Abstract
The binding of chemoattractants to cognate G protein-coupled receptors activates a variety of signaling cascades that provide spatial and temporal cues required for chemotaxis. When subjected to uniform stimulation, these responses are transient, showing an initial peak of activation followed by a period of adaptation, in which activity subsides even in the presence of stimulus. A tightly regulated balance between receptor-mediated stimulatory and inhibitory pathways controls the kinetics of activation and subsequent adaptation. In Dictyostelium, the adenylyl cyclase expressed during aggregation (ACA), which synthesizes the chemoattractant cAMP, is essential to relay the signal to neighboring cells. Here, we report that cells lacking phosphoinositide 3-kinase (PI3K) activity are deficient in signal relay. In LY294002-treated cells, this defect is because of a loss of ACA activation. In contrast, in cells lacking PI3K1 and PI3K2, the signal relay defect is because of a loss of ACA adaptation. We propose that the residual low level of 3-phosphoinositides in pi3k1-/2- cells is sufficient to generate the initial peak of ACA activity, yet is insufficient to sustain the inhibitory phase required for its adaptation. Thus, PI3K activity is poised to regulate both ACA activation and adaptation, thereby providing a link to ensure the proper balance of counteracting signals required to maintain optimal chemoresponsiveness.
INTRODUCTION
Chemotaxis is a fundamental process in which cells migrate directionally by sensing and responding to extracellular gradients of chemoattractants. This process is exhibited in a variety of biological responses, including wound healing, angiogenesis, and embryogenesis as well as during the recruitment of neutrophils to sites of inflammation. Chemotaxis is also essential for the survival of the social amoebae Dictyostelium discoideum. Neutrophils and Dictyostelium cells migrate using amoeboid movements, characterized by leading edge pseudopod extensions and back retractions/contractions (Chung et al., 2001; Iijima et al., 2002; Weiner, 2002; Parent, 2004). It has been determined that these cells are extremely sensitive and can detect spatial gradients in which the chemoattractant concentration differs by as little as 1% between the front and the back of the cell (Mato et al., 1975; Zigmond, 1977). Furthermore, these cells are responsive over a wide dynamic range of chemoattractant concentrations, which can vary over at least 4 orders of magnitude. This dynamic responsiveness requires a tightly regulated balance between stimulatory and inhibitory responses, which are both initiated upon chemoattractant receptor activation (Zigmond and Sullivan, 1979; Dinauer et al., 1980a,b). Temporal modulation of stimulatory and inhibitory inputs give rise to transient responses composed of an initial peak of activation followed by a period of adaptation, during which the system is refractory to further signals, and finally deadaptation, which resets the system and allows cells to respond to additional chemotactic signals (Parent and Devreotes, 1999; Devreotes and Janetopoulos, 2003). Intriguingly, although the mechanisms that control the activation process have been extensively characterized, most of the adaptive mechanisms remain poorly understood.
For neutrophils and Dictyostelium, the binding of chemoattractants to G protein-coupled receptors stimulates a variety of responses, including an increase in the levels of cAMP (Harvath et al., 1991; Dai et al., 1994; Saran et al., 2002). In Dictyostelium, the signaling pathways that lead to the activation of the adenylyl cyclase expressed during aggregation (ACA), which converts ATP into cAMP, have been extensively studied (Kriebel and Parent, 2004). In this organism, cAMP acts as a chemoattractant by binding to specific seven-transmembrane cAMP receptors, cARs. Stimulation of the receptors leads to the activation of ACA and the synthesis of more cAMP, a fraction of which is secreted to initiate a signal relay loop that propagates the signal to neighboring cells. As chemotaxis proceeds, Dictyostelium cells begin to migrate in a head-to-tail manner, generating long streams of cells (Pitt et al., 1992; Kriebel et al., 2003). We have previously shown that this streaming process not only depends on the presence of ACA itself but also on its cellular distribution to the rear of cells (Kriebel et al., 2003). We propose that the spatial restriction of ACA provides a compartment from which cAMP is secreted, thereby locally attracting neighboring cells.
The activation of ACA shows an absolute requirement for the pleckstrin homology (PH) domain-containing protein cytosolic regulator of adenylyl cyclase (CRAC) (Insall et al., 1994; Lilly and Devreotes, 1995). On receptor stimulation, Gβγ subunits activate phosphoinositide 3-kinase (PI3K) in a Ras-dependent manner, leading to the production of 3-phosphoinositides (3-PI) to which CRAC binds via its PH domain (Funamoto et al., 2002; Comer et al., 2005). After the addition of a uniform dose of chemoattractant, CRAC is rapidly and transiently recruited to the plasma membrane around the entire periphery of the cell. In contrast, when cells are exposed to a gradient of chemoattractant, the membrane recruitment is restricted to the side of the cell facing the highest concentration of attractant (Parent et al., 1998). This dynamic distribution is part of an elegant mechanism that cells use to compartmentalize signal transduction events. In both neutrophils and Dictyostelium, PI3K signaling has been shown to localize to the leading edge of chemotaxing cells, whereas the enzyme that degrades the products of PI3K, the tumor suppressor PTEN, localizes to the back and sides (Servant et al., 2000; Funamoto et al., 2002; Iijima and Devreotes, 2002; Li et al., 2005). Disruption of the balance between these two enzyme activities, either by pharmacological inhibition or targeted gene disruption, results in defects in cell polarity, spatial actin organization, gradient sensing, and efficiency of chemotaxis (Chung et al., 2001; Iijima et al., 2002; Stephens et al., 2002). Although the mechanism by which CRAC activates ACA remains to be determined, recent findings have shown that the PI3K-mediated activation of CRAC independently regulates ACA activation and chemotaxis, suggesting that these two processes are subject to coordinated regulation (Comer et al., 2005).
Interestingly, the activation of ACA requires another cytosolic regulator called Pianissimo (Pia) (Chen et al., 1997). In contrast to CRAC, Pia harbors no known protein motifs, but several Pia homologues in organisms that range from yeast to mammals have been identified. The mammalian Pia homologue, called Rictor or mAVO3, is part of the target of rapamycin (TOR) complex 2 (TORC2), along with TOR and LST8, and has been shown to regulate cytoskeletal rearrangements (Sarbassov et al., 2004). We have recently shown that in Dictyostelium the components of TORC2, which also include the AVO1 homologue ras interacting protein 3 (RIP3), function together in a preformed complex to regulate ACA activity (Lee et al., 1999, 2005). Moreover, reconstitution experiments established that TORC2 can form in the absence of CRAC. Given previous data showing that ACA activity can be reconstituted in lysates derived from cells lacking both Pia and CRAC only when both proteins are added back (Chen et al., 1997), these findings suggest that ACA activation requires an input from both TORC2 and CRAC.
In this article, we show that PI3K is central to both activation and adaptation of ACA in response to chemoattractant stimulation. Our results are consistent with a model in which PI3K coordinately regulates pathways that control chemotaxis, ACA activation, and ACA adaptation. Control of all three pathways by PI3K provides an efficient mechanism for a strict regulation and ensures the proper balance of these counteracting inputs on ACA activation.
MATERIALS AND METHODS
Reagents
All chemicals were purchased from Sigma Chemical (St. Louis, MO) or VWR (West Chester, PA), unless otherwise indicated. Monoclonal anti-green fluorescent protein (GFP) antibody was purchased from Covance Research Products (Berkeley, CA). Enhanced chemiluminescence detection reagent and the cAMP radioimmunoassay kit were purchased from GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom). Micropipettes for chemotaxis assays were from Eppendorf (Hamburg, Germany). [α-32P]ATP (800 Ci/mmol) was purchased from MP Biomedicals (Irvine, CA). Precast NuPage Bis-Tris protein electrophoresis gradient gels were purchased from Invitrogen (Carlsbad, CA).
Cell Lines
The wild-type Ax3 and pten-/pi3k1-/2- cell lines (Janetopoulos et al., 2005) were a kind gift from Peter Devreotes (Johns Hopkins University School of Medicine, Baltimore, MD). The pi3k1-/2- (Zhou et al., 1995; Buczynski et al., 1997) and PI3K2/pi3k1-/2- (Funamoto et al., 2002) cell lines were a kind gift from Rick Firtel (University of California, San Diego, San Diego, CA).
Cell Growth and Differentiation
Cells were cultured in shaking suspension in HL5 medium and differentiated to the chemotaxis competent aggregation stage essentially as described previously (Devreotes et al., 1987; Parent et al., 1998). In brief, cells from log phase suspension culture were harvested and differentiated for 4.5-7 h in suspension at 2 × 107 cells/ml in development buffer (DB; 5 mM sodium phosphate buffer, pH 6.2, 2 mM MgSO4, and 0.2 mM CaCl2) with exogenous pulses of 75 nM cAMP every 6 min. To verify that cells differentiated to the chemotaxis competent stage, we performed Western blot analysis of the early developmental markers, chemoattractant receptor (cAR1), and the aggregation stage adenylyl cyclase (ACA).
Microscopy
For the micropipette chemotaxis assay and CRAC translocation assay, cells were viewed with an inverted Zeiss Axiovert 200 microscope (Carl Zeiss, Thornwood, NY) equipped with automated filter wheels (Ludl Electronic Products, Hawthorne, NJ). Images were recorded with a CoolSnap HQ charge-coupled device camera (Roper Scientific, Trenton, NJ) operated by IPLab software (Scanalytics, Fairfax, VA). All images in a series were processed identically using IPLab and/or Adobe Photoshop. Fluorescent images were viewed with the appropriate filter set and nonfluorescent cells were viewed using either Varel or differential interference contrast optics.
Chemotaxis Assays
To analyze the migration of groups of individual cells, the micropipette chemotaxis assay was carried out essentially as described previously (Parent et al., 1998). In this assay, cells undergo starvation induced differentiation to the chemotaxis competent aggregation stage (4-7 h), and their directed migration is observed in a gradient supplied by a micropipette filled with the chemoattractant cAMP at a concentration of 10 μM. The chemotaxis was recorded with a 15-s interval between successive frames. Chemotaxis of a large population of cells was observed with the under agarose assay, as described previously (Nelson et al., 1975; Comer et al., 2005). Briefly, 3-4 ml of 0.5% agarose in DB containing 1 mM caffeine was poured into 35-mm Petri dishes. The agarose was allowed to cool completely, and then three equidistant wells were cut out of the agarose with a wide-bore Pasteur pipet. The middle well was filled with 1 μM cAMP in DB, and the gradient was allowed to establish for 30 min before plating cells. Differentiated cells were suspended at 2 × 107 cells/ml in DB containing 2 mM caffeine and plated into the outer wells. After the cells were allowed to chemotax for ∼1 h, the wells were carefully overlaid with phosphate buffer and visualized on a Leica stereoscope. Each assay was performed in triplicate, and the results are representative of at least three independent experiments on different days, and wild-type cells were always assayed along with the tester cell line.
CRAC Translocation Assay
The chemoattractant-mediated recruitment of CRAC (CRAC-GFP) to the plasma membrane was observed on a fluorescence microscope as described previously (Parent et al., 1998). Briefly, CRAC-GFP-expressing cells were stimulated with 20 μM cAMP and fixed (1% formaldehyde, 0.125% glutaral-dehyde, and 0.01% Triton X-100 in phosphate buffer) after 5 s of stimulation. Cells were viewed as described above.
Adenylyl Cyclase and cAMP Accumulation Assays
Activation of adenylyl cyclase via chemoattractant receptor stimulation was measured essentially as described previously (Parent and Devreotes, 1995; Parent et al., 1998). Briefly, differentiated cells were stimulated with 50 μM cAMP and filter lysed at the indicated time points into Tris buffer containing [α-32P]ATP diluted with unlabeled ATP to a final concentration of ∼150 Ci/mol. The reaction was allowed to proceed for 1 min, stopped with SDS/ATP, and the radiolabeled cAMP was purified by sequential Dowex-AG 50W X-4 and alumina column chromatography (Salomon, 1979). Adenylyl cyclase measurements by this assay exhibit considerable variation from experiment to experiment. Although the relative extent of activation of different cell lines or treatment conditions is highly reproducible between experiments, the absolute activity can vary significantly (2- to 3-fold at times) from day to day. Therefore we, and others in the field, have chosen to present adenylyl cyclase activation data as representative of results from at least three to five or more independent experiments, each performed in duplicate on a given day. Furthermore, comparisons between different cell lines or treatment conditions are made on samples assayed on the same day, using the same reagent mixture.
To measure activation of adenylyl cyclase by global stimulation of both heterotrimeric G proteins and small GTPases, lysates were incubated with 100 μM guanosine 5′-O-(3-thio)triphosphate (GTPγS) and, after 5 min, aliquots of the lysate were assayed for adenylyl cyclase activity for 2 min. For the mixing experiments, cell lines were mixed, lysed together, and otherwise assayed exactly as described above. To prepare cytosolic and membrane extracts for the in vitro complementation experiment, cells were suspended at 8 × 107 cells/ml in simple lysis buffer (SLB; 10 mM Tris, pH 7.5, 0.2 mM EGTA, and 200 mM sucrose), filter lysed, and centrifuged at 9500 × g for 20 min. The supernatant fraction was denoted the cytosolic extract and was used without further dilution. The pellet fraction was resuspended in an equivalent volume of SLB and designated the membrane fraction. The in vitro complementation was performed in essentially the same manner as the direct GTPγS-stimulated adenylyl cyclase activation assay, except that cells were lysed directly into a tube on ice containing the cytosolic or membrane extract, as indicated. After 5 min, the adenylyl cyclase activity of the mixture was assayed for 2 min, as described.
Chemoattractant-mediated cAMP production was measured in differentiated cells after the addition of 5 μM 2′deoxy-cAMP in the presence of 10 mM DTT and 200 μM 3-isobutyl-1-methylxanthine using the cAMP radioimmunoassay kit from GE Healthcare.
RESULTS
Disruption of PI3K Activity Impairs Streaming
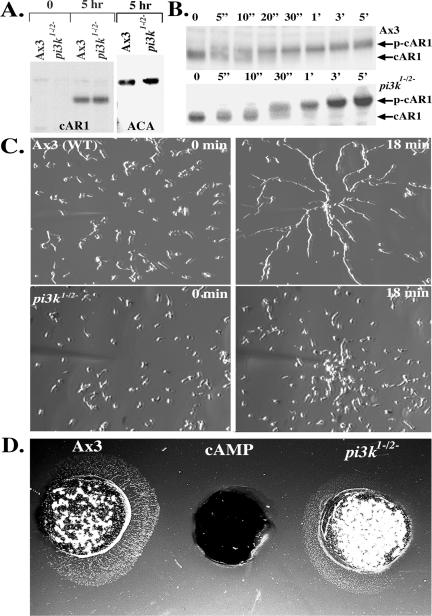
In previous work examining the role of 3-PIs in CRAC function, we introduced a point mutation in the predicted 3-PI binding motif within the CRAC PH domain (R42C-CRAC) and found that this mutant does not support chemotaxis and shows minimal ACA activation (Comer et al., 2005). To gain insight into this finding, we more carefully investigated the role of PI3K action in these processes. Although there are six PI3K isoforms in Dictyostelium (Janetopoulos et al., 2005), most of the chemoattractant-mediated PI3K activity arises from PI3K1 and PI3K2 (Huang et al., 2003). We therefore used cells in which the PI3K1 and PI3K2 isoforms have been disrupted (pi3k1-/2-) for our studies (Zhou et al., 1995; Buczynski et al., 1997). When we plated pi3k1-/2- cells on nonnutrient agar, we observed that these cells aggregate and differentiate, but they make misshapen terminal differentiation structures, as described previously (Zhou et al., 1995). To study this phenotype further, we recorded the differentiation process and found that the normal oscillatory pattern of periodic waves present in wild-type cells is disrupted in pi3k1-/2- cells. Although wild-type cells display regular wave patterns, which arise as cells respond to passing waves of synchronized chemoattractant release from cells in an aggregation center (Parent and Devreotes, 1996; Sawai et al., 2005), the wave patterns of the pi3k1-/2- cells are irregular and do not propagate far from the aggregation center (our unpublished data). To determine whether this defect is simply caused from aberrant expression of key signaling components, we assessed the expression of the chemoattractant receptor, cAR1, and ACA in pi3k1-/2- cells and found that they were comparable with wild-type cells (Figure 1A). We next investigated whether cAMP stimulation affects the extent or kinetics of cAR1 phosphorylation in pi3k1-/2- cells. On cAMP stimulation, cAR1 is rapidly phosphorylated on a cluster of C-terminal serine residues, giving rise to a 3- to 5-fold reduction in the cAMP binding affinity of cAR1 (Caterina et al., 1995). We found that cAMP-induced cAR1 phosphorylation is normal in pi3k1-/2- cells (Figure 1B). These results show that the wave propagation defect observed in pi3k1-/2- cells is not a consequence of defects at the level of cAMP detection by cAR1 or at alterations in the extent of cAMP-mediated cAR1 phosphorylation by downstream kinases.
Figure 1.
Reduction of PI3K activity impairs cell streaming during chemotaxis. (A) Expression of cAR1 and ACA in pi3k1-/2- cells. After 5 h of differentiation, samples of pi3k1-/2- and wild-type Ax3 cells were processed for Western analyses using antibodies against cAR1 (top left) and ACA (top right). The data presented are representative of three independent experiments. (B) cAR1 is functional in pi3k1-/2- cells. Western blot depicting the chemoattractant-dependent mobility shift of cAR1 in wild-type Ax3 (top) and pi3k1-/2- cells (bottom). Numbers on top of blot represent time after chemoattractant addition. The data presented are representative of three independent experiments. (C) pi3k1-/2- cells do not stream during chemotaxis. Cells differentiated for 6-7 h were subjected to a point source of 10 μM cAMP (see Materials and Methods for details). Images were recorded by time-lapsed imaging through a 10× Varel objective, with a 15-s delay between successive frames. The axenic wild type Ax3 cells (top; see Video 1) quickly align head to tail in long streams of cells as they migrate toward the chemoattractant source. The pi3k1-/2- cells (bottom; see Video 2) migrate without forming streams. The data presented are representative of four independent experiments. (D) Under agarose analysis of chemotaxis and streaming. A well containing 1 μM cAMP was allowed to establish a concentration gradient for 30 min. Differentiated wild-type Ax3 and pi3k1-/2- cells were plated on either side of the well containing the chemoattractant. After ∼1 h, the wells were overlaid with phosphate buffer, and images were collected on a Leica DM IL stereoscope. Image is representative of four independent experiments, each performed in triplicate.
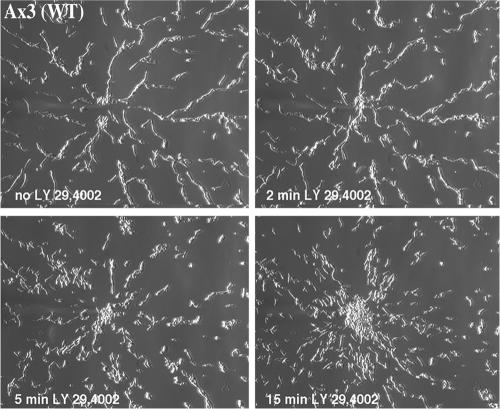
To further assess the developmental defect of the pi3k1-/2- cells, we more carefully examined the chemotactic behavior of pi3k1-/2- cells and found that they failed to align in a head-to-tail manner during chemotaxis, as suggested by Zhou et al. (1998). Figure 1C shows images of wild-type and pi3k1-/2- cells migrating in a gradient of cAMP generated by a micropipette filled with 10 μM cAMP. Images taken just before and 18 min after the micropipette is activated are shown. Although wild-type cells polarize and migrate in long streams of cells (see Video 1), we found that pi3k1-/2- cells migrate exclusively as individual cells to the micropipette tip (see Video 2). As observed previously, we noted that the pi3k1-/2- cells display reduced polarity and do not migrate with the same directionality as wild-type cells (Funamoto et al., 2001), a behavior that is more pronounced in cells that are differentiated for 4-5 h (our unpublished data). To observe a larger population of cells, we examined the behavior of pi3k1-/2- cells in the under agarose chemotaxis assay (Nelson et al., 1975; Comer et al., 2005). Again, we found that, in contrast to wild-type cells, pi3k1-/2- cells do not align and migrate as a diffuse halo of individual chemotaxing cells (Figure 1D). To further test the role of PI3K in signal relay and stream formation, we subjected wild-type cells to the PI3K inhibitor LY294002 during the process of streaming (Figure 2 and Video 3). In this experiment, we allowed cells to migrate toward the micropipette and, after ∼18 min, added 30 μM LY294002. Again, we observed that inhibition of PI3K function leads to profound streaming defects. Remarkably, we found that LY294002 acts very rapidly, dissociating streams within 5 min. Together, these results show that PI3K activity is required for streaming during Dictyostelium chemotaxis.
Figure 2.
LY294002 inhibits streaming. Wild-type Ax3 cells were plated for the micropipette chemotaxis assay and streams were allowed to form. After frame 48 (12 min; no LY294002 panel on top left), LY294002 was added to 30 μM from a 1000× stock in dimethyl sulfoxide (DMSO) and the recording was continued for an additional 15 min (see Video 3). Control experiments showed no effect upon addition of DMSO alone (see Video 3). The data presented are representative of three independent experiments.
Disruption of PI3K Activity Impairs PH Domain Translocation
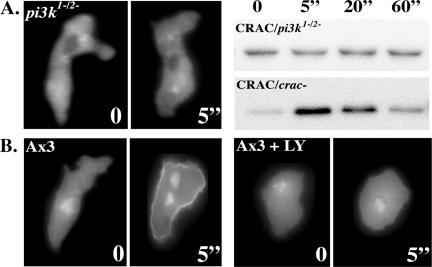
We previously demonstrated that R42C-CRAC fails to translocate to the plasma membrane upon chemoattractant stimulation and does not support chemotaxis, suggesting that PI3K action, mediated via CRAC translocation, is necessary for chemotaxis (Comer et al., 2005). Nevertheless, pi3k1-/2- cells and LY294002-treated wild-type Ax3 cells can still undergo chemotaxis, albeit with reduced polarity and directionality (Figures 1C and 2; Funamoto et al., 2001). Thus, we next examined whether chemoattractant-mediated translocation of CRAC to the plasma membrane is impaired in pi3k1-/2- cells or in LY294002-treated wild-type cells. We observed that, as for other PH domain-containing proteins (Meili et al., 1999; Servant et al., 2000; Funamoto et al., 2001), the recruitment of CRAC-GFP to the membrane is undetectable in pi3k1-/2- cells, whether observed microscopically (Figure 3A, left) or in a biochemical translocation assay (Figure 3A, right). Likewise, membrane translocation of CRAC-GFP was undetectable in LY294002-treated cells (Figure 3B). Thus, although the R42C-CRAC mutation and the reduction of PI3K activity both impair CRAC translocation beyond the detection limits of our assays, they confer distinct chemotaxis phenotypes. Although these results would seem to be in discrepancy, we submit that the R42C-CRAC mutant gives a more definitive chemotaxis phenotype because the mutation abolishes the interaction with PI3K products, which is tantamount to complete inhibition of PI3K activity. In contrast, it has been shown that in pi3k1-/2- cells, steady-state phosphatidylinositol-(3,4,5)-trisphosphate [PI(3,4,5)P3] levels are reduced to <50% (Zhou et al., 1998), whereas chemoattractant-induced PI(3,4,5)P3 production is reduced to ∼4% that of wild-type cells (Huang et al., 2003). Likewise, although Akt/PKB activation is greatly reduced in pi3k1-/2- cells, it is not abolished (Meili et al., 1999). Because chemotaxis is very sensitive, we propose that this residual PI3K response is sufficient to promote low levels of CRAC translocation, which are undetectable by our assay, but sufficient to drive the highly sensitive process of gradient sensing and chemotaxis. One consequence of this hypothesis is that there is likely to be signal amplification downstream of CRAC translocation. Thus, cells can recover from the low levels of PI3K-mediated CRAC translocation in pi3k1-/2- cells and upon LY294002 treatment because signal amplification renders cells resilient to significant perturbations in the chemotaxis signaling machinery.
Figure 3.
Disruption of PI3K activity impairs CRAC translocation. (A) Chemoattractant stimulated translocation of CRAC-GFP to the plasma membrane is impaired in pi3k1-/2- cells. No detectable translocation was observed in either the microscope assay (left) or the biochemical assay (right) upon stimulation of pi3k1-/2- cells with 20 μM cAMP (bottom). (B) Wild-type cells display a robust chemoattractant stimulated CRAC-GFP translocation response (left) that is inhibited by treatment with 30 μM LY294002 (right). The numbers represent time after chemoattractant addition. See Materials and Methods for details.
PI3K Regulates Adenylyl Cyclase Activation and Adaptation, but Not Its Cellular Distribution
Although ACA is properly expressed in pi3k1-/2- cells, we reasoned that the streaming defect of these cells may arise by a defect in regulating its activity or its cellular distribution. Indeed, we demonstrated previously that cells lacking ACA are unable to stream, although they can sense and migrate directionally toward a point source of cAMP (Pitt et al., 1992; Kriebel et al., 2003). We also established that ACA is highly enriched at the back of polarized, chemotaxing cells (Kriebel et al., 2003) and proposed that this provides a spatially restricted compartment from which cAMP is locally secreted to specifically attract neighboring cells, thus comprising a primary mechanism for signal relay and streaming. To determine whether the streaming defect of PI3K-deficient cells is caused by mislocalization of ACA, we examined its cellular distribution in pi3k1-/2- cells. For these experiments, we expressed ACA-YFP in pi3k1-/2- cells (ACA-YFP/pi3k1-/2-) or treated aca- cells expressing ACA-YFP (ACA-YFP/aca-) with LY294002. We found that, in both cases, ACA-YFP is properly enriched at the back of migrating cells (Figure 4, A and B). We conclude that the streaming defect caused by PI3K deficiency does not arise from disrupted cellular distribution of ACA.
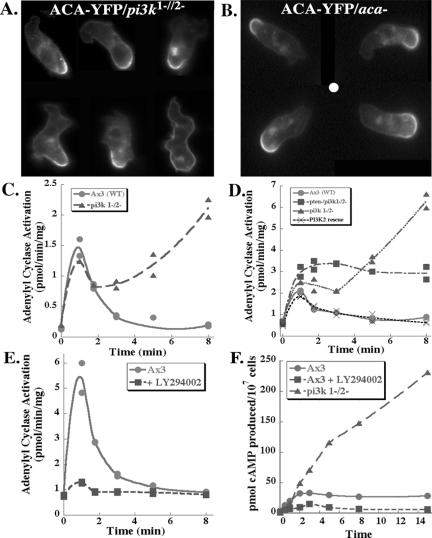
Figure 4.
PI3K regulates ACA activation and adaptation, but not its cellular distribution. (A) ACA-YFP is properly enriched at the back of pi3k1-/2- cells. Montage of migrating pi3k1-/2- cells expressing ACA-YFP. Cells were subjected to a chemoattractant gradient supplied by a micropipette containing 10 μM cAMP. For each frame, the cells are migrating toward the top. (B) ACA-YFP is properly enriched at the back of LY294002-treated ACA-YFP/aca- cells. ACA-YFP/aca- cells were treated with 30 μM LY294002 for 30 min and allowed to migrate in a gradient of chemoattractant generated by a micropipette filled with 10 μM cAMP. A montage of images is presented. The white circle indicates the position of the micropipette. (C) The adaptation phase of the chemoattractant receptor-mediated ACA activation response is impaired in pi3k1-/2- cells. ACA activity of pi3k1-/2- and wild-type Ax3 cells was measured as de novo synthesis of 32P-labeled cAMP after the addition of the chemoattractant cAMP to a final concentration of 50 μM, as described under Materials and Methods. Data are representative of results from >10 independent experiments, each performed in duplicate, from three independent isolates of the pi3k1-/2- knockout cells. (D) Reexpression of PI3K2 in pi3k1-/2- cells (PI3K2 rescue) restores the adaptation phase of the ACA response, whereas deletion of PTEN in the pi3k1-/2- background only partially rescues the defect. Assays were performed as in C, and the graph is representative of three to five independent experiments performed in duplicate. (E) ACA activation is severely impaired in LY294002-treated wild-type cells. Cells were treated with 30 μM LY294002 for 30 min on ice before the ACA activation assay, which was otherwise performed as described in C and D. (F) cAMP production is dramatically increased in pi3k1-/2- cells. Cells were treated with 5 μM 2′deoxy-cAMP and total cAMP produced was measured at the various time points. The graph is a representative of two independent experiments.
We next assessed the activity of ACA in PI3K-deficient cells. For these experiments, we used an activation trap assay, in which cells are stimulated with chemoattractant and at a specific time, rapidly lysed and assayed for adenylyl cyclase activity. When we examined receptor-mediated activation of ACA in pi3k1-/2- cells, we found that they exhibited a dramatic defect in adaptation of the response (Figure 4C). Wild-type cells exhibit a peak of activation at ∼1 min poststimulation, followed by a period of adaptation that reduces the activity to basal levels within 3-5 min. In contrast, pi3k1-/2- cells show a normal initial peak of activation, followed by a slight dip in activity and a dramatic loss of adaptation that results in a subsequent rise in activity, which often exceeds the initial peak, within 5-8 min poststimulation. The activity continues to rise for at least 12 min, and eventually begins to subside, although we have measured significant activity even 30 min after stimulation (our unpublished data). We further found that the reexpression of PI3K2 in pi3k1-/2- cells rescues the ability to properly attenuate the ACA response (Figure 4D) and restores streaming during chemotaxis (our unpublished data), consistent with previous observations showing that reexpression of PI3K2 in pi3k1-/2- cells rescues PKB activation (Funamoto et al., 2002). These findings show that PI3K activity controls the adaptation phase of the chemoattractant-mediated activation of ACA.
To further study the balance between 3-PI production and degradation in chemoattractant signaling to ACA, we examined the ACA response in cells deficient in both PI3K and PTEN activity. Deletion of the 3-PI-degrading enzyme PTEN in the pi3k1-/2- background results in cells that have an elevated basal level of CRAC at the plasma membrane, but they do not recruit more CRAC upon chemoattractant stimulation (Janetopoulos et al., 2005). These data suggest that, despite elevated basal levels of 3-PIs and membrane-associated CRAC, these cells do not exhibit proper temporal and spatial regulation of 3-PIs upon chemoattractant stimulation. We found that chemoattractant stimulation of these cells gives rise to a normal peak of ACA activation and a partial rescue of the adaptation defect observed in pi3k1-/2- cells (Figure 4D). This result has several implications. First, it shows that fluctuations in 3-PI production are not necessary for the ACA activation when there is an elevated basal level of CRAC at the plasma membrane. Second, it demonstrates that ACA activation must require a secondary input, such as TORC2, in addition to PI3K-mediated CRAC recruitment, because, in spite of the fact that there is a significant amount of CRAC at the plasma membrane in unstimulated cells, the basal ACA activity is not elevated. Finally, the partial rescue of the ACA adaptation response in these cells suggests that the balance of PI3K-mediated activation and adaptation pathways is partially restored by slowing the degradation of the small amount of 3-PIs that are synthesized in the pi3k1-/2- background. We therefore wanted to test whether inhibition of all PI3K isoforms with LY294002 would give the same ACA adaptation defect. Surprisingly, in contrast to the pi3k1-/2- cells but in agreement with our results using R42C-CRAC/crac- cells, LY294002 treatment significantly inhibited ACA activation (Figure 4E), suggesting that PI3K activity is indeed separately required for both activation and adaptation of ACA signaling. Furthermore, LY294002 treatment of pi3k1-/2- cells also ablated ACA activation (our unpublished data). Although we cannot rule out the possibility that treatment with LY294002 inhibits ACA activity via a non-PI3K pathway, such as through inhibition of TOR signaling (Brunn et al., 1996), our previous observation that crac- cells expressing a point mutation in the 3-PI binding motif within the CRAC PH domain are strongly impaired in their ability to activate ACA supports a direct role for PI3K. From these results, we infer that the loss of streaming during chemotaxis of wild-type cells treated with LY294002 is likely the result of too little ACA activity, as in the streaming defect reported previously for aca- cells (Kriebel et al., 2003). We propose that the streaming defect in the pi3k1-/2- cells, in contrast, is because of misregulation of the adaptation response, resulting in locally high concentrations of chemoattractant, which neighboring cells are unable to integrate into a proper streaming response. This hypothesis is further supported by our finding that net cAMP production is dramatically increased in pi3k1-/2- cells and significantly reduced in LY294002-treated wild-type cells (Figure 4F).
Distinct G Protein-mediated Pathways Control Chemoattractant Signaling
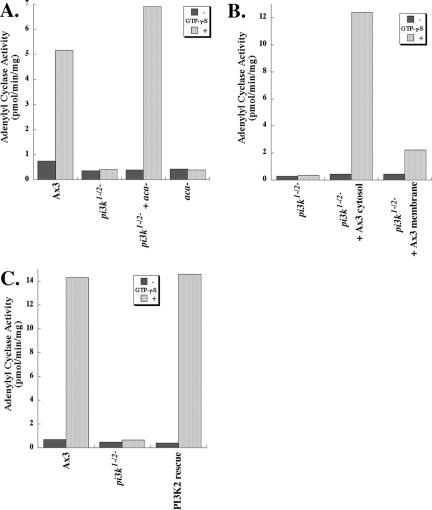
Another means of activating ACA is by direct G protein stimulation of cell lysates with the nonhydrolyzable GTP analogue GTPγS, which simultaneously activates the entire cellular complement of GTP binding proteins, both heterotrimeric G proteins and small GTPases. In wild-type cells, GTPγS stimulation results in a net activation of ACA, as observed previously (Figure 5A). Surprisingly, however, we found that the addition of GTPγS to pi3k1-/2- lysates does not increase ACA activity (Figure 5A). We infer from these findings that GTPγS stimulation is not functionally equivalent to chemoattractant receptor stimulation. To test whether the GTPγS defect of the pi3k1-/2- cells can be reconstituted, we mixed aca- cells with the pi3k1-/2- cells before lysis. In these experiments, the aca- cells, which are devoid of G protein-coupled adenylyl cyclase activity, are thought to supply the PI3Ks required to reconstitute the lost activity. We found that the mixed pi3k1-/2-/aca- lysate is fully capable of GTPγS-mediated ACA activation (Figure 5A). Furthermore, we observed that wild-type cytosol, but not wild-type membranes, restores GTPγS-mediated ACA activity to pi3k1-/2- lysates (Figure 5B), showing that the wild-type cytosol effectively restores the cytosolic PI3K activity, as observed previously for the reconstituted translocation of CRAC (Huang et al., 2003). Finally, we again found that reexpression of PI3K2 in the pi3k1-/2- cells restores GTPγS-mediated ACA activation (Figure 5C). We conclude that the loss of PI3K2 impairs the ability of GTPγS to activate ACA.
Figure 5.
Loss of PI3K activity leads to a loss of GTPγS-stimulated ACA activation. (A) pi3k1-/2- cells show no GTPγS-mediated activation of ACA. Graph depicting the GTPγS-mediated activation of ACA is shown. Lysates were assayed for 2 min after a 5-min incubation with GTPγS as described under Materials and Methods. The data presented are representative of at least five independent experiments. (B) Supplementation of pi3k1-/2- lysates with wild-type Ax3 cytosol, but not membrane fraction, restores responsiveness to GTPγS. See Materials and Methods for details. The data presented are representative of three independent experiments. (C) Reexpression of PI3K2 in pi3k1-/2- cells restores GTPγS-mediated stimulation of ACA activity. The data presented are representative of four independent experiments.
DISCUSSION
Sensory adaptation was recognized many years ago as important for chemoresponsiveness of both Dictyostelium cells (Devreotes and Steck, 1979) and mammalian leukocytes (Zigmond and Sullivan, 1979). Selective adaptation seems to be important for neutrophils to integrate conflicting chemotactic signals in a complex environment of multiple chemat-tractants (Foxman et al., 1999) and is important for both chemotaxis and signal relay in Dictyostelium (Devreotes and Steck, 1979; Van Haastert, 1983). However, although Gα9 has recently been identified as a general modulator of inhibitory chemotactic responses (Brzostowski et al., 2004), the mechanisms that regulate adaptation remain largely unknown. Progress in this area has undoubtedly been hampered by the complexity of the chemoattractant signaling system. Moreover, the phenotype of Dictyostelium adaptation mutants may be subtle and difficult to identify in a mutagenesis screen. For example, the pi3k1-/2- cells, in spite of their profound ACA adaptation defect, develop relatively normal terminal differentiation structures (Zhou et al., 1995).
Two opposing receptor-mediated biochemical processes—an excitatory and an inhibitory response—have been proposed to mediate the transient nature of the ACA response (Parent and Devreotes, 1999). When both responses reach a steady state, ACA activity returns to basal levels. We present evidence that supports a role for PI3K in both the excitation and inhibition pathways that control ACA in response to chemoattractant stimulation. Analysis of the chemoattractant receptor-mediated ACA activation in pi3k1-/2- cells reveals a profound adaptation defect, implying a key role for PI3K in the mechanism of adaptation. Our finding that deletion of PTEN in the pi3k1-/2- background, which elevates basal 3-PI levels, partially suppresses the adaptation defect suggests a direct role for 3-PIs in this process. In contrast, treatment of wild-type cells with a PI3K inhibitor or expressing a mutant of CRAC that has lost the capacity to bind to 3-PIs in crac- cells drastically reduces activation of the ACA response, pointing to an essential role for 3-PIs in ACA activation. These results are consistent with a model in which PI3K controls both the excitation and the inhibition of the ACA response through independent pathways (see model, Figure 6).
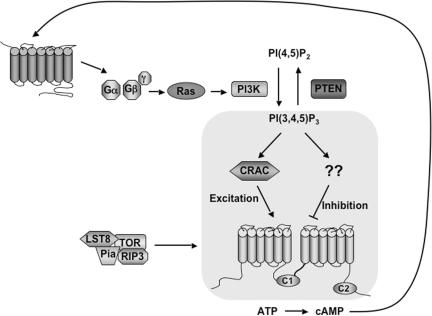
Figure 6.
Model for PI3K action in chemoattractant signaling to ACA. PI3K activity can simultaneously control the activation and adaptation phases of the chemoattractant-mediated ACA response through branching pathways. We propose that the excitatory pathway is more sensitive and/or kinetically fast compared with the inhibitory pathway, requiring minimal PI3K input for a robust response. Thus, in pi3k1-/2- cells, which only display ∼4% PI3K activity compared with wild-type cells, ACA is still activated. This excitation pathway functions through the PI3K-dependent action of CRAC. TORC2 is also required for ACA activation, but at this time it is not clear how it interacts with the PI3K pathway in this response. In contrast, the inhibition branch is less sensitive and/or kinetically slower. Thus, in pi3k1-/2- cells, ACA activation is normal, but the adaptation response is severely impaired. General inhibition of PI3K activity with LY294002 severely impairs ACA activation, thus adaptation is moot. An alternative, though not mutually exclusive, interpretation of our data is that specific PI3K isoforms independently control the ACA activation and adaptation pathways or that distinct activities of PI3K, i.e., protein kinase and lipid kinase, are involved. Global stimulation of the entire cellular complement of G proteins, both heterotrimeric and small GTPases, with GTPγS stimulates ACA activity in wild-type cells, but it does not lead to ACA activation in pi3k1-/2- cells, raising the possibility that G protein action downstream of PI3K may control the adaptation/inhibition branch, as outlined in Discussion.
We envision four nonmutually exclusive mechanisms for coordinating the output from PI3K signaling to the excitation and inhibition pathways controlling ACA. The first possibility is that the excitation branch is more sensitive than the inhibition branch. Thus, because the inhibition branch would require a greater input from PI3K, inhibition would be more affected than excitation in conditions of reduced PI3K activity and a loss of the adaptation response would occur before the activation is affected. Indeed, the chemoattractant-induced PI(3,4,5)P3 production in pi3k1-/2- cells is greatly reduced compared with wild-type cells, yet pi3k1-/2- cells show a robust initial peak of ACA activation. The second possibility is that the two branches have distinct kinetics, and the temporal delay in the inhibition pathway is exaggerated in the pi3k1-/2- cells. Third, PI3K isoforms other than PI3K1 and PI3K2 could be responsible for the initial activation peak, whereas these two predominant isoforms would specifically control the inhibition branch. Finally, because PI3Ks are known to exhibit both lipid and protein kinase activities (Carpenter et al., 1993; Dhand et al., 1994; Naga Prasad et al., 2005), it is possible that the inhibition pathway leading to ACA adaptation depends on the protein kinase activity of PI3K, which could be regulated independently of the lipid kinase activity. At the moment, it is not known whether the Dictyostelium PI3Ks display protein kinase activity.
Our finding that stimulation of cell lysates with GTPγS does not promote ACA activation in pi3k1-/2- cells provides further insight into the mechanisms controlling the adaptive response. Our data are consistent with at least two hypotheses. First, it is possible that pleiotropic defects in the pi3k1-/2- cells impair the GTPγS-mediated activation of ACA, despite the ability of these cells to activate ACA through the G protein-coupled chemoattractant receptor. Alternatively, our results may suggest the presence of a small GTPase that functions downstream of PI3K to attenuate the ACA activation response. This putative GTPase would need to be kinetically slower and/or less sensitive than the receptor-coupled heterotrimeric G protein and the Ras GTPase that has been proposed to activate PI3K (Funamoto et al., 2002; Sasaki et al., 2004). Thus, in wild-type cells, activation of the upstream Gβγ and Ras would dominate with a net activation of ACA upon GTPγS stimulation. In contrast, in the pi3k1-/2- cells, the efficiency of the Gβγ and Ras signals is reduced because of the low PI3K activity in the excitation branch, and thus, a GTPase functioning in the inhibition branch dominates, resulting in a shift of the balance between excitation and inhibition, thus yielding little or no net activation. The identification of the GTPase and its associated regulators as mediators of ACA inhibition presents a considerable challenge. In Dictyostelium, there are at least six Ras proteins, some with redundant functions, and greater than 20 Ras guanine nucleotide exchange factors (Wilkins and Insall, 2001; Kae et al., 2004; Wilkins et al., 2005). Nevertheless, as these challenges are met, elucidation of the mechanisms of adaptation will provide fundamental insight into chemotaxis and the integration of chemoattractant signal transduction responses.
Reduction of PI3K activity affects multiple chemoattractant-mediated processes, pointing to a central role for PI3Ks in the integration of chemoattractant signaling. Previous studies have shown that pi3k1-/2- Dictyostelium cells and neutrophils from PI3Kγ-null mice have reduced directionality, polarity and speed, but chemotaxis can still occur (Hirsch et al., 2000; Li et al., 2000; Chung et al., 2001; Funamoto et al., 2001; Iijima et al., 2002; Stephens et al., 2002; Weiner, 2002). Similar effects on chemotaxis were obtained upon PI3K inhibition with LY294002 or wortmannin (Funamoto et al., 2001; Wang et al., 2002). These observations are consistent with our hypothesis that, in addition to the upstream actin-dependent signal amplification loops that lead to the polarization of PI3K activity (Weiner et al., 2002), further downstream amplification of the PI3K response is likely to occur, such that even small PI3K inputs can be amplified into functional responses. This exquisite regulation may help explain the wide dynamic range of chemotactic responsiveness of Dictyostelium. These cells not only migrate directionally in very shallow gradients supplied by a source of 10-9 M cAMP (Mato and Konijn, 1975), they can also chemotax in a gradient applied over a constant background concentration of 10-5 M cAMP (Van Haastert, 1983). It therefore seems that PI3Ks are involved in regulating multiple effectors of chemoattractant signaling and that these effectors display distinct sensitivities to PI3K products.
Our findings suggest a novel mechanism by which PI3K activity regulates the excitation and inhibition pathways that control ACA in response to chemoattractant stimulation. We propose that such a mechanism, in which activation and adaptation are controlled by the same input, allows for a stringent regulation of the excitation/inhibition cycle. By linking two opposing responses, inhibition and excitation responses would always be proportional, ensuring the proper balance that is required to maintain the oscillatory circuit of chemoresponsiveness. We envision that similar coordinated controls operate in higher eukaryotes.
Supplementary Material
Acknowledgments
We thank Peter Devreotes and Rick Firtel for providing cell lines used in this study. We also thank Chris Janetopoulos for valuable discussions and for sharing unpublished observations. Finally, we thank members of the Parent laboratory as well as members of the laboratories of Alan Kimmel and Tian Jin for helpful discussions. This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research and by the National Institute of General Medical Sciences Pharmacology Research Associate program.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-08-0781) on November 2, 2005.
Abbreviations used: ACA, adenylyl cyclase expressed during aggregation; CRAC, cytosolic regulator of adenylyl cyclase; GTPγS, guanosine 5′-3-O-(thio)triphosphate.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Brunn, G. J., Williams, J., Sabers, C., Wiederrecht, G., Lawrence, J. C., Jr., and Abraham, R. T. (1996). Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO J. 15, 5256-5267. [PMC free article] [PubMed] [Google Scholar]
- Brzostowski, J. A., Parent, C. A., and Kimmel, A. R. (2004). A Gα-dependent pathway that antagonizes multiple chemoattractant responses that regulate directional cell movement. Genes Dev. 18, 805-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczynski, G., Grove, B., Nomura, A., Kleve, M., Bush, J., Firtel, R. A., and Cardelli, J. (1997). Inactivation of two Dictyostelium discoideum genes, DdPIK1 and DdPIK2, encoding proteins related to mammalian phosphatidylinositide 3-kinases, results in defects in endocytosis, lysosome to postlysosome transport, and actin cytoskeleton organization. J. Cell Biol. 136, 1271-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter, C. L., Auger, K. R., Duckworth, B. C., Hou, W. M., Schaffhausen, B., and Cantley, L. C. (1993). A tightly associated serine/threonine protein kinase regulates phosphoinositide 3-kinase activity. Mol. Cell. Biol. 13, 1657-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina, M. J., Hereld, D., and Devreotes, P. N. (1995). Occupancy of the Dictyostelium cAMP receptor, cAR1, induces a reduction in affinity which depends upon COOH-terminal serine residues. J. Biol. Chem. 270, 4418-4423. [DOI] [PubMed] [Google Scholar]
- Chen, M. Y., Long, Y., and Devreotes, P. N. (1997). A novel cytosolic regulator, Pianissimo, is required for chemoattractant receptor and G protein-mediated activation of the 12 transmembrane domain adenylyl cyclase in Dictyostelium. Genes Dev. 11, 3218-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, C. Y., Funamoto, S., and Firtel, R. A. (2001). Signaling pathways controlling cell polarity and chemotaxis. Trends Biochem. Sci. 26, 557-566. [DOI] [PubMed] [Google Scholar]
- Comer, F. I., Lippincott, C. K., Masbad, J. J., and Parent, C. A. (2005). The PI3K-mediated activation of CRAC independently regulates adenylyl cyclase activation and chemotaxis. Curr. Biol. 15, 134-139. [DOI] [PubMed] [Google Scholar]
- Dai, Y., Holgate, S. T., Church, M. K., and Shute, J. K. (1994). Modulation of the chemotactic responsiveness of guinea pig neutrophils to hrIL-8 and fMLP. J. Leukoc. Biol. 56, 776-783. [DOI] [PubMed] [Google Scholar]
- Devreotes, P., Fontana, D., Klein, P., Sherring, J., and Theibert, A. (1987). Transmembrane signaling in Dictyostelium. Methods Cell Biol. 28, 299-331. [DOI] [PubMed] [Google Scholar]
- Devreotes, P., and Janetopoulos, C. (2003). Eukaryotic chemotaxis: distinctions between directional sensing and polarization. J. Biol. Chem. 278, 20445-20448. [DOI] [PubMed] [Google Scholar]
- Devreotes, P. N., and Steck, T. L. (1979). Cyclic 3′,5′ AMP relay in Dictyostelium discoideum. II. Requirements for the initiation and termination of the response. J. Cell Biol. 80, 300-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhand, R., et al. (1994). PI 3-kinase is a dual specificity enzyme: autoregulation by an intrinsic protein-serine kinase activity. EMBO J 13, 522-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinauer, M. C., Steck, T. L., and Devreotes, P. N. (1980a). Cyclic 3′,5′-AMP relay in Dictyostelium discoideum IV. Recovery of the cAMP signaling response after adaptation to cAMP. J. Cell Biol. 86, 545-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinauer, M. C., Steck, T. L., and Devreotes, P. N. (1980b). Cyclic 3′,5′-AMP relay in Dictyostelium discoideum V. Adaptation of the cAMP signaling response during cAMP stimulation. J. Cell Biol. 86, 554-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxman, E. F., Kunkel, E. J., and Butcher, E. C. (1999). Integrating conflicting chemotactic signals. The role of memory in leukocyte navigation. J. Cell Biol. 147, 577-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funamoto, S., Meili, R., Lee, S., Parry, L., and Firtel, R. A. (2002). Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell 109, 611-623. [DOI] [PubMed] [Google Scholar]
- Funamoto, S., Milan, K., Meili, R., and Firtel, R.A. (2001). Role of phosphatidylinositol 3′ kinase and a downstream pleckstrin homology domain-containing protein in controlling chemotaxis in Dictyostelium. J. Cell Biol. 153, 795-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvath, L., Robbins, J. D., Russell, A. A., and Seamon, K. B. (1991). cAMP and human neutrophil chemotaxis. Elevation of cAMP differentially affects chemotactic responsiveness. J. Immunol. 146, 224-232. [PubMed] [Google Scholar]
- Hirsch, E., Katanaev, V. L., Garlanda, C., Azzolino, O., Pirola, L., Silengo, L., Sozzani, S., Mantovani, A., Altruda, F., and Wymann, M. P. (2000). Central role for G protein-coupled phosphoinositide 3-kinase γ in inflammation. Science 287, 1049-1053. [DOI] [PubMed] [Google Scholar]
- Huang, Y. E., Iijima, M., Parent, C. A., Funamoto, S., Firtel, R. A., and Devreotes, P. (2003). Receptor-mediated Regulation of PI3Ks Confines PI(3,4,5)P3 to the Leading Edge of Chemotaxing cells. Mol. Biol. Cell 14, 1913-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima, M., and Devreotes, P. (2002). Tumor suppressor PTEN mediates sensing of chemoattractant gradients. Cell 109, 599-610. [DOI] [PubMed] [Google Scholar]
- Iijima, M., Huang, Y. E., and Devreotes, P. (2002). Temporal and spatial regulation of chemotaxis. Dev. Cell 3, 469-478. [DOI] [PubMed] [Google Scholar]
- Insall, R., Kuspa, A., Lilly, P. J., Shaulsky, G., Levin, L. R., Loomis, W. F., and Devreotes, P. (1994). CRAC, a cytosolic protein containing a pleckstrin homology domain, is required for receptor and G protein-mediated activation of adenylyl cyclase in Dictyostelium. J. Cell Biol. 126, 1537-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janetopoulos, C., Borleis, J., Vazquez, F., Iijima, M., and Devreotes, P. (2005). Temporal and spatial regulation of phosphoinositide signaling mediates cytokinesis. Dev. Cell 8, 467-477. [DOI] [PubMed] [Google Scholar]
- Kae, H., Lim, C. J., Spiegelman, G. B., and Weeks, G. (2004). Chemoattractant-induced Ras activation during Dictyostelium aggregation. EMBO Rep. 5, 602-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriebel, P. W., Barr, V. A., and Parent, C. A. (2003). Adenylyl cyclase localization regulates streaming during chemotaxis. Cell 112, 549-560. [DOI] [PubMed] [Google Scholar]
- Kriebel, P. W., and Parent, C. A. (2004). Adenylyl cyclase expression and regulation during the differentiation of Dictyostelium discoideum. IUBMB Life 56, 541-546. [DOI] [PubMed] [Google Scholar]
- Lee, S., Comer, F. I., Sasaki, A., McLeod, I. X., Duong, Y., Okumura, K., Yates Iii, J. R., Parent, C. A., and Firtel, R. A. (2005). TOR complex 2 integrates cell movement during chemotaxis and signal relay in Dictyostelium. Mol. Biol. Cell 16, 4572-4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S., Parent, C. A., Insall, R., and Firtel, R. A. (1999). A novel Ras-interacting protein required for chemotaxis and cyclic adenosine monophosphate signal relay in Dictyostelium. Mol. Biol. Cell 10, 2829-2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z., et al. (2005). Regulation of PTEN by Rho small GTPases. Nat. Cell Biol. 7, 399-404. [DOI] [PubMed] [Google Scholar]
- Li, Z., Jiang, H., Xie, W., Zhang, Z., Smrcka, A. V., and Wu, D. (2000). Roles of PLC-β2 and -β3 and PI3Kγ in chemoattractant-mediated signal transduction. Science 287, 1046-1049. [DOI] [PubMed] [Google Scholar]
- Lilly, P. J., and Devreotes, P. N. (1995). Chemoattractant and GTP gamma S-mediated stimulation of adenylyl cyclase in Dictyostelium requires translocation of CRAC to membranes. J. Cell Biol. 129, 1659-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mato, J. M., and Konijn, T. M. (1975). Chemotaxis and binding of cyclic AMP in cellular slime molds. Biochim. Biophys. Acta 385, 173-179. [DOI] [PubMed] [Google Scholar]
- Mato, J. M., Losada, A., Nanjundiah, V., and Konijn, T. M. (1975). Signal input for a chemotactic response in the cellular slime mold Dictyostelium discoideum. Proc. Natl. Acad. Sci. USA 72, 4991-4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meili, R., Ellsworth, C., Lee, S., Reddy, T. B., Ma, H., and Firtel, R. A. (1999). Chemoattractant-mediated transient activation and membrane localization of Akt/PKB is required for efficient chemotaxis to cAMP in Dictyostelium. EMBO J. 18, 2092-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naga Prasad, S. V., Jayatilleke, A., Madamanchi, A., and Rockman, H. A. (2005). Protein kinase activity of phosphoinositide 3-kinase regulates β-adrenergic receptor endocytosis. Nat. Cell Biol. 7, 785-796. [DOI] [PubMed] [Google Scholar]
- Nelson, R., Quie, P., and Simmons, R. (1975). Chemotaxis under agarose: a new and simple method for measuring chemotaxis and spontaneous migration of human polymorphonuclear leukocytes and monocytes. J. Immunol. 115, 1650-1656. [PubMed] [Google Scholar]
- Parent, C. A. (2004). Making all the right moves: chemotaxis in neutrophils and Dictyostelium. Curr. Opin. Cell Biol. 16, 4-13. [DOI] [PubMed] [Google Scholar]
- Parent, C. A., Blacklock, B. J., Froehlich, W. M., Murphy, D. B., and Devreotes, P. N. (1998). G protein signaling events are activated at the leading edge of chemotactic cells. Cell 95, 81-91. [DOI] [PubMed] [Google Scholar]
- Parent, C. A., and Devreotes, P. N. (1995). Isolation of inactive and G protein-resistant adenylyl cyclase mutants using random mutagenesis. J. Biol. Chem. 270, 22693-22696. [DOI] [PubMed] [Google Scholar]
- Parent, C. A., and Devreotes, P. N. (1996). Molecular genetics of signal transduction in Dictyostelium. Annu. Rev. Biochem. 65, 411-440. [DOI] [PubMed] [Google Scholar]
- Parent, C. A., and Devreotes, P. N. (1999). A cell's sense of direction. Science 284, 765-770. [DOI] [PubMed] [Google Scholar]
- Pitt, G. S., Milona, N., Borleis, J., Lin, K. C., Reed, R. R., and Devreotes, P. N. (1992). Structurally distinct and stage-specific adenylyl cyclase genes play different roles in Dictyostelium development. Cell 69, 305-315. [DOI] [PubMed] [Google Scholar]
- Salomon, Y. (1979). Adenylate cyclase assay. Adv. Cyclic Nucleotide Res. 10, 35-55. [PubMed] [Google Scholar]
- Saran, S., Meima, M. E., Alvarez-Curto, E., Weening, K. E., Rozen, D. E., and Schaap, P. (2002). cAMP signaling in Dictyostelium. Complexity of cAMP synthesis, degradation and detection. J. Muscle Res. Cell Motil. 23, 793-802. [DOI] [PubMed] [Google Scholar]
- Sarbassov, D. D., Ali, S. M., Kim, D. H., Guertin, D. A., Latek, R. R., Erdjument-Bromage, H., Tempst, P., and Sabatini, D. M. (2004). Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 14, 1296-1302. [DOI] [PubMed] [Google Scholar]
- Sasaki, A. T., Chun, C., Takeda, K., and Firtel, R. A. (2004). Localized Ras signaling at the leading edge regulates PI3K, cell polarity, and directional cell movement. J. Cell Biol. 167, 505-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai, S., Thomason, P. A., and Cox, E. C. (2005). An autoregulatory circuit for long-range self-organization in Dictyostelium cell populations. Nature 433, 323-326. [DOI] [PubMed] [Google Scholar]
- Servant, G., Weiner, O. D., Herzmark, P., Balla, T., Sedat, J. W., and Bourne, H. R. (2000). Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science 287, 1037-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens, L., Ellson, C., and Hawkins, P. (2002). Roles of PI3Ks in leukocyte chemotaxis and phagocytosis. Curr. Opin. Cell Biol. 14, 203-213. [DOI] [PubMed] [Google Scholar]
- Van Haastert, P. J. (1983). Sensory adaptation of Dictyostelium discoideum cells to chemotactic signals. J. Cell Biol. 96, 1559-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, F., Herzmark, P., Weiner, O. D., Srinivasan, S., Servant, G., and Bourne, H. R. (2002). Lipid products of PI(3)Ks maintain persistent cell polarity and directed motility in neutrophils. Nat. Cell Biol. 4, 513-518. [DOI] [PubMed] [Google Scholar]
- Weiner, O. D. (2002). Regulation of cell polarity during eukaryotic chemotaxis: the chemotactic compass. Curr. Opin. Cell Biol. 14, 196-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner, O. D., Neilsen, P. O., Prestwich, G. D., Kirschner, M. W., Cantley, L. C., and Bourne, H. R. (2002). A PtdInsP(3)- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nat. Cell Biol. 4, 509-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins, A., and Insall, R. H. (2001). Small GTPases in Dictyostelium: lessons from a social amoeba. Trends Genet. 17, 41-48. [DOI] [PubMed] [Google Scholar]
- Wilkins, A., Szafranski, K., Fraser, D. J., Bakthavatsalam, D., Muller, R., Fisher, P. R., Glockner, G., Eichinger, L., Noegel, A. A., and Insall, R. H. (2005). The Dictyostelium genome encodes numerous RasGEFs with multiple biological roles. Genome Biol. 6, R68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, K., Pandol, S., Bokoch, G., and Traynor-Kaplan, A. E. (1998). Disruption of Dictyostelium PI3K genes reduces [32P]phosphatidylinositol 3,4 bisphosphate and [32P]phosphatidylinositol trisphosphate levels, alters F-actin distribution and impairs pinocytosis. J. Cell Sci. 111, 283-294. [DOI] [PubMed] [Google Scholar]
- Zhou, K., Takegawa, K., Emr, S. D., and Firtel, R. A. (1995). A phosphatidylinositol (PI) kinase gene family in Dictyostelium discoideum: biological roles of putative mammalian p110 and yeast Vps34p PI 3-kinase homologs during growth and development. Mol. Cell. Biol. 15, 5645-5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond, S. H. (1977). Ability of polymorphonuclear leukocytes to orient in gradients of chemotactic factors. J. Cell Biol. 75, 606-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond, S. H., and Sullivan, S. J. (1979). Sensory adaptation of leukocytes to chemotactic peptides. J. Cell Biol. 82, 517-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.