Abstract
Background
Antioxidant supplementation with vitamin E had no effect in the prevention of cardiovascular diseases (CVD) in three recent large, randomized clinical trials. In order to reassess critically the role of vitamin E in CVD prevention, it is important to establish whether these results are related to a lack of antioxidant action.
Methods
We examined the in vivo antioxidant effect of vitamin E (300 mg/day for about three years) in 144 participants in the Primary Prevention Project (females and males, aged ≥ 50 y, with at least one major CV risk factor, but no history of CVD). Urinary 8-epi-PGF2α (isoprostane F2α-III or 15-F2t-isoP), a validated biomarker of lipid peroxidation, was measured by mass spectrometry.
Results
Urinary excretion of 8-epi-PGF2α [pg/mg creatinine, median (range)] was 141 (67–498) in treated and 148 (76–561) in untreated subjects (p = 0.10). Taking into account possible confounding variables, multiple regression analysis confirmed that vitamin E had no significant effect on this biomarker. Levels of 8-epi-PGF2α were in the normal range for most subjects, except smokers and those with uncontrolled blood pressure or hyperglycemia.
Conclusions
Prolonged vitamin E supplementation did not reduce lipid peroxidation in subjects with major cardiovascular risk factors. The observation that the rate of lipid peroxidation was near normal in a large proportion of subjects may help explain why vitamin E was not effective as an antioxidant in the PPP study and was ineffective for CVD prevention in large scale trials.
Keywords: Vitamin E, cardiovascular prevention, lipid peroxidation, F2-isoprostane, hypertension
Background
The "oxidative hypothesis" of atherosclerosis proposes that oxidative modification of lipids in low-density lipoproteins (LDL) contributes to atherogenesis [1,2]. Antioxidants that are effective against lipid peroxidation should therefore reduce atherosclerosis and hence afford protection from cardiovascular diseases (CVD)[1]. In contrast to a) epidemiological evidence that antioxidants taken with the diet or as supplements reduce cardiovascular (CV) risk [3], and b) experimental data supporting its anti-atherogenic properties [4], vitamin E failed to show any beneficial effect in recent large intervention studies [5]. In two large-scale trials, long-term supplementation with vitamin E (300–400 lU/day) failed to reduce cardiovascular events in post-myocardial infarction patients (GISSI-Prevenzione [6]) and in subjects at high CV risk (HOPE [7]). In the trial conducted by our group (Primary Prevention Project, PPP [8]), vitamin E (300 mg/day) taken over three years also showed no effect on the incidence of cardiovascular events in individuals with one or more major risk factors (see Methods). Therefore, the question whether vitamin E had an effective in vivo antioxidant action in the populations under study in these trials is under debate [9-12].
When these studies were designed, the antioxidant efficacy of vitamin E in humans had not been demonstrated in vivo because of the lack of reliable methods [13]. Measurement of urinary or circulating F2-isoprostanes (iPF2 or F2-isoP) is now accepted as a reliable tool for evaluating the rate of lipid peroxidation in vivo[9,14-16]. Using urinary excretion of 8-epi-PGF2α (also termed iPF2α-III or 15-F2t-isoP) as a biomarker, it has been shown that short-term administration of vitamin E (600 mg/day for 14 days) reduced in vivo lipid peroxidation in some clinical settings where oxidative stress is abnormally high, e.g., diabetes mellitus (-37%), hypercholesterolemia (-58%) and cystic fibrosis (-42%) [17-19]. In contrast, vitamin E had no antioxidant activity in conditions where lipid peroxidation was normal [20]. No data are available on the antioxidant effect of longer-term supplementation with vitamin E in subjects at moderate/high cardiovascular risk.
Using a highly selective and validated mass spectrometric assay for 8-epi-PGF2α[21], we measured in vivo lipid peroxidation in PPP trial participants who had taken vitamin E daily for about three years vs those who did not take vitamin E.
Methods
PPP is a large, randomized, controlled 2 × 2 factorial trial on primary prevention of CVD [8]. It was designed to test the efficacy of long-term administration of vitamin E (synthetic α-tocopherol, 300 mg/day) and/or aspirin (100 mg/day) in preventing cardiovascular events in subjects of both sexes aged ≥ 50 years, with at least one of the following cardiovascular risk factors: old age (≥ 65 yr); hypertension (systolic blood pressure ≥ 160 mm Hg or diastolic blood pressure ≥ 95 mm Hg on at least three separate occasions); hypercholesterolemia (≥ 250 mg/dL on at least two separate occasions); diabetes mellitus (≥ 140 mg/dL fasting venous plasma glucose, on at least two separate occasions); chronic drug treatment for any of the three latter conditions; obesity (body mass index ≥ 30 kg/m2); and premature myocardial infarction before 55 years of age in at least one parent or sibling. Patients with a history of cardiovascular events or diseases were not included. Table 1 shows the frequency of these CV risk factors in our sample.
Table 1.
Major cardiovascular risk factors in the 144 subjects.
| RISK FACTOR* | n(%) |
| Old age ≥ 65 y | 38 (26) |
| Male sex | 63 (44) |
| Smoking | 24 (17) |
| Hypertension | 136 (94) |
| Diabetes | 12(8) |
| Hypercholesterolemia | 43 (30) |
| Obesity | 32 (22) |
| Family history of premature myocardial infraction | 17 (12) |
*See Methods for definition
Overnight urine was obtained from subjects consecutively presenting at five participating centers for a scheduled follow-up visit after at least one year of randomized treatment. With a sample size of 70 individuals per arm, the study had a 90% power (1-β) to detect, with α = 0.05, a difference of at least 25% in urinary 8-epi-PGF2α between treated and untreated individuals. Two groups of 72 subjects treated or not treated with vitamin E were studied. Clinical and biochemical variables were reassessed yearly and on the occasion of urine collection.
Urinary 8-epi-PGF2α was selectively measured as we have described previously [21] using immunoaffinity chromatography for selective extraction/purification and a stable isotope dilution assay with gas chromatography-negative ion chemical ionization mass spectrometry for quantitation, with 2H4-8-epi-PGF2α as the internal standard. Urinary excretion of 8-epi-PGF2α was expressed as pg/mg creatinine. Creatinine was measured highly selectively by stable-isotope dilution HPLC-electrospray-tandem mass spectrometry, using 2H3-creatinine as the internal standard. Urine was stored at -20°C until analyzed. Analyses were done on blind-coded samples.
Means were compared by the non-parametric Mann-Whitney U test to avoid assumptions about the distribution of the variables. Levels of 8-epi-PGF2α were expressed as median (range) values. Multiple regression analysis was used to 1) evaluate the effect of vitamin E on urinary excretion of 8-epi-PGF2α, taking into account the following potential confounding variables (age, sex, aspirin treatment, smoking, systolic and diastolic blood pressure, blood glucose, blood cholesterol and obesity), and 2) assess whether any of these variables was independently associated with lipid peroxidation. Linear correlation analysis was also used. Probability values of p ≤ 0.05 (two tails) were considered to be statistically significant.
Results
1. Antioxidant Effect of Vitamin E
In vivo lipid peroxidation was not reduced significantly by vitamin E (Figure 1), as indicated by the similar urinary excretion of 8-epi-PGF2α in the supplemented group and in the controls [141 (67–498) vs 148 (76–561) pg/mg creatinine, p = 0.10]. These subjects had mean ± SD follow-up durations of 2.8 ± 1.0 and 2.7 ± 1.1 years, respectively. Baseline characteristics were well balanced across the two study groups, except for a slight difference in blood levels of glucose and cholesterol (Table 2). For this reason, we excluded with reasonable confidence a potential bias due to different baseline levels of urinary 8-epi-PGF2α. Multiple regression analysis, which takes into account possible confounding variables at the time of urine collection (age, sex, smoking, blood glucose, blood cholesterol, systolic and diastolic blood pressure, body mass index, aspirin treatment), confirmed that vitamin E had no significant overall effect on urinary 8-epi-PGF2α (β = -0.14, p = 0.12, Table 3).
Figure 1.
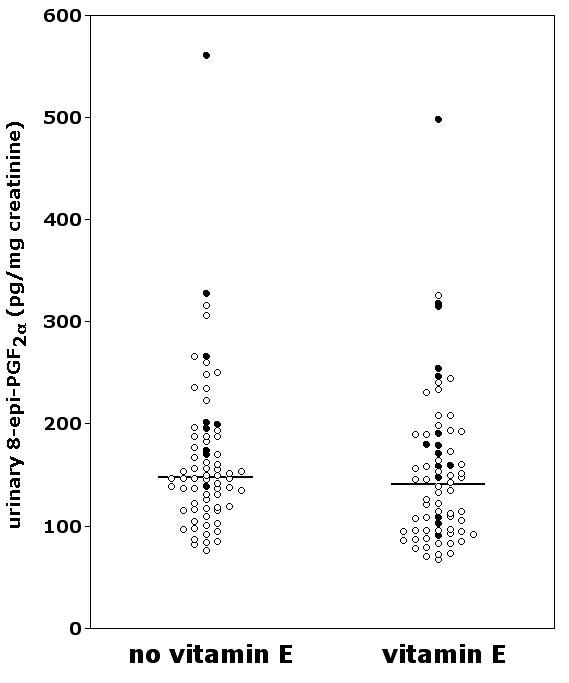
Urinary excretion of 8-epi-PGF2α in PPP participants supplemented or not with vitamin E. Smokers are indicated by filled circles. The horizontal lines represent the median.
Table 2.
Baseline characteristics of the two study groups
| VITAMIN E (n = 72) | NO VITAMIN E (n = 72) | |
| Age (y) | 59 ± 6 | 61 ± 7 |
| Sex (M/F) | 32/40 | 31/41 |
| Body mass index (kg/m2) | 27 ± 4 | 27 ± 4 |
| Smokers (yes/no) | 15/57 | 9/63 |
| Systolic blood pressure (mm Hg) | 146 ± 16 | 145 ± 15 |
| Diastolic blood pressure (mm Hg) | 88 ± 8 | 87 ± 7 |
| Blood glucose (mg/dL) | 91 ± 32 | 103 ± 35** |
| Total blood cholesterol (mg/dL) | 243 ± 47 | 225 ± 45* |
| Aspirin treatment (yes/no) | 36/36 | 34/38 |
Values are expressed as mean ± SD or number. *p < 0.05, **p < 0.01
Table 3.
Multiple regression analysis: variables associated with urinary excretion of 8-epi-PGF2α
| all subjects (n = 144) | nonsmokers (n = 120) | |||
| VARIABLE | β | p | β | p |
| Smoking | 0.46 | <0.0001 | – | – |
| Vitamin E treatment | -0.14 | 0.12 | -0.10 | 0.32 |
| Systolic blood pressure (mmHg) | 0.13 | 0.19 | 0.26 | 0.02 |
| Obesity | 0.12 | 0.21 | 0.15 | 0.15 |
| Sex (M) | 0.11 | 0.22 | 0.13 | 0.21 |
| Aspirin treatment | -0.10 | 0.24 | -0.07 | 0.45 |
| Blood glucose (mg/dL) | 0.09 | 0.35 | 0.17 | 0.11 |
| Diastolic blood pressure (mmHg) | -0.05 | 0.63 | -0.07 | 0.50 |
| Age (y) | 0.04 | 0.67 | 0.09 | 0.38 |
| Blood cholesterol (mg/dL) | 0.03 | 0.74 | 0.11 | 0.30 |
Smoking was the only strong determinant of lipid peroxidation in the overall sample (Table 3, discussed below). Since it is known that vitamin E does not reduce excessive lipid peroxidation in smokers [22,23], we investigated whether the presence of smokers in our sample might mask an antioxidant effect of vitamin E in the nonsmokers (n = 120; 63 untreated, 57 treated with vitamin E). The levels of 8-epi-PGF2α in nonsmokers, however, were not significantly reduced by prolonged vitamin E supplementation [122 (67–326) vs 146 (76–316), p = 0.09)]. Multivariate analysis confirmed that vitamin E did not reduce lipid peroxidation in this sample (β = -0.10, p = 0.32, Table 3).
2. Lipid Peroxidation in Subjects at CV Risk
Since vitamin E has thus far proved effective as an in vivo antioxidant in humans when lipid peroxidation is excessive [17-19,24], but not when it is normal [20], we addressed the question whether in subjects eligible for primary prevention of CVD, lipid peroxidation was increased enough to decrease appreciably with vitamin E. As a whole, this sample of patients with at least one CVD risk factor did not have an abnormal rate of lipid peroxidation. The levels of urinary 8-epi-PGF2α in untreated nonsmokers [146 (76–316) pg/mg creatinine] were similar to those of controls in other studies where this biomarker was selectively measured by mass spectrometry [20,22]. They were also similar to those we found in healthy nonsmoking volunteers [139 (71–256) pg/mg creatinine; mean ± SD age, 37 ± 11y; n = 20, unpublished data].
To better characterize the level of CV risk in our sample, we calculated a global CV risk score for each subject. We used Framingham's multiple-risk-factor assessment equation, a function assessing the risk of developing coronary heart disease on the basis of the presence and the level of major CV risk factors [25]. As shown in Figure 2, urinary 8-epi-PGF2α did not correlate with risk level in untreated or treated subjects.
Figure 2.
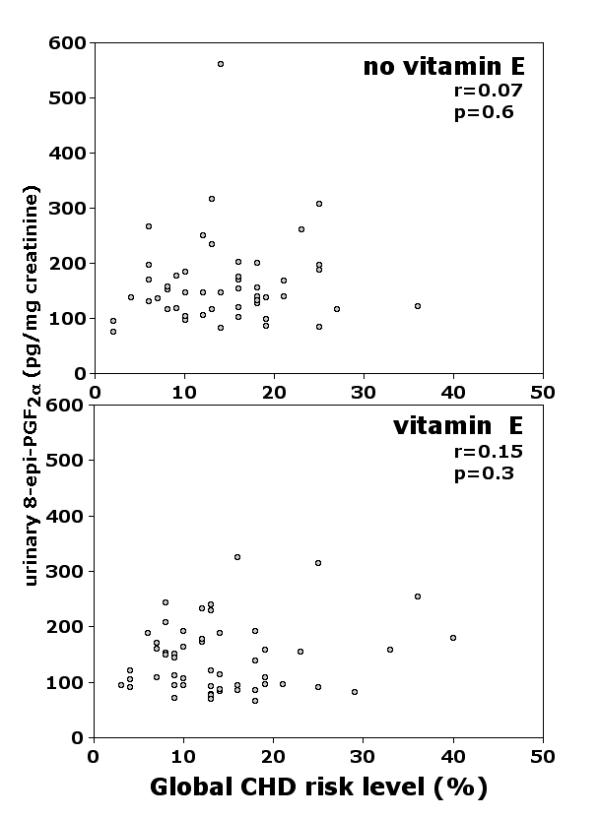
Correlation between global CV risk level and urinary excretion of 8-epi-PGF2α in PPP participants without (upper panel) or with (lower panel) vitamin E supplementation. Risk levels, calculated according to Framingham's multiple-risk-factor assessment equation, represent coronary heart disease (CHD) individual risk over the next ten years [25].
We therefore analyzed more closely factors possibly associated with lipid peroxidation in our sample, which is rather heterogeneous but is fairly representative of a population with major risk factors for cardiovascular diseases.
3. Factors Associated with Lipid Peroxidation in Subjects at CV Risk
Smoking
Multiple regression analysis of the whole group of 144 subjects showed cigarette smoking was the only strong determinant of excessive lipid peroxidation (β = 0.46; p < 0.0001, Table 3). Urinary excretion of 8-epi-PGF2α was higher in smokers (n = 24; 14 ± 6 cigarettes daily) than nonsmokers (n = 120) [185 (91–561) vs 138 (67–326) pg/mg creatinine; p = 0.003]. Vitamin E did not significantly reduce levels of 8-epi-PGF2α in smoking PPP participants [179 (91–498) vs 199 (138–561) pg/mg creatinine; p = 0.44; n = 15 and 9, respectively].
Other factors
The presence of smoking, a strong determinant of lipid peroxidation, very likely hampered the detection of other clinically important variables possibly associated with urinary 8-epi-PGF2α in our initial sample. We therefore investigated the relationship between these variables and 8-epi-PGF2α levels in the 120 nonsmokers.
Systolic blood pressure
Lipid peroxidation appeared to be related to systolic blood pressure in nonsmokers (β = 0.26; p = 0.02, Table 3). To confirm this in a less heterogeneous sample, we analyzed a subgroup of subjects who had hypertension as the only risk factor (n = 45). All except one were under antihypertensive treatment, with mean ± SD systolic and diastolic blood pressure 145 ± 16 (range 107–187) and 87 ± 8 (range 64–105) mm Hg, respectively. In this subgroup, the correlation between systolic blood pressure and urinary excretion of 8-epi-PGF2α was highly significant (r = 0.50, p = 0.0005. Figure 3), and subjects with systolic blood pressure ≥ 140 mm Hg had higher excretion of 8-epi-PGF2α than those with <140 mm Hg [151 (140–187) vs 127 (107–139) pg/mg creatinine, n = 27 and 18, respectively, p = 0.004]. Vitamin E did not significantly affect 8-epi-PGF2α excretion [109 (67–240) vs 138 (76–266) pg/mg creatinine in 21 treated and 24 untreated hypertensive subjects; p = 0.19].
Figure 3.
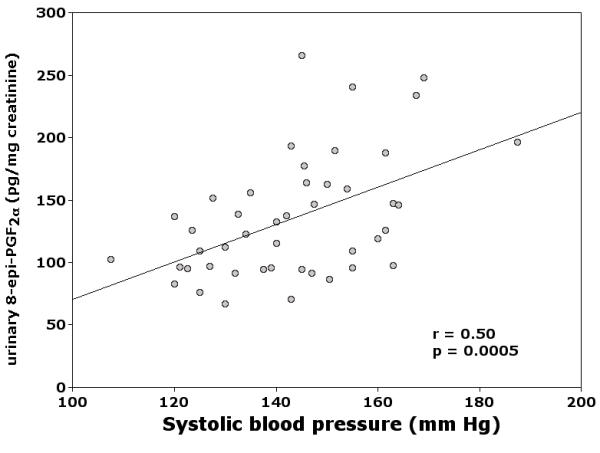
Correlation between systolic blood pressure and urinary excretion of 8-epi-PGF2α in 45 PPP participants with hypertension as the only risk factor.
Hyperglycemia
In nonsmoking participants, blood glucose measured at the time of urine collection (mean ± SD: 108 ± 24 mg/dL, n = 94) did not appear to be related to lipid peroxidation, based on multiple regression analysis (β = 0.17, p = 0.11. Table 3). However, subjects with blood glucose ≥ 140 mg/dL had higher excretion of 8-epi-PGF2α than those with <140 mg/dL [180 (121–326) vs 140 (70–266), n = 10 and 84, respectively, p = 0.009)], likely because only high blood glucose levels may be associated with excessive lipid peroxidation.
Other factors
In nonsmoking PPP participants, blood cholesterol at the time of urine collection (mean ± SD: 231 ± 40 mg/dL, range 144–351) was not significantly associated with lipid peroxidation (Table 3). Aspirin administration (n = 72) did not affect urinary excretion of 8-epi-PGF2α (Table 3), as already reported [14,26]. We found no evidence (Table 3) of increased lipid peroxidation in relation to age, sex or obesity.
Discussion
Long-term supplementation with vitamin E-at a dose largely exceeding that of a vitamin E-rich diet (300 mg/day)-did not substantially reduce lipid peroxidation in people with one or more major cardiovascular risk factors. This may help explain why vitamin E was not effective for CVD prevention in the PPP study. As opposed to other studies showing an antioxidant effect of vitamin E in small, uncontrolled subgroups of patients, our observations were obtained in a sample of subjects that more realistically represent a population with cardiovascular risk factors. In fact, as usually occurs in clinical practice, most candidates for cardiovascular prevention are treated-although not necessarily controlled-for their modifiable risk factors. The lack of antioxidant effect in our sample may be explained by the rather surprising finding that lipid peroxidation was normal in a large proportion of these subjects.
We investigated whether oxidative stress was increased in high-risk subjects, given that this category could, in principle, be more sensitive to antioxidant therapy. However, we found no evidence of a correlation between CV risk score and urinary excretion of 8-epi-PGF2α in untreated subjects, suggesting that lipid peroxidation was not associated with their global CV risk. Although, on average, lipid peroxidation was normal in our population, it was clearly increased in relation to extreme conditions reportedly associated with oxidative stress. Lipid peroxidation was, in fact, significantly higher in smokers, in agreement with consistent evidence of elevated oxidative stress in cigarette smokers, mostly obtained using 8-epi-PGF2α and/or other F2-isoprostanes as biomarkers [21,22,27]. We also confirmed previous observations that excessive lipid peroxidation in smokers cannot be reduced by vitamin E [22,23].
A secondary, but original finding of this study is the direct relationship between urinary excretion of an F2-isoprostane and systolic blood pressure in treated hypertensive patients with different degrees of blood pressure control. Whether this relationship also exists in untreated hypertensive patients should be investigated. An association between oxidative stress and arterial hypertension has been suggested by several clinical and experimental studies [28-32]. The hypothesis that free radical-mediated mechanisms may play a role in the pathophysiology of hypertension has recently gained support from observations that antioxidants lower blood pressure in hypertensive patients [30,33-36]. However, vitamin E in particular does not seem effective [37], possibly because it was not antioxidant in these circumstances.
As to other factors reportedly related to in vivo lipid peroxidation, our findings agree with previous evidence. In particular, we confirmed that enhanced urinary excretion of 8-epi-PGF2α is associated with impaired glycemic control in diabetic patients [17]. We did not find a correlation between urinary excretion of 8-epi-PGF2α and blood cholesterol, which, on average, was slightly elevated in our sample. Such a correlation was, in fact, found in subjects with homozygote familial hypercholesterolemia and in subjects with very high blood cholesterol, but not in those with normal cholesterol levels [24].
Conclusions
Prolonged supplementation with 300 lU/day vitamin E did not reduce lipid peroxidation in subjects with one or more major cardiovascular risk factors. On average, however, lipid peroxidation was near-normal in this population. These data may help explain the overall lack of benefit of vitamin E in recent cardiovascular prevention trials [6-8]. They also suggest the need to reassess whether lipid peroxidation is indeed an epidemiologically relevant determinant of cardiovascular diseases and, consequently, to reconsider the utility of antioxidants as a general preventive measure.
Competing Interests
None declared.
Collaborative Group
Participating physicians: Marina Bosisio Pioltelli (general practitioner, Monza), Alberto Capra (Ospedale Civile, Voghera), Mario Cristofari (Ospedale di Desio), Gaetana Palumbo (Ospedale S. Carlo Borromeo, Milano) and Susanna Rossi (Ospedale di Rovereto)
Coordinating group: Chiara Chiabrando, Fausto Avanzini, Roberto Fanelli and Maria Carla Roncaglioni (Istituto Mario Negri, Milano)
Acknowledgments
Acknowledgements
Claudia Rivalta was supported by the Fondazione "Angela and Angelo Valenti."
Contributor Information
Chiara Chiabrando, Email: chiabrando@marionegri.it.
Fausto Avanzini, Email: avanzini@marionegri.it.
Claudia Rivalta, Email: rivalta@marionegri.it.
Fabio Colombo, Email: fabio@marionegri.it.
Roberto Fanelli, Email: fanelli@marionegri.it.
Gaetana Palumbo, Email: geetana.palumbo@tiscalinet.it.
Maria Carla Roncaglioni, Email: roncaglioni@marionegri.it.
PPP Collaborative Group on the antioxidant effect of vitamin E, Email: chiabrando@marionegri.it.
References
- Diaz MN, Frei B, Vita JA, Keaney JF., Jr Antioxidants and atherosclerotic heart disease. N Engl J Med. 1997;337:408–416. doi: 10.1056/NEJM199708073370607. [DOI] [PubMed] [Google Scholar]
- Jialal I, Fuller CJ, Huet BA. The effect of alpha-tocopherol supplementation on LDL oxidation. A dose-response study. Arterioscler Thromb Vasc Biol. 1995;15:190–198. doi: 10.1161/01.atv.15.2.190. [DOI] [PubMed] [Google Scholar]
- Marchioli R. Antioxidant vitamins and prevention of cardiovascular disease: laboratory, epidemiological and clinical trial data. Pharmacol Res. 1999;40:227–238. doi: 10.1006/phrs.1999.0480. [DOI] [PubMed] [Google Scholar]
- Pratico D, Tangirala RK, Rader DJ, Rokach J, FitzGerald GA. Vitamin E suppresses isoprostane generation in vivo and reduces atherosclerosis in ApoE-deficient mice. Nat Med. 1998;4:1189–1192. doi: 10.1038/2685. [DOI] [PubMed] [Google Scholar]
- Steinberg D. Is there a potential therapeutic role for vitamin E or other antioxidants in atherosclerosis? Curr Opin Lipidol. 2000;11:603–607. doi: 10.1097/00041433-200012000-00006. [DOI] [PubMed] [Google Scholar]
- Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999;354:447–455. doi: 10.1016/S0140-6736(99)07072-5. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:154–160. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- Collaborative Group of the Primary Prevention Project Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Lancet. 2001;357:89–95. doi: 10.1016/S0140-6736(00)03539-X. [DOI] [PubMed] [Google Scholar]
- Witztum JL. To E or not to E-how do we tell? Circulation. 1998;98:2785–2787. doi: 10.1161/01.cir.98.25.2785. [DOI] [PubMed] [Google Scholar]
- Halliwell B. The antioxidant paradox. Lancet. 2000;355:1179–1180. doi: 10.1016/S0140-6736(00)02075-4. [DOI] [PubMed] [Google Scholar]
- Hooper L, Ness AR, Smith GD. Antioxidant strategy for cardiovascular diseases. Lancet. 2001;357:1705–1706. doi: 10.1016/S0140-6736(00)04876-5. [DOI] [PubMed] [Google Scholar]
- Roncaglioni MC, Tombesi M, Chiabrando C, Bertele' V, Tognoni G. Antioxidant strategy for cardiovascular diseases. Lancet. 2001;357:1706. doi: 10.1016/S0140-6736(00)04836-4. [DOI] [Google Scholar]
- Meagher EA, FitzGerald GA. Indices of lipid peroxidation in vivo: strengths and limitations. Free Radic Biol Med. 2000;28:1745–1750. doi: 10.1016/S0891-5849(00)00232-X. [DOI] [PubMed] [Google Scholar]
- Delanty N, Reilly M, Pratico D, FitzGerald DJ, Lawson JA, FitzGerald GA. 8-Epi PGF2α: specific analysis of an isoeicosanoid as an index of oxidant stress in vivo. Br J Clin Pharmacol. 1996;42:15–19. doi: 10.1046/j.1365-2125.1996.03804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrono C, FitzGerald GA. Isoprostanes: potential markers of oxidant stress in atherothrombotic disease. Arterioscler Thromb Vasc Biol. 1997;17:2309–2315. doi: 10.1161/01.atv.17.11.2309. [DOI] [PubMed] [Google Scholar]
- Pratico D, Lawson JA, Rokach J, FitzGerald GA. The isoprostanes in biology and medicine. Trends Endocrinol Metab. 2001;12:243–247. doi: 10.1016/S1043-2760(01)00411-8. [DOI] [PubMed] [Google Scholar]
- Davi G, Ciabattoni G, Consoli A, Mezzetti A, Faico A, Santarone S, Pennese E, Vitacolonna E, Bucciarelli T, Costantini F, Capani F, Patrono C. In vivo formation of 8-iso-prostaglandin F2α and platelet activation in diabetes mellitus: effects of improved metabolic control and vitamin E supplementation. Circulation. 1999;99:224–229. doi: 10.1161/01.cir.99.2.224. [DOI] [PubMed] [Google Scholar]
- Davi G, Alessandrini P, Mezzetti A, Minotti G, Bucciarelli T, Costantini F, Cipollone F, Bon GB, Ciabattoni G, Patrono C. In vivo formation of 8-Epi-prostaglandin F2α is increased in hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1997;17:3230–3235. doi: 10.1161/01.atv.17.11.3230. [DOI] [PubMed] [Google Scholar]
- Ciabattoni G, Davi G, Collura M, Iapichino L, Pardo F, Ganci A, Romagnoli R, Maclouf J, Patrono C. In vivo lipid peroxidation and platelet activation in cystic fibrosis. Am J Respir Crit Care Med. 2000;162:1195–1201. doi: 10.1164/ajrccm.162.4.9911071. [DOI] [PubMed] [Google Scholar]
- Meagher EA, Barry OP, Lawson JA, Rokach J, FitzGerald GA. Effects of vitamin E on lipid peroxidation in healthy persons. JAMA. 2001;285:1178–1182. doi: 10.1001/jama.285.9.1178. [DOI] [PubMed] [Google Scholar]
- Bachi A, Zuccato E, Baraldi M, Fanelli R, Chiabrando C. Measurement of urinary 8-Epiprostaglandin F2α, a novel index of lipid peroxidation in vivo, by immunoaffinity extraction/gas chromatography-mass spectrometry. Basal levels in smokers and nonsmokers. Free Radic Biol Med. 1996;20:619–624. doi: 10.1016/0891-5849(95)02087-X. [DOI] [PubMed] [Google Scholar]
- Reilly M, Delanty N, Lawson JA, FitzGerald GA. Modulation of oxidant stress in vivo in chronic cigarette smokers. Circulation. 1996;94:19–25. doi: 10.1161/01.cir.94.1.19. [DOI] [PubMed] [Google Scholar]
- Patrignani P, Panara MR, Tacconelli S, Seta F, Bucciarelli T, Ciabattoni G, Alessandrini P, Mezzetti A, Santini G, Sciulli MG, Cipollone F, Davi G, Gallina P, Bon GB, Patrono C. Effects of vitamin E supplementation on F2-isoprostane and thromboxane biosynthesis in healthy cigarette smokers. Circulation. 2000;102:539–545. doi: 10.1161/01.cir.102.5.539. [DOI] [PubMed] [Google Scholar]
- Reilly MP, Pratico D, Delanty N, DiMinno G, Tremoli E, Rader D, Kapoor S, Rokach J, Lawson J, FitzGerald GA. Increased formation of distinct F2 isoprostanes in hypercholesterolemia. Circulation. 1998;98:2822–2828. doi: 10.1161/01.cir.98.25.2822. [DOI] [PubMed] [Google Scholar]
- Anderson KM, Wilson PW, Odell PM, Kannel WB. An updated coronary risk profile. A statement for health professionals. Circulation. 1991;83:356–362. doi: 10.1161/01.cir.83.1.356. [DOI] [PubMed] [Google Scholar]
- Wang Z, Ciabattoni G, Creminon C, Lawson J, Fitzgerald GA, Patrono C, Maclouf J. Immunological characterization of urinary 8-epi-prostaglandin F2α excretion in man. J Pharmacol Exp Ther. 1995;275:94–100. [PubMed] [Google Scholar]
- Morrow JD, Frei B, Longmire AW, Gaziano JM, Lynch SM, Shyr Y, Strauss WE, Oates JA, Roberts LJ., 2nd Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N Engl J Med. 1995;332:1198–1203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- Russo C, Olivieri O, Girelli D, Faccini G, Zenari ML, Lombardi S, Corrocher R. Anti-oxidant status and lipid peroxidation in patients with essential hypertension. J Hypertens. 1998;16:1267–1271. doi: 10.1097/00004872-199816090-00007. [DOI] [PubMed] [Google Scholar]
- Romero JC, Reckelhoff JF. State-of-the-Art lecture. Role of angiotensin and oxidative stress in essential hypertension. Hypertension. 1999;34:943–949. doi: 10.1161/01.hyp.34.4.943. [DOI] [PubMed] [Google Scholar]
- Schnackenberg CG, Wilcox CS. Two-week administration of tempol attenuates both hypertension and renal excretion of 8-Iso prostaglandin F2α. Hypertension. 1999;33:424–428. doi: 10.1161/01.hyp.33.1.424. [DOI] [PubMed] [Google Scholar]
- Touyz RM. Oxidative stress and vascular damage in hypertension. Curr Hypertens Rep. 2000;2:98–105. doi: 10.1007/s11906-000-0066-3. [DOI] [PubMed] [Google Scholar]
- Berry C, Brosnan MJ, Fennell J, Hamilton CA, Dominiczak AF. Oxidative stress and vascular damage in hypertension. Curr Opin Nephrol Hypertens. 2001;10:247–255. doi: 10.1097/00041552-200103000-00014. [DOI] [PubMed] [Google Scholar]
- Galley HF, Thornton J, Howdle PD, Walker BE, Webster NR. Combination oral antioxidant supplementation reduces blood pressure. Clin Sci (Colch) 1997;92:361–365. doi: 10.1042/cs0920361. [DOI] [PubMed] [Google Scholar]
- Kitiyakara C, Wilcox CS. Antioxidants for hypertension. Curr Opin Nephrol Hypertens. 1998;7:531–538. doi: 10.1097/00041552-199809000-00008. [DOI] [PubMed] [Google Scholar]
- Duffy SJ, Gokce N, Holbrook M, Huang A, Frei B, Keaney JF, Jr, Vita JA. Treatment of hypertension with ascorbic acid. Lancet. 1999;354:2048–2049. doi: 10.1016/S0140-6736(99)04410-4. [DOI] [PubMed] [Google Scholar]
- Ortiz MC, Manriquez MC, Romero JC, Juncos LA. Antioxidants block angiotensin II-induced increases in blood pressure and endothelin. Hypertension. 2001;38:655–659. doi: 10.1161/01.hyp.38.3.655. [DOI] [PubMed] [Google Scholar]
- Palumbo G, Avanzini F, Alli C, Roncaglioni MC, Ronchi E, Cristofari M, Capra A, Rossi S, Nosotti L, Costantini C, Cavalera C. Effects of vitamin E on clinic and ambulatory blood pressure in treated hypertensive patients. Collaborative Group of the Primary Prevention Project (PPP) – Hypertension study. Am J Hypertens. 2000;13:564–567. doi: 10.1016/S0895-7061(00)00244-2. [DOI] [PubMed] [Google Scholar]


