Abstract
In this study, we investigated the role of phospholipase D (PLD) in mediating Arf6 function in cells. Expression of Arf6 mutants that are defective in activating PLD, Arf6N48R and Arf6N48I, inhibited membrane recycling to the plasma membrane (PM), resulting in an accumulation of tubular endosomal membranes. Additionally, unlike wild-type Arf6, neither Arf6 mutant could generate protrusions or recruit the Arf6 GTPase activating protein (GAP) ACAP1 onto the endosome in the presence of aluminum fluoride. Remarkably, all of these phenotypes, including accumulated tubular endosomes, blocked recycling, and failure to make protrusions and recruit ACAP effectively, could be recreated in either untransfected cells or cells expressing wild-type Arf6 by treatment with 1-butanol to inhibit the formation of phosphatidic acid (PA), the product of PLD. Moreover, most of the defects present in cells expressing Arf6N48R or N48I could be reversed by treatment with agents expected to elevate PA levels in cells. Together, these observations provide compelling evidence that Arf6 stimulation of PLD is required for endosomal membrane recycling and GAP recruitment.
INTRODUCTION
Arf GTP-binding proteins function in the regulation of membrane traffic and organization of membrane structure. Arf6, a widely studied family member, regulates peripheral membrane dynamics and the cortical actin cytoskeleton at the plasma membrane (PM) (Donaldson, 2003; Sabe, 2003). Thus, in a number of systems, Arf6 has been shown to influence endosomal membrane traffic (D'Souza-Schorey et al., 1995; Radhakrishna and Donaldson, 1997; Naslavsky et al., 2003; Powelka et al., 2004), regulated secretion (Caumont et al., 1998; Vitale et al., 2002; Aikawa and Martin, 2003; Lawrence and Birnbaum, 2003; Zheng and Bobich, 2004), disassembly of cell adhesions (Palacios et al., 2001), cell migration (Santy and Casanova, 2001; Palacios and D'Souza-Schorey, 2003), and formation of PM protrusions and ruffles (Radhakrishna et al., 1996; D'Souza-Schorey et al., 1997; Franco et al., 1999; Brown et al., 2001).
Identifying targets of Arf6-GTP will lead to a clearer understanding of how Arf6 possesses such a wide range of activities. There is compelling evidence that Arf6 functions to regulate enzymes involved in membrane lipid modification. Arfs were purified as activators of phosphatidylinositol 4-phosphate 5-kinase (PIP 5-kinase), the enzyme that generates phosphatidylinositol 4, 5-bisphosphate (PIP2) (Honda et al., 1999). It was Arf6 in particular that colocalized with and affected PIP 5-kinase activity in cells and was associated with changes in the actin cytoskeleton (Honda et al., 1999). Indeed, we (Brown et al., 2001) and others (Schafer et al., 2000; Aikawa and Martin, 2003; Krauss et al., 2003; Lawrence and Birnbaum, 2003) have demonstrated that Arf6 activation of PIP 5-kinase and subsequent PIP2 generation leads to the cytoskeletal changes and membrane traffic alterations observed upon Arf6 activation. In addition, Arf6 activation of PIP 5-kinase has been implicated in regulated exocytic events: dense core granule and synaptic vesicle exocytosis (Vitale et al., 2002; Aikawa and Martin, 2003; Zheng and Bobich, 2004), and Glut4 vesicle translocation upon stimulation (Lawrence and Birnbaum, 2003).
Arfs also activate phospholipase D (PLD), an enzyme that catalyzes the hydrolysis of phosphatidylcholine to generate phosphatidic acid (PA) (Brown et al., 1993; Cockcroft et al., 1994). PLD is activated in response to various signaling receptors; the resulting PA can affect Raf kinase and mammalian target of rapamycin pathways, regulate the activity of PIP 5-kinase, and alter membrane structure (McDermott et al., 2004). At least two isoforms of PLD exist in mammalian cells, PLD1 and PLD2. Both isoforms share a dependency on PIP2 for activity, but they differ in localization and mechanisms of regulation (Colley et al., 1997). Although there is some discrepancy about localization of endogenous enzymes (Freyberg et al., 2003), epitope-tagged PLD1 localizes to juxtanuclear membranes and translocates to the PM during signaling (Brown et al., 1998; Du et al., 2003), whereas PLD2 localizes to the PM and endosomal membranes (O'Luanaigh et al., 2002; Du et al., 2004). PLD2 has a much higher basal activity than PLD1 (McDermott et al., 2004); however, recent data have suggested PLD2 may also be subject to stimulation (Hiroyama and Exton, 2005a). Arf6 activation of PLD has been shown to be critical for changes in the actin cytoskeleton associated with mast cell ruffling (O'Luanaigh et al., 2002) and cell migration (Santy and Casanova, 2001). Arf6 stimulation of PLD has also been implicated in dense core granule exocytosis (Vitale et al., 2002) and for translocation of vesicles containing glucose transporter 4 (Glut4) to the PM (Huang et al., 2005).
An effector domain mutant of Arf1, N52R, was described that lacked the ability to stimulate PLD1 yet was able to stimulate PIP 5-kinase (Jones et al., 1999; Skippen et al., 2002). Furthermore, an equivalent mutation in Arf6, N48I, was shown to inhibit dense core vesicle secretion in PC12 cells (Vitale et al., 2002). Biochemical characterization showed that Arf6N48I could be activated by ARNO and inactivated by Git1, an Arf6 guanine nucleotide exchange factor (GEF) and GTPase activating protein (GAP), respectively. Like Arf1N52R, Arf6N48I remained able to stimulate PIP 5-kinase. Thus, mutation of asparagine 48 to either arginine or isoleucine generates an Arf mutant that seems to function normally with respect to GEF, GAP, and PIP 5-kinase activity but is selectively impaired in PLD activation.
We have been studying the role of Arf6 in the regulation of endosomal membrane traffic and cortical actin rearrangements. In HeLa cells, Arf6 is present at the PM and on endosomal membranes that contain PM proteins, such as the major histocompatibility protein class I (MHCI) that enter cells independently of clathrin endocytosis. PIP 5-kinase and PIP2 are present at the PM and also on these endosomal membranes, where the PIP2 level corresponds to the degree that Arf6 is activated (Brown et al., 2001). Recycling of MHCI back to the PM occurs via tubular endosomal membranes and requires a number of factors, including the activation of Arf6 (Radhakrishna and Donaldson, 1997; Powelka et al., 2004). Because Arf6 activation of PLD is required for regulated exocytosis of dense core granules (Vitale et al., 2002), we examined whether Arf6 stimulation of PLD was also required for endosomal recycling and other Arf6 functions.
MATERIALS AND METHODS
Cells, Reagents, and Antibodies
HeLa cells were grown in DMEM containing 10% fetal calf serum. Cytochalasin D, phorbol 12-myristate 13-acetate (PMA), propranolol, 1-butanol, 2-butanol, sodium fluoride, latrunculin A, and saponin were purchased from Sigma-Aldrich (St. Louis, MO). Aluminum chloride was purchased from General Chemical Company (New York, NY). Mouse monoclonal antibody (mAb) to MHCI (W6/32) was used as described previously (Naslavsky et al., 2003). Alexa secondary antibodies (Alexa 488, Alexa 594, anti-rabbit, and anti-mouse) were from Invitrogen (Carlsbad, CA). Mouse mAb to the FLAG epitope (M5) was purchased from Sigma-Aldrich. FuGENE 6 transfection reagent was purchased from Roche Diagnostics (Indianapolis, IN).
Plasmids and Transient Transfections
Arf6 and mutants were in the pXS plasmid (Radhakrishna and Donaldson, 1997). The isoleucine and arginine mutations at position 48 of Arf6 were introduced using the QuikChange kit (Stratagene, Cedar Creek, TX) and checked by sequencing. N-terminally FLAG-tagged ACAP1 was as described previously (Jackson et al., 2000). For transfection, HeLa cells were plated on glass coverslips and transfected the following day with FuGENE 6 (Roche Diagnostics). Cells were washed in phosphate-buffered saline (PBS) 24 h after transfection, and all experiments (with the exception of the MHC recycling experiment) were conducted 48 h after transfection. For the MHC recycling experiment, the cells were transfected the morning after being plated on coverslips and were washed with PBS that afternoon. The recycling experiment was conducted the following day.
Treatments
Cells were incubated in DMEM containing 10% serum for ∼20 min in 37°C before drugs were added at the appropriate concentrations. The final concentrations of cytochalasin D, propranolol, and PMA were 100 nM, 0.1 mM, and 200 nM, respectively. Cells were exposed to the appropriate drug treatment for 30 min at 37°C. For the cells undergoing recovery, the media containing the drug treatment was replaced with media alone, and the cells were allowed to recover for 90 min. In the butanol treatments, 0.3% 1-butanol or 2-butanol in media was allowed to preequilibrate at 37°C for 20 min before adding to cells. For aluminum fluoride treatment, 30 mM NaF and 50 μM AlCl3 were added to complete media.
Immunofluorescence and Confocal Microscopy Analysis
Cells underwent treatments 48 h after transfection and were subsequently fixed for 10 min with 2% formaldehyde in PBS and then blocked in 10% fetal bovine serum in PBS (PBS/serum) for a minimum of 30 min. The cells were incubated with primary antibody in 0.2% saponin in PBS/serum for 1 h and then rinsed with PBS/serum. Next, the cells were incubated for 1 h with secondary antibody in 0.2% saponin in PBS/serum, rinsed with PBS/serum, followed by PBS. Images were acquired with a ZEISS LSM 510 confocal microscope with a Plan Apo 63× objective and prepared in Adobe Photoshop (Adobe Systems, Mountain View, CA).
MHC1 Recycling Assay
Cells were transfected with Arf6 WT, N48R, or N48I, then washed with PBS 8 h later. The recycling experiment was carried out 24 h after transfection (Weigert et al., 2004). W6/32 anti-MHCI antibody was bound to cells on ice for 30 min; unbound antibody was then washed away with PBS, and cells were incubated with 1 μM latrunculin A at 37°C for 30 min. Surface antibody not internalized was then removed by exposing cells to a low pH buffer (0.5% acetic acid, 0.5 M NaCl, pH 3.0) for 1 min at room temperature. Cells were washed twice with PBS, twice with serum-free media, and then placed in complete media at 37°C for 30 min to allow for MHCI antibody recycling to the plasma membrane. The surface pools of MHCI antibody were again removed by low pH buffer so that subsequent staining with Alexa 594-GAM revealed internal pools only. During the last 10 min of W6/32 antibody uptake and for the 30 min recycling of MHCI, some cells were treated with either 0.3% 1-butanol or 2-butanol. The PMA treatment was administered during the 30-min recycling phase.
The amount of MHCI in the internal pool was measured according to published protocols (Weigert et al., 2004). Briefly, to estimate the amount of internalized MHCI, 30 cells per coverslip were randomly selected and imaged with a 510 LSM confocal microscope (Carl Zeiss, Thornwood, NY) with a 40× Plan Apo objective, with the pinhole opened to the maximum setting. All images were taken with identical settings that were optimized for the signals to be in the dynamic range. The total fluorescence of each cell was measured using the LSM Image examiner version 3.01 (Carl Zeiss). Recycled MHCI antibody was measured by taking the difference of internal pool signal intensity of MHCI antibody from uptake (time 0) and chase (30 min), minus the background (recycled MHCI at time 0). The wild-type (WT) Arf6-overexpressing cells were taken to be the standard against which the treated cells, and the cells overexpressing the mutant forms of Arf6 were measured.
RESULTS
Expression of Arf6N48R/I Causes an Accumulation of Endosomal Membranes
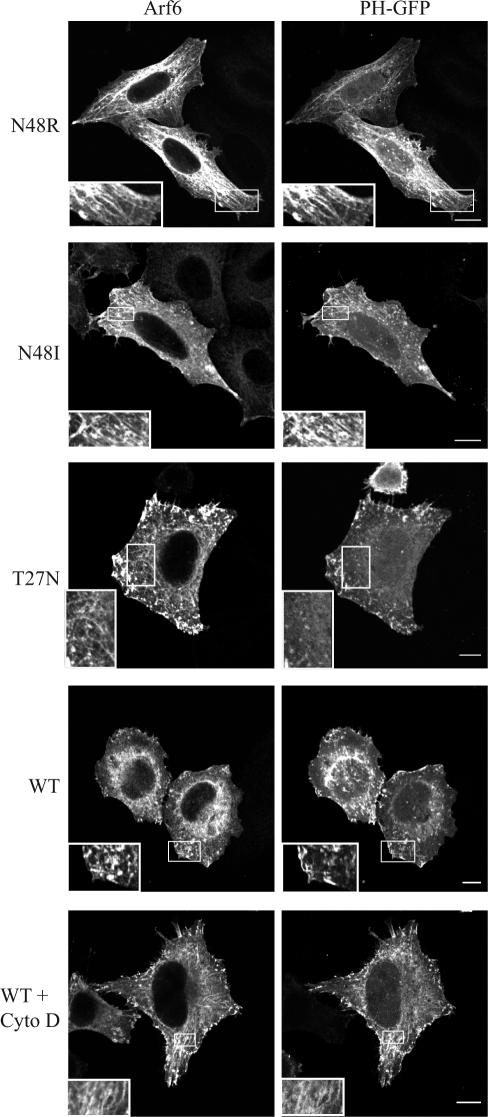
To investigate whether Arf6 activation of PLD is important for Arf6 function in cells, we examined the effects of expressing PLD activation-defective mutants of Arf6, N48R and N48I, in HeLa cells. We assessed the steady-state distribution of MHCI, a PM protein that travels through the Arf6-associated endosomal pathway, to determine whether the endosomal system was altered. When we expressed Arf6N48R or N48I in cells, we observed sparse labeling of the PM and prominent distribution of the mutant proteins on tubular endosomal membranes that colocalized with antibody staining for MHCI (Figure 1, A and B, insets). In some Arf6N48R-expressing cells, vesicular structures were also apparent. This contrasted with the distribution of wild-type Arf6, which was more visible at the PM and edges of the cells with only some labeling of endosomal structures (Figure 1C). The tubular endosomal structures that contain MHCI are observed in untransfected HeLa cells as well as those expressing wild-type Arf6 (Radhakrishna and Donaldson, 1997). Although all HeLa cells have tubular endosomes, they are only visible in ∼40% of cells at any given time because of the dynamic nature of the endosome (Figure 1E). However, >90% of cells expressing the N48 mutants had prominent tubular endosomes (Figure 1E). This accumulation of tubular endosomes was also observed in cells expressing wild-type Arf6 that were treated with cytochalasin D to inhibit actin polymerization (Figure 1D) as was observed previously in both Arf6-transfected and untransfected HeLa cells (Radhakrishna and Donaldson, 1997; Weigert et al., 2004). The addition of cytochalasin D to cells expressing wild-type Arf6 increased the number of cells exhibiting tubular endosomes by >80%, similar to that observed in cells expressing N48R or N48I (Figure 1E). The accumulation of endosomes containing MHCI was only observed in cells expressing the Arf6 PLD mutants because neither the expression of Arf1 nor of the corresponding PLD mutant N52R caused any change in peripheral MHCI-containing endosomes (Supplemental Figure 1).
Figure 1.
Cells expressing Arf6N48R or N48I or treatment of cells expressing wild-type Arf6 with 1-butanol accumulate tubular endosomes. HeLa cells were transfected with Arf6N48R (A), Arf6N48I (B), or Arf6 (C, D, and F). Some Arf6-expressing cells were incubated with 100 nM cytochalasin D (D), 0.3% 1-butanol (F), or 2-butanol for 30 min. The cells were then fixed and immunolabeled with antibodies to Arf6 and MHCI. The juxtanuclear labeling of MHCI visible in a number of cells represents biosynthetic MHCI in the Golgi complex. Insets show colocalization of MHCI with Arf6. (E) Quantification of cells displaying tubular endosomal phenotype as a percentage of 100 transfected cells counted per experiment. Shown are the mean values with standard deviations from three experiments. Bars, 10 μm.
Because both mutants are defective in the ability to activate PLD, we examined whether cells expressing wild-type Arf6 treated with agents that inhibit PLD activity would generate a similar morphological phenotype. Because there are no specific inhibitors of PLD, alcohols are commonly used to assess the role of PLD in biological function. Primary alcohols replace water in the transphosphatidylation reaction catalyzed by PLD resulting in the formation of phosphatidylalcohol at the expense of PA (Yang et al., 1967). Cells expressing wild-type Arf6 treated with 1-butanol to inhibit the production of PA showed dramatically increased tubular endosome formation (Figure 1F, inset); the fraction of cells expressing tubular endosomes was increased from ∼45% to >70% (Figure 1E). Butanol treatment of untransfected cells also caused an increase in MHCI-containing tubular endosomes, although they were thinner and more difficult to discern (our unpublished observations). In contrast, treatment with 2-butanol, a secondary alcohol, which does not participate in the transphosphatidylation reaction, did not have this effect (Figure 1E). Thus, inhibition of PA generation with 1-butanol recreates the effects of expression of the N48 mutants.
Expression of Arf6N48R Inhibits Membrane Recycling
The accumulation of tubular endosomes in HeLa cells suggests that there may be an inhibition in recycling of membrane back to the PM (Radhakrishna and Donaldson, 1997; Weigert et al., 2004). Endosomal membrane recycling is dependent on Arf6 activities. Expression of a dominant-negative, GTP-binding-defective mutant of Arf6, T27N, leads to the accumulation of both Arf6 and MHCI on tubular endosomes and a block in recycling of MHCI and other Arf6-associated cargo proteins back to the PM (Radhakrishna and Donaldson, 1997; Powelka et al., 2004). Because expression of Arf6N48R caused an accumulation of tubular endosomes containing MHCI, we examined whether this mutant also inhibited recycling of MHCI back to the PM. Cells were incubated with antibody to MHCI for 30 min to load the endosomal compartment and then the amount of MHCI reappearing at the PM after 30-min chase was determined (see Materials and Methods). In this assay, between 40 and 50% of internalized MHCI is recycled in control preparations.
Cells expressing Arf6N48R were impaired in recycling of MHCI back to the PM. For N48R, recycling was diminished to 40% of the recycling exhibited by control cells (Figure 2A). An inhibition of recycling, although less potent, was also observed in cells expressing Arf6N48I (our unpublished data; see below). Thus, when expressed, Arf6N48R seemed to act as a dominant negative by inhibiting the activity of the endogenous Arf6 protein, leading to the decreased ability to recycle membrane through the endosomal compartment. To obtain further support for PLD's role in endosomal membrane recycling, we examined MHCI recycling in untransfected HeLa cells in the presence of 1-butanol to inhibit PA production. Treatment of HeLa cells with 1-butanol during the 30-min recycling phase inhibited recycling of MHCI to 10% of that observed in control (untransfected cells). By contrast, treatment of cells with 2-butanol, diminished recycling to 80% of control cells (Figure 2B). A similar response to the alcohol treatment was observed in HeLa cells expressing Arf6 (Figure 2B). Together, these findings indicate that Arf6 activation of PLD is required for the efficient recycling of MHCI back to the PM.
Figure 2.
Expression of Arf6N48R or treatment of cells with 1-butanol, but not 2-butanol, inhibits MHCI recycling to the plasma membrane. (A) HeLa cells were transfected with Arf6 or Arf6N48R. Cells were loaded with antibody to MHCI for 30 min according to protocol described in Materials and Methods. Recycling of MHCI was measured by comparing the emptying of the MHCI internal pool within cells after a 30-min chase. Arf6-overexpressing cells were used as the standard against which the mutants were compared and set with an arbitrary value of 100%. (B) The presence of 0.3% 1-butanol during the last 10 min of antibody internalization and subsequent 30-min recycling inhibited MHCI antibody recycling, measured by internal pool emptying. Shown are means and SD from three independent experiments.
Endosomal Membranes That Accumulate in Cells Expressing Arf6N48R or N48I Have Elevated PIP2 Levels
The accumulation of tubular endosomes and block of recycling of MHCI back to the PM is a phenotype also observed in cells expressing Arf6T27N (Radhakrishna and Donaldson, 1997; Powelka et al., 2004). However, Vitale et al. (2002) had demonstrated that Arf6N48I could be activated by ARNO (Vitale et al., 2002), so we looked for indications that Arf6 N48R and N48I could become activated in HeLa cells. As an indication of Arf6 activation, we monitored the PIP2 content of the endosomes by coexpressing the PLC delta pleckstrin homology (PH)-green fluorescent protein (GFP) chimera to detect membrane PIP2. Arf6 activation leads to stimulation of PIP 5-kinase and production of PIP2 at the PM and on endosomes (Honda et al., 1999; Brown et al., 2001). In untreated cells expressing wild-type Arf6, little PIP2 was present on the internal endosomes; however, treatment of cells with cytochalasin D blocked endosomal recycling and led to an accumulation of PH-GFP, and presumably PIP2, on tubular endosomes (Figure 3, bottom 2 rows) as observed previously (Brown et al., 2001). We also found that the tubules generated by cells expressing Arf6N48R and N48I labeled extensively with PH-GFP (Figure 3, insets), indicating that substantial PIP2 was present on the tubules. By contrast, PH-GFP did not label the tubules and endosomal membranes that accumulated in cells expressing Arf6T27N, indicating diminished PIP2 levels on these membranes. Indeed, cells expressing Arf6T27N showed membrane-associated PIP2 only on the PM (Figure 3), likely because of the basal activity of PIP 5-kinases at the PM. The distinct labeling of the N48R-induced tubular membranes with PH-GFP is an indication that the mutants of Arf6 can be activated and stimulate the activity of PIP 5-kinase to generate PIP2. Hence, the block in membrane recycling may be because of the failure of Arf6N48R to stimulate PLD, although we cannot rule out other deficiencies that might be attributed to Arf6N48R.
Figure 3.
Tubular endosomes that accumulate in cells expressing Arf6N48R and N48I contain PIP2. HeLa cells were cotransfected with Arf6N48R, Arf6N48I, Arf6T27N, or wild-type Arf6 and PH-GFP for 20 h. Some cells expressing WT Arf6 were treated with cytochalasin D (Cyto D) (200 nM) for 30 min and then fixed, and Arf6 was detected with an antibody. Insets reveal Arf6 tubular endosomes. Bars, 10 μm.
Reagents That Bypass Arf-stimulated PLD Can Rescue Phenotypes of Arf6N48R and N48I
To demonstrate that the Arf6N48R- and N48I-induced accumulation of tubular endosomes and inhibition in recycling is because of failure to stimulate PLD, we sought to produce PA through other means to restore a normal morphology and function to cells expressing these mutants. Phorbol esters such as PMA can stimulate PLD through activation of protein kinase C (PKC) (Hu and Exton, 2003). This stimulation of PLD activity can occur independently of Arf proteins (Hiroyama and Exton, 2005b). Another mechanism to elevate cellular PA levels is by inhibiting PA phosphohydrolase, the enzyme that converts PA to diacylglycerol, by treatment of cells with propranolol (Koul and Hauser, 1987). Treating cells expressing Arf6N48R or N48I with either PMA or propranolol eliminated the tubular endosome distribution of Arf6 (Figure 4A). For N48I, PMA treatment restored the Arf6 distribution to normal in most cells, whereas for N48R, the vacuolar structures sometimes observed in these cells persisted (Figure 4A). PMA treatment decreased tubular endosomes in cells expressing N48R or N48I by >50%, to the level observed in untreated, wild-type cells (Figure 4B). The fraction of wild-type cells exhibiting tubular endosomes was also decreased by PMA treatment. These effects were reversible because removal of PMA and recovery for 90 min led to an increase in cells with tubular endosomes for the mutants and wild-type Arf6 (Figure 4A) almost to the levels originally observed (Figure 4B).
Figure 4.
Accumulation of tubular endosomes in Arf6N48R- and N48I-expressing cells is rescued by PMA and propranolol treatments. (A) HeLa cells transfected with either Arf6N48R or N48I were treated with 200 nM PMA for 30 min. Some treated cells were then fixed and stained for Arf6, whereas others were washed with media and allowed to recover in media for 90 min before fixation. (B) Quantification of cells displaying a tubular phenotype as a percentage of transfected cells counted. Data collected from 100 cells from at least two experiments. (C) Tubular endosome accumulations of Arf6N48R and N48I are rescued by propranolol treatment. HeLa cells transfected with Arf6N48R or N48I were treated with 0.1 mM propranolol for 30 min. Some cells were fixed, whereas others were allowed to recover by washout with media for 90 min before fixing. (D) Quantification of cells displaying tubular endosomes as a percentage of 100 transfected cells counted from at least two experiments. Mean and SE from two independent experiments. Bars, 10 μm.
Treatment of cells with propranolol had a more dramatic effect on reducing the presence of tubular endosomes in cells expressing Arf6N48R and N48I (Figure 4C); cells with tubular endosomes were essentially absent after propranolol treatment (Figure 4D). Even cells expressing wild-type Arf6 saw a dramatic decrease in the percentage of cells displaying tubular endosomes (Figure 4D). This effect was also reversible because removal of propranolol and recovery in regular media for 90 min caused a return of tubular endosomes to the levels seen initially for N48R, N48I, and the wild-type protein (Figure 4D).
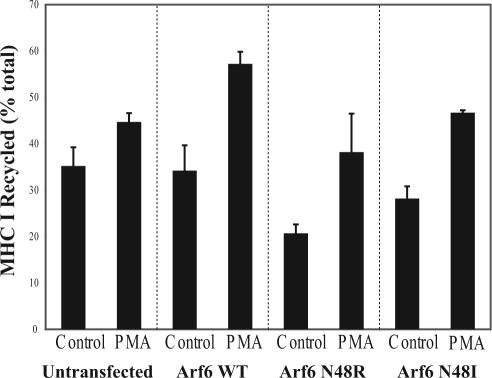
The loss of tubular endosomes in Arf6N48R- and N48I-expressing cells treated with PMA or propranolol suggested that these alternative means for generating PA might also relieve the block in MHCI recycling observed for these mutants. To assess whether this was so, we measured the effect of PMA on MHCI recycling in cells expressing N48R and N48I. As shown in Figure 5, MHCI recycling was inhibited in both N48R- and N48I-expressing cells. However, if PMA was added during recycling it enhanced the rate of recycling of MHCI in N48R- and N48I-expressing cells to the same level as that observed in untransfected cells treated with PMA. There was also an increase in the amount of MHCI that was recycled when cells expressing wild-type Arf6 were treated with PMA. These findings indicate that the recycling block observed in Arf6N48R- and N48I-expressing cells can be relieved by stimulating PLD through PKC. In contrast, propranolol treatment, although successful at eliminating tubular endosomes, did not rescue the block to recycling observed with Arf6N48R (our unpublished observations).
Figure 5.
PMA treatment rescues inhibition of MHCI recycling in Arf6N48R- and N48I-expressing cells. Untransfected cells or cells expressing Arf6, Arf6N48R, or Arf6N48I were loaded with antibody to MHCI for 30 min. Cells were treated with 200 nM PMA during the 30-min recycling phase. The surface MHCI antibody was stripped, and the extent of MHCI recycling was determined as described in Materials and Methods. The fraction of internalized MHCI that was recycled at 30 min is shown for two independent experiments (mean ± SE).
GAP Recruitment during Aluminum Fluoride (AlF)-induced Activation of Arf6 Is Dependent on PLD Activation
Another indicator of Arf6 function in cells is the ability of Arf6, when overexpressed, to generate cell surface protrusions in response to treatment with AlF (Radhakrishna and Donaldson, 1997). We examined whether Arf6 stimulation of PLD was required for AlF-induced protrusions in cells expressing wild-type Arf6 or the N48 mutants. Cells expressing wild-type Arf6 formed protrusions after 30 min of treatment, whereas cells expressing Arf6N48R were unchanged after AlF treatment (Supplemental Figure 2A). We also found that 1-butanol treatment of cells expressing wild-type Arf6 to inhibit PA production blocked AlF protrusions (Supplemental Figure 2A). Thus, Arf6 activation of PLD and generation of PA seems to be required for events that allow protrusion formation after AlF treatment.
Although the details remain unclear concerning the mechanism of protrusion formation after AlF treatment, we suspect it occurs, to a large extent, through formation of a complex composed of AlF, Arf6, and its GAP (Jackson et al., 2000). Thus, in cells overexpressing Arf6, all the endogenous GAP becomes sequestered in this complex upon addition of AlF, freeing surplus Arf6 to become activated and generate the protrusive structures we observe (Jackson et al., 2000). Consistent with this model, overexpression of ACAP1, an Arf6 GAP, blocks AlF-induced protrusion formation and ACAP1 accumulates on the tubular endosomes (Figure 6A). By contrast, Arf6T27N, which cannot become activated, does not recruit ACAP1 (Jackson et al., 2000). The failure of the mutant Arf6 to form protrusions raises the possibility that GAP recruitment to these mutants might be defective. Indeed, on assaying GAP recruitment in response to AlF treatment, we found that wild-type Arf6 recruited ACAP1 (Figure 6A) but N48R did not and N48I did so only weakly (Figure 6, B and C). Remarkably, propranolol pretreatment restored ACAP1 recruitment in cells expressing N48R and N48I (Figure 6, B and C), essentially back to levels seen with wild-type Arf6 (Figure 6B). Thus, consistent with previous biochemical observations (Vitale et al., 2002), these Arf6 point mutants seem to be able to physically interact with GAP protein. When cells expressing wild-type Arf6 were pretreated with 1-butanol before the addition of AlF, ACAP1 was not effectively recruited to the tubular endosomes (Figure 6, A and B), whereas cells pretreated with 2-butanol were less affected, supporting a role for PA generation in GAP recruitment. Although propranolol could rescue the recruitment of ACAP1 onto the tubular endosomes, we did not observe rescue of protrusions in cells expressing N48R and N48I treated with AlF and propranolol.
Figure 6.
GAP recruitment is defective in cells expressing Arf6N48R and N48I, and in cells treated with 1-butanol, and is rescued with propranolol treatment. HeLa cells that were cotransfected with Arf6 (A), Arf6N48R (B), or Arf6N48I (B) and ACAP1 were treated with 30 mM NaF and 50 μM AlCl3 for 30 min and then fixed and probed with antibodies to Arf6 and FLAG epitope to detect ACAP1. Some cells were pretreated with 0.1 mM propranolol for 10 min, 0.3% 1-butanol, or 0.3% 2-butanol before AlF was added for 30 min; the cells were then fixed and stained described in text. (C) Quantification of ACAP1 recruitment to membranes in 100 transfected cells from at least two separate experiments. Bars, 10 μm.
DISCUSSION
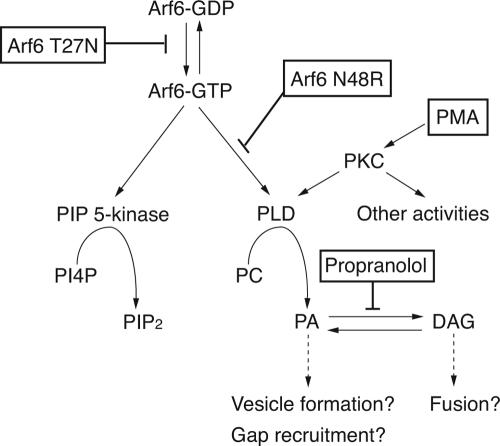
In this study, we investigated the extent to which activation of PLD was essential for Arf6 functions. Using mutants of Arf6, previously characterized as being defective in their ability to activate PLD (Jones et al., 1999; Skippen et al., 2002; Vitale et al., 2002), we found that Arf6 activation of PLD was required for recycling of endosomal membrane back to the PM. This recycling defect could be recreated in untransfected cells treated with 1-butanol and rescued by activating PLD through alternative means (Figure 7). Additionally, we observed that PA generation is required for the GAP recruitment onto the endosome during AlF treatment. Again this defect in GAP recruitment was recreated in cells treated with 1-butanol and rescued by elevating PA. This ability to recreate and rescue the phenotypes caused by Arf6N48R and N48I with agents that inhibit or enhance the production of PA favors the idea that the phenotype is because of a failure to activate PLD and not some other activity. Attempts were made to measure basal PLD activity in cells; however, we did not detect a significant decrease in PLD activity in cells expressing Arf6N48R compared with that observed in cells expressing Arf6 or untransfected cells (our unpublished observations). The inability to measure differences in whole cells could be because of Arf6 affecting only a small pool of PLD in cells. Together, these observations provide compelling evidence that Arf6 stimulation of PLD is essential for events taking place on the tubular endosome that are necessary for fusion of membrane back to the PM.
Figure 7.
Scheme for Arf6 activation of PIP 5-kinase and PLD. Arf6-GTP can activate PIP 5-kinase and PLD leading to the generation of PIP2 and PA, respectively. Arf6T27N inhibits activation of Arf6 and Arf6N48R inhibits activation of PLD but not of PIP 5-kinase. PMA-induced PKC stimulation of PLD, and propranolol inhibition of PA phosphohydrolase lead to alternative means for generation of PA, although PKC may have other activities.
The block in recycling that is observed in cells expressing Arf6N48R and N48I is distinct from the block observed in cells expressing Arf6T27N (Figure 7). Arf6T27N cannot bind GTP, causes a block in recycling (Radhakrishna and Donaldson, 1997; Powelka et al., 2004), and the tubular endosomes that accumulate in cells expressing Arf6T27N are devoid of PIP2. By contrast, the endosomal membranes that accumulate in cells expressing Arf6N48R or N48I are enriched in PIP2. Collectively, these observations suggest that Arf6N48R and N48I can be activated and stimulate PIP 5-kinase, but because they fail to activate PLD, recycling is inhibited. It is not clear why we consistently observed a more severe phenotype in cells expressing N48R as opposed to N48I because both mutants are defective in activation of PLD in vitro (Jones et al., 1999; Vitale et al., 2002). It is possible that the N48R mutation inhibits other Arf6 interactions in addition to PLD.
What then could be the role of PA in endosomal recycling and regulated exocytosis? Generation of PA in the membrane could lead to changes in membrane curvature that might facilitate vesicle fission or fusion (Huttner and Zimmerberg, 2001). Although PA can stimulate PIP 5-kinase directly, the abundant PIP2 on the tubular endosome suggests this is not the mechanism for PA's effect in these experiments. Other proteins are regulated by PA; indeed, we have shown previously that the activities of ACAP1 and ACAP2, both Arf6 GAPs, are markedly stimulated by PA in the presence of PIP2 (Jackson et al., 2000). The failure of the N48 mutants to recruit ACAP1 argues that PA generation is crucial for GAP function on the endosome. Furthermore, because the defect in GAP function manifests as an inhibition of recycling, the possibility exists that GAP must bind to the tubular endosomes and inactivate Arf6 during normal endosomal recycling. Although endosomal recycling specifically requires activation of Arf6 (Radhakrishna and Donaldson, 1997; Powelka et al., 2004), it is possible that GAP recruitment, either for inactivation or another function, is involved in the recycling. Indeed, the peripheral ArfGAPs are all multidomain proteins that could serve other functions in additional to Arf inactivation during membrane recycling (Randazzo and Hirsch, 2004).
In addition to a requirement for PA for endosomal membrane recycling, our observations suggest that there may also be a need for PA-derived 1,2-sn-diacylglycerol (DAG) (Figure 7). Although we found that alternative routes of PA generation, either through PKC activation of PLD or propranolol inhibition of PA phosphohydrolase, would cause a breakdown of the tubular endosomes that accumulate in cells expressing N48R (Figure 4), propranolol treatment failed to rescue the recycling of MHCI back to the PM. The failure of propranolol to rescue might indicate a requirement for DAG for fusion of the released vesicles back to the PM (Figure 7). This seems plausible because DAG promotes vesicle fusion in a number of systems (Goni and Alonso, 1999). Additionally, it is possible that activated PKC may have other effects on MHCI recycling besides stimulation of PLD.
The requirement for Arf6 stimulation of PLD for endosomal membrane recycling could also explain the PLD involvement in PM ruffling and stimulation of cell migration. Cells expressing Arf6N48R and N48I were flat, did not demonstrate any PM cortical actin activity, and could not make protrusions upon AlF treatment. Reports of PLD requirements for antigen-stimulated ruffling in mast cells (O'Luanaigh et al., 2002), macrophage phagocytosis (Iyer et al., 2004), actin changes associated with myogenesis (Komati et al., 2005), and increased Madin-Darby canine kidney cell motility (Santy and Casanova, 2001) may be because of the inhibition of membrane recycling observed in the present study.
We do not know which PLD isoform is mediating these Arf6 effects. PLD1 has been studied more intensively as an Arf-responsive enzyme than has PLD2. However, in HeLa cells, overexpressed PLD1 is localized primarily to lysosomal structures. In COS cells, PLD1 undergoes a complex trafficking journey that includes residence at the PM (Du et al., 2003). Furthermore, a PLD1 mutant unable to be palmitoylated is constitutively at the PM (Sugars et al., 1999). In contrast, PLD2 is present throughout the Arf6 tubular endosome system in addition to the PM (our unpublished observations). Although PLD2 is generally not considered to be regulated by Arf, recent studies suggest that PLD2 can indeed be activated by Arf6 in cells (Hiroyama and Exton, 2005a). If PLD2 were the Arf6 target, then the site where Arf6 activates PLD2 could occur at any point in the membrane recycling pathway.
Our findings demonstrate that stimulation of PLD is necessary for Arf6-dependent membrane recycling. The parallels of this constitutive, endocytic recycling pathway described here with that of regulated exocytosis are striking. Arf6 regulation of PIP 5-kinase and PLD have been implicated in regulated exocytosis of dense core granules (Caumont et al., 1998; Vitale et al., 2002), vesicles containing Glut4 transporter (Lawrence and Birnbaum, 2003; Huang et al., 2005), and secretion in neutrophil HL60 cells (O'Luanaigh et al., 2002; Skippen et al., 2002). Intriguingly, insulin-stimulated Glut4 exocytosis also involves Rab11 function (Zeigerer et al., 2002) and actin polymerization (Tsakiridis et al., 1994), activities that are also required for the Arf6-mediated recycling pathway (Powelka et al., 2004; Weigert et al., 2004). Hence, understanding the recycling pathway that Arf6 regulates will provide insight into regulated secretory pathways.
Supplementary Material
Acknowledgments
We thank Drs. Akira Honda, Edward Korn, and Roberto Weigert for discussions and comments on the manuscript. We also thank Drs. Ze Ping and Michael Beaven (National Heart, Lung, and Blood Institute) for performing PLD assays on our samples.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-06-0523) on November 9, 2005.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Aikawa, Y., and Martin, T.F.J. (2003). ARF6 regulates a plasma membrane pool of phosphatidylinositol(4,5)bisphosphate required for regulated exocytosis. J. Cell Biol. 162, 647-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, F. D., Rozelle, A. L., Yin, H. L., Balla, T., and Donaldson, J. G. (2001). Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic. J. Cell Biol. 154, 1007-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, F. D., Thompson, N., Saqib, K. M., Clark, J. M., Powner, D., Thompson, N. T., Solari, R., and Wakelam, M. J. (1998). Phospholipase D1 localises to secretory granules and lysosomes and is plasma-membrane translocated on cellular stimulation. Curr. Biol. 8, 835-838. [DOI] [PubMed] [Google Scholar]
- Brown, H. A., Gutowski, S., Moomaw, C. R., Slaughter, C., and Sternweis, P. C. (1993). ADP-ribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase D activity. Cell 75, 1137-1144. [DOI] [PubMed] [Google Scholar]
- Caumont, A. S., Galas, M. C., Vitale, N., Aunis, D., and Bader, M. F. (1998). Regulated exocytosis in chromaffin cells. Translocation of ARF6 stimulates a plasma membrane-associated phospholipase D. J. Biol. Chem. 273, 1373-1379. [DOI] [PubMed] [Google Scholar]
- Cockcroft, S., Thomas, G. M., Fensome, A., Geny, B., Cunningham, E., Gout, I., Hiles, I., Totty, N. F., Truong, O., and Hsuan, J. J. (1994). Phospholipase D: a downstream effector of ARF in granulocytes. Science 263, 523-526. [DOI] [PubMed] [Google Scholar]
- Colley, W. C., Sung, T. C., Roll, R., Jenco, J., Hammond, S. M., Altshuller, Y., Bar-Sagi, D., Morris, A. J., and Frohman, M. A. (1997). Phospholipase D2, a distinct phospholipase D isoform with novel regulatory properties that provokes cytoskeletal reorganization. Curr. Biol. 7, 191-201. [DOI] [PubMed] [Google Scholar]
- D'Souza-Schorey, C., Boshans, R. L., McDonough, M., Stahl, P. D., and Van Aelst, L. (1997). A role for POR1, a Rac1-interacting protein, in ARF6-mediated cytoskeletal rearrangements. EMBO J. 16, 5445-5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza-Schorey, C., Li, G., Colombo, M. I., and Stahl, P. D. (1995). A regulatory role for ARF6 in receptor-mediated endocytosis. Science 267, 1175-1178. [DOI] [PubMed] [Google Scholar]
- Donaldson, J. G. (2003). Multiple roles for Arf 6, sorting, structuring, and signaling at the plasma membrane. J. Biol. Chem. 278, 41573-41576. [DOI] [PubMed] [Google Scholar]
- Du, G., Altshuller, Y. M., Vitale, N., Huang, P., Chasserot-Golaz, S., Morris, A. J., Bader, M. F., and Frohman, M. A. (2003). Regulation of phospholipase D1 subcellular cycling through coordination of multiple membrane association motifs. J. Cell Biol. 162, 305-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, G., Huang, P., Liang, B. T., and Frohman, M. A. (2004). Phospholipase D2 localizes to the plasma membrane and regulates angiotensin II receptor endocytosis. Mol. Biol. Cell 15, 1024-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco, M., Peters, P. J., Boretto, J., van Donselaar, E., Neri, A., D'Souza-Schorey, C., and Chavrier, P. (1999). EFA6, a sec7 domain-containing exchange factor for ARF6, coordinates membrane recycling and actin cytoskeleton organization. EMBO J. 18, 1480-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyberg, Z., Siddhanta, A., and Shields, D. (2003). “Slip, sliding away”: phospholipase D and the Golgi apparatus. Trends Cell Biol. 13, 540-546. [DOI] [PubMed] [Google Scholar]
- Goni, F. M., and Alonso, A. (1999). Structure and functional properties of diacylglycerols in membranes. Prog. Lipid Res. 38, 1-48. [DOI] [PubMed] [Google Scholar]
- Hiroyama, M., and Exton, J. H. (2005a). Localization and regulation of phospholipase D2 by ARF6. J. Cell. Biochem. 95, 149-164. [DOI] [PubMed] [Google Scholar]
- Hiroyama, M., and Exton, J. H. (2005b). Studies of the roles of ADP-ribosylation factors and phospholipase D in phorbol ester-induced membrane ruffling. J. Cell. Physiol. 202, 608-622. [DOI] [PubMed] [Google Scholar]
- Honda, A., et al. (1999). Phosphatidylinositol 4-phosphate 5-kinase alpha is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell 99, 521-532. [DOI] [PubMed] [Google Scholar]
- Hu, T., and Exton, J. H. (2003). Mechanisms of regulation of phospholipase D1 by protein kinase Calpha. J. Biol. Chem. 278, 2348-2355. [DOI] [PubMed] [Google Scholar]
- Huang, P., Altshuller, Y. M., Chunqiu Hou, J., Pessin, J. E., and Frohman, M. A. (2005). Insulin-stimulated plasma membrane fusion of Glut4 glucose transporter-containing vesicles is regulated by phospholipase D1. Mol. Biol. Cell 16, 2614-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner, W. B., and Zimmerberg, J. (2001). Implications of lipid microdomains for membrane curvature, budding and fission. Curr. Opin. Cell Biol. 13, 478-484. [DOI] [PubMed] [Google Scholar]
- Iyer, S. S., Barton, J. A., Bourgoin, S., and Kusner, D. J. (2004). Phospholipases D1 and D2 coordinately regulate macrophage phagocytosis. J. Immunol. 173, 2615-2623. [DOI] [PubMed] [Google Scholar]
- Jackson, T. R., Brown, F. D., Nie, Z., Miura, K., Foroni, L., Sun, J., Hsu, V. W., Donaldson, J. G., and Randazzo, P. A. (2000). ACAPs are arf6 GTPase-activating proteins that function in the cell periphery. J. Cell Biol. 151, 627-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, D. H., Bax, B., Fensome, A., and Cockcroft, S. (1999). ADP ribosylation factor 1 mutants identify a phospholipase D effector region and reveal that phospholipase D participates in lysosomal secretion but is not sufficient for recruitment of coatomer I. Biochem. J. 341, 185-192. [PMC free article] [PubMed] [Google Scholar]
- Komati, H., Naro, F., Mebarek, S., De Arcangelis, V., Adamo, S., Lagarde, M., Prigent, A. F., and Nemoz, G. (2005). Phospholipase D is involved in myogenic differentiation through remodeling of actin cytoskeleton. Mol. Biol. Cell 16, 1232-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koul, O., and Hauser, G. (1987). Modulation of rat brain cytosolic phosphatidate phosphohydrolase: effect of cationic amphiphilic drugs and divalent cations. Arch. Biochem. Biophys. 253, 453-461. [DOI] [PubMed] [Google Scholar]
- Krauss, M., Kinuta, M., Wenk, M. R., De Camilli, P., Takei, K., and Haucke, V. (2003). ARF6 stimulates clathrin/AP-2 recruitment to synaptic membranes by activating phosphatidylinositol phosphate kinase type Igamma. J. Cell Biol. 162, 113-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence, J. T., and Birnbaum, M. J. (2003). ADP-ribosylation factor 6 regulates insulin secretion through plasma membrane phosphatidylinositol 4,5-bisphosphate. Proc. Natl. Acad. Sci. USA 100, 13320-13325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott, M., Wakelam, M. J., and Morris, A. J. (2004). Phospholipase D. Biochem. Cell Biol. 82, 225-253. [DOI] [PubMed] [Google Scholar]
- Naslavsky, N., Weigert, R., and Donaldson, J. G. (2003). Convergence of non-clathrin- and clathrin-derived endosomes involves Arf6 inactivation and changes in phosphoinositides. Mol. Biol. Cell 14, 417-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Luanaigh, N., Pardo, R., Fensome, A., Allen-Baume, V., Jones, D., Holt, M. R., and Cockcroft, S. (2002). Continual production of phosphatidic acid by phospholipase D is essential for antigen-stimulated membrane ruffling in cultured mast cells. Mol. Biol. Cell 13, 3730-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios, F., and D'Souza-Schorey, C. (2003). Modulation of Rac1 and ARF6 activation during epithelial cell scattering. J. Biol. Chem. 278, 17395-17400. [DOI] [PubMed] [Google Scholar]
- Palacios, F., Price, L., Schweitzer, J., Collard, J. G., and D'Souza-Schorey, C. (2001). An essential role for ARF6-regulated membrane traffic in adherens junction turnover and epithelial cell migration. EMBO J. 20, 4973-4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powelka, A. M., Sun, J., Li, J., Gao, M., Shaw, L. M., Sonnenberg, A., and Hsu, V. W. (2004). Stimulation-dependent recycling of integrin beta1 regulated by ARF6 and Rab11. Traffic 5, 20-36. [DOI] [PubMed] [Google Scholar]
- Radhakrishna, H., and Donaldson, J. G. (1997). ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J. Cell Biol. 139, 49-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishna, H., Klausner, R. D., and Donaldson, J. G. (1996). Aluminum fluoride stimulates surface protrusions in cells overexpressing the ARF6 GTPase. J. Cell Biol. 134, 935-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo, P. A., and Hirsch, D. S. (2004). Arf GAPs: multifunctional proteins that regulate membrane traffic and actin remodelling. Cell Signal 16, 401-413. [DOI] [PubMed] [Google Scholar]
- Sabe, H. (2003). Requirement for Arf6 in cell adhesion, migration, and cancer cell invasion. J. Biochem. 134, 485-489. [DOI] [PubMed] [Google Scholar]
- Santy, L. C., and Casanova, J. E. (2001). Activation of ARF6 by ARNO stimulates epithelial cell migration through downstream activation of both Rac1 and phospholipase D. J. Cell Biol. 154, 599-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer, D. A., D'Souza-Schorey, C., and Cooper, J. A. (2000). Actin assembly at membranes controlled by ARF6. Traffic 1, 892-903. [DOI] [PubMed] [Google Scholar]
- Skippen, A., Jones, D. H., Morgan, C. P., Li, M., and Cockcroft, S. (2002). Mechanism of ADP ribosylation factor-stimulated phosphatidylinositol 4,5-bisphosphate synthesis in HL60 cells. J. Biol. Chem. 277, 5823-5831. [DOI] [PubMed] [Google Scholar]
- Sugars, J. M., Cellek, S., Manifava, M., Coadwell, J., and Ktistakis, N. T. (1999). Fatty acylation of phospholipase D1 on cysteine residues 240 and 241 determines localization on intracellular membranes. J. Biol. Chem. 274, 30023-30027. [DOI] [PubMed] [Google Scholar]
- Tsakiridis, T., Vranic, M., and Klip, A. (1994). Disassembly of the actin network inhibits insulin-dependent stimulation of glucose transport and prevents recruitment of glucose transporters to the plasma membrane. J. Biol. Chem. 269, 29934-29942. [PubMed] [Google Scholar]
- Vitale, N., Chasserot-Golaz, S., Bailly, Y., Morinaga, N., Frohman, M. A., and Bader, M. F. (2002). Calcium-regulated exocytosis of dense-core vesicles requires the activation of ADP-ribosylation factor (ARF)6 by ARF nucleotide binding site opener at the plasma membrane. J. Cell Biol. 159, 79-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert, R., Yeung, A. C., Li, J., and Donaldson, J. G. (2004). Rab22a regulates the recycling of membrane proteins internalized independently of clathrin. Mol. Biol. Cell 15, 3758-3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, S. F., Freer, S., and Benson, A. A. (1967). Transphosphatidylation by phospholipase D. J. Biol. Chem. 242, 477-484. [PubMed] [Google Scholar]
- Zeigerer, A., Lampson, M. A., Karylowski, O., Sabatini, D. D., Adesnik, M., Ren, M., and McGraw, T. E. (2002). GLUT4 retention in adipocytes requires two intracellular insulin-regulated transport steps. Mol. Biol. Cell 13, 2421-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, Q., and Bobich, J. A. (2004). ADP-ribosylation factor6 regulates both [3H]-noradrenaline and [14C]-glutamate exocytosis through phosphatidylinositol 4,5-bisphosphate. Neurochem. Int. 45, 633-640. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.