Abstract
AKAP121 focuses distinct signaling events from membrane to mitochondria by binding and targeting cAMP-dependent protein kinase (PKA), protein tyrosine phosphatase (PTPD1), and mRNA. We find that AKAP121 also targets src tyrosine kinase to mitochondria via PTPD1. AKAP121 increased src-dependent phosphorylation of mitochondrial substrates and enhanced the activity of cytochrome c oxidase, a component of the mitochondrial respiratory chain. Mitochondrial membrane potential and ATP oxidative synthesis were enhanced by AKAP121 in an src- and PKA-dependent manner. Finally, siRNA-mediated silencing of endogenous AKAP121 drastically impaired synthesis and accumulation of mitochondrial ATP. These findings indicate that AKAP121, through its role in enhancing cAMP and tyrosine kinase signaling to distal organelles, is an important regulator in mitochondrial metabolism.
INTRODUCTION
Protein kinase A (PKA) is an essential mediator in most cAMP-dependent signaling pathways. A family of proteins named A-kinase anchor proteins (AKAPs) has been identified that enhance cAMP-dependent PKA signaling pathways (Rubin, 1994; Gray et al., 1998; McKnight et al., 1998; Feliciello et al., 2001; Houslay and Adams, 2003; Tasken and Aandahl, 2004; Taylor et al., 2004; Wong and Scott, 2004). AKAP121 (also called D-AKAP1), AKAP149, and AKAP84 arise from a single gene by alternative pre-mRNA splicing (Lin et al., 1995; Trendelenburg et al., 1996; Chen et al., 1997; Huang et al., 1997, 1999; Furusawa et al., 2002). AKAP121 and AKAP84 tether PKA to the mitochondrial outer surface. This localization is mediated by the interaction of AKAP121 and AKAP84 with β tubulin, an integral component of mitochondrial outer membrane (Cardone et al., 2002). AKAP121 is widely expressed in several tissues and its accumulation is regulated at the transcriptional level by the cAMP/PKA pathway (Feliciello et al., 1998). Anchoring of PKA to mitochondria supports cAMP signaling and suppresses apoptosis (Harada et al., 1999; Affaitati et al., 2003). AKAP121, via a KH domain at its COOH-terminus, binds at least two mRNAs that encode mitochondrial proteins (Ginsberg et al., 2003; Ranganathan et al., 2005). This multicomponent system, reminiscent of other AKAP complexes at cell membranes, ensures efficient translation and import of nuclear-encoded mitochondrial proteins. It is suggested that PKA may phosphorylate some of these proteins cotranslationally, as well as acting on AKAP121 itself to regulate the stability of the RNA-AKAP121 complex (Ginsberg et al., 2003; Feliciello et al., 2005).
In addition, AKAP121 and AKAP84 bind the central core of PTPD1, a classical nonreceptor protein tyrosine phosphatase (Moller et al., 1994). PTPD1 binds to and activates src, enhancing EGF-dependent mitogenic signaling (Cardone et al., 2004). By translocating PTPD1 to the outer membrane of mitochondria, AKAP121 inhibits PTPD1-dependent EGF signaling to the nucleus. These data suggest a model whereby AKAP121, by targeting PTPD1/src complex to mitochondria, may shift the focus of tyrosine kinase signaling from membrane to specific distal organelles, such as mitochondria (Feliciello et al., 2005).
We tested this hypothesis and found that AKAP121, indeed, targets src tyrosine kinase to mitochondria. By manipulating the localization and expression of AKAP121, we were able to modulate cAMP- and src-dependent signaling to mitochondria, affecting phosphorylation of mitochondrial substrates, activity of components of the respiratory chain, mitochondrial membrane potential (ΔΨm) and oxidative synthesis of ATP.
MATERIALS AND METHODS
Cell Lines
The human embryonic kidney cell line HEK293 was cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal calf serum (FCS) in an atmosphere of 5% CO2. Where indicated, HEK293 cells were stably transfected with a CMV-G418R vector expresssing AKAP121 and selected for 4 wk in medium containing G418 (800 μg/ml). Resistant clones were isolated, screened for expression of the transgene, and pooled (10 and 5 independent positive clones for PTPD1 and CMV, respectively). Pools were expanded and grown in medium supplemented with 400 μg/ml G418. GC2 cells were derived from primary mouse preleptotene spermatocytes by stable cotransfection with transgenes encoding SV40 large T-antigen and a temperature-sensitive variant of the p53 transcriptional regulator protein (Wolkolwicz et al., 1996). GC2 cells were grown at 37°C in DMEM supplemented with 10% FCS. Primary cultures of human fibroblasts were obtained from cutaneous biopsy of healthy subjects. Primary human fibroblasts and NIH3T3 cells were propagated in DMEM medium supplemented with 10% calf serum.
Antibodies and Chemicals
Polyclonal anti-AKAP121 antibody was purchased from Santa Cruz (C-20; Santa Cruz, CA). We also used an anti-AKAP121 polyclonal antibody that has been previously described (Chen et al., 1997). Anti-PTPD1 polyclonal antibody was prepared as previously described (Moller et al., 1994). Mouse monoclonal anti-src antibody was purchased from Oncogene Research Products (Boston, MA); anti-COXII from Molecular Probes (Eugene, OR); anti-hemoagglutinin epitope (HA.11) from Covance (Madison, WI); anti-tubulin from Sigma (St. Louis, MO); anti-SOD monoclonal antibody from Walter Occhiena; anti-AKT from Santa Cruz; CPT-cAMP from Sigma; and H89 and PP2 from Calbiochem (La Jolla, CA).
Plasmids and Transfection
Mouse pCEP4-AKAP121 cDNA was a gift of Dr C. Rubin (Albert Einstein College of Medicine, New York). An AKAP84 mutant lacking the first 30 amino acids was generated by PCR using specific oligonucleotides. The PCR product was subcloned in the CMV vector. cDNA coding the kinase-inactive form of Src (Lys259 changed to methionine) was cloned into pSG5 (Barone and Courtneidge, 1996) and was kindly provided by Prof. A. Migliaccio (Second University of Naples, Italy). The vector encoding for human PTPD1 was previously described (Moller et al., 1994). A small DNA insert (∼70 base pairs) encoding for short hairpin RNA targeting mouse AKAP121 (nucleotides 301–321, ATG + 1) was subcloned in pRNA-H1/neo vector (GenScript, Piscataway, NJ). Blast search confirmed that this sequence specifically recognizes mouse. A scrambled sequence subcloned in the same vector was used as experimental control. siRNA vectors were transiently transfected using the lipofectamine protocol. All plasmids were purified using QIAGEN tip columns (Qiagen, Chatsworth, CA) and sequenced using the CEQ2000 DNA Analysis System and a Beckman automated sequencer (Fullerton, CA).
Immunoprecipitation and Immunoblot Analysis
Cells were homogenized in lysis buffer (20 mM Tris-HCl, pH 7.4, 0.15 M NaCl, 10 mM EDTA, 0.25% Triton X-100, 0.05% Tween-20, 0.02% sodium azide) containing aprotinin (5 μg/ml), leupeptin (10 μg/ml), pepstatin (2 μg/ml), and 0.5 mM phenylmethylsulfonyl fluoride. The lysates were cleared by centrifugation at 15,000 × g for 15 min. Cell lysates (2 mg) were immunoprecipitated with the indicated antibodies. An aliquot of cell lysate (100 μg) or immunoprecipitates were resolved by SDS-PAGE gel and transferred to Immobilon P membrane. The immunoblot analysis was performed as previously described (Cardone et al., 2004). Chemoluminescent (ECL) signals were quantified by scanning densitometry (Molecular Dynamics, Sunnyvale, CA). Highly purified mitochondria and supernatant fraction were isolated as described (Hovius et al., 1990).
Immunofluorescence Analysis
Cells were rinsed with phosphate-buffered saline (PBS) and fixed in 3.7% formaldehyde for 20 min. After permeabilization with 0.5% Triton X-100 in PBS for 5 min, the cells were incubated with PBS 1×/0.1 mg/ml bovine serum albumin for 1 h at room temperature. Double immunofluorescence was carried out with the following antibodies: anti-superoxide dismutase monoclonal (1/200), anti-AKAP121 goat polyclonal (1/200; Santa Cruz, sc-6439), anti-PTPD1 rabbit polyclonal (1/200), and anti-AKAP121/84 rabbit polyclonal (1/100). Fluorescein- or rhodamine-tagged anti-rabbit and anti-mouse IgG secondary antibodies were used. Coverslips were analyzed by confocal microscopy.
Assays for Cytochrome c Oxidase Activity and Mitochondrial ATP Synthesis
The activity of cytochrome c oxidase on purified mitochondria was determined by spectrophotometric measurement of the rate of reduced cytochrome c oxidation at 550 nm (Couperstein and Lazarov, 1951). Cytochrome c (type VI, Sigma) was reduced by dithiothreitol (DTT) at a final concentration of 0.5 mM. Cytochrome c reduction was assessed measuring A550/A565 ratio. In our conditions, this ratio was between 15 and 20. The mitochondrial fraction was suspended in 60 mM of phosphate buffer (pH 7.4) containing 0.6% lauryl maltoside and centrifuged for 10 min at 10,000 × g. The assay was performed in a total volume of 1 ml of phosphate buffer (60 mM) containing 0.1 mg of mitochondrial proteins, 30 μM final concentration of reduced cytochrome c. The decrease in absorbance at 550 nm was measured for 1 min with 15 seconds integration time (Stieglerova et al., 2000).
Assay for Oxidative ATP Synthesis
Cells were harvested by trypsinization 48 h after transfection, washed twice in PBS, and counted in a hemo-cytometer. A replicate for each sample was prepared that had been treated for 1 h with 4 μg/ml rotenone (Sigma). The emission recorded from samples treated with rotenone was defined as baseline luminescence corresponding to a nonmitochondrial source of ATP. Assays were performed using the ATP luminescence assay kit HS II (Roche, Nutley, NJ) according to manufacturer's instructions, using 3000 cells per sample. Light emission was recorded in a single measure of 2 s using a Lumat LB 9507 luminometer (Berthold Technologies, Bad Wildbad, Germany).
Quantitative Analysis of Mitochondrial DNA
Mitochondrial DNA content relative to nuclear genome from control (CMV) and AKAP121 expressing cells was evaluated by PCR using oligonucleotide primers specific for nuclear (β globin) and mitochondrial genes (NADH dehydrogenase and cytochrome b). The primer sequences used were: cytochrome b: FW: 5′ CCTAGGCGACCCAGACAATTAT; rev: 5′-TCATTCGGGCTTGATGTGG; NADHd: FW: 5′-CAGCCATTCTCATCCAAACC; rev: 5′-ATTATGATGCGACTGT GAGTGC; β globin: FW: 5-AGCCTGACCAACATGGTGAAAC; rev: 5′-AGCCACCTGAATAGCTGGGACT. PCR reactions were carried out on the Applied Biosystem 7000 Real time PCR System (Foster City, CA) using Syber Green method. All reactions were performed in a 30-μl mixture containing 1× SYBR reaction buffer, 10 μM primers, and 20 ng of whole cellular DNA. PCR products range between 70 and 150 base pairs. Southern blot analysis was performed as described (Maniatis, 1989). Briefly, 20 μg of total genomic DNA was digested with EcoRI restriction enzyme, separated on 1% agarose gel, transferred to N-Hybond membrane, and sequentially hybridized with the mitochondrial and nuclear (β globin) cDNA probes. The mitochondrial probe spanning the nucleotides 7392–8625 of human mitochondrial genome was obtained with the following oligonucleotide primers: FW: 5′ GGATGCCCCCCACCCTAC; rev: 5′-GGAGGTGGGGATCAATAGAGG.
Imaging Mitochondrial Membrane Potential
The ΔΨm was assessed using the fluorescent dye tetra-methyl rhodamine ethyl ester (TMRE) in the “redistribution mode.” Cells transfected with CMV and AKAP121 were loaded with TMRE, 20 nM, for 30 min in a medium containing 156 mM NaCl, 3 mM KCl, 2 mM MgSO4, 1.25 mM KH2PO4, 2 mM CaCl2, 10 mM glucose, and 10 mM HEPES. The pH was adjusted to 7.35 with NaOH (Abramov et al., 2004). At the end of the incubation, cells were washed in the same medium containing 20 nM dye and allowed to equilibrate. A decline of mitochondria-localized intensity of fluorescence was indicative of mitochondrial membrane depolarization. Confocal images were obtained using a Zeiss inverted 510 confocal laser scanning microscopy (Thornwood, NY) and a 63× oil immersion objective. The illumination intensity of 543 Xenon laser, used to excite TMRE fluorescence, was kept to a minimum of 0.5% of laser output to avoid phototoxicity.
RESULTS
AKAP121 Assembles a Tyrosine Kinase/Phosphatase Signaling Complex on Mitochondria
We previously showed that AKAP121 binds and targets PTPD1 to subcellular organelles. Because PTPD1 associates with src, we hypothesized that AKAP121 may form a scaffold complex that includes not only PTPD1 and PKA, but src as well. In this context, PTPD1 may act as molecular bridge between the anchor protein and src. To test this notion, we performed coimmunoprecipitation experiments using total lysates prepared from HEK293 cells. This cell line expresses endogenous PTPD1 and low levels of AKAP121 (Figure 1A). Cells were transiently transfected with AKAP121 vector with or without PTPD1 vector. Forty-eight hours after transfection, total lysates were prepared and subjected to immunoprecipitation. Figure 1A shows that AKAP121 was precipitated by anti-src antibody only in AKAP121-transfected cells, and the amount of precipitated AKAP121 significantly increased when it was coexpressed with hemoagglutinin (HA)-tagged PTPD1. Conversely, anti-AKAP121 antibody precipitated significant amounts of PTPD1 and src (Figure 1B).
Figure 1.
PTPD1-src complex binds to AKAP121. (A) HEK293 cells were transiently transfected with expression vector encoding for AKAP121 in the presence or absence of HA-PTPD1 vector. Cell lysates were immunoprecipitated with anti-src antibody. Immunoprecipitates and aliquots (80 μg) of lysates were resolved on SDS-PAGE and immunoblotted with anti-HA, anti-PTPD1, anti-AKAP121, and anti-src specific antibodies. (B) Lysates from control or AKAP121/PTPD1-transfected cells were immunoprecipitated with anti-AKAP121 antibody. Precipitates were resolved on SDS-PAGE and sequentially immunoblotted with anti-AKAP121, anti-HA, and anti-src antibodies.
To determine if AKAP121 targets src tyrosine kinase on mitochondria in intact cells, we performed immunostaining analysis using specific antibodies directed against src, PTPD1 and AKAP121. Primary mouse pre-leptotene spermatocytes (GC2) express high levels of endogenous AKAP121 and PTPD1. Both proteins partly colocalize on mitochondria (Cardone et al., 2004). We analyzed src localization in GC2 cells by double immunostaining with anti-AKAP121 and anti-src antibody. The signals were collected and analyzed by confocal microscopy. As expected, AKAP121 selectively localized on mitochondria (Figure 2b) (Cardone et al., 2004). Some src immunostaining overlapped that of AKAP121 (Figure 2, a–c), suggesting that both proteins colocalize on mitochondria. Similarly, src immunostaining partly overlapped that of superoxide dismutase (Figure 2, d–f), a protein that selectively localizes to the mitochondrial matrix. However, src staining was also evident at the cell periphery (membrane) as well as at the perinuclear and nuclear regions, where no mitochondria or AKAP121 were detectable. We confirmed this finding using different anti-AKAP121 or anti-src antibodies (our unpublished data; see Materials and Methods). We performed similar experiments examining src localization in human fibroblast primary cultures that express endogenous AKAP121. Growing fibroblasts were labeled in vivo with Texas red-conjugated mitotracker, which selectively accumulates into mitochondria, fixed in formaldehyde, and immunostained with anti-src antibody. Src staining partly overlapped with that of mitotracker (Figure 2, g–i). Additionally, we asked whether exogenous AKAP121 expression in the HEK293 cell line, which costitutively expresses low AKAP121 levels, would target src to mitochondria. Indeed, HEK293 cells stably transfected with AKAP121 showed significant amounts of src concentrated on mitochondria (Figure 2, j–l).
Figure 2.
AKAP121 targets src to mitochondria. (a–c) double immunostaining of GC2 cells with anti-AKAP121 (red) and anti-src antibodies (green); (d–f) double immunostaining of GC2 cells with anti-SOD (red) and anti-src (green) antibodies; (g–i) primary culture of human fibroblasts labeled in vivo with mitotracker (red), fixed, and immunostained with anti-src (green) antibody; (j–l) HEK293 cells stably expressing transfected with AKAP121 vector were labeled in vivo with mitotracker (red), fixed, and immunostained with anti-src (green) antibody.
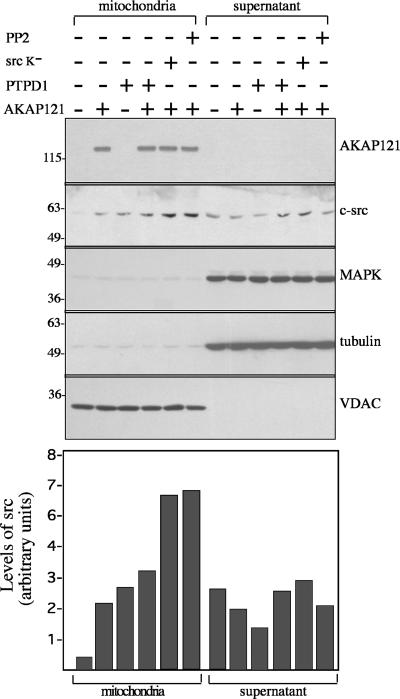
The ability of AKAP121 to direct src to mitochondria was also demonstrated in HEK293 cells by cofractionation experiments. HEK293 were transiently transfected with expression vectors encoding PTPD1 and AKAP121. Forty-eight hours after transfection, mitochondrial and supernatant fractions were isolated, size-fractionated on SDS-PAGE, and immunoblotted with the indicated antibodies. As shown in Figure 3, AKAP121 copurified with the mitochondria-enriched fraction, as did the mitochondrial voltage-dependent anion channel (VDAC), whereas tubulin and MAPK were found exclusively in supernatant fractions. In untransfected control cells most of endogenous c-src protein was found in the supernatant. Expression of AKAP121, PTPD1, or both significantly increased the amount of c-src recovered in the mitochondrial fraction. Note that coexpression of AKAP121 and PTPD1 translocates an amount of src to mitochondria roughly equivalent to AKAP121 or PTPD1 alone. PTPD1 and src are not only localized to mitochondria. Significant amounts of these enzymes have been found associated with other organelles and cell structures. This suggests that interaction with specific targeting sites is critically dependent on the absolute levels and binding affinity of AKAP121, src, and PTPD1.
Figure 3.
AKAP121 and src copurify with mitochondria. HEK293 cells were transiently transfected with vectors encoding AKAP121, PTPD1, and, where indicated, with a kinase-dead mutant of c-src (src K–). Treatment with src inhibitor PP2 was perfomed 30 min before harvesting. Forty-eight hours after transfection, cells were harvested and lysed. Mitochondrial and cytosolic fractions were prepared as described in Materials and Methods, size-fractionated on SDS-PAGE, and immunoblotted with the indicated antibodies. The bottom panel shows a quantitative analysis of src content of mitochondrial and supernatant fractions. VDAC and MAPK were used as loading controls. The data are expressed as arbitrary units and represent the mean of two independent experiments that yielded similar results.
Src activity was not required for mitochondrial localization promoted by AKAP121. Thus, cotransfection of HEK293 with AKAP121 and an src kinase dead (src K–) increased src levels in the mitochondrial fraction. A similar increase was seen when AKAP121-transfected cells were treated with the src inhibitor, PP2. We conclude that AKAP121 recruits c-src to mitochondria independently on its kinase activity.
AKAP121 Enhances src-dependent Phosphorylation and Activation of Mitochondrial Substrates
Some src is normally found in the mitochondrial matrix, where it phosphorylates and stimulates the activity of cytochrome c oxidase (COX), a component of the mitochondrial respiratory chain (Miyazaki et al., 2003). We wanted to determine if src localization by AKAP121/PTPD1 on mitochondria correlated with enhanced phosphorylation of mitochondrial src substrates. HEK293 cells were transiently transfected with AKAP121 and PTPD1 vectors for 24 h and subsequently harvested and lysed. Mitochondrial fractions were prepared and subjected to immunoblot analysis with anti-phosphotyrosine antibody. As shown in Figure 4A, expression of AKAP121 or PTPD1, to a lesser degree, markedly enhanced tyrosine phosphorylation of mitochondrial proteins. In contrast to src localization, stimulation of tyrosine phosphorylation by AKAP121 required src activity. Thus, treatment with PP2 or expression of src K– reduced phosphorylation to control levels. The phosphorylation of some mitochondrial substrates in cells transfected with AKAP121 and PTPD1 was reduced, compared with cells expressing AKAP121 alone. We have evidence that PTPD1 is not only localized to mitochondria, but in the absence of AKAP121, a significant fraction is linked to the actin cytoskeleton. In this compartment, PTPD1 regulates EGF-dependent src-FAK signaling (Carlucci, Gedressi, Avvedimento, Garbi, and Feliciello, unpublished results).
Figure 4.
AKAP121 increases src-dependent phosphorylation and activity of components of the mitochondrial respiratory chain. (A) HEK293 cells were transiently transfected with vectors encoding AKAP121, PTPD1, and kinase-inactive form of Src (src K–). Where indicated, cells were treated with the src inhibitor PP2, 30 min before harvesting. Mitochondrial fractions were immunoblotted with anti-phosphotyrosine antibody. A representative set of autoradiograms is shown. (B) HEK293 cells were transiently transfected with control (CMV), AKAP121, or AKAP84Δ1–30 vectors and harvested 48 h after transfection. Where indicated, PP2 was added to the medium 30 min before harvesting. COX activity was assayed with purified mitochondria. Inset, immunoblot analysis of cell lysates with anti-cytochrome c oxidase II subunit (1, CMV; 2, AKAP121; 3, AKAP84Δ1–30; AKAP121 +PP2). The data represent the mean ± SEM of five independent experiments. (C) Semiquantitative PCR of total genomic DNA extracted from HEK293 cells stably or transiently transfected with the AKAP121 expression vector. Mitochondrial (cytochrome B, Nadhd) and nuclear (β globin) genomic DNAs were amplified as described in Materials and Methods. The data are shown as fold increase of mitochondrial versus nuclear genomic DNA. * p < 0.05 vs. CMV-transfected cells; ** p < 0.01 vs. CMV-transfected cells. (D) Southern blot analysis of total genomic DNA extracted from stably transfected HEK293 cells. Mitochondrial (mito) and nuclear (β globin) cDNAs were used as probes. Indicated are the fold increases of mitochondrial versus β globin DNA. Values from control, CMV cells (0-time point) were set as 1.
We then monitored the activity of COX. HEK293 cells were transiently transfected with the indicated expression vectors. Forty-eight hours from transfection, cells were harvested, lysed, and mitochondrial COX activity was assayed. Figure 4B shows that AKAP121 increased COX activity by ca. 30%. HEK293 cells transiently expressing AKAP84, the smaller splice variant of AKAP121, also showed increased COX activity compared with their control HEK293 cells (our unpublished data). To prove that AKAP-mediated targeting of src to mitochondria was required for COX activity, we tested an AKAP84 mutant carrying a deletion of the mitochondrial targeting (MT) domain (Δ1–30). This mutant retained the ability to bind PTPD1/src and PKA, but failed to target these proteins to mitochondria (Cardone et al., 2004). AKAP84Δ1–30 was transiently transfected in HEK293 cells, and COX activity was assayed. The expression of mutant protein was comparable to that of wild-type AKAP121 (our unpublished data). Figure 4B shows that AKAP84Δ1–30 acted as dominant negative, reducing COX activity by ca. 40% relative to controls. Stimulation of COX activity by AKAP121 depended on active src kinase. Treatment of the transfected cells with PP2 reduced COX activity below control levels. The amount of COX protein, as shown by Western blot, was unaffected by transfection and treatment with PP2 (Figure 4B, inset). This indicates that the number of mitochondria per cell remained constant during these treatments.
Elevated oxidative respiratory chain activity is associated with increased mitochondrial DNA content (Deveaud et al., 2004). We therefore asked whether AKAP121 promoted mitochondrial DNA accumulation. Using semiquantitative PCR, we monitored the accumulation of two mitochondrial genes, NADH dehydrogenase and cytochrome B. The nuclear β globin gene was used as an internal control. Figure 4C shows that AKAP121 increased the levels of NADH dehydrogenase and cytochrome B by ca. 50%, compared with control cells transfected with the CMV vector (CMV). To confirm this finding, we performed Southern blot analysis on total cellular DNA using as probes mitochondrial and β globin cDNAs. Control cells and cells expressing AKAP121 were serum-deprived overnight, transferred to 10% FCS, and harvested at the indicated times. As shown in Figure 4D, AKAP121 increased both basal and serum-stimulated mitochondrial DNA levels by ∼2–3-fold.
AKAP121 Regulates ΔΨm and Oxidative ATP Synthesis
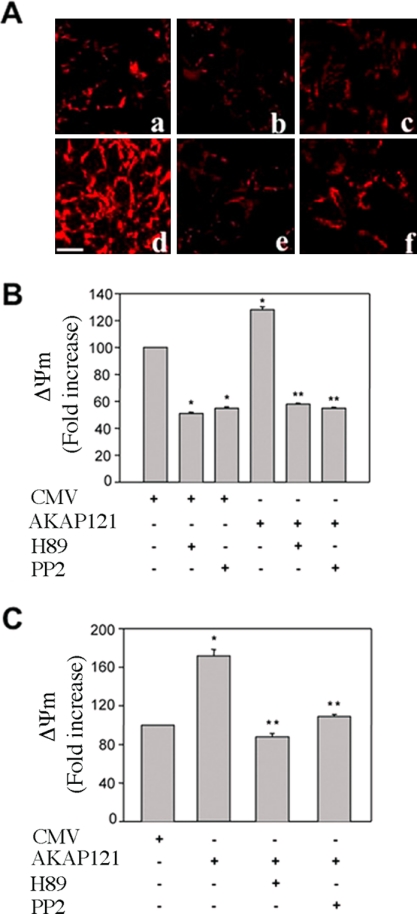
Electron flux through the respiratory chain is used to reduce free oxygen at the level of complex IV. Hydrogen then moves from the mitochondrial matrix to the intermembrane space. This generates an electrochemical gradient, expressed as ΔΨm (Saris and Carafoli, 2005). The ΔΨm is fundamental for the efficient production of ATP and requires the coordinated activity of several enzyme complexes, including COX. Because AKAP121-src stimulated COX activity, we asked if this anchor protein enhanced the ΔΨm under basal or stress conditions using a fluorescence assay (Abramov et al., 2004). Indeed, mitochondria in HEK293 transiently transfected with AKAP121 were hyperpolarized in comparison to control cells (Figure 5A, a and d). Hyperpolarization was inhibited by 30-min exposure to H89 (10 μM), an inhibitor of PKA and other kinases (Figure 5Ae). Similarly, treatment with PP2 (10 μM) for 30 min reduced polarization (Figure 5Af). Treatment with H89 or PP2 also decreased the intensity of mitochondria localized fluorescence in control cells (Figure 5, A and B). Similar results were obtained in HEK293 cells stably transfected with AKAP121 (Figure 5C).
Figure 5.
AKAP121 increases mitochondrial membrane potential. (A) Confocal images showing TMRE fluorescence were collected from HEK293 cells transiently transfected with CMV (a–c) or AKAP121 (d–f) expression vectors. Forty-eight hours after transfection, cells were treated for 30 min either with PP2 (10 μM) or H89 (10 μM) and subjected to TMRE analysis. (a and d) Control cells; (b and e) cells treated with H89 (10 μM); (c and f) cells treated with PP2. (B) Cumulative data are expressed as mean ± SE of changes in TMRE fluorescence and represent fold increase over control (CMV, untreated cells) that was set as 100. The intensity of fluorescence was evaluated in single cell by Meta-Morph software analysis (Universal Imaging, West Chester, PA). * p < 0.05 vs CMV-transfected cells, ** p < 0.05 vs AKAP 121-transfected cells. (C) Cumulative data from HEK293 cells stably transfected with CMV or AKAP121 are expressed as mean ± SE of changes in TMRE fluorescence and represent fold increase over control (CMV, untreated cells) that was set as 100. PP2 and H89 treatment were performed as in B. * p < 0.05 vs PCMV-transfected cells. ** p < 0.05 vs AKAP121-transfected cells. Statistical analysis was performed by ANOVA and Newman Keuls methods. Bar, 20 μm.
We then compared the response of cells transiently transfected with CMV or AKAP121 and exposed to serum deprivation (SD) or to chemical hypoxia. Cells were serum deprived for 18 h or subjected to chemical hypoxia by exposure to oligomycin (5 μg/ml) and 2 deoxyglucose (2DG, 2 mM) for 45 min, followed by 15 h of reoxygenation. Either treatment dramatically reduced ΔΨm in control cells (Figure 6A, b and c, and B). In contrast, these treatments only slightly reduced the intensity of mitochondria localized fluorescence in AKAP121-transfected cells (Figure 6A, e and f, and B). The localized fluorescence intensity of cells expressing AKAP121 was significantly higher than in control cells.
Figure 6.
AKAP121 protects cells from chemical hypoxia and serum deprivation. (A) Confocal images collected from HEK293 cells transiently transfected with CMV (a–c) or AKAP 121 (d–f). Cells were exposed to chemical hypoxia or serum deprivation. (a, d) Control cells; (b, e) cells treated with 2DG (2 mM) and olygomicine (5 μg/ml) for 45 min followed by reoxygenation for 24 h; (c and f) cells exposed to serum deprivation for 18 h. (B) Cumulative data are expressed as mean ± SE of changes in TMRE fluorescence and represent fold increase over control (CMV, untreated cells) that was set as 100. * p <0.05 vs CMV-transfected cells, ** p < 0.05 vs AKAP 121-transfected cells. Bar, 20 μm.
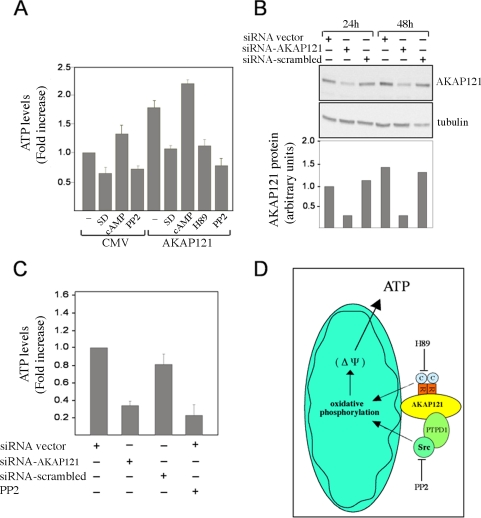
AKAP121, by facilitating the electrochemical gradient along the inner mitochondrial membrane, should enhance ATP synthesis. Figure 7A shows that cells transfected with AKAP121 had higher ATP levels than controls. H89 abrogated this increase. Treatment with CPT-cAMP, a cAMP analog, increased the ATP levels in control cells and, to a lesser extent, in AKAP121 expressing cells, compared with untreated cells. This suggests that AKAP121 increased mitochondrial PKA signaling close to maximum levels. In fact, in serum-deprived AKAP121 cells, the effects of cAMP on mitochondrial PKA signaling were more evident (Affaitati et al., 2003; Ginsberg et al., 2003). Taken together, these data imply that PKA up-regulates ATP synthesis and this effect is enhanced by AKAP121. ATP accumulation was also dependent on src activity. Thus, ATP concentrations were lowered by PP2 in both control and, more dramatically, in AKAP121-transfected cells. Western blot analysis showed that neither PP2 nor H89 reduced expression of AKAP121 (our unpublished data).
Figure 7.
AKAP121 controls oxidative ATP synthesis. (A) HEK293 cells were transiently transfected with AKAP121 or control vector (CMV). Twenty-four hours after transfection, cells were treated with PP2 (10 μM), H89 (10 μM), or CPT-cAMP (250 μM) for 30 min, or serum deprived (SD) for 18 h and harvested. ATP accumulation was evaluated as described in the Materials and Methods. The data are expressed as fold increase over the control (CMV) that was set as 1 and represent the mean ± SEM of four independent experiments. (B) HEK293 cells were transiently transfected with siRNA control vector, or with the vector targeting AKAP121 (siRNAAKAP121) or a scrambled sequence (siRNAscrambled). Cells were harvested 24 and 48 h after transfection. Cell lysates were sequentially immunoblotted with anti-AKAP121 and anti-tubulin antibodies. (C) Assay of oxidative ATP synthesis in cells from B harvested at 48 h from transfection. The data represent fold increase over basal value from control cells (siRNA vector) that was set as 1 and are expressed as SEM of three independent experiments. (D) Model of AKAP121 effects on oxidative phosphorylation and mitochondrial membrane potential.
We then examined the effects of serum deprivation on ATP accumulation. Twenty-four hours after transfection, cells were serum-deprived for 18 h and their mitochondrial ATP concentration was determined. Serum-deprivation significantly reduced ATP levels in control cells and in cells expressing AKAP121. These findings, along with the data reported in Figure 6B, suggest that growth factors stimulate oxidative phosphorylation and that AKAP121 increases mitochondrial robustness and resistance to oxidative stresses.
To confirm that AKAP121 regulates the mitochondrial respiratory chain, we silenced endogenous AKAP121 by DNA vector-based siRNA and measured ATP levels. As control, we used the same vector carrying a scrambled RNA sequence. Mouse fibroblasts (NIH3T3), which express AKAP121, were transiently transfected and the efficiency of silencing was evaluated by immunoblot analysis (Figure 7B). Expression of siRNAAKAP121 decreased endogenous AKAP121 levels ca. 2.5-fold. The control vector, which expresses siRNAscrambled, did not reduce AKAP121 concentrations. Consistent with our hypothesis, the synthesis of mitochondrial ATP was suppressed by siRNAAKAP121 but not siRNAscrambled. The extent of inhibition by siRNA was comparable to that provoked by src inhibition with PP2 (Figure 7C).
DISCUSSION
Our data shows that mitochondrial AKAP121 forms a signaling complex that includes, in addition to PKA, PTPD1 and src. AKAP121 increases cAMP and src signaling to mitochondria. Thus, tyrosine phosphorylation of some mitochondrial substrates, activity of COX, ΔΨm, and ATP synthesis were enhanced by AKAP121 in an src- and PKA-dependent manner.
AKAPs, by colocalizing signaling enzymes and their substrate(s), are proposed to ensure efficient propagation of transduction events generated at distal sites to specific intracellular compartments (Rubin, 1994; Feliciello et al., 1997; Gray et al., 1998; McKnight et al., 1998; Feliciello et al., 2001; Houslay and Adams, 2003; Tasken and Aandahl, 2004; Taylor et al., 2004; Wong and Scott, 2004). AKAP121 clearly plays this role in mitochondria. By localizing PKA at the outer membrane of mitochondria, AKAP121 increases PKA-dependent phosphorylation/inactivation of proapoptotic protein BAD and enhances cell survival (Harada et al., 1999; Affaitati et al., 2003). AKAP121 also facilitates PKA-dependent phosphorylation and activation of StAR, a mitochondrial steroidogenic factor that localizes in mitochondria of adrenal and testicular Leydig cells. In doing so, AKAP121 increases the biosynthesis of steroid hormones in a PKA-dependent manner (Stocco, Dyson, Jones, and Gottesman, unpublished results).
Signaling enzymes other than PKA are also bound and targeted by AKAP121. The amino-terminus of AKAP121 interacts with the central core of PTPD1, localizing the phosphatase on mitochondria (Cardone et al., 2004). PTPD1 is an effector for EGF signal transduction from the membrane to the nucleus. AKAP121 binding diverts PTPD1 to mitochondria and down-regulates this transduction pathway. We report here that AKAP121, via PTPD1, targets src to mitochondria. AKAP121 enhances src-dependent tyrosine phosphorylation of some mitochondrial substrates, facilitating the mitochondrial respiratory chain and increasing ATP synthesis. This conclusion is based both on AKAP121 overproduction and AKAP121 knockdown by siRNA.
The importance of tyrosine kinase signaling in mitochondrial function is supported by several lines of evidence (Abram and Courtneidge, 1999; Ko et al., 2002; Boerner et al., 2004; Augereau et al., 2005; Salvi et al., 2005). Tyrosine phosphorylation of mitochondrial proteins is stimulated in vitro by ATP and H2O2 (Augereau et al., 2005). ATP production at state 3 likewise enhances phosphorylation; this stimulation is ablated by PP2. Phosphorylation and activation of COX by mitochondrial src is postulated to play an important role in osteoclast function and bone remodeling (Miyazaki et al., 2003). The 39-kDa subunit of complex I is tyrosine-phosphorylated, and subunits of complexes II, III, and IV may also be tyrosine kinase substrates (Augereau et al., 2005). Platelet-derived growth factor (PDGF) signaling is linked to tyrosine phosphorylation of the c and δ subunits of the mitochondrial ATP synthase complex. This accounts for the enhanced activity of ATP synthase seen in a variety of PDGF-treated cells, including cortical neurons, mouse fibroblasts, and kidney cells (Evtodienko et al., 2000; Ko et al., 2002; Boerner et al., 2004). Moreover, serum deprivation has been linked to loss of mitochondrial respiratory control (Gottlieb et al., 2002).
Components of the respiratory chain can be also phosphorylated and regulated by PKA (Yang et al., 1998; Ludwig et al., 2001, Papa et al., 2002). A functional interplay between cAMP, tyrosine kinase, and mitochondrial COX has been recently described (Lee et al., 2005). These authors found that high cAMP levels induced phosphorylation of COX subunit I at tyrosine304 and inhibited COX activity. The responsible tyrosine kinase has not been identified.
We propose that AKAP121 is a nodal point where PKA and src signaling integrate, increasing the rate and magnitude of signaling to mitochondria. The mechanism by which src bound to AKAP121-PTPD1 phosphorylates substrates located within the mitochondrial matrix is still unknown. One possibility is that src may translocate inside mitochondria through the outer/inner mitochondrial transport system (Endo et al., 2003). AKAP121 increases the absolute levels of src anchored at the outer membrane of mitochondria. This facilitates transport of src, as well as PKA, inside the organelle, where both kinases normally reside (Yang et al., 1998; Miyazaki et al., 2003; Papa et al., 2003). This regulation is critical for mitochondrial physiology and explains the essential role of AKAP121 in cell survival, steroidogenesis, and oxidative phosphorylation. Identification of the critical mitochondrial substrates of PKA and src, and the functional relationship between these two signaling enzymes on mitochondria will, of course, require further study.
In this article we also present evidence that mitochondrial DNA content is increased by AKAP121. In this regard, our preliminary data suggest that cAMP and PKA are functionally linked to this process. In Saccharomyces cerevisiae, mitochondrial activity (citric acid cycle and oxidative respiration) and mtDNA content are coregulated. Mitochondrial activities and mtDNA are down-regulated by growth in glucose, whereas growth in a nonfermentable carbon source stimulates oxidative phosphorylation and increases mtDNA content. The cAMP/PKA signal transduction pathway positively regulates both reactions (Robertson et al., 2000; Cho et al., 2001; Griffioen and Thevelein, 2002). We have shown that AKAP121 concomitantly regulates mtDNA content and oxidative ATP synthesis. We cannot, however, ascertain which of the two events is the primary regulator of mammalian mitochondrial metabolism.
In summary, we demonstrate for the first time that AKAP121 regulates src events on mitochondria and highlight a unique role of this protein in the regulation of oxidative metabolism (Figure 7D). This mechanism increases the complexity of the symbiotic relationship developed million years ago between primordial eukaryotic cells and the aerobic bacteria that are thought to be mitochondrial ancestors. In view of the ubiquitous expression of AKAP121, our findings reveal an efficient mechanism that may be used in most or all mammalian cells to adapt physiologically to rapid changes in carbon source availability.
Acknowledgments
We thank Dr. Luigi Nezi for help in confocal microscopy. We give special thanks to Dr. Max Gottesman (Columbia University) for insightful discussions and for critical reading of the manuscript. This work was supported by grants from Associazione Italiana per la Ricerca sul Cancro (AIRC); MURST (Italian Ministry of University and Research). AKAP121 and PTPD1 vectors were kindly provided by Dr. C. Rubin (Albert Einstein College of Medicine, New York) and Dr. Axel Ullrich (Max-Planck-Institut fur Biochemie, Martinsried, Germany), respectively.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05–09–0827) on October 26, 2005.
References
- Abram, C. L., and Courtneidge, S. A. (1999). Src family tyrosine kinases and growth factor signalling. Exp. Cell Res. 254, 1–13. [DOI] [PubMed] [Google Scholar]
- Abramov, A. Y., Canevari, L., and Duchen, M. R. (2004). Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. J. Neurosci. 24, 565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Affaitati, A., Cardone, L., Carlucci, A., de Cristofaro, T., Ginsberg, M. D., Varrone, S., Gottesman, M. E., Avvedimento, V. E., and Feliciello, A. (2003). Essential role of A-Kinase Anchor Protein 121 for cAMP signalling to mitochondria. J. Biol. Chem. 278, 4286–4294. [DOI] [PubMed] [Google Scholar]
- Augereau, O., Claverol, S., Boudes, N., Basurko, M. J., Bonneu, M., Rossignol, R., Mazat, J. P., Letellier, T., Dachary-Prigent, J. (2005) Identification of tyrosine-phosphorylated proteins of the mitochondrial oxidative phosphorylation machinery. Cell Mol. Life Sci. May 28; [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- Barone, M. V., and Courtneidge, S. A. (1996). Myc but not Fos rescue of PDGF signalling block caused by kinase-inactive Src. Nature 378, 509–512. [DOI] [PubMed] [Google Scholar]
- Boerner, J. L., Demory, M. L., Silva, C., and Parsons, S. J. (2004). Phosphorylation of Y845 on the epidermal growth factor receptor mediates binding to the mitochondrial protein cytochrome c oxidase subunit II. Mol. Cell. Biol. 24, 7059–7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardone, L., Carlucci, A., Affaitati, A., Livigni, A., DeCristofaro, T., Garbi, C., Varrone, S., Ullrich, A., Gottesman, M. E., Avvedimento, E. V., and Feliciello, A. (2004): Mitochondrial AKAP121 binds and targets protein tyrosine phosphatase D1, a novel positive regulator of src signalling. Mol. Cell. Biol. 24, 4613–4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardone, L., de Cristofaro, T., Affaitati, A., Garbi, C., Ginsberg, M. D., Saviano, M., Varrone, S., Gottesman, M. E., Avvedimento, V. E., and Feliciello, A. (2002). A-Kinase Anchor Protein 84/121 are targeted on mitochondria and mitotic spindles by overlapping amino-terminal motifs. J. Mol. Biol. 320, 663–675. [DOI] [PubMed] [Google Scholar]
- Chen, Q., Lin, R. Y., and Rubin, C. S. (1997). Organelle-specific targeting of protein kinase AII (PKAII). Molecular and in situ characterization of murine A-kinase anchor proteins that recruit regulatory subunits of PKAII to the cytoplasmic surface of mitochondria. J. Biol. Chem. 272, 15247–51527. [DOI] [PubMed] [Google Scholar]
- Cho, J. H., Lee, Y. K., and Chae, C. B. (2001). The modulation of the biological activities of mitochondrial histone Abf2p by yeast PKA and its possible role in the regulation of mitochondrial DNA content during glucose repression. Biochim. Biophys. Acta 1522, 175–186. [DOI] [PubMed] [Google Scholar]
- Couperstein, S. J., and Lazarov, A. (1951). A micro-spectrophotometric method for the determination of cytochrome c oxidase. J. Biol. Chem. 665–670. [PubMed]
- Deveaud, C., Beauvoit, B., Salin, B., Schaeffer, J., and Rigoulet, M. (2004). Regional differences in oxidative capacity of rat white adipose tissue are linked to the mitochondrial content of mature adipocytes. Mol. Cell. Biochem. 267, 157–166. [DOI] [PubMed] [Google Scholar]
- Endo, T., Yamamoto, H., and Esaki, M. (2003). Functional cooperation and separation of translocators in protein import into mitochondria, the double-membrane bounded organelles. J. Cell Sci. 116, 3259–3267. [DOI] [PubMed] [Google Scholar]
- Evtodienko, Y. V., Azarashvili, T. S., Teplova, V. V., Odinokova, I. V., and Saris, N. (2000). Regulation of oxidative phosphorylation in the inner membrane of rat liver mitochondria by calcium ions. Biochemistry 65, 1023–1026. [PubMed] [Google Scholar]
- Feliciello, A., Gottesman, M. E., and Avvedimento, E. V. (2005). cAMP-PKA signalling to the mitochondria: protein scaffolds, mRNA and phosphatases. Cell Signal. 17, 279–287. [DOI] [PubMed] [Google Scholar]
- Feliciello, A., Gottesman, M. E., and Avvedimento, V. E. (2001). The biological functions of A-Kinase Anchor Proteins. J. Mol. Biol. 308, 99–114. [DOI] [PubMed] [Google Scholar]
- Feliciello, A., Li, Y., Avvedimento, E. V., Gottesman, M. E., and Rubin, C. S. (1997). A-kinase anchor protein 75 increases the rate and magnitude of cAMP signalling to the nucleus. Curr. Biol. 7, 1011–1014. [DOI] [PubMed] [Google Scholar]
- Feliciello, A., Rubin, C. S., Avvedimento, V. E., and Gottesman, M. E. (1998). Expression of A Kinase Anchor Protein121 is regulated by hormones in thyroid and testicular germ cells. J. Biol. Chem. 273, 23361–23366. [DOI] [PubMed] [Google Scholar]
- Furusawa, M., Taira, T., Iguchi-Ariga, S. M., and Ariga, H. (2002). AMY-1 interacts with S-AKAP84 and AKAP95 in the cytoplasm and the nucleus, respectively, and inhibits cAMP-dependent protein kinase activity by preventing binding of its catalytic subunit to A-Kinase-anchoring protein (AKAP) complex. J. Biol. Chem. 277, 50885–50892. [DOI] [PubMed] [Google Scholar]
- Ginsberg, M. D., Feliciello, A., Jones, J. K., Avvedimento, E. V., and Gottesman, M. E. (2003). PKA-dependent binding of mRNA to the mitochondrial AKAP121 protein. J. Mol. Biol. 327, 885–897. [DOI] [PubMed] [Google Scholar]
- Gottlieb, E., Armour, S. M., and Thompson, C. B. (2002). Mitochondrial respiratory control is lost during growth factor deprivation. Proc. Natl. Acad. Sci. USA 99, 12801–12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray, P. C., Scott, J. D., and Catterall, W. A. (1998). Regulation of ion channels by cAMP-dependent protein kinase and A-kinase anchoring proteins. Curr. Opin. Neurobiol. 8, 330–334. [DOI] [PubMed] [Google Scholar]
- Griffioen, G., and Thevelein, J. M. (2002). Molecular mechanisms controlling the localisation of protein kinase A. Curr. Genet. 41, 199–207. [DOI] [PubMed] [Google Scholar]
- Harada, H., Becknell, B., Wilm, M., Mann, M., Huang, L. J., Taylor, S. S., Scott, J. D., and Korsmeyer, S. J. (1999). Phosphorylation and inactivation of BAD by mitochondria-anchored protein kinase A. Mol. Cell 4, 413–422. [DOI] [PubMed] [Google Scholar]
- Houslay, M. D., and Adams, D. R. (2003). PDE4 cAMP phosphodiesterases: modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem. J. 370, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovius, R., Lambrechts, H., Nicolay, K., and de Kruijff, B. (1990). Improved methods to isolate and subfractionate rat liver mitochondria. Lipid composition of the inner and outer membrane. Biochim. Biophys. Acta 1021, 217–226. [DOI] [PubMed] [Google Scholar]
- Huang, L. J., Durick, K., Weiner, J. A., Chun, J., and Taylor, S. S. (1997). Identification of a novel protein kinase A anchoring protein that binds both type I and type II regulatory subunits. J. Biol. Chem. 272, 8057–8064. [DOI] [PubMed] [Google Scholar]
- Huang, L. J., Wang, L., Ma, Y., Durick, K., Perkins, G., Deerinck, T. J., Ellisman, M. H., and Taylor, S. S. (1999). NH2-Terminal targeting motifs direct dual specificity A-kinase-anchoring protein 1 (D-AKAP1) to either mitochondria or endoplasmic reticulum. J. Cell Biol. 145, 951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko, Y. H., Pan, W., Inoue, C., and Pedersen, P. L. (2002). Signal transduction to mitochondrial ATP synthase: evidence that PDGF-dependent phosphorylation of the δ-subunit occurs in several cell lines, involves tyrosine, and is modulated by lysophosphatidic acid. Mitochondrion 1, 339–348. [DOI] [PubMed] [Google Scholar]
- Lee, I., Salomon, A. R., Ficarro, S., Mathes, I., Lottspeich, F., Grossman, L. I., and Huttemann, M. (2005). cAMP-dependent tyrosine phosphorylation of subunit I inhibits cytochrome c oxidase activity. J. Biol. Chem. 280, 6094–6100. [DOI] [PubMed] [Google Scholar]
- Lin, R. Y., Moss, S. B., and Rubin, C. S. (1995). Characterization of S-AKAP84, a novel developmentally regulated A kinase anchor protein of male germ cells. J. Biol. Chem. 270, 27804–27811. [DOI] [PubMed] [Google Scholar]
- Ludwig, B., Bender, E., Arnold, S., Huttemann, M., Lee, I., and Kadenbach, B. (2001). Cytochrome C oxidase and the regulation of oxidative phosphorylation. Chembiochemistry 2, 392–403. [DOI] [PubMed] [Google Scholar]
- Maniatis, T. (1989). Molecular cloning. A Laboratory Manual, 2nd ed., Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- McKnight, G. S., Cummings, D. E., Amieux, P. S., Sikorski, M. A., Brandon, E. P., Planas, J. V., Motamed, K., and Idzerda, R. L. (1998). Cyclic AMP, PKA, and the physiological regulation of adiposity. Rec. Prog. Horm. Res. 53, 139–159. [PubMed] [Google Scholar]
- Miyazaki, T., Neff, L., Tanaka, S., Horne, W. C., and Baron, R. (2003). Regulation of cytochrome c oxidase activity by c-Src in osteoclasts. J. Cell Biol. 160, 709–718.12615910 [Google Scholar]
- Moller, N. P., Moller, K. B., Lammers, R., Kharitonenkov, A., Sures, I., and Ullrich, A. (1994). Src kinase associates with a member of a distinct subfamily of protein-tyrosine phosphatases containing an ezrin-like domain. Proc. Natl. Acad. Sci. USA 91, 7477–7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa, S., Scacco, S., Sardanelli, A. M., Petruzzella, V., Vergari, R., Signorile, A., and Technikova-Dobrova, Z. (2002). Complex I and the cAMP cascade in human physiopathology. Biosci. Rep. 22, 3–16. [DOI] [PubMed] [Google Scholar]
- Ranganathan, G., Pokrovskaya, I, Ranganathan, S., and Kern, P. A. (2005). Role of A kinase anchor proteins in the tissue specific regulation of lipoprotein lipase. Mol. Endocrinol. [Epub ahead of print]. [DOI] [PubMed]
- Robertson, L. S., Causton, H. C., Young, R. A., and Fink, G. R. (2000). The yeast A kinases differentially regulate iron uptake and respiratory function Proc. Natl. Acad. Sci. USA 97, 5984–5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin, C. S. (1994). A kinase anchor proteins and the intracellular targeting of signals carried by cyclic AMP. Biochim. Biophys. Acta 1224, 467–479. [PubMed] [Google Scholar]
- Salvi, M., Brunati, A. M., and Toninello, A. (2005). Tyrosine phosphorylation in mitochondria: a new frontier in mitochondrial signalling. Free Radic. Biol. Med. 38, 1267–1277. [DOI] [PubMed] [Google Scholar]
- Saris, N., and Carafoli, E. (2005). A historical review of cellular calcium handling, with emphasis on mitochondria. Biochemistry 70, 231–239. [DOI] [PubMed] [Google Scholar]
- Stieglerova, A., Drahota, Z., Ostadal, B., and Houstek, J. (2000). Optimal conditions for determination of cytochrome c oxidase activity in the rat heart. Physiol. Res. 49, 245–250. [PubMed] [Google Scholar]
- Tasken, K., and Aandahl, E. M. (2004). Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol. Rev. 84, 137–167. [DOI] [PubMed] [Google Scholar]
- Taylor, S. S., Yang, J., Wu, J., Haste, N. M., Radzio-Andzelm, E., and Anand, G. (2004). PKA: a portrait of protein kinase dynamics. Biochim. Biophys. Acta 1697, 259–269. [DOI] [PubMed] [Google Scholar]
- Trendelenburg, G., Hummel, M., Riecken, E. O., and Hanski, C. (1996). Molecular characterization of AKAP149, a novel A kinase anchor protein with a KH domain. Biochem. Biophys. Res. Commun. 225, 313–319. [DOI] [PubMed] [Google Scholar]
- Yang, W. L., Iacono, L., Tang, W. M., and Chin, K. V. (1998). Novel function of the regulatory subunit of protein kinase A: regulation of cytochrome c oxidase activity and cytochrome c release. Biochemistry 37, 14175–14180. [DOI] [PubMed] [Google Scholar]
- Wolkolwicz, M. J., Coonrod, S. M., Reddi, P. P., Millan, J. C., Hofmann, M. C., and Herr, J. R. (1996). Refinement of the differentiated phenotype of the spermatogenic cell line GC-2spd(ts). Biol. Reprod. 55, 923–932. [DOI] [PubMed] [Google Scholar]
- Wong, W., and Scott, J.D. AKAP signalling complexes: focal points in space and time. (2004) Nat. Rev. Mol. Cell. Biol. 5, 959–970. [DOI] [PubMed] [Google Scholar]