Abstract
Cells respond to DNA replication stress by triggering cell cycle checkpoints, repair, or death. To understand the role of the DNA damage response pathways in determining whether cells survive replication stress or become committed to death, we examined the effect of loss of these pathways on cellular response to agents that slow or arrest DNA synthesis. We show that replication inhibitors such as excess thymidine, hydroxyurea, and camptothecin are normally poor inducers of apoptosis. However, these agents become potent inducers of death in S-phase cells upon small interfering RNA-mediated depletion of the checkpoint kinase Chk1. This death response is independent of p53 and Chk2. p21-deficient cells, on the other hand, produce a more robust apoptotic response upon Chk1 depletion. p21 is normally induced only late after thymidine treatment. In Chk1-depleted cells p21 induction occurs earlier and does not require p53. Thus, Chk1 plays a primary role in the protection of cells from death induced by replication fork stress, whereas p21 mediates through its role in regulating entry into S phase. These findings are of potential importance to cancer therapy because we demonstrate that the efficacy of clinically relevant agents can be enhanced by manipulation of these signaling pathways.
INTRODUCTION
Cells respond to DNA damage by triggering cell cycle arrest, DNA repair, or death. DNA damage response pathways are frequently disrupted during tumor development, leading to genetic instability, loss of cell cycle checkpoint controls, and defects in the induction of apoptosis (Kastan and Bartek, 2004). The related PIKK kinases ataxia-telangiectasia mu-tated (ATM) and ATM- and Rad3-related (ATR) are major coordinators of this damage response (Shiloh, 2003). ATM is central to the response to DNA double-strand breaks (DSBs) and is required to delay DNA synthesis and the onset of mitosis after the induction of this type of damage by agents such as ionizing radiation (IR) (Lim et al., 2000; Falck et al., 2001, 2002). ATR and its downstream phosphorylation target Chk1 are generally activated in response to UV and agents that stall DNA replication forks (Cha and Kleckner, 2002; Ward et al., 2004) but are also activated by DSBs. In the DSB response, ATR joins later and maintains the phosphorylation state of specific substrates (Shiloh, 2003). After DNA synthesis arrest, the ATR-mediated response is essential to maintain the integrity of replication forks and to prevent futile replication origin firing (Feijoo et al., 2001; Zachos et al., 2003; Syljuasen et al., 2005).
Excess thymidine (TdR) also triggers a rapid ATM-mediated protein kinase cascade followed by the ATR-mediated response (Bolderson et al., 2004). Both the ATM and ATR-mediated responses are required for cell survival after thymidine treatment, and AT-cells are defective in homologous recombination repair induced by this agent. Exposure of cells in culture to thymidine leads to an increased level of dTTP that acts as a feedback inhibitor of ribonucleotide reductase (Eriksson et al., 1979). As a result of this inhibition thymidine starves cells of dCTP and slows DNA replication but does not arrest it, leading to an accumulation of cells that slowly traverse S phase (an effect known as thymidine block (Bjursell and Reichard, 1973). However, thymidine induces little detectable DNA damage in the form of DSBs (Lundin et al., 2002; Bolderson et al., 2004), and its effects on DNA replication are readily reversible. In cells treated with more stringent inhibitors of ribonucleotide reductase and replication such as hydoxyurea (HU; Bianchi et al., 1986), the ATR-mediated response seems to play a more prominent role. After treatment with HU, ATR and the ATR-interacting protein (ATRIP) are recruited to single-stranded DNA coated with the replication protein A (RPA) complex. The interaction between these proteins seems to be required for the activation of Chk1 (Zou and Elledge, 2003) which then may suppress the firing of new replication origins (Syljuasen et al., 2005), promote the reactivation of stalled or collapsed forks via homologous recombination repair (HRR; Sorensen et al., 2005), and stimulate the phosphorylation of downstream targets such as CDC25C to inhibit mitotic entry (Funari et al., 1997; Sanchez et al., 1997).
Given the increased sensitivity of cells lacking ATM- or ATR-mediated DNA damage response pathways to the effects of thymidine on S-phase transition and colony formation, we sought to determine how loss of these pathways affected the ultimate fate of cells treated with this agent or other inhibitors of DNA replication. We show that thymidine is normally a poor inducer of apoptosis in cultured tumor cells. However small interfering RNA (siRNA)-mediated ablation of Chk1 (but not Chk2) causes thymidine-treated cells entering S phase (as well as those treated with other inhibitors of DNA replication) to rapidly undergo apoptosis. This death response is p53 independent, but cells that lack both Chk1 and p21 show a strikingly robust death response and reduced cell survival. Thus, the Chk1 pathway plays a key role in protecting cells entering S phase from undergoing apoptosis with thymidine (or other DNA replication inhibitors) from undergoing apoptosis, whereas p21 mediates this role by preventing entry into S phase.
MATERIALS AND METHODS
Cell Lines and Cultures
The HCT116 human colon cancer cell lines (wt, p53-/- and p21 -/-) were generously provided by Dr. Bert Vogelstein (Johns Hopkins University, Baltimore, MD). The + 3 derivative of HCT116 was obtained from Dr. C. Richard Boland (University of California, San Diego, San Diego, CA). Remaining cell lines were obtained from American Type Culture Collection (Manassas, VA). Cells were maintained in DMEM supplemented with 10% fetal bovine serum (FBS). For experiments using thymidine, dialyzed FBS was used to remove deoxynucleosides in the serum that might alter the response to this agent. For colony-forming assays, 500-1000 cells were plated in 10-cm dishes before thymidine (Sigma-Aldrich, St. Louis, MO) treatment in triplicate. Cells were grown 6-15 d before staining with 0.4% methylene blue/50% methanol (Fisher Scientific, Pittsburgh, PA). Colonies of >50 cells were scored. The surviving fraction was determined by dividing the average number of colonies for each treatment by the average number of colonies in the control.
siRNA Transfection
Chk1 siRNAs were designed to correspond to the Chk1 DNA sequence (Blackburn and Smythe, unpublished data) and purchased from Dharmacon (Lafayette, CO). The Chk2 siRNA (GAACCUGAGGACCAAGAA) was obtained from Eurogentec (Southampton, United Kingdom). Control duplex RNA (Dharmacon) corresponding to an unknown protein, with a G-C ratio of 42%, was used in controls. siRNA duplexes were transfected into cells using Oligofectamine (Invitrogen, Paisley, United Kingdom) according to manufacturer's instructions. Twenty-four hours after transfection, cells were washed with phosphate-buffered saline (PBS) before further treatment.
Cell Cycle Analysis
After treatment, floating (obtained from the medium and a PBS wash) and adherent (obtained after trypsinization) cells were pooled and pelleted by centrifugation. Cell pellets were washed with PBS, fixed in 70% ice-cold ethanol, and stored for up 2 wk at -20°C. Cells were washed twice with PBS followed by incubation in 50 μg/ml propidium iodide (PI) (Sigma-Aldrich) and 100 μg/ml RNase A (Sigma-Aldrich) for 30 min. Stained nuclei were analyzed on a FACScan (BD Biosciences, Franklin Lakes, NJ) using CellQuest software.
Detection of Apoptosis
Apoptotic cells were assessed by flow cytometry using fluorescein isothiocyanate (FITC)-Annexin V and PI according to the manufacturer's instructions (BD Biosciences). The percentage of early apoptotic (% Annexin V+/PI-) cells is presented.
Simultaneous Analysis of Apoptosis and Cell Cycle
Caspase-3 Activation Versus Cell Cycle. Unfixed cells were assayed for active caspase-3 immediately after treatment using the CaspGLOW fluorescein active caspase-3 staining kit according to the manufacturer's instructions (MBL, Woburn, MA). Cells were then resuspended in PBS containing 50 mg/ml PI, 100 mg/ml RNAse A, and 0.1% (vol/vol) Triton X-100. After a 30-min incubation the cell suspensions were analyzed by flow cytometry for active caspase-3 and DNA content simultaneously.
Loss of Mitochondrial Membrane Potential Versus Cell Cycle. Mitochondrial membrane potential was assessed with tetramethyl rhodamine (TMRM) ethyl ester (Rasola and Geuna, 2001; Invitrogen). Working solution (20 μM) was prepared in mitochondrial buffer (80 mM KCl, 10 mM Tris-HCl, 3 mM MgCl2, 1 mM EDTA, 5 mM KH2PO4, and 10 mM sodium succinate, pH 7,4). Cells were harvested, washed in PBS, and incubated in mitochondrial buffer with digitonin (at the ratio digitonin:protein, 0.12) for 5 min at room temperature. Then, cells were incubated with 200 nM TMRM, 0.5 μg/ml 7-anime-actinomycin D (7AAD; Sigma-Aldrich) and 100 μg/ml RNAse for 15 min. The TMRM and 7AAD signals (excitation, 488 nm; emission, 585 nm) were analyzed by flow cytometry.
Detection of Phosphorylated Histone H3
Treated cells were fixed with 70% ice-cold ethanol and stored at -20°C. Fixed cells were washed twice with PBS and incubated for 15 min in PBS, 0.1% bovine serum albumin (BSA), and 0.25% Triton X-100. After centrifugation, the cell pellet was suspended in 100 μl of PBS containing 1% BSA and 0.75 μg of a polyclonal antibody recognizing the phosphorylated form of histone H3 (Upstate Biotechnology, Lake Placid, NY) and incubated for 3 h at room temperature. The cells were rinsed with PBS containing 1% BSA and incubated with FITC-conjugated goat anti-rabbit immunoglobulin antibody (DakoCytomation Denmark, Glostrup, Denmark) diluted at a ratio of 1:30 in PBS containing 1% BSA. After a 30-min incubation at room temperature in the dark, the cells were stained with PI solution (PBS with 5 μg/ml PI and 100 μg/ml RNAse A), and cellular fluorescence was measured by a flow cytometer.
Western Blotting
Cell extracts were prepared as described previously (Bolderson et al., 2004), resolved on 10% SDS-PAGE gels, and blotted onto nitrocellulose (Whatman Schleicher and Schuell, Dassel, Germany). Proteins were detected with the ECL detection system (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom) using anti-p53 (DO-1; Santa Cruz Biotechnology, Santa Cruz, CA); anti-phospho-p53 (ser15) (Oncogene Research Products, Nottingham, United Kingdom); anti-p21 (BD Biosciences PharMingen, San Diego, CA); anti-total Chk1, anti-total Chk2, anti-phospho-Chk1 (ser345), anti-phospho-Chk2 (Thr68) (all from Cell Signaling Technology, Beverly, MA); or anti-β-actin (Sigma-Aldrich).
Reverse Transcription (RT)-PCR
Total RNA was extracted using GenElute mammalian total RNA kit (Sigma-Aldrich). One microgram of RNA was used as a template for each RT-PCR using SuperScript II reverse transcriptase (Invitrogen) for the RT reaction and 2× PCR Master Mix (Abgene, Epsom, United Kingdom) for the PCR reaction. The conditions for the RT-PCR reaction were as follows: cDNA synthesis at 42°C for 50 min, pre-PCR denaturation at 94°C for 2 min, denaturation at 94°C for 1 min, annealing at 60°C for 30 s., and extension at 72°C for 1.5 min for 23 cycles. Amplified products were separated on a 1% agarose gel and relative band intensities were quantified using Image Gauge 3.3 software (Fuji Photo Film, Tokyo, Japan). Primer sequences were 5-CAGAGGAGGCGCCATGTCAG-3 and 5-CCTGTCGGCGGATTAGGG for p21WAF1/Cip1 and 5-GGGAAATCGTGCGTGACATTAAG-3 and 5-TGTGTTGGCGTACAGGTCTT TG-3for β-actin.
RESULTS
Loss of Colony-forming Ability in Cells Treated with Thymidine or Low Levels of Camptothecin (CPT) Is Not the Consequence of Apoptosis
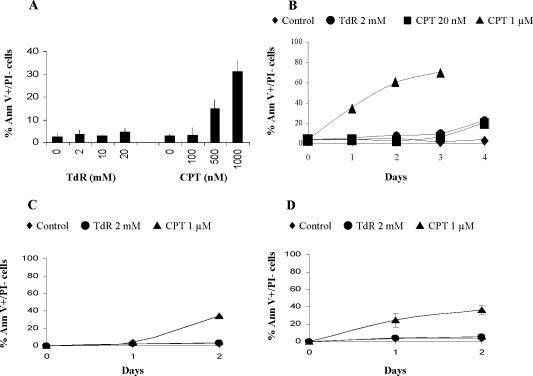
To determine whether the sensitivity of cells to thymidine was the consequence of induction of cell death, we measured the frequency of apoptotic cells induced by this agent in a collection of tumor cell lines. In particular, we examined the response of mismatch repair (MMR)-deficient tumor cell lines that show increased sensitivity to this agent (Mohindra et al., 2002). After a 24-h treatment with up to 20 mM thymidine (which is as much as 100-fold greater than the dose of this agent required to reduce plating efficiency of these cell lines to 10% or D10), there was no significant increase in the frequency of apoptotic cells as measured by the Annexin V assay (Table 1 and Figure 1A). After a 4-d exposure of one of these lines (HCT116) to 2 mM thymidine, a slight increase in the frequency of Annexin V+ cells or cells with a sub-G1 DNA content was detected (Figure 1B). Thymidine also failed to induce apoptosis in the thymidine-resistant, MMR-proficient colon cancer cell line SW480 after 24- or 48-h treatments (Table 1 and Figure 1C). Thus, thymidine is a poor inducer of apoptosis in all types of cells tested even at concentrations that substantially reduce colony formation.
Table 1.
MMR status, P53 status, and induction of apoptosis by TdR or CPT in several cancer cell lines
| Apoptosisa
|
|||||||
|---|---|---|---|---|---|---|---|
| Cell line | MMR status | P53 status | Control | TdR 2 mM | TdR 20 mM | CPT 20 nM | CPT 1 μM |
| SW480 | Proficient | Mutant | 1.9 ± 1.7 | 1.3 ± 0.7 | 2.7 ± 0.25 | 1.2 ± 0.6 | 4.7 ± 2.9 |
| HCT 116 | hMLH1− | Wild type | 3.5 ± 1.9 | 2.9 ± 0.8 | 5.0 ± 0.2 | 3.5 ± 0.6 | 33.6 ± 1.4 |
| RKO | hMLH1− | Wild type | 2.9 ± 1.0 | 5.8 ± 3.0 | 3.6 | 2.7 | 36.7 ± 7.2 |
| DLD-1 | hMSH6− | Mutant | 1.8 ± 0.5 | 2.8 ± 0.6 | 2.8 | 3.2 ± 0.5 | 3.8 |
| SKUT-1 | hMSH2− | Mutant | 1.4 | 1.4 | 1.4 | 1.6 | 1.6 |
| HEC-A1 | hPMS2− | ND | 5.3 | 6.0 | 5.3 | 4.8 | 9.7 |
ND, not determined.
Percentage of Annexin V+/PI− cells (media and SD) after 24-h treatment with the indicated agents.
Figure 1.
Effects of TdR or CPT on the induction of apoptosis. (A) HCT 116 cells were treated with the indicated concentrations of thymidine or CPT for 24 h and then analyzed for the binding of Annexin V/PI (percentages of Annexin V+/PI- cells are represented). (B-D) HCT 116 (B), SW480 (C), or HCT 116 + 3 cells (D) were treated with the indicated agents for 1 to 4 d, and the induction of apoptosis was analyzed by Annexin V/PI binding.
We compared the response of these cell lines to thymidine with that induced by CPT, which induces DSBs at DNA replication forks (Avemann et al., 1988). As reported previously (Han et al., 2002), 20 nM CPT (a concentration that is about 3-fold higher than the D10 for CPT) does not increase the frequency of Annexin V+ cells in HCT116 (Figure 1A) or in the other tumor cell lines tested (Table 1). However, high concentrations (>500 nM) produce a robust apoptotic response in HCT116 cells within 24 h and by 96 h, 70-85% are apoptotic (Figure 1B). Another MMR-deficient colon cancer cell line (RKO, which like HCT116 is deficient in hMLH1) shows a similar apoptotic response (Table 1). However, not all thymidine-sensitive MMR-deficient tumor cell lines respond in the same manner because several fail to induce apoptosis within a 24-h treatment with high concentrations of CPT (Table 1). This induction of apoptosis in the hMLH1-deficient tumor cells is not the consequence of the hMLH1 deficiency as an HCT116 derivative corrected for the MMR defect (Koi et al., 1994) retained the rapid apoptotic response after treatment with high levels of CPT (Figure 1D). About 40% of SW480 cells undergo apoptosis after a 2-d treatment with 1 μM CPT (Figure 1C).
Chk1 Depletion Ablates Thymidine Block by Committing S-Phase Cells to Apoptosis
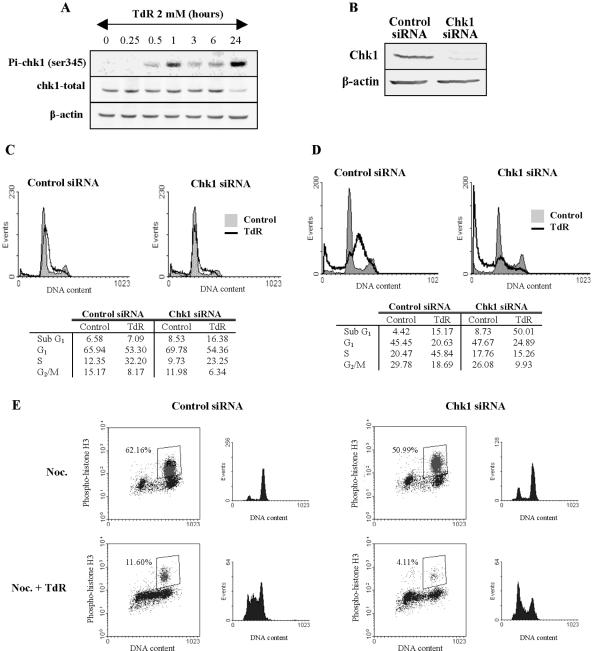
Considering recent reports that Chk1 ablation can affect the induction of cell death in response to DNA damage either positively or negatively (Urist et al., 2004; Cho et al., 2005; Xiao et al., 2005), we next determined the role of Chk1 in the cellular response to thymidine. Chk1 phosphorylation is detectable 30 min after thymidine treatment of HCT116, and the level of phospho-Chk1 (ser345) seems strongest in these cells at 1 and 24 h posttreatment (Figure 2A). The level of the Chk1 protein is maintained through 6 h of treatment, although it begins to decrease by 24 h. To examine the effect of Chk1 depletion, HCT116 cells were exposed to Chk1 siRNA or a control siRNA preparation for 24 h before treatment with 2 mM thymidine for a further 24-48 h. We first confirmed that treatment with the Chk1 siRNA effectively reduced the level of the Chk1 protein in HCT116 cells by Western blotting (Figure 2B). This depletion of Chk1 had only a small effect upon the accumulation of cells with a sub-G1 DNA content or the distribution of cells in other phases of the cell cycle after 24 or 48 h relative to cells treated with the control siRNA (Figure 2, C and D). Thymidine-treated cells exposed to the control siRNA showed the distinctive accumulation of S-phase cells after 24 or 48 h. In striking contrast, cells treated with the Chk1 siRNA did not accumulate in S or G2 phases at either 24 or 48 h (Figure 2, C and D). Instead, a higher level of cells with a sub-G1 DNA content was evident at 24 h (Figure 2C) and up to 50% of the cells had a sub-G1 DNA content at 48 h (Figure 2D).
Figure 2.
Chk1 depletion ablates TdR block by committing S-phase cells to apoptosis. (A) Appearance of phosho-Chk1 (ser345) and total Chk1 levels of HCT 116 cells treated with 2 mM thymidine for the indicated times. β-Actin levels in the extracts are included as loading controls. (B) Chk1 levels 24 h after the transfection of indicated siRNAs in HCT116 cells. β-Actin levels are presented as a loading control. (C and D) Effect of siRNA transfection on cell cycle distribution of HCT116 cells treated with 2 mM thymidine for 24 (C) or 48 (D) hours. (E) HCT 116 siRNA-transfected cells were treated with 2 mM thymidine for 48 h and with 0.2 μg/ml nocodazole (Noc) for the last 20 h of this period to trap cycling cells in mitosis. Cell were then harvested, fixed, and analyzed by flow cytometry for the level of phospho-Histone H3 (ser10) and DNA content simultaneously. Separated cell cycle profiles for each condition were also displayed in the panels on right.
It was recently reported that Chk1-depleted cells treated with the metabolic inhibitor 5-fluorouracil died in M phase after abrogation of an S-phase checkpoint by Chk1 siRNA (Xiao et al., 2005). To determine whether Chk1 depletion eliminated thymidine-triggered S-phase arrest, we examined the phosphorylation of histone H3, a specific M-phase marker, after thymidine treatment of Chk1-depleted cells. Control or Chk1-depleted HCT116 cells were treated with 2 mM thymidine for 48 h or left untreated as described above. In addition nocodazole was added to these cultures for the last 20 h of the experiment to trap cells in mitosis. Phosphohistone H3 levels in harvested cells were measured by flow cytometry. Control or Chk1-depleted cells that were not exposed to thymidine accumulated in G2/M and 62% of the control and 51% of Chk1 siRNA-treated cells showed phospho-histone H3 staining (Figure 2E). In contrast, parallel cultures treated with thymidine showed a reduced accumulation of cells in G2 and only 12% of cells treated with the control and 4% of cells treated with the Chk1 siRNAs showed phospho-histone H3 staining. Thus, Chk1-depleted cells treated with thymidine do not seem to die of mitotic catastrophe as they do not reach mitosis in the presence or absence of Chk1.
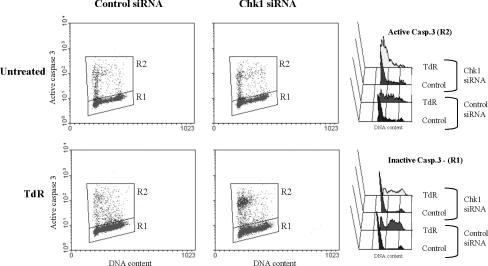
Given the loss of S-phase cells after thymidine treatment of Chk1-depleted cells, we next determined whether death was induced in S-phase cells. To accomplish this, we measured levels of activated caspase-3 in thymidine-treated cells together with the DNA content (Figure 3). After treatment with 2 mM thymidine activated caspase-3 was found in control cells throughout the cell cycle. In contrast in Chk1-depleted cultures, cells containing activated caspase-3 had a DNA content characteristic of cells in early S phase. Similar results were obtained when we measured the DNA content of cells with low or high mitochondrial potential in control or Chk1-depleted cells treated with thymidine (Supplemental Figure 1). The DNA content of Chk1-depleted cells with a low mitochondrial membrane potential (characteristic of apoptotic cells; Sun et al., 1999; Rasola and Geuna, 2001) was again characteristic of cells in early S phase with relatively few showing a late S/G2 DNA content. Those treated with the control siRNA were more evenly distributed over the cell cycle. Thus, Chk1 depletion seems to result in the induction of apoptosis in thymidine-treated cells early in S phase.
Figure 3.
Thymidine treatment of Chk1-depleted cells induces apoptosis predominantly in cells in early S phase. HCT 116 cells were transfected with control or Chk1 siRNA and treated or not with 2 mM TdR for 48 h. Cells were then analyzed for caspase-3 activation and DNA content simultaneously. Cells presenting inactive and active forms of caspase-3 are gated and computed (R1 and R2 gates, respectively) in the cytogram panels on the left, and their corresponding cell cycle profiles are depicted in the panels on the right.
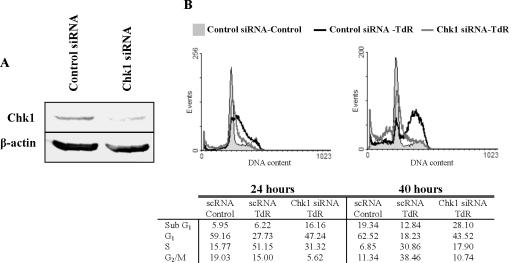
This induction of death by thymidine after Chk1 depletion is not restricted to the thymidine-sensitive HCT116 cells. When thymidine-resistant SW480 cells depleted of Chk1 (Figure 4A) were treated with 2 mM thymidine, a similar loss of S-phase cells and accumulation of cells with a sub-G1 DNA content was observed (Figure 4B).
Figure 4.
Effect of Chk1 silencing on cell cycle distribution of SW480 cells treated with thymidine. (A) Chk1 protein level 24 h after the transfection of indicated siRNAs. β-Actin levels are presented as a loading control. (B) Cell cycle analysis of SW480 cells transfected with control or Chk1 siRNAs and treated with 2 mM TdR for 24 or 40 h.
Chk1 Depletion Induces Death in Cells Treated with Other DNA Synthesis Inhibitors
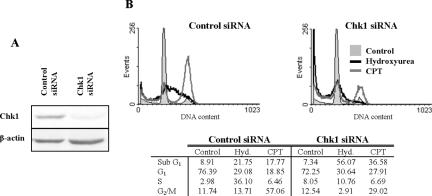
We next determined whether the induction of death after Chk1 silencing occurred in cells treated with other DNA synthesis inhibitors. Chk1-depleted HCT116 cultures (Figure 5A) treated for 48 h with 2 mM HU (which is ∼4-fold higher than the D10 for HU) showed a similar loss of cells in S phase, whereas those treated with 20 nM CPT failed to show an accumulation of cells in G2. In both types of cultures, a corresponding increase in the fraction of cells with a sub-G1 DNA content was observed (Figure 5B). Thus, Chk1 seems to be essential for the prevention of cell death in response to multiple agents that affect DNA synthesis.
Figure 5.
Induction of apoptosis in Chk1- depleted HCT116 cells treated with hydroxyurea or CPT. (A) Western blot analysis of Chk1 in extracts of HCT116 cells treated with control or Chk1 siRNAs for 24 h. (B) Cell cycle distribution of siRNA-treated HCT116 cells exposed to hydroxyurea or 20 nM CPT for 48 h.
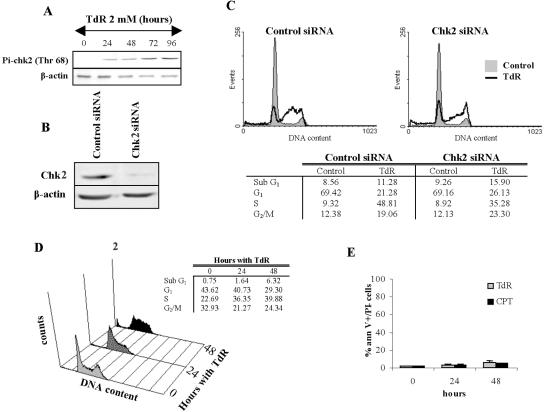
Chk2-deficient Cells Do Not Undergo Apoptosis after Thymidine or CPT Treatment
Chk2 is rapidly activated after thymidine treatment through an ATM-dependent phosphorylation of Thr68 (Bolderson et al., 2004). Chk2 has also been shown to be required for the induction of cell death after treatment with some types of DNA-damaging agents (Hirao et al., 2002; Stevens et al., 2003; Urist et al., 2004). Chk2 phosphorylation occurs in HCT116 cells within 24 h of thymidine treatment (Figure 6A). To investigate the role of Chk2 in the induction of apoptosis by thymidine, we depleted HCT116 cells of Chk2 by siRNA treatment (Figure 6B). Unlike cells depleted of Chk1, Chk2-depleted cells accumulated in S phase after thymidine treatment and had no significant increase in the frequency of cells with a sub-G1 DNA content (Figure 6C). We further investigated the role of Chk2 by determining the effect of thymidine on DLD-1 cells which (like the colon cancer cell line HCT15) carry the R145W and A247D mutations of Chk2 that compromise the stability of the protein and its ability to respond to DNA damage (Falck et al., 2001; Lee et al., 2001; Bolderson, Phear, and Meuth, unpublished data). DLD-1 cells do not undergo apoptosis after thymidine or CPT treatment (Table 1). Exposure of DLD-1 cells to 2 mM thymidine for 24 h produced the distinctive accumulation of cells in S phase and very low levels of apoptosis (as indicated by cells with a sub-G1 DNA content; Figure 6D). Measurement of Annexin V+ cells in cultures treated with 2 mM thymidine for 48 h confirmed this result (Figure 6E). Even after a 4-d exposure to 10 mM thymidine, the fraction of apoptotic cells was <10% as measured by flow cytometry (cells with a sub-G1 DNA content) and Annexin V assays (our unpublished data).
Figure 6.
Cells deficient in Chk2 do not undergo apoptosis after thymidine treatment. (A) Appearance of phosho-Chk2 (Thr68) in HCT 116 cells treated with 2 mM TdR for the indicated times. β-Actin levels in the extracts are included as loading controls. (B) Western blot analysis of Chk2 in extracts of HCT116 cells treated with control or Chk2 siRNAs for 24 h. (C) Effect of siRNA transfection on cell cycle distribution of HCT116 cells treated with 2 mM thymidine for 48 h. (D) DLD-1 cells were treated during the indicated periods of time with 2 mM thymidine and analyzed for cell cycle distribution. Cell cycle profiles and percentage of cells in each phase of a representative experiment are shown. (E) DLD-1 cells were treated for 24 or 48 h with 2 mM thymidine or 20 nM CPT and then analyzed for the binding of Annexin V/PI (percentages of Annexin V+/PI- cells are represented).
To determine whether loss of both Chk1 and Chk2 function enhanced the apoptotic effect of thymidine, we depleted DLD-1 cells of Chk1 by siRNA treatment. Like HCT116 and SW480, DLD-1 cells depleted of Chk1 showed an increase in the level cells with a sub-G1 DNA content after a 48-h treatment with thymidine relative to cells exposed to the control siRNA (our unpublished data). The increase in sub-G1 cells after Chk1 depletion of DLD-1 was similar to that seen in the Chk2-proficient cell lines. Thus, loss of both checkpoint kinases did not further enhance the apoptotic effects of thymidine.
Induction of Cell Death by Thymidine in Chk1-depleted Cells Is Independent of p53 but Is Enhanced in p21-deficient Cells
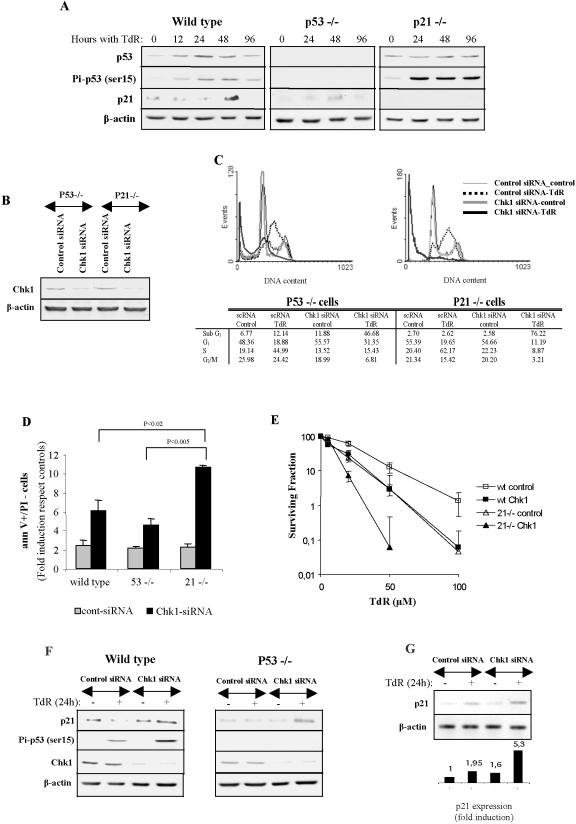
We next determined whether loss of p53 or p21 affected the induction of apoptosis in Chk1-depleted cells. Western blot analysis of extracts prepared from HCT116 cells treated with thymidine revealed that both the level of p53 and phosphorylation at ser15 increased within 12 h of thymidine treatment. The level of p21 seemed to decrease in the first 24 h but was then higher at 48 h posttreatment (Figure 7A). p53-/- HCT116 cells showed no induction of p53 after thymidine treatment (Figure 7A). However, a very low level of p21 was detectable in these cells at 48 h after treatment. Similar results have previously been reported for CPT-treated HCT116 cells (Han et al., 2002). In the p21-/- cells, an induction of p53 together with a robust phosphorylation of p53 at ser15 was evident, but no p21 was detected (Figure 7A).
Figure 7.
Effect of Chk1 silencing on p53-/- and p21-/- cells. (A) Western blot analysis of p53, phospho-p53 (ser15), and p21 in wild-type, p53-/-, and p21-/- HCT116 cells treated with 2 mM thymidine for the indicated times. (B) Chk1 and β-actin protein levels 24 h after transfection of control or Chk1 siRNAs. (C) Cell cycle analysis of siRNA-transfected HCT116 p53-/- or p21-/- cells treated with 2 mM thymidine for 48 h. (D) Induction of apoptosis (as measured by Annexin V binding) in wild-type, p53-/-, and p21-/- HCT116 cells transfected with control or Chk1 siRNAs after 48 h treatment with 2 mM thymidine. Values represent the increase in the percentage of apoptotic cells in thymidine treated cells relative to cells not exposed to thymidine. (E) Survival of wild-type or p21-/- HCT116 cells treated with control or Chk1 siRNAs in increasing concentrations of thymidine in a colony-forming assay. (F) Western blot analysis of p21, phospho-p53 (ser15), chk1, and β-actin in wild-type (left) or p53-/- HCT 116 cells (right) transfected with control or Chk1 siRNAs and treated or not with 2 mM thymidine for 24 h. (G) Chk1-depleted cells treated with thymidine have an increased level of the p21 transcript. p21 mRNA levels were determined by RT-PCR analysis as described in Materials and Methods in HCT 116 cells transfected with control or Chk1 siRNAs and treated with thymidine as described above. p21 expression was quantified and expressed as fold induction relative to the control. β-Actin was used to normalize the amount of loaded RNA.
Control and Chk1 depleted cultures of the p53-/- and p21-/- derivatives of HCT116 were obtained after a 24-h siRNA treatment as described above (Figure 7B). After exposure to thymidine for 48 h, cells were fixed and analyzed by flow cytometry. p53-/- HCT116 cells responded similarly to the parental strain with respect to the depletion of S-phase cells after thymidine treatment and the accumulation of cells with a sub-G1 DNA content (Figure 7C). In contrast, p21-/- cells showed a further significant increase in the fraction of cells with a sub-G1 DNA content (Figure 8C) and in Annexin V+ cells (Figure 7D). This was accompanied by a decrease in the fraction of cells in G1 as well as S and G2. We confirmed this result using HCT116 cells depleted of p21 and Chk1 by siRNA treatment (Supplemental Figure 2). Thus, loss of p21 function enhances the induction of cell death after Chk1 depletion in thymidine-treated cells. This effect was not evident in thymidine-treated p21-/- cells transfected with the control siRNA (Figure 7D). The importance of p21 function for survival after thymidine treatment was further demonstrated in colony-forming assays (Figure 7E). HCT116 cells deficient in p21 had a significantly reduced survival in thymidine, and this was further depressed in cells treated with the Chk1 siRNA.
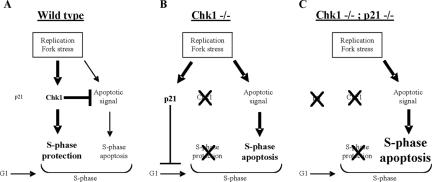
Figure 8.
Role of Chk1 and p21 in prevention of cell death after replication fork stress. (A) DNA synthesis inhibitors activate Chk1 that protects replication forks and prevents S-phase apoptosis. (B) The lack of Chk1-induced protection favors the induction of apoptosis in the S phase. In this case, p21 activation triggers an alternative protective mechanism by preventing cells from entering S phase. (C) Depletion of both Chk1 and p21 eliminates the protection of G1 phase cells and results in apoptosis of virtually the entire cell population.
Although these results suggested that p21 cooperates with Chk1 to protect cells from apoptosis after replication fork stress induced by thymidine, p21 is normally induced only late after thymidine treatment. Therefore, we next determined whether p21 was more rapidly induced in Chk1-depleted cells. Cell extracts were prepared from cultures treated with control or Chk1 siRNAs for 24 h in the presence or absence of thymidine and analyzed by Western blotting. This revealed that p21 levels were elevated by 24 h in cultures treated with the Chk1 siRNA and thymidine, whereas those treated with the control siRNA seemed to have a slightly lower level of p21 after the 24-h thymidine treatment (Figure 7F). Phospho-p53 (ser15) levels were also slightly elevated after thymidine treatment of Chk1-depleted cells. In p53-/-, cells an induction of p21 was still evident in thymidine-treated cells depleted of Chk1 (Figure 7F). The increase in p21 level was accompanied by an increase in the level of the p21 transcript (Figure 7G) but does not seem to be the result of stabilization caused by phosphorylation of the protein (our unpublished data). Thus, p21 is elevated at earlier times after thymidine treatment of Chk1-depleted cells, but this response is not dependent upon the p53-mediated DNA damage response.
DISCUSSION
In fission and budding yeast, S-phase checkpoints are required to maintain the integrity of stalled replication forks and to suppress futile origin firing. These checkpoints depend upon the Chk2 homologues Cds1 and Rad53 (Santocanale and Diffley, 1998; Kim and Huberman, 2001). Recent reports provide evidence that Chk1 fulfills this role in vertebrate cells (Feijoo et al., 2001; Zachos et al., 2003; Syljuasen et al., 2005). Furthermore depletion or inhibition of Chk1 in mammalian cells triggers a rapid phosphorylation of ATR targets, induction of H2AX foci, and an accumulation of DNA breaks (Syljuasen et al., 2005). Despite these effects, our findings, together with other recent reports (Zachos et al., 2003; Cho et al., 2005; Xiao et al., 2005), show that the induction of apoptosis in Chk1-depleted cells occurs only when replication forks in such cells are impeded by excess thymidine or other replication inhibitors (see model in Figure 8). Several observations support our conclusion that apoptosis occurs in early S phase in thymidine-treated cells depleted of Chk1: 1) S-phase cells are specifically lost in these cultures, 2) caspase-3 induction (an early marker of apoptosis) and loss of mitochondrial membrane potential occur predominantly in Chk1-depleted cells with an early S-phase DNA content after thymidine treatment, and 3) no phospho-histone H3 is evident in these cells. It is unclear why the disruption of replication fork progression in Chk1-depleted cells should serve as a signal for apoptosis. It seems unlikely that the inappropriate firing of replication origins caused by Chk1 depletion per se is responsible. Initiation and unwinding of DNA by the replicative DNA helicase are not dependent upon DNA replication (Pacek and Walter, 2004) and therefore are likely to occur in the presence or absence of the inhibitors. Interestingly, the uncoupling of unwinding of DNA from elongation has been shown to amplify Chk1 phosphorylation (Byun et al., 2005). We speculate that such uncoupling caused by the replication inhibitors at origins inappropriately fired in the absence of Chk1 may amplify a signal that triggers apoptosis. It is also possible that the loss of HRR, resulting from Chk1 depletion, may cause further accumulation of such lesions. However, loss of RAD51 homologues that are required for this repair does not increase the level of apoptosis after treatment with low levels of CPT (Hinz et al., 2003) or thymidine (Rodriguez and Meuth, unpublished data).
Although Chk1 seems to be the primary determinant for protection of cells suffering replication fork stress from the induction of apoptosis, p21 also plays an antiapoptotic role in Chk1-depleted cells (see model in Figure 8). Normally, p21 is down-regulated at early times after thymidine treatment; however, p21 levels are markedly increased in Chk1- depleted cells. In p21-/- cells Chk1 depletion significantly increased the fraction of apoptotic cells and reduced colony formation in the presence of thymidine relative to p21+/+ cells. We propose that p21 retards cell death in the absence of Chk1 through its role as a cyclin-dependent kinase (CDK) inhibitor by slowing S-phase entry where cell death is induced. Consistent with this proposal is our observation that G1 cells are retained after thymidine treatment of Chk1- depleted p21+/+ cells, but these cells are lost together with the S and G2 cells in the p21-/- cells. Previous reports have indicated that p21 may play either a pro- or antiapoptotic role depending on the agent and the cells used (Gorospe et al., 1997; Geller et al., 2004). In the response to thymidine (as well as other replication inhibitors tested such as HU or CPT), p21 has little effect on the apoptotic response until cells are depleted of Chk1.
Both Chk1 and Chk2 have been shown to be required for the induction of apoptosis after various forms of DNA damage, including that induced by CPT (Urist et al., 2004). Tissues and cells derived from Chk2-deficient mice are defective in apoptosis induced by IR as an apparent result of the suppression of p53 activation after DNA damage in Chk2- deficient cells (Hirao et al., 2002; Jack et al., 2002; Takai et al., 2002). It has also been shown that Chk1 and Chk2 promote apoptosis through the stabilization of E2F-1 that, in turn, induces p73 (Stevens et al., 2003; Urist et al., 2004). The antiapoptotic role for Chk1 and lack of Chk2 involvement in the response to replication fork inhibitors found here would seem to contradict these reports. However, much higher levels of some replication fork inhibitors were used to demonstrate the proapoptotic role of Chk1. For example, Urist et al. (2004)used 300 nM CPT to demonstrate the requirement for Chk1 in the induction of apoptosis (Urist et al., 2004), whereas 20 nM CPT was used in our work. Thus, we propose that Chk1 may play an antiapoptotic role in response to weaker replication fork stresses (like that obtained after treatment with low concentrations of CPT or thymidine), whereas more catastrophic damage (such as DSB accumulation that occurs in these higher levels of CPT) may lead Chk1 to activate apoptosis via the p53 and/or E2F-1/p73 pathways.
Although relatively little is known about the role of other proteins responding to DNA replication fork stress in the induction of cell death, a recent report has shown that cells with defects of the BLM helicase (which is defective in patients with the genome instability disorder Bloom syndrome; BS) also rapidly induce apoptosis after DNA replication fork damage (Davalos and Campisi, 2003). In contrast to the effect of Chk1 depletion, apoptosis induced by these agents in immortalized BS fibroblasts is p53 dependent. Previous reports have suggested that loss of repair pathways may also protect cells from the induction of apoptosis. MMR repair-deficient cells are protected from the induction of apoptosis by DNA alkylating agents (Toft et al., 1999; Zhang et al., 1999) and hamster cells defective in the Rad51 paralog XRCC3 have a reduced apoptotic response after treatment with CPT (Hinz et al., 2003). Studies of the interaction of these repair pathways with Chk1 in the induction of apoptosis after DNA replication stress are in progress.
The observations presented here may also have clinical significance. The use of Chk1 inhibitors in cancer chemotherapy has been widely proposed (Hapke et al., 2001; Li and Zhu, 2002; Zhou and Sausville, 2003). However, the recent report of extensive cell death in adult cells deficient in Chk1 has presented some obstacles to such a strategy (Lam et al., 2004). We show that Chk1 depletion can convert an agent that is a poor inducer of apoptosis into an effective inducer of cell death. Furthermore, Chk1 depletion increases the efficacy of clinically relevant inhibitors of replication (such as CPT). The acute sensitivity of MMR-deficient tumor cells to a combined therapy of thymidine and/or low levels of CPT in combination with Chk1 inhibitors might provide a very targeted therapy for this subset of tumors. Such therapies employing Chk1 inhibitors could be further enhanced by inhibition of p21 function. Because the death pathway induced by this combination does not depend on a functional p53, this strategy may also be useful for the treatment of tumors carrying mutations of p53.
Supplementary Material
Acknowledgments
We are grateful to Thomas Helleday for comments on the manuscript. This work was supported by a program grant to M. M. from Yorkshire Cancer Research.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-07-0594) on November 9, 2005.
Abbreviations used: ATM, ataxia-telangiectasia mutated; ATR, ATM- and Rad3-related; CPT, camptothecin; DSB, double-strand break; HRR, homologous recombination repair; HU, hydroxyurea; IR, ionizing radiation; MMR, mismatch repair; RPA, replication protein A.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Avemann, K., Knippers, R., Koller, T., and Sogo, J. M. (1988). Camptothecin, a specific inhibitor of type I DNA topoisomerase, induces DNA breakage at replication forks. Mol. Cell. Biol. 8, 3026-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi, V., Pontis, E., and Reichard, P. (1986). Changes of deoxyribonucleoside triphosphate pools induced by hydroxyurea and their relation to DNA synthesis. J. Biol. Chem. 261, 16037-16042. [PubMed] [Google Scholar]
- Bjursell, G., and Reichard, P. (1973). Effects of thymidine on deoxyribonucleoside triphosphate pools and deoxyribonucleic acid synthesis in Chinese hamster ovary cells. J. Biol. Chem. 248, 3904-3909. [PubMed] [Google Scholar]
- Bolderson, E., Scorah, J., Helleday, T., Smythe, C., and Meuth, M. (2004). ATM is required for the cellular response to thymidine induced replication fork stress. Hum. Mol. Genet. 13, 2937-2945. [DOI] [PubMed] [Google Scholar]
- Byun, T. S., Pacek, M., Yee, M. C., Walter, J. C., and Cimprich, K. A. (2005). Functional uncoupling of MCM helicase and DNA polymerase activities activates the ATR-dependent checkpoint. Genes Dev. 19, 1040-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha, R. S., and Kleckner, N. (2002). ATR homolog Mec1 promotes fork progression, thus averting breaks in replication slow zones. Science 297, 602-606. [DOI] [PubMed] [Google Scholar]
- Cho, S. H., Toouli, C. D., Fujii, G. H., Crain, C., and Parry, D. (2005). Chk1 is essential for tumor cell viability following activation of the replication checkpoint. Cell Cycle 4, 131-139. [DOI] [PubMed] [Google Scholar]
- Davalos, A. R., and Campisi, J. (2003). Bloom syndrome cells undergo p53-dependent apoptosis and delated assembly of BRCA1 and NBS1 repair complexes at stalled replication forks. J. Cell Biol. 162, 1197-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson, S., Thelander, L., and Akeerman, M. (1979). Allosteric regulation of calf thymus ribonucleoside diphosphate reductase. Biochemistry 18, 2948-2952. [DOI] [PubMed] [Google Scholar]
- Falck, J., Malland, N., Syljuasen, R. G., Bartek, J., and Lukas, J. (2001). The ATM-Chk2-Cdc25A checkpoint pathway guards against radioresistant DNA synthesis. Nature 410, 842-847. [DOI] [PubMed] [Google Scholar]
- Falck, J., Petrini, J. H., Williams, B. R., Lukas, J., and Bartek, J. (2002). The DNA damage-dependent intra-S phase checkpoint is regulated by parallel pathways. Nat. Genet. 30, 290-294. [DOI] [PubMed] [Google Scholar]
- Feijoo, C., Hall-Jackson, C., Wu, R., Jenkins, D., Leitch, J., Gilbert, D. M., and Smythe, C. (2001). Activation of mammalian Chk1 during DNA replication arrest: a role for Chk1 in the intra-S phase checkpoint monitoring replication origin firing. J. Cell Biol. 154, 913-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funari, B., Rhind, N., and Russell, P. (1997). Cdc25 mitotic inducer targeted by chk1 DNA damage checkpoint kinase. Science 277, 1495-1497. [DOI] [PubMed] [Google Scholar]
- Geller, J. I., Szekely-Szucs, K., Petak, I., Doyle, B., and Houghton, J. A. (2004). p21 (Cip1) is a critical mediator of the cytotoxic action of thymidylate synthase inhibitors in colorectal carcinoma cells. Cancer Res. 64, 6296-6303. [DOI] [PubMed] [Google Scholar]
- Gorospe, M., Cirielli, C., Wang, X., Seth, P., Capogrossi, M. C., and Holbrook, N. J. (1997). p21(Waf1/Cip1) protects against p53-mediated apoptosis of human melanoma cells. Oncogene 14, 929-935. [DOI] [PubMed] [Google Scholar]
- Han, Z., Wei, W., Dunaway, S., Darnowski, J. W., Calabresi, P., Sedivy, J., Hendrickson, E. A., Balan, K. V., Pantazis, P., and Wyche, J. H. (2002). Role of p21 in apoptosis and senescence of human colon cancer cells treated with camptothecin. J. Biol. Chem. 277, 17154-17160. [DOI] [PubMed] [Google Scholar]
- Hapke, G., Yin, M. B., and Rustum, Y. M. (2001). Targeting molecular signals in chk1 pathways as a new approach for overcoming drug resistance. Cancer Metastasis Rev. 20, 109-115. [DOI] [PubMed] [Google Scholar]
- Hinz, J. M., Helleday, T., and Meuth, M. (2003). Reduced apoptotic response to camptothecin in CHO cells deficient in XRCC3. Carcinogenesis 24, 249-253. [DOI] [PubMed] [Google Scholar]
- Hirao, A., et al. (2002). Chk2 is a tumor suppressor that regulates apoptosis in both an ataxia telangiectasia mutated (ATM)-dependent and an ATM-independent manner. Mol. Cell. Biol. 22, 6521-6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack, M. T., Woo, R. A., Hirao, A., Cheung, A., Mak, T. W., and Lee, P. W. (2002). Chk2 is dispensable for p53-mediated G1 arrest but is required for a latent p53-mediated apoptotic response. Proc. Natl. Acad. Sci. USA 99, 9825-9829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastan, M. B., and Bartek, J. (2004). Cell-cycle checkpoints and cancer. Nature 432, 316-323. [DOI] [PubMed] [Google Scholar]
- Kim, S. M., and Huberman, J. A. (2001). Regulation of replication timing in fission yeast. EMBO J. 20, 6115-6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koi, M., Umar, A., Chauhan, D. P., Cherian, S. P., Carethers, J. M., Kunkel, T. A., and Boland, C. R. (1994). Human chromosome 3 corrects mismatch repair deficiency and microsatellite instability and reduces N-methyl-N′-nitro-N-nitrosoguanidine tolerance in colon tumor cells with homozygous hMLH1 mutation. Cancer Res. 54, 4308-4312. [PubMed] [Google Scholar]
- Lam, M. H., Liu, Q., Elledge, S. J., and Rosen, J. M. (2004). Chk1 is haploin-sufficient for multiple functions critical to tumor suppression. Cancer Cell 6, 45-59. [DOI] [PubMed] [Google Scholar]
- Lee, S. B., et al. (2001). Destabilization of CHK2 by a missense mutation associated with Li-Fraumeni Syndrome. Cancer Res. 61, 8062-8067. [PubMed] [Google Scholar]
- Li, Q., and Zhu, G. D. (2002). Targeting serine/threonine protein kinase B/Akt and cell-cycle checkpoint kinases for treating cancer. Curr. Top. Med. Chem. 2, 939-971. [DOI] [PubMed] [Google Scholar]
- Lim, D. S., Kim, S. T., Xu, B., Maser, R. S., Lin, J., Petrini, J. H., and Kastan, M. B. (2000). ATM phosphorylates p95/nbs1 in an S-phase checkpoint pathway. Nature 404, 613-617. [DOI] [PubMed] [Google Scholar]
- Lundin, C., Erixon, K., Arnaudeau, C., Schultz, N., Jenssen, D., Meuth, M., and Helleday, T. (2002). Different roles for nonhomologous end joining and homologous recombination following replication arrest in mammalian cells. Mol. Cell. Biol. 22, 5869-5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohindra, A., Hays, L. E., Phillips, E. N., Preston, B. D., Helleday, T., and Meuth, M. (2002). Defects in homologous recombination repair in mismatch-repair-deficient tumour cell lines. Hum. Mol. Genet. 11, 2189-2200. [DOI] [PubMed] [Google Scholar]
- Pacek, M., and Walter, J. C. (2004). A requirement for MCM7 and Cdc45 in chromosome unwinding during eukaryotic DNA replication. EMBO J. 23, 3667-3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasola, A., and Geuna, M. (2001). A flow cytometry assay simultaneously detects independent apoptotic parameters. Cytometry 45, 151-157. [DOI] [PubMed] [Google Scholar]
- Sanchez, Y., Wong, C., Thoma, R. S., Richman, R., Wu, Z., Piwnica-Worms, H., and Elledge, S. J. (1997). Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science 277, 1497-1501. [DOI] [PubMed] [Google Scholar]
- Santocanale, C., and Diffley, J. F. (1998). A Mec-1- and Rad53-dependent checkpoint controls late-firing origins of DNA replication. Nature 395, 615-618. [DOI] [PubMed] [Google Scholar]
- Shiloh, Y. (2003). ATM and related protein kinases: safeguarding genome integrity. Nat. Rev. Cancer 3, 155-168. [DOI] [PubMed] [Google Scholar]
- Sorensen, C. S., Hansen, L. T., Dziegielewski, J., Syljuasen, R. G., Lundin, C., Bartek, J., and Helleday, T. (2005). The cell-cycle checkpoint kinase Chk1 is required for mammalian homologous recombination repair. Nat. Cell Biol. 7, 195-201. [DOI] [PubMed] [Google Scholar]
- Stevens, C., Smith, L., and La Thangue, N. B. (2003). Chk2 activates E2F-1 in response to DNA damage. Nat. Cell Biol. 5, 401-409. [DOI] [PubMed] [Google Scholar]
- Sun, X.-M., MacFarlane, M., Zhuang, J., Wolf, B. B., Green, D. R., and Cohen, G. M. (1999). Distinct caspase cascades are initiated in receptor-mediated and chemical-induced apoptosis. J. Biol. Chem. 274, 5053-5060. [DOI] [PubMed] [Google Scholar]
- Syljuasen, R. G., Sorensen, C. S., Hansen, L. T., Fugger, K., Lundin, C., Johansson, F., Helleday, T., Sehested, M., Lukas, J., and Bartek, J. (2005). Inhibition of Human Chk1 Causes Increased Initiation of DNA Replication, Phosphorylation of ATR Targets, and DNA Breakage. Mol. Cell. Biol. 25, 3553-3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai, H., et al. (2002). Chk2-deficient mice exhibit radioresistance and defective p53-mediated transcription. EMBO J. 21, 5195-5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft, N. J., Winton, D. J., Kelly, J., Howard, L. A., Dekker, M., te Riele, H., Arends, M. J., Wyllie, A. H., Margison, G. P., and Clarke, A. R. (1999). Msh2 status modulates both apoptosis and mutation frequency in the murine small intestine. Proc. Natl. Acad. Sci. USA 96, 3911-3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urist, M., Tanaka, T., Poyurovsky, M. V., and Prives, C. (2004). p73 induction after DNA damage is regulated by checkpoint kinases Chk1 and Chk2. Genes Dev. 18, 3041-3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, I. M., Minn, K., and Chen, J. (2004). UV-induced ataxia-telangiectasia-mutated and Rad3-related (ATR) activation requires replication stress. J. Biol. Chem. 279, 9677-9680. [DOI] [PubMed] [Google Scholar]
- Xiao, Z., Xue, J., Sowin, T. J., Rosenberg, S. H., and Zhang, H. (2005). A novel mechanism of checkpoint abrogation conferred by Chk1 downregulation. Oncogene 24, 1403-1411. [DOI] [PubMed] [Google Scholar]
- Zachos, G., Rainey, M. D., and Gillespie, D. A. (2003). Chk1-deficient tumour cells are viable but exhibit multiple checkpoint and survival defects. EMBO J. 22, 713-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H., Richards, B., Wilson, T., Lloyd, M., Cranston, A., Thorburn, A., Fishel, R., and Meuth, M. (1999). Apoptosis induced by overexpression of hMSH2 or hMLHM1. Cancer Res. 59, 3021-3027. [PubMed] [Google Scholar]
- Zhou, B. B., and Sausville, E. A. (2003). Drug discovery targeting Chk1 and Chk2 kinases. Prog. Cell Cycle Res. 5, 413-421. [PubMed] [Google Scholar]
- Zou, L., and Elledge, S. J. (2003). Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science 300, 1542-1548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.