Abstract
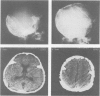
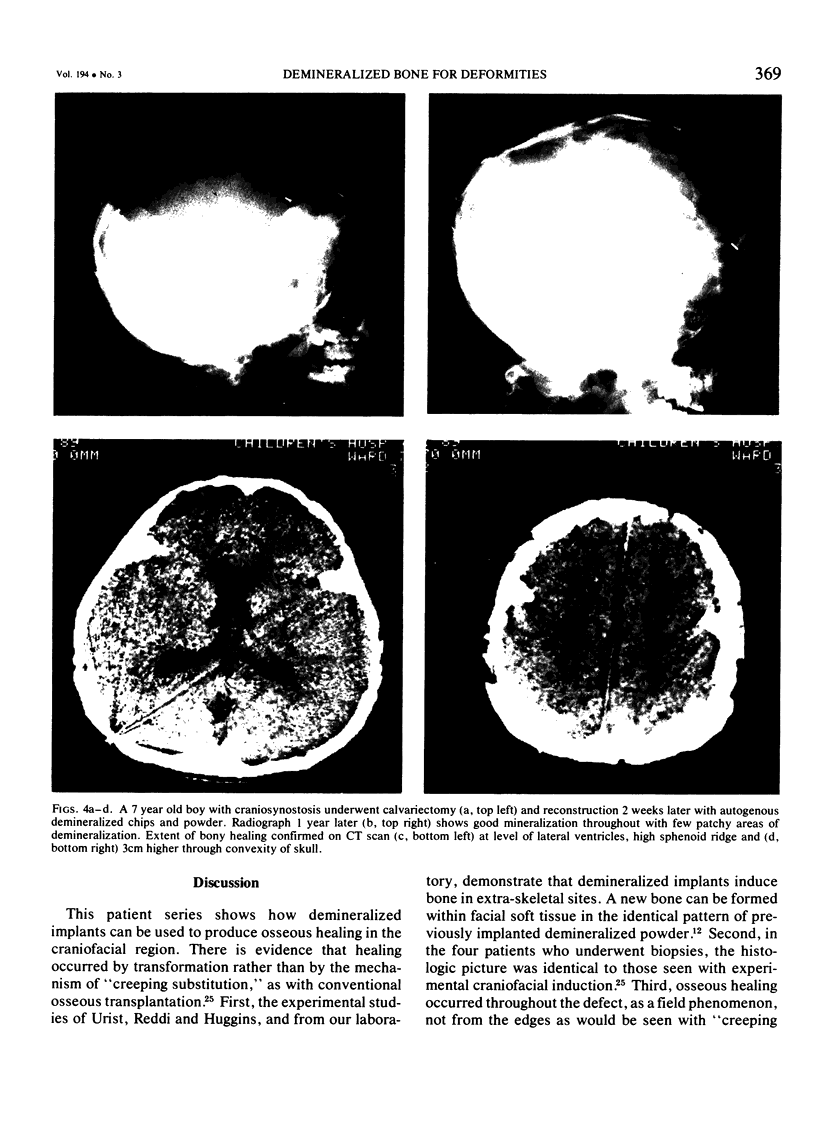
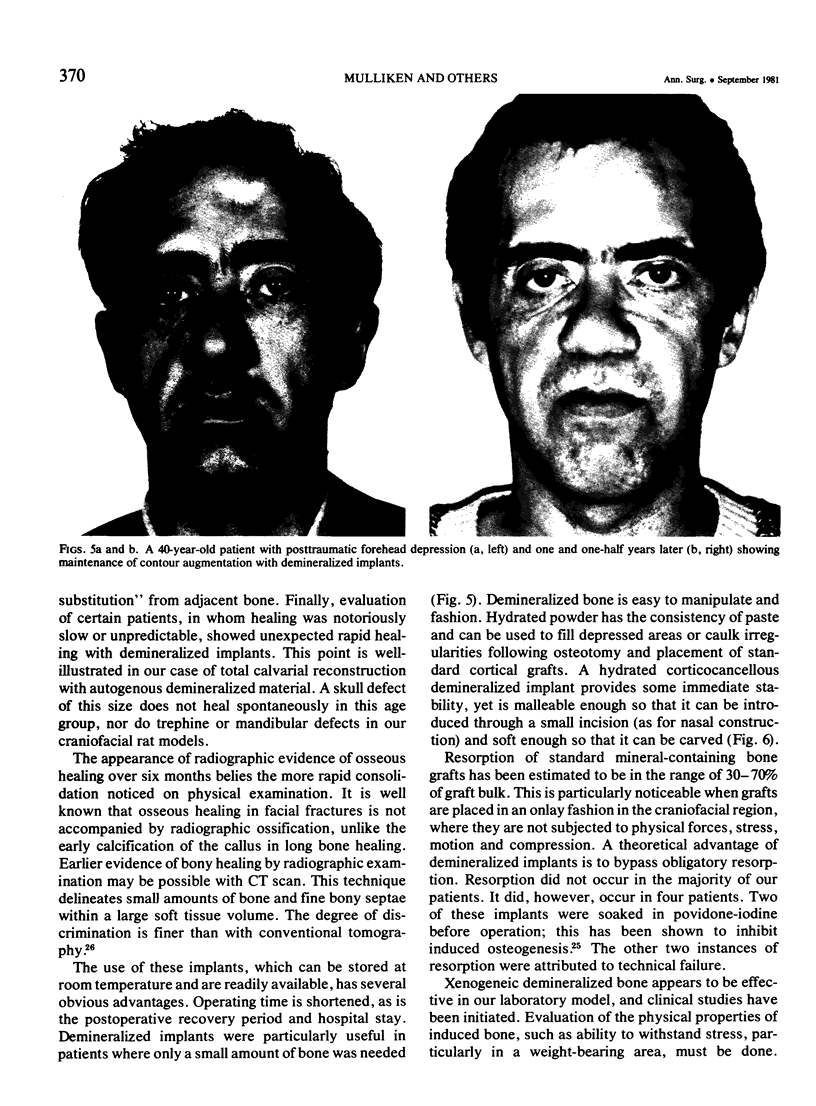
Two major problems in maxillocraniofacial surgery are the limited amount of fresh autogenous bone, the standard material for bone grafting, and the resorption of the grafted bone. Experimental studies with demineralized, devitalized bone matrix have shown induction of endochondral ossification. Fifty-five demineralized allogeneic implants have been used in 44 patients over the past two years for a variety of congenital (n = 37) and acquired (n = 7) defects. The allogeneic bone was obtained from cadavers, prepared as powders, chips or blocks, and was demineralized. After having been sterilized by irradiation, they were used to augment contour, fill defects, or construct bone within soft tissue. Of implanted sites that could be evaluated by physical examination, 31 of 31 were solid by three months. By radiographic examination three of 19 were healed by three months, and an additional 11 were positive by six months. Induced bone was seen in four of four biopsy specimens. Infection occurred in four of 44 patients (9%), comparable with conventional grafts. Implant resorption occurred in four instances. Allogeneic demineralized implants offer several advantages over conventional bone grafting, such as avoidance of a harvesting operation, ease of manipulation, and potentially unlimited material in banked form. In addition, healing by induced osteogenesis may bypass the resorption seen with healing of mineral-containing grafts.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrektsson T. Repair of bone grafts. A vital microscopic and histological investigation in the rabbit. Scand J Plast Reconstr Surg. 1980;14(1):1–12. doi: 10.3109/02844318009105731. [DOI] [PubMed] [Google Scholar]
- Bassett C. A. Clinical implications of cell function in bone grafting. Clin Orthop Relat Res. 1972 Sep;87:49–59. [PubMed] [Google Scholar]
- Boyne P. J. Review of the literature on cryopreservation of bone. Cryobiology. 1968 May-Jun;4(6):341–357. doi: 10.1016/s0011-2240(68)80134-8. [DOI] [PubMed] [Google Scholar]
- COHEN J. Cathode ray sterilization of bone grafts. AMA Arch Surg. 1955 Nov;71(5):784–789. doi: 10.1001/archsurg.1955.01270170142026. [DOI] [PubMed] [Google Scholar]
- Glowacki J., Altobelli D., Mulliken J. B. Fate of mineralized and demineralized osseous implants in cranial defects. Calcif Tissue Int. 1981;33(1):71–76. doi: 10.1007/BF02409414. [DOI] [PubMed] [Google Scholar]
- Glowacki J., Kaban L. B., Murray J. E., Folkman J., Mulliken J. B. Application of the biological principle of induced osteogenesis for craniofacial defects. Lancet. 1981 May 2;1(8227):959–962. doi: 10.1016/s0140-6736(81)91730-x. [DOI] [PubMed] [Google Scholar]
- HAM A., GORDON S. The origin of bone that forms in association with cancellous chips transplanted into muscle. Br J Plast Surg. 1952 Oct;5(3):154–160. doi: 10.1016/s0007-1226(52)80015-3. [DOI] [PubMed] [Google Scholar]
- HEIPLE K. G., CHASE S. W., HERNDON C. H. A COMPARATIVE STUDY OF THE HEALING PROCESS FOLLOWING DIFFERENT TYPES OF BONE TRANSPLANTATION. J Bone Joint Surg Am. 1963 Dec;45:1593–1616. [PubMed] [Google Scholar]
- KREUZ F. P., HYATT G. W., TURNER T. C., BASSETT A. L. The preservation and clinical use of freeze-dried bone. J Bone Joint Surg Am. 1951 Oct;33-A(4):863–passim. [PubMed] [Google Scholar]
- Kaban L. B., Glowacki J. Induced osteogenesis in the repair of experimental mandibular defects in rats. J Dent Res. 1981 Jul;60(7):1356–1364. doi: 10.1177/00220345810600071201. [DOI] [PubMed] [Google Scholar]
- Langer F., Czitrom A., Pritzker K. P., Gross A. E. The immunogenicity of fresh and frozen allogeneic bone. J Bone Joint Surg Am. 1975 Mar;57(2):216–220. [PubMed] [Google Scholar]
- Mulliken J. B., Glowacki J. Induced osteogenesis for repair and construction in the craniofacial region. Plast Reconstr Surg. 1980 May;65(5):553–560. doi: 10.1097/00006534-198005000-00001. [DOI] [PubMed] [Google Scholar]
- Reddi A. H., Huggins C. B. Cyclic electrochemical inactivation and restoration of competence of bone matrix to transform fibroblasts. Proc Natl Acad Sci U S A. 1974 May;71(5):1648–1652. doi: 10.1073/pnas.71.5.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddi A. H., Huggins C. B. Influence of geometry of transplanted tooth and bone on transformation of fibroblasts. Proc Soc Exp Biol Med. 1973 Jul;143(3):634–637. doi: 10.3181/00379727-143-37381. [DOI] [PubMed] [Google Scholar]
- Reddi A. H., Huggins C. Biochemical sequences in the transformation of normal fibroblasts in adolescent rats. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1601–1605. doi: 10.1073/pnas.69.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddi A. H., Huggins C. Lactic-malic dehydrogenase quotients during transformation of fibroblasts into cartilage and bone. Proc Soc Exp Biol Med. 1971 May;137(1):127–129. doi: 10.3181/00379727-137-35527. [DOI] [PubMed] [Google Scholar]
- SHARRARD W. J., COLLINS D. H. The fate of human decalcified bone grafts. Proc R Soc Med. 1961 Dec;54:1101–1102. [PMC free article] [PubMed] [Google Scholar]
- Urist M. R. Bone: formation by autoinduction. Science. 1965 Nov 12;150(3698):893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- Urist M. R., Dawson E. Intertransverse process fusion with the aid of chemosterilized autolyzed antigen-extracted allogeneic (AAA) bone. Clin Orthop Relat Res. 1981 Jan-Feb;(154):97–113. [PubMed] [Google Scholar]
- Urist M. R., Mikulski A., Boyd S. D. A chemosterilized antigen-extracted autodigested alloimplant for bone banks. Arch Surg. 1975 Apr;110(4):416–428. doi: 10.1001/archsurg.1975.01360100058011. [DOI] [PubMed] [Google Scholar]
- WILLIAMS J. B., IRVINE J. W., Jr Preparation of the inorganic matrix of bone. Science. 1954 May 28;119(3100):771–772. doi: 10.1126/science.119.3100.771. [DOI] [PubMed] [Google Scholar]