Abstract
The effect of nonspecific proteolysis on the structure of single isolated mitotic newt chromosomes was studied using chromosome elastic response as an assay. Exposure to either trypsin or proteinase K gradually decondensed and softened chromosomes but without entirely eliminating their elastic response. Analysis of chromosome morphology revealed anisotropic decondensation upon digestion, with length increasing more than width. Prolonged protease treatment resulted only in further swelling of the chromosome without complete dissolution. Mild trypsinization induced sensitivity of chromosome elasticity to five- and six-base-specific restriction enzymes. These results, combined with previous studies of effects of nucleases on mitotic chromosome structure, indicate that mild proteolysis gradually reduces the density of chromatin-constraining elements in the mitotic chromosome, providing evidence consistent with an anisotropically folded “chromatin network” model of mitotic chromosome architecture.
INTRODUCTION
The scheme by which chromatin is folded into chromosomes and the mechanism by which that folding is modulated during the cell cycle are poorly understood (Swedlow and Hirano, 2003). The study of eukaryote DNA organization at scales larger than the nucleosome challenges traditional methods of structural biology for a few reasons. First, large-scale chromatin structure is at present thought to be determined to a large degree by sequence-nonspecific DNA–protein interactions, e.g., histone–DNA and high mobility group (HMG)–DNA interactions. Second, whole chromosomes are remarkably soft yet elastic objects (Houchmandzadeh et al., 1997; Houchmandzadeh and Dimitrov, 1999; Poirier and Marko, 2003; Almagro et al., 2004), indicating that although their spatial organization is well defined, the chromatin inside them enjoys a large amount of conformational freedom. Third, at short length scales (<0.5 μm) chromatin in vivo behaves as a polymer with a time-varying and fluctuating structure (Marshall et al., 1997; Bystricky et al., 2004), whereas over larger length scales, there is significant cell-to-cell variation of chromosome and nuclear structure (Trask et al., 1993). All these results indicate that large-scale chromatin structure must be thought of, at least in part, statistically, rather than exclusively in terms of precise interactions and spatial relations of individual macromolecules and particular DNA sequences.
During eukaryote cell division, chromosomes become condensed into their compact mitotic form. This process is essential to the proper segregation of the duplicated chromatids to the two daughter cells, and it is likely that mitotic condensation and entanglement resolution are closely related phenomena (Hirano, 1995). An initial step toward understanding mitotic chromosome condensation is the study of mitotic chromosome structure, which is the broad objective of this article.
Even the rough outline of how the mitotic chromosome is spatially organized remains a subject of current research. The textbook “scaffold-loop” model of mitotic chromosome structure (Paulson and Laemmli, 1977; Laemmli et al., 1978; Marsden and Laemmli, 1979; Earnshaw and Laemmli, 1983, 1984), based on “chromatin loops” attached at their bases to a protein-rich, connected “scaffold” has gradually evolved into a conception of the mitotic chromatid as a “network” or “gel” of chromatin, stabilized by many isolated chromatin–chromatin cross-linkers. The beginning of this evolution began two decades ago when Earnshaw and Heck (Earnshaw and Heck, 1985) showed that topoisomerase II, a major mitotic chromosome scaffold component (Earnshaw et al., 1985), is organized into localized “islets”; they concluded that the mitotic scaffold was in fact disconnected.
Subsequent experiments indicated that topoisomerase II is not essential to maintaining mitotic chromosome structure (Hirano and Mitchison, 1993), and eventually led to a focus on the structural maintenance of chromosomes protein (SMC)-containing “condensin” protein complexes, which seem to act as organizers of mitotic chromatin (Hirano, 2005). Condensins are essential for establishing and maintaining mitotic chromosome structure (Hirano and Mitchison, 1994), properly assembling other nonhistone proteins (topoisomerase II and ICENP) on mitotic chromosomes (Hudson et al., 2003), and allowing chromosomes to refold after having been disrupted by buffer changes (Hudson et al., 2003).
Two distinct condensins, termed I and II, exist in vertebrate cells and have distinct functions (Ono et al., 2003). In vertebrate cells, the two condensins act in a temporally distinct manner (Hirota et al., 2004; Ono et al., 2004): condensin II is found in the interphase nucleus, plays an important role during early prophase condensation, and may stabilize the final coiling and stiffening of the metaphase chromosome (Ono et al., 2003), whereas condensin I is cytoplasmic, acting on chromosomes after nuclear envelope breakdown. In normal metaphase vertebrate chromosomes, the two species of condensins assemble into two adjacent axial helical patterns along the chromatids (Ono et al., 2004).
These biological advances have been complemented by biophysical work. A recent study showed that it was possible to mechanically disrupt, and then completely cleave, whole mitotic chromosomes using only nucleases (Poirier and Marko, 2002). Those experiments, done under buffer conditions where native chromosome structure is well preserved, made clear that the mechanical integrity of the mitotic chromosome comes from the continuity of DNA in chromatin itself. Notably, that study found that restriction enzymes with four-base specificity were capable of dissolving the mitotic newt chromosome. Because only sporadic cuts are necessary to dissolve the whole chromosome, it is not possible for the chromosome to be organized by a connected, underlying, non-DNA scaffold. Accounting for the reduced access to DNA in chromatin, these experiments suggest that successive points of tethering of DNA in the mitotic chromosome are at least a few kilobases apart.
However, the biophysical experiments also indicated that nuclease-insensitive elements (most likely proteins, possibly condensins) play an important role in holding the chromosome together, because it was observed that sufficiently rarely cutting restriction enzymes (five-base and higher specificity) had no detectable effect on chromosome elasticity (Poirier and Marko, 2002). The picture emerging from the recent experiments is one where the mitotic chromosome has the structure of a “chromatin network” (or a “chromatin gel”) with isolated “chromatin cross-linker” elements serving to stabilize mitotic chromosome structure. This “network” hypothesis explains the null effect of five-base and higher specificity restriction enzymes by supposing that the relatively rare cuts made by such enzymes occur further apart along DNA than do the cross-links, rendering their effect on chromosome elasticity undetectable. Importantly, the micromanipulation experiments showed that condensins, or any other chromatin-chromatin linkers, are not connected together by direct interactions but only by intervening stretches of chromatin.
This type of micromanipulation-digestion experiment is reminiscent of the classic studies of enzyme effects on meiotic prophase “lampbrush” chromosomes (Callan and Macgregor, 1958; Macgregor and Callan, 1962; Gall, 1963; Gould et al., 1976; Hartley and Callan, 1978). Nuclease experiments of this type provided definitive evidence for the “unineme hypothesis” that each chromatid contains a single, long DNA (Callan and Macgregor, 1958; Macgregor and Callan, 1962). In definitive and quantitative experiments by Gall, analysis of DNA digestions showed that only one DNA cut was necessary to cleave each chromatid (Gall, 1963). That experiment was based on the fact that the roughly millimeter-long meiotic prophase lampbrush chromosomes consist of highly extended DNA, with giant DNA loops along the chromatids directly observable as individual fibers in the light microscope. Later experiments even examined the effect of restriction enzymes on particular lampbrush loops (Hartley and Callan, 1978). We note that the micromanipulation-nuclease digestion experiments of Poirier and Marko (2002) and this report are focused on the much more (roughly 100-fold) dense mitotic chromosomes, which contain highly folded and cross-linked chromatin, and which require a much higher level of DNA cutting to be cleaved.
The experiments of this article are aimed at defining in more detail the interplay between protein and DNA in mitotic chromosome structure. We use the mechanical, elastic response of whole mitotic chromosomes as an assay for changes in chromosome structure. The basic idea is to use the response of the chromosome to applied force, to quantify changes in chromatin structure induced by various enzymes. Our experiments are performed in the extracellular medium using slightly prometaphase chromosomes removed from mitotic cells. We first report experiments on proteolysis by trypsin and proteinase K, which establish that mitotic chromosomes gradually unfold and soften during protein digestion, but also show that unlike with nucleases, protein digestion does not induce strongly irreversible (plastic) behavior or dissolution of the chromosome. Our results are qualitatively consistent with protease digestion experiments on whole metaphase genomes (Maniotis et al., 1997) and on micromechanical experiments on Xenopus high-speed egg extract-assembled chromatids digested with proteases (Almagro et al., 2004). However, our new experiments reveal that the more compacted chromosomes from cells decondense anisotropically during digestion. The anisotropy, which takes the form of an excess lengthening, may reflect protease-induced opening of the highest level of chromatid folding described by (Kireeva et al., 2004). That highest level of folding may be mediated by the condensin II complexes; note that condensin II is present at reduced levels in Xenopus egg extracts (Ono et al., 2003).
Finally, we report experiments that combine protease and nuclease treatments. We recall that restriction enzymes with five- and six-base specificities do not generate a reduction in mitotic chromosome elasticity, suggesting that the chromatin in mitotic chromosomes is tethered, or cross-linked, every ∼15 kb (Poirier and Marko, 2002). We find that protease treatment induces a subsequent effect of the five-base-specific restriction enzymes HincII and HindII, and the six-base-specific restriction enzyme StuI. This result is consistent with the chromatin-cross-link hypothesis, providing additional data supporting the “network” model of the mitotic chromosome based on isolated chromatin cross-linking protein elements.
MATERIALS AND METHODS
Cell Culture, Chromosome Extraction, and Micromanipulation
Sample dishes were prepared using thin glass microscope slides onto which rubber O-rings (∼25 mm in diameter) were affixed using wax. Newt TVI (Notophthalmus viridescens) cells were grown (TVI cell line; Reese et al., 1976) in these dishes in cell culture medium, in the open air, and at room temperature. The cell culture medium consisted of 50% (vol/vol) L-15 with l-glutamine (Cellgro; Mediatech, Herndon, VA), 8% (vol/vol) premium fetal bovine serum (Cambrex Bio Science Walkersville, Walkersville, MD), 1% (vol/vol) penicillin/streptomycin (Cambrex Bio Science Walkersville), and 1 ng/ml fungizone (Cambrex Bio Science Walkersville) in molecular biology grade purified water (Cambrex Bio Science Walkersville). The medium was replaced daily. When the sample dish was ∼80% confluent, it was ready for use in the micromanipulation experiments.
The sample was positioned on an inverted microscope (IX70; Olympus, Tokyo, Japan) with a motorized stage (1-μm step size). Micropipettes were fabricated from borosilicate glass capillaries (1 mm o.d., 0.75 mm i.d.; WPI, Sarasota, FL) using a pipette puller (P-97 puller; Sutter Instruments, Novato, CA) and a home-built forge. The taper of the micropipettes could be adjusted over a wide range to produce repeatable deflection force constants anywhere between 0.01 and 10000 nN/μm. Two different types of micropipette were fabricated. One type of micropipette had an ∼12-mm taper, a force constant of ∼100 pN/μm, and a 2-μm end opening; this type of pipette will be described as “floppy” because it was used here as a force sensor in the chromosome stretching experiments. The second type of micropipette had an ∼5 mm taper, a 2-μm end opening, and a much higher force constant, used for either stretching or spraying the chromosome. These pipettes were filled with various solutions using a pneumatic vacuum pump and then positioned into the sample dish, close to the glass surface upon which the cells were growing, by using a 10×, 0.3 NA air objective lens. The two pipettes for holding the ends of the chromosome were attached to motorized XYZ micromanipulators (40-nm step size, Sutter Instruments MP-285). These could be controlled using either a joystick or via a purpose-written LabView program. The third pipette, used for spraying enzymes onto the chromosome, was attached to a coarser mechanical micromanipulator (Taurus, World Precision Instruments).
Chromosome extraction was carried out using a 60×, 1.4 numerical aperture oil immersion objective. One of the micropipettes was filled with a 0.05% solution of Triton X-100 (Fisher Scientific, Pittsburgh, PA) in 60% phosphate-buffered saline (PBS). This pipette was positioned close to the edge of a cell identified to be in prometaphase. Controlled spraying using this pipette produced a hole in the cell membrane through which the chromosomes could flow out into solution. At this point, a second pipette filled with 60% PBS was used to catch one end of a chromosome that seemed to be loosely attached to the other chromosomes. This second “floppy” pipette was used as a force sensor to measure tension within the chromosome as a function of extension. The other end of the chromosome was next detached from the other chromosomes and sucked into the “stiff” moveable pipette. Hence, a single isolated chromosome would be suspended between the two micropipettes in the extracellular medium (pH 7.5, ≈100 mM net univalent salt, Na+ and K+) ready for manipulation experiments. A schematic of the experimental configuration is shown in Figure 1. All experiments were carried out at room temperature.
Figure 1.
A simplified schematic of the experimental setup. Cells and extracted chromosomes are imaged through a 60× contact objective. Cell and chromosome manipulations are from above by using micropipettes. A single chromosome is extracted from a cell and suspended between a stiff moving pipette and a floppy fixed pipette. Images are acquired via the charge-coupled device camera as shown in the phase contrast image (bar, 5 μm). As the stiff pipette moves (see arrow), the deflection of the fixed floppy pipette is used as a force sensor. Force versus chromosome length data are plotted using the recorded images (see stretch data for a native chromosome). A third pipette is introduced into the sample dish to spray the chromosomes with various biochemicals.
Chromosome Elasticity Assay for Structural Changes Induced Biochemically
The tension in the chromosome as a function of extension could be measured by acquiring images of the two pipettes as the stiff pipette was moved at a rate of 0.08 μm/s (0.04-μm step size) in the same direction as the chromosome length axis (Figure 1, phase contrast image). The experiments were PC-controlled using LabView programs. Using two-dimensional image autocorrelation to calculate pipette positions, the chromosome length was taken as the distance between the two pipettes and the deflection of the floppy pipette was used to measure the tension within the chromosome. Deflection of the floppy pipette could be converted to force by calibration of the pipette against a known standard (Poirier et al., 2000). Position changes of the pipettes were located to ∼10-nm position resolution, corresponding to ∼1-pN force resolution for 100 pN/μm pipettes. Image acquisition was at video rate using an NTSC camera (Panasonic WV-BP310) and a frame grabber (IMAQ; National Instruments, Austin, TX) onto the PC.
Combined biochemical–micromechanical experiments were carried out by the incorporation of the third spray pipette into the experimental dish. This pipette was moved into position by careful manual control using a micromanipulator (Taurus; WPI). This pipette could be filled with a range of different biochemicals to test their effect on the mechanical properties of the isolated single chromosomes. Spraying was carried out using a gravity-feed system.
Enzyme Digestion of Whole Chromosomes
A number of different enzyme solutions were prepared for the digestion experiments. Trypsin (Sigma-Aldrich, St. Louis, MO) was prepared at a concentration of 100 nM in 60% PBS. Proteinase K (Promega, Madison, WI) was prepared at a concentration of 500 nM in 60% PBS, 1 mM CaCl2. These protease solutions were made using sterile, filtered PBS (Cambrex Bio Science Walkersville) and molecular biology grade water (Cambrex Bio Science Walkersville). The blunt-cutting restriction enzymes used were HincII [GT(T/C) ↓ (A/G)AC] (New England Biolabs, Beverly, MA), HindII [GT(T/C) ↓ (A/G)AC] (Roche Diagnostics, Indianapolis, IN), and StuI [AGG ↓ CCT] (New England Biolabs). HincII was prepared at a concentration of 0.5 U/μl in 50 mM Tris-HCl, 100 mM NaCl, 10 mM MgCl2, and 1 mM dithiothreitol (DTT), pH 7.9. HindII was prepared at a concentration of 0.125 U/μl in 10 mM Tris-HCl, 50 mM NaCl, 10 mM MgCl2, and 1 mM DTT, pH 7.5. StuI was prepared at a concentration of 2.5 U/μl in 10 mM Tris-HCl, 50 mM NaCl, 10 mM MgCl2, and 1 mM DTT, pH 7.9.
RESULTS
Combined Biochemical–Mechanical Experiments Can Reveal Roles of Specific Molecules in Organizing Mitotic Chromosomes
Newt TVI cells were cultured in sample dishes until ∼80% confluent. The sample was then mounted onto the microscope, and a single chromosome was extracted from a slightly prometaphase cell (fully condensed, but not yet captured on spindle; for details, see Materials and Methods). The ends of the chromosome were aspirated into two micropipettes. One of these was a moveable “stiff” pipette (essentially unable to be bent by the nanonewton-scale forces of this study) mounted on a micromanipulator, which was used to stretch the chromosome. The other pipette was “floppy” (force constant of roughly 0.1 nN/μm = 100 pN/μm) and held at fixed position. This second, floppy pipette was used as a force sensor to detect tension in the chromosome as a function of extension. An overview of the experimental setup is given in Figure 1. The TVI chromosomes are well suited for micromanipulation experiments because they are large (10–20 μm in length and ∼2 μm in width) and during mitosis become easily visible under the light microscope.
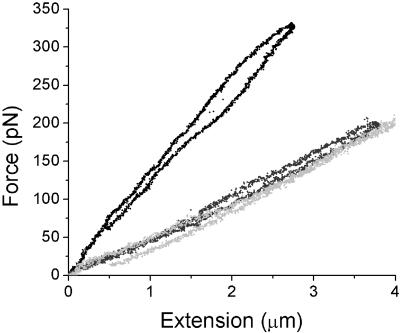
The two pipettes holding the chromosome were positioned as shown in the phase contrast image (Figure 1). The starting position was chosen to be close to the native chromosome length; hence, at a zero stretch and zero force. The tension in the chromosome as a function of extension could then be measured by acquiring images of the two pipettes as the stiff pipette was slowly moved in the direction shown by the arrow (Figure 1). A stretch–relax cycle for a native chromosome results in a force versus chromosome length data as shown (Figure 1). The chromosome stretch data show a linear force versus extension response, and the slope of the force-extension provides the spring constant of the chromosome (force per length; in this study we use units of piconewtons per micrometer [pN/μm]). As observed in previous studies (Poirier et al., 2000), for sufficiently slow pipette motions, the same force is observed during extension and relaxation, i.e., the native mitotic chromosomes display reversible elastic response. Furthermore, for extensions to less than doubling of native length (as in Figure 1), the elastic response is nearly linear.
Combined biochemical–micromechanical experiments were carried out by the use of a third “spray” pipette into the experimental dish. A basic study of this type was previously used to ascertain effects of various salt conditions on mitotic chromosomes (Maniotis et al., 1997; Poirier et al., 2002b). Those experiments revealed abrupt decondensation effects for >200 mM univalent and divalent salt concentrations. Additionally hypercondensation was observed for exposure to 5–100 mM concentrations of Mg2+ and Ca2+ (Poirier et al., 2002b). Importantly, it was demonstrated that exposure of chromosomes to moderate concentrations of divalent salts (10 mM MgCl2 for 200 s) only caused a temporary change in chromosome elasticity, while spraying was being done (Poirier et al., 2002b). When sprays of salt solutions were ended, chromosome elasticity returned to its native linear, reversible form. This behavior is important to the interpretation of enzyme spray experiments described in this study, where divalent ions are often present at this concentration.
In contrast to the salt experiments, use of enzymes that digest DNA have profound and irreversible effects on chromosome mechanical properties. Treatment of chromosomes with sufficiently frequently cutting nucleases (micrococcal nuclease and four-base restriction enzymes) rapidly degrades their elasticity. Low exposures to these nucleases dramatically reduced the force necessary to extend a chromosome, and chromosomes became plastic instead of elastic, displaying irreversible lengthening as a result of extension. Longer exposures to nonspecific nuclease led to complete dissolution of the chromosome (Poirier and Marko, 2002).
Trypsin and Proteinase K Cause Gradual Unfolding of Mitotic Chromosomes without Completely Dissolving Them
Two different protein-cutting enzymes, trypsin and proteinase K, were chosen to study the effect of protein digestion on mitotic chromosomes. Proteinase K, a member of the serine protease family, is known to cleave peptide bonds on the carboxyl-terminal side of aliphatic and aromatic amino acids. Trypsin, also a member of the serine proteases, cleaves on the carboxyl-terminal side of lysine and arginine amino acid residues, but cleavage does not occur when proline is on the carboxyl side of the cleavage site. Hence, we were able to compare a protease with a very broad range of specificity (proteinase K) to one that was more specific (trypsin).
Elasticity and image data were recorded for a number of single isolated chromosomes treated with these nonspecific proteases, trypsin (15 experiments) and proteinase K (9 experiments). For both enzymes, a gradual swelling of the chromosome was observed (Figure 2, a and b). This morphological change is apparent as reduced phase contrast in the image data and represents a gradual unfolding of the chromosome structure. Both the length and width of the chromosome gradually increase to a point where the chromosome becomes greatly reduced in phase contrast.
Figure 2.
(a) Phase contrast images following the morphological changes of a chromosome exposed to a 100 nM trypsin spray (bar, 5 μm). (b) Phase contrast images of a chromosome exposed to successive 500 nM proteinase K sprays (bar, 5 μm). Swelling of the chromosome in both a and b is evident. (c) Force-extension data corresponding to the chromosome shown in a. A gradual decrease in elasticity is observed for successive trypsin exposures. (d) Force-extension data corresponding to the data shown in b. Again, a gradual decrease in elasticity is observed. All stretch–relax cycles are at a rate of 0.08 μm/s.
Even with prolonged treatment, with spray times of >20 min, it was found that these enzymes were unable to completely sever the chromosome. Figure 3 shows a time course of images, acquired during the exposure of a chromosome to 500 nM proteinase K. After an exposure time of 22 min, the chromosome is almost invisible in the phase contrast image. However, the enhanced image shows that the chromosome remains connected, with the same overall shape as the native chromosome.
Figure 3.
Phase contrast images of an extensive proteinase K treatment. After more than 20 min, the chromosome seems to have disappeared (1320-s data). Enhancing the contrast of this image data reveals that the chromosome is still connected.
Chromosomes Remain Elastic during Protease Treatment, but with a Progressively Reduced Force Constant
Figure 2, c and d, shows elasticity data for both trypsin- and proteinase K-digested mitotic chromosomes. In both cases, a gradual decrease in the elastic force constant of the chromosome is observed as digestion proceeds. Even after extensive protease digestion, the chromosomes retain a reversible elastic response upon successive stretch–relax cycles. This result will be crucial for the experiments outlined below, where we will use elastic response to read out effects of other enzymes after protease treatment.
No experiment with protease ever led to cutting of a chromosome. Furthermore, it was observed that during stretching of extensively digested chromosomes to lengths >20 times their native length, the two micropipettes always remained connected.
Proteolysis Induces Anisotropic Unfolding of Chromosomes, with Proportionally More Lengthening than Widening
In most of the protease digestion experiments, protease-treated chromosomes increased more in length than in width, relative to their native morphology. Measurements of chromosome lengths and widths were made from images collected at various points during the digestion experiments. Widths were taken from the half-maxima of amplitudes of cross-sectional profiles taken through the chromosome images. Through phase-contrast imaging, we are sensitive to the total nonbuffer mass distribution. Alternatively, one might attempt to use fluorescent dyes to visualize the chromosome; however, in these experiments such an approach would be susceptible to systematic errors due to the modifications of DNA physical properties generated by DNA dyes. In preliminary experiments, we have found that modifications of native mitotic chromosome mechanical properties are generated by the dyes ethidium bromide, SYTO, Hoechst 33258, and 4,6-diamidino-2-phenylindole (DAPI) (Poirier and Kawamura, unpublished data). Given that fluorescent DNA dyes can modify mechanical properties of whole chromosomes, it is possible that they will affect the morphology of protease-digested chromosomes, and enzyme accessibility and activity in chromatin. We therefore elected to determine chromosome morphologies using phase contrast imaging.
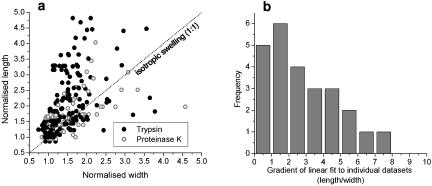
The measured length and width measurements were divided by the native (initial) chromosome lengths and widths, to obtain normalized values. This analysis revealed that swelling due to protease treatment is usually highly anisotropic (Figure 4a). For the majority of chromosomes studied, the increase in length relative to the initial (native) length was greater than the width increase relative to the initial width (Figure 4a, above dashed line). Interestingly, for a minority of the chromosomes studied, width was observed to increase more than length (Figure 4a, below dashed line).
Figure 4.
(a) Plot of width normalized to native (initial) width, versus length normalized to native length, for progressive trypsin and proteinase K treatments (15 trypsin experiments and 9 proteinase K experiments). The dotted line indicates behavior expected for isotropic chromosome swelling. (b) Histogram of anisotropies observed in each protein digestion experiment. The distribution is broad with a peak between 1 and 2 but with a slow decay for large length/width ratios. The average anisotropy observed was length/width = 3. The number of experiments where lengthening exceeded widening was three times that where widening exceeded lengthening.
The anisotropy (length-to-width ratio) for each digestion experiment can be described by a single anisotropy value, via the slope of the normalized length versus normalized width. Figure 4b shows the distribution of slopes obtained in our experiments. Experiments where normalized length increased more than normalized width change dominated (20 experiments with length/width larger than 1 vs. 5 with length/width less than 1). Note that although the peak (most frequently observed) anisotropy is between 1 and 2, the distribution is very broad, with an average anisotropy of ∼3.
Trypsinization Induces a Detectable Effect of Subsequent Digestion by the Infrequently Cutting Restriction Enzyme HincII
In previous studies, it was found that chromosome digestion by blunt-cutting restriction enzymes (REs) having recognition sites of five- and six-base lengths had no observable effect on chromosome elasticity (Poirier and Marko, 2002). We were therefore interested in investigating whether chromosomes pretreated with protease would show a different response to these restriction enzymes. Trypsin rather than proteinase K was used for these combined protease–RE experiments because this enzyme has fewer cleavage targets and therefore was a better candidate for inducing controllable proteolysis.
For combined trypsin–nuclease experiments, we needed to ascertain that trypsin activity ceased after the end of our pipette spray. Although this was expected on the basis of the subpicoliter spray volumes from the small pipette tip, we wished to rule out the possibility that trypsin might somehow remain associated with the chromosome for a long time after the spray. Thus, we carried out control experiments as shown in Figure 5. First, a native state measurement was made (top curve). Then, after a 90-s trypsin spray, we carried out one chromosome elasticity measurement immediately, and then a second measurement 140 s later (bottom curves). The two posttrypsin force–extension curves are nearly identical, showing that trypsin activity ceases when spraying stops. This particular experiment was carried out three times with the same result, and we have observed on many other occasions that successive force curves measured after the end of trypsin spraying coincide. This result is important to the following experiments because it ensures that changes in chromosome elasticity after the end of trypsin spraying are not due to remnant trypsin on or near the chromosome. Also, Figure 5 emphasizes the reversible and stable elastic response of a trypsinized chromosome.
Figure 5.
Trypsin activity ceases when trypsin spray stops. Upper curve (black) shows initial, native chromosome response before trypsin treatment. Lower curves (gray) show two measurements after the end of one 90-s 100 nM trypsin exposure; one was made immediately after the trypsin spray, whereas the other was made 140 s later. No change in the force-extension curve occurs over the time period after the trypsin treatment, indicating that trypsin activity ceases when the trypsin spray ceases. Also, this experiment emphasizes that the trypsinized chromosome is stable against repeated extension–relaxation cycles.
Figure 6, a–c, shows images, elasticity, and force-constant data from a combined trypsin–RE experiment, where first trypsin, and then the five-base cutter HincII were used. The force-extension data (Figure 6b) and the force constants derived from linear fits to the elasticity data (Figure 6c) show that trypsin digestion results in an initial reduction in the chromosome spring constant (∼40% of the starting value) as expected from the results reported above. After this initial proteolysis step, subsequent digestion by HincII (5-base-specific RE) reduces the spring constant still further (final value is ∼17% of the starting value). Thus, trypsin treatment induces an effect of HincII on chromosome elasticity.
Figure 6.
(a) Morphological changes are shown after 180 s of exposure of the chromosome to trypsin followed by a 360-s exposure of the chromosome to HincII. (b) Force versus chromosome length data for the combined trypsin-HincII digestion experiment shown in a at various points in the digestion. (c) Chromosome elasticity (taken from the gradient of the curves shown in b) is plotted as a function of cumulative exposure time to trypsin followed by HincII. Error bars indicate the range of spring constants measured in a series of at least three successive measurements, indicating the stability of the chromosome between each enzyme exposure step in the experiment. (d) In a control experiment the native chromosome is exposed first to HincII with no reduction in elasticity (see second data point at 120 s). After trypsin treatment the chromosome elasticity becomes sensitive to HincII digestion. (e) A plot of elasticity versus spray time for a chromosome digested by trypsin followed by StuI. (f) A control experiment shows no effect of StuI on the native chromosome (see second data point at 60 s). Elasticity versus cumulative spray time data are shown for the digestion of a chromosome by StuI, followed by trypsin, followed by StuI.
The morphological changes shown in Figure 6a demonstrate that the initial exposure to trypsin results in lengthening and widening of the chromosome. However, during the subsequent HincII digestion, further increase in width is no longer evident, but lengthening of the chromosome is observed (evident in the images of Figure 6a and in the extensions where the force-extension data of Figure 6b extrapolate to zero force). A second feature of the HincII digestion data is that unlike for the four-base-specificity REs, HincII gives rise to a gradual reduction in elastic constant, with the chromosome retaining a reversible elastic response after prolonged HincII exposure. We note that throughout these experiments, the measured chromosome spring constants (e.g., Figure 6c) are stable after each enzyme spray; the error bars in the spring constant versus time plots of Figure 6 are derived from a series of at least three successive force-extension measurements made after each spray. The narrow spread of these measurements reflects the fact that in between enzyme sprays, chromosome spring constants are stable, in accord with the data of Figure 5.
Combined trypsin–HincII experiments of this type were carried out on two different chromosomes. The effects in both experiments were the same as described above. This experiment also was carried out with the RE HindII, which has exactly the same target cleavage site as HincII, with the same results.
Initial HincII Digestion of the Native Chromosome Does Not Affect Its Elasticity
In a separate control experiment, chromosomes were treated with HincII first (Figure 6d, second data point, spray time = 120 s). In agreement with previously published results (Poirier and Marko, 2002), no reduction in chromosome elastic constant was observed. On the contrary, after HincII exposure, a slight increase in force constant was observed, most likely due to the binding of divalent cations present within the spray buffer. After this HincII treatment, trypsin generated the expected reduction in force constant (Figure 6d, third data point, spray time = 150 s). The first part of Figure 6d amounts to a control for experiments where trypsin was used first. We then continued with further spraying with further HincII and observed a further decrease in force constant (Figure 6d, fourth-seventh data points, spray time = 300-1200 s). This type of experiment was carried out on two different chromosomes with the same results.
Trypsin Induces Reduction in Elastic Constant by the Six-Base Cutter StuI
The same types of experiments were also carried out with StuI, a restriction enzyme having a rare, six-base recognition sequence. This enzyme was previously reported to have no detectable effect on native chromosome elasticity (Poirier and Marko, 2002). As for HincII, we found that after treatment of the chromosome with trypsin, subsequent digestion with StuI could be detected as a reduction in force constant (Figure 6e). This effect was observed for two different chromosomes. Digestion experiments in the reverse order show that StuI does not induce a reduction in elastic modulus of native chromosomes (Figure 6f, second data point at spray time = 60 s), in accord with previously published data (Poirier and Marko, 2002). Only after trypsinization was a reduction in spring constant observed (Figure 6f, fourth-sixth data points for spray times = 190–430 s). This type of experiment was carried out on two different chromosomes with the same results. Thus, experiments with StuI showed the same qualitative results as those with HincII.
DISCUSSION
We briefly summarize the main results. Single mitotic chromosomes, from newt TVI cells, when treated with protein-digesting enzymes, undergo a gradual decondensation without the elimination of reversible elasticity. This basic effect was observed for both trypsin and proteinase K and is qualitatively in agreement with experiments on in vitro-assembled Xenopus chromatids (Almagro et al., 2004). The decondensation of the chromosome was gradual with increasing exposure time to protease. Although proteinase K has a broader range in cleavage sites, the effects of trypsin and proteinase K were found to be qualitatively the same. In particular, both enzymes led to decondensation that was usually very anisotropic, with length expanding faster than width (normalized by the initial length and width) as digestion proceeded. We note that we have verified that after microspraying with protease, the chromosome displays reversible elastic response with no change of spring constant on the few-minute time scales relevant to our experiments (Figure 5).
In previous experiments using frequently cutting nucleases (Poirier and Marko, 2002), reversible elasticity was almost immediately lost, and sufficient digestion dissolved and severed chromosomes. By contrast, prolonged protein digestion never completely severed the newt chromosomes, even when the chromosome volume expanded by more than a factor of 10. Both morphological and mechanical changes observed indicate that both proteases have a sufficient number of cleavage sites to disrupt protein interactions responsible for much of the compaction of chromatin in the mitotic chromosome.
Experiments using infrequently cutting REs revealed that pretreatment of the chromosome with trypsin could induce an effect with REs that had no observable effect on native chromosomes. A reduction in chromatin elasticity was observed upon exposure of trypsin-treated chromosomes to the five-base-specific REs HincII and HindII and the six-base-specific enzyme StuI. Although changing the elastic response, these REs had little effect on chromosome morphology in that no further swelling was observed.
Relationship of Our Results to Previous Studies
Our proteolysis results are consistent with two previous studies. (Maniotis et al., 1997) carried out trypsin and proteinase K digestions on whole metaphase genomes extracted with microneedles from HeLa cells. They observed that the mitotic chromosomes decondensed but remained connected, in accord with our observations. Almagro et al. (2004) carried out experiments similar to those reported here, on in vitro reconstituted Xenopus chromatids, using proteases and carrying out stretching force measurements. In those experiments proteolysis induced decondensation and an associated reduction in force constant, again qualitatively consistent with the effects we observe. Almagro et al. (2004) argue that SMCs are the primary targets for proteolysis, that SMCs are the primary cross-linking elements, and that the results of their micromechanical experiments are in accord with the distribution of SMCs along the central axis of the chromatids observed in immunofluorescence studies (Ono et al., 2003; Kireeva et al., 2004).
Some comments regarding the relation of the unreplicated, embryonic chromatids assembled by the in vitro Xenopus high-speed extract system, to chromosomes from cells, are in order. We note that the reconstituted chromatids are far more floppy than chromosomes from somatic cells (Poirier et al., 2002a) and contain low levels of condensin II. In addition, it has been very recently reported that linker histone plays an important role in establishing proper mitotic chromosome condensation and that the unreplicated chromatids assembled using Xenopus high-speed extracts are deficient in linker histone (Maresca et al., 2005). These differences suggest a biochemical basis for the observations that chromatids assembled using high-speed extracts differ in structure and physical properties from normal somatic chromosomes. Nevertheless, our current results establish that the effects of proteases on micromechanical properties of metaphase chromosomes from cells are qualitatively similar to those in the Xenopus in vitro system. We note that the anisotropy and nuclease-susceptibility after proteolysis have not been measured for embryonic Xenopus egg-extract chromosomes.
Which Proteins Are Likely Candidate Digestion Targets in These Experiments?
An important consideration for further discussion of our experiments is the question of which chromosomal proteins are likely to be digested by these proteases. Previous experiments have established that the biochemical preparation of tail-less oligosomes can be achieved by the incubation of 0.5 mg/ml oligosomes in 50 mM Tris-HCl, pH 8.0, 50 mM NaCl, and 1 mM EDTA with 0.02 mg/ml trypsin for 3 min at 37°C (de la Barre et al., 2000). This amount of trypsinization leaves the core histone proteins intact but does digest away the histone tails. In comparison, our experiments use a 0.0024 mg/ml (100 nM) trypsin spray at room temperature, notably approximately eightfold less in concentration. The cumulative spray time used in the proteolysis experiment shown in Figure 2a was 390 s and spray times in the combined trypsin-restriction enzyme experiments are typically <60 s.
At the concentrations and exposure times used in our digestion experiments its is likely that the core histone proteins remain well protected by the DNA that wraps around them and that other less well-protected proteins will be digested. The likely digestion targets are exposed regions of the condensing SMCs (Almagro et al., 2004), topoisomerase II, linker histones, HMG proteins as well as the core histone tails.
Protease Induction of Effects of Rare-cutting Restriction Enzymes Are Consistent with a Cross-linked Mitotic Chromosome
Our previous study of nucleases (Poirier and Marko, 2002) showed that the non-DNA content of the newt mitotic chromosome was not connected together. This conclusion followed from experiments that showed that the sequence-nonspecific micrococcal nuclease, and four-base-specificity nucleases both were able to first reduce the chromosome force constant, and then completely sever the chromosome. Subsequent experiments (Almagro et al., 2004) have verified this for the Xenopus in vitro reconstituted chromatid system. Thus, the interior of the mitotic chromosome can be thought of as a “network” of chromatin, with intermittent non-DNA cross-links constraining the mass of chromatin fibers into the condensed chromatid (Figure 7a). It should be noted that the cross-links need not be uniformly distributed through the chromatid (Poirier and Marko, 2002), e.g., there may well be a greater density of cross-links along central chromatid axis regions than in the outer “halo” region, as suggested by a number of lines of work (Poirier and Marko, 2002; Ono et al., 2003; Almagro et al., 2004; Kireeva et al., 2004).
Figure 7.
Cross-linked chromatin fiber model for mitotic chromosome structure. (a) Nuclease experiments indicate that the non-DNA component of the mitotic chromosome must be disconnected, i.e., chromatin fibers (black) are cross-linked by non-DNA elements (gray ovals). (b) Proteolysis (gray arrow) must either digest proteins along chromatin fibers (top sketch) or eliminate cross-links (bottom sketch). In either case, after proteolysis, more restriction enzyme targets per remaining interconnect are accessible. If a sufficient number of targets are available for restriction enzyme digestion, a significant reduction in chromosome elasticity will occur after restriction enzyme digestion. (c) Hierarchically folded chromosome, showing the long prophase chromosome (top left) folded or coiled (bottom middle) by the action of cross-links (black bars). The interior of the long prophase chromosome can be considered to be a network of the form shown in a. Proteolysis will break the high-level coil-stabilizing cross-links, leading to strongly anisotropic unfolding, whereas simultaneous cleavage of proteins inside the chromosome leads to swelling. A varying amount of these two effects can lead to a range of observed anisotropies.
A very rough estimate of the intercross-link sequence distance can be made from the fact that five-base-specificity REs, which cut bare random-sequence DNA once per 1024 nucleotides, do not affect the native chromosome. Given the inaccessibility of ∼90% of the DNA in chromatin (the wrapped DNA) to restriction enzymes (Polach and Widom, 1995, 1999), we estimate that the cross-links are spaced by ∼15 kb (Poirier and Marko, 2002). If the intercross-link spacing was much larger than this, the five-base cutters would cleave a significant fraction of the network connections and reduce the chromosome force constant, or sever the chromosome.
The “network” model provides a simple interpretation of our combined protease-nuclease experiments. The initial protease treatment can be expected to have two effects. One effect is digestion of nucleosome tails and other exposed proteins along the intercross-linked chromatin, which will likely expose more RE targets (the important point here is that this will certainly not decrease the number of accessible targets). The other effect is breakage of some of the cross-link points. Both of these effects will increase the average number of accessible RE targets between the remaining cross-link points (Figure 7b). Therefore, the cross-linked-chromatin fiber model can be expected to display the protease-induced sensitivity to rarer-cutting REs that we have observed.
An interesting question concerns experiments where REs were used before proteases, for example, with HincII (Figure 6d). Given the accessibility of four-base RE targets in the native chromosome (Poirier and Marko, 2002), it seems likely that some HincII cuts are made during the first spray (second data point at spray time = 120 s; Figure 6d). It is possible that these cuts are “invisible” to our elastic assay, due to the high cross-linking density of the native chromosome. If this is the case, subsequent trypsin sprays (third data point at spray time = 150 s; Figure 6d) may “reveal” the effects of these initial cuts, and possibly only a few additional HincII targets would be available for subsequent digestion. At present, our experiments are not precise enough to solidly settle this question.
Anisotropic Protease-induced Decondensation of the Mitotic Chromosome Suggests That It Is Anisotropically Folded
In most (75%) of our protease digestion experiments, the relative length change exceeded the relative width change. This generally anisotropic unfolding triggered by protein digestion (Figure 4) must have its origin in anisotropy of the folding scheme itself. There exists evidence for an overall high-level coiling or folding that transforms the long, thin prophase chromosome (Figure 7c, top left) into a shorter and slightly wider late prophase/prometaphase chromosome (Figure 7c, bottom middle) (Kireeva et al., 2004; Kleckner et al., 2004). This high-level coiling suggests an explanation for the anisotropy that we observe during our proteolysis experiments, as follows.
The high-level folding or coiling could be accomplished by a relatively small number of cross-link elements added to the long prophase chromosome, e.g., essentially on its surface (Figure 7c, bottom middle, black bars). The cross-links stabilizing the highest level of folding are, following the observations of Kireeva et al. (2004), added to the prophase chromosome after its condensation via a denser set of lower level cross-links. Given this, the decondensation observed in our experiments may be due to a combination of anisotropic high-level unfolding, accomplishing largely chromatid lengthening, with the more isotropic swelling of the lower level chromatin network inside the long prophase fiber (Figure 7c, top right). In our proteolysis experiments, these two contributions to decondensation occur simultaneously, although possibly at rather different rates. The variation we observe in the anisotropy of proteolysis-induced decondensation could be simply a result of differing densities or accessibilities of cross-links stabilizing the high-level folding, possibly dependent on the precise point in the mitotic cycle when the chromosomes were extracted from the cell.
In conclusion, our observations support the view that the mitotic chromosome is a dense network of chromatin fibers held together by protein-based cross-linking elements. The strong and generally anisotropic decondensation in response to protein digestion suggests that there are at least a subset of the protein cross-linkers that are responsible for maintaining anisotropic folding or coiling of the chromosome. The general decondensation in response to nonspecific proteolysis of mitotic chromosomes by trypsin and proteinase K, plus the result that chromosomes become sensitive to rare-cutting REs after protein digestion, are consistent with proteolysis leading to a coarsening of these putative cross-links.
A number of previous studies indicate that condensin SMCs play a major role in establishing and maintaining mitotic chromosome structure (Hirano and Mitchison, 1994; Hudson et al., 2003; Ono et al., 2003, 2004; Almagro et al., 2004; Hirota et al., 2004; Kireeva et al., 2004); however, we note that conditional knockout data of Hudson et al. (2003) indicates that mitotic condensation proceeds surprisingly well in the absence of SMC2 condensin subunits, although the resulting chromosomes are less stable than native chromosomes. The question of whether SMCs are the predominant cross-linker proteins of the mitotic chromosome is therefore as yet unresolved. An experiment where SMCs are specifically lysed, preferably at known sequence positions, is needed to answer this question.
Acknowledgments
This research was supported by National Science Foundation Grants MCB-0240998 and DMR-0203963.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05–04–0321) on October 12, 2005.
Abbreviations used: DTT, dithiothreitol; PBS, phosphate-buffered saline; RE, restriction enzyme; SMC, structural maintenance of chromosomes protein.
References
- Almagro, S., Riveline, D., Hirano, T., Houchmandzadeh, B., and Dimitrov, S. (2004). The mitotic chromosome is an assembly of rigid elastic axes organized by structural maintenance of chromosomes (SMC) proteins and surrounded by a soft chromatin envelope. J. Biol. Chem. 279, 5118–5126. [DOI] [PubMed] [Google Scholar]
- Bystricky, K., Heun, P., Gehlen, L., Langowski, J., and Gasser, S. M. (2004). Long-range compaction and flexibility of interphase chromatin in budding yeast analyzed by high-resolution imaging techniques. Proc. Natl. Acad. Sci. USA 101, 16495–16500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callan, H. G., and Macgregor, H. C. (1958). Action of deoxyribonuclease on lampbrush chromosomes. Nature 181, 1479–1480. [DOI] [PubMed] [Google Scholar]
- de la Barre, A. E., Gerson, V., Gout, S., Creaven, M., Allis, C. D., and Dimitrov, S. (2000). Core histone N-termini play an essential role in mitotic chromosome condensation. EMBO J. 19, 379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw, W. C., Halligan, B., Cooke, C. A., Heck, M. M., and Liu, L. F. (1985). Topoisomerase II is a structural component of mitotic chromosome scaffolds. J. Cell Biol. 100, 1706–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw, W. C., and Heck, M. M. (1985). Localization of topoisomerase II in mitotic chromosomes. J. Cell Biol. 100, 1716–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw, W. C., and Laemmli, U. K. (1983). Architecture of metaphase chromosomes and chromosome scaffolds. J. Cell Biol. 96, 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw, W. C., and Laemmli, U. K. (1984). Silver staining the chromosome scaffold. Chromosoma 89, 186–192. [DOI] [PubMed] [Google Scholar]
- Gall, J. G. (1963). Kinetics of deoxyribonuclease action on chromosomes. Nature 198, 36–38. [DOI] [PubMed] [Google Scholar]
- Gould, D. C., Callan, H. G., and Thomas, C. A., Jr. (1976). The actions of restriction endonucleases on lampbrush chromosomes. J. Cell Sci. 21, 303–313. [DOI] [PubMed] [Google Scholar]
- Hartley, S. E., and Callan, H. G. (1978). RNA transcription on the giant lateral loops of the lampbrush chromosomes of the American newt Notophthalmus viridescens. J. Cell Sci. 34, 279–288. [DOI] [PubMed] [Google Scholar]
- Hirano, T. (1995). Biochemical and genetic dissection of mitotic chromosome condensation. Trends Biochem. Sci. 20, 357–361. [DOI] [PubMed] [Google Scholar]
- Hirano, T. (2005). SMC proteins and chromosome mechanics: from bacteria to humans. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 360, 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano, T., and Mitchison, T. J. (1993). Topoisomerase II does not play a scaffolding role in the organization of mitotic chromosomes assembled in Xenopus egg extracts. J. Cell Biol. 120, 601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano, T., and Mitchison, T. J. (1994). A heterodimeric coiled-coil protein required for mitotic chromosome condensation in vitro. Cell 79, 449–458. [DOI] [PubMed] [Google Scholar]
- Hirota, T., Gerlich, D., Koch, B., Ellenberg, J., and Peters, J. M. (2004). Distinct functions of condensin I and II in mitotic chromosome assembly. J. Cell Sci. 117, 6435–6445. [DOI] [PubMed] [Google Scholar]
- Houchmandzadeh, B., and Dimitrov, S. (1999). Elasticity measurements show the existence of thin rigid cores inside mitotic chromosomes. J. Cell Biol. 145, 215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houchmandzadeh, B., Marko, J. F., Chatenay, D., and Libchaber, A. (1997). Elasticity and structure of eukaryote chromosomes studied by micromanipulation and micropipette aspiration. J. Cell Biol. 139, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson, D. F., Vagnarelli, P., Gassmann, R., and Earnshaw, W. C. (2003). Condensin is required for nonhistone protein assembly and structural integrity of vertebrate mitotic chromosomes. Dev. Cell 5, 323–336. [DOI] [PubMed] [Google Scholar]
- Kireeva, N., Lakonishok, M., Kireev, I., Hirano, T., and Belmont, A. S. (2004). Visualization of early chromosome condensation: a hierarchical folding, axial glue model of chromosome structure. J. Cell Biol. 166, 775–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner, N., Zickler, D., Jones, G. H., Dekker, J., Padmore, R., Henle, J., and Hutchinson, J. (2004). A mechanical basis for chromosome function. Proc. Natl. Acad. Sci. USA 101, 12592–12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli, U. K., Cheng, S. M., Adolph, K. W., Paulson, J. R., Brown, J. A., and Baumbach, W. R. (1978). Metaphase chromosome structure: the role of nonhistone proteins. Cold Spring Harb. Symp. Quant. Biol. 42, 351–360. [DOI] [PubMed] [Google Scholar]
- Macgregor, H. C., and Callan, H. G. (1962). The actions of enzymes on lampbrush chromosomes. Q. J. Microscop. Sci. 103, 173–203. [Google Scholar]
- Maniotis, A. J., Bojanowski, K., and Ingber, D. E. (1997). Mechanical continuity and reversible chromosome disassembly within intact genomes removed from living cells. J. Cell Biochem. 65, 114–130. [PubMed] [Google Scholar]
- Maresca, T. J., Freedman, B. S., and Heald, R. (2005). Histone H1 is essential for mitotic chromosome architecture and segregation in Xenopus laevis egg extracts. J. Cell Biol. 169, 859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden, M. P., and Laemmli, U. K. (1979). Metaphase chromosome structure: evidence for a radial loop model. Cell 17, 849–858. [DOI] [PubMed] [Google Scholar]
- Marshall, W. F., Straight, A., Marko, J. F., Swedlow, J., Dernburg, A., Belmont, A., Murray, A. W., Agard, D. A., and Sedat, J. W. (1997). Interphase chromosomes undergo constrained diffusional motion in living cells. Curr. Biol. 7, 930–939. [DOI] [PubMed] [Google Scholar]
- Ono, T., Fang, Y., Spector, D. L., and Hirano, T. (2004). Spatial and temporal regulation of Condensins I and II in mitotic chromosome assembly in human cells. Mol. Biol. Cell 15, 3296–3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono, T., Losada, A., Hirano, M., Myers, M. P., Neuwald, A. F., and Hirano, T. (2003). Differential contributions of condensin I and condensin II to mitotic chromosome architecture in vertebrate cells. Cell 115, 109–121. [DOI] [PubMed] [Google Scholar]
- Paulson, J. R., and Laemmli, U. K. (1977). The structure of histone-depleted metaphase chromosomes. Cell 12, 817–828. [DOI] [PubMed] [Google Scholar]
- Poirier, M., Eroglu, S., Chatenay, D., and Marko, J. F. (2000). Reversible and irreversible unfolding of mitotic newt chromosomes by applied force. Mol. Biol. Cell 11, 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier, M. G., Eroglu, S., and Marko, J. F. (2002a). The bending rigidity of mitotic chromosomes. Mol. Biol. Cell 13, 2170–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier, M. G., and Marko, J. F. (2002). Mitotic chromosomes are chromatin networks without a mechanically contiguous protein scaffold. Proc. Natl. Acad. Sci. USA 99, 15393–15397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier, M. G., and Marko, J. F. (2003). Micromechanical studies of mitotic chromosomes. Curr. Top Dev. Biol. 55, 75–141. [DOI] [PubMed] [Google Scholar]
- Poirier, M. G., Monhait, T., and Marko, J. F. (2002b). Reversible hypercondensation and decondensation of mitotic chromosomes studied using combined chemical-micromechanical techniques. J. Cell. Biochem. 85, 422–434. [DOI] [PubMed] [Google Scholar]
- Polach, K. J., and Widom, J. (1995). Mechanism of protein access to specific DNA sequences in chromatin: a dynamic equilibrium model for gene regulation. J. Mol. Biol. 254, 130–149. [DOI] [PubMed] [Google Scholar]
- Polach, K. J., and Widom, J. (1999). Restriction enzymes as probes of nucleosome stability and dynamics. Methods Enzymol. 304, 278–298. [DOI] [PubMed] [Google Scholar]
- Reese, D. H., Yamada, T., and Moret, R. (1976). An established cell line from the newt Notophthalmus viridescens. Differentiation 6, 75–81. [DOI] [PubMed] [Google Scholar]
- Swedlow, J. R., and Hirano, T. (2003). The making of the mitotic chromosome: modern insights into classical questions. Mol. Cell 11, 557–569. [DOI] [PubMed] [Google Scholar]
- Trask, B. J., Allen, S., Massa, H., Fertitta, A., Sachs, R., van den Engh, G., and Wu, M. (1993). Studies of metaphase and interphase chromosomes using fluorescence in situ hybridization. Cold Spring Harb. Symp. Quant. Biol. 58, 767–775. [DOI] [PubMed] [Google Scholar]