Abstract
Scd5p regulates endocytosis and cortical actin organization as a targeting subunit for the Ser/Thr protein phosphatase-1 (PP1) in yeast. To identify localization signals in Scd5p required for cell surface recruitment, visualization of GFP-tagged Scd5 truncations and deletions was performed. Scd5p contains a PP1 binding site, a 3-repeat region of 20 amino acids (3R), and a 9-repeat region of 12 amino acids (9R). We found that the 9R is critical for cortical localization of Scd5p, but cortical recruitment is not essential for Scd5p's function in actin organization and endocytosis. We propose that Scd5p can target PP1 to endocytic factors in the cytoplasm that have been disassembled and/or inactivated by phosphorylation. We also found that Scd5p undergoes nuclear-cytoplasmic shuttling in a Crm1p-dependent manner. Scd5p-ΔCT lacking the 9R region and its nuclear export signal (NES) accumulates in the nucleus, causing cortical actin and endocytic defects. Cytoplasmic localization and function of Scd5p-ΔCT is restored by NES addition. However, removal of Scd5p's nuclear localization signal prevents nuclear entry, but endocytosis and actin organization remain relatively normal. These results indicate that nuclear-cytoplasmic shuttling is not required for regulation of Scd5p's cortical function and suggest that Scd5p has an independent nuclear function.
INTRODUCTION
Cargo selection, invagination of the plasma membrane, and formation and scission of clathrin-coated vesicles are sequential steps of receptor-mediated endocytosis. During this process, many proteins are recruited from the cytosol to endocytic sites at the plasma membrane, such as the adaptor complex AP-2, the coat protein clathrin, and a large number of other accessory proteins (Kirchhausen, 2000). Many of these additional factors, including epsin, Eps15, AP180/CALM, amphiphysin, and intersectin, interact with AP-2 and clathrin, but their precise functions during endocytosis are still being elucidated.
In budding yeast, most endocytic factors localize at the cell periphery in cortical spots that often contain actin (for review see Engqvist-Goldstein and Drubin, 2003). Mutations in many proteins of the endocytic machinery cause defects in actin organization, and many cortical actin patch-associated proteins, including the Arp2/3 complex and its activators, are important for internalization (Engqvist-Goldstein and Drubin, 2003). The actin polymerization machinery likely functions at endocytic sites to provide force for plasma membrane invagination, vesicle scission, and/or movement into the cytoplasm (Merrifield et al., 2002; Kaksonen et al., 2003; Yarar et al., 2005).
Recruitment of endocytic factors to sites of internalization is mediated by multiple protein-protein and protein-lipid interactions that work in a cooperative manner. In yeast, as in more complex eukaryotes, epsins Ent1/2p contain epsin N-terminal homology (ENTH) domains that interact with phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2); Aguilar et al., 2003). Similarly, two other endocytic proteins Sla2p and Yap180a/b have ENTH-like domains (ANTH) that are predicted to bind to phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2; Itoh and Takenawa, 2004; Legendre-Guillemin et al., 2004), suggesting that phospholipid binding may serve to anchor the endocytic complex to the plasma membrane and stabilize subsequent interactions at endocytic sites (Aguilar et al., 2003). All of these ENTH/ANTH domain-containing proteins interact with clathrin (Wendland and Emr, 1998; Wendland et al., 1999; Henry et al., 2002). Epsins and Yap180's are clathrin recruitment factors, whereas Sla2p is required for endocytic progression and release of clathrin and other patch components (Kaksonen et al., 2003; Newpher et al., 2005). The NPF motifs of Ent1/2p and Yap180a/b bind to the Eps15 homology (EH) domain of Pan1p (Wendland and Emr, 1998; Wendland et al., 1999), which is an Arp2/3 complex activator of actin polymerization (Duncan et al., 2001). Pan1p forms the core of an endocytic complex by multiple interactions with additional proteins, the EH domain protein End3p, and the intersectin-like SH3 domain protein Sla1p, which also binds to Sla2p and Rvs167p (Tang et al., 2000; Gourlay et al., 2003; Stamenova et al., 2004).
Endocytosis in yeast is regulated by the kinases, Ark1p and Prk1p. Prk1p negatively regulates Pan1p (Zeng and Cai, 1999; Duncan et al., 2001; Toshima et al., 2005), and other Prk1p targets include Sla1p, Ent1/2p, Yap180a/b, and Scd5p (Tang et al., 2000; Watson et al., 2001; Henry et al., 2003; Huang et al., 2003). Inhibition of both Ark1p and Prk1p causes an endocytic defect and aberrant actin assembly leading to accumulation of large actin aggregates and other cortical patch proteins in the cytoplasm (Cope et al., 1999; Watson et al., 2001; Sekiya-Kawasaki et al., 2003).
Scd5p, identified in a screen for multicopy suppressors of clathrin deficiency (Nelson and Lemmon, 1993; Nelson et al., 1996), plays a crucial role in endocytosis and cortical actin organization (Henry et al., 2002). Binding of the Ser/Thr protein phosphase-1 (PP1/Glc7p) is important for Scd5p's function, suggesting that Scd5p is a targeting subunit for PP1 to regulate endocytosis and cortical actin, possibly countering Ark1p and Prk1p kinases (Chang et al., 2002; Henry et al., 2002). Like many other endocytic proteins, Scd5p localizes to cortical patches, some of which contain cortical actin. In addition, Scd5p is physically associated with cortical patch endocytic factors, Sla2p and Rvs167p (Henry et al., 2002), implying that these interactions may contribute to the cortical association of Scd5p. However, the precise sequences in Scd5p necessary for its surface association have not been determined. Here we report our identification of a region of Scd5p required for cortical patch localization. However, we find that recruitment of Scd5p to the cell surface is not essential for its function in endocytosis and actin organization, suggesting that Scd5p's function at the cortex may be redundant with other endocytic factors or Scd5p/PP1 can act on its targets from the cytosol. Unexpectedly, we found that Scd5p constitutively shuttles between the cytoplasm and the nucleus in a Crm1p-dependent manner, and Scd5p's internalization and actin patch functions are greatly impaired when both its nuclear export and cortical localization signals are removed. However, nuclear-cytoplasmic shuttling is not required for Scd5p's cortical functions, suggesting that Scd5p is multifunctional and has an additional role in the nucleus.
MATERIALS AND METHODS
Strains, Media, and Growth Conditions
Strains used in this study are listed in Table 1. Yeast were grown in YEPD (1% yeast extract, 2% peptone, and 2% glucose) or selective dropout medium as described previously (Nelson and Lemmon, 1993). Synthetic medium containing 5-fluoroorotic acid (5-FOA) was prepared as described in (Boeke et al., 1984). Yeast transformation was performed using the lithium acetate method (Gietz et al., 1995). Yeast mating, sporulation, and tetrad analysis were carried out as described (Guthrie and Fink, 1991). Plasmid shuffling by 5-FOA selection was used to transfer SCD5 or GFP-SCD5 wild-type or mutant plasmids to scd5Δ::TRP1 cells carrying YEpSCD5 (SCD5, URA3, 2μ; Nelson et al., 1996).
Table 1.
Strains used in this study
| Strain No.a | Genotype |
|---|---|
| SL3004 | MATα leu2 ura3 trp1-901 his3 ade2-101 lys2-801 gal4 gal80 |
| SL3799 | MATa ark1Δ:HIS3 prk1Δ:URA3 ura3-52 his3-Δ200 leu2-3,112 lys2-801 |
| SL4397 | MATa/MATα leu2/leu2 ura3-52/ura3-52 trp1/trp1 his3-Δ200/his3-Δ200 scd5Δ::TRP1/scd5Δ::TRP1 pJSC3[CEN, LEU2, scd5-ΔCT] |
| SL4403 | MATa/MATα leu2/leu2 ura3-52/ura3-52 trp1/trp1 his3-Δ200/his3-Δ200 scd5Δ::TRP1/scd5Δ::TRP1 pKRH24[CEN, LEU2, scd5-Δ3R] |
| SL4418 | MATa/MATα leu2/leu2 ura3-52/ura3-52 trp1/trp1 his3-Δ200/his3-Δ200 scd5Δ::TRP1/scd5Δ::TRP1 pCC545[CEN, LEU2, SCD5] |
| SL4436 | MATa leu2 ura3-52 trp1 his3-Δ200 scd5Δ::TRP1 bar1Δ::HIS3-MX4 pCC545[CEN, LEU2, SCD5] |
| SL4619 | MATα leu2 ade2-1 ura3-1 trp1-1 can1-100 xpo1Δ::LEU2(crm1Δ) pKW440[CEN, HIS3, XPO1(CRM1)] |
| SL4620 | MATα leu2 ade2-1 ura3-1 trp1-1 can1-100 xpo1Δ::LEU2(crm1Δ) pKW457[CEN, HIS3, xpo1-1(crm1-1)] |
| SL4642 | MATa leu2 ura3-52 trp1 his3-Δ200 scd5Δ::TRP1 pJSC36[CEN, LEU2, GFP-scd5-ΔCT] |
| SL4645 | MATa leu2 ura3-52 trp1 his3-Δ200 scd5Δ::TRP1 pJSC35[CEN, LEU2, GFP-scd5-Δ3R] |
| SL4675 | MATa leu2 ura3-52 trp1 his3-Δ200 pJSC42[CEN, LEU2, GFP-CT] |
| SL4683 | MATa leu2 ura3-52 trp1 his3-Δ200 scd5Δ::TRP1 pJSC46[CEN, LEU2, GFP-scd5Δ9R] |
| SL4687 | MATa leu2 ura3-52 trp1 his3-Δ200 scd5Δ::TRP1 pJSC41[CEN, LEU2, GFP-scd5-ΔNLS] |
| SL4702 | MATa leu2 ura3-52 trp1 his3-Δ200 scd5Δ::TRP1 pJSC31[CEN, LEU2, GFP-SCD5] |
| SL4704 | MATa/MATα leu2/leu2 ura3-52/ura3-52 trp1/trp1 his3-Δ200/his3-Δ200 scd5Δ::TRP1/scd5Δ::TRP1 pJSC45[CEN, LEU2, scd5-Δ9R] |
| SL4706 | MATa leu2 ura3-52 trp1 his3-Δ200 scd5Δ::TRP1 pCC545[CEN, LEU2, SCD5] |
| SL4708 | MATa leu2 ura3-52 trp1 his3-Δ200 scd5Δ::TRP1 pKRH24[CEN, LEU2, scd5-Δ3R] |
| SL4709 | MATa leu2 ura3-52 trp1 his3-Δ200 scd5Δ::TRP1 pJSC45[CEN, LEU2, scd5-Δ9R] |
| SL4714 | MATa leu2 ura3-52 trp1 his3-Δ200 scd5Δ::TRP1 pJSC49[CEN, LEU2, GFP-scd5-Δ33] |
| SL4725 | MATa leu2 ura3-52 trp1 his3-Δ200 scd5Δ::TRP1 pJSC53[CEN, LEU2, GFP-scd5LLL>AAA] |
| SL4726 | MATa leu2 ura3-52 trp1 his3-Δ200 scd5Δ::TRP1 pJSC52[CEN, LEU2, scd5LLL>AAA] |
| SL4727 | MATa/MATα leu2/leu2 ura3-52/ura3-52 trp1/trp1 his3-Δ200/his3-Δ200 scd5Δ::TRP1/scd5Δ::TRP1 pJSC52[CEN, LEU2, scd5LLL>AAA] |
| SL4738 | MATa leu2 ura3-52 trp1 his3-Δ200 scd5Δ::TRP1 bar1Δ::HIS3-MX4 pKRH24[CEN, LEU2, scd5-Δ3R] |
| SL4741 | MATa leu2 ura3-52 trp1 his3-Δ200 scd5Δ::TRP1 bar1Δ::HIS3-MX4 pJSC45[CEN, LEU2, scd5-Δ9R] |
| SL4751 | MATa leu2 ura3-52 trp1 his3-Δ200 scd5Δ::TRP1 pJSC3[CEN, LEU2, scd5-ΔCT] |
| SL4759 | MATa leu2 ura3-52 trp1 his3-Δ200 scd5Δ::TRP1 bar1Δ::HIS3-MX4 pJSC52[CEN, LEU2, scd5LLL>AAA] |
| SL4764 | MATa leu2 ura3-52 trp1 his3-Δ200 scd5Δ::TRP1 bar1Δ::HIS3-MX4 pJSC3[CEN, LEU2, scd5-ΔCT] |
| SL4770 | MATa leu2 ura3-52 trp1 his3-Δ200 scd5Δ::TRP1 pJSC57[CEN, LEU2, scd5-ΔCT+NES] |
| SL4772 | MATa/MATα leu2/leu2 ura3-52/ura3-52 trp1/trp1 his3-Δ200/his3-Δ200 scd5Δ::TRP1/scd5Δ::TRP1 pJSC57[CEN, LEU2, scd5-ΔCT+NES] |
| SL4774 | MATa leu2 ura3-52 trp1 his3-Δ200 scd5Δ::TRP1 pJSC59[CEN, LEU2, GFP-scd5-ΔCT+NES] |
| SL4784 | MATa leu2 ura3-52 trp1 his3-Δ200 scd5Δ::TRP1 bar1Δ::HIS3-MX4 pJSC57[CEN, LEU2, scd5-ΔCT+NES] |
| SL5143 | MATa leu2 ura3-52 trp1 his3-Δ200 scd5Δ::TRP1 pJSC34[CEN, URA3, GFP-SCD5] pTMN2[CEN, LEU2, GAL:GST-CT] |
| SL5151 | MATa leu2 ura3-52 trp1 his3-Δ200 scd5Δ::TRP1 pJSC34[CEN, URA3, GFP-SCD5] pTB338[CEN, LEU2, GAL:GST] |
| SL5227 | MATa leu2 ura3-52 trp1 his3-Δ200 scd5Δ::TRP1 bar1Δ::HIS3-MX4 pOTT4[CEN, LEU2, scd5-ΔNLS] |
| SL5228 | MATa leu2 ura3-52 trp1 his3-Δ200 scd5Δ::TRP1 bar1Δ::HIS3-MX4 pOTT6[CEN, LEU2, SCD5] |
| SL5231 | MATa bar1-1 his3-Δ200 leu2 ura3-52 pTB338[CEN, LEU2, GAL:GST] |
| SL5232 | MATa bar1-1 his3-Δ200 leu2 ura3-52 pTMN2[CEN, LEU2, GAL:GST-CT] |
| YPJ96—4A | MATa trp1-901 leu2-3,112 ura3-52 his3-Δ200 gal4Δ ade2 gal80Δ GAL2-ADE2 LYS2::GAL1-HIS3 met2::GAL7-lacZ |
All strains are from this laboratory except for SL3799 (David Drubin), SL4619 and SL4620 (Alan Tartakoff), and YPJ96-4A (Elizabeth Craig).
Plasmids
All regions mutagenized or sequences amplified by PCR were verified by DNA sequencing.
pCC545 (CEN, LEU2, SCD5; Henry et al., 2002) was modified to generate pKRH24 (scd5-Δ3R) and pJSC45 (scd5-Δ9R) by gap repair. PCR products replacing the central 3-repeat region (3R, aa 405–529) or the 9-repeat region (9R, aa 527–747) of SCD5 with a HIS3 cassette flanked by HindIII sites were amplified from pFA6a-HIS3MX6 (Longtine et al., 1998) using primers 5′-GAATGGCAATACTCTACCACCTAGCAATGTGAATAACACCACTGTTCCACAGAAGCTTCGTACGCTGCAGGTCGAC-3′, 5′-GTTGCTGTTGATTGCTTTGAGGAAGAGTAATGTTCGGAGAATTCAGAAATGCAAGCTTATCGATGAATTCGAGCTCG-3′ or 5′-CGATGTTTCAGGCGCAATTTACGAACCAATCTTCATCTCCACAAAGTAAGCTTCGTACGCTGCAGGTCGAC-3′, 5′-CACTAGTATTTTGGAATGAACTTATGCCATTACTGGCGTTATTGCCAAGCTTATCGATGAATTCGAGCTCG-3′, respectively. The PCR products were cotransformed with BlpI-cut pCC545 for gap repair in yeast. The plasmids were shuttled into and purified from bacteria, digested with HindIII to remove the HIS3 cassette, and religated generating pKRH24 and pJSC45.
Clones containing a scd5-Δ33 truncation mutation (pJSC50) or a scd5LLL>AAA mutation (pJSC52) were created in two steps. pBluescript SK+ containing 4.6-kb BamHI-ClaI SCD5 fragment was subjected to site-directed mutagenesis by the Stratagene (La Jolla, CA) quickchange method using primer 5′-CCATTCACTTAAGATGTTAATAG-3′ to create a stop codon at position 839 of the Scd5p coding sequence (scd5-Δ33) or primer 5′-CAAGCGATATTGCGGGTAACGCGCAGTCAGCGCAGCAGCAAGTC-3′ to alter three leucines to alanines in the NES (scd5LLL>AAA). BlpI-HpaI fragments (0.79-kb) containing these mutations were then gap-repaired into PstI-cut pCC545 to generate pJSC50 and pJSC52, respectively.
pJSC3 (CEN, LEU2, scd5-Δ338) has been described previously (Henry et al., 2002). scd5-Δ338 is also referred to here as scd5-ΔCT.
To construct pJSC57 (scd5-ΔCT+NES) by gap repair, a PCR product replacing aa 527–835 of SCD5 with the HIS3MX6 cassette flanked by HindIII sites was amplified using primers, 5′-CGATGTTTCAGGCGCAATTTACGAACCAATCTTCATCTCCACAAAGTAAGCTTCGTACGCTGCAGGTCGAC-3′ and 5′-CAAAATATCGCTTGAATTGGATCTATTAACATCTGCAGTGAATGGAAAAAGCTTATCGATGAATTCGAGCTCG-3′ and pFA6a-HIS3MX6 as a template. The PCR product was cotransformed with BlpI-cut pCC545 for gap repair. HindIII-digestion and religation of the plasmid-generated pJSC57.
pJSC31(CEN, LEU2, GFP-SCD5) was constructed in two steps. First, a HindIII site was inserted in frame directly after the first codon of SCD5 in pCC545 by gap repair. A PCR product containing the HIS3 cassette with flanking HindIII sites and SCD5 sequences was amplified from pFA6a-HIS3MX6 using primers, 5′-TTAGCTAAGATTTTATAGATCATTGCAGGCTCGATAAATTTTACATGGTAACGAAACATGAAGCTTCGTACGCTGCAGGTCGAC-3′ and 5′-CTTTTCTGCTTGGTCCCCGCTGCTTAAGTCCAATCCCGGAACATTAAGCCAATCAAACGAAAGCTTATCGATGAATTCGAGCTCG-3′. pCC545 was gap-repaired with the PCR product in yeast, followed by HindIII digestion and ligation of the plasmid to generate a HindIII site after the first codon of SCD5. Second, a GFP(S65T) coding sequence was PCR-amplified from pFA6a-GFP(S65T)-HIS3MX6 using primers, 5′-TTAGCTAAGATTTTATAGATCATTGCAGGCTCGATAAATTTTACATGGTAACGAAACATGAAGCTTGGTCGACGGATCCCCGGGTTA-3′ and 5′-CTTTTCTGCTTGGTCCCCGCTGCTTAAGTCCAATCCCGGAACATTAAGCCAATCAAACGAAAGCTTACCAGCACCAGCACCTTTGTATAGTTCATCCATGCC-3′. The PCR product was then gaprepaired into HindIII cut pCC545 that was engineered in step 1 above, to generate pJSC31.
pJSC33 (2μ, URA3, GFP-SCD5) was made by cutting YEpSCD5 (Nelson et al., 1996) with AflII and gap repairing with a KpnI fragment containing the N-terminal GFP coding segment from pJSC31. pJSC34 (URA3, CEN, GFP-SCD5) was made by gap repairing the SacI-BlpI GFP-SCD5 fragment from pJSC31 into XbaI cut YCp-SCD5 (Nelson et al., 1996).
The scd5-ΔNLS mutation was constructed in two steps. First, a PCR product was amplified for gap repair to replace the potential NLS (aa 257–268) of SCD5 with the HIS3MX6 cassette and flanking AgeI sites using primers, 5′-GGTGATCAAAAGGTCGATTTTGACTCATTTGCTTCATTGCTGTTGACTGGTAAGACAACCGGTCGTACGCTGCAGGTCGAC-3′ and 5′-TTGATTGAGGTTTGGAGGGTCTTGAAATGTTATATGCTCTGAAAACCTAACCTTCTTACCGGTATCGATGAATTCGAGCTCG-3′. Second, pJSC33 was cotransformed with the PCR product selecting for His+. The resultant clone with HIS3MX6 replacing the NLS was digested with AgeI and religated generating pJSC40 (2μ, URA3, GFP-scd5-ΔNLS).
To obtain pJSC41 (LEU2, CEN, GFP-scd5-ΔNLS), SpeI cut pJSC31 was gap repaired with the AflII-PstI fragment from pJSC40. pJSC44 (URA3, CEN, GFP-scd5-ΔNLS) was generated by gap repair transfer of GFP-scd5-ΔNLS into AflII-BlpI cut pJSC34. To make pOTT4 (LEU2, CEN, scd5-ΔNLS) and pOTT6 (LEU2, CEN, SCD5) the GFP coding sequences in pJSC41 and pJSC31, respectively, were excised by cutting with HindIII and religating.
pJSC35 (LEU2, CEN, GFP-scd5-Δ3R) was constructed by gap-repairing SpeI-cut pJSC31 (GFP-SCD5) with the 2.5-kb AflII-PstI Δ3R fragment from pKRH24.
pJSC46 (LEU2, CEN, GFP-scd5-Δ9R) was generated by gap-repairing SpeI-cut pJSC31 with the 3.8-kb AflII-ClaI Δ3R fragment from pJSC45.
For construction of pJSC49 (LEU2, CEN, GFP-scd5-Δ33) and pJSC53 (LEU2, CEN, GFP-scd5LLL>AAA), PstI-cut pJSC31 was gap-repaired with 0.8-kb BlpI-HpaI fragments from pJSC50 and pJSC52, respectively.
pJSC36 (LEU2, CEN, GFP-scd5-ΔCT) was generated by gap-repairing SpeI-cut pJSC31 with a 2.5-kb AflII-PstI fragment from pJSC3.
To generate pJSC59 (GFP-scd5-ΔCT+NES), SpeI-PstI cut pJSC31 was gap-repaired with 3.6-kb AflII-SacII fragments from pJSC57. To construct a clone for expression of the GFP fusion to the C-terminal (CT) fragment of Scd5p (aa 526–872), a PCR product for gap repair was amplified from pFA6a-GFP(S65T)-HIS3MX6 using primers, 5′-CTGCATCTTCTCGTTAGCTAAGATTTTATAGATCATTGCAGGCTCGATAAATTTTCATGGTAACGAAACATGTCGTTTGATTGGCTTGGTCGACGGATCCCCGGG-3′ and 5′-GCTTTGAGGAAGAGTAATGTTCGGAGAATTCAGAAATGCTGGTCCTGTACTAGCACCAGCACCAGCACCAGCACCTTTGTATAGTTCATCCATGCC-3′. Cotransformation of the PCR product with NcoI-cut pCC545 generated GFP-CT (pJSC42), in which inframe insertion of GFP followed by a 4× Gly-Ala linker replaces codons 7–525 of SCD5.
To generate the GAL1:GST-CT expression construct, a PCR product encoding amino acids 510–872 of Scd5p flanked by BamHI and SalI sites was amplified using primers 5′-CGGGATCCTCGATGTTTCAGGCGC-3′ and 5′-GCGTCGACGCTCAATAATCAAGACAA-3′ and cloned into the BamHI-SalI sites of pTB338 (2μ, LEU2, GAL1:GST; gift of Mike Hall).
To construct pJSC54 (2μ, URA3, GBD-scd5-ΔCT-NLS), SpeI cut pNT1 (GBD-scd5-Δ338/ΔCT; Henry et al., 2002) was gap-repaired with a DNA fragment containing the ΔNLS mutation. Other two hybrid plasmids have been described previously (Chang et al., 2002; Henry et al., 2002).
Growth Assays
Cells were grown to log phase in YEPD at 25°C. Cultures were serial-diluted by one fifth starting at 5 × 106 cells/ml and spotted on YEPD plates by using a multiprong replicator. Plates were incubated at 25 and 37°C for 2–3 d.
Immunoblotting
Cells were grown to log phase in YEPD medium and cultures were kept at 25°C or preshifted to 37°C for 90 min. Cells (6 × 107) were harvested, washed with ice-cold water, resuspended in 0.2 ml of ice-cold RIPA buffer (50 mM Tris-HCl, pH 7.5, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 1 mM sodium pyrophosphate, 150 mM NaCl) with addition of 1 mM phenylmethylsulfonyl fluoride and a 1× protease inhibitor cocktail (Stepp et al., 1995), and lysed by glass bead homogenization (Chang et al., 2002). Twenty microliters of cell extracts were separated on SDS-gels (7.5%) and transferred to nitrocellulose. Equal loading of protein extracts was confirmed by amido black staining. Western blots were probed with anti-Scd5p antibodies (1:6000, Clarence Chan). These were detected by IRDye800-conjugated goat anti-rabbit IgG (1:10,000, Rockland, Gilbertsville, PA) using the Odyssey Infrared Imaging system (LI-COR, Lincoln, NE).
Two-hybrid Analysis
Bait plasmids (URA3, pGBD-fusions) were transformed into YPJ96-4A and prey plasmids (LEU2, pGAD-fusions) were transformed into SL3004. YPJ96-4A and SL3004 containing these plasmids were mated pairwise on a YEPD plate for 2 d, and the diploids containing both plasmids were selected on C-LEU-URA. Cells containing both baits and preys were grown in liquid C-LEU-URA medium to a concentration of 0.5 × 107 cells/ml and equal numbers of cells were spotted on C-LEU-URA and C-LEU-URA-ADE to monitor expression of the GAL2-ADE2 reporter gene.
Microscopy
Most of the fluorescence microscopy was performed using an Olympus BX61 microscope (Melville, NY) equipped with Nomarski differential interference contrast (DIC) optics, a UPlan Apo 100×/NA 1.35 objective, a Roper Cool-SNAP HQ CCD camera (Tucson, AZ), Sutter Lambda 10 + 2 automated excitation and emission filter wheels (Novato, CA) and a 175 W Xenon remote source lamp with liquid light guide. Images were acquired using Slidebook 4.0 software (Intelligent Imaging Innovations/3I, Denver, CO) and prepared for figures in Adobe Photoshop (San Jose, CA).
For analysis of actin, yeast cells were grown to early log phase in YEPD and cultures were kept at 25°C or preshifted to 37°C as indicated. For galactose induction of GST-CT, cells were growth adapted in–leucine synthetic medium plus 2% raffinose as the carbon source. Then log phase cultures were generated in raffinose and cells were induced for indicated times (3–4 h) in fresh medium containing 2% raffinose and 2% galactose. Cells were fixed with 3.7% formaldehyde and stained with Alexa-568-phalloidin (Molecular Probes, Eugene, OR) as described previously (Adams and Pringle, 1984). When visualization of both GFP and actin was required, the initial fixation in growth medium was for 10 min, followed by a second fixation period of 60 min. In this case actin was stained with Alexa-594-phalloidin. A series of Z-sectioned images (0.25–0.3 μM) were acquired and subsequently deconvolved by the nearest neighbor algorithm and projected into a single plane using Slidebook. For Figure 1E a Zeiss Axioplan-2 microscope equipped with a Hamamatsu C4742–95 cooled CCD camera (Bridgewater, NJ), DIC, 100× plan neofluor (NA 1.3) and QED acquisition software was used as described previously (Henry et al., 2002).
Figure 1.
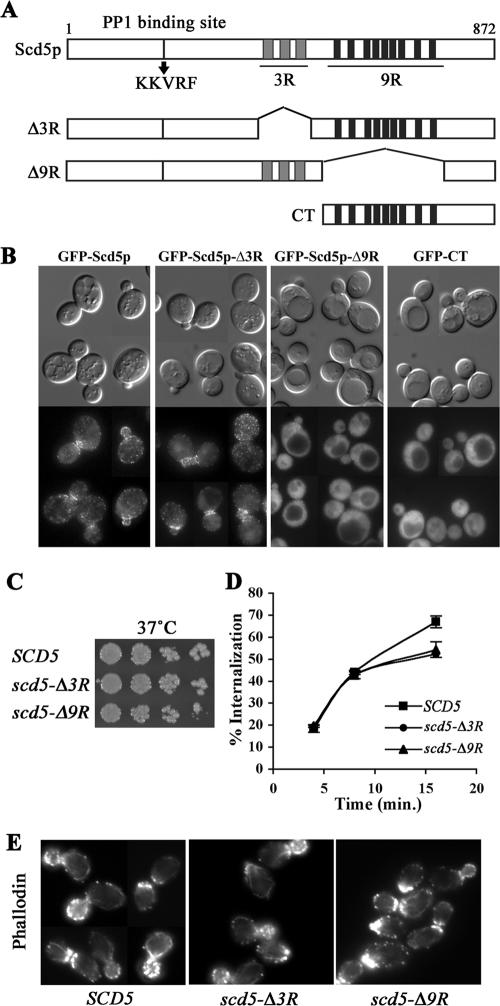
The 9R is necessary for cortical localization of Scd5p, but growth, actin organization, and endocytosis are relatively normal in scd5-Δ9R. (A) Schematic diagram of Scd5p and deletion mutants. Scd5p contains a PP1-binding site (KKVRF), three repeats of 20 amino acids (3R; gray boxes), and nine repeats of 12 amino acids (9R; black boxes). (B) Localization of GFP-tagged Scd5p and deletion mutants: scd5Δ cells expressing GFP-Scd5p (SL4702), GFP-Scd5p-Δ3R (SL4645) or GFP-Scd5p-Δ9R (SL4683), and SCD5 cells expressing GFP-CT (SL4675) were examined by fluorescence microscopy at 25°C. Similar results were observed after shift to 37°C (unpublished data). (C) Growth: wild-type (SL4706), scd5-Δ3R (SL4708), and scd5-Δ9R (SL4709) cells were serial diluted, spotted on YEPD plates, and grown for 3 d at 37°C. (D) Receptor-mediated endocytosis: wild-type SCD5 (SL4436), scd5-Δ3R (SL4738), and scd5-Δ9R (SL4741) cells were preincubated at 37°C for 15 min and then pulsed with 35S-labeled-α-factor. Samples were collected at indicated time points for determination of percent of cell-associated α-factor internalized. (E) Actin staining: wild-type (SL4418), scd5-Δ3R (SL4403), and scd5-Δ9R (SL4704) cells were grown to log-phase in YEPD at 25°C and shifted to 37°C for 2 h before fixation. Filamentous actin was stained with Alexa-568 phalloidin.
For localization of GFP-tagged proteins alone, cells were grown to early log phase in selective dropout medium and temperature was shifted as indicated or cultures were induced on galactose for GST-CT expression as described above. To costain for nuclear DNA, 0.1–0.5 μg/ml DAPI was added to the overnight growth medium. Cells were visualized with the Olympus BX61 fluorescence microscope. Z-sectioned images were acquired, deconvolved and projected into a single plane as described above. Note that Figure 4 images were taken on the Zeiss Axioplan-2.
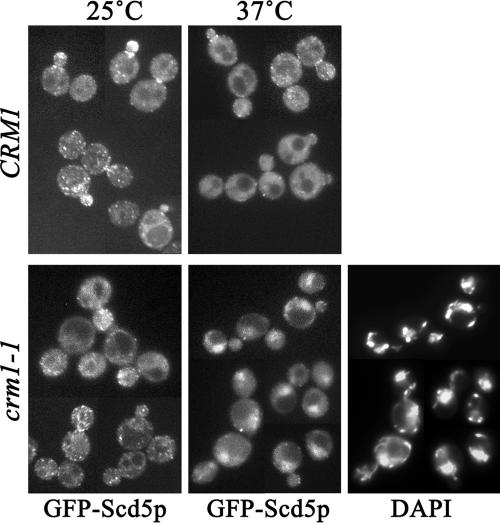
Figure 4.
Scd5p constitutively undergoes nuclear-cytoplasmic shuttling via a Crm1p-dependent pathway. GFP-Scd5p was expressed in wild-type CRM1 (SL4619), or crm1-1 (SL4620) strains. Cells were grown at 25°C, shifted to 25 or 37°C for 30 min, and examined by fluorescence microscopy. DAPI staining was used to localize nuclear DNA.
Endocytosis Assays
Lucifer yellow (LY) uptake was carried out as described previously (Munn et al., 1995). Cells were grown at 25°C in YEPD or selective medium to early log phase and cultures were kept at 25°C or preshifted to 37°C for 15 min before addition of LY (Sigma, St. Louis, MO). For GST-CT expression cells were induced for 3 h on galactose before LY addition. After incubation for 1 h in LY, cells were washed in a NaF and NaN3 buffer to block further internalization as described in Munn et al. (1995) and observed immediately by fluorescence microscopy.
The 35S-α-factor internalization assay was performed as described previously (Dulic et al., 1991). Cells were incubated at 25 or 37°C for 15 min before addition of radiolabeled α-factor. For GST or GST-CT inductions, cells transformed with pTB338 or pTMN2, respectively, were grown on–leucine + raffinose selective medium as described above and induced on galactose for 4 h. Cells were then pelleted and resuspended in the same medium containing 1% yeast extract to reduce background binding to cells. After 15-min preincubation, 35S-α-factor was added and assays were performed as usual. The results are the averages of three independent experiments ± SD.
RESULTS
The 9R Region Is Necessary for Cortical Localization of Scd5p
To define the domain(s) necessary for the cortical association of Scd5p we performed localization analysis by fluorescence microscopy using GFP-tagged Scd5 deletion or truncation mutant proteins. In nearly all cases mutant proteins were expressed as the sole source of Scd5p under control of its own promoter. Expression levels were essentially the same as that of endogenous Scd5p (unpublished data). When a mutant protein could not complement the essentiality of scd5Δ, the GFP-tagged protein was expressed over endogenous SCD5.
Scd5p contains a protein phosphatase-1 (PP1) binding site (KKVRF), a central 3R region of 20 amino acids, and a C-terminal 9R region of 12 amino acids (Figure 1A). The 3R region has consensus motifs that are phosphorylated by Prk1p (Henry et al., 2003; Huang et al., 2003), which is thought to disrupt interactions of other cortical patch/endocytic factors, such as Pan1p, Sla1p, and End3p (Zeng et al., 2001). Thus, we thought reversible phosphorylation of the 3R region might also regulate interaction of Scd5p with other endocytic factors, thereby affecting its cortical association. However, deletion of the 3R region had no effect on cortical localization of Scd5p (Figure 1B), nor did mutation of the three major Prk1p target Thr residues to Ala or Glu to mimic dephospho- or phospho-3R, respectively, have any effect (unpublished data). In contrast, deletion of the 9R region significantly reduced punctate structures at the cell cortex (Figure 1B). GFP-Scd5p-Δ9R was mostly dispersed in the cytoplasm, indicating that the 9R, but not the 3R region, is important for the surface recruitment of Scd5p. Supporting the role of the 9R region for association with cortical actin patch components, we found that GFP-Scd5p or GFP-Scd5p-Δ3R localized to the large actin aggregates found in ark1Δ prk1Δ cells, whereas GFP-Scd5p-Δ9R was largely excluded (see Supplementary Figure 1).
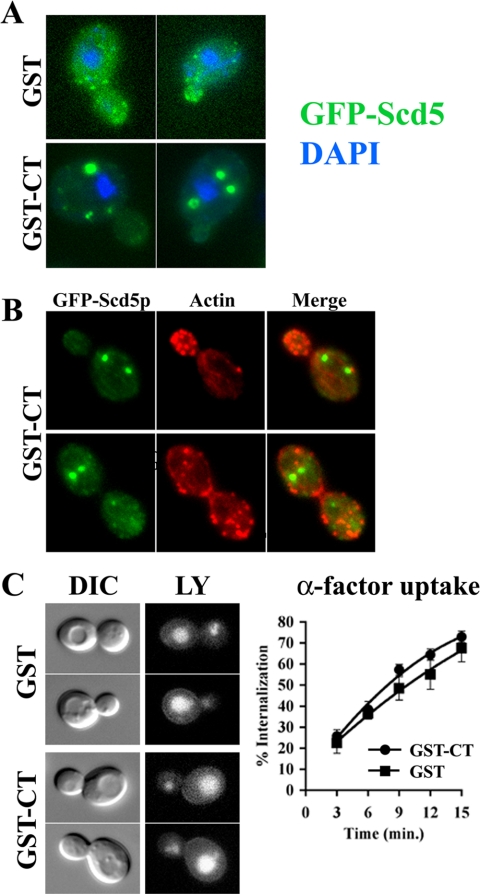
We next constructed a GFP-tagged carboxyl-terminal domain (CT) of Scd5p (residues 526–872; Figure 1A) to determine whether the CT containing the 9R is sufficient for cortical association. GFP-CT did not complement the lethality of scd5Δ cells, so it was expressed in the presence of endogenous SCD5. Although the 9R region was required for the membrane recruitment of Scd5p, GFP-CT was not targeted to the cell cortex and was completely dispersed through the cytoplasm (Figure 1B). It is possible that that endogenous Scd5p preferentially competes with the C-terminal fragment of Scd5p for cortical localization. Indeed overexpression of a GST-CT fusion displaced GFP-Scd5p from the cortex, sometimes causing it to form inclusions in the cytoplasm (Figure 2A). These aggregates of GFP-Scd5p were not associated with the nucleus nor did they contain significant actin (Figure 2, A and B)
Figure 2.
Overexpression of the C-terminal domain causes loss of Scd5p cortical localization, but actin and endocytosis are normal. (A) Localization of GFP-Scd5p in GST-CT-overexpressing cells. GFP-SCD5 cells transformed with pTB338 [GAL1:GST] (SL5151) or pTMN2 [GAL1:GST-CT](SL5143) were grown to log phase in–leucine/2% raffinose medium with 0.5 μg/ml DAPI to log phase. Then cultures were induced on galactose at 30°C for 3.5 h and examined by fluorescence microscopy. (B) Localization of actin and GFP-Scd5p in GST-CT-overexpressing cells. GFP-SCD5 cells transformed with pTMN2 [GAL1:GST-CT](SL5143) were induced on galactose at 30°C for 3.5 h and then fixed and stained for simultaneous actin (Alexa-594 phalloidin) and GFP visualization. (C) Endocytosis: LY uptake in SL5151 (GST) or SL5143 (GST-CT) was performed after 3 h galactose induction (left panel). 35S-α-factor internalization was performed in wild-type cells transformed with pTB338/GST (SL5231) or pTMN2/GST-CT (SL5232) after 4-h induction on galactose (right panel).
Effects of Deletion of the 3R or the 9R on Growth, Actin Organization, and Endocytosis
Although GFP-Scd5p-Δ3R properly localizes at the cell cortex, deletion of the 3R, which is subjected to phosphorylation by Prk1p, could influence Scd5p's function. In addition, loss of the membrane recruitment of Scd5p due to the 9R deletion could cause severe actin and endocytic defects. Therefore, we examined whether deletion of the 3R or the 9R affects growth, actin organization, and endocytosis.
For phenotypic analysis, untagged truncation and deletion mutations were expressed as the sole source of Scd5p. Surprisingly, both scd5-Δ9R and scd5-Δ3R cells grew normally at 25°C (unpublished data) and at 37°C (Figure 1C) and displayed normal polarized actin patches and actin cables at 25°C (unpublished data) and 37°C (Figure 1E). Fluid-phase endocytosis of LY into the vacuole was also efficient in scd5-Δ3R and scd5-Δ9R cells at both temperatures (unpublished data). Similar results were observed for receptor-mediated endocytosis of 35S-labeled α-factor at 25°C (unpublished data), although internalization of α-factor at 37°C slowed slightly at later times of uptake as compared with wild-type SCD5 cells (Figure 1D). These data imply that the 3R or the 9R are dispensable for Scd5p's role in actin organization and endocytosis. Moreover, overexpression of the CT region, which displaced GFP-Scd5p from the cortex, also did not affect actin organization or endocytosis (Figure 2, B and C), further indicating cortical localization of Scd5p is not required for its actin and endocytic functions.
Scd5p Undergoes Constitutive Nuclear-cytoplasmic Shuttling via a Crm1p-dependent Pathway
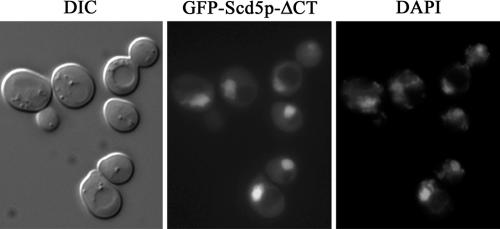
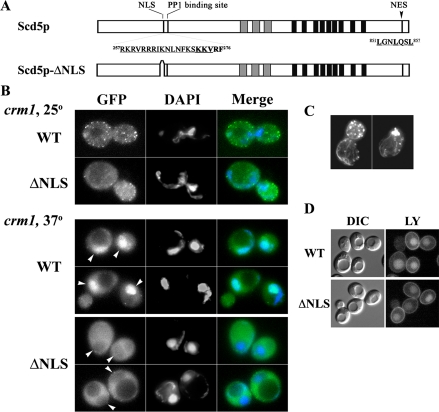
Previously we characterized scd5-ΔCT/Δ338, which encodes Scd5p with a C-terminal truncation of 338 amino acids (Henry et al., 2002). Cells expressing scd5-ΔCT showed abnormal actin structures and severe endocytic defects. On the basis of the above observations, we predicted that this protein would be mislocalized to the cytoplasm because of lack of the 9R cortical localization region. Unexpectedly, GFP-Scd5p-ΔCT was found predominantly in the nucleus colocalizing with DAPI-stained DNA (Figure 3). This result suggests that loss of Scd5p from the cytoplasm may be a major cause of the severe actin and endocytic defects of scd5-ΔCT cells.
Figure 3.
GFP-Scd5p-Δ338/ΔCT predominantly localizes in the nucleus. SL4642 (GFP-scd5-Δ338/ΔCT) was grown at 25°C to logphase in the presence of 0.1 μg/ml DAPI to reveal nuclear DNA and imaged by fluorescence microscopy.
Nuclear accumulation of GFP-Scd5p-ΔCT indicates that Scd5p may enter the nucleus and a C-terminal region of Scd5p may contain a signal for nuclear export. Although nuclear localization of GFP-Scd5p was never observed if the gene was expressed under control of its endogenous promoter in low copy, GFP-Scd5p was often found in the nucleus in addition to at the cortex when overexpressed from a multicopy (2μ) plasmid (unpublished data). Nuclear import and export for proteins larger than 40–50 kDa have been shown to be highly selective and regulated processes (Kaffman and O'Shea, 1999). Therefore, it is unlikely that overexpression of Scd5p (98 kDa) simply allowed passive diffusion through the nuclear pore complex (NPC). Interestingly, a previous genome-wide two-hybrid screen identified Scd5p as an interacting protein of Crm1p, a nuclear export transporter (Ito et al., 2001). Thus to test whether Scd5p undergoes Crm1-dependent nuclear-cytoplasmic shuttling, we examined GFP-Scd5p localization in a temperature-sensitive crm1-1 strain (Stade et al., 1997). Indeed GFP-Scd5p appeared in the nucleus upon shift of crm1-1 cells to 37°C, but not at 25°C and not in CRM1 cells at either temperature (Figure 4). Nuclear accumulation of GFP-Scd5p was seen as early as 15 min after shift to 37°C (unpublished data) and was more pronounced after 30-min incubation (Figure 4). This result provides strong evidence that Scd5p enters the nucleus and is exported in a Crm1p-dependent manner, but the rate of export is likely faster or more efficient than the rate of import at steady state.
Scd5p Contains a Functional NES at the C-terminus
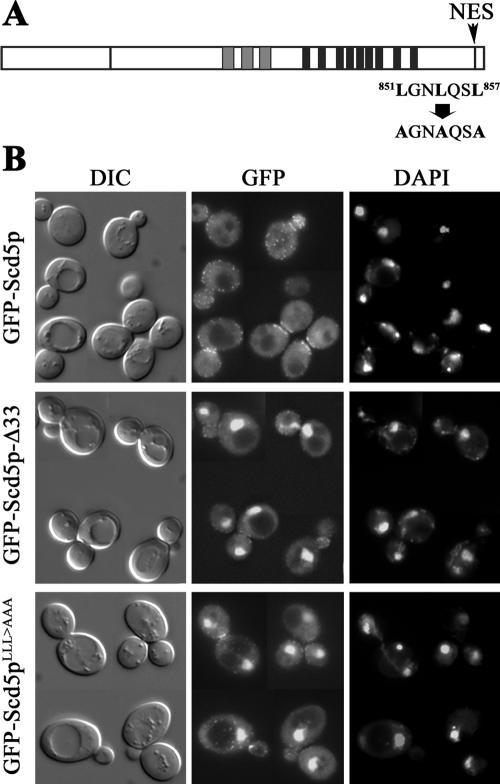
In general, the export receptor Crm1p recognizes a NES comprised of leucine- or other hydrophobic residue-rich motifs (e.g., LxxLxxLxL; Stade et al., 1997). Sequence analysis of Scd5p revealed a classic leucine-rich NES at the C-terminus (residues 851–857, LGNLQSL; Figure 5A). To test whether this short sequence can function as an NES, we first deleted the C-terminal 33 amino acids (residues 840–872) that possess the putative NES (GFP-Scd5p-Δ33) and expressed this mutant protein as the sole source of Scd5p. The expression level of GFP-Scd5p-Δ33 was similar to that of wild-type GFP-Scd5p (unpublished data). Fluorescence microscopy showed overlapping localization of GFP-Scd5p-Δ33 with DAPI-stained nuclei (Figure 5B), suggesting that the C-terminal leucine-rich sequence of Scd5p contains a functional NES. However, cortical localization at the cell periphery was also still observed. To further confirm that the leucine residues in the putative NES are critical for nuclear export of Scd5p, we mutagenized the three leucines to alanines (LLL>AAA; Figure 5A). Similar to GFP-Scd5p-Δ33, GFP-Scd5pLLL>AAA was found in the nucleus as well as at the cell cortex (Figure 5B). Therefore, Scd5p constitutively undergoes nuclear-cytoplasmic shuttling and it has a C-terminal NES that mediates Crm1p-dependent nuclear export.
Figure 5.
Scd5p contains a functional NES at the C-terminus. (A) Scd5p is shown with a potential leucine-rich nuclear export signal (LGNLQSL) at the C-terminus. (B) Cells expressing GFP-Scd5p-Δ33 (SL4714), which has a deletion of the C-terminal 33 amino acids, or GFP-Scd5pLLL>AAA (SL4725), which contains Leu-to-Ala mutations of the NES, were grown at 25°C in the presence of 0.1 μg/ml DAPI and examined by fluorescence microscopy.
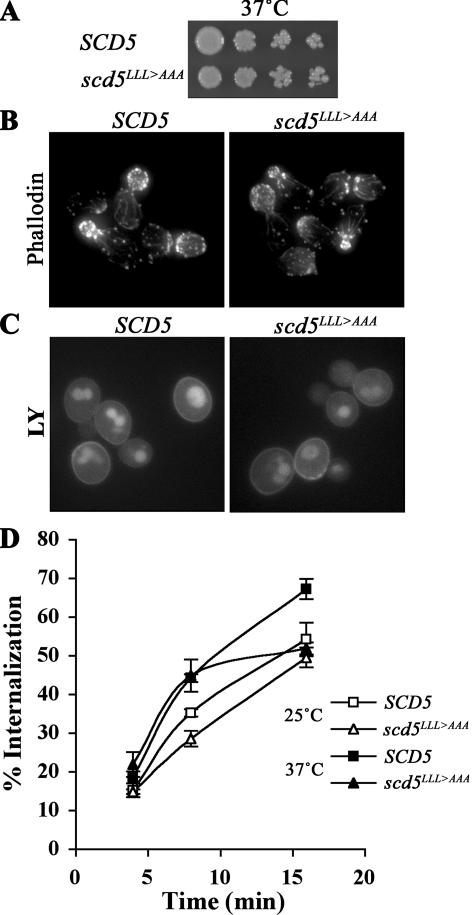
Growth, Actin Organization, and Endocytosis Are Relatively Normal in scd5LLL>AAA Cells
We next investigated whether the nuclear export defect of Scd5p would hamper Scd5p's function in actin organization and endocytosis. scd5LLL>AAA cells showed wild-type growth, relatively normal structures of actin patches and actin cables, and endocytosis of LY to the vacuole at 25°C (unpublished data) and at 37°C (Figure 6, A–C) was efficient. Similarly, α-factor internalization in scd5LLL>AAA cells was nearly identical to wild type, although at later times after shifting to 37°C endocytosis was slowed relative to SCD5 (Figure 6D). As shown above, although GFP-Scd5pLLL>AAA accumulated in the nucleus, it also localized at cortical patches and in the cytoplasm (Figure 5B), suggesting that this cortical/cytoplasmic pool of Scd5pLLL>AAA is sufficient to maintain relatively normal actin organization and endocytosis.
Figure 6.
Growth, actin, and endocytosis are relatively normal in scd5LLL>AAA cells. (A) Growth assay. SCD5 (SL4706) and scd5LLL>AAA (SL4726) were grown in YEPD medium at 25°C, diluted and spotted on YEPD, and grown for 3 d at 37°C. (B) Actin staining: wild-type (SL4418) and scd5LLL>AAA (SL4727) cells were grown to log-phase in YEPD at 25°C and shifted to 37°C for 2 h before fixation and actin staining. (C) LY uptake: wild-type (SL4706) and scd5LLL>AAA (SL4726) cells were preincubated at 37°C for 15 min before addition of LY. Cells were further incubated at 37°C for 1 h and immediately visualized using fluorescence microscopy. (D) Receptor-mediated endocytosis: wild-type (SL4436) and scd5LLL>AAA (SL4759) cells were preincubated at 37°C for 15 min and then 35S-labeled-α-factor was added. Samples were collected at indicated time points for determination of percent of cell-associated α-factor internalized.
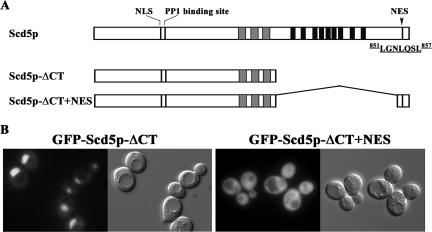
Addition of the NES Restores Cytoplasmic Localization and Suppresses the Growth, Actin, and Endocytic Defects of Scd5p-ΔCT
GFP-Scd5p-ΔCT is found mostly in the nucleus (Figure 3) because it lacks both the NES for export and the Scd5p cortical localization information of the 9R domain. Therefore, we tested whether addition of the NES to the C-terminal truncation of Scd5p could restore cytosolic localization. We constructed a GFP-tagged Scd5p-ΔCT+NES by fusing the coding region for residues 836–872 to the coding sequence for N-terminal amino acids 1–526 (Figure 7A). Confirming the role of the C-terminal NES for nuclear export, GFP-Scd5p-ΔCT+NES almost completely localized to the cytoplasm (Figure 7B). However, GFP-Scd5p-ΔCT+NES was not observed in cortical patches. Furthermore, neither GFP-Scd5p-ΔCT nor GFP-Scd5p-ΔCT+NES were in aggregates of ark1Δ prk1Δ cells (Supplementary Figure 1), supporting the role of the 9R region for cortical recruitment.
Figure 7.
Addition of the NES increases cytoplasmic localization of GFP-Scd5p-ΔCT. (A) Schematic diagram of Scd5p with its nuclear export signal at the C-terminus and the Scd5p-ΔCT and Scd5p-ΔCT+NES mutants. (B) scd5Δ cells expressing GFP-Scd5p-ΔCT (SL4642) or GFP-Scd5p-ΔCT+NES (SL4774) were examined by fluorescence microscopy at 25°C. Similar results were observed at 37°C.
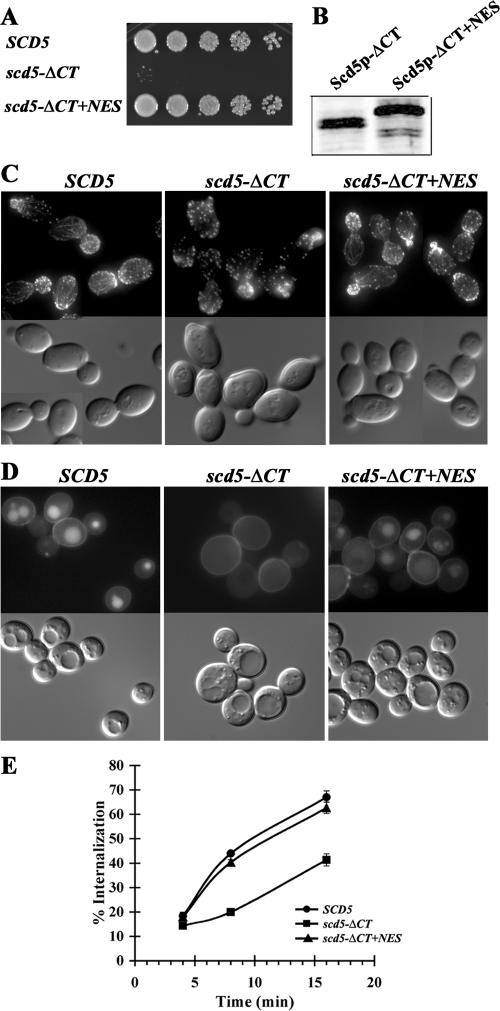
If the growth, actin, and endocytic phenotypes that we previously observed for scd5-ΔCT (Nelson et al., 1996; Henry et al., 2002) resulted from being trapped in the nucleus, we hypothesized that these defects might be rescued by addition of the NES. Consistent with this, although scd5-ΔCT was temperature-sensitive for growth, cells expressing Scd5p-ΔCT+NES grew well at 37°C (Figure 8A). The difference was not due to differences in levels of the proteins, because Scd5p-ΔCT+NES was expressed similarly to Scd5p-ΔCT (Figure 8B). Even at 37°C, actin structures were relatively normal in scd5-ΔCT+NES and the cells no longer had a thickened cell wall, which is notable in the scd5-ΔCT DIC image (Figure 8C). Finally, fluid phase and receptor-mediated endocytosis were reconstituted to near wild-type efficiency in scd5-ΔCT+NES cells (Figure 8, D and E).
Figure 8.
Growth, actin, and endocytic defects of scd5-ΔCT are suppressed by addition of the NES. (A) Growth: wild-type (SL4706), scd5-ΔCT (SL4751), and scd5-ΔCT+NES (SL4770) cells were grown in YEPD medium at 25°C, diluted and spotted on YEPD, and grown for 2 d at 37°C. (B) Western blot analysis. scd5-ΔCT (SL4751) and scd5-ΔCT+NES (SL4770) cells were grown at 25°C and shifted to 37°C for 1.5 h. Equal amounts of proteins from cell extracts were analyzed by immunoblotting with anti-Scd5p antibodies. (C) Actin staining: wild-type (SL4418), scd5-ΔCT (SL4397), and scd5-ΔCT+NES (SL4772) cells were grown to log-phase in YEPD at 25°C and shifted to 37°C for 2 h before fixation. Filamentous actin was stained with Alexa-568 phalloidin. Note thickened cell wall rim in the ΔCT DIC images. (D) LY accumulation: wild-type (SL4706), scd5-ΔCT (SL4751), and scd5-ΔCT+NES (SL4770) cells were preincubated at 37°C for 15 min before addition of LY. Cells were further incubated at 37°C for 1 h and immediately visualized using fluorescence microscopy. (E) Receptor-mediated endocytosis: wild-type (SL4436), scd5-ΔCT (SL4764), and scd5-ΔCT+NES (SL4784) cells were preincubated at 37°C for 15 min and then 35S-labled-α-factor was added. Samples were collected at indicated time points for determination of percent of cell-associated α-factor internalized.
These results indicate that the defects seen in the scd5-ΔCT mutant are caused by mislocalization to the nucleus. Furthermore, they support the conclusion that cytoplasmic, but not necessarily cortical localization, of Scd5p is required for its actin and endocytic roles.
Nuclear-cytoplasmic Shuttling of Scd5p Is Not Required for Its Function in Actin Organization and Endocytosis
A major question concerns whether Scd5p's roles in actin organization and endocytosis require or are regulated by nuclear-cytoplasmic shuttling. Although the cytoplasmic localization and functions of Scd5p-ΔCT were restored by addition of the NES, Scd5p-ΔCT+NES was still able to enter and exit the nucleus, and thus this mutant did not address this issue. To assess whether entry into the nucleus is required for Scd5p's cortical functions, we sought a mutant version of the protein that could not enter the nucleus.
In general, nuclear import of proteins larger than 40–50 kDa is mediated by the action of nuclear import receptors (Kaffman and O'Shea, 1999). We identified a potential bipartite Arg- or Lys-rich NLS in Scd5p (Figure 9A) and constructed a mutant in which amino acid residues 257–269 were deleted (Scd5p-ΔNLS). This region is close to the site where PP1 (encoded by GLC7 in yeast) binds Scd5p (KKVRF residues 272–276; Figure 9A), which we previously demonstrated is important for Scd5p's function in actin organization and endocytosis (Chang et al., 2002). However, two-hybrid analysis showed that deletion of the NLS had no effect on Glc7p binding, whereas the Scd5p PP1 binding site mutation (KKVRF(272–276) to AKAAA; Scd5p-PP1Δ2) greatly reduced interaction with Glc7p, as shown previously (Chang et al., 2002; Supplementary Figure 2). Therefore any changes in the localization or function of Scd5p caused by the NLS deletion is not likely due to effects on PP1 binding.
Figure 9.
Deletion of the NLS in Scd5p prevents nuclear localization, but has no effect on actin organization and endocytosis. (A) Schematic diagram of Scd5p and Scd5p-ΔNLS. (B) SL4620 (crm1-1) was transformed with pJSC34 (URA3, CEN, GFP-SCD5; WT) or pJSC44 (URA3, CEN, GFP-scd5-ΔNLS) (ΔNLS). Cells were pregrown at 25°C in–uracil medium with 0.5 μg/ml DAPI and shifted to 25 or 37°C for 1 h. Arrows point to nuclear regions in GFP panels at 37°C. Note that Scd5p accumulates in the nucleus, whereas there is a zone of exclusion of Scd5p-ΔNLS from the nucleus in crm1-1 at 37°C. (C) SL4687 (GFP-scd5-ΔNLS) was shifted to 37°C for 2.5 h before fixation and staining with Alexa-phalloidin. Similar results were obtained without temperature shift. (D) LY uptake was analyzed in SL5227 (scd5-ΔNLS) and SL5228 (SCD5) with a 15-min preshift to 37°C before initiating internalization. α-factor internalization was also identical for the WT and ΔNLS cells at 25 or 37°C (unpublished data).
To determine whether the putative NLS is a functional import signal, we localized a GFP-tagged version of the mutant protein, taking advantage of the crm1-1 nuclear export mutant. GFP-Scd5p-ΔNLS still localized to cortical patches, in both wild-type cells (unpublished data) or in crm1-1 cells at 25°C (Figure 9A). Although shift of crm1-1 cells to 37°C caused strong accumulation of wild-type GFP-Scd5p in the nucleus, as seen above in Figure 4, GFP-Scd5p-ΔNLS was excluded, even after incubation for 1 h at the nonpermissive temperature (Figure 9B). However, scd5-ΔNLS cells did not show temperature-sensitive growth (unpublished data) or actin organization defects (Figure 9C), and fluid phase endocytosis (Figure 9D) and α-factor uptake (unpublished data) were normal.
Overall, these results indicate that although Scd5p enters the nucleus, nuclear-cytoplasmic shuttling does not regulate Scd5p's function in actin organization and endocytosis. Therefore, Scd5p may have another cytosolic function regulated by nuclear-cytoplasmic shuttling or it may have a nuclear function.
DISCUSSION
Previously we showed that Scd5p, a cortical actin patch-associated protein, has a critical role in actin organization and endocytosis (Henry et al., 2002). A number of findings lead us to propose that recruitment of Scd5p to the cortex is not absolutely required for these functions, and that, instead, cytoplasmic localization may be sufficient. First, deletion of the 9R region of Scd5p significantly reduced cortical patch localization but did not significantly affect cortical actin patches and endocytosis. Moreover, displacement of GFP-Scd5p from the cell cortex by overexpression of the CT region also did not affect these functions. Finally, translocation of Scd5p-ΔCT/Δ338 from the nucleus to cytoplasm by adding back the NES rescued growth, actin, and endocytic defects, but did not restore translocation to the cortex. We cannot completely rule out that transient cortical association occurs in cells lacking the 9R domain; however, the lack of significant association of Scd5p-Δ9R or Scd5p-ΔCT+NES with the actin aggregates in ark1Δ prk1Δ cells argues against this. Therefore, although Scd5p is recruited to cortical patches through the 9R, its function in actin organization and endocytosis may be relatively efficient when performed in the cytoplasm.
It is commonly accepted that most endocytic factors are recruited to sites at the plasma membrane for their activity. Therefore, mislocalization of such proteins from the endocytic membrane often causes severe actin and endocytic defects (Yang et al., 1999; Zeng et al., 2001; Warren et al., 2002). How can Scd5p function in the cytoplasm then? One attractive possibility is that Scd5p targets PP1/Glc7p to dephosphorylate other endocytic proteins that are disassembled from cortical endocytic complexes by phosphorylation. Many endocytic factors, including Pan1p, Sla1p, Ent1/2p, Yap1801p, and Scd5p, are phosphorylated by Prk1p, one of the actin-regulating kinases (Watson et al., 2001; Zeng et al., 2001; Henry et al., 2003; Huang et al., 2003). In addition, Prk1p-mediated phosphorylation inhibits interactions of Pan1p with Sla1p and End3p (Zeng et al., 2001). Therefore, it has been proposed that endocytic complexes recruited to the plasma membrane for cargo selection and membrane invagination are disassembled and/or inactivated by phosphorylation before or at the time of vesicle scission and release (Zeng et al., 2001; Kaksonen et al., 2003; Sekiya-Kawasaki et al., 2003).
Previously we showed that a mutation of the PP1-binding site of Scd5p (scd5-pp1Δ2) severely impaired actin organization and endocytosis (Chang et al., 2002). A number of PP1 regulatory subunits containing the consensus PP1 binding site (R/K-x0–1-V/I-x-F) target PP1/Glc7p to physiological substrates or subcellular locations to perform specific dephosphorylation in vivo (Hubbard et al., 1990; Stark, 1996; Egloff et al., 1997). Furthermore, a glc7 mutant has been shown to affect cortical actin organization (Andrews and Stark, 2000). Consistent with these results, deletion of PRK1 suppresses the phenotypes of scd5-Δ338/ΔCT and scd5-pp1Δ2 (Henry et al., 2003; Huang et al., 2003). We speculate that Scd5p-PP1 can reverse Prk1p phosphorylation by targeting PP1 to phosphorylated and disassembled or inactivated endocytic factors in the cytoplasm, thus modulating their activity and competence for reassembly during a new round of endocytosis. This would be similar to the role of Ca2+-dependent dephosphorylation of endocytic factors, such as dynamin, amphiphysin, synaptojanin, AP180, Epsin, and Eps15, to allow a burst of clathrin-dependent endocytosis at nerve terminals after synaptic vesicle release (Slepnev et al., 1998; Cousin and Robinson, 2001). The influx of Ca2+ during neurotransmission is thought to activate the phosphatase calcineurin for compensatory recycling of synaptic vesicle membranes.
Scd5p Undergoes Nuclear-cytoplasmic Shuttling in a Crm1p-dependent Manner
Our current study reports an unexpected observation that Scd5p constitutively shuttles in and out of the nucleus. Usually, the active trafficking of proteins larger than 40–50 kDa through the nuclear pore complex is mediated by nuclear import and export receptors, which recognize specific signals, termed NLS and NES, respectively (Kaffman and O'Shea, 1999). Although Scd5p, a 98-kDa protein, showed predominantly cytoplasmic and cortical localization at steady state, it accumulated in the nucleus when overexpressed (J. Chang, unpublished observations), when expressed in crm1-1 cells at 37°C or when the candidate NES was removed or mutated. This implies that the Crm1p-mediated export of Scd5p is faster or more efficient than the nuclear import pathway.
Why does Scd5p shuttle in and out of the nucleus? A number of recent studies in animal cells have shown that some endocytic factors, whose major functions are thought to be at the plasma membrane, also undergo nuclear-cytoplasmic shuttling (Hyman et al., 2000; Vecchi et al., 2001; Poupon et al., 2002; Scott et al., 2002; Mills et al., 2005). Such proteins include Eps15, epsin, CALM, α-adaptin, Huntingtin interacting protein 1 (HIP1), and β-arrestin-2. Similarly, the yeast phosphoinositide kinase Mss4p necessary for production of PI(4,5)P2 at the plasma membrane undergoes nuclear cytoplasmic shuttling (Audhya and Emr, 2003). Our observations for Scd5p provide additional evidence that this novel feature of endocytic factors is conserved between yeast and animals.
At the present time, the precise roles of the nuclear-cytoplasmic shuttling of these endocytic factors are still poorly understood. It is hypothesized that shuttling of Mss4p is a mechanism to regulate PI(4,5)P2 production at the plasma membrane needed for actin organization and endocytosis (Audhya and Emr, 2003). We found that deletion of the NLS basic sequence in Scd5p prevented nuclear import but had little effect on actin and endocytic functions. This suggests that nuclear cytoplasmic shuttling is not required for regulation of Scd5p's cortical/endocytic functions. Another possibility is that endocytic proteins in the nucleus may act as transcriptional modulators, because epsin interacts with the transcription factor PLZF (Hyman et al., 2000), Eps15 and CALM positively regulate transcription of a Gal4-based reporter gene (Vecchi et al., 2001), and HIP1 (related to yeast endocytic factor Sla2p) enters the nucleus and coactivates androgen-dependent transcription upon hormone stimulation (Mills et al., 2005). A recent study has shown that inhibition of clathrin-mediated endocytosis increases the level of dynamin, a GTPase required for CCV formation, suggesting that the nuclear-cytoplasmic shuttling of endocytic proteins could provide an efficient mechanism to regulate genes whose products are involved in endocytosis (Iversen et al., 2003; Benmerah, 2004). Alternatively, nuclearcytoplasmic shuttling of endocytic components may serve to relocate binding partners to regulate their function. The constitutive nuclear-cytoplasmic shuttling of β-arrestin 2 was shown to be required for the cytoplasmic relocation of JNK3 and Mdm2, an ubiquitin ligase (Scott et al., 2002; Wang et al., 2003). Likewise, Scd5p in the nucleus may modulate transcription or alter subcellular localization of nuclear binding partners.
Our studies cannot rule out that Scd5p has another cytosolic function regulated by nuclear-cytoplasmic shuttling. Another intriguing possibility is that Scd5p is multifunctional and has an independent PP1 targeting function in the nucleus. PP1 has many different nuclear functions, such as in cell cycle progression at G2/M, kinetochore attachment, meiosis, 3′-end primary transcript processing, transcription termination from polII promoters, and mRNA export (Hisamoto et al., 1994; Sassoon et al., 1999; Andrews and Stark, 2000; Bailis and Roeder, 2000; Nedea et al., 2003; Peng et al., 2003; Gilbert and Guthrie, 2004). However, in many cases the targeting subunits involved in these nuclear roles of PP1 have not been identified. Therefore, important work for the future will be to determine whether Scd5p has a nuclear function.
Supplementary Material
Acknowledgments
We thank Clarence Chan, Marion Carlson, Elizabeth Craig, Alan Tartakoff, David Drubin, and Michael Hall who provided strains, plasmids, and antibodies. We acknowledge the expert technical assistance of O. Theresa Torres and Thomas Newpher for creation of the GST-CT fusion construct. We also thank Beatriz Fontoura, Thomas Newpher, and John Collette for helpful discussions and for critical reading of this manuscript. K.R.H. was supported by an individual National Research Service Award Minority Predoctoral Fellowship (F31 GM20082). This work was funded by National Institutes of Health Grant R01 GM55796 (S.K.L.) and SAF2002-04707 from the Ministerio de Ciencia y Technologia (M.G.).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05–10–0936) on October 26, 2005.
Abbreviations used: PP1, protein phosphatase-1; 3R, three repeat; 9R, nine repeat; NES, nuclear export signal; NLS, nuclear localization signal; CT, carboxy-terminal domain; PI(4,5)P2, phosphatidylinositol-4,5-bisphosphate; ENTH, epsin N-terminal homology; ANTH, AP180 N-terminal homology; 5-FOA, 5 fluoroorotic acid; YEPD, yeast extract/peptone/dextrose; DIC, differential interference contrast; LY, Lucifer yellow; GST, glutathione-S-transferase.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Adams, A. E., and Pringle, J. R. (1984). Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J. Cell Biol. 98, 934–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar, R. C., Watson, H. A., and Wendland, B. (2003). The yeast epsin Ent1 is recruited to membranes through multiple independent interactions. J. Biol. Chem. 278, 10737–10743. [DOI] [PubMed] [Google Scholar]
- Andrews, P. D., and Stark, M. J. (2000). Type 1 protein phosphatase is required for maintenance of cell wall integrity, morphogenesis and cell cycle progression in Saccharomyces cerevisiae. J. Cell Sci. 113, 507–520. [DOI] [PubMed] [Google Scholar]
- Audhya, A., and Emr, S. D. (2003). Regulation of PI4,5P2 synthesis by nuclear-cytoplasmic shuttling of the Mss4 lipid kinase. EMBO J. 22, 4223–4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailis, J. M., and Roeder, G. S. (2000). Pachytene exit controlled by reversal of Mek1-dependent phosphorylation. Cell 101, 211–221. [DOI] [PubMed] [Google Scholar]
- Benmerah, A. (2004). Endocytosis: signaling from endocytic membranes to the nucleus. Curr. Biol. 14, R314–R316. [DOI] [PubMed] [Google Scholar]
- Boeke, J. D., LaCroute, F., and Fink, G. R. (1984). A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol. Gen. Genet. 197, 345–346. [DOI] [PubMed] [Google Scholar]
- Chang, J. S., Henry, K., Wolf, B. L., Geli, M., and Lemmon, S. K. (2002). Protein phosphatase-1 binding to Scd5p is important for regulation of actin organization and endocytosis in yeast. J. Biol. Chem. 277, 48002–48008. [DOI] [PubMed] [Google Scholar]
- Cope, M. J., Yang, S., Shang, C., and Drubin, D. G. (1999). Novel protein kinases Ark1p and Prk1p associate with and regulate the cortical actin cytoskeleton in budding yeast. J. Cell Biol. 144, 1203–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin, M. A., and Robinson, P. J. (2001). The dephosphins: dephosphorylation by calcineurin triggers synaptic vesicle endocytosis. Trends Neurosci. 24, 659–665. [DOI] [PubMed] [Google Scholar]
- Dulic, V., Egerton, M., Elguindi, I., Raths, S., Singer, B., and Riezman, H. (1991). Yeast endocytosis assays. Methods Enzymol. 194, 697–710. [DOI] [PubMed] [Google Scholar]
- Duncan, M. C., Cope, M. J., Goode, B. L., Wendland, B., and Drubin, D. G. (2001). Yeast Eps15-like endocytic protein, Pan1p, activates the Arp2/3 complex. Nat. Cell Biol. 3, 687–690. [DOI] [PubMed] [Google Scholar]
- Egloff, M. P., Johnson, D. F., Moorhead, G., Cohen, P. T., Cohen, P., and Barford, D. (1997). Structural basis for the recognition of regulatory subunits by the catalytic subunit of protein phosphatase 1. EMBO J. 16, 1876–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engqvist-Goldstein, A. E., and Drubin, D. G. (2003). Actin assembly and endocytosis: from yeast to mammals. Annu. Rev. Cell Dev. Biol. 19, 287–332. [DOI] [PubMed] [Google Scholar]
- Gietz, R. D., Schiestl, R. H., Willems, A. R., and Woods, R. A. (1995). Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast 11, 355–360. [DOI] [PubMed] [Google Scholar]
- Gilbert, W., and Guthrie, C. (2004). The Glc7p nuclear phosphatase promotes mRNA export by facilitating association of Mex67p with mRNA. Mol. Cell 13, 201–212. [DOI] [PubMed] [Google Scholar]
- Gourlay, C. W., Dewar, H., Warren, D. T., Costa, R., Satish, N., and Ayscough, K. R. (2003). An interaction between Sla1p and Sla2p plays a role in regulating actin dynamics and endocytosis in budding yeast. J. Cell Sci. 116, 2551–2564. [DOI] [PubMed] [Google Scholar]
- Guthrie, C., and Fink, G. R. (eds.) (1991). Guide to Yeast Genetics and Molecular Biology, San Diego: Academic Press.
- Henry, K. R., D'Hondt, K., Chang, J., Newpher, T., Huang, K., Hudson, R. T., Riezman, H., and Lemmon, S. K. (2002). Scd5p and clathrin function are important for cortical actin organization, endocytosis, and localization of Sla2p in yeast. Mol. Biol. Cell 13, 2607–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry, K. R., D'Hondt, K., Chang, J. S., Nix, D. A., Cope, M. J., Chan, C. S., Drubin, D. G., and Lemmon, S. K. (2003). The actin-regulating kinase Prk1p negatively regulates Scd5p, a suppressor of clathrin deficiency, in actin organization and endocytosis. Curr. Biol. 13, 1564–1569. [DOI] [PubMed] [Google Scholar]
- Hisamoto, N., Sugimoto, K., and Matsumoto, K. (1994). The Glc7 type 1 protein phosphatase of Saccharomyces cerevisiae is required for cell cycle progression in G2/M. Mol. Cell. Biol. 14, 3158–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, B., Zeng, G., Ng, A. Y., and Cai, M. (2003). Identification of novel recognition motifs and regulatory targets for the yeast actin-regulating kinase Prk1p. Mol. Biol. Cell 14, 4871–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard, M. J., Dent, P., Smythe, C., and Cohen, P. (1990). Targetting of protein phosphatase 1 to the sarcoplasmic reticulum of rabbit skeletal muscle by a protein that is very similar or identical to the G subunit that directs the enzyme to glycogen. Eur. J. Biochem. 189, 243–249. [DOI] [PubMed] [Google Scholar]
- Hyman, J., Chen, H., Di Fiore, P. P., De Camilli, P., and Brunger, A. T. (2000). Epsin 1 undergoes nucleocytosolic shuttling and its eps15 interactor NH(2)-terminal homology (ENTH) domain, structurally similar to Armadillo and HEAT repeats, interacts with the transcription factor promyelocytic leukemia Zn(2)+ finger protein (PLZF). J. Cell Biol. 149, 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, T., Chiba, T., Ozawa, R., Yoshida, M., Hattori, M., and Sakaki, Y. (2001). A comprehensive two-hybrid analysis to explore the yeast protein interactome. Proc. Natl. Acad. Sci. USA 98, 4569–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh, T., and Takenawa, T. (2004). Regulation of endocytosis by phosphatidylinositol 4,5-bisphosphate and ENTH proteins. Curr. Top. Microbiol. Immunol. 282, 31–47. [DOI] [PubMed] [Google Scholar]
- Iversen, T. G., Skretting, G., van Deurs, B., and Sandvig, K. (2003). Clathrincoated pits with long, dynamin-wrapped necks upon expression of a clathrin antisense RNA. Proc. Natl. Acad. Sci. USA 100, 5175–5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaffman, A., and O'Shea, E. K. (1999). Regulation of nuclear localization: a key to a door. Annu. Rev. Cell Dev. Biol. 15, 291–339. [DOI] [PubMed] [Google Scholar]
- Kaksonen, M., Sun, Y., and Drubin, D. G. (2003). A pathway for association of receptors, adaptors, and actin during endocytic internalization. Cell 115, 475–487. [DOI] [PubMed] [Google Scholar]
- Kirchhausen, T. (2000). Clathrin. Annu. Rev. Biochem. 69, 699–727. [DOI] [PubMed] [Google Scholar]
- Legendre-Guillemin, V., Wasiak, S., Hussain, N. K., Angers, A., and McPherson, P. S. (2004). ENTH/ANTH proteins and clathrin-mediated membrane budding. J. Cell Sci. 117, 9–18. [DOI] [PubMed] [Google Scholar]
- Longtine, M. S., McKenzie, A., 3rd, Demarini, D. J., Shah, N. G., Wach, A., Brachat, A., Philippsen, P., and Pringle, J. R. (1998). Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953–961. [DOI] [PubMed] [Google Scholar]
- Merrifield, C. J., Feldman, M. E., Wan, L., and Almers, W. (2002). Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nat. Cell. Biol. 4, 691–698. [DOI] [PubMed] [Google Scholar]
- Mills, I. G., Gaughan, L., Robson, C., Ross, T., McCracken, S., Kelly, J., and Neal, D. E. (2005). Huntingtin interacting protein 1 modulates the transcriptional activity of nuclear hormone receptors. J. Cell Biol. 170, 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn, A. L., Stevenson, B. J., Geli, M. I., and Riezman, H. (1995). end5, end6, and end 7, mutations that cause actin delocalization and block the internalization step of endocytosis in Saccharomyces cerevisiae. Mol. Biol. Cell 6, 1721–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedea, E., He, X., Kim, M., Pootoolal, J., Zhong, G., Canadien, V., Hughes, T., Buratowski, S., Moore, C. L., and Greenblatt, J. (2003). Organization and function of APT, a subcomplex of the yeast cleavage and polyadenylation factor involved in the formation of mRNA and small nucleolar RNA 3′-ends. J. Biol. Chem. 278, 33000–33010. [DOI] [PubMed] [Google Scholar]
- Nelson, K. K., Holmer, M., and Lemmon, S. K. (1996). SCD5, a suppressor of clathrin deficiency, encodes a novel protein with a late secretory function in yeast. Mol. Biol. Cell 7, 245–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, K. K., and Lemmon, S. K. (1993). Suppressors of clathrin deficiency: overexpression of ubiquitin rescues lethal strains of clathrin-deficient Saccharomyces cerevisiae. Mol. Cell. Biol. 13, 521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newpher, T. M., Smith, R. P., Lemmon, V., and Lemmon, S. K. (2005). In vivo dynamics of clathrin and its adaptor-dependent recruitment to the actinbased endocytic machinery in yeast. Dev. Cell 9, 87–98. [DOI] [PubMed] [Google Scholar]
- Peng, W. T. et al. (2003). A panoramic view of yeast noncoding RNA processing. Cell 113, 919–933. [DOI] [PubMed] [Google Scholar]
- Poupon, V., Polo, S., Vecchi, M., Martin, G., Dautry-Varsat, A., Cerf-Bensussan, N., Di Fiore, P. P., and Benmerah, A. (2002). Differential nucleocytoplasmic trafficking between the related endocytic proteins Eps15 and Eps15R. J. Biol. Chem. 277, 8941–8948. [DOI] [PubMed] [Google Scholar]
- Sassoon, I., Severin, F. F., Andrews, P. D., Taba, M. R., Kaplan, K. B., Ashford, A. J., Stark, M. J., Sorger, P. K., and Hyman, A. A. (1999). Regulation of Saccharomyces cerevisiae kinetochores by the type 1 phosphatase Glc7p. Genes Dev. 13, 545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, M. G., Le Rouzic, E., Perianin, A., Pierotti, V., Enslen, H., Benichou, S., Marullo, S., and Benmerah, A. (2002). Differential nucleocytoplasmic shuttling of beta-arrestins. Characterization of a leucine-rich nuclear export signal in beta-arrestin2. J. Biol. Chem. 277, 37693–37701. [DOI] [PubMed] [Google Scholar]
- Sekiya-Kawasaki, M. et al. (2003). Dynamic phosphoregulation of the cortical actin cytoskeleton and endocytic machinery revealed by real-time chemical genetic analysis. J. Cell Biol. 162, 765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slepnev, V. I., Ochoa, G. C., Butler, M. H., Grabs, D., and Camilli, P. D. (1998). Role of phosphorylation in regulation of the assembly of endocytic coat complexes. Science 281, 821–824. [DOI] [PubMed] [Google Scholar]
- Stade, K., Ford, C. S., Guthrie, C., and Weis, K. (1997). Exportin 1 (Crm1p) is an essential nuclear export factor. Cell 90, 1041–1050. [DOI] [PubMed] [Google Scholar]
- Stamenova, S. D., Dunn, R., Adler, A. S., and Hicke, L. (2004). The Rsp5 ubiquitin ligase binds to and ubiquitinates members of the yeast CIN85-endophilin complex, Sla1-Rvs167. J. Biol. Chem. 279, 16017–16025. [DOI] [PubMed] [Google Scholar]
- Stark, M. J. (1996). Yeast protein serine/threonine phosphatases: multiple roles and diverse regulation. Yeast 12, 1647–1675. [DOI] [PubMed] [Google Scholar]
- Stepp, J. D., Pellicena-Palle, A., Hamilton, S., Kirchhausen, T., and Lemmon, S. K. (1995). A late Golgi sorting function for Saccharomyces cerevisiae Apm1p, but not for Apm2p, a second yeast clathrin AP medium chain-related protein. Mol. Biol. Cell 6, 41–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, H. Y., Xu, J., and Cai, M. (2000). Pan1p, End3p, and S1a1p, three yeast proteins required for normal cortical actin cytoskeleton organization, associate with each other and play essential roles in cell wall morphogenesis. Mol. Cell. Biol. 20, 12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toshima, J., Toshima, J. Y., Martin, A. C., and Drubin, D. G. (2005). Phosphoregulation of Arp2/3-dependent actin assembly during receptor-mediated endocytosis. Nat. Cell Biol. 7, 246–254. [DOI] [PubMed] [Google Scholar]
- Vecchi, M., Polo, S., Poupon, V., van de Loo, J. W., Benmerah, A., and Di Fiore, P. P. (2001). Nucleocytoplasmic shuttling of endocytic proteins. J. Cell Biol. 153, 1511–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, P., Wu, Y., Ge, X., Ma, L., and Pei, G. (2003). Subcellular localization of beta-arrestins is determined by their intact N domain and the nuclear export signal at the C terminus. J. Biol. Chem. 278, 11648–11653. [DOI] [PubMed] [Google Scholar]
- Warren, D. T., Andrews, P. D., Gourlay, C. W., and Ayscough, K. R. (2002). Sla1p couples the yeast endocytic machinery to proteins regulating actin dynamics. J. Cell Sci. 115, 1703–1715. [DOI] [PubMed] [Google Scholar]
- Watson, H. A., Cope, M. J., Groen, A. C., Drubin, D. G., and Wendland, B. (2001). In vivo role for actin-regulating kinases in endocytosis and yeast epsin phosphorylation. Mol. Biol. Cell 12, 3668–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland, B., and Emr, S. D. (1998). Pan1p, yeast Eps15, functions as a multivalent adaptor that coordinates protein-protein interactions essential for endocytosis. J. Cell Biol. 141, 71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland, B., Steece, K. E., and Emr, S. D. (1999). Yeast epsins contain an essential N-terminal ENTH domain, bind clathrin and are required for endocytosis. EMBO J. 18, 4383–4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, S., Cope, M. J., and Drubin, D. G. (1999). Sla2p is associated with the yeast cortical actin cytoskeleton via redundant localization signals. Mol. Biol. Cell 10, 2265–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarar, D., Waterman-Storer, C. M., and Schmid, S. L. (2005). A dynamic actin cytoskeleton functions at multiple stages of clathrin-mediated endocytosis. Mol. Biol. Cell 16, 964–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, G., and Cai, M. (1999). Regulation of the actin cytoskeleton organization in yeast by a novel serine/threonine kinase Prk1p. J. Cell Biol. 144, 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, G., Yu, X., and Cai, M. (2001). Regulation of yeast actin cytoskeleton-regulatory complex Pan1p/Sla1p/End3p by serine/threonine kinase Prk1p. Mol. Biol. Cell 12, 3759–3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.