Abstract
Background
Agave tequilana Weber var. azul, is the only one variety permitted by federal law in México to be used for tequila production which is the most popular contemporary alcoholic beverage made from agave and recognized worldwide. Despite the economic, genetic, and ornamental value of the plant, it has not been subjected to detailed cytogenetic research, which could lead to a better understanding of its reproduction for future genetic improvement.
The objective of this work was to study the meiotic behavior in pollen mother cells and its implications on the pollen viability in Agave tequilana Weber var. azul.
Results
The analysis of Pollen Mother Cells in anaphase I (A-I) showed 82.56% of cells with a normal anaphase and, 17.44% with an irregular anaphase. In which 5.28% corresponded to cells with side arm bridges (SAB); 3.68% cells with one bridge and one fragment; 2.58% of irregular anaphase showed cells with one or two lagging chromosomes and 2.95% showed one acentric fragment; cells with two bridges and cells with two bridges and one acentric fragment were observed in frequencies of 1.60% and 1.35% respectively. In anaphase II some cells showed bridges and fragments too. Aberrant A-I cells had many shrunken or empty pollen grains (42.00%) and 58.00 % viable pollen.
Conclusion
The observed meiotic irregularities suggest that structural chromosome aberrations have occurred, such as heterozygous inversions, sister chromatid exchanges, deletions and duplications which in turn are reflected in a low pollen viability.
Background
The genus Agave, is distributed in the tropical and subtropical areas of the world and represents a large group of succulent plants, with 197 taxa from 136 species, and its center of origin is probably limited to Mexico [1]. The subgenus Agave and particularly the sections Rigidae and Sisalanae, are cultivated because of their commercial importance for diverse purposes: a) alcoholic beverages such as tequila and mezcal; b) natural long and hard fibers; and c) steroidal compounds and medicinal principles [2-4].
The genus Agave is a semelparus perennial that produces flowers only once towards the end of its life cycle (6–8 years for A. tequilana). This genus has been the object of cytological investigations only after 1933, since then, chromosome counts have been made on a large number of species. The basic chromosome number is x = 30 and species ranging from diploid to hexaploid [5-7]. Cave [8] reported regular meiosis in five diploid, two tetraploid and one hexaploid species, and irregular meiosis in two polyploids, where bridges and fragments were observed at anaphase I. Brandham [9] carried out a cytological investigation in Agave stricta, for which he described a meiotic behavior heterozygous for a paracentric inversion, that produced a number of aberrations: one bridge and one fragment, two loops and one fragment, two bridges and two fragments, and two loops with two fragments at anaphase I.
More recently, Castorena-Sánchez et al [10] reported the first karyotype for Agave tequilana (2x = 60), where chromosomes were classified and arm ratios, chromatin length and their variability are discussed. On the other hand, the nuclear DNA content, chromatin structure, and DNA composition were investigated in four Agave species including A. tequilana [11].
Agave tequilana Weber var. azul, is the only one variety permitted by federal law in México to be used for tequila production [12] which is the most popular contemporary alcoholic beverage made from agave and recognized worldwide. Despite the economic, genetic, and ornamental value of the plant, it has not been subjected to detailed cytogenetic research, which could lead to a better understanding of its reproduction for future genetic improvement.
The objective of this work was to study the meiotic behavior in pollen mother cells and its implications on the pollen viability in Agave tequilana Weber var. azul.
Results
The analysis of Pollen Mother Cells (PMC's) at metaphase I (M-I) showed 62.3% of the cells with thirty normal bivalents, (Fig. 1A), and 37.7% of PMC's presented an aberrant meiotic behavior at the same phase (Fig. 1B).
Figure 1.
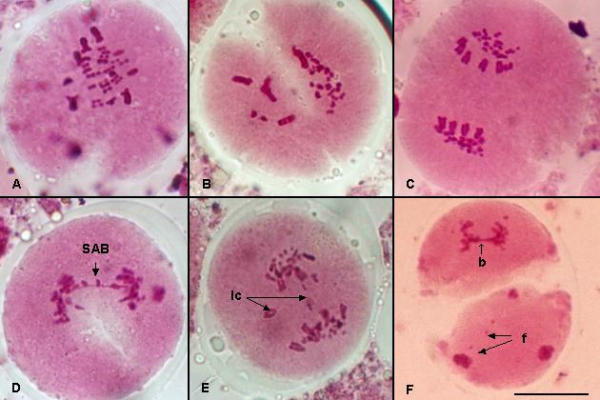
PMC's showing meiotic regular and irregular phases in Agave tequilana Weber var. azul. A) M I with 30 normal bivalents. B) Aberrant M I. C) Normal A – I. D) A – I with one side arm bridge (SAB). E) Lagging chromosomes at A I (lc = lagging chromosomes). F) Dyad showing bridge and fragments at A – II (b = bridge; f = fragment). Bar for all microphotographs = 17 μm
The chromosome complement of this species (and within the genus) is of a markedly bimodal persistence, having 10 large and 50 from medium to small chromosomes in the diploid. Thus, 5 large and 25 small bivalents can be seen at normal M-I (Fig. 1A).
The analysis of PMC's in anaphase I (A-I) showed 82.56% of cells with a normal anaphase (Fig. 1C) and, 17.44% with an irregular anaphase. In which 5.28% corresponded to cells with side arm bridges (SAB); 3.68% cells with one bridge and one fragment; 2.58% of irregular anaphase showed cells with one or two lagging chromosomes and 2.95% showed one acentric fragment; cells with only two bridges and cells with two bridges and one acentric fragment were observed in frequencies of 1.60% and 1.35% respectively (Fig, 1D, 1E, Table 1). Also, in anaphase II some cells showed bridges and fragments (Fig 1F). Aberrant meiotic cells produced 42% of shrunken or empty pollen grains, while the rest of normal dividing cells produced viable pollen (Fig. 2).
Table 1.
Normal and irregular anaphase I (A-I) of Agave tequilana Weber var. azul
| PMC's | Total 814 | %100.00 |
| Regular | 672 | 85.56 |
| Cells with one or two lagging chromosomes | 21 | 2.58 |
| Cells with one bridge (SAB) | 43 | 5.28 |
| Cells with one bridge and fragment | 30 | 3.68 |
| Cells with two bridges (SAB) | 13 | 1.60 |
| Cells with two bridges and fragment | 11 | 1.35 |
| Cells with acentric fragment | 24 | 2.95 |
Figure 2.
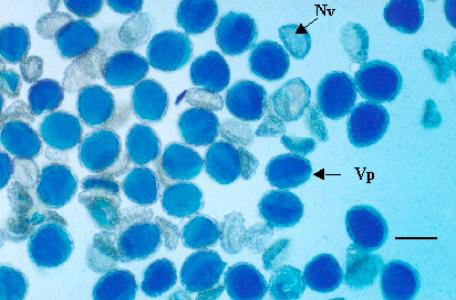
Pollen grains viability of Agave tequilana var. azul. Vp = viable pollen. Nv = non viable pollen. Bar = 0.5 mm
Discussion
Our cytogenetic studies confirmed that Agave tequilana Weber var. azul is a diploid species with 2n = 60 chromosomes, and thirty bivalents from pollen mother cells confirm the basic chromosome number of x = 30 [10].
The presence of bridges with or without fragment reflected structural changes such as heterozygous inversions, chromatid exchanges, deletions and duplications [14-16]. PMC's in A II with the presence of bridges and segment in each of the two cells of a dyad (Fig. 1F), could be an evidence of heterozygous inversions with 3 crossover points within the inversion loop [17], where similar aberrations were reported for Agave stricta [9].
The observed subchromatic or side arm bridges (SAB) could be originated from an abnormal function at the places where the chiasmata are formed [9,14]. In this study it could be observed that SAB involved long chromosomes as it was previously described for Agave stricta (Fig. 1D) [9]. Abnormal disjunction in some chromosomes, showed irregular anaphase I (2.58%) with one or two lagging chromosomes (Fig. 1D). In this study acentric fragments were also recorded (2.95%) as it was observed in Gibasis schiedeana where acentric fragments probably resulted from sister chromatid reunion [18].
It could be observed that an asynchronous movement of chromosomes was evident at the beginning of the rainy season. The occurrence of desynchronization in the movement of chromosomes and the advance of the meiotic process produced aberrant metaphase I behavior as it was observed in the 37.7% of the total of PMC's studied. For example, dyads were formed when the homologous chromosomes (bivalents) remained coupled (Fig. 1B). This aberrant meiotic behavior could also have an influence in the low viability of pollen.
On the other hand, Piven et al [19] working with henequen (A. fourcroydes Lem.) showed meiotic and postmeiotic alterations in the formation of the male gametophyte, which could be due to its polyploid nature (5x = 150), and these alterations might be responsible for the high sterility of pollen grains of this species (66.4%), on the contrary, A. tequilana is a diploid with 2n = 60, however, it had a high percentage (42.00%) of nonviable pollen grains.
Conclusions
The observed meiotic irregularities suggest that structural chromosome aberrations have occurred, such as heterozygous inversions, sister chromatid exchanges, deletions and duplications which in turn are reflected in a low pollen viability.
Methods
Inflorescences from 7 plants were collected from commercial plantations (Santa Fe, Jalisco, México). Fresh anthers from young buds (± 1.8 cm) were selected, and squashed in 1% aceto-orceine, and 320 pollen mother cells (PMC's) at metaphase I (M – I) and 814 PMC's at anaphase I (A – I) were analyzed.
Pollen viability was estimated by staining a sample of 1,583 pollen grains with 1% blue aniline in lactophenol [13] and the percentage of viable stained grains was recorded. The best cells for chromosome analysis and pollen samples were photographed using an Olimpus BH2 microscope coupled with an automatic photograph camera.
Authors' contributions
DR-R participated with the laboratory methods and chromosome analysis. BR-G was involved in the design and coordination, and drafted the manuscript.
Acknowledgments
Acknowledgements
The authors wish to thank Tequilas del Señor, S. A. de C. V. for providing the biological materials from their agave plantations and to Dr. Fernando Santacruz-Ruvalcaba for his advise and assistance in field work. D. Ruvalcaba-Ruiz is a graduate student at Posgrado en Procesos Biotecnológicos. U. de G. – CIATEJ, A. C., and financially supported by CONACYT-México.
Contributor Information
Domingo Ruvalcaba-Ruiz, Email: druvalcaba_1@yahoo.es.
Benjamin Rodríguez-Garay, Email: brodriguez@ciatej.net.mx.
References
- Gentry HS. Agaves of Continental North America. Tucson, The University of Arizona Press. 1982.
- Blunden G, Carabot C, Jewers K. Steroidal sapogenins from leaves of some species of Agave and Fureraea. Phytochemistry. 1980;19:2489–2490. doi: 10.1016/S0031-9422(00)91065-3. [DOI] [Google Scholar]
- Banerjee S, Sharma AK. Structural differences of chromosomes in diploid Agave. Cytologia. 1988;53:415–420. [Google Scholar]
- Cedeño GM. Tequila production. Crit Rev Biotechnol. 1995;15:1–11. doi: 10.3109/07388559509150529. [DOI] [PubMed] [Google Scholar]
- McKelvey SD, Sax K. Taxonomic and cytological relationships of Yucca and Agave. J Arnold Arbor Harv Univ. 1933;14:76–81. [Google Scholar]
- Whitaker TW. Chromosome constitution in certain monocotyledons. J Arnold Arbor Harv Univ. 1934;15:135–143. [Google Scholar]
- Darlington CD, Wylie AP. Chromosome atlas of flowering plants. London, George Allen and Unwin Ltd. 1955.
- Cave MS. Cytological observations on some genera of the Agavaceae. Madroño. 1964;17:163–169. [Google Scholar]
- Brandham PE. Inversion heterozygosity and sub-chromatid exchanges in Agave stricta. Chromosoma. 1969;26:270–286. [Google Scholar]
- Castorena-Sánchez R, Escobedo M, Quiroz A. New cytotaxonomical determinants recognized in six taxa of Agave in the sections Rigidae and Sisalanae. Can J Bot. 1991;69:1257–1264. [Google Scholar]
- Cavallini A, Natali L, Cionini G, Castoreña-Sánchez I. Cytophotometric and biochemical analyses of DNA in pentaploid and diploid Agave species. Genome. 1996;39:266–271. doi: 10.1139/g96-036. [DOI] [PubMed] [Google Scholar]
- Diario Oficial de la Federación Norma Oficial Mexicana NOM-006-SCFI-1993. Bebidas Alcohólicas-Tequila-Especificaciones Secretaría de Comercio y Fomento Industrial México, DF. 1993. pp. 48–52.
- Hauser EJP, Morrison JH. The cytochemical reduction of nitro tetrazolium as an index of pollen viability. Am J Bot. 1964;51:748–752. [Google Scholar]
- Brandham PE. Chromosome behaviour in the Aloineae. III Correlations between spontaneous chromatid and sub-chromatid aberrations. Chromosoma. 1970;31:1–17. [Google Scholar]
- Kenton A. Chromosome evolution in the Gibaris linearis alliance (Commelinaceae). I. The Robertsonian differentiation of G. venustula and G. speciosa. Chromosoma. 1981;84:291–304. [Google Scholar]
- Palomino Gq. Vázquez: Cytogenetic studies in mexican populations of species Crotolaria L. (Leguminosae-Papiloideae). Cytologia. 1991;56:343–351. [Google Scholar]
- Schulz-Schaeffer J. Cytogenetics: Plants, Animals, Humans. New York, Springer-Verlag. 1980.
- Martínez J, Palomino G. Evidence of heterozygous chromosome interchange and chromatid exchange in an autotetraploid cytotype of Gibasis schiedeana (Tradescantieae-Commelinaceae). Cytologia. 1997;61:215–223. [Google Scholar]
- Piven NM, Barredo-Pool FA, Borges-Argáez IC, Herrera-Alamillo MA, Mayo-Mosqueda A, Herrera-Herrera JL, Robert ML. Reproductive biology of henequen (Agave fourcroydes) and its wild ancestor Agave angustifolia (Agavaceae). I Gametophyte development. Am J Bot. 2001;88:1966–1976. [PubMed] [Google Scholar]


