Abstract
Background
Agitated or violent patients constitute 10% of all emergency psychiatric treatment. Management guidelines, the preferred treatment of clinicians and clinical practice all differ. Systematic reviews show that all relevant studies are small and none are likely to have adequate power to show true differences between treatments. Worldwide, current treatment is not based on evidence from randomised trials. In Brazil, the combination haloperidol-promethazine is frequently used, but no studies involving this mix exist.
Methods
TREC-Rio (Tranquilização Rápida-Ensaio Clínico [Translation: Rapid Tranquillisation-Clinical Trial]) will compare midazolam with haloperidol-promethazine mix for treatment of agitated patients in emergency psychiatric rooms of Rio de Janeiro, Brazil. TREC-Rio is a randomised, controlled, pragmatic and open study. Primary measure of outcome is tranquillisation at 20 minutes but effects on other measures of morbidity will also be assessed.
TREC-Rio will involve the collaboration of as many health care professionals based in four psychiatric emergency rooms of Rio as possible. Because the design of this trial does not substantially complicate clinical management, and in several aspects simplifies it, the study can be large, and treatments used in everyday practice can be evaluated.
Background
Agitated or violent patients constitute 10% of all emergency psychiatric treatment [16]. The majority of these people have severe psychiatric illnesses such as schizophrenia, affective disorder or substance abuse [16]. Less frequently, organic illness or serious psychological stresses underlie the aggression.
Guidelines recommend that patients should be 'verbally tranquillised' for the doctor to proceed with a diagnostic history, and undergo physical examination and laboratory tests before starting any pharmacological treatment [10]. A violent patient, however, may not allow this kind of management and doctors and nurses have to work with very limited background knowledge. Although some patients who are prone to violent episodes may be well known to the psychiatric services, many represent a considerable problem for the team faced with the challenge of initiating treatment before any firm diagnosis is possible. The psychiatric team has a responsibility to ensure the safety of everyone involved.
Rapid and safe tranquillisation of aggressive/violent patients is sometimes unavoidable. Medication is given, often under duress, with the aim of safely and swiftly making the patient less agitated and hostile. Drugs may be given orally, intravenously (IV), or by injections into the muscle (intramuscular/IM). With the acutely disturbed person, oral medication is often not possible. Although some psychiatric units prefer IV administration [7], its use can present additional difficulties and risks. IV administration may be problematic in aggressive and violent people who are difficult to contain whilst a controlled injection is given. IV administration may also cause cardiac and respiratory problems not seen with intramuscular injections [2,16] and is best employed where good medical support is readily available [7]. Finally, IM injections can be easier to administer in the acute situation but the onset of tranquillisation may be less swift and predictable than with an IV injection.
Guidelines
Exactly which drug, or combination of drugs, is best to use for the purpose of rapid tranquillisation of aggressive mentally ill people is still a matter of debate. High profile consensus guidelines do not give clear recommendations (see Table 1).
Table 1.
Guidelines and their recommendations for emergency management
| Date | Source | Guideline | Pharmacological recommendation |
| 1999 | USA | Expert Consensus Guidelines Series [10] | Give conventional antipsychotics for patients who require IM medications |
| 1998 | UK | Royal College of Psychiatrists [22] | Use rapid acting antipsychotics oral, IM, IV or rapid acting benzodiazepines such as lorazepam |
Surveys of favoured treatments
The advice of these guidelines, however, may not fit with the preferred treatment of clinicians. Groups of doctors in the UK [7] and USA [4] have been asked to list their preferred pharmacological management of acutely aggressive patients. Medical Directors in the USA frequently recommended the use of the high potency antipsychotic, haloperidol, combined with the benzodiazepine, lorazepam. In 1996, a group of doctors in England preferred the use of chlorpromazine (Table 2).
Table 2.
Pharmacological treatments and outcomes favoured by clinicians
| Study | Favoured regimen | Number of Doctors |
| USA 1999 [4] | Haloperidol + lorazepam +/- benztropine | 11 (55%) |
| Droperidol | 4 (20%) | |
| Benzodizepine (unspecified) alone | 3 (15%) | |
| Droperidol + lorazepam + diphenhydramine | 1 (5%) | |
| Haloperidol + benztropine | 1 (5%) | |
| Use of physical restraints | ||
| Common | 14 (70%) | |
| Usually not used | 6 (30%) | |
| Route of administration | ||
| Preferred IM or IV | 14 (70%) | |
| Preferred IM | 3 (15%) | |
| Unknown | 3 (15%) | |
| UK 1994 [7] | Chlorpromazine | 14 (50%) |
| Haloperidol | 8 (29%) | |
| Haloperidol + chlorpromazine | 2 (7%) | |
| Droperidol | 1 (4%) | |
| 'Neuroleptic' | 1 (4%) | |
| Haloperidol + diazepam | 1 (4%) | |
| Haloeridol + lorazepam | 1 (4%) | |
| Desired end point | ||
| Sedated but mobile | 12 (43%) | |
| Not sedated but calm | 9 (32%) | |
| Asleep | 7 (25%) | |
| Route of administration | ||
| Preferred IM | 26 (93%) | |
| Preferred IV | 2 (7%) |
Surveys of practice
Neither guidelines nor the management preferred by clinicians may reflect real-world practice. Surveys undertaken in Emergency Rooms can shed some light on what is actually being used for acutely disturbed people. Table 3 shows the results from two European studies undertaken in general hospitals emergency rooms. In the UK [20], intravenous treatments were common and the doses employed high. In France [18], were aggression due to intoxication was common, mostly loxapine IM was used. As the European studies suggested that very different clinical practices were being undertaken, and it is unclear how these results reflect what is happening in Brazil, a survey was designed and completed in March 2000 [13].
Table 3.
Studies of clinical practice
| Incidents | Physical restraint | IV:IM | Drug of choice | Frequency of use | mean dose in mg (range) | Second injection | Complications / comments | |
| UK 1997 [20] | 3.3 / week / 100,000 catchment area population ~5 people per week | 64% (nurses) | 1:1 | Diazepam | Most frequent | 27 (10–80) | 1 hypotention | |
| Haloperidol | (exact data not presented) | 22 (10–60) | 26% | 1 cardiorespiratory arrest (60 mg haloperidol + 80 mg DZ) | ||||
| Chlorpromazine | 162 (50–400) | 1 tachycardia, 1 hypotention | ||||||
| Droperidol | 14 (10–20) | |||||||
| Paraldehyde | 1 respiratory distress | |||||||
| Amytal | ||||||||
| Lorazepam | ||||||||
| Nitrazepam | Least frequent | |||||||
| France 1999 [18] | 5.6 / 1000 contacts | 86% (nurses) | 0:80 | Loxapine | 80% | 200 mg | 2 with acute dystonia | |
| Droperidol | 5% | |||||||
| Chlorazepate | 5% | |||||||
| Cyamemazine | < 2% | 6% | Mostly people with substance abuse | |||||
| Diazepam | ||||||||
| Sultopride | ||||||||
| Meprobamate | ||||||||
| Brazil 2000 [13] | 2.1 / week / 100,000 catchment area population ~74 people per week | Majority (restraints and nurses) | 0:74 | Haloperidol + promethazine | 61% | 5 (2.5–10) + 50 (25–100) | 0% | |
| Haloperidol + Promethazine + Diazepam | 15% | 5 (2.5–10) + 50 (25–100) + 10 | ||||||
| Diazepam | 9% | 10 | ||||||
| Haloperidol + Promethazine + Chlorpromazine | 7% | 5 + 50 + 25 | ||||||
| Chlorpromazine + Diazepam + Promethazine | 1% | 25 + 10 | ||||||
| Chlorpromazine + Promethanzine | 1% | 25 + 50 | ||||||
| Chlorpromazine | 1% | 25 | ||||||
| Diazepam + Promethazine | 1% | 10 + 5 | ||||||
| Haloperidol + Diazepam | 1% | 5 + 10 | ||||||
| Promethazine | 1% | 50 |
The Rio de Janeiro survey [13]
The county of Rio de Janeiro has about 5.8 m habitants and four public hospitals are responsible for the care of about 70% of the population (Hospital Phillippe Pinel, Centro Psiquiátrico Rio de Janeiro, Centro Psiquiátrico Pedro II and Hospital Jurandir Manfredini). The period of the survey covered emergency consultations from Saturday 25th March 2000 to Friday 31st March 2000, inclusive. The Emergency Room notes were inspected and medical records sought for additional information on use of emergency intramuscular sedation. As the main focus of the survey was the management of people with psychotic illness, whenever a primary diagnosis of substance abuse was made, data were not recorded.
Table 3 includes the results of this work and sets it against the European studies. In Hospital Jurandir Manfredini, 133 patients attended the Emergency Room in this period, but data on medication used could not be collected and subsequently are not presented in the table. During the seven-day period in the three other Rio's hospitals 764 patients attended at the emergency rooms and at least 74 (9.7%) received emergency sedative intramuscular drugs. Intravenous sedation was not used and no patient received extra parenteral doses for the same episode. Complications or adverse events due to the use of medication, as well as the use of physical restraints, were not recorded, but it is likely that some people were subject to four-point restraint, as it is accepted practice in these hospitals. A haloperidol-promethazine mix was the most popular combination and was used in over 80% of these emergencies, alone or in combination with other drugs.
Systematic reviews
Several systematic reviews have been undertaken to investigate whether the use of any particular drug regimen is supported by good evidence. These systematic reviews are objective appraisals of randomised trials, incorporate extensive searches and, where possible, meta-analyses.
In the field of acute sedation, zuclopenthixol acetate (Clopixol Acuphase) has been subject to the largest evaluation within randomised trials (Table 4). It is a relatively expensive treatment, widely advertised to be of value for management of acute disturbance, and is used in Europe.
Table 4.
ZUCLOPENTHIXOL ACETATE versus haloperidol, chlorpromazine or clothiapine – 5 trials, 413 people randomised [11,6]
| Main outcomes | Zu. Ac. | Control | RR (95% CI) |
| Not sedated by 2 hours | 6/20 | 10/20 | 0.6 (0.3–1.3) |
| Needing another injection | 12/20 | 8/20 | 1.5 (0.8–2.9) |
| Mental state: no improvement – by 36 hours | 11/103 | 11/85 | 0.86 (0.4–1.9) |
| Leaving the study early | 6/192 | 7/166 | 0.75 (0.3–2.3) |
Although the total number of people randomised into zuclopenthixol acetate trials is greater than for other compounds used for rapid tranquillisation, data are not decisive. None of the results is statistically significant in favour of either approach. Data are poorly reported and clinically relevant results rare. There are not enough data to support its use over other, less expensive, treatments.
Although haloperidol is the most frequently recommended drug to manage acute aggressive behavior, only 40 people were randomised within a single trial comparing haloperidol (IV) to placebo for acutely disturbed psychiatric patients (Table 5). Data suggest that haloperidol aids improvement.
Table 5.
Haloperidol versus placebo – 1 trial, 40 participants [15]
| Main outcomes | Hal'dol | Placebo | RR (95% CI) |
| Not improved by 2 hours | 2/29 | 4/11 | 0.19 (0.04–0.9) |
| Needing another injection | Majority in both groups | ||
| Mental state: asleep – by 2 hours | 1/29 | 0/11 | - |
| Leaving the study early | 0/29 | 0/11 | - |
| Adverse effects: needing antiparkinsonian medication | 6/29 | 0/11 | - |
No trials have been identified investigating the value of the haloperidol-promethazine mix.
Benzodiazepines are also indicated by key guidelines, but there are little data on the comparison of these drugs versus placebo for the acute management of disturbed people (Table 6). No clinically useful conclusions can be drawn from these data.
Table 6.
Benzodiazepines versus placebo – 1 trial, 12 participants
| Main outcomes | Benz. | Placebo | RR (95% CI) |
| Not improved | 1/6 | 5/6 | 0.2 (0.03–1.2) |
| Leaving the study early | 0/6 | 0/6 | - |
Although two studies were found comparing typical antipsychotics and benzodiazepines to placebo, in the great majority of the emergency situations it is not desirable, safe or ethical to provide no treatment.
When comparing benzodiazepines with typical antipsychotics, data suggest that benzodiazepines are more likely to produce 'improvement' by 1.5 hours, but patients may also be at greater risk of needing additional injections (Table 7). More patients given haloperidol are asleep by three hours than those allocated to benzodiazepines. No trials present useful data on the use of midazolam; only one trial exists (n = 15)[27] and it is not possible to analyse these data.
Table 7.
Benzodiazepines versus typical antipsychotics – 7 trials, 206 participants
| Main outcomes | Benz. | Control | RR (95% CI) |
| Not improved by 90 minutes | 20/72 | 32/75 | 0.64 (0.4–0.98) |
| Needing another injection | 24/31 | 31/35 | 0.66 (0.42–1.02) |
| Mental state: asleep – by 3 hours | 31/74 | 21/74 | 1.6 (0.99–2.5) |
| Leaving the study early | 14/101 | 16/105 | 0.87 (0.5–1.5) |
| Adverse effects: needing antiparkinsonian medication | 4/31 | 9/35 | 0.50 (0.2–1.5) |
Only 96 people were randomised to trials investigating the value of a benzodiazepine-haloperidol mix for acutely disturbed people (Table 8). The combination, largely with lorazepam, is no better than haloperidol for all the outcomes measured, except for 'being asleep by three hours', which favours the benzodiazepine-haloperidol mix.
Table 8.
Benzodiazepine-haloperidol mix versus haloperidol alone – 3 trials, 96 participants
| Main outcomes | B-H mix | Hal'dol | RR (95% CI) |
| Not improved by 90 minutes | 8/32 | 13/35 | 0.67 (0.3–1.4) |
| Needing another injection | 27/32 | 31/35 | 0.95 (0.8–1.2) |
| Mental state: asleep – by 3 hours | 20/32 | 11/35 | 2.0 (1.1–3.5) |
| Leaving the study early | 0/49 | 0/47 | - |
| Adverse effects: needing antiparkinsonian medication | 3/32 | 9/35 | 0.36 (0.1–1.2) |
Setting
Eighty percent of people across the world live in low or middle income countries and approximately 1–2% of people suffer from severe mental illnesses [14]. There is no evidence that psychiatric emergencies are less prevalent in these countries, therefore, most episodes of aggression for severely mentally ill people take place in the low or middle income countries.
Although new preparations of atypical antipsychotic drugs may be made available for use in the acute emergency, these are unlikely to affect the care of the majority of people in need of tranquillisation. Typical antipsychotics or benzodiazepines are relatively inexpensive, accessible interventions for people right across the world. As these treatments are prevalent in many countries, it is important that a definitive study is undertaken to fully investigate and understand their relative advantages and drawbacks. From the systematic reviews listed above, it can be seen that all pharmacological treatments of the psychiatric emergency have been inadequately investigated.
The TREC-Rio study was designed in collaboration with those working in a busy Brazilian psychiatric care setting. The great majority of clinical trials are explanatory; they are small, short, evaluate rigid care regimens, measure outcomes in ways that are of little clinical value and are difficult to relate to everyday practice.[24] Pragmatic trials, on the other hand, evaluate care that can be used in everyday practice and measure outcomes that are of general concern [12]
Size
Two main factors determine the number of people who should be recruited to in order for the trial to provide clear answers. They are the frequency of the investigated event and the size of the effect of treatment. It is important to avoid results that are erroneous. The probability of producing so called 'false-positive' results (type I error – α) and 'false-negative' findings (type II error – β) is minimised by having adequate sample size. The aim of TREC-Rio is to investigate whether people do better if they get haloperidol-promethazine or midazolam. The main outcome to monitor in the TREC-Rio trial is the proportion of patients who are tranquillised at 20 minutes in each group.
In such a stressful situation, even a small advantage for an intervention could represent a worthwhile benefit and so, TREC-Rio has been planned so that even a 15% difference in the proportion of tranquillised patients within the 20 minutes could be detected. TREC-Rio expects to involve 300 patients in a six-month period. A sample size of 300 people would have at least a 75% chance (1 – β error or power) of detecting an absolute difference of 15% between the proportion of tranquillised patients in each group, at 5% level of significance (α error) (Table 9).
Table 9.
Sample size needed to detect an absolute difference of 15% in the proportion of tranquillised patients (α = 5%, power = 80%).
| Haloperidol + promethazine (% tranquilised) | Midazolam (% tranquilised) | N |
| 5 | 20 | 152 |
| 10 | 25 | 200 |
| 15 | 30 | 242 |
| 20 | 35 | 276 |
| 25 | 40 | 304 |
| 30 | 45 | 326 |
| 35 | 50 | 340 |
Ethical considerations
The Helsinki Declaration [26], the European Directive on Clinical Trials [9], and the Nuffield Council documents on bioethics [19] state that trials in non-consenting patients are permitted on two conditions: i. no other context exists in which to answer the question; and ii. all trial participants get clear therapeutic benefit from whichever arm they are randomised to.
Aggressive patients in a situation of psychiatric emergency are not able to give consent for their participation in a study. Drugs are usually given against the will of the patient. So, in the same way that doctors are responsible for the choice of a treatment, they take responsibility for the recruitment of a patient into the study.
However, TREC-Rio will not involve administering an inactive compound to those who clearly need sedation/tranquillisation. Both treatments can calm the patient and there is no 'experimental' intervention. What is still uncertain is the speed for the onset of action, the duration of the effects and the different kinds of adverse reactions. TREC-Rio will answer clinical questions to help the care of these people be more informed. TREC-Rio will also produce widely applicable findings, so that the treatment of people beyond Rio de Janeiro should also be safer.
TREC-Rio has been approved by the ethics committees of institutions in charge of research and local ethics committees of each hospital involved.
A patient/carer information leaflet about TREC-Rio is available for all for whom a TREC-Rio box is opened. Carers will always be free to decide that their relative should not be entered. Not being involved in TREC-Rio will not affect the person's standard of care.
Methods
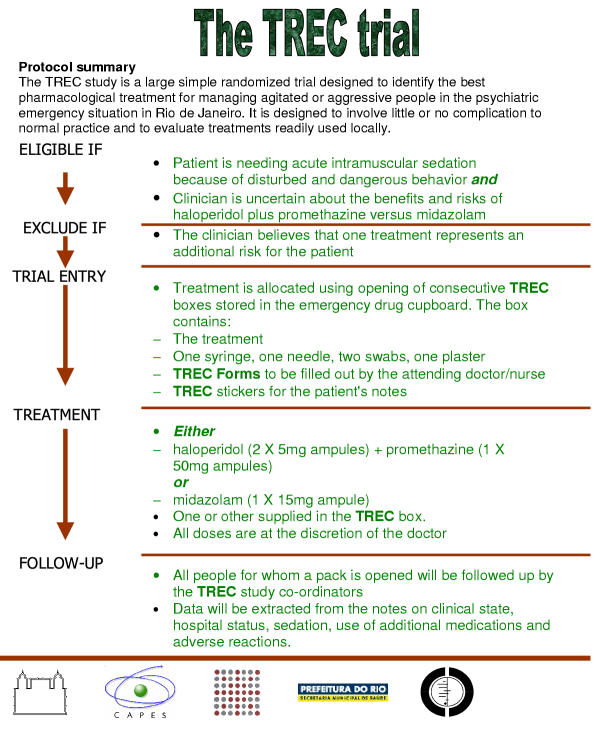
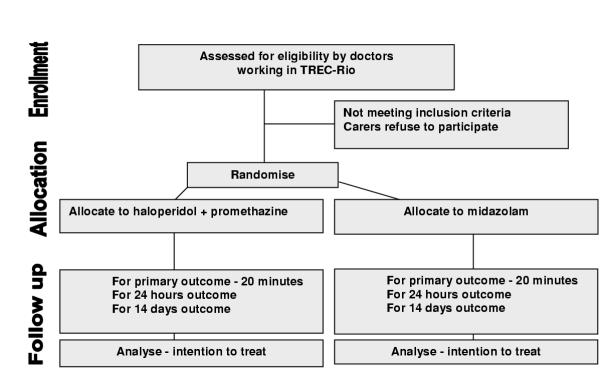
The TREC-Rio study is a pragmatic study; it is randomised, controlled and open. The protocol is summarised in Figure 1 and a Consort diagram is provided in Figure 2.
Figure 1.
Protocol summary
Figure 2.
CONSORT DIAGRAM for TREC-Rio
TREC-Rio is designed to fit into everyday practice
For the trialists to be able to detect important differences between the treatments, it will be necessary to treat hundreds of patients and this will only be possible if many professionals collaborate in each centre involved. The TREC-Rio trial is designed to not interfere with the routine care of people in participating centres. The process of randomisation is very similar to the normal procedure of beginning the treatment and the eligibility criteria are simple. Drugs will be provided in emergency sealed boxes. Data collection will be limited to the minimum necessary, and will involve little more than extraction of routine information by a person designated to spend time on the TREC-Rio trial. It is not envisaged that busy doctors and nurses will be spending time filling out complicated forms and all trial materials. The interventions will be supplied in a TREC-box that will be opened in the emergency situation.
Randomisation
A fundamental step in such a trial is the randomisation; the distribution of the treatments in a way that is not a function of a clinical decision, but of pure chance. Randomisation will be undertaken in the UK. Microsoft Excel 'RAND' function will be used to choose even numbered block sizes less than ten. Again using this function, the order of use of these block sizes will be randomised. Which drug regimen was represented by which number within the block was then selected, again at random. Finally a table of random numbers will be used to randomise within the blocks. Tables of TREC-box number by contents will be constructed and will be supplied to a Brazilian colleague. The tables will list the contents of the boxes in groups of ten, not disclosing the block sizes used. The Brazilian colleague, always working independently of the TREC-Rio team, will ensure that the correct drugs are in the TREC-box before it is sealed. Concealment of allocation will be ensured by not disclosing the randomly varied block sizes to the colleagues packing the boxes, the supply of tables to those colleagues that gives no suggestion that blocks are even being employed, the independence of those packing the boxes from the other researchers or the clinicians, and the identical nature of the packed boxes.
These easy-to-use boxes will be constructed of cardboard, identical and consecutively numbered. The final check to ensure that nothing has gone wrong with the randomisation will be by the principal investigator filling in a form for each block of ten opened boxes. She will record which intervention was in the box and these data will be returned to the UK so that any inconsistencies can quickly become known.
TREC-Rio is blinded for the initial ratings only
Because the TREC-Rio study evaluates care in the emergency situation, it is imperative that the doctors and nurses know which intervention is being given. The study is blind only up until the time that the TREC-Rio trial box is opened. Therefore, it is crucial that the evaluation of the severity of a person's disturbance and the first impression on the possible cause for the disturbed behavior are recorded before this box is opened. Once the box is opened, doctors and nurses will have knowledge of the drug to be used. It is perfectly feasible that the knowledge that one drug has been given will influence the care beyond the actual effects of the medication. Keeping the study open is not only practical in the emergency situation, but also desirable as the evaluation of care being undertaken is as near real-world circumstances as is possible.
Participants
Aggressive/violent patients who arrive at the emergency services of the public psychiatric hospitals of Rio de Janeiro-Brazil can be recruited for TREC-Rio.
Patients are eligible for trial entry if:
It is clear that they need acute intramuscular sedation because of agitation and disturbed and dangerous behaviour
and
The clinician is uncertain about the benefits and risks of the comparator medications.
For the purposes of this trial, people are considered to be agitated if uncontrollably and severely restless so as to cause concern for safety in carer, or aggressive if they present with threatening verbal behaviour, physical aggression against objects, self-aggression or physical aggression against other people.
People are not eligible for trial entry if:
The clinician believes that one treatment represents an additional risk for the patient.
Interventions
Placebo controlled studies in this area are difficult to justify (see section on ethics). TREC-Rio will evaluate the existing care in the health services of Rio de Janeiro and this care involves the use of medication that is considered both safe and effective. Currently, this protocol includes a comparison of an intramuscular haloperidol-promethazine mix with an intramuscular rapid acting benzodiazepine, midazolam. In the future, other centers may wish to compare interventions such as a haloperidol-benzodiazepine mix with lorazepam or zuclopenthixol acetate.
The haloperideol-promethazine mix is an obvious choice as standard treatment for TREC-Rio. A pragmatic randomised trial should not substantially interfere with routine practice and, in Brazil, this combination was given to 61% of patients needing sedation in the public psychiatric rooms in Rio de Janeiro [13]. It is perceived as effective, safe, and with adverse effects that are readily recognised by both medical and nursing staff. It is easy to administer by intra-muscular injection and has never been evaluated within a randomised control trial. Haloperidol and promethazine are both included in WHO's Model List of Essential Drugs [25].
The comparison intervention in TREC-Rio is a rapidly acting intramuscular benzodiazepine. Only lorazepam and midazolam are indicated for IM use, as all other benzodiazepines are slowly and erratically absorbed by this method. Lorazepam is not available for IM use in Brazil, however, midazolam is widely used in Brazil as premedication prior to surgical procedures in general emergency rooms and its use for the management of acutely disturbed people is being reported. The use of midazolam for rapid tranquillisation in psychiatry has not been subject to rigorous evaluation within a large and well-designed randomised controlled trial.
All drugs, haloperidol, promethazine and midazolam are included in Rio de Janeiro's list of essential drugs.
Haloperidol – risks and benefits
Haloperidol is a highly potent, widely used, neuroleptic that is indicated to help promote adequate levels of tranquillisation when administered IM. Doses used are usually 5–10 mg and its onset of action is by 60–90 minutes. The half-life of haloperidol varies between 13 and 40 hours, although effects may occur even two days after administration. Adverse effects include akathisia (manifested as restlessness) in 20% and acute dystonic reactions (rigid muscles and involuntary movements) for about 2% of patients. Neuroleptic malignant syndrome (hypothermia, rigid muscles and alteration in the level of consciousness developing 24–72 hours after administration) is an idiosyncratic serious reaction occurring in 0.02–3.2% of people [23]. Akathisia and acute dystonia are usually treated with the administration of antimuscarinic agents, although the optimal management of neuroleptic malignant syndrome is unclear. despite these adverse effects, which may happen even after a single injection, haloperidol is the elected treatment, widely available and used in the emergency situations.
Promethazine – risks and benefits
Promethazine is an antihistamine combined as an IM injection with haloperidol for the management of acutely disturbed people in both Brazil and India. The rational for this combination lies in the main sedative effects of promethazine and its antimuscarinic properties. Doses are usually between 25–50 mg but, as adjunctive sedative for emergency use, may reach 100 mg IM [23]. The onset of action is about 1–2 hours after intramuscular administration and half-life is 5–14 hours. The main adverse reactions of promethazine are gastrointestinal disturbances, dry mouth and blurred vision. Paradoxical reactions such as CNS stimulation and extrapyramidal symptoms have also been reported. Overdose may lead to coma and convulsions, progressing to respiratory failure or possibly cardiovascular collapse.
Midazolam – risks and benefits
Doses of midazolam for IM sedation varies between 3–10 mg, depending on clinical condition of the patient and previous exposure to other drugs. The onset of action is rapid, and occurs in 15–30 minutes after IM administration. Midazolam's half-life is 2–3 hours, the duration of action being generally up to 120 minutes. Few adverse effects are associated with IM use of midazolam. Amnesia for the incident is likely to occur. Respiratory depression and paradoxical reactions are only rarely associated with IV use of the drug but with intramuscular administration important changes in respiratory function have not been observed. IM midazolam, however, can cause confusion in about 0.3% of people. Flumazenil, a benzodiazepine antagonist, can be used to reverse the sedation induced by midazolam [23]. Flumazenil, a benzodiazepine antagonist, can be used to reverse the central effects induced by midazolam. The initial dose is 200 μ g given intravenously in 30 seconds, but additional doses may be needed, up to 3 mg. The onset of action occurs a few minutes after IV injection and can last up to 3 hours. The half-life is about 50 minutes. The adverse effects to flumazenil resemble those of withdrawal symptoms to benzodiazepines (nausea, headache, dizziness, blurred vision). Flumazenil should not be used in the presence of tryciclic intoxication or for patients who have used benzodiazepines for seizures for a long time.
Procedures
All trial materials, and guidelines for their use, are provided in the TREC-Rio folder supplied by the co-ordinating centre. What follows here is a brief summary of all of trial procedures.
Whenever possible, carers accompanying the disturbed person should have an opportunity to see the information leaflet (Appendix 1, see Additional file 1) before randomisation. Randomisation proceeds using a local pack system. Identical sealed treatment packs are provided.
As soon as the person enters the study, the clinician completes the trial entry form on the top of the next consecutive pack (Appendix 2, see Additional file 1). This must be completed before the treatment pack is opened. It records brief baseline details about the person and the number of the treatment pack. The treatment packs must be used in order in which they are removed, the lowest number first. Once the trial entry form has been completed the person is in the trial, even if the doctor changes his/her management and the treatment pack is not opened.
Trial packs
As soon as the person has been allocated a treatment pack, the pack is opened and the trial treatment inside given. Each pack contains:
1 × ampoule of midazolam 15 mg
1 × syringe
1 × needle
2 × swabs
1 × TREC-Rio follow-up form (Appendix 3, see Additional file 1)
2 × TREC-Rio stickers for the drug prescription form and medical notes
or
2 × ampoules of haloperidol 5 mg
1 × ampoule of promethazine 50 mg
1 × syringe
1 × needle
2 × swabs
1 × TREC-Rio follow-up forms (Appendix 3, see Additional file 1)
2 × TREC-Rio stickers for the drug prescription form and medical notes
All doses used are at the discretion of the attending clinician. Ampoules will be clearly labelled and the clinician will be in no doubt as regards the treatment being given. If the contents of a trial pack are destroyed, or unfit for use, the person should not be randomised a second time and the equivalent material should be obtained from the usual hospital supplies.
In the event of continuing aggression despite the TREC-Rio medication, ongoing emergency management would be up to the discretion of the clinicians. Another pack is not opened and the doctor is free to use any standard interventions.
Toxicity and serious unexpected events
After trial entry, clinical events are recorded, as usual, in the patients' notes. Complications and adverse events should be managed as usual. A serious unexpected event form (Appendix 5, see Additional file 1) is provided, and will be sent to the TREC-Rio Co-ordinator as soon it is completed.
Outcome and follow-up
It is crucial that follow-up is complete and accurate for everyone entered into the study. As a pragmatic study, causing minimal interference with routine care, TREC will not employ any rating scale outcomes. It is likely that completion of scales would be inaccurate, and incomplete, validity and reliability would be in question, and clinical utility problematic. The main outcome of TREC-Rio is tranquillisation by 20 minutes. This primary outcome was requested by the nursing and medical staff of the relevant hospitals. By asking the relevant clinical staff to select the primary outcome for TREC-Rio we hoped to ensure maximum compliance with the trial protocol. Therefore, upon injection of the patient, a timer is started, and this rings at 20 minutes and then again at 40, 60 and 120 minutes. At each period the attending nurse rates whether the person is tranquil, asleep, has shown adverse effects or needs additional treatment (see Appendix 2, see Additional file 1). This attending nurse is not blinded. The person is considered tranquillised when they are felt to be calm and peaceful, but not asleep. They should not be agitated or restless, nor displaying threatening verbal behaviour, physical aggression against objects, self-aggression or physical aggression to other people. Blinding this rater for every participant would have added additional complexity to the study that would have made the trial much less acceptable to the emergency room staff. More importantly, it would have completely changed the emphasis of TREC-Rio. We are interested in evaluating the real-world practice of giving two different drug regimens in the psychiatric emergency setting. In the real world situation health care professionals know what treatment is being given. In addition, for 10% of participants an additional rater, blind to allocated treatment, will, unknown to the health professionals looking after the patient, time the period between injection and tranquillisation and / or sleep exactly. These data will be used to validate the rating of the follow up form (see Appendix 4, see Additional file 1).
Additional data are then recorded at 24 hours and finally at two weeks (see Appendix 6, see Additional file 1). All data is extracted from routine notes. If the person is transferred to another hospital, the co-ordinating centre will contact every hospital to find out further details on what happened after transfer.
Data collection, entry and analysis
All data for TREC-Rio will be collated from the TREC-box forms and routine notes of each emergency room or ward (see Appendix 6, see Additional file 1). These data will be entered by the principal investigator into especially created forms in Epi-Info v 6.0 [5].
Analysis will take place within this package and SPSS [21]. Dummy tables for this analysis are prepared before recruitment of the first patient (see Appendix 7, see Additional file 1). All analysis will be based on groups as randomly allocated; this will be an intention-to-treat analysis. For the principal comparisons statistical significance will be taken at a 5% level and for subsidiary comparisons at the 1% level, to minimise the impact of multiple comparisons. Relative risk, risk difference, number needed to treat and respective 95% confidence intervals will be estimated for tranquillisation by 20, 40 and 60 minutes.
For the continuous outcome mean difference will be assessed. As in most experiments, this study carried out a randomisation of a non random sample instead of a random sampling of a specific population. In order to be coherent with the adopted design, the statistical significance of the means difference will be evaluated by a randomisation model (not a population model), and a design-based permutation test will be used instead of an approximate test to preserve the type I error rate [3,17]. Permutation test will be performed using StatXact 3.0 for Windows [8]. For a subgroup of 10% of patients, quality of data on time to tranquillisation will be evaluated by two independent observers. The agreement of this measurement will be assessed using Kappa statistics.
Trial organisation
The TREC-Rio Co-ordinating Group: The co-ordinating centre of the Rio de Janeiro arm is based at Fundação Osvaldo Cruz, Rio de Janeiro, Brazil. The Co-ordinating Group has overall responsibility for the design of the proposed trial and is responsible for all aspects of day to day trial administration. The Co-ordinating team is also responsible for preparing reports for the steering committee. Membership: Gisele Huf, Evandro SF Coutinho, Clive E Adams.
The TREC-Rio Steering Committee: The overall progress of the trial, adherence to protocol, patient safety and the consideration of new information will be monitored by a scientific and administrative steering committee. At the end of the proposed study period, the Steering Committee will consider the extension of the study, to allow the detection of other important effects. The membership of this committee is: Dr. Marco Antônio Brasil (chair), Dr. Gisele Huf, Dr. Evandro Coutinho, Prof. Clive Adams, Dr. Hugo Marques Fagundes Jr., Dr. José Ramón R. A. Lopez, Dr. Maurício Lima, Dr. Mário Barreira Campos, Dr. Suely Rozenfeld and Rosaura Maria Braz.
Data monitoring
Should recruitment to TREC-Rio be slow (take more than one year) or very swift (more than 300 in the expected six month recruitment period), an independent data monitoring committee (DMC) will, in confidence, monitor results. Should recruitment to the TREC-Rio be slow or go beyond 300, interim results will be supplied, in strict confidence to the chair of DMC as frequently as requested. Meetings of the committee will be arranged periodically as considered appropriate by the chair of the committee. In the light of the interim data, and of any other evidence or advice they wish to seek, the data monitoring committee will inform the chair of the steering committee if, in their view: i. there is proof beyond reasonable doubt that for any particular group or subgroup treatment with one or other regiment is clearly indicated or contraindicated or: ii. it is evident that no clear outcome will be obtained. Proof beyond reasonable doubt may be taken as the difference of at least three standard deviations and at least one of the primary outcomes.
The data monitoring committee may communicate certain interim analysis to the steering committee or suggest certain protocol changes, but the steering committee will remain responsible for deciding which changes to adopt. The membership of this committee is: Claudio Jose Struchiner (chair), Luiz B. Camacho, Jair de Jesus Mari.
Funding
No participating centre will directly receive funds for involvement in TREC-Rio. By design, funding for the overall project is minimal. All funding is intramural and everyone involved is undertaking this project as part of their usual funded employment. This support s jointly funded by Fundação Osvaldo Cruz, Cochrane Schizophrenia Group, British Council and CAPES – Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. Drugs to be used in the trial will be supplied by Secretaria Municipal de Saúde do Rio de Janeiro.
Proposed policy for publication and authorship
The success of the TREC-Rio trial depends on active collaboration of a large number of people in each of the participating hospitals. For this reason, authorship of any presentations or reports related to the trial will be in the name of the TREC-Rio Collaborative Group. Inevitably, for general publication it is not possible to name everybody that has contributed to a study such as this. Certificates of collaboration will be provided to those who have made a substantial contribution but whose name is not on the final report.
The results will be presented in confidence to the collaborators before publication. Once the final report has been published, collaborators will have access to the data in the hospital for additional descriptive analysis. Outcome by treatment group will not be presented for individual centres in the main reports of the TREC-Rio trial.
Authors' contributions
GH drafted the protocol, searched for relevant studies and worked with the other authors to refine the methods. ESFC helped draft the protocol, search for relevant studies and worked with the other authors to refine the methods. CEA helped draft the protocol, search for relevant studies, undertake the additional systematic reviews, and worked with the other authors to refine the methods.
Competing interests
GH felt that midazolam would have benefits over the haloperidol – promethazine mix. ESFC and CEA did not know. None of the authors are aware of any conflicts of interest.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
Appendix 1. – Information leaflet Appendix 2. TREC-BOX entry form Appendix 3. Primary outcome form Appendix 4. Primary outcome validation form Appendix 5. Serious event form Appendix 6. TREC-Rio Main data collection form Appendix 7. Dummy tables
Contributor Information
Gisele Huf, Email: gisele@ensp.fiocruz.br.
Evandro SF Coutinho, Email: evandro@ensp.fiocruz.br.
Clive E Adams, Email: ceadams@cochrane-sz.org.
References
- Armitage P, Berry G, Matthews JNS. Malden, MA: Blackwell Science. 4 2001. Statistical methods in medical research. [Google Scholar]
- Atakan Z, Davies T. ABC of mental health. Mental health emergencies. BMJ. 1997;314:1740–1742. doi: 10.1136/bmj.314.7096.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger VW. Pros and cons of permutation tests in clinical trials. Stat Med. 2000;19:1319–28. doi: 10.1002/(SICI)1097-0258(20000530)19:10<1319::AID-SIM490>3.3.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Binder RL, McNiel DE. Emergency psychiatry: contemporary practices in managing acutely violent patients in 20 psychiatric emergency rooms. Psychiatr Serv. 1999;50:1553–1554. doi: 10.1176/ps.50.12.1553. [DOI] [PubMed] [Google Scholar]
- Centres for Disease Control and Prevention Epi Info (Web page). http://www.cdc.gov/epiinfo/index.htm 10th September 2002.
- Coutinho E, Fenton M, Adams C, et al. Zuclopenthixol acetate in psychiatric emergencies: looking for evidence from clinical trials. Schizophr Res. 2000;46:111–118. doi: 10.1016/S0920-9964(99)00226-1. [DOI] [PubMed] [Google Scholar]
- Cunnane JG. Drug management of disturbed behaviour by psychiatrists. Psychiatric Bulletin. 1994;18:138–139. [Google Scholar]
- Cytel Software Corporation StatXact 3.0 for Windows (Web page). http://www.cytel.com/new.pages/SX.2.html 10th September 1997.
- European Union EUR-Lex: Community legislation in force – Document 301L0020 (Web page). http://europa.eu.int/eur-lex/en/lif/dat/2001/en_301L0020.html 03/04/2002.
- Expert Consensus Guideline Group Treatment of schizophrenia 1999. The expert consensus guideline series. J Clin Psychiatry. 1999;60 Suppl 11:3–80. [PubMed] [Google Scholar]
- Fenton M, Coutinho ES, Campbell C. Zuclopenthixol acetate in the treatment of acute schizophrenia and similar serious mental illnesses (Cochrane Review). Cochrane Database Syst Rev. 2001;3:CD000525. doi: 10.1002/14651858.CD000525. [DOI] [PubMed] [Google Scholar]
- Hotopf M, Churchill R, Lewis G. Pragmatic randomised controlled trials in psychiatry. Br J Psychiatry. 1999;175:217–223. doi: 10.1192/bjp.175.3.217. [DOI] [PubMed] [Google Scholar]
- Huf G, da Silva Freire Coutinho E, Fagundes HM, Jr, et al. Current practices in managing acutely disturbed patients at three hospitals in Rio de Janeiro-Brazil: a prevalence study. BMC Psychiatry. 2002;2:4. doi: 10.1186/1471-244X-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablensky A, Sartorius N, Ernberg G, et al. Schizophrenia: manifestations, incidence and course in different cultures. A world health organization ten-country study. Psychol Med Monogr Suppl. 1992:1–97. doi: 10.1017/s0264180100000904. [DOI] [PubMed] [Google Scholar]
- Joy CB, Adams CE, Lawrie SM. Haloperidol versus placebo for schizophrenia. Cochrane Database Syst Rev. 2001:CD003082. doi: 10.1002/14651858.CD003082. [DOI] [PubMed] [Google Scholar]
- Kaplan HI, Sadock BJ, Grebb JA. Kaplan and Sadock's Synopsis of Psychiatry. Baltimore, USA Williams & Wilkins. 1994.
- Ludbrook J, Dudley H. Why permutation tests are superior to t and F test in biomedical research. American Statistician. 1998;52:127–32. [Google Scholar]
- Moritz F, Bauer F, Boyer A, et al. [Patients in a state of agitation at the admission service of a Rouen hospital emergency department]. Presse Med. 1999;28:1630–1634. [PubMed] [Google Scholar]
- Nuffield Council Nuffield Council on Bioethics (Web page). http://www.nuffieldbioethics.org/home/ 03/04/2002.
- Pilowsky LS, Ring H, Shine PJ, et al. Rapid tranquillisation. A survey of emergency prescribing in a general psychiatric hospital. Br J Psychiatry. 1992;160:831–835. doi: 10.1192/bjp.160.6.831. [DOI] [PubMed] [Google Scholar]
- SPSS Inc. SPSS for Windows (Web page). http://www.spss.com 10th September 2002.
- The Royal College of Psychiatrists Management of Imminent Violence: Clinical practice guidelines to support mental health services. London Royal College of Psychiatrists. 1998.
- The Royal Pharmaceutical Society of Great Britain The British National Formulary (Web page). http://bnf.org/ January 2001.
- Thornley B, Adams C. Content and quality of 2000 controlled trials in schizophrenia over 50 years. BMJ. 1998;317:1181–1184. doi: 10.1136/bmj.317.7167.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Expert Committee World Health Organization/EDM/PAR/Procedures for Updating the WHO Model List of Essential Drugs (Web page). http://www.who.int/medicines/organization/par/edl/infedlmain.shtml 03/04/2002.
- World Medical Association World Medical Association Recommendations Guiding Physicians in Biomedical Research Involving Human Subjects (Web page). http://www.wma.net/e/policy/17-c_e.html 03/04/2002.
- Wyant M, Diamond BI, O'Neal E, et al. The use of midazolam in acutely agitated psychiatric patients. Psychopharmacol Bull. 1990;26:126–129. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1. – Information leaflet Appendix 2. TREC-BOX entry form Appendix 3. Primary outcome form Appendix 4. Primary outcome validation form Appendix 5. Serious event form Appendix 6. TREC-Rio Main data collection form Appendix 7. Dummy tables