Surg. 1978 Sep;188(3):423–430. doi: 10.1097/00000658-197809000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD J. M. Studies of the absorption and metabolism of glucose following injury; the systemic response to injury. Ann Surg. 1955 Mar;141(3):321–326. doi: 10.1097/00000658-195503000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James J. H., Escourrou J., Fischer J. E. Blood-brain neutral amino acid transport activity is increased after portacaval anastomosis. Science. 1978 Jun 23;200(4348):1395–1397. doi: 10.1126/science.663619. [DOI] [PubMed] [Google Scholar]
- Molinoff P. B., Landsberg L., Axelrod J. An enzymatic assay for octopamine and other beta-hydroxylated phenylethylamines. J Pharmacol Exp Ther. 1969 Dec;170(2):253–261. [PubMed] [Google Scholar]
- Odessey R., Khairallah E. A., Goldberg A. L. Origin and possible significance of alanine production by skeletal muscle. J Biol Chem. 1974 Dec 10;249(23):7623–7629. [PubMed] [Google Scholar]
- Ryan N. T., Blackburn G. L., Clowes H. A., Jr Differential tissue sensitivity to elevated endogenous insulin levels during experimental peritonitis in rats. Metabolism. 1974 Nov;23(11):1081–1089. doi: 10.1016/0026-0495(74)90075-4. [DOI] [PubMed] [Google Scholar]
- Ryan N. T., George B. C., Egdahl D. H., Egdahl R. H. Chronic tissue insulin resistance following hemorrhagic shock. Ann Surg. 1974 Oct;180(4):402–407. doi: 10.1097/00000658-197410000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. R., Rossi-Fanelli F., Freund H., Fischer J. E. Sulfur-containing amino acids in experimental hepatic coma in the dog and the monkey. Surgery. 1979 Jun;85(6):677–683. [PubMed] [Google Scholar]
- Vaidyanath N., Oswald G., Trietley G., Weissenhofer W., Moritz E., McMenamy R. H., Birkhahn R., Yuan T. F., Border J. R. Turnover of amino acids in sepsis and starvation: Effect of glucose infusion. J Trauma. 1976 Feb;16(2):125–135. doi: 10.1097/00005373-197602000-00008. [DOI] [PubMed] [Google Scholar]



