Abstract
Because missense mutations in genetic diseases of membrane proteins often result in endoplasmic reticulum (ER) retention of functional proteins, drug-induced rescue of their cell surface expression and understanding the underlying mechanism are of clinical value. To study this, we tested chemical chaperones and sarco(endo)plasmic reticulum Ca2+ ATPase pump inhibitors on Madin-Darby canine kidney cells expressing nine ER-retained vasopressin type-2 receptor (V2R) mutants involved in nephrogenic diabetes insipidus. Of these nine, only V2R-V206D showed improved maturation and plasma membrane rescue with glycerol, dimethyl sulfoxide (DMSO), thapsigargin/curcumin, and ionomycin but not with other osmolytes or growth at 27°C. This revealed that rescue is mutant specific and that this mutant is prone to rescue by multiple compounds. Rescue did not involve changed expression of molecular chaperones calnexin, heat-shock protein (HSP) 70, or HSP90. V2R antagonist SR121463B treatment revealed that V2R-V206D and V2R-S167T were rescued and matured to a greater extent, suggesting that the rescuing activity of a pharmacological versus chemical chaperone is broader and stronger. Calcium measurements showed that rescue of V2R-V206D by thapsigargin, curcumin, and ionomycin was because of increased cytosolic calcium level, rather than decreased endoplasmic reticulum calcium level. The molecular mechanism underlying rescue by DMSO, glycerol, and SR121463B is different, because with these compounds intracellular calcium levels were unaffected.
INTRODUCTION
In 50% of congenital diseases, such as cystic fibrosis (Skach, 2000), long QT syndrome (Finlayson et al., 2004), or nephrogenic diabetes insipidus (NDI) (Deen et al., 2000), the underlying cause is the presence of missense mutations in the gene involved. Cell culture expression of the corresponding mutants revealed that the majority of these proteins are misfolded and retained by molecular chaperones of the quality control machinery in the endoplasmic reticulum (ER), after which they are usually targeted for proteasomal degradation. Despite the mutation, several studies have reported that these so-called class II mutants may be functional on a molecular level, which implicates that compounds that restore the routing of such mutants to the cell surface may be useful as a therapeutic.
A group of compounds known as “chemical chaperones” is able to facilitate the escape of mutant proteins from the ER quality control mechanism, leading to their translocation to the proper subcellular location (Welch and Brown, 1996). Among these compounds are osmolytes, such as glycerol, dimethyl sulfoxide (DMSO), and trimethyl-amine-N-oxide (TMAO) (Sato et al., 1996; Tamarappoo et al., 1999; Yang et al., 1999; Song and Chuang, 2001) that may facilitate rescue by stabilizing a mutant protein's conformation or by inducing a stress response, leading to up-regulation of molecular chaperones (Welch and Brown, 1996; Diamant et al., 2001). Also, inhibitors of sarco(endo)plasmic reticulum Ca2+ ATPases (SERCAs), such as thapsigargin and curcumin, have been reported to induce plasma membrane rescue (Egan et al., 2002; Delisle et al., 2003; Egan et al., 2004). Because these SERCA pumps help maintaining high endoplasmic reticulum calcium level ([Ca2+]ER) levels, the inhibitors cause decreased ER calcium levels, which are thought to affect the action of ER molecular chaperones, thereby allowing mutant proteins to leave the ER (Brostrom and Brostrom, 2003). Finally, factors that increase expression of heat-shock proteins (HSPs), such as 4-phenyl butyric acid (4-PBA) or growth at reduced temperature, are sometimes able to rescue the cell surface expression of ER-retained proteins (Choo-Kang and Zeitlin, 2001). Although rescued cell surface expression has been reported for several chemical chaperones it is still unclear whether rescue is mutation specific, and whether certain mutants are more prone to be rescued. In addition, the mechanisms by which chemical chaperones rescue the cell surface expression of ER-retained mutants are still largely unknown.
NDI is a disease in which the kidney is unable to concentrate urine in response to vasopressin, resulting in polyuria and polydipsia. The autosomal dominant and recessive inheritable forms are caused by mutations in the gene encoding the water channel aquaporin-2, whereas X-linked recessive NDI involves mutations in the vasopressin V2 receptor (V2R) gene (Deen et al., 2000). Recently, we showed that in polarized Madin-Darby canine kidney (MDCK) cells, stably expressed human V2R coupled to green fluorescent protein (wtV2R-GFP) is mainly localized in the basolateral membrane and is regulated as can be anticipated to occur in vivo (Robben et al., 2004). Also, we found that nine V2R missense mutants in NDI were predominantly retained in the ER when stably expressed in polarized MDCK cells (Robben et al., 2005). To address the issues mentioned above, we tested seven chemical chaperones and incubation at decreased temperature for their ability to rescue the plasma membrane expression of these nine V2R mutants. In addition, we set out to determine the molecular mechanism underlying the role of cytosolic and ER calcium levels in the rescue of V2R mutants.
MATERIALS AND METHODS
Materials
Glycerol was from Invitrogen (Carlsbad, CA); DMSO, TMAO, curcumin, thapsigargin, and probenecid were from Sigma-Aldrich (St. Louis, MO); 4-PBA was from Sigma-Aldrich (Gillingham, United Kingdom); and FURA-2-acetoxymethyl ester (AM), 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA)-AM, and Pluronic were from Invitrogen (Leiden, The Netherlands). SR121463B was kindly provided by Dr. C. Serradeil-Le Gal (Sanofi Synthélabo, Toulouse, France).
Expression Constructs
Using the QuikChange site-directed mutagenesis kit (Stratagene, Heidelberg, Germany), mutations were introduced into the human V2R cDNA sequence, using pEGFP-N1-V2R (Schulein et al., 1998) as a template; the primers GTCGCACCTATCTCACGTGGATTGCCCTGATG (V206L), CCGTCGCACATATGAAACCTGGATTGCCCTG (V206E), and CGTCGCACCTACAGAACCTGGATTGCCCTG (V206R); and their complementary antisense primers. After digestion of mutagenized clones with PstI/HindIII, the mutation-containing fragments were isolated and cloned into the corresponding sites of pEGFP-N1-V2R. Sequence analysis of selected clones confirmed that only the desired mutations were introduced.
Cell Culture and Chemical Chaperone Treatments
MDCK type I cells stably expressing the green fluorescent protein (GFP) tagged wild-type (wt)-V2R or its mutants V2R-L44P, -Δ62-64, -R113W, -I130F, -S167T, -S167L, -G201D, -T204N, or -V206D were generated and maintained as described. Stable transfection of MDCK I cells, isolation, analysis, and selection of clones was performed as described previously (Robben et al., 2004, 2005). For translocation studies, cells were seeded on Costar filters at a density of 300,000 cells/cm2 and grown for 3 d. Next, cells were treated for 16 h with 4% glycerol, 1% DMSO, TMAO, 5 mM 4-PBA, or 1 μM SR121463B at 37°C or grown for 16 h at 27°C. Alternatively, cells were treated for 2 h at 37°C with 1 μM thapsigargin, curcumin, or ionomycin in culture medium unless indicated otherwise.
Immunoblotting and Immunocytochemistry
Poly-acrylamide gel electrophoresis, Western blotting, and immunodetection were performed as described previously (Deen et al., 2002; Robben et al., 2004). Immunocytochemistry (ICC), confocal laser scanning microscopy (CLSM), and data quantification was performed as described previously (Robben et al., 2005). Mouse anti-HSP70 and -HSP90 antibodies were kindly supplied by Dr. David Toft (Mayo Clinic, Rochester, MN). Rabbit anti-calnexin antibodies were kindly supplied by Dr. I. Braakman (UMC Utrecht, Utrecht, The Netherlands)
Calcium Measurements
To measure cytosolic calcium ([Ca2+]cyt,) levels, cells were seeded in 35/22-mm glass bottom dishes (Wilco Wells, Amsterdam, The Netherlands) at a density of 100,000 cells/cm2 and grown overnight. Subsequently, the cells were loaded with 3 μM FURA-2 in the presence of 100 nM Pluronic and 500 μM probenecid in culture medium and incubated for 30 min at 37°C. Because the phenol red in DMEM interferes with the FURA-2 measurement, measurements were performed in HEPES/Tris buffer. The cells were then washed in HEPES/Tris buffer (132.6 mM NaCl, 5.8 mM glucose, 10 mM HEPES, 4.2 mM KCl, and 1 mM MgCl2), followed by addition of 500 μM HEPES/Tris buffer with 500 μM probenecid, supplemented with either 1 mM EGTA or different concentrations of CaCl2 and one of the chemical chaperones. The FURA-2 fluorescence emission ratio at 492 nm was monitored as a measure of [Ca2+]cyt after alternating excitation at 340 and 380 nm using MetaFluor software (Molecular Devices, Sunnyvale, CA). Averaged data of 25 individual cells were used per experiment. Measurements were performed on an inverted microscope (Axiovert 200 M; Carl Zeiss, Jena, Germany) equipped with a Zeiss 40×/1.3 numerical aperture F Fluar objective coupled to a CoolSNAP HQ monochrome charge-coupled device camera (Roper Scientific, Vianen, The Netherlands). Experiments were performed at least in three times.
RESULTS
Chemical Chaperones and Their Specificity
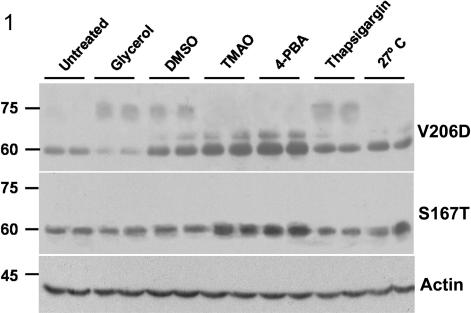
Recently, we reported that the V2R mutants -L44P, -del62-64, -I130F, -S167T, -S167L, and -V206D were fully ER retained when stably expressed in polarized MDCK cells, whereas R113W, -G201D, and -T204N were partially ER retained (Robben et al., 2005). Consistently, the ER-retained proteins are expressed as 60- to 63-kDa immature proteins, whereas the mature forms were expressed as 75-kDa proteins. As the maturation state of the V2R mutants is an indication for their level of plasma membrane localization, these mutant-expressing cell lines were incubated with different chemical chaperones and subjected to V2R immunoblotting to test for increased receptor maturation. MDCK-V2R-V206D cells showed an increased amount of mature V2R when treated with 4% glycerol or 1% DMSO (vol/vol) for 16 h, or with 1 μM thapsigargin for 2 h (Figure 1, top). Incubation with TMAO, 4-PBA, or growth at 27°C for 16 h increased the amount of immature V2R proteins, but it did not induce receptor maturation. For the mutants V2R-S167T (Figure 1, middle), -L44P, -del62-64, -R113W, -I130F, -S167L, -G201D, and -T204N (our unpublished data), however, no significant (p < 0.05) increases in maturation were observed. Immunodetection of β-actin revealed equal levels for all lanes (Figure 1, bottom), which indicated that the cells were not affected by the given treatments. These effects were observed for two independent clones per mutant, indicating that the effects were V2R mutant dependent and not because of clonal differences. Together, our data show that chemical chaperones induce maturation of only a limited number of V2R mutants (i.e., only V2R-V206D) and that the maturation of this particular mutant can be induced by different chemical chaperones.
Figure 1.
Maturation of V2R-V206D upon treatment with chemical chaperones. Confluent V2R-V206D- or V2R-S167T-expressing MDCK cells were untreated, incubated for 16 h with 4% glycerol, 1% DMSO, 1% TMAO, 5 mM 4-PBA or at 27°C, or treated for 2 h with 1 μM thapsigargin, lysed in Laemmli sample buffer, loaded on a 10% polyacrylamide gel, and subjected to immunoblotting. V2R-V206D or -S167T was detected using anti-GFP antibodies (top and middle, respectively). To ensure equal loading of the samples, blots were incubated with β-actin antibodies (bottom). Duplicate samples are shown. Mass indications in kilodaltons are given on the left.
Chemical Chaperones Induce Translocation of V2R-V206D to the Plasma Membrane
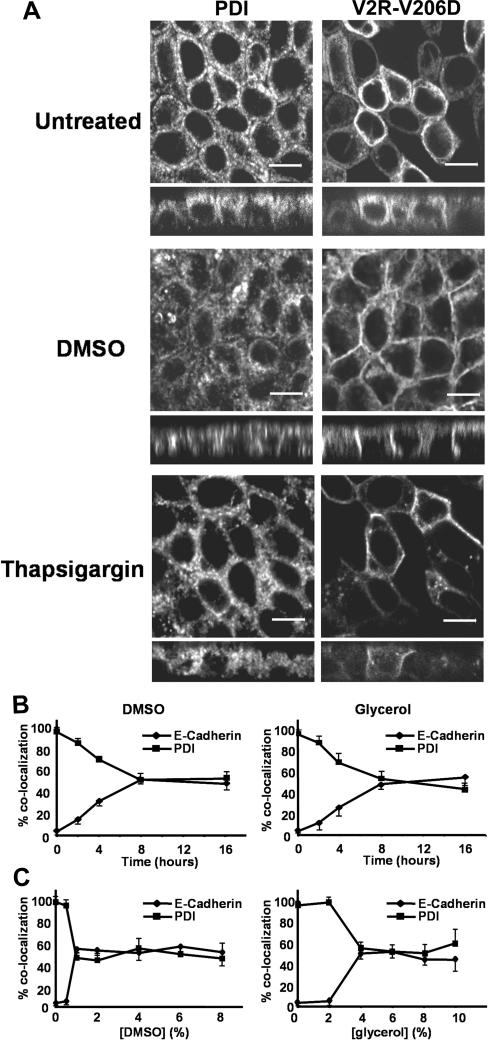
To determine whether chemical chaperones and thapsigargin rescue the cell surface expression of V2R-V206D, cells grown and treated as described above were subjected to CLSM, after immunocytochemistry. Colocalizations with the organelle marker proteins protein disulfide isomerase (PDI) (ER) and E-cadherin (basolateral membrane) were semiquantified using densitometry. As reported previously (Robben et al., 2005), V2R-V206D in untreated cells is almost completely localized in the ER (94.5 ± 5.3%; Figure 2A, top), whereas no V2R-V206D is found in the basolateral membrane. When treated with DMSO (Figure 2A, middle) or glycerol (our unpublished data), the localization of V2R-V206D indeed shifted from the ER to the basolateral membrane, because PDI colocalization decreased to 44.2 ± 6.4 or 54.5 ± 7.4%, respectively, whereas E-cadherin colocalization (our unpublished data) increased to 52.5 ± 7.1 or 49.6 ± 8.3%, respectively.
Figure 2.
Rescued cell surface expression of V2R-V206D upon treatment with chemical chaperones. (A) MDCK-V2R-V206D cells were seeded on filters, grown to confluence, and left untreated, or incubated with 1% DMSO or 1 μM thapsigargin (indicated). Cells were subsequently fixed and subjected to immunocytochemistry using anti-PDI antibodies to stain the ER, followed by confocal analysis. Signals for PDI are shown on the left, whereas signals for the GFP-tagged V2R-V206D are shown on the right. Bar, 10 μm. (B) Confluent MDCK-V2R-V206D cells were treated for increasing time periods with 1% DMSO or glycerol (indicated), followed by fixation, immunocytochemical staining, and confocal analysis as described under A. The percentage of colocalization of V2R-V206D with E-cadherin (basolateral membrane marker) or PDI (endoplasmic reticulum marker) was quantified for each time point (n > 3) and plotted as a function of time. (C) Confluent MDCK-V2R-V206D cells were treated for 16 h with the indicated concentrations of DMSO (left) or glycerol (right), followed by fixation, staining, and analysis as described under B. The percentage of colocalization of V2R-V206D with E-cadherin or PDI was quantified for each concentration (n > 3) and plotted.
On treatment with 1 μM thapsigargin for 2 h (Figure 2A, bottom), localization of V2R-V206D to the ER was reduced to 46.2 ± 6.5%, whereas the remainder colocalized with E-cadherin in the basolateral membrane (53.8 ± 7.0%; our unpublished data). This effect was slightly weaker with 1 μM curcumin, because 2-h treatment with this drug decreased the ER localization of V2R-V206D to 64.6 ± 7.3%, whereas its basolateral membrane localization increased to 32.8 ± 6.7% (our unpublished data). Within this time frame, the rescuing effect with DMSO and glycerol was much lower. To determine the speed of V2R-V206D rescue by DMSO and glycerol, we performed a time-response curve, and semiquantified the corresponding CLSM data (Figure 2B). In time, colocalization with PDI gradually decreases, which is accompanied with increased colocalization with E-cadherin. For DMSO, the values obtained for t = 8 h were not significantly (p > 0.05) different from the values for t = 16 h described above. For glycerol, however, a significantly increased rescue effect was observed in this time period (p = 0.03).
In all cases, the translocation effect was observed for ∼70% of the cells expressing V2R-V206D. The remaining cells did not show any translocation effect. There was no correlation between the expression levels of individual cells and the occurrence of rescue. Also, consistent with the absence of maturation, none of the other V2R mutants showed any rescued cell surface expression with any of the chemical chaperones or thapsigargin (our unpublished data). Incubation with 2, 4, 6, or 8% DMSO, or with 6, 8, or 10% glycerol, also induced translocation of V2R-V206D, but semiquantification of CLSM data (Figure 2C) revealed that the observed effect was not significantly increased compared with treatment with 1% DMSO or 4% glycerol. At concentrations above 10% DMSO or 12% glycerol, the compounds induced severe morphological changes or showed cytotoxic effects (as revealed by the reduced number of remaining cells; our unpublished data).
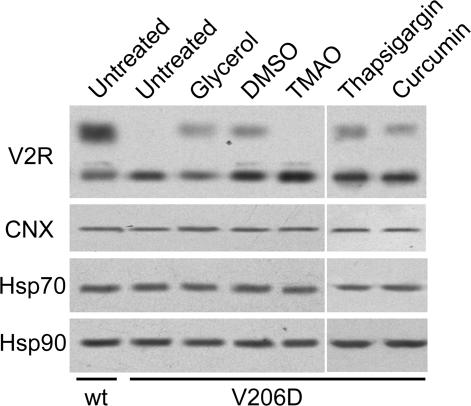
As shown in Figure 3, no differences in expression were observed for calnexin, HSP70, or HSP90 between MDCK cells expressing wtV2R or V2R-V206D. In addition, their expression was not altered in response to treatment with the rescue-inducing osmolytes glycerol and DMSO, or with TMAO.
Figure 3.
The expression of molecular chaperones is not affected by osmolyte treatment. MDCK-wtV2R or -V2R-V206D cells were left untreated or incubated for 16 h with 4% glycerol, 1% DMSO, or 1% TMAO, or 2 h with 1 μM thapsigargin or 1 μM curcumin (indicated), lysed, and subjected to immunoblotting as described in the legend of Figure 1. As primary antibodies, rabbit-anti-GFP (top), rabbit-anti-calnexin (CNX), mouse-anti-HSP70 (HSP70), or mouse-anti-HSP90 (HSP90) were used. Per lane, 10 μg of total cell lysate was loaded.
Chemical Chaperones Versus Cell-permeable Ligands
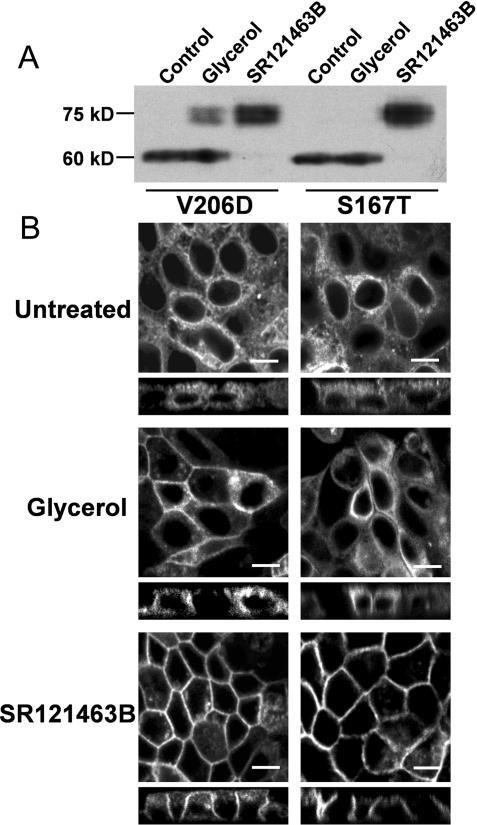
Recently, Morello et al. (2000) showed that the plasma membrane expression of several, but not all, V2R mutants in human embryonic kidney 293 cells could be rescued by the cell-permeable V2R antagonist SR121463B (Morello et al., 2000). To determine whether V2R mutants show a different sensitivity in rescue toward this pharmacological chaperone compared with a chemical chaperone, we treated MDCK cells expressing V2R-V206D or V2R-S167T for 16 h with 1 μM SR121463B or 4% glycerol. As shown in Figure 4A, treatment with SR121463B drastically induced the maturation of both V2R mutants, which coincided with the disappearance of the 60-kDa band. In contrast, glycerol treatment increases receptor maturation of V2R-V206D only. In line with these data, immunocytochemistry revealed that, whereas both mutants localized to the ER when untreated (Figure 4B, top) and glycerol rescued the plasma membrane expression of V2R-V206 only (Figure 4B, middle), SR121463B rescued the basolateral plasma membrane localization of both V2R-V206D and -S167T (Figure 4B, bottom). These data indicate that SR121463B has a more pronounced rescuing effect on more V2R mutants than glycerol.
Figure 4.
Rescue of V2R mutants by pharmacological and chemical chaperones. (A) Confluent V2R-V206D- or -S167T-expressing MDCK cells (indicated) were left untreated or incubated for 16 h with 4% glycerol, or 1 μM SR121463B. Subsequently, cells were lysed and analyzed by immunoblotting as described in the legends of Figure 1. (B) V2R-V206D or -S167T expressing MDCK cells (indicated) were seeded on filters, grown to confluence, and subsequently left untreated, or incubated for 16 h with 4% glycerol, or 1 μM SR121463B. Cells were subsequently fixed and analyzed by confocal microscopy. Bar, 10 μm.
The V206D Mutation Specifically Induces ER Retention
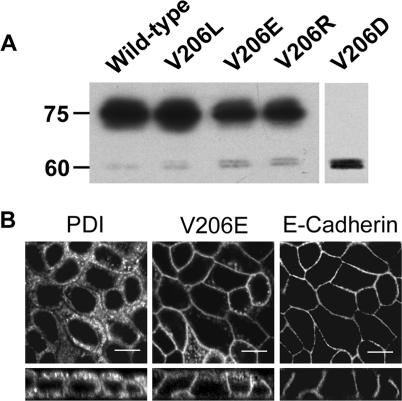
To elucidate whether the ability to restore the plasma membrane localization of V2R-V206D depends on the charge of the introduced mutation, V206 was changed into a negatively charged glutamic acid (V206E), a hydrophobic leucine (V206L), or a positively charged arginine (V206R), and stably expressed in MDCK type I cells. Immunoblot analysis of expressing clones, however, showed that all these mutants were mainly expressed as mature 75-kDa proteins (Figure 5A), suggesting that these mutants were hardly ER retained. Indeed, immunocytochemistry on two clones of each cell line revealed that V2R-V206E (Figure 5B), V2R-V206R, and V2R-V206L (our unpublished data) mainly localize in the basolateral membrane and colocalized only to a minor extent with PDI. These data indicated that the ER retention of V2R-V206D is specific for aspartic acid and that no information could be obtained on the amino acid specificity of rescue by chemical chaperones.
Figure 5.
V2R-V206L, -V206E, and -V206R are expressed as mature proteins. (A) MDCK cell lines stably expressing either wild-type V2R, V2R-V206L, -V206E, -V206R, or -V206D (indicated) were grown to confluence, lysed, and analyzed by immunoblotting for GFP. The masses of the immature (60-kDa) and mature (75-kDa) V2R proteins are indicated on the left. (B) MDCK cells stably expressing the GFP-tagged V2R-V206E were grown to confluence, fixed, immunocytochemically stained using anti-PDI and anti-E-cadherin antibodies, and analyzed by CLSM as described in the legend of Figure 2. Bar, 10 μm.
Cytosolic Calcium Is Involved in the Rescue of V2R-V206D
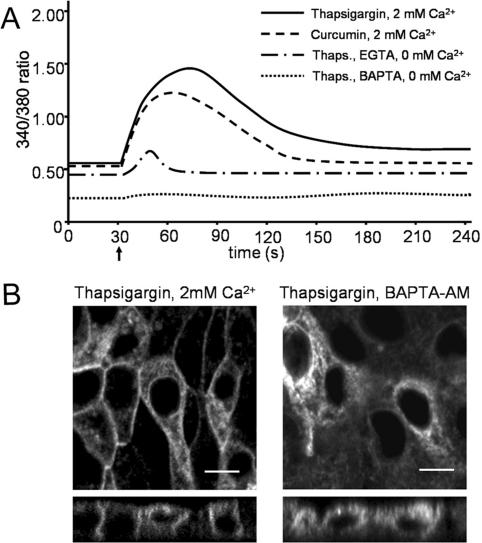
Thapsigargin-induced emptying of ER Ca2+ stores, resulting in decreased free [Ca2+]ER, has been postulated to be the determining factor causing rescue of the ΔF508 mutant of the cystic fibrosis transmembrane conductance regulator (CFTR) (Egan et al., 2002). To determine how alterations in intracellular Ca2+ levels affect translocation of V2R-V206D to the plasma membrane, we determined the changes in [Ca2+]cyt upon drug treatment using the Ca2+-sensitive fluorophore FURA-2-AM. Addition of thapsigargin to MDCK-V2R-V206D cells in HEPES/Tris buffer with 2 mM CaCl2 showed a rapid increase of [Ca2+]cyt, which peaked after ∼30 s, followed by a gradual decrease to a steady-state level that was slightly increased compared with the starting situation (Figure 6A). Addition of curcumin showed similar results, although the peak value and the final plateau phase were decreased compared with thapsigargin. In Ca2+-free buffer with 1 mM EGTA, base levels of [Ca2+]cyt were lower than in Ca2+-containing the buffer, which did not change with the addition of thapsigargin, except for a small peak right after addition, which is likely caused by Ca2+ diffusing from the ER lumen. Supplementary incubation with the cytosolic Ca2+ chelator BAPTA-AM resulted in a low [Ca2+]cyt throughout the experiment.
Figure 6.
Cytosolic calcium responses to treatment with SERCA inhibitors. (A) MDCK type 1 cells were seeded at 100,000 cells/cm2 in glass-bottom culture dishes and grown for 24 h. Cytosolic calcium levels were measured using FURA-2. Cells were incubated in HEPES/Tris buffer with the indicated levels of Ca2+, or EGTA with or without BAPTA (indicated), to allow the baseline to settle. Thirty seconds after the start of the measurement (indicated with an arrow), 1 μM thapsigargin or curcumin (indicated) was added to the cells followed by measurement of the 340/380-nm ratio. Each data series comprises the averaged data of 25 cells that were measured simultaneously. The figures show data of a representative experiment (n = 3). (B) Confluent MDCK-V2R-V206D cells were incubated for 2 h in HEPES/Tris buffer with 1 μM thapsigargin, supplemented with 2 mM Ca2+ (left), or 5 mM BAPTA-AM and without Ca2+ (right). Subsequently, cells were fixed and analyzed by confocal microscopy. Bar, 10 μM.
Immunocytochemistry revealed a similar rescue effect of V2R-V206D with thapsigargin (Figure 6B, left) and curcumin in HEPES/Tris buffer with 2 mM Ca2+ as in DMEM. Without Ca2+ and independent of the presence of BAPTA, however, no V2R-V206D rescue was observed (Figure 6B, right).
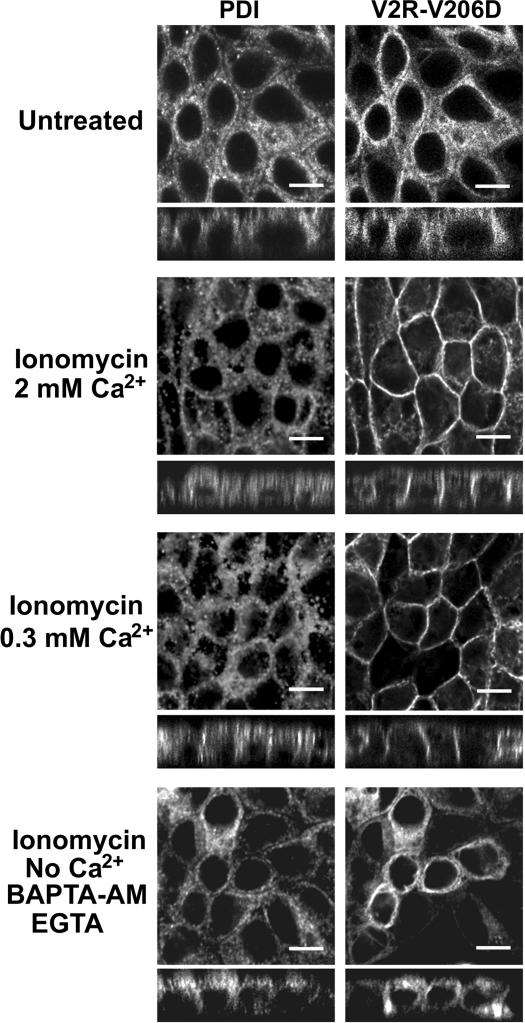
These experiments could indicate that increased [Ca2+]cyt instead of reduced [Ca2+]ER mediated rescue of V2R-V206D to the plasma membrane. To further investigate the roles of [Ca2+]ER and [Ca2+]cyt in rescue of V2R-V206D, we used the ionophore ionomycin. This drug renders cell membranes permeable to Ca2+, which allowed us to clamp [Ca2+]ER. In eukaryotic cells, the resting [Ca2+]ER is ∼300 μM Ca2+ (Arnaudeau et al., 2001; Demaurex and Frieden, 2003). Incubation of MDCK-V2R-V206D cells in buffer with 2 mM Ca2+ did not affect its ER localization (Figure 7, top), but addition of 1 μM ionomycin for 2 h induced a clear translocation of V2R-V206D to the basolateral membrane (Figure 7, second top panel). As shown in Figure 7 (third top panel), incubation of the cells for 2 h with 1 μM ionomycin in buffer with 300 μM Ca2+ also induced a translocation of V2R-V206D to the basolateral membrane. In Ca2+-free buffer with EGTA and/or BAPTA-AM, however, ionomycin was not able to rescue the cell surface expression of V2R-V206D (Figure 7, bottom).
Figure 7.
Effect of ionomycin and calcium on the localization of V2R-V206D. Confluent cell layers of MDCK cells stably expressing GFP-tagged V2R-V206D were incubated for 2 h in HEPES/Tris buffer with 2 mM Ca2+, 2 mM Ca2+, and 1 μM ionomycin, or 300 μM Ca2+ and 1 μM ionomycin, or in buffer without Ca2+ in the presence of 1 μM ionomycin, 5 mM BAPTA-AM, and 1 mM EGTA (indicated). Subsequently, the cells were fixed, immunocytochemically stained, and analyzed by CLSM as described in the legend of Figure 2. Bar, 10 μm.
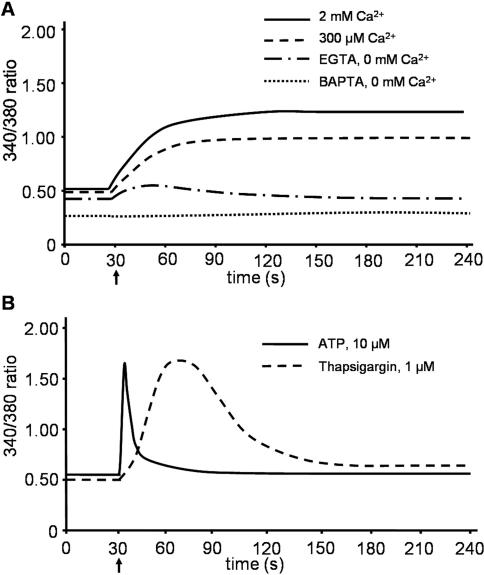
Corresponding FURA-2 measurements showed a steady state increase in [Ca2+]cyt with ionomycin when either 2 mM or 300 μM Ca2+, which reached a plateau phase after ∼30 s (Figure 8A). In Ca2+-free buffer with 1 mM EGTA, a small increase in the [Ca2+]cyt was observed, which decreased to below base level after ∼2 min. In Ca2+-free buffer with BAPTA-AM and EGTA, [Ca2+]cyt again remained low throughout the experiment.
Figure 8.
Cytosolic calcium levels in MDCK cells upon treatment with ionomycin. MDCK cells were grown as described in the legend of Figure 4. (A) Cells were incubated in HEPES/Tris buffer containing the indicated levels of Ca2+, or EGTA with or without BAPTA (indicated), to allow the baseline to settle. Thirty seconds after the start of the measurement (indicated with an arrow), ionomycin was added to the cells, followed by measurement of the 340/380-nm ratio. (B) Cells were incubated in HEPES/Tris buffer containing 2 mM Ca2+ to allow the baseline to settle. 30 s after the start of the measurement (indicated with an arrow), 1 μM thapsigargin or 10 μM ATP (indicated) was added. Each data series comprises the averaged data of 25 cells that were measured simultaneously. The figures show data of a representative experiment (n = 3).
Several hormones, such as ATP, also induce an increase in cytosolic calcium via the phospholipase C pathway. To compare the intracellular calcium flux patterns of such a hormone with those induced by thapsigargin, curcumin, and ionomycin, we added ATP to MDCK cells and measured intracellular calcium. As shown in Figure 8B, ATP seemed to induce an increase in intracellular calcium, but with less total Ca2+ mobilization, and different kinetics compared with the drugs mentioned above. As anticipated, ATP did not induce a translocation of V2R-V206D to the plasma membrane (our unpublished data).
DISCUSSION
Rescue of V2R Mutants Is Mutation Specific and Induced by Several Chemical Chaperones
We have previously shown that in polarized cells, many V2R mutants involved in the X-linked NDI are retained in the ER. Out of nine of these mutants, only V2R-V206D showed a rescued plasma membrane expression and increased receptor maturation upon treatment with chemical chaperones or calcium-affecting drugs, whereas all other mutants tested did not (Figures 1 and 2). This illustrates that plasma membrane rescue is not a general phenomenon for a particular protein but is specific for a limited set of mutations in a particular protein. This is in line with the results of Delisle et al. (2003) who reported that the potassium channel mutants HERG-G601S and -F805C, found in long QT syndrome, were rescued in their cell surface expression by thapsigargin, whereas the localization of HERG-N470D was not changed. Although we tested only one pharmacological chaperone, this class of compounds seems to induce a stronger level of rescue of V2R mutants, because the level of maturation of V2R-V206D and V2R-S167T with SR121463B was better than obtained with the most optimal concentrations of the chemical chaperones glycerol or DMSO (Figure 4). Moreover, the pharmacological chaperone seems to act on more V2R mutants than chemical chaperones or SERCA inhibitors, because it also rescued the basolateral expression of V2R-S167T. This is in line with the finding of Morello et al. (2000) who found that eight of 15 V2R mutants were rescued by SR121463B, although they used different V2R mutants and did not test chemical chaperones. In addition to their possible clinical applicability, this makes cell-permeable antagonists, and the mechanism by which they facilitate rescue, promising subjects for more detailed investigation, which will be the subject of further studies.
The V2R-V206E/R/L mutants were only ER retained to a minor extent (Figure 4), which may indicate that V206D is a subtle mutation, thereby allowing proper folding upon slight structural changes induced by chemical chaperones. However, as V2R-R113W, -G201D, and -T204N, are only partially ER retained (Robben et al., 2005), it is likely that these mutants are also not severely misfolded. It is, therefore, striking that the latter three mutants did not respond to any of the chemical chaperones. Likely, the relationship between the location and type of a mutation in the V2R, and the mutant's ability to be rescued by chemical chaperones has to await the atomic structure of the V2R.
Our study furthermore revealed that V2R-V206D cell surface expression is rescued by the chemical chaperones DMSO and glycerol, the SERCA inhibitors thapsigargin and curcumin, and the Ca2+ ionophore ionomycin, whereas none of these compounds changed the localization of any of the other V2R mutants. These data indicate that if a particular mutant is rescued by one chemical chaperone, it seems to be more prone to be rescued by others.
Mechanism of Rescue by Chemical Chaperones
Rescue of ER retention of mutant membrane proteins in eukaryotic cells by osmolytes, such as DMSO and glycerol, has been postulated to be due to increased expression or changed functionality of stress-sensitive molecular chaperones (Fuller and Cuthbert, 2000; Choo-Kang and Zeitlin, 2001). Despite rescue of V2R-V206D, however, glycerol and DMSO treatment did not affect the expression level of the ER lectin calnexin (Figure 3), which has been suggested to be involved in ER retention of V2R mutants (Morello et al., 2001). Also, although increased HSP70 expression levels have been shown to promote rescue of CFTR-ΔF508 cells (Choo-Kang and Zeitlin, 2001), its level remained unaltered with V2R-V206D. In addition, HSP90 expression was unchanged. Although our data reveal that a changed expression of these molecular chaperones does not contribute to V2R-V206D rescue, it does not exclude effects of a changed activity of these proteins or the involvement of other folding proteins in this process.
Besides these explanations, DMSO and glycerol have been suggested to increase the relative hydration around a polypeptide, thereby inducing a tighter packing of the protein and a stabilization of the protein's conformation (Welch and Brown, 1996; Shearer and Hampton, 2004). However, although the effects on 3-hydroxy-3-mehtylglutaryl-CoA reductase in yeast are accomplished in minutes, rescue of V2R-V206D by DMSO and glycerol is only fully effective after 8-16 h, whereas there is hardly any rescue noticeable after 2 h (Figure 2B). Although we cannot exclude it, a direct effect of the osmolytes on V2R-V206D to explain its rescue is therefore rather unlikely. It remains to be established which mechanism underlies rescue of V2R-V206D plasma membrane expression by chemical chaperones.
Mechanisms of Rescue by ER Calcium-modifying Drugs
The mechanism underlying rescue by thapsigargin, curcumin, or ionomycin seems to be different in that these compounds efficiently rescued the cell surface expression of V2R-V206D within 2 h. Also, these drugs induced a raise in intracellular calcium levels (Figures 6 and 8), which was not observed with DMSO, glycerol, or SR121463B (our unpublished data).
Low cytosolic calcium levels are mainly maintained by the SERCA ATPases, which pump leaked calcium back into the ER, and by plasma membrane calcium ATPases (PMCAs), which pump calcium out of the cells. From the combined FURA-2 measurements and V2R-V206D translocation studies, the following information can be deduced: First, cells incubated with thapsigargin, ionomycin, or curcumin in the absence of extracellular calcium show a small and transient increase in intracellular calcium in contrast to cells with extracellular calcium. Cell surface expression of V2R-V206D is only obtained under the latter condition, which indicates that an influx of extracellular calcium is needed for the rescue of V2R-V206D.
Second, if the mechanism by which V2R-V206D is rescued is identical for thapsigargin, ionomycin, and curcumin, our data indicate that this is rather because of increased cytosolic, instead of decreased ER calcium levels. Inhibition of SERCA pumps by thapsigargin, and to a lower extent curcumin, result in a cytosolic calcium spike of ∼90 s, which likely results from an initial extracellular calcium entry and a reduced ER calcium entry. The following decrease of cytosolic calcium to the observed slightly increased basal levels is likely caused by activated PMCAs. Under this condition, however, [Ca2+]ER will be decreased. The calcium ionophore ionomycin renders the ER and plasma membranes permeable for calcium (Visch et al., 2004). Under this condition, PMCAs are not able to compensate the extracellular calcium influx, which is shown by the sustained high cytosolic calcium levels (Figure 8A). Therefore, [Ca2+]cyt and [Ca2+]ER will be similar to the extracellular [Ca2+]. Extracellular calcium levels similar (300 μM) or well above (2 mM) ER resting levels coincided with a rescued cell surface expression of V2R-V206D, which indicated that this rescue was due to increased [Ca2+]cyt levels. The absence of V2R-V206 rescue with ATP indicates that a short transient increase in cytosolic calcium is not sufficient to mediate rescue. It remains unclear, however, whether the larger calcium peak, the increased basal calcium level or both are involved in this rescue.
Because the V206D mutation is exposed to the ER luminal side, it is unlikely that altered [Ca2+]cyt levels directly affect the mutation. More likely, the observed increased [Ca2+]cyt levels may affect cytosolic folding proteins, such as HSP70 or HSP90, to facilitate V2R-V206D folding or induce its release from the ER. If so, this was not mediated by changed expression levels of these proteins, because these levels were unchanged (Figure 3). Possibly, the functionality of the transmembrane ER protein calnexin may be directly or indirectly affected by changes in [Ca2+]cyt, because this molecular chaperone was shown to have prolonged interaction with an ER retained V2R mutant compared with wtV2R (Morello et al., 2001).
Interestingly, thapsigargin and curcumin also rescue the cell surface expression of CFTR-ΔF508 (Egan et al., 2002, 2004). In contrast to V2R-V206D, however, CFTR-ΔF508 was rescued in the presence of BAPTA-AM, which indicates that cytosolic calcium has no role in CFTR-ΔF508 rescue and that CFTR-ΔF508 rescue is mediated through another mechanism. However, in the experiments of Egan et al. (2002,2004), a 1-h thapsigargin and BAPTA-AM treatment was followed by a 1.5-h recovery time. Because thapsigargin, but not BAPTA-AM, is difficult to wash out, it cannot be excluded that CFTR-ΔF508 rescue occurred in the last 1.5 h due to thapsigargin alone. Unfortunately, we were not able to obtain CFTR-ΔF508 or CFTR expression in MDCK cells to test this further.
In conclusion, we have shown that plasma membrane rescue by chemical chaperones and altered Ca2+ levels only occurs for V2R-V206D but that its cell surface expression is rescued by the chemical chaperones glycerol and DMSO. In addition, the SERCA-inhibitors thapsigargin and curcumin as well as the Ca2+ ionophore ionomycin are able to induce translocation of V2R-V206D to the plasma membrane by increasing [Ca2+]cyt, rather than by decreasing [Ca2+]ER. Increased insight in the molecular mechanisms that facilitate restoration of the plasma membrane localization of class II mutants may aid in developing therapies for diseases caused by such mutant proteins.
Acknowledgments
We thank Dr. B. Wieringa (Department of Cell Biology, Nijmegen Centre for Molecular Life Sciences) for the rabbit anti-GFP antiserum, Dr. I. Braakman for the rabbit anti-PDI and calnexin antibodies, and Dr. David Toft for the mouse anti-HSP70 and -HSP90 antibodies. In addition, we thank Drs. P. Willems, W. Koopman, and H. J. Visch (Department of Biochemistry, Nijmegen Centre for Molecular Life Sciences) for useful suggestions and assistance setting up the Ca2+ measurements. This project is supported by a grant from the Dutch Kidney Foundation (PC 104) to P.M.T.D. and N.V.A.M.K. and from the European Union (QLK3-CT-2001-00987) to P.M.T.D.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-06-0579) on November 2, 2005.
Abbreviations used: 4-PBA, 4-phenyl butyric acid; [Ca2+]cyt, cytosolic calcium level; [Ca2+]ER, endoplasmic reticulum calcium level; CFTR, cystic fibrosis transmembrane conductance regulator; CLSM, confocal laser scanning microscopy; DMSO, dimethylsulfoxide; NDI, nephrogenic diabetes insipidus; HSP, heat-shock protein; ICC, immunocytochemistry; TMAO, tri-methyl-amine-N-oxide; V2R, vasopressin V2 receptor.
References
- Arnaudeau, S., Kelley, W. L., Walsh, J. V., Jr., and Demaurex, N. (2001). Mitochondria recycle Ca2+ to the endoplasmic reticulum and prevent the depletion of neighboring endoplasmic reticulum regions. J. Biol. Chem. 276, 29430-29439. [DOI] [PubMed] [Google Scholar]
- Brostrom, M. A., and Brostrom, C. O. (2003). Calcium dynamics and endoplasmic reticular function in the regulation of protein synthesis: implications for cell growth and adaptability. Cell Calcium 34, 345-363. [DOI] [PubMed] [Google Scholar]
- Choo-Kang, L. R., and Zeitlin, P. L. (2001). Induction of HSP70 promotes delta-F508 CFTR trafficking. Am. J. Physiol. 281, L58-L68. [DOI] [PubMed] [Google Scholar]
- Deen, P.M.T., Marr, N., Kamsteeg, E. J., and Van Balkom, B.W.M. (2000). Nephrogenic diabetes insipidus. Curr. Opin. Nephrol. Hypertens. 9, 591-595. [DOI] [PubMed] [Google Scholar]
- Deen, P.M.T., Van Balkom, B.W.M., Savelkoul, P.J.M., Kamsteeg, E. J., van Raak, M., Jennings, M. L., Muth, T. R., Rajendran, V., and Caplan, M. J. (2002). Aquaporin-2, COOH terminus is necessary but not sufficient for routing to the apical membrane. Am. J. Physiol. 282, F330-F340. [DOI] [PubMed] [Google Scholar]
- Delisle, B. P., Anderson, C. L., Balijepalli, R. C., Anson, B. D., Kamp, T. J., and January, C. T. (2003). Thapsigargin selectively rescues the trafficking defective LQT2 channels G601S and F805C. J. Biol. Chem. 278, 35749-35754. [DOI] [PubMed] [Google Scholar]
- Demaurex, N., and Frieden, M. (2003). Measurements of the free luminal ER Ca2+ concentration with targeted “cameleon” fluorescent proteins. Cell Calcium 34, 109-119. [DOI] [PubMed] [Google Scholar]
- Diamant, S., Eliahu, N., Rosenthal, D., and Goloubinoff, P. (2001). Chemical chaperones regulate molecular chaperones in vitro and in cells under combined salt and heat stresses. J. Biol. Chem. 276, 39586-39591. [DOI] [PubMed] [Google Scholar]
- Egan, M. E., Glockner-Pagel, J., Ambrose, C., Cahill, P. A., Pappoe, L., Balamuth, N., Cho, E., Canny, S., Wagner, C. A., Geibel, J., and Caplan, M. J. (2002). Calcium-pump inhibitors induce functional surface expression of delta F508-CFTR protein in cystic fibrosis epithelial cells. Nat. Med. 8, 485-492. [DOI] [PubMed] [Google Scholar]
- Egan, M. E., Pearson, M., Weiner, S. A., Rajendran, V., Rubin, D., Glockner-Pagel, J., Canny, S., Du, K., Lukacs, G. L., and Caplan, M. J. (2004). Curcumin, a major constituent of turmeric, corrects cystic fibrosis defects. Science 304, 600-602. [DOI] [PubMed] [Google Scholar]
- Finlayson, K., Witchel, H. J., McCulloch, J., and Sharkey, J. (2004). Acquired QT interval prolongation and HERG: implications for drug discovery and development. Eur. J. Pharm. 500, 129-142. [DOI] [PubMed] [Google Scholar]
- Fuller, W., and Cuthbert, A. W. (2000). Post-translational disruption of the delta F508 cystic fibrosis transmembrane conductance regulator (CFTR)-molecular chaperone complex with geldanamycin stabilizes delta F508 CFTR in the rabbit reticulocyte lysate. J. Biol. Chem. 275, 37462-37468. [DOI] [PubMed] [Google Scholar]
- Morello, J. P., et al. (2000). Pharmacological chaperones rescue cell-surface expression and function of misfolded V2 vasopressin receptor mutants. J. Clin. Investig. 105, 887-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello, J. P., Salahpour, A., Petaja-Repo, U. E., Laperriere, A., Lonergan, M., Arthus, M. F., Nabi, I. R., Bichet, D. G., and Bouvier, M. (2001). Association of calnexin with wild type and mutant AVPR2 that causes nephrogenic diabetes insipidus. Biochemistry 40, 6766-6775. [DOI] [PubMed] [Google Scholar]
- Robben, J. H., Knoers, N.V.A.M., and Deen, P.M.T. (2004). Regulation of the vasopressin V2 receptor by vasopressin in polarized renal collecting duct cells. Mol. Biol. Cell 15, 5693-5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robben, J. H., Knoers, N.V.A.M., and Deen, P.M.T. (2005). Characterization of vasopressin V2 receptor mutants in nephrogenic diabetes insipidus in a polarized cell model. Am. J. Physiol. 289, F265-F272. [DOI] [PubMed] [Google Scholar]
- Sato, S., Ward, C. L., Krouse, M. E., Wine, J. J., and Kopito, R. R. (1996). Glycerol reverses the misfolding phenotype of the most common cystic fibrosis mutation. J. Biol. Chem. 271, 635-638. [DOI] [PubMed] [Google Scholar]
- Schulein, R., Lorenz, D., Oksche, A., Wiesner, B., Hermosilla, R., Ebert, J., and Rosenthal, W. (1998). Polarized cell surface expression of the green fluorescent protein-tagged vasopressin V2 receptor in Madin-Darby canine kidney cells. FEBS Lett. 441, 170-176. [DOI] [PubMed] [Google Scholar]
- Shearer, A. G., and Hampton, R. Y. (2004). Structural control of endoplasmic reticulum-associated degradation: effect of chemical chaperones on 3-hydroxy-3-methylglutaryl-CoA reductase. J. Biol. Chem. 279, 188-196. [DOI] [PubMed] [Google Scholar]
- Skach, W. R. (2000). Defects in processing and trafficking of the cystic fibrosis transmembrane conductance regulator. Kidney Int. 57, 825-831. [DOI] [PubMed] [Google Scholar]
- Song, J. L., and Chuang, D. T. (2001). Natural osmolyte trimethylamine N-oxide corrects assembly defects of mutant branched-chain alpha-ketoacid decarboxylase in maple syrup urine disease. J. Biol. Chem. 276, 40241-40246. [DOI] [PubMed] [Google Scholar]
- Tamarappoo, B. K., Yang, B., and Verkman, A. S. (1999). Misfolding of mutant aquaporin-2 water channels in nephrogenic diabetes insipidus. J. Biol. Chem. 274, 34825-34831. [DOI] [PubMed] [Google Scholar]
- Visch, H. J., et al. (2004). Inhibition of mitochondrial Na+-Ca2+ exchange restores agonist-induced ATP production and Ca2+ handling in human Complex I deficiency. J. Biol. Chem. 279, 40328-40336. [DOI] [PubMed] [Google Scholar]
- Welch, W. J., and Brown, C. R. (1996). Influence of molecular and chemical chaperones on protein folding. Cell Stress Chap. 1, 109-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, D. S., Yip, C. M., Huang, T.H.J., Chakrabartty, A., and Fraser, P. E. (1999). Manipulating the amyloid-beta aggregation pathway with chemical chaperones. J. Biol. Chem. 274, 32970. [DOI] [PubMed] [Google Scholar]