Abstract
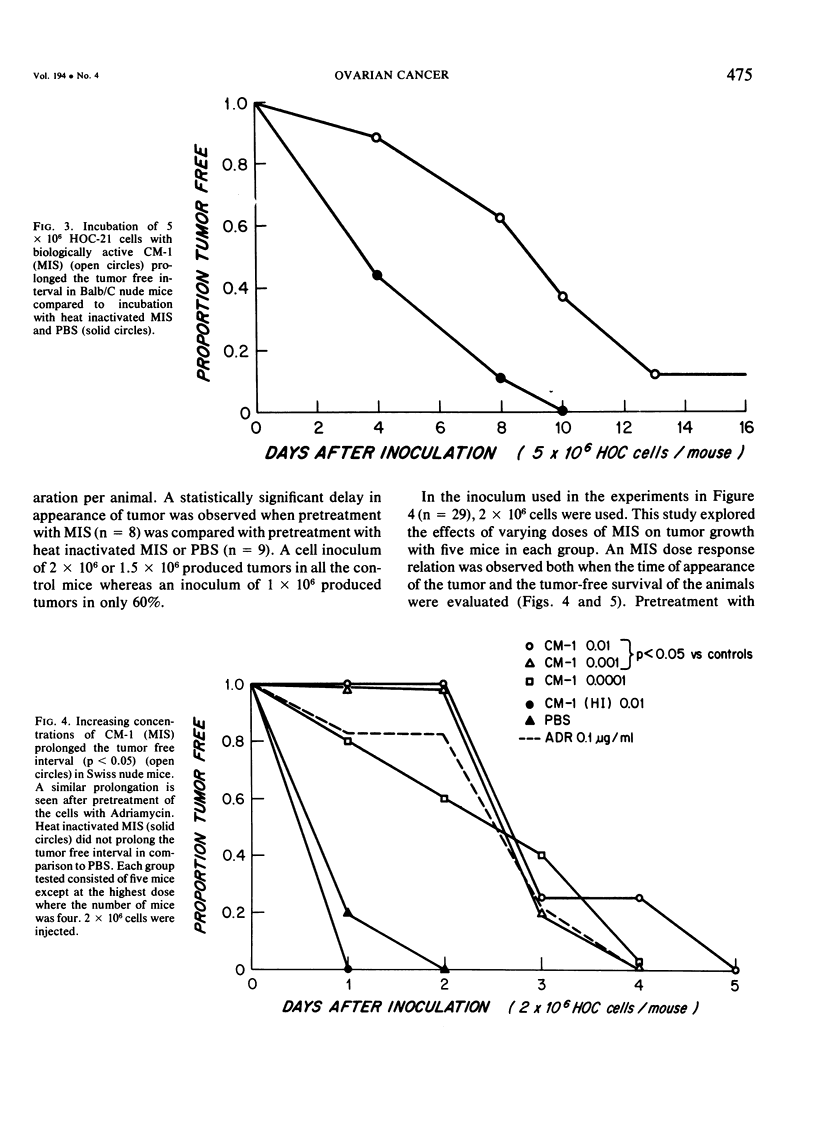
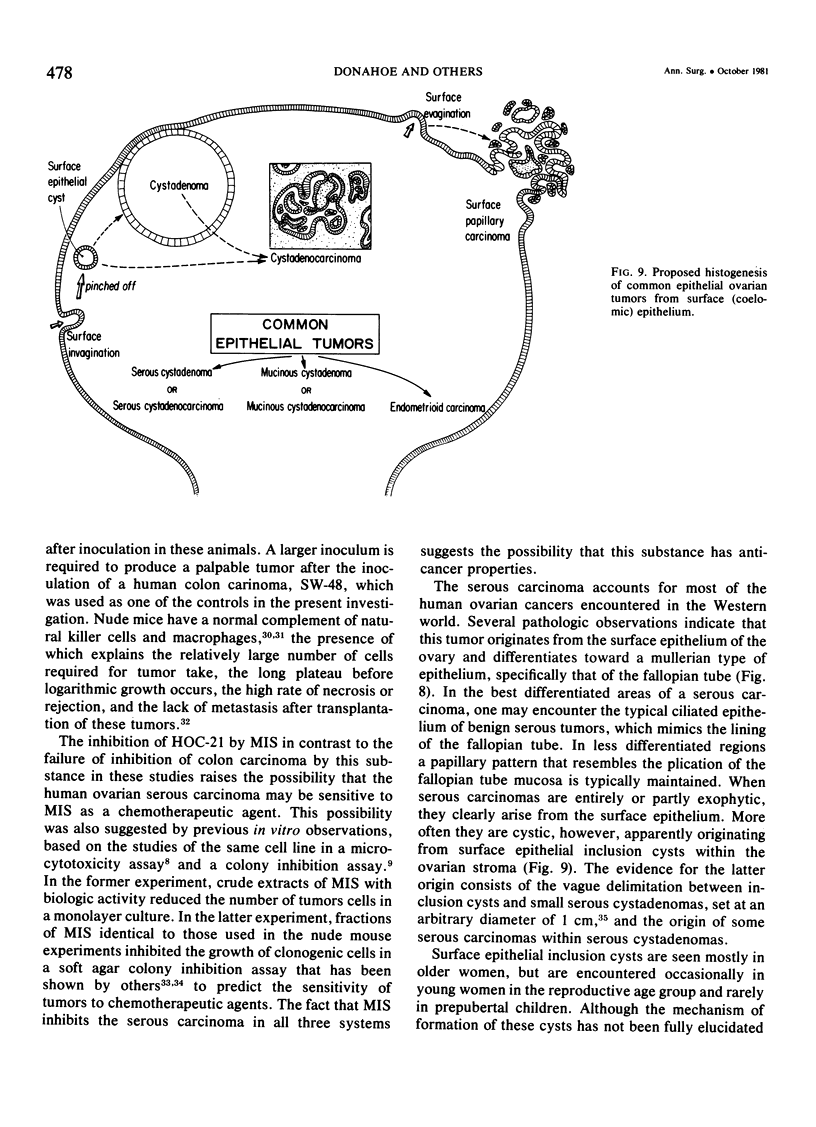
Mullerian inhibiting substance (MIS) was investigated for its ability to inhibit growth of a human ovarian cancer in nude mice. Biologically active preparations from newborn calf testes, obtained after sequential ion exchange chromatography, delayed or prevented growth of a human ovarian cancer (HOC-21) when 2 X 10(6) cells were preincubated with them prior to subcutaneous injection of the tumor cells into Balb/C homozygous nude mice. Preincubation of a human colon carcinoma cells (SW-48) with similar preparations of MIS failed to inhibit growth of the tumor cells in nude mice. Human serous carcinomas are thought to arise from the ovarian surface epithelium, a derivative of the coelomic epithelium of the urogenital ridge, which invaginates to form the mullerian duct early in embryonic life. The neoplastic cells of serous tumors simulate morphologically the lining cells of the fallopian tube, which are derivatives of mullerian duct epithelium. This study provides physiologic confirmation of the mullerian nature of this type of tumor and suggests that MIS may ultimately prove to be effective in its therapy.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auerbach R., Morrissey L. W., Sidky Y. A. Regional differences in the incidence and growth of mouse tumors following intradermal or subcutaneous inoculation. Cancer Res. 1978 Jun;38(6):1739–1744. [PubMed] [Google Scholar]
- Budzik G. P., Swann D. A., Hayashi A., Donahoe P. K. Enhanced purification of Mullerian inhibiting substance by lectin affinity chromatography. Cell. 1980 Oct;21(3):909–915. doi: 10.1016/0092-8674(80)90454-7. [DOI] [PubMed] [Google Scholar]
- Donahoe P. K., Ito Y., Hendren W. H., 3rd A graded organ culture assay for the detection of Mullerian inhibiting substance. J Surg Res. 1977 Aug;23(2):141–148. doi: 10.1016/0022-4804(77)90202-5. [DOI] [PubMed] [Google Scholar]
- Donahoe P. K., Swann D. A., Hayashi A., Sullivan M. D. Müllerian duct regression in the embryo correlated with cytotoxic activity against human ovarian cancer. Science. 1979 Aug 31;205(4409):913–915. doi: 10.1126/science.472712. [DOI] [PubMed] [Google Scholar]
- Hanna N., Fidler I. J. Expression of metastatic potential of allogenic and xenogeneic neoplasms in young nude mice. Cancer Res. 1981 Feb;41(2):438–444. [PubMed] [Google Scholar]
- Hanna N., Fidler I. J. Role of natural killer cells in the destruction of circulating tumor emboli. J Natl Cancer Inst. 1980 Oct;65(4):801–809. doi: 10.1093/jnci/65.4.801. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leibovitz A., Stinson J. C., McCombs W. B., 3rd, McCoy C. E., Mazur K. C., Mabry N. D. Classification of human colorectal adenocarcinoma cell lines. Cancer Res. 1976 Dec;36(12):4562–4569. [PubMed] [Google Scholar]
- Picard J. Y., Tran D., Josso N. Biosynthesis of labelled anti-müllerian hormone by fetal testes: evidence for the glycoprotein nature of the hormone and for its disulfide-bonded structure. Mol Cell Endocrinol. 1978 Oct;12(1):17–30. doi: 10.1016/0303-7207(78)90098-9. [DOI] [PubMed] [Google Scholar]
- Picon R. Action du testicule foetal sur le développement in vitro des canaux de Müller chez le rat. Arch Anat Microsc Morphol Exp. 1969 Jan-Mar;58(1):1–19. [PubMed] [Google Scholar]
- Poste G., Fidler I. J. The pathogenesis of cancer metastasis. Nature. 1980 Jan 10;283(5743):139–146. doi: 10.1038/283139a0. [DOI] [PubMed] [Google Scholar]
- Povlsen C. O., Rygaard J. Heterotransplantation of human adenocarcinomas of the colon and rectum to the mouse mutant Nude. A study of nine consecutive transplantations. Acta Pathol Microbiol Scand A. 1971;79(2):159–169. doi: 10.1111/j.1699-0463.1971.tb03324.x. [DOI] [PubMed] [Google Scholar]
- Povlsen C. O., Rygaard J. Heterotransplantation of human epidermoid carcinomas to the mouse mutant nude. Acta Pathol Microbiol Scand A. 1972;80(6):713–717. doi: 10.1111/j.1699-0463.1972.tb00340.x. [DOI] [PubMed] [Google Scholar]
- Price J. M., Donahoe P. K., Ito Y., Hendren W. H., 3rd Programmed cell death in the Müllerian duct induced by Müllerian inhibiting substance. Am J Anat. 1977 Jul;149(3):353–375. doi: 10.1002/aja.1001490304. [DOI] [PubMed] [Google Scholar]
- Price J. M., Donahoe P. K., Ito Y. Involution of the female Mullerian duct of the fetal rat in the organ-culture assay for the detection of Mullerian Inhibiting Substance. Am J Anat. 1979 Oct;156(2):265–284. doi: 10.1002/aja.1001560207. [DOI] [PubMed] [Google Scholar]
- Radisavljevic S. V. The pathogenesis of ovarian inclusion cysts and cystomas. Obstet Gynecol. 1977 Apr;49(4):424–429. [PubMed] [Google Scholar]
- Rygaard J., Povlsen C. O. Heterotransplantation of a human malignant tumour to "Nude" mice. Acta Pathol Microbiol Scand. 1969;77(4):758–760. doi: 10.1111/j.1699-0463.1969.tb04520.x. [DOI] [PubMed] [Google Scholar]
- Salmon S. E., Hamburger A. W., Soehnlen B., Durie B. G., Alberts D. S., Moon T. E. Quantitation of differential sensitivity of human-tumor stem cells to anticancer drugs. N Engl J Med. 1978 Jun 15;298(24):1321–1327. doi: 10.1056/NEJM197806152982401. [DOI] [PubMed] [Google Scholar]
- Sebesteny A., Hill A. C. Hepatitis and brain lesions due to mouse hepatitis virus accompanied by wasting in nude mice. Lab Anim. 1974 Sep;8(3):317–326. doi: 10.1258/002367774780943733. [DOI] [PubMed] [Google Scholar]
- Swann D. A., Donahoe P. K., Ito Y., Morikawa Y., Hendren W. H. Extraction of Mullerian inhibiting substance from newborn calf testis. Dev Biol. 1979 Mar;69(1):73–84. doi: 10.1016/0012-1606(79)90275-6. [DOI] [PubMed] [Google Scholar]
- Yamada T. The cellular biology of a newly established cell line of human ovarian adenocarcinoma in vitro. Keio J Med. 1974 Jun;23(2):53–70. doi: 10.2302/kjm.23.53. [DOI] [PubMed] [Google Scholar]




