Abstract
KCa3.1 is an intermediate conductance Ca2+-activated K+ channel that is expressed predominantly in hematopoietic cells, smooth muscle cells, and epithelia where it functions to regulate membrane potential, Ca2+ influx, cell volume, and chloride secretion. We recently found that the KCa3.1 channel also specifically requires phosphatidylinositol-3 phosphate [PI(3)P] for channel activity and is inhibited by myotubularin-related protein 6 (MTMR6), a PI(3)P phosphatase. We now show that PI(3)P indirectly activates KCa3.1. Unlike KCa3.1 channels, the related KCa2.1, KCa2.2, or KCa2.3 channels do not require PI(3)P for activity, suggesting that the KCa3.1 channel has evolved a unique means of regulation that is critical for its biological function. By making chimeric channels between KCa3.1 and KCa2.3, we identified a stretch of 14 amino acids in the carboxy-terminal calmodulin binding domain of KCa3.1 that is sufficient to confer regulation of KCa2.3 by PI(3)P. However, mutation of a single potential phosphorylation site in these 14 amino acids did not affect channel activity. These data together suggest that PI(3)P and these 14 amino acids regulate KCa3.1 channel activity by recruiting an as yet to be defined regulatory subunit that is required for Ca2+ gating of KCa3.1.
INTRODUCTION
KCa3.1 subunits (also known as IKCa and KCNN4) are components of an intermediate conductance Ca2+-activated K+ channel that is found predominantly in peripheral tissues such as blood cells, epithelia, and smooth muscle cells where it functions to couple alterations in cytosolic Ca2+ to K+ flux (Bond et al., 1999; Jensen et al., 2001; Wulff et al., 2003). KCa3.1 channels thereby play an important physiological role to set the membrane potential at negative values close to the K+ equilibrium potential (Bond et al., 1999; Jensen et al., 2001; Wulff et al., 2003; Stocker, 2004). This effect on membrane potential can have diverse physiological responses in a variety of cell types, including water movement and volume regulation in red blood cells, mitogen activation of T-lymphocytes, Cl– secretion of exocrine epithelial cells, and control of proliferation by a variety of cells such as T- and B-lymphocytes, vascular smooth muscle cells, keratinocytes, and some cancer cell lines (Khanna et al., 1999; Ghanshani et al., 2000; Fanger et al., 2001; Koegel and Alzheimer, 2001; Kohler et al., 2003; Maher and Kuchel, 2003; Ouadid-Ahidouch et al., 2004; Wulff et al., 2004). Based on these activities, pharmacological modulation of KCa3.1 channels has been proposed to treat proliferative diseases such as restenosis after angioplasty and cancer, transplant rejection as well as secretory diarrheas, sickle cell anemia, and cystic fibrosis (Rufo et al., 1997; Jensen et al., 2001; Maher and Kuchel, 2003; Wulff et al., 2003).
Over the past several years, the mechanism of activation of KCa3.1 and the three related small conductance Ca2+-activated K+ channels, KCa2.1, KCa2.2, and KCa2.3 channels (also known as SK1, SK2, and SK3), has been determined. These channels are all structurally similar and possess six transmembrane domains with cytosolic N and C termini. Functional channels, however, are heteromeric complexes with constitutively bound calmodulin (CaM), and channel opening occurs only after Ca2+ binds to CaM. The C termini of all of the Ca2+-activated K+ channels contain a proximal 100-amino acid calmodulin binding domain (CBD) (Xia et al., 1998; Keen et al., 1999; Maylie et al., 2004). The model proposed for Ca2+-dependent gating is that when Ca2+ binds to calmodulin, cross-linking of two adjacent C-terminal tails occurs, which results in channel opening (Schumacher et al., 2001; Schumacher and Adelman, 2002; Maylie et al., 2004).
We recently found that, in addition to requiring Ca2+ for activation, the KCa3.1 channel uniquely also depends on the lipid phosphatidylinositol-3 phosphate [PI(3)P] for activation (Srivastava et al., 2005). Interestingly, the PI(3)P dependence does not seem to exist for the related KCa2.2 channel. Our initial insight that KCa3.1 channels are regulated by PI(3)P followed the finding that the CT coil-coil (CC) domain of KCa3.1 bound the CC domain in myotubularin-related protein 6 (MTMR6), a PI(3)P-specific phosphate (Laporte et al., 2003; Taylor and Dixon, 2003; Srivastava et al., 2005). These studies demonstrated that KCa3.1 channel activity was inhibited >90% in cells overexpressing MTMR6 or in cells treated with the PI3 kinase inhibitors wortmannin and 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). Moreover, the finding that addition of PI(3)P, but not other phosphoinositides, to the pipette solution restored KCa3.1 channel activity indicated that channel inhibition by both MTMR6 and wortmannin is mediated via decreased PI(3)P levels.
Fourteen different MTMs have been identified in mammalian cells (Laporte et al., 2003; Taylor and Dixon, 2003). The founding member of this family of phosphatases, MTM1, was identified as the gene mutated in a congenital muscular dystrophy. MTMR2 and MTMR13 were identified as genes mutated in a subset of patients with Charcot Marie Tooth Syndrome type 4B (Laporte et al., 1996; Bolino et al., 2000). The finding that MTMR6 inhibits KCa3.1 suggests that MTMR6 plays an important and unexpected role in negatively regulating biological responses mediated by KCa3.1 by dephosphorylating PI(3)P in the membrane adjacent to KCa3.1. It is unlikely that the congenital diseases that result from loss of MTM1 or MTMR2 are due to abnormal regulation of KCa3.1 because inhibition of KCa3.1 is specific to MTMR6; neither MTM1 nor MTMR2 inhibits KCa3.1 (Srivastava et al., 2005). In this study, we sought to determine the mechanism whereby PI(3)P regulates KCa3.1.
MATERIALS AND METHODS
Constructs
CHO-KCa3.1 cells are a stable cell line that overexpressed KCa3.1 and have been described previously (Srivastava et al., 2005). Human KCa3.1 and human KCa2.3 were amplified by PCR and cloned in frame with green fluorescent protein (GFP) into the vector pEGFP (BD Biosciences Clontech, Palo Alto, CA). KCa3.1-KCa2.3 chimeric channels were made by overlapping PCR and cloned into pEGFP. The following chimeric channels were generated: KCa3.1-KCa2.3/CT: AA 1–273 KCa3.1, AA 536–736 KCa2.3; KCa2.3-KCa3.1/CT: AA 1–535 KCa2.3, AA 274–427 KCa3.1; KCa2.3-KCa3.1/CC, AA 1–642 KCa2.3, AA 376–427 KCa3.1; KCa2.3-KCa3.1/355–427; AA 1–616 KCa2.3, AA 355–427 KCa3.1; and KCa2.3-KCa3.1/369–427: AA 1–631 KCa2.3, AA 369–427 KCa3.1. Accession numbers for the various constructs are: human KCa3.1, NP_002241; rat SK2, NP_062187; and human KCa2.3, NP_00240.
Cell Culture, Transfection, and Patch Clamping
The GFP-tagged constructs were transfected into Chinese hamster ovary (CHO) cells using FuGENE (Roche Diagnostics, Indianapolis, IN) and whole cell-patch clamping was performed 48 h after transfection on GFP-positive cells as described previously (Srivastava et al., 2005). For electro-physiological studies, CHO cells transfected with GFP-tagged constructs were plated on 18-mm coverslips at the time of transfection. After 48 h, a coverslip was positioned in a recording chamber, and patch clamping was performed at room temperature using a pipette solution containing 100 mM K+-gluconate, 30 mM KCl, 10 mM HEPES, 1.15 mM MgCl2, 5 mM EGTA, and 4.27 mM CaCl2, pH 7.2, with 1 N KOH (calculated free Ca2+, 1 μM) and a bath solution containing 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, and 10 mM glucose. Patch-clamp pipettes had resistances ranging between 2.2 and 3.5 MΩ. Current-voltage (I-V) relationships were measured using ramp voltage-clamp protocols (at 15-s intervals) from a holding potential of–70 to–120 mV, followed by ramp depolarization to +60 mV (symmetrical ramp rate of 0.18 mV ms–1). The current-voltage relationship was obtained by plotting the current during the depolarizing ramp phase as a function of the corresponding voltage. Membrane currents were filtered (–3 dB at 1 kHz) and digitized at 10 kHz (pClamp 9.2 with Digidata 1200 ADC interface; Molecular Devices, Sunnyvale, CA). Cell capacitance and pipette series resistances were compensated (usually >80%), and these were obtained using the “membrane test” function of Clampex. Whole cell current density was expressed as picoamperes per picofarad.
The identity of the currents were verified by 1) their sensitivity to cytosolic Ca2+; 2) the dependence of the reversal potential on the extracellular K+ concentration; 3) their absence in parental CHO cells; 4) voltage-independent gating; and 4) their sensitivity to either apamin, a specific inhibitor of KCa2.3 or Tram-34, a specific inhibitor of KCa3.1.
Inhibition of PI3K and Treatment with PI(3)P
To determine whether PI3-kinase inhibition affects channel activity, transfected cells were treated with the PI3K inhibitor wortmannin (1 μM) 45 min before patch clamping, and I-V relationship was performed as described above. Channels that were inhibited by wortmannin were determined to be due to inhibition of PI(3)P by showing that channel activity could be rescued by the addition of 100 nM PI(3)P into the pipette solution during patch clamping. PI(3)P [C41H45Na3O16P2 (C6) or C17H29Na3O16P2 (C2)] were purchased from Echelon Biosciences (Salt Lake City, UT) and used according to specifications. PI(3)P was resuspended in water and flash frozen in liquid nitrogen and used at a concentration of 100 nM in the pipette solution as described previously.
Inside-Out Patch Clamping to Record KCa3.1 Channels
CHO cells stably transfected with KCa3.1 were used for single-channel recordings. These were performed using the inside-out (I/O) patch-clamp mode, and data were obtained using standard patch-clamp techniques with an Axopatch-200B amplifier (Molecular Devices). Currents were recorded at room temperature (21–23°C), low-pass filtered (–3-dB cut-off frequency at 1 kHz), and recorded on computer disk at a sampling frequency of 5 kHz (Clampex; Molecular Devices). The pipette resistance was 6–8 MΩ when filled with a solution containing 145 mM KCl, 1 mM MgCl2, 1 mM CaCl2, and 5 mM HEPES, pH 7.4. The bath solution contained 141 mM KCl, 5 mM HEPES, 1 mM EGTA, 1.01 mM MgCl2, 0.244 or 0.62 mM CaCl2, and 10 mM glucose, pH 7.3. The Ca2+ activity was calculated to be 33 or 170 nM (Win-MAX Chelator software) (Bers et al., 1994). PI(3)P (0.2–10 μM) was added to the bath solution as needed. Data were analyzed using pClamp9.0 (Molecular Devices) and Origin 7.0 (OriginLab, Northampton, MA) software.
RESULTS
KCa3.1 Is Activated Indirectly by Membrane-bound PI(3)P
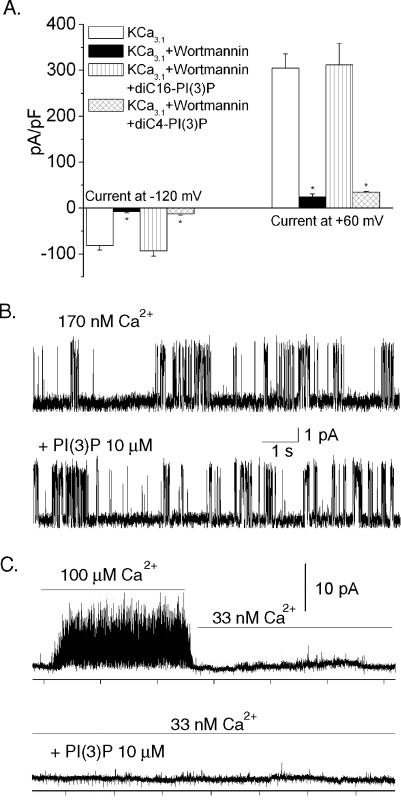
We previously found that KCa3.1 channels are inhibited by PI(3)P depletion and that channel activity in these cells could be rescued by including PI(3)P in the pipette solution (Srivastava et al., 2005). To determine whether activation of KCa3.1 channels requires membrane-localized PI(3)P, we compared C17H29Na3O16P2 [diC4-PI(3)P, soluble] with C41H45Na3O16P2 [diC16-PI(3)P, membrane bound] in their ability to restore KCa3.1 channel activity in cells treated with the PI3 kinase inhibitor wortmannin. These studies showed that addition of C41H45Na3O16P2, but not C17H29Na3O16P2, in the pipette solution rescued KCa3.1 channel activity in cells treated with wortmannin, indicating that membrane-bound PI(3)P is required for KCa3.1 channel activity (Figure 1A).
Figure 1.
KCa3.1 channels require membrane-bound PI(3)P for activity and are not directly activated by P(3)P in isolated, I/O membrane patches. (A) CHO cells overexpressing KCa3.1 (CHO-KCa3.1) were treated with wortmannin, and whole cell patch clamping was performed in the absence (control) or presence of wortmannin. To assess whether soluble PI(3)P was sufficient for KCa3.1 channel activity, current activity was assessed in wortmannin-treated cells containing either C17H29Na3O16P2 [diC4-PI(3)P, soluble] or C41H45Na3O16P2 [diC16-PI(3)P, membrane bound] in the pipette solution. Bar graph summary of results at–120 and +60 mV are shown. (B and C) Control recording of I/O patches recorded in a bath solution containing 170 nm Ca2+ without (top tracing) and after the addition of 10 μM PI(3)P (bottom tracing). PI(3)P did not affect single channel activity (n = 3). (C) The recording conditions were the same as in B but the Ca2+ activity was 33 nM. When switching to a higher Ca2+ (100 μM), channels were activated which verifies the presence of functional channels in the patch (top left). Addition of PI(3)P (10 μM) to the low Ca2+ bath (33 nM) did not produce single channel activity (bottom tracing). Similar results were observed in three other patches. All recordings in B and C were at–80 mV.
To determine whether membrane bound PI(3)P directly or indirectly regulates KCa3.1 channel activity, we next performed single-channel recording using I/O membrane patches and examined whether application of PI(3)P to the cytosolic face of the channel increased KCa3.1 channel activity. KCa3.1 channels had an open probability that depended on the Ca2+ activity of the bath solution. Addition of PI(3)P (up to 10 μM) did not increase channel activity (Figure 1B). It is possible that PI(3)P functions to increase Ca2+ sensitivity of the KCa3.1 channel. Therefore, we also measured single-channel activity in excised patches at lower cytosolic Ca2+ activities. In these experiments, I/O patches were initially exposed to high Ca2+ (100 μM) to verify the presence of KCa3.1 channels in the patch. The bath solution was then switched to a solution with lower Ca2+ activity (33 nM), and phosphatidylinositol phosphates (PIPs) were then added to the low Ca2+ solution. PI(3)P also failed to activate KCa3.1 channels in the low Ca2+ solution (Figure 1C). Experiments were also performed by exposing excised patches to various solutions, respectively, containing (10 μM each) PI(3)P (n=4), phosphatidylinositol-3,4-bisphosphate [PI(3,4)P2] (n=4), or phosphatidylinositol trisphosphate [PI(3,4,5)P3] (n=3) (our unpublished data). Similar to the results in Figure 1, no activation of KCa3.1 channels was observed by addition of any of these PIPs (our unpublished data). Thus, these data suggest that PI(3)P does not directly activate the KCa3.1 channel.
KCa2.1, KCa2.2, and KCa2.3 Channels Are Not Inhibited by PI(3)P Depletion
In contrast to KCa3.1 channels, we previously found that PI(3)P depletion with wortmannin had no effect on the related KCa2.2 channel (Srivastava et al., 2005). We now find that PI(3)P depletion with wortmannin similarly has no effect on KCa2.3 channels (Figure 2) or KCa2.1 channels (our unpublished data). To determine the PI(3)P sensitivity of channels, CHO cells were transfected with GFP-tagged KCa3.1 or KCa2.3, and patch clamp in the whole cell configuration was performed on GFP-positive cells that were untreated or treated with wortmannin (Srivastava et al., 2005). The KCa3.1 channel current was identified as the current blocked by Tram-34, and this current component was significantly reduced in cells pretreated with wortmannin (1 μM for 45 min) (Figure 2, A and B). In contrast, GFP-tagged KCa2.3 channel current (identified as the current component blocked by apamin) was not inhibited by wortmannin pretreatment (Figure 2, C and D; n = 8). Thus, of the KCa family members, KCa3.1 is uniquely dependent upon PI(3)P for channel activation.
Figure 2.
Wortmannin inhibits KCa3.1 but not KCa2.3 channels. CHO cells were transfected with GFP-KCa3.1 or GFP-KCa2.3, and patch clamping in the whole cell configuration was performed on GFP-positive cells in the absence or presence of wortmannin (1 μM). Shown are the I-V plots. (A) I-V plot of KCa3.1 cells before and after treatment with Tram-34 (1 μM for 3–5 min), demonstrating the identity of the current as being formed by KCa3.1 channels. The K+ current displays the reversal potential of–73 mV (not corrected for the liquid junction potential, calculated to be ∼13 mV), which is similar to the calculated reversal potential of–84 mV at an extracellular K+ of 5 mM. (B) Currents recorded from the same cells treated with 1 μM wortmannin. (C) I-V plots of KCa2.3 cells before and after treatment with apamin, demonstrating that the current measured is KCa2.3. (D) Currents from same cells treated with 1 μM wortmannin (n = 8).
The C Terminus of KCa3.1 Is Necessary and Sufficient for Channel Activation by PI(3)P
Chimeric channels between KCa2.2 and KCa3.1 and between KCa2.3 and KCa3.1 have previously provided important insights into regulation of this family of channels (Gerlach et al., 2000, 2001; Wulff et al., 2001). The finding that KCa3.1, but not KCa2.3, is inhibited by wortmannin and MTMR6 (the latter data are unpublished) provides a unique opportunity to determine the region(s) of KCa3.1 responsible for conferring regulation by PI(3)P and MTMR6 by generating a series of KCa2.3/KCa3.1 chimeric channels.
We first assessed whether the C terminus of KCa3.1 mediates channel regulation by PI(3)P. Chimeric GFP-KCa2.3/KCa3.1 channels were generated in which the C-terminal cytosolic region of KCa3.1 and KCa2.3 were swapped to generate KCa2.3-KCa3.1(CT) and KCa3.1-KCa2.3(CT). Chimeric constructs were transfected into CHO cells, and whole cell patch clamping was performed on GFP-positive cells that were untreated or treated with wortmannin. Currents produced by the KCa2.3 chimera containing the KCa3.1 C terminus were inhibited by wortmannin (Figure 3, A and B). These studies demonstrated that the C terminus of KCa3.1 was necessary and sufficient for channel regulation by PI(3)P. In contrast, we found that the chimeric KCa3.1 channel containing the KCa2.3 C terminus [KCa3.1-KCa2.3/CT] was constitutively active, but it was not inhibited by wortmannin (Figure 3, C–E). The ability of wortmannin to inhibit KCa2.3-KCa3.1/CT was due to loss of PI(3)P because wortmannin-inhibited channel activity could be rescued by including PI(3)P in the pipette solution (our unpublished data).
Figure 3.
The CT cytosolic domain of KCa3.1 confers regulation by PI(3)P. Chimeric KCa2.3/KCa3.1 channel were generated as described in Materials and Methods in which the CT cytosolic portion of KCa2.3 and KCa3.1 was replaced with similar regions of the other channel to generate GFP-KCa3.1-KCa2.3(CT) and GFP-KCa2.3-KCa3.1(CT). CHO cells were transfected with each construct, and whole cel patch clamping was performed on GFP-positive cells as described in Figure 2. I-V plots of KCa2.3-KCa3.1(CT) before (A) or after (B) treatment with 1 μM wortmannin. I-V plots of KCa3.1-KCa2.3(CT) before (C) or after (D) treatment with 1 μM wortmannin. Although both channels are constitutively active in the presence of PI(3)P, only KCa2.3-KCa3.1(CT), which contains the CT of KCa3.1, is inhibited by wortmannin. Bar graph summary is shown in E.
A Stretch of 14 Amino Acids in the KCa3.1 C Terminus Mediates Activation of KCa3.1 by PI(3)P
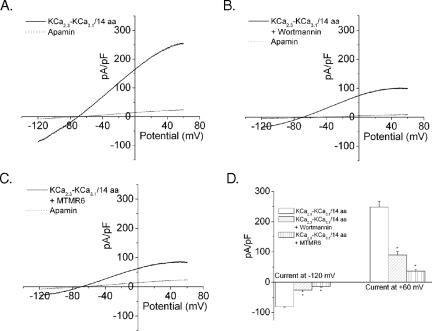
Alignment of the C termini of KCa2.1, KCa2.2, KCa2.3, and KCa3.1 is shown in Figure 4A. Each of the C termini contains a membrane-proximal CBD that mediates activation of these channels by calcium and a more carboxy terminus CC domain. To narrow down the region in KCa3.1 that mediates activation by PI(3)P, a further series of chimeric KCa2.3/KCa3.1 constructs were generated in which various regions in the CT of KCa2.3 were replaced with corresponding regions from KCa3.1. The boundaries between chimeric channels tested are shown in Figure 4A. As before, PI(3)P sensitivity of the chimeric channels was assessed by its ability to be inhibited by wortmannin pretreatment as well as whether wortmannin-inhibited current could be rescued by including PI(3)P in the pipette solution. We found that the CC domain of KCa3.1 did not mediate its unique regulation by PI(3)P because a KCa3.1 chimera containing the KCa2.3 CC domain was still sensitive to wortmannin (Figure 4B). Using a number of different chimeras, we narrowed down the region of KCa3.1 that mediates regulation by PI(3)P to a 14-amino acid stretch within the C-terminal CBD of KCa3.1 (Figure 4, A and B). To determine whether these 14 amino acids are sufficient to confer regulation by PI(3)P, we replaced the corresponding residues of KCa2.3 with these 14 amino acids [KCa2.3–KCa3.1/14aa]. This mutant channel was active under baseline conditions, but unlike wild-type KCa2.3, it was inhibited by wortmannin treatment (Figure 5, A and B). In addition, KCa2.3-KCa3.1/14aa was inhibited by MTMR6 overexpression (Figure 5C). Thus, substitution of only 14 amino acids from KCa3.1 onto KCa2.3 was sufficient to change KCa2.3 into a channel that resembled KCa3.1 with respect to its sensitivity to PI(3)P.
Figure 4.
(A) Alignment of C terminus of KCa2.1, KCa2.2, KCa2.3, and KCa3.1. Amino acids shown are human KCa2.1:350–536, rat KCa2.2: 383–580, human KCa2.3: 536–736, and human KCa3.1: 274–427. The CBD and CC domains are shown, respectively, by filled and open boxes below the sequence. The position of the boundaries used to generate chimeric channels are marked by arrows and labeled. Fourteen amino acids swapped between KCa2.3 and KCa3.1 are located between the two chimeras marked by arrows as KCa2.3-KCa3.1/355–427 and KCa2.3-KCa3.1/369–427. (B) Bar graph summary of results at–120 and +60 mV of the various chimeras shown in Figure 4A. These results identified a stretch of 14 amino acids in KCa3.1 that separated a channel that was dependent upon PI(3)P for activity from a channel that was still active in the absence of PI(3)P.
Figure 5.
Fourteen amino acids in the CT of KCa3.1 is sufficient to confer regulation by PI(3)P. GFP-KCa2.3 containing the substitution of 14 amino acids in CT portion of the CBD of KCa3.1 [KCa2.3-KCa3.1/14aa] was transfected into CHO cells and analyzed as described in Figures 1 and 2. I-V plots of KCa2.3-KCa3.1/14aa before (A) or after (B) treatment with 1 μM wortmannin. (C) Whole cell patch clamping in CHO cells cotransfected with GFP-KCa2.3/KCa3.1/14aa and MTMR6. (D) Bar graph summary of results. In all experiments, the current was inhibited by apamine, indicating that the current measured was KCa2.3.
PI(3)P Activation of KCa2.3-KCa3.1/14aa Is Not Mediated by Direct Phosphorylation of the Channel
PI(3)P could indirectly regulate KCa3.1, for example, by recruiting a kinase that contains a PX domain to the plasma membrane, which is then able to phosphorylate and activate KCa3.1. Several serine/threonine kinases with PX domains that bind PI(3)P have been identified (Liu et al., 2000; Xing et al., 2004). KCa3.1 contains a single potential phosphorylation site in these 14 amino acids, serine 367, which is a threonine in all the other mammalian SK channels (Figure 4A). To determine whether serine 367 is important for KCa3.1 channel activity, serine 367 was mutated to alanine [KCa3.1(S367A)], and channel activity was determined in the presence and absence of wortmannin. Our prediction was that if PI(3)P was required for the constitutive phosphorylation of serine 367, KCa3.1(S367A) would be constitutively inactive and resemble the KCa3.1 channel activity in CHO cells expressing wild-type KCa3.1 [KCa3.1(WT)] and treated with wortmannin. We found that in the absence of wortmannin, channel activity of KCa3.1(S367A) was similar to channel activity of KCa3.1(WT) (Figure 6A). These findings indicate that phosphorylation of S367 is not required for KCa3.1 channel activity.
Figure 6.
Mutation of the potential phosphorylation site at serine 367 of KCa2.3 did not inhibit channel activity. Serine 367 in KCa.1 was mutated to alanine and glutamic acid by site-directed mutagenesis to generate GFP-KCa3.1(S367A) and GFP-KCa3.1 (S367Q). Both constructs were transfected into CHO cells, and whole cell patch clamping was performed as described in text. I-V plots are shown. (A) GFP-KCa3.1(S367A). (B) GFP-KCa3.1(S367A) treated with 1 μM wortmannin. (C) GFP-KCa3.1(S367E). (D) GFP-KCa3.1(S367E) treated with 1 μM wortmannin. Bar graph summary is shown in E.
In contrast to KCa3.1(WT), the S367A mutant was insensitive to wortmannin (Figure 6B). The finding that KCa3.1(S367A) is no longer inhibited by wortmannin raised the possibility that inhibition of KCa3.1 is mediated by a phosphorylation event at residue S367 and that PI(3)P is required for constitutive KCa3.1 channel activity by stimulating perpetual dephosphorylation of S367, perhaps by recruiting a phosphatase to the plasma membrane. Previous studies have indicated that substitution of a serine for a negatively charged amino acid such as glutamic acid can mimic the effect of phosphorylation under some circumstances. To test this possibility, we mutated S367 to glutamic acid (S367E). If phosphorylation of S367 was inhibitory, KCa3.1(S367E) may be constitutively inactive if 367E mimicked phosphoserine at this site. However, KCa3.1(S367E) channel activity resembled KCa3.1(WT) in that it was active in the absence of wortmannin, and unlike KCa3.1(S367A), was inhibited by wortmannin (Figure 6, C and D). Thus, these data together suggest that phosphorylation of S367 does not play a role in the regulation of KCa3.1 channel activity. We can, however, not rule out the possibility that phosphorylation plays a role in negatively regulating the KCa3.1 channel because glutamic acid does not always mimic phosphoserine.
DISCUSSION
We found that, unlike KCa3.1, the three related Ca2+-activated K+ channels, KCa2.1, KCa2.2, and KCa2.3, do not require PI(3)P for channel activity. Several other factors also distinguish KCa3.1 from KCa2.1, KCa2.2, and KCa2.3 channels. For example, KCa3.1 is expressed predominantly in T- and B-cells, epithelial cells, and smooth muscle cells where they function to stimulate cell proliferation and activation by regulating membrane potential and Ca2+ influx, whereas KCa2.1, KCa2.2, and KCa2.3 channels are expressed predominantly in the nervous system where they function to regulate membrane potential, neuronal firing, and Ca2+ influx (Ghanshani et al., 2000; Jensen et al., 2001; Kohler et al., 2003; Maylie et al., 2004; Stocker, 2004; Wulff et al., 2004). In addition, only KCa2.1, KCa2.2, and KCa2.3 channels are found in invertebrates. These data would indicate that KCa3.1 channels have evolved unique functions in higher organisms and with these different functions have evolved unique means for regulation. We propose that the requirement of KCa3.1 for PI(3)P provides a means to rapidly turn on or turn off KCa3.1 channel activity, which may play important roles in the function of KCa3.1 channels in these cells. This would enable KCa3.1 channel activity to be rapidly activated by increasing PI3 kinase activity leading to PI(3)P generation adjacent to KCa3.1 or to be rapidly inhibited via the recruitment of MTMs (MTMR6 subfamily members) via their CC domains to KCa3.1, leading to dephosphorylation of PI(3)P and KCa3.1 channel inhibition.
One other difference between KCa3.1 and KCa2.1, KCa2.2, and KCa2.3 channels is their regulation by kinases (Khanna et al., 1999; Gerlach et al., 2000; Gerlach et al., 2001). Although KCa3.1 channels are activated by serine/threonine kinases, KCa2.1, KCa2.2, and KCa2.3 channels are not. Interestingly, many of the requirements for KCa3.1 channel activation by kinases are remarkably similar to our findings for PI(3)P activation of KCa3.1. Gerlach et al. found that the same 14 amino acids that we found are required for PI(3)P activation of KCa3.1 are also required for kinase activation of KCa3.1 (Gerlach et al., 2000, 2001. In fact, we used these data to help guide the construction of some of the chimeric channels studied herein. Based on these findings, we tested whether PI(3)P functions to activate KCa3.1 by recruiting a kinase with a PX domain that binds PI(3)P to the plasma membrane where it can then phosphorylate and activate KCa3.1 (Liu et al., 2000; Xing et al., 2004). However, in agreement with a previous study (Gerlach et al., 2001), KCa3.1 is not activated by direct phosphorylation because mutation of the only serine (serine 367) in these 14 amino acids did not inhibit channel activity. Surprisingly, mutation of 367 to alanine resulted in a channel that was constitutively active, but no longer inhibited by wortmannin. This finding suggests that S367 plays a role in negatively regulating KCa3.1 channel activity, and one function of PI(3)P may be to antagonize this inhibition. The mechanism whereby S367 functions to negatively regulate KCa3.1 is currently unknown. Our data cannot exclude the possibility that constitutive phosphorylation of S367 negatively regulates KCa3.1.
Our current working model is that PI(3)P together with (at least some of) these 14 amino acids in the C-terminal CBD of KCa3.1 is required to recruit another protein that functions as a subunit for the active KCa3.1 channel. We can in theory explain all of the above-mentioned results if direct phosphorylation of this putative regulatory subunit increases KCa3.1 channel activity. This hypothesis is consistent with PI(3)P indirectly activating KCa3.1 as well as the same 14 amino acids mediating regulation by PI(3)P and kinases; PI(3)P and these 14 amino acids would be required to recruit the putative regulatory subunit to the active channel that would then be the target for phosphorylation. It should be pointed out that no other subunit that associates with KCa3.1 has been identified to date. However, the requirement for PI(3)P would suggest that this subunit likely contains a PI(3)P binding domain (such as a FYVE or PX domain) that is required for membrane targeting (Stenmark and Aasland, 1999; Gillooly et al., 2001; Ellson et al., 2002).
In addition to requiring PI(3)P, KCa3.1 channel activity also requires Ca2+ (Srivastava et al., 2005). Thus, any proposed model for PI(3)P regulation of KCa3.1 channels must also take into account a concomitant requirement for Ca2+. Important insight into the role for Ca2+ in the activation of channels in this family has come from crystallographic studies of KCa2.2 and calmodulin (Schumacher et al., 2001). These studies demonstrated that in the absence of Ca2+, the C-terminal lobe of calmodulin is constitutively bound to the amino-terminal region of the CBD of one KCa2.2 channel, resulting in a calmodulin/CBD monomer. Binding of Ca2+ to the N-terminal lobe of calmodulin then stimulates its binding to the C-terminal region of an adjacent KCa2.2 CBD, resulting in a calmodulin/CBD dimer that has then been proposed to transmit a rotary force to the S6 pore region leading to opening of the channel (Schumacher et al., 2001; Schumacher and Adelman, 2002; Maylie et al., 2004). Because the functional KCa2.2 channel is tetrameric, these data would suggest that in the presence of Ca2+ the active channel is composed of two calmodulin/CBD dimers. It is intriguing that the region of KCa3.1 that mediates its activation by PI(3)P is also within the same region of the CBD that interacts with N-terminal lobe of calmodulin. Thus, it is possible that in the absence of PI(3)P, Ca2+ is unable to stimulate binding of the N-terminal lobe of calmodulin to an adjacent KCa3.1 CBD and therefore calmodulin/CBD dimers do not form. PI(3)P, possibly by recruiting a regulatory subunit, may then function to relieve this inhibition by enabling Ca2+ to now stimulate binding of the N-terminal lobe of calmodulin to an adjacent KCa3.1 CBD. Validation of this hypothesis will ultimately require the identification and characterization of this putative regulatory subunit.
Acknowledgments
This work is supported by National Institutes of Health Grant D-49207. We thank Dr. K. George Chandy (University of California, Irvine, Irvine, CA) and Heike Wulff (University of California, Davis, Davis, CA) for Tram-34 and KCa2.3 cDNA, Dr. John Adleman (Vollum Institute, Oregon Health and Science University, Portland, OR) for KCa3.2 cDNA, and Dr. Len Kazcmarek (Yale University, New Haven, CT) for the human KCa3.1 cDNA.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05–08–0763) on October 26, 2005.
References
- Bers, D. M., Patton, C. W., and Nuccitelli, R. (1994). A practical guide to the preparation of Ca2+ buffers. Methods Cell Biol. 40, 3–29. [DOI] [PubMed] [Google Scholar]
- Bolino, A., et al. (2000). Charcot-Marie-Tooth type 4B is caused by mutations in the gene encoding myotubularin-related protein-2. Nat. Genet 25, 17–19. [DOI] [PubMed] [Google Scholar]
- Bond, C. T., Maylie, J., and Adelman, J. P. (1999). Small-conductance calcium-activated potassium channels. Ann. N.Y. Acad. Sci. 868, 370–378. [DOI] [PubMed] [Google Scholar]
- Ellson, C. D., Andrews, S., Stephens, L. R., and Hawkins, P. T. (2002). The PX domain: a new phosphoinositide-binding module. J. Cell Sci. 115, 1099–1105. [DOI] [PubMed] [Google Scholar]
- Fanger, C. M., Rauer, H., Neben, A. L., Miller, M. J., Wulff, H., Rosa, J. C., Ganellin, C. R., Chandy, K. G., and Cahalan, M. D. (2001). Calcium-activated potassium channels sustain calcium signaling in T lymphocytes. Selective blockers and manipulated channel expression levels. J. Biol. Chem. 276, 12249–12256. [DOI] [PubMed] [Google Scholar]
- Gerlach, A. C., Gangopadhyay, N. N., and Devor, D. C. (2000). Kinasedependent regulation of the intermediate conductance, calcium-dependent potassium channel, hIK1. J. Biol. Chem. 275, 585–598. [DOI] [PubMed] [Google Scholar]
- Gerlach, A. C., Syme, C. A., Giltinan, L., Adelman, J. P., and Devor, D. C. (2001). ATP-dependent activation of the intermediate conductance, Ca2+-activated K+ channel, hIK1, is conferred by a C-terminal domain. J. Biol. Chem. 276, 10963–10970. [DOI] [PubMed] [Google Scholar]
- Ghanshani, S., Wulff, H., Miller, M. J., Rohm, H., Neben, A., Gutman, G. A., Cahalan, M. D., and Chandy, K. G. (2000). Up-regulation of the IKCa1 potassium channel during T-cell activation. Molecular mechanism and functional consequences. J. Biol. Chem. 275, 37137–37149. [DOI] [PubMed] [Google Scholar]
- Gillooly, D. J., Simonsen, A., and Stenmark, H. (2001). Cellular functions of phosphatidylinositol 3-phosphate and FYVE domain proteins. Biochem. J. 355, 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, B. S., Strobaek, D., Olesen, S. P., and Christophersen, P. (2001). The Ca2+-activated K+ channel of intermediate conductance: a molecular target for novel treatments? Curr. Drug Targets 2, 401–422. [DOI] [PubMed] [Google Scholar]
- Keen, J. E., Khawaled, R., Farrens, D. L., Neelands, T., Rivard, A., Bond, C. T., Janowsky, A., Fakler, B., Adelman, J. P., and Maylie, J. (1999). Domains responsible for constitutive and Ca(2+)-dependent interactions between calmodulin and small conductance Ca(2+)-activated potassium channels. J. Neurosci. 19, 8830–8838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna, R., Chang, M. C., Joiner, W. J., Kaczmarek, L. K., and Schlichter, L. C. (1999). hSK4/hIK1, a calmodulin-binding KCa channel in human T lymphocytes. Roles in proliferation and volume regulation. J. Biol. Chem. 274, 14838–14849. [DOI] [PubMed] [Google Scholar]
- Koegel, H., and Alzheimer, C. (2001). Expression and biological significance of Ca2+-activated ion channels in human keratinocytes. FASEB J. 15, 145–154. [DOI] [PubMed] [Google Scholar]
- Kohler, R., et al. (2003). Blockade of the intermediate-conductance calcium-activated potassium channel as a new therapeutic strategy for restenosis. Circulation 108, 1119–1125. [DOI] [PubMed] [Google Scholar]
- Laporte, J., Bedez, F., Bolino, A., and Mandel, J. L. (2003). Myotubularins, a large disease-associated family of cooperating catalytically active and inactive phosphoinositides phosphatases. Hum. Mol. Genet. 12, R285–R292. [DOI] [PubMed] [Google Scholar]
- Laporte, J., Hu, L. J., Kretz, C., Mandel, J. L., Kioschis, P., Coy, J. F., Klauck, S. M., Poustka, A., and Dahl, N. (1996). A gene mutated in X-linked myotubular myopathy defines a new putative tyrosine phosphatase family conserved in yeast. Nat. Genet 13, 175–182. [DOI] [PubMed] [Google Scholar]
- Liu, D., Yang, X., and Songyang, Z. (2000). Identification of CISK, a new member of the SGK kinase family that promotes IL-3-dependent survival. Curr. Biol. 10, 1233–1236. [DOI] [PubMed] [Google Scholar]
- Maher, A. D., and Kuchel, P. W. (2003). The Gardos channel: a review of the Ca2+-activated K+ channel in human erythrocytes. Int. J. Biochem. Cell Biol. 35, 1182–1197. [DOI] [PubMed] [Google Scholar]
- Maylie, J., Bond, C. T., Herson, P. S., Lee, W. S., and Adelman, J. P. (2004). Small conductance Ca2+-activated K+ channels and calmodulin. J. Physiol. 554, 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouadid-Ahidouch, H., Roudbaraki, M., Delcourt, P., Ahidouch, A., Joury, N., and Prevarskaya, N. (2004). Functional and molecular identification of intermediate-conductance Ca(2+)-activated K(+) channels in breast cancer cells: association with cell cycle progression. Am. J. Physiol. 287, C125–C134. [DOI] [PubMed] [Google Scholar]
- Rufo, P. A., Merlin, D., Riegler, M., Ferguson-Maltzman, M. H., Dickinson, B. L., Brugnara, C., Alper, S. L., and Lencer, W. I. (1997). The antifungal antibiotic, clotrimazole, inhibits chloride secretion by human intestinal T84 cells via blockade of distinct basolateral K+ conductances. Demonstration of efficacy in intact rabbit colon and in an in vivo mouse model of cholera. J. Clin. Investig. 100, 3111–3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher, M., and Adelman, J. P. (2002). Ion channels: an open and shut case. Nature 417, 501–502. [DOI] [PubMed] [Google Scholar]
- Schumacher, M. A., Rivard, A. F., Bachinger, H. P., and Adelman, J. P. (2001). Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+/calmodulin. Nature 410, 1120–1124. [DOI] [PubMed] [Google Scholar]
- Srivastava, S., Li, Z., Lin, L., Liu, G., Ko, K., Coetzee, W. A., and Skolnik, E. Y. (2005). The phosphatidylinositol 3-phosphate phosphatase myotubularin-related protein 6 (MTMR6) is a negative regulator of the Ca2+-activated K+ channel KCa3.1. Mol. Cell. Biol. 25, 3630–3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark, H., and Aasland, R. (1999). FYVE-finger proteins–effectors of an inositol lipid. J. Cell Sci. 112, 4175–4183. [DOI] [PubMed] [Google Scholar]
- Stocker, M. (2004). Ca(2+)-activated K+ channels: molecular determinants and function of the SK family. Nat. Rev. Neurosci. 5, 758–770. [DOI] [PubMed] [Google Scholar]
- Taylor, G. S., and Dixon, J. E. (2003). PTEN and myotubularins: families of phosphoinositide phosphatases. Methods Enzymol. 366, 43–56. [DOI] [PubMed] [Google Scholar]
- Wulff, H., Beeton, C., and Chandy, K. G. (2003). Potassium channels as therapeutic targets for autoimmune disorders. Curr. Opin. Drug Discov. Devel. 6, 640–647. [PubMed] [Google Scholar]
- Wulff, H., Gutman, G. A., Cahalan, M. D., and Chandy, K. G. (2001). Delineation of the clotrimazole/TRAM-34 binding site on the intermediate conductance calcium-activated potassium channel, IKCa1. J. Biol. Chem. 276, 32040–32045. [DOI] [PubMed] [Google Scholar]
- Wulff, H., Knaus, H. G., Pennington, M., and Chandy, K. G. (2004). K+ channel expression during B cell differentiation: implications for immunomodulation and autoimmunity. J. Immunol. 173, 776–786. [DOI] [PubMed] [Google Scholar]
- Xia, X. M., et al. (1998). Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature 395, 503–507. [DOI] [PubMed] [Google Scholar]
- Xing, Y., Liu, D., Zhang, R., Joachimiak, A., Songyang, Z., and Xu, W. (2004). Structural basis of membrane targeting by the Phox homology domain of cytokine-independent survival kinase (CISK-PX). J. Biol. Chem. 279, 30662–30669. [DOI] [PubMed] [Google Scholar]