Abstract
Neurons and glia are thought to arise from multipotent and self-renewing stem cells, which comprise the majority of neuroepithelial cells in the ventricular zone (VZ) of the early embryonic CNS. However, this idea remains to be tested rigorously, because CNS stem cells have been identified only by using in vitro assays, from which their abundance in vivo cannot be directly inferred. In the hematopoietic system, stem cells are characterized by using prospective isolation and direct in vivo transplantation. Here we have used this approach to ask whether most VZ progenitors behave as stem cells in vivo. The best-studied region of the embryonic CNS for addressing this problem is, arguably, the ventral spinal cord, within which progenitors in the motoneuron progenitor (pMN) domain sequentially generate motoneurons (MNs) and oligodendrocyte precursors (OPs). Virtually all VZ cells in pMN express the transcription factor Olig2. If most of these cells are stem cells, then they should maintain neurogenic potential, even at later, gliogenic stages. To test this hypothesis, we have prospectively isolated Olig2+ cells from murine embryonic day (E)9.5 and E13.5 spinal cord and directly transplanted them to E2 chick spinal cord. Transplanted E9.5 cells generate both neurons, including MNs and OPs, whereas E13.5 cells generate. The observation that most Olig2+ progenitors do not maintain neurogenic potential into the period of gliogenesis argues that they do not self-renew. These results do not support the commonly held view that most neuroepithelial cells in the embryonic CNS VZ are stem cells in vivo.
Keywords: CNS stem cells, neuroepithelial cells, in vivo transplantation
Stem cells are defined as undifferentiated progenitors that can self-renew and give rise to one or more differentiated derivatives. The multipotency and self-renewal of hematopoietic stem cells (HSCs) have been established by using direct in vivo transplantation of prospectively isolated progenitor cells (1). In contrast, the multipotency and self-renewal of stem cells in the CNS have been established primarily by using in vitro assays (reviewed in refs. 2 and 3). Such experiments have led to an operational definition of CNS stem cells (CNS-SCs) as self-renewing, clonogenic progenitors of neurons and glia (4). These cells can be passaged over many generations, in the presence of high concentrations of mitogens such as FGF-2, while retaining multipotency (5-7). Because these cells express markers, such as nestin (8, 9), that are expressed by most or all neuroepithelial cells in the embryonic ventricular zone (VZ), it has been inferred that most neuroepithelial cells (or, at later stages, radial glial cells) are multipotent stem cells in vivo (10-14). However, because evidence increasingly suggests that the culture conditions used to grow CNS progenitors in vitro may alter their developmental properties (15-17), it has become important to directly test this inference, without resorting to in vitro assays.
Here, we have asked whether most neuroepithelial cells are multipotent and self-renewing in vivo by direct transplantation of a population of candidate stem cells isolated from a well defined domain of the embryonic VZ. The best studied region of the embryonic CNS for addressing this questions is, arguably, the ventral spinal cord, where progenitors expressing the transcription factor Olig2 (18-20) sequentially generate motoneurons (MNs) and oligodendrocyte precursors (OPs) (reviewed in refs. 21 and 22). Single Olig2+ cells can form multipotent, self-renewing neurospheres (17) and, therefore, can behave as stem cells in vitro. Self-renewal of CNS-SCs is defined as the maintenance of neuronal and glial potential over multiple generations. According to this definition, if most Olig2+ progenitors self-renew in vivo, then they should retain neuronal potential, even at stages when they are fated to generate only OPs. But if Olig2+ cells do not maintain neuronal potential at later, gliogenic stages, then it follows that they do not self-renew.
To distinguish between these alternatives, we prospectively isolated Olig2+ cells at different stages and directly transplanted them into chick embryonic spinal cord, at a stage permissive for MN differentiation (Fig. 1A). Such a transplantation assay should reveal whether Olig2+ cells retain neuronal potential at a stage when they are fated to generate only OPs in vivo. We find that Olig2+ cells isolated at embryonic day 13.5 (E13.5) generate only glial derivatives after transplantation. By contrast, Olig2+ cells from E9.5 embryos readily generate MNs in the same assay. Thus, most Olig2+ cells do not maintain neuronal capacity and, therefore, do not self-renew. Because essentially all VZ cells in the MN progenitor domain at E9.5 express Olig2, these results argue against the commonly held view that most CNS neuroepithelial cells are stem cells in vivo.
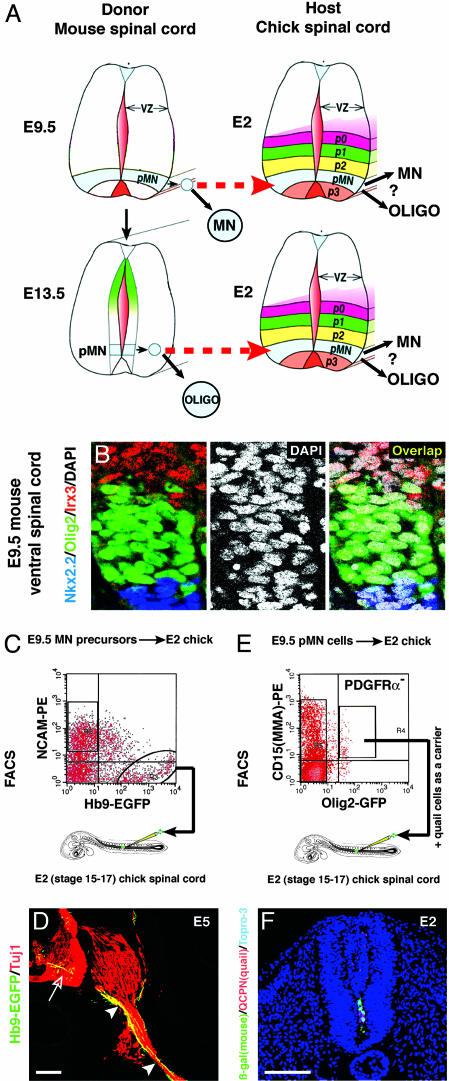
Fig. 1.
(A) Schematic, illustrating experimental design. Murine-equivalent isochronic (E9.5 Olig2+ cells → chick E2 spinal cord) or heterochronic (E13.5 Olig2+ cells → chick E2 spinal cord) transplants are shown. Modified from ref. 45. (B Left) The pMN domain, marked by Olig2 expression (green), is bounded dorsally by P2 (marked by Irx3, red) and ventrally by P3 (labeled by Nkx2.2, blue) (46). (Middle) All nuclei labeled by DAPI (white). (Right) Virtually all (>99.9%) cells in pMN (DAPI+, Irx3-, and Nkx2.2-) are Olig2+. (C) FACS-isolated Hb9-GFP+/NCAM- cells from E9.5 Hb9::EGFP embryonic mouse spinal cord were transplanted into chick E2 spinal cord. Negative selection for NCAM removed already-differentiated Hb9+ MNs. (D) Transplanted Hb9-GFP+ cells differentiate to MNs (open arrow) that project axons (labeled by anti-Tuj1 antibody, red, arrowheads) from the ventral root of the chick spinal cord. (E) FACS plots of Olig2+/CD15+/PDGFRα- cells at E9.5. The PDGFRα- subpopulation is not shown. (F) Location of murine E9.5 donor cells (β-gal+, green) and quail carrier cells (QCPN+, red) in chick spinal cord sectioned immediately after transplantation. Transplanted E13.5 donor cells were located at similar position (data not shown). Topro-3 (blue) stains all cell nuclei. (Scale bars, 100 μm.)
Results
Olig2-Expressing Cells Are Candidate Stem Cells in the Embryonic Spinal Cord. To investigate whether Olig2+ cells are candidate stem cells in the MN progenitor (pMN) domain, we first determined their abundance in the VZ and their expression of CNS-SC markers. At E9.5, when VZ cells in the pMN domain are generating MNs, the vast majority (>99.9%) of these cells in situ express Olig2, as determined by quantification of triply immunostained sections (Fig. 1B; 3,554 Olig2+ cells/3,558 DAPI+, Irx3-/low, Nkx2.2-/low cells, n = 2 embryos counted). The majority of these cells coexpress the CNS-SC markers Sox2 (23) (98 ± 1%), nestin (9) (92 ± 6%), and CD133 (24) (97 ± 2%), and the proliferation marker PCNA and lack expression of markers of differentiated neurons or OPs (NeuN or PDGFRα, respectively; see Fig. 6 and Table 2, which are published as supporting information on the PNAS web site). Similarly, at E13.5, when MN generation has ceased and OPs are being generated, Olig2+ cells comprise >99% (99.37 ± 0.37%, n = 1,068 cells counted in two embryos) of VZ cells between the domain bounded dorsally by Pax6 and that bounded ventrally by Nkx2.2. These Olig2+ cells coexpressed CNS-SC markers, such as Sox2, nestin, and RC2, a marker of radial glia (12, 13) (see Fig. 7 and Table 2, which are published as supporting information on the PNAS web site).
In the hematopoietic system, stem and progenitor cells are prospectively isolated by using surface markers. In contrast, Olig2 is a nuclear protein. However, Olig2+ cells can be isolated from murine embryos expressing GFP from the Olig2 locus (ref. 17 and B.G.N. and T.M.J., unpublished work). To further enrich for Olig2+ cells in the VZ, we isolated Olig2-GFP+ cells that coexpressed CD15/MMA or CD133 (Fig. 1E, Table 2), surface markers expressed by CNS-SCs (24, 25). CD15/MMA expression provided an additional ≈1.5-fold enrichment for VZ-derived Olig2+ cells (Figs. 6H and 7H). In addition, we selected against the OP surface marker PDGFRα (Figs. 6G and 7 G and P-R). By using Olig2-GFP, anti-PDGFRα, and CD15, ≈0.4% of E9.5 total spinal cord cells (Fig. 1E), and ≈0.2% of E13.5 spinal cord cells, were isolated (Fig. 7H). Immediately after sorting, the isolated cells were positive for Sox2, nestin, and CD133 and negative for NeuN (Table 2 and Fig. 6). Taken together, these data indicate that Olig2+ cells prospectively isolated from the pMN domain express markers characteristic of CNS-SCs and that these cells constitute the vast majority of VZ cells in the pMN domain at E9.5 and E13.5. Therefore, Olig2+ cells in the pMN domain would appear to be good candidates for stem cells, if such cells are, indeed, an abundant component of the VZ.
FACS-Isolated Suspensions of Murine MN Precursors Can Survive and Differentiate After Direct Transplantation into Chick Embryonic Spinal Cord. To examine the developmental potential of prospectively isolated Olig2+ cells, a suitable recipient for transplantation is required. Because transplantation into the E9.5 murine spinal cord is technically difficult, we used the E2 chick embryonic spinal cord as a more accessible host (Fig. 1 A). Transplanted embryoid bodies derived from murine ES cells have been shown to differentiate to MNs in this chick host (26). To determine whether this system can also be used for transplantation of MN precursor cells isolated directly from murine embryos by FACS, we first transplanted cells expressing GFP from the Hb9 promoter (26), which marks newly generated postmitotic MNs (27, 28). After incubation for 3 days to E5 (stage 27), mouse EGFP+ (Hb9+) neurons were observed in the ventral spinal cord, and many of these neurons projected axons out of the ventral roots (Fig. 1D). Thus, FACS-isolated murine MN precursors can differentiate into neurons when transplanted into the E2 chick spinal cord.
Prospectively Isolated E9.5 Olig2-GFP+ Cells Differentiate to both MNs and OPs After Direct Transplantation into Chick Embryonic Spinal Cord. We next asked whether E9.5 Olig2-GFP+ progenitors can differentiate to MNs in this transplantation assay. Because Olig2 expression is extinguished upon MN differentiation (20), to mark the progeny of transplanted Olig2-GFP+ cells, we crossed Olig2GFP/+ mice to Rosa26 mice, which carry a ubiquitously expressed lacZ transgene (29). For grafting, isolated Olig2+, PDGFRα-, CD15+ cells were mixed with carrier cells isolated from quail E2 ventral spinal cord (in a 1:4 ratio) and injected into the lumen of E2 (stage 11-12) chick spinal cord (Fig. 1E). To assess the number and distribution of transplanted cells, some host embryos were fixed and analyzed 2 h after injection. Cells expressing β-galactosidase or QCPN (a quail cell nuclei-specific antibody to mark quail carrier cells) were observed in the lumen of the spinal cord (Fig. 1F). Serial sectioning and counting of every section indicated that 120 ± 48 murine cells were injected per embryo (mean± SEM, n = 3 experiments).
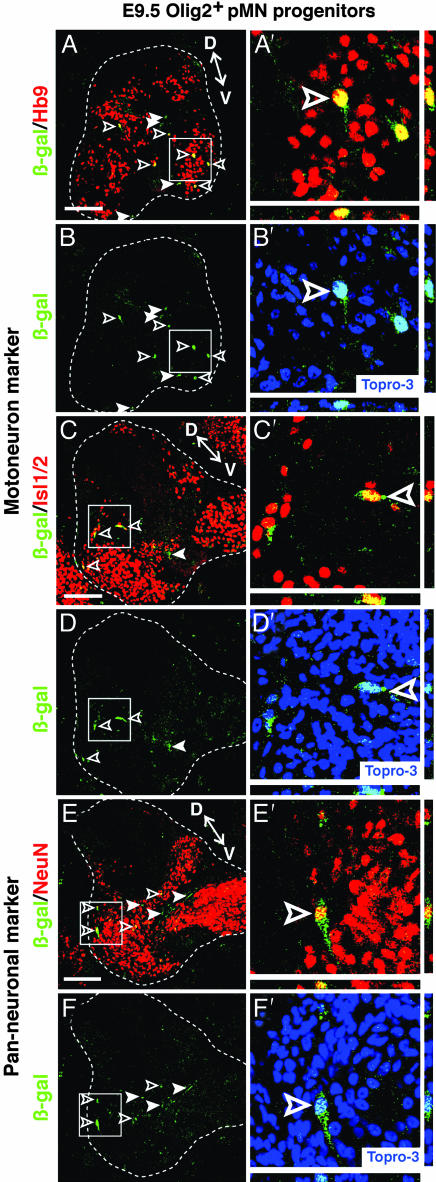
Analysis at E5 (3 days posttransplantation) with two different MN-specific nuclear markers, Hb9 (Fig. 2 A-B′) and Isl1/2 (Fig. 2 C-D′), revealed numerous β-gal+ cells that coexpressed these markers in the host spinal cord (Fig. 2 A′ and C′). β-gal+ cells also coexpressed the panneuronal markers NeuN (Fig. 2 E-F′) and Cyn-1 (30) (see Fig. 8 A-C′, which is published as supporting information on the PNAS web site). Quantification of three transplanted embryos sectioned in their entirety and triple-labeled for β-gal, Hb9, and Cyn-1 indicated that ≈35% of β-gal+ cells expressed the MN-specific marker Hb9 (Table 1). Virtually all of these Hb9+ cells coexpressed Cyn-1 (Table 1). An additional ≈15% of the β-gal+ cells were Cyn-1+ and Hb9- (Table 1). Thus, ≈50% of surviving transplanted cells differentiated to neurons, and >70% of these neurons expressed Hb9. The remaining 30% of neurons may represent interneurons or classes of MNs that do not express Hb9 (30). Qualitatively similar results were obtained in 37 chimeric embryos from 18 independent experiments.
Fig. 2.
Transplanted, uncultured E9.5 Olig2+/CD15+/PDGFRα- cells express MN-specific and panneuronal markers. (A/A′-D/D′) Triple labeling with anti-β-gal antibody (green), Topro-3 (blue) and the indicated markers (red) shows that transplanted murine donor cells (β-gal+, green) coexpress the MN markers Hb9. (A/A′-B/B') and Isl1/2 (C/C′-D/D′) and the panneuronal marker NeuN (E/E′-F/F′). Open arrowheads indicate coexpressing cells and white arrowheads indicate β-gal+ marker- cells. The boxed regions in (A-F) are magnified in (A′-F′), respectively. The nuclear marker Topro-3 (blue) is provided for reference in (B′, D′, and F′). Confirmation of colabeling by z-series analysis is shown below and to the right of the images. D, dorsal region; V, ventral region. See Fig. 4 for analysis of glial differentiation by E9.5 transplanted cells. (Scale bars, 100 μm.)
Table 1. Neuronal differentiation of transplanted Olig2+ cells.
| Cells per one embryo | E9.5 donor | E13.5 donor |
|---|---|---|
| Hb9+/Cyn-1+/β-gal+ cells | 34 ± 4 | 0 |
| Hb9−/Cyn-1+/β-gal+ cells | 14 ± 5 | 0 |
| Total β-gal+ cells | 97 ± 38* | 108 ± 20* |
Three operated embryos were fixed after 3 days of incubation (at E5), sectioned, and processed for triple labeling with anti-Hb9, anti-Cyn-1, and anti-β-gal antibodies. Transplanted cells were counted on all serial sections from each embryo. Data represent mean ± SEM per one embryo.
Difference not statistically significant (P = 0.55).
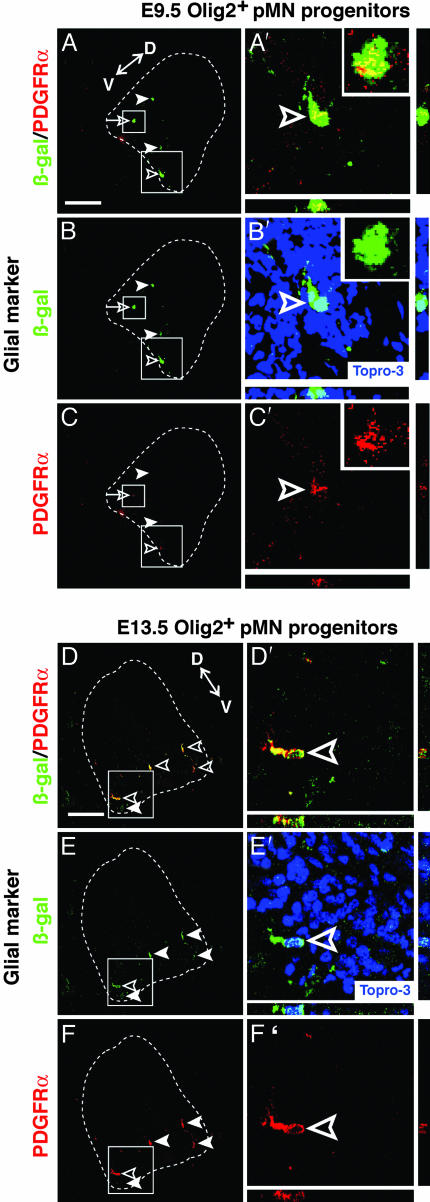
To investigate whether any E9.5 Olig2+ cells differentiated to glia, we incubated some transplanted embryos to E6, a stage when chick oligodendrocyte differentiation begins (31). Close to half of the β-gal+ cells (40 ± 8%, mean ± SD of four sections per embryo, n = 2 embryos) expressed the OP marker PDGFRα (Fig. 4 A-C′ Insets). Expression of GFAP was not detected at this stage (data not shown), consistent with the timing of astrocyte differentiation in vivo. Thus, freshly isolated E9.5 Olig2-GFP+/CD15+/PDGFRα- cells can generate both MNs and OPs after in vivo transplantation.
Fig. 4.
Transplanted, uncultured E9.5 and E13.5 Olig2+/CD15+/PDGFRα- cells generate glial cells. Triple labeling of chick embryos incubated to E5 or E6 with anti-β-gal antibody, Topro-3, and glial markers (red) show that some (≈40%) murine E9.5 donor cells (β-gal+, green) weakly express the OP marker PDGFRα (A/A′-C/C′). A higher percentage (≈70%) of E13.5 donor cells strongly express PDGFRα (D/D′-F/F′). The anti-PDGFRα antibody is murine-specific and therefore does not detect endogenous chick OPs. Topro-3 (blue) nuclear staining is shown for reference in (B′ and E′). Open arrowheads, coexpressing cells; arrowheads, β-gal single-positive cells. The boxed regions are magnified with z-series views to the right and below (A′-F′). (A′-C′ Insets) Higher magnification details of β-gal+/PDGFRα+ cells in the smaller boxed region (A-C, open arrows). D, dorsal region; V, ventral region. (Scale bar, 100 μm.)
E13.5 Olig2+ Cells Generate Glia but Not Neurons After Transplantation into a Neurogenic Environment. Having established that murine E9.5 Olig2+ cells transplanted into the chick spinal cord at a murine-equivalent isochronic stage can differentiate to both MNs and OPs, we next performed a heterochronic transplantation experiment. We grafted isolated E13.5 Olig2-GFP+, PDGFRα-, CD15+ cells (Fig. 7H) into chick hosts of the same age as used for transplantation of E9.5 cells (Fig. 1 A). Selection against PDGFRα+ cells was important at E13.5, because Olig2 is expressed by OPs at this stage (Fig. 7G and P-R). Quantification of β-gal+ cells 2 h after transplantation indicated that 107 ± 18 E13.5 cells per embryo were injected. A similar number of β-gal+ cells was detected after 3 days of incubation (108 ± 20 cells per embryo).
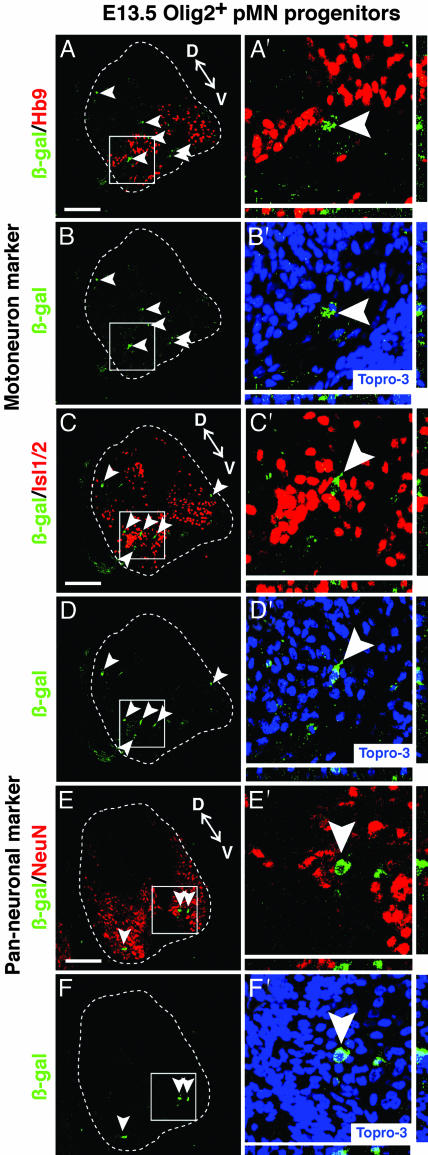
Strikingly, unlike the E9.5 Olig2+ cells, transplanted E13.5 Olig2+ cells did not generate any Hb9+ MNs (Fig. 3 A′ and C′). Furthermore, none of these cells expressed panneuronal markers, such as NeuN or Cyn-1 (Fig. 3E′ and Fig. 8D′). Quantification of all β-gal+ cells on every section from each of three embryos sectioned in toto confirmed this result (Table 1). Qualitatively similar results were obtained in each of 29 chimeric embryos from 15 independent experiments. By extrapolation, therefore, not a single neuron was observed among ≈3,000 β-gal+ cells (assuming ≈100 β-gal+ cells per embryo). The failure of these cells to differentiate to neurons was striking, given their proximity to host cells expressing MN markers (Fig. 3 A′, C′, and E′, and Fig. 8D′, arrowheads).
Fig. 3.
Transplanted, uncultured E13.5 Olig2+/CD15+/PDGFRα- cells do not differentiate to neurons. Triple labeling with anti-β-gal antibody, Topro-3 (blue) and neuronal markers (red) shows that transplanted murine donor cells (β-gal+, green) do not coexpress either Hb9 (A/A′-B/B′) or Isl1/2 (C/C′-D/D′) or the panneuronal markers NeuN (E/E′-F/F′). Arrowheads indicate β-gal single-positive cells. The boxed regions are magnified in (A′-F′) with z-series views to the right and below. D, dorsal region; V, ventral region. (Scale bar, 100 μm.)
The robust neuronal differentiation obtained with E9.5 donors (50% of transplanted cells), coupled with the fact that similar numbers of E9.5 and E13.5 cells engrafted and survived (Table 1), suggested that we should have been able to detect neuronal differentiation by the transplanted E13.5 cells, even at an incidence ≈10-fold below that of E9.5 cells. The fact that we did not suggests that E13.5 Olig2+ VZ-derived progenitors lack the capacity to generate MNs or, indeed, any other class of neurons in this permissive host environment. This behavior was not due to the exclusion of CD15/MMA- cells from the Olig2-GFP+ fraction, as similar results were obtained by using Olig2-GFP+/PDGFRα- cells without additional surface marker selection. In contrast to the lack of neuronal differentiation, staining for glial markers revealed that a high proportion (70 ± 13%) of β-gal+ cells expressed PDGFRα (Fig. 4 D-F′). In addition, some β-gal+ cells exhibited weak expression of the astrocyte marker, GFAP (data not shown). These data suggest that by E13.5, Olig2+ cells possess glial, but not neuronal, differentiation potential.
To determine whether the lack of neuronal differentiation by transplanted E13.5 progenitors might reflect age-specific damage suffered during the isolation procedure, we isolated and transplanted E13.5 dorsal spinal cord VZ cells, which generate dorsal interneurons at this stage (32), using the same dissociation and sorting procedure, but selecting for Olig2-GFP-/CD15+ cells. Many of these transplanted cells differentiated into neurons expressing NeuN or Cyn-1 (see Fig. 9 A-D′, which is published as supporting information on the PNAS web site; 24.9 ± 7.3% of β-gal+ cells were Cyn-1+ per section, mean ± SD. of three sections per embryo, n = 2 experiments) as well as into glia (see Fig. 9 E-F′). Therefore, the lack of neurogenesis by transplanted E13.5 Olig2+ cells does not reflect the inability of progenitors in other regions of the VZ at E13.5 to differentiate into neurons, after FACS-isolation and transplantation into chick spinal cord. These control data provide further evidence in support of the conclusion that Olig2+ progenitors isolated from E13.5 spinal cord are intrinsically restricted to glial fates.
Discussion
The idea that most neuroepithelial cells in the embryonic CNS are multipotent, self-renewing stem cells in vivo has been indirectly inferred from in vitro studies and from the expression of various markers. But, to our knowledge, there are no data that directly test this inference without the use of cell culture manipulations. Retroviral lineage-tracing studies in the chick spinal cord (33) have indicated that some MN progenitors are multipotent, but such retrospective labeling experiments cannot distinguish whether these progenitors underwent self-renewal or rapidly generated separate lineages of committed neuronal and nonneuronal precursors. Time-lapse imaging of cortical radial glial cells in vivo has indicated that they divide asymmetrically, to generate neuronal and radial glial progeny (reviewed in refs. 12 and 13), but these observations cannot resolve whether the nonneuronal daughters of such divisions maintain the developmental potentials available to the parent cell. The only case in which self-renewal of embryonic neural progenitors has been directly tested by prospective isolation and transplantation experiments is in the peripheral nervous system, where progenitors in the fetal sciatic nerve (34) or gut (35) have been shown to retain multipotency (at the population level) for several days or longer (36) after migration from the neural crest.
Here, we have addressed this question in the CNS, by prospective isolation and transplantation of Olig2+ cells from the embryonic spinal cord. We find that transplanted Olig2+ cells isolated from E9.5 embryos readily generate MNs and cells that express panneuronal but not MN markers, suggesting that the host environment is permissive for the generation of multiple classes of neurons. The E9.5 Olig2+ cells also generate OPs. By contrast, if these cells are isolated at E13.5 they generate glial cells, but no neurons, in the same transplantation assay. Progenitors isolated from dorsal regions of the E13.5 spinal cord, when neurogenesis is ongoing in vivo (32), do generate neurons in this assay, indicating that the failure of E13.5 Olig2+ cells to generate neurons is not a generic deficiency of all progenitors at this stage.
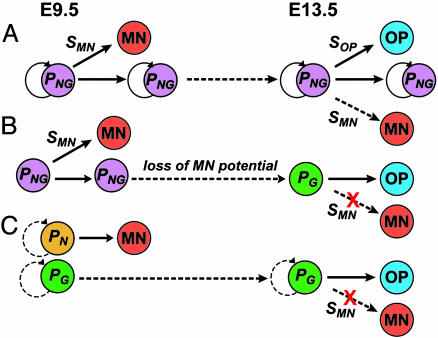
Taken together, these data suggest that Olig2+ cells at E13.5 are intrinsically restricted to a glial fate. Thus, the neurogenic potential of the earlier Olig2+ population is not maintained during the switch to gliogenesis, implying that most Olig2+ MN progenitors do not self-renew in vivo and, therefore, are not stem cells. Because Olig2+ cells constitute 99.9% of the neuroepithelial cells in pMN, then if multipotent, self-renewing progenitors of MNs and OPs indeed exist in this domain (Fig. 5A), they must be extremely rare. Alternatively, MNs and OPs may be generated by a common Olig2+ progenitor that rapidly loses MN potential (Fig. 5B) or by separate populations of committed Olig2+ MN and OP progenitors (Fig. 5C). In the first scenario (Fig. 5B), individual Olig2+ progenitors are initially multipotent (33), but do not self-renew. In the second case (Fig. 5C), individual Olig2+ cells may self-renew but are unipotent. In either case, Olig2+ cells are not both multipotent and self-renewing in vivo, and, thus, do not fit the accepted definition of a neural stem cell (2, 3, 37, 38). We cannot exclude that a small subpopulation of Olig2+ cells may be stem cells that escaped detection or failed to engraft, in our cross-species assay. We also cannot exclude the existence of very rare (<≈0.1%) Olig2- stem cells in the pMN domain. Either scenario, however, would still require a major revision of the commonly (although not universally (2)) held view that the majority of embryonic VZ cells are multipotent, self-renewing stem cells (8, 10, 12-14).
Fig. 5.
Stem cell (A) and non-stem-cell (B and C)-based models for MN and OP generation. PNG, PG, PN indicate progenitors with neuronal and glial, glial, or neuronal potential, respectively. Circular arrows indicate self-renewal. SMN and SOP indicate environmental signals for MN and OP differentiation, respectively. Dashed arrow/SMN in E13.5 indicates predicted behavior of E13.5 progenitors transplanted to an environment containing MN differentiation signals. In B, individual progenitors may be bifatent (shown), or multipotential but unifatent (not shown). Such cells may divide transitorily before losing MN potential. Self-renewal of unipotent cells in model C is possible (dashed circular arrow) but not obligatory.
The results obtained in the pMN domain could represent a special case or could be generalizable to other regions of the CNS. In the cortex, progenitors at late stages of neurogenesis become intrinsically restricted to upper-laminar fates (39), suggesting that they do not maintain the capacity to generate earlier-formed cortical neuron subtypes. By contrast, in the forebrain subventricular zone and hippocampus, neurogenesis persists into adulthood (reviewed in ref. 3), and, therefore, some progenitor cells in these areas must maintain neurogenic capacity (40). However these cases appear to represent the exception rather than the rule (41, 42). The ability of embryonic CNS progenitors to self-renew in vitro in serum-free medium containing high concentrations of growth factors (5-7) may, therefore, represent the “capture” and propagation of a normally transitory or very rare progenitor cell, analogous to the ability to derive “embryonic stem cells” from primordial germ cells or inner-cell mass cells in vitro (43, 44). Alternatively, such culture conditions may induce multipotency and self-renewal in progenitors that do not behave as stem cells in vivo (15-17). The ability to expand and differentiate neural stem cells in vitro is certainly useful, but our results suggest that such cells are not representative of the majority of progenitor cells in the embryonic CNS VZ in vivo.
Materials and Methods
Mice. Olig2GFP/+ mice were generated by homologous recombination in ES cells according to standard procedures. Characterization of Hb9::EGFP (26), and Rosa26 mice has been reported in ref. (29).
In Vivo Transplantation of Single-Cell Suspensions. Fertile white eggs were incubated to E2 (stage 15-17) and grafted as described in ref. 26. FACS-isolated murine cells were mixed with quail carrier cells (1:4 ratio) and transplanted into E2 chick spinal cord in which a small suction lesion had been created. Operated embryos were incubated for an additional 3-4 days. Embryos were fixed in 4% paraformaldehyde/PBS at 4°C and sectioned (10 μm).
Supporting Information. Detail protocols of FACS isolation of spinal cord neuroepithelial cells and immunohistochemistry are available in Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Supplementary Material
Acknowledgments
We thank R. Diamond and S. L. Adams for FACS assistance; S. Pease, B. Kennedy, and the staff of the Transgenic Animal Facility at California Institute of Technology (Caltech) for assistance with mouse breeding and care; the Biological Imaging Center at Caltech for imaging assistance; I. Weissman (Stanford University) for antibody CD133; G. Mosconi for laboratory management; J.-S. Chang and M. Martinez for technical support; G. Mancuso for administrative assistance; C. Hochstim, L. Gabay, S. Lowell, M. Yui, and P. Lwigle for helpful discussion; and Anderson lab members for technical help and discussion. This work was supported by funding from the Howard Hughes Medical Institute and the National Institiutes of Health. T.M.J. and D.J.A. are Investigators of the Howard Hughes Medical Institute.
Author contributions: Y.-s.M. and D.J.A. designed research; Y.-s.M., B.D., and A.L. performed research; B.G.N., H.W., and T.M.J. contributed new reagents/analytic tools; Y.-s.M., B.D., and A.L. analyzed data; and Y.-s.M., T.M.J., and D.J.A. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: CNS-SC, CNS stem cell; En, embryonic day n; HSC, hematopoietic stem cell; MN, motoneuron; OP, oligodendrocyte precursor; VZ, ventricular zone.
References
- 1.Morrison, S. J., Uchida, N. & Weissman, I. L. (1995) Annu. Rev. Cell Dev. Biol. 11, 35-71. [DOI] [PubMed] [Google Scholar]
- 2.Temple, S. (2001) Nature 414, 112-117. [DOI] [PubMed] [Google Scholar]
- 3.Gage, F. (2000) Science 287, 1433-1438. [DOI] [PubMed] [Google Scholar]
- 4.Davis, A. A. & Temple, S. (1994) Nature 372, 263-266. [DOI] [PubMed] [Google Scholar]
- 5.Palmer, T. D., Takahashi, J. & Gage, F. H. (1997) Mol. Cell. Neurosci. 8, 389-404. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds, B. A. & Weiss, S. (1996) Dev. Biol. 175, 1-13. [DOI] [PubMed] [Google Scholar]
- 7.Johe, K. K., Hazel, T. G., Muller, T., Dugich-Djordjevic, M. M. & McKay, R. D. (1996) Genes Dev. 10, 3129-3140. [DOI] [PubMed] [Google Scholar]
- 8.Frederiksen, K. & McKay, R. D. (1988) J. Neurosci. 8, 1144-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lendahl, U., Zimmerman, L. B. & McKay, R. D. G. (1990) Cell 60, 585-595. [DOI] [PubMed] [Google Scholar]
- 10.McKay, R. D. G. (1989) Cell 58, 815-821. [DOI] [PubMed] [Google Scholar]
- 11.Alvarez-Buylla, A., Garcia-Verdugo, J. M. & Tramontin, A. D. (2001) Nat. Rev. Neurosci. 2, 287-293. [DOI] [PubMed] [Google Scholar]
- 12.Ever, L. & Gaiano, N. (2005) Curr. Opin. Neurobiol. 15, 29-33. [DOI] [PubMed] [Google Scholar]
- 13.Gotz, M. & Barde, Y. A. (2005) Neuron 46, 369-372. [DOI] [PubMed] [Google Scholar]
- 14.Pevny, L. & Placzek, M. (2005) Curr. Opin. Neurobiol. 15, 7-13. [DOI] [PubMed] [Google Scholar]
- 15.Kondo, T. & Raff, M. (2000) Science 289, 1754-1757. [DOI] [PubMed] [Google Scholar]
- 16.Shihabuddin, L. S., Horner, P. J., Ray, J. & Gage, F. H. (2000) J. Neurosci. 20, 8727-8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gabay, L., Lowell, S., Rubin, L. L. & Anderson, D. J. (2003) Neuron 40, 485-499. [DOI] [PubMed] [Google Scholar]
- 18.Lu, Q. R., Yuk, D., Alberta, J. A., Zhu, Z., Pawlitzky, I., Chan, J., McMahon, A. P., Stiles, C. D. & Rowitch, D. H. (2000) Neuron 25, 317-329. [DOI] [PubMed] [Google Scholar]
- 19.Zhou, Q., Wang, S. & Anderson, D. J. (2000) Neuron 25, 331-343. [DOI] [PubMed] [Google Scholar]
- 20.Novitch, B. G., Chen, A. I. & Jessell, T. M. (2001) Neuron 31, 773-789. [DOI] [PubMed] [Google Scholar]
- 21.Jessell, T. M. (2000) Nat. Rev. Genet. 1, 20-29. [DOI] [PubMed] [Google Scholar]
- 22.Kessaris, N., Pringle, N. & Richardson, W. D. (2001) Neuron 31, 677-680. [DOI] [PubMed] [Google Scholar]
- 23.Ellis, P., Fagan, B. M., Magness, S. T., Hutton, S., Taranova, O., Hayashi, S., McMahon, A., Rao, M. & Pevny, L. (2004) Dev. Neurosci. 26, 148-165. [DOI] [PubMed] [Google Scholar]
- 24.Uchida, N., Buck, D. W., He, D., Reitsma, M. J., Masek, M., Phan, T. V., Tsukamoto, A. S., Gage, F. H. & Weissman, I. L. (2000) Proc. Natl. Acad. Sci. USA 97, 14720-14725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Capela, A. & Temple, S. (2002) Neuron 35, 865-875. [DOI] [PubMed] [Google Scholar]
- 26.Wichterle, H., Lieberam, I., Porter, J. A. & Jessell, T. M. (2002) Cell 110, 385-397. [DOI] [PubMed] [Google Scholar]
- 27.Arber, S., Han, B., Mendelsohn, M., Smith, M., Jessell, T. M. & Sockanathan, S. (1999) Neuron 23, 659-674. [DOI] [PubMed] [Google Scholar]
- 28.Thaler, J., Harrison, K., Sharma, K., Lettieri, K., Kehrl, J. & Pfaff, S. L. (1999) Neuron 23, 675-687. [DOI] [PubMed] [Google Scholar]
- 29.Zambrowicz, B. P., Imamoto, A., Fiering, S., Herzenberg, L. A., Kerr, W. G. & Soriano, P. (1997) Proc. Natl. Acad. Sci. USA 94, 3789-3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanabe, Y., William, C. & Jessell, T. M. (1998) Cell 95, 67-80. [DOI] [PubMed] [Google Scholar]
- 31.Poncet, C., Soula, C., Trousse, F., Kan, P., Hirsinger, E., Pourquie, O., Duprat, A.-M. & Cochard, P. (1996) Mech. Dev. 60, 13-32. [DOI] [PubMed] [Google Scholar]
- 32.Altman, J. & Bayer, S. A. (1984) Adv. Anat. Embryol. Cell Biol. 85, 1-166. [DOI] [PubMed] [Google Scholar]
- 33.Leber, S. M., Breedlove, S. M. & Sanes, J. R. (1990) J. Neurosci. 10, 2451-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrison, S. J., White, P. M., Zock, C. & Anderson, D. J. (1999) Cell 96, 737-749. [DOI] [PubMed] [Google Scholar]
- 35.Bixby, S., Kruger, G. M., Mosher, J. T., Joseph, N. M. & Morrison, S. J. (2002) Neuron 35, 643-656. [DOI] [PubMed] [Google Scholar]
- 36.Kruger, G. M., Mosher, J. T., Bixby, S., Joseph, N., Iwashita, T. & Morrison, S. J. (2002) Neuron 35, 657-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKay, R. (1997) Science 276, 66-71. [DOI] [PubMed] [Google Scholar]
- 38.Weissman, I. L. (2000) Cell 100, 157-168. [DOI] [PubMed] [Google Scholar]
- 39.Frantz, G. D. & McConnell, S. K. (1996) Neuron 17, 55-61. [DOI] [PubMed] [Google Scholar]
- 40.Merkle, F. T., Tramontin, A. D., Garcia-Verdugo, J. M. & Alvarez-Buylla, A. (2004) Proc. Natl. Acad. Sci. USA 101, 17528-17532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Altman, J. & Das, G. D. (1966) J. Comp. Neurol. 126, 337-390. [DOI] [PubMed] [Google Scholar]
- 42.Rakic, P. (2002) Nat. Rev. Neurosci. 3, 65-71. [DOI] [PubMed] [Google Scholar]
- 43.Robertson, E. J. (1987) in Teratocarcinomas and Embryonic Stem Cells: A Practical Approach, ed. Robertson, E. J. (IRL, Oxford), pp. 71-112.
- 44.Resnick, J. L., Bixler, L. S., Cheng, L. & Donovan, P. J. (1992) Nature 359, 550-551. [DOI] [PubMed] [Google Scholar]
- 45.Anderson, D. J. (2001) Neuron 30, 19-35. [DOI] [PubMed] [Google Scholar]
- 46.Briscoe, J., Pierani, A., Jessell, T. M. & Ericson, J. (2000) Cell 101, 435-445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.