Abstract
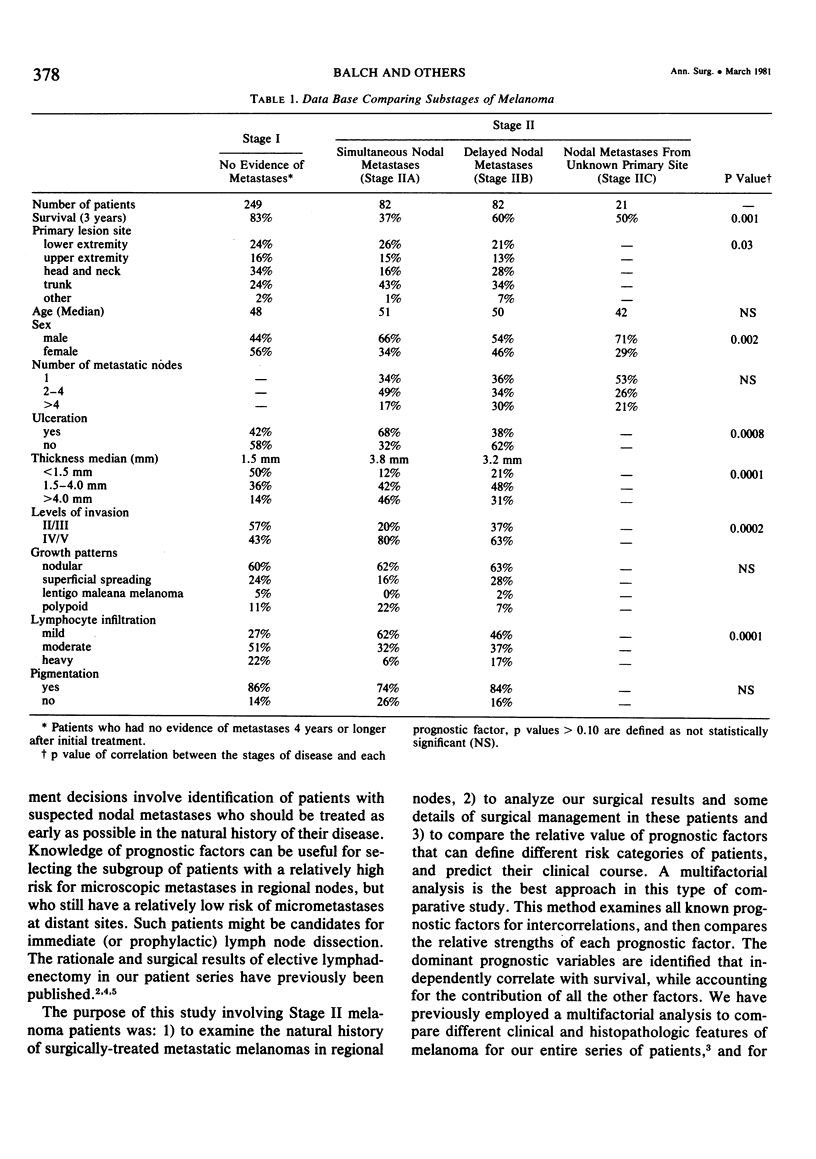
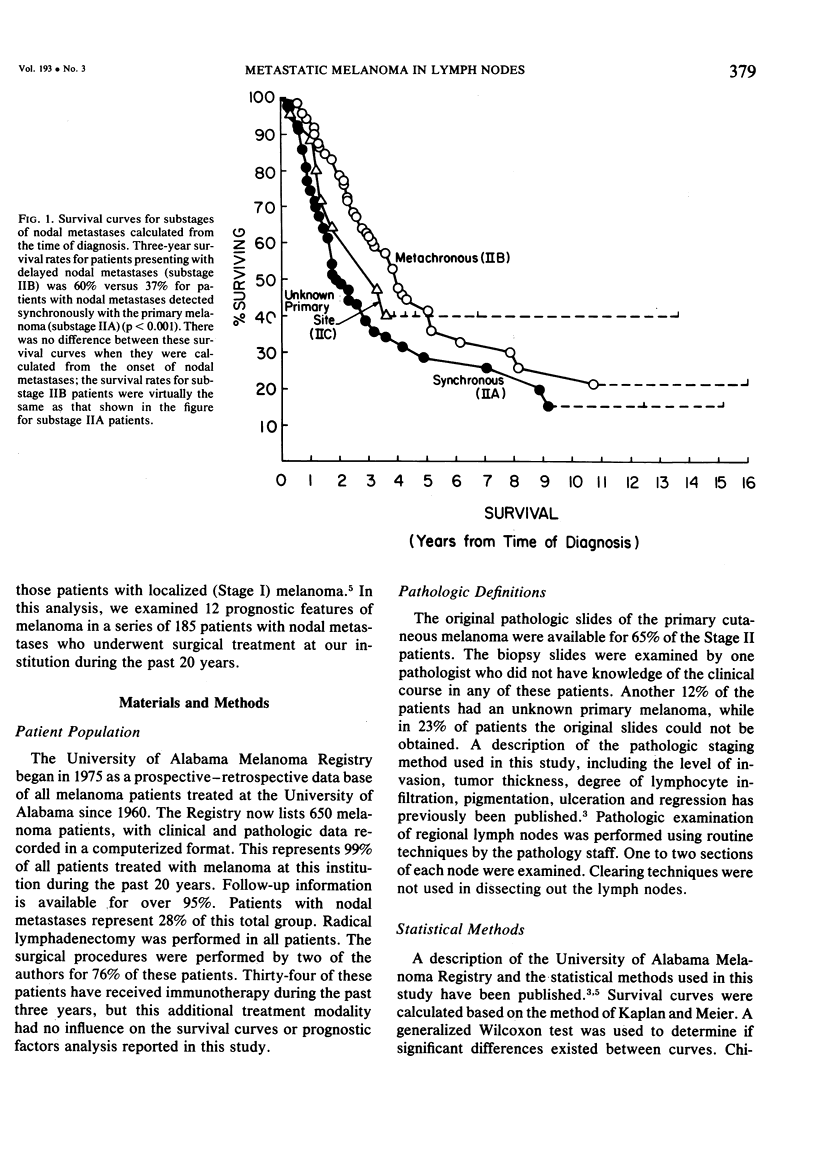
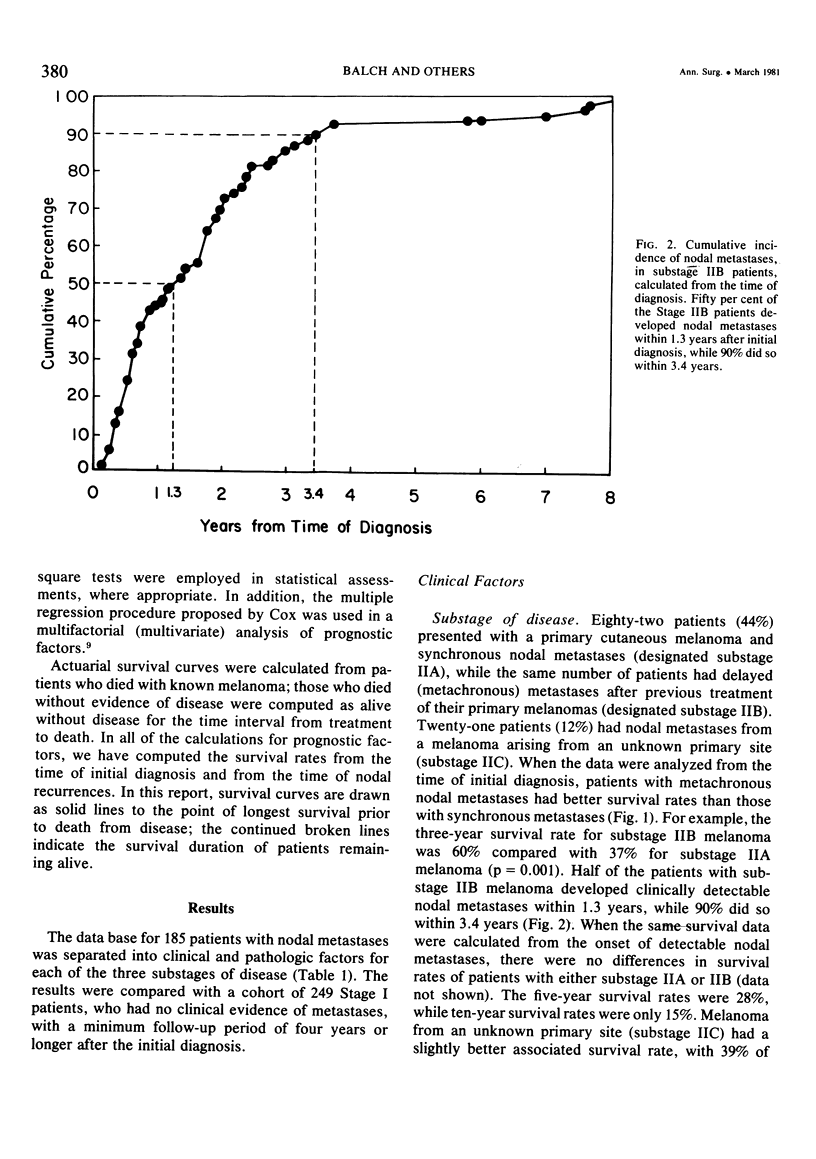
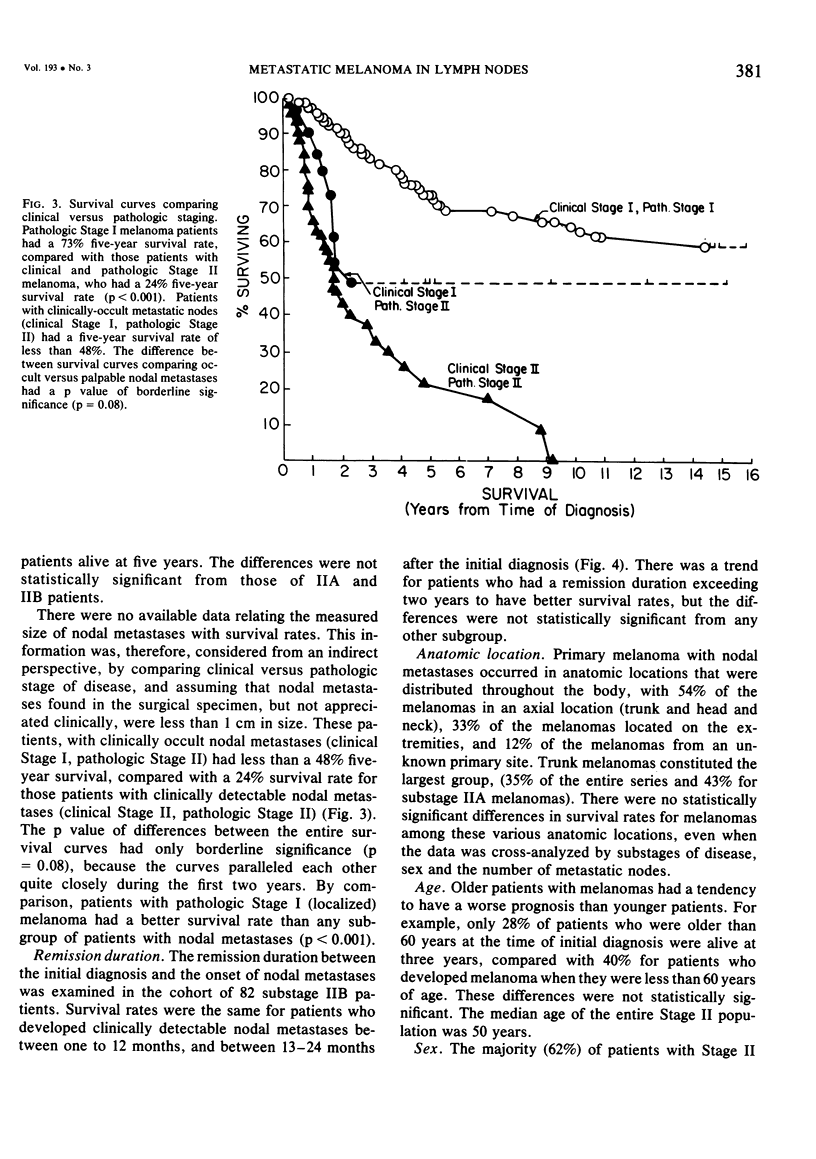
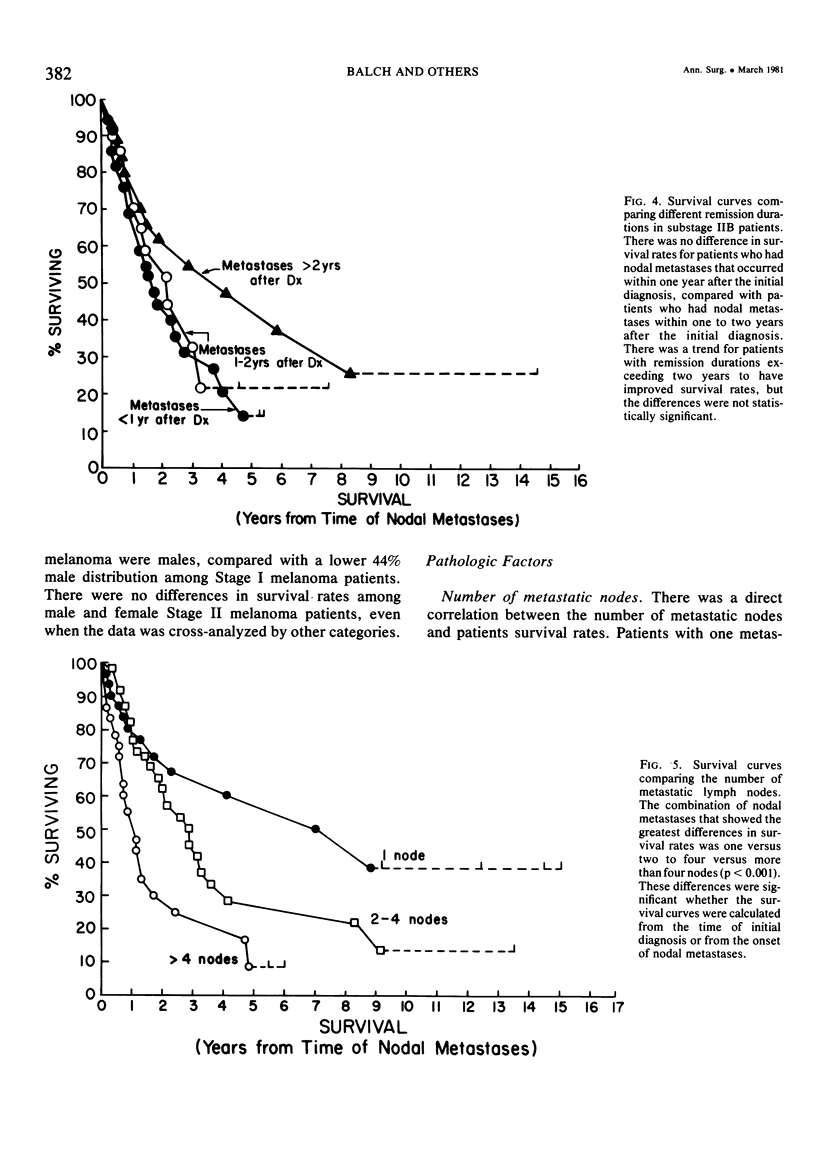
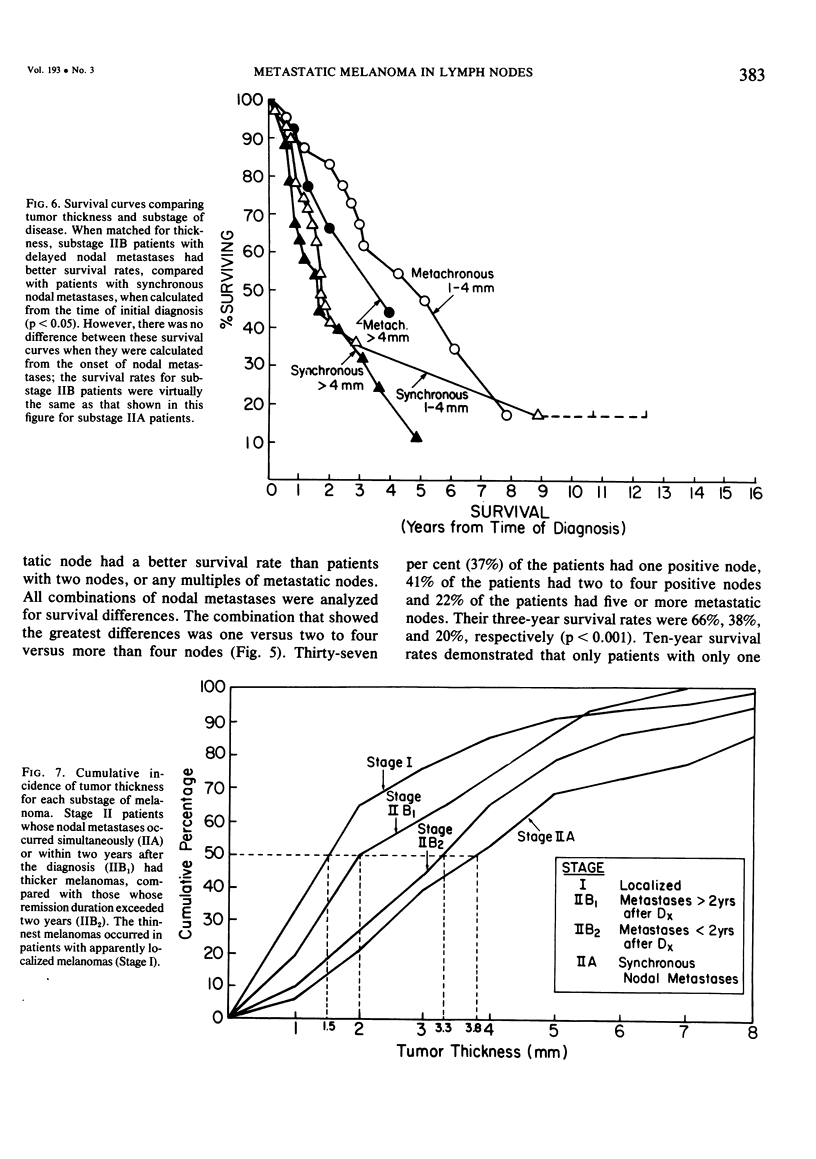
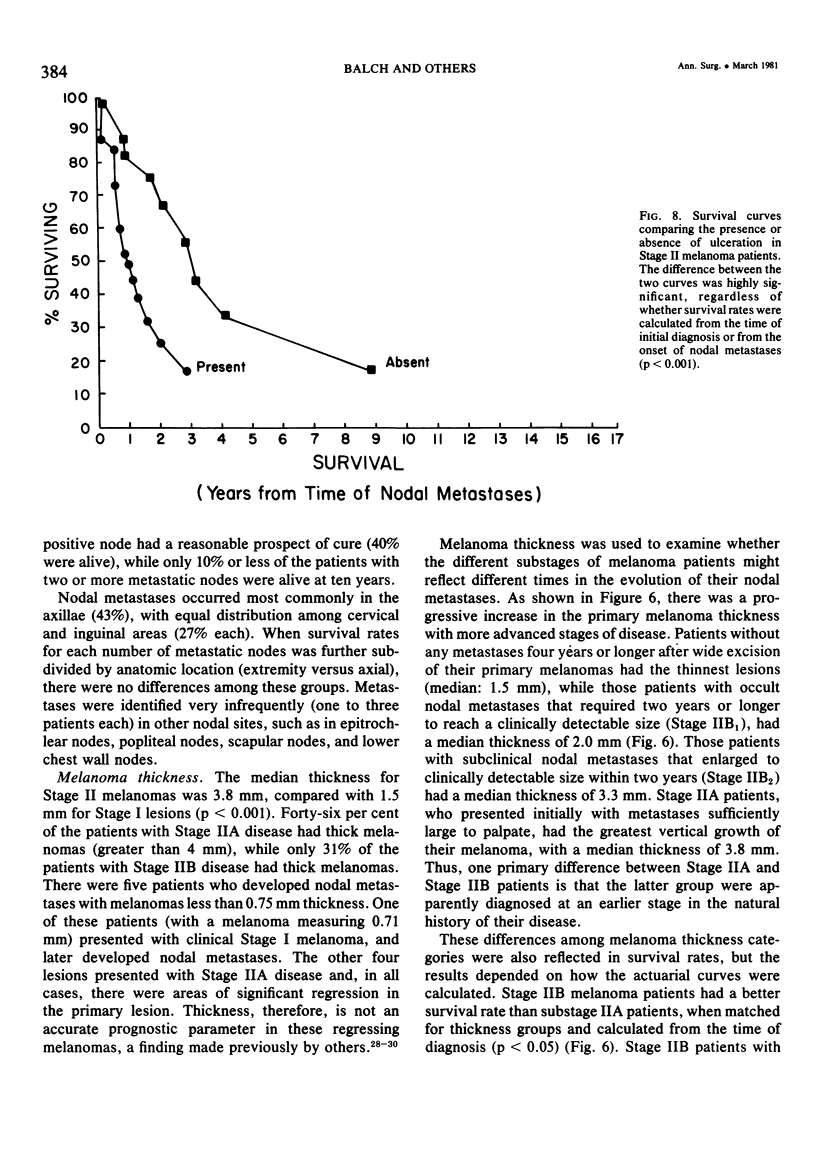
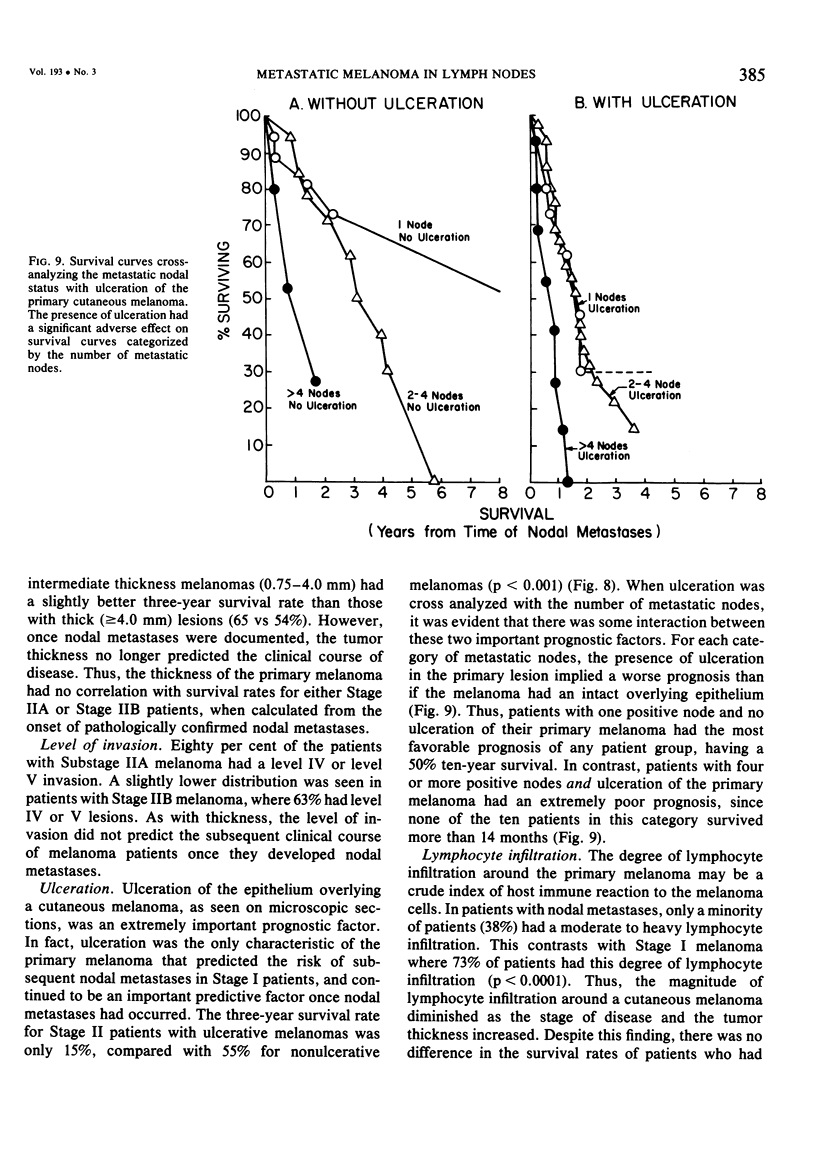
Twelve prognostic features of melanoma were examined in a series of 185 patients with nodal metastases (Stage II), who underwent surgical treatment at our institution during the past 20 years. Forty-four per cent of the patients presented with synchronous nodal metastases (substage IIA), 44% of the patients had delayed nodal metastases (substage IIB), and 12% of the patients had nodal metastases from an unknown primary site (substage IIC). The patients with IIB (delayed) metastases had a better overall survival rate than patients with IIA (synchronous) metastases, when calculated from the time of diagnosis. These differences could be explained on the basis of tumor burden at the time of initial diagnosis (microscopic for IIB patients versus macroscopic for IIA patients). Once nodal metastases became evident in IIB patients, their survival rates were the same as for substage IIA patients, when calculated from the onset of nodal metastases. The survival rates for both subgroups was 28% at five years and 15% for ten years. Substage IIC patients (unknown 1° site) had better five-year survival rates (39%), but the sample size was small and the differences were not statistically significant. A multifactorial analysis was used to identify the dominant prognostic variables from among 12 clinical and pathologic parameters. Only two factors were found to independently influence survival rates: 1) the number of metastatic nodes (p = 0.005), and the presence or absence of ulceration (p = 0.0019). Additional factors considered that had either indirect or no influence on survival rates (p > 0.10) were: anatomic location, age, sex, remission duration, substage of disease, tumor thickness, level of invasion, pigmentation, and lymphocyte infiltration. All combinations of nodal metastases were analyzed from survival differences. The combination that showed the greatest differences was one versus two to four versus more than four nodes. Their five-year survival rates were 58%, 27% and 10%, respectively (p < 0.001). Ulceration of the primary cutaneous melanoma was associated with a <15% five-year survival rate, while nonulcerative melanomas had a 30% five-year survival rate (p < 0.001). The combination of ulceration and multiple metastatic nodes had a profound adverse effect on survival rates. While tumor thickness was the most important factor in predicting the risk of nodal metastases in Stage I patients (p < 10-8), it had no predictive value on the patient's clinical course once nodal metastases had occurred (p = 0.507). The number of metastatic nodes and the presence of ulceration are important factors to account for when comparing surgical results, and when analyzing the efficacy of adjunctive systemic treatments.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch C. M., Murad T. M., Soong S. J., Ingalls A. L., Halpern N. B., Maddox W. A. A multifactorial analysis of melanoma: prognostic histopathological features comparing Clark's and Breslow's staging methods. Ann Surg. 1978 Dec;188(6):732–742. doi: 10.1097/00000658-197812000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch C. M., Murad T. M., Soong S. J., Ingalls A. L., Richards P. C., Maddox W. A. Tumor thickness as a guide to surgical management of clinical stage I melanoma patients. Cancer. 1979 Mar;43(3):883–888. doi: 10.1002/1097-0142(197903)43:3<883::aid-cncr2820430316>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Balch C. M., Soong S. J., Murad T. M., Ingalls A. L., Maddox W. A. A multifactorial analysis of melanoma. II. Prognostic factors in patients with stage I (localized) melanoma. Surgery. 1979 Aug;86(2):343–351. [PubMed] [Google Scholar]
- Balch C. M. Surgical management of regional lymph nodes in cutaneous melanoma. J Am Acad Dermatol. 1980 Nov;3(5):511–524. doi: 10.1016/s0190-9622(80)80118-6. [DOI] [PubMed] [Google Scholar]
- Balch C. M., Wilkerson J. A., Murad T. M., Soong S. J., Ingalls A. L., Maddox W. A. The prognostic significance of ulceration of cutaneous melanoma. Cancer. 1980 Jun 15;45(12):3012–3017. doi: 10.1002/1097-0142(19800615)45:12<3012::aid-cncr2820451223>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Cady B., Legg M. A., Redfern A. B. Contemporary treatment of malignant melanoma. Am J Surg. 1975 Apr;129(4):472–482. doi: 10.1016/0002-9610(75)90195-6. [DOI] [PubMed] [Google Scholar]
- Cohen M. H., Ketcham A. S., Felix E. L., Li S. H., Tomaszewski M. M., Costa J., Rabson A. S., Simon R. M., Rosenberg S. A. Prognostic factors in patients undergoing lymphadenectomy for malignant melanoma. Ann Surg. 1977 Nov;186(5):635–642. doi: 10.1097/00000658-197711000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DASGUPTA T., BOWDEN L., BERG J. W. MALIGNANT MELANOMA OF UNKNOWN PRIMARY ORIGIN. Surg Gynecol Obstet. 1963 Sep;117:341–345. [PubMed] [Google Scholar]
- Durkin K., Haagensen C. D. An improved technique for the study of lymph nodes in surgical specimens. Ann Surg. 1980 Apr;191(4):419–429. doi: 10.1097/00000658-198004000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilber F. R., Morton D. L., Holmes E. C., Sparks F. C., Ramming K. P. Adjuvant immunotherapy with BCG in treatment of regional-lymph-node metastases from malignant melanoma. N Engl J Med. 1976 Jan 29;294(5):237–240. doi: 10.1056/NEJM197601292940501. [DOI] [PubMed] [Google Scholar]
- Fee H. J., Robinson D. S., Sample W. F., Graham L. S., Holmes E. C., Morton D. L. The determination of lymph shed by colloidal gold scanning in patients with malignant melanoma: a preliminary study. Surgery. 1978 Nov;84(5):626–632. [PubMed] [Google Scholar]
- Fortner J. G., Woodruff J., Schottenfeld D., Maclean B. Biostatistical basis of elective node dissection for malignant melanoma. Ann Surg. 1977 Jul;186(1):101–103. doi: 10.1097/00000658-197707000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano A. E., Moseley H. S., Morton D. L. Clinical aspects of unknown primary melanoma. Ann Surg. 1980 Jan;191(1):98–104. doi: 10.1097/00000658-198001000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith H. S., Shah J. P., Kim D. H. Prognostic significance of lymph node dissection in the treatment of malignant melanoma. Cancer. 1970 Sep;26(3):606–609. doi: 10.1002/1097-0142(197009)26:3<606::aid-cncr2820260317>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Goldsmith H. S. The debate over immediate lymph node dissection in melanoma. Surg Gynecol Obstet. 1979 Mar;148(3):403–405. [PubMed] [Google Scholar]
- Gumport S. L., Harris M. N. Results of regional lymph node dissection for melanoma. Ann Surg. 1974 Jan;179(1):105–108. doi: 10.1097/00000658-197401000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta T. K. Results of treatment of 269 patients with primary cutaneous melanoma: a five-year prospective study. Ann Surg. 1977 Aug;186(2):201–209. doi: 10.1097/00000658-197708000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huvos A. G., Shah A. P., Miké V. Prognostic factors in cutaneous malignant melanoma. A comparative study of long term and short term survivors. Hum Pathol. 1974 May;5(3):347–357. doi: 10.1016/s0046-8177(74)80117-6. [DOI] [PubMed] [Google Scholar]
- Karakousis C. P., Seddiq M. K., Moore R. Prognostic value of lymph node dissection in malignant melanoma. Arch Surg. 1980 Jun;115(6):719–722. doi: 10.1001/archsurg.1980.01380060021006. [DOI] [PubMed] [Google Scholar]
- Little J. H., Davis N. C. Secondary malignant melanoma in lymph nodes: incidence, time of occurrence, and mortality. Aust N Z J Surg. 1978 Feb;48(1):9–13. doi: 10.1111/j.1445-2197.1978.tb05795.x. [DOI] [PubMed] [Google Scholar]
- McCarthy J. G., Haagensen C. D., Herter F. P. The role of groin dissection in the management of melanoma of the lower extremity. Ann Surg. 1974 Feb;179(2):156–159. doi: 10.1097/00000658-197402000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer C. M., Lecklitner M. L., Logic J. R., Balch C. E., Bessey P. Q., Tauxe W. N. Technetium-99m sulfur-colloid cutaneous lymphoscintigraphy in the management of truncal melanoma. Radiology. 1979 Apr;131(1):205–209. doi: 10.1148/131.1.205. [DOI] [PubMed] [Google Scholar]
- Milton G. W., Shaw H. M., Farago G. A., McCarthy W. H. Tumour thickness and the site and time of first recurrence in cutaneous malignant melanoma (stage I). Br J Surg. 1980 Aug;67(8):543–546. doi: 10.1002/bjs.1800670804. [DOI] [PubMed] [Google Scholar]
- Schmoeckel C., Braun-Falco O. Prognostic index in malignant melanoma. Arch Dermatol. 1978 Jun;114(6):871–873. [PubMed] [Google Scholar]
- Sugarbaker E. V., McBride C. M. Melanoma of the trunk: the results of surgical excision and anatomic guidelines for predicting nodal metastasis. Surgery. 1976 Jul;80(1):22–30. [PubMed] [Google Scholar]
- Veronesi U., Adamus J., Bandiera D. C., Brennhovd I. O., Caceres E., Cascinelli N., Claudio F., Ikonopisov R. L., Javorskj V. V., Kirov S. Inefficacy of immediate node dissection in stage 1 melanoma of the limbs. N Engl J Med. 1977 Sep 22;297(12):627–630. doi: 10.1056/NEJM197709222971202. [DOI] [PubMed] [Google Scholar]
- Veronesi U., Cascinelli N., Preda F. Prognosis of malignant melanoma according to regional metastases. Am J Roentgenol Radium Ther Nucl Med. 1971 Feb;111(2):301–309. doi: 10.2214/ajr.111.2.301. [DOI] [PubMed] [Google Scholar]
- Wanebo H. J., Fortner J. G., Woodruff J., MacLean B., Binkowski E. Selection of the optimum surgical treatment of stage I melanoma by depth of microinvasion: Use of the combined microstage technique (Clark-Breslow). Ann Surg. 1975 Sep;182(3):302–315. doi: 10.1097/00000658-197509000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]


