Abstract
Thirteen cats, newly diagnosed with hyperthyroidism, were treated with a transdermal formulation of methimazole at a dose of 5 mg (0.1 mL) (concentration of 50 mg/mL) applied to the internal ear pinna every 12 h for 28 d. Baseline hematologic and biochemical values, along with serum thyroxine (T4) levels, were obtained on presentation (day 0). Cats were evaluated at 14 d (D14) and 28 d (D28) following transdermal therapy. At each visit, a physical examination, a complete blood cell count, a serum biochemical analysis, and a serum T4 evaluation were performed. Ten cats completed the study. Clinical improvement, as well as a significant decrease in T4, was noted in all cats. Serum T4 measured at D14 and D28 were significantly lower at 27.44 nmol/L, s = 37.51 and 14.63 nmol/L, s = 10.65, respectively (P < 0.0001), as compared with values at D0 (97.31 nmol/L, s = 37.55). Only 1 cat showed a cutaneous adverse reaction along with a marked thrombocytopenia. The results of this prospective clinical study suggest that transdermal methimazole is an effective and safe alternative to conventional oral formulations.
Résumé
Efficacité clinique et sécurité du méthimazole transdermique dans le traitement de l’hyperthyroïdisme félin. Treize chats avec un récent diagnostic d’hyperthyroïdisme ont été traités avec une forme transdermique de méthimazole à la dose de 5 mg (0,1 mL) (concentration de 50 mg/mL) appliqué à l’intérieur du pavillon de l’oreille aux 12 heures pendant 28 jours. Les valeurs hématologiques et biochimiques de départ ainsi que les niveaux de thyroxine sérique (T4) étaient celles du jour 0. Les chats ont été évalués au jour 14 (J14) et 28 (J28) de la thérapie transdermique. À chaque visite, un examen physique, une formule sanguine complète, une biochimie sérique et une évaluation de la T4 sérique ont été effectués. Dix chats ont complété l’étude. On a constaté une amélioration clinique et une diminution significative de T4 chez tous les chats. Aux J14 et J28, la T4 sérique était significativement plus base (27,44 nmol/L, s = 37,51 et 14,63 nmol/L, s = 10,65, respectivement, P < 0,0001) qu’au jour 0 (97,31 nmol/L, s = 37,55). Un seul chat a présenté une réaction cutanée indésirable ainsi qu’une thronbocytopénie marquée. Les résultats de cette étude prospective laissent entrevoir que le méthimazole transdermique constitue une alternative efficace et sécuritaire aux présentations orales traditionnelles.
(Traduit par Docteur André Blouin)
Introduction
Hyperthyroidism is the most commonly diagnosed endocrinopathy in middle-aged and older cats, with a prevalence of 0.33% (1–3). This disease, characterized by an excessive production of thyroxine (T4) and triiodothyronine (T3), results from a functional benign adenomatous hyperplasia of the thyroid gland in 98% of affected cats (1,2). The exact etiology remains unknown; however, multiple nutritional and environmental causes have been suspected. Among these, potential goitrogens found in canned food, such as iodine, soybean, phthalates, polyphenols, and polychlorinated biphenyls, have been incriminated (4). Sustained, excessive production of active thyroid hormones results in detrimental systemic effects, which can lead to death if left untreated (1,2).
There are currently 3 options available for the treatment of hyperthyroidism: pharmacological therapy, surgical ablation of one or both thyroid glands, and radioactive iodine therapy, resulting in the destruction of active thyroid tissue (2). Only surgical ablation and radioactive iodine therapy can lead to a permanent resolution of the hyperthyroid state. Pharmacological therapy reversibly inhibits thyroid hormone production and offers a practical and convenient alternative for many patients. Other treatments options, such as the use of oral calcium ipodate and large doses of oral stable iodine, as well as percutaneous ethanol injection in the thyroid tissue, are also reported (2).
In North America, 2 thiourylenes are currently available for the pharmacological treatment of feline hyperthyroidism, methimazole and propylthiouracil. Because of the high rate of adverse reactions (20% to 25%), propylthiouracil is no longer recommended in feline patients, thus leaving methimazole as the most commonly used drug for this condition (5). Methimazole is actively concentrated in the thyroid gland and interferes with hormonal synthesis by inhibition of the enzyme thyroid peroxidase (2). Methimazole does not affect the transport of iodide through the iodide pump or the release of “preformed” hormones (1,2). Following oral administration, the bioavailability of methimazole varies from 27% to 100%, with an average of 80%, and the drug achieves a peak serum level in 4 to 6 h after administration (6,7). Although the plasma half-life of methimazole is 6 h in the cat, the duration of its pharmacodynamic effect can be greater than 20 h, due to its ability to accumulate in the thyroid gland (1,2,6–8). Methimazole has been recommended in hyperthyroid patients prior to surgical ablation of the gland in order to produce a euthyroid state, thus reducing surgical risk by counteracting the deleterious systemic effects of hyperthyroidism. Medical treatment is also useful to assess the impact of a return to a euthyroid state on kidney function, prior to permanent therapy (9). Finally, methimazole is used for long-term management of patients which are not candidates for surgical ablation or radioactive iodine therapy, such as patients with a high anesthetic risk, patients in renal failure, or when access to radioiodine is limited. It is currently recommended that oral methimazole be administered at a dose of 2.5 to 5 mg per cat every 8 to 12 h (1,2). Following this regimen, treated animals will usually reach a euthyroid state within 7 to 14 d. The dose of methimazole can then be adjusted accordingly to maintain a normal serum T4.
Side effects of methimazole treatment are frequent and usually occur within the first 3 mo of therapy (1,2). Vomiting, anorexia, and lethargy, as well as hematologic changes, can be observed in 15% of treated patients (1). Vomiting and anorexia are often caused by the bitter taste of the product (1). Facial excoriations and hepatopathy have been reported in 2% of cases (1,2). Other less common side effects that have been noted in cats are positive antinuclear antibody and myasthenia gravis (1,2).
Recently, a transdermal formulation of methimazole, using a pleurolecithin organogel (PLO), has become available through compounding pharmacies. In a recent study performed on 6 normal cats, variable serum levels of methimazole following the application of a transdermal dose of 5 mg, were reported (10): only 2 out of the 6 cats had a detectable serum concentration of methimazole and only 1 cat showed a near 100% absorption. Pharmacokinetics of methimazole in hyperthyroid cats has not been described to date. In contrast, a pharmacodynamic study of transdermal methimazole gel showed a positive response to therapy, including normalization of serum T4 in hyperthyroid cats (11): 13 hyperthyroid cats were treated with variable doses of transdermal methimazole ranging from 2.5 mg/cat, q24h to 10 mg/cat, q24h. Nine of 10 patients showed a marked decrease in serum T4 after 4 wk and 7/8 after 6 mo of therapy. No side effects in either study were reported by the authors (10,11).
The objective of this prospective clinical study was to determine the pharmacodynamic effect and clinical safety in hyperthyroid cats of a transdermal formulation of methimazole (Transdermal methimazole [5 mg/0.1 mL] Giroux Laboratories, Saint-Hyacinthe, Quebec).
Material and methods
Thirteen newly diagnosed hyperthyroid cats that had been presented to the University of Montreal’s Centre Hospitalier Universitaire Vétérinaire were included in the study. The diagnosis of hyperthyroidism was based on the presence of ≥ 2 of the following criteria: typical clinical signs (polyuria, polydipsia, polyphagia, weight loss, hyperactivity), palpation of a thyroid nodule, and a serum total thyroxine (TT4) exceeding 55 nmol/L. The study subjects were comprised of 11 domestic shorthairs, and 2 Persians. All the cats had been neutered and there were 8 females and 5 males. The mean age was 11 y with a range of 9 to 17 y. A complete blood (cell) count (CBC) and biochemical profile, as well as a serum TT4 determined by radio immunological assay (Test TT4, Immulite; Inter Medico, Markham, Ontario), were performed on each patient prior to therapy. In addition, thoracic radiographs and echocardiographs were obtained from patients with abnormal cardiac sounds on auscultation. Indirect systolic blood pressure, measured by Doppler ultrasonographs (Doppler; Parks Medical Electronics, Aloha, Oregon, USA) was recorded in some patients. All cats were treated with 0.1 mL of PLO containing 5 mg of methimazole (Transdermal methimazole, 50 mg/mL, Giroux Laboratories) which was applied by the owner to the internal ear pinna q12h for 28 d. Owners were instructed to alternate the pinna for each application and to remove any gel residue with wet gauze before applying the next dose. Owners were also instructed to put on gloves for the application of the gel. The gel was dispensed in 1-mL syringes, at a concentration of 50 mg/ mL, and was kept at room temperature, shaded from light. As the duration of stability of the LPO-methimazole gel was unknown, it was decided to renew the product every 14 d. Owners signed a consent form that explained the project and the off-label use of transdermal methimazole. The cats were rechecked on days 14 (D14) and 28 (D28), at which time a complete physical examination, a CBC, and a biochemical profile, as well as a serum TT4, were performed. No doses were modified throughout the study. At each recheck, the owners were questioned on the ease of product application and asked to report any problems they had encountered with the gel.
The study was approved by the Université de Montréal Institutional Animal Use and Care Committee.
Statistical analysis
Descriptive statistics (mean, standard deviation [s], median, minimum, maximum) were performed for serum TT4. The response to therapy was evaluated by using the Kruskal-Wallis one-way ANOVA. If significance was reached, a Kruskal-Wallis multiple comparison Z procedure was used to pinpoint which time was different according to the Bonferroni adjustment. The statistical analysis was performed using NCSS software (Number Cruncher Statistical System [NCSS], 2001, Kaysville, Utah, USA). Probability values of less than 0.05 were considered significant.
Results
Thirteen cats were enrolled in the study. Two cats were euthanized after D14 for reasons unrelated to hyperthyroidism and 1 cat was withdrawn from the study after D14 because of an adverse cutaneous reaction to the transdermal methimazole. Given that values from the 13 cats were available for D0 and D14, these values were included in the statistical analysis, while values for D28 were obtained for the remaining 10 cats only. The clinical signs and abnormalities noted on the physical examination are listed in Table 1. The hematologic changes, as well as the biochemical changes, are presented in Table 2. Thoracic radiographs were taken in 6 cats and considered normal in 1 cat. Other findings included cardiomegaly (5/6), pulmonary hyperinflation (2/6), vascular congestion (1/6), and a prominent aortic arch (1/6). Echocardiographs were taken in 5 cats: they showed mild hypertrophic changes in 4 cats and no abnormal findings in 1 cat.
Table 1.
Clinical signs and abnormalities found on physical examination of the 13 cats initially selected for study
| Clinical signs | Number of cats | Physical abnormalities | Number of cats |
|---|---|---|---|
| Vomiting | 7 | Palpable thyroid nodule | 12 |
| Polyuria/polydipsia | 6 | Cardiac murmur | 5 |
| Polyphagia | 4 | Poor body condition | 3 |
| Weight loss | 4 | Alopecia | 2 |
| Lethargy | 4 | Hypertension | 1a |
| Diarrhea | 2 | ||
| Vocalization/agitation | 2 | ||
| Hair loss | 2 | ||
| Dysorexia | 1 | ||
| Asymptomatic | 2 |
180 mmHg
Table 2.
Hematologic and biochemical changes of the 13 cats initially selected for study
| Parameter | Number of cats | Range | Reference range |
|---|---|---|---|
| Normal hematology | 9 | — | — |
| Normal biochemical profile | 4 | — | — |
| Neutrophilic leukocytosis | 1 | 13.9 × 109/L | 2.50 to 12.5 × 109/L |
| Mild leucopenia | 2 | 2.4 to 4.4 × 109/L | 5.50 to 19.50 × 109/L |
| Polycythemia | 1 | 0.51 L/L | 0.24 to 0.45 L/L |
| Increased BUN | 3 | 11.08 to 20.15 mmol/L | 4.1 to 10.8 mmo/L |
| Increased ALT | 6 | 64 to 198 U/L | 16 to 63 U/L |
| Increased ALKP | 6 | 51 to 115 U/L | < 50 U/L |
| Hyperphosphoremia | 3 | 2.00 to 2.23 mmol/L | 0.96 to 1.96 mmol/L |
Excluding the 2 asymptomatic cats, all cats showed improvement in their clinical condition with the transdermal methimazole therapy. One cat developed severe facial erythema of the internal pinna of both ears. This erythema was not typical of the facial excoriations occasionally reported with the use of oral methimazole (8). Concurrently, a marked thrombocytopenia developed in this same patient, with platelet values of 4.0 × 109/L (reference range: 150 to 700 × 109/L). This cat was withdrawn from the study. Platelet counts taken 10 d later on this patient were in the normal range. No significant hematological changes were observed on D14 and D28 in all remaining cats. Improvement in biochemical parameters (ALT, alkaline phosphatase, BUN, and serum phosphorus) were observed in 5 patients on D14 and D28. One cat showed an increased BUN, from 20.15 to 27.27 mmol/L (reference range: 4.1 to 10.8 mmol/L), and an increased serum creatinine at 187 μmol/L (reference range: 51 to 180 μmol/L) on D14. Urine specific gravity in this patient on D14 was 1.023.
Sera TT4 were significantly decreased on D14 and D28 as compared with those on D0 in all cats, with ranges of 48.66 to 182.07 nmol/L at D0, 3.80 to 125.90 nmol/L at D14, and 2.10 to 30.03 nmol/L at D28 (reference range: 19 to 45 nmol/L) (Table 3, Figure 1).
Table 3.
Descriptive statistics for serum total thyroxin (TT4) concentrations (nmol/L) at baseline and after transdermal administration of methimazole.
| Serum total thyroxin (TT4) concentrations (nmol/L)
|
|||
|---|---|---|---|
| Statistical analysis | Day 0 (n = 13) | Day 14 (n = 13) | Day 28 (n = 10) |
| Mean, s | 97.31, s = 37.55 | 27.44, s = 37.51a | 14.63, s = 10.65a |
| Median | 96.19 | 8.93 | 10.23 |
| Minimum | 48.66 | 3.80 | 2.10 |
| Maximum | 182.07 | 125.90 | 30.03 |
n — number of cats; s — standard deviation
Value significantly different from baseline (P < 0.0001)
Figure 1.
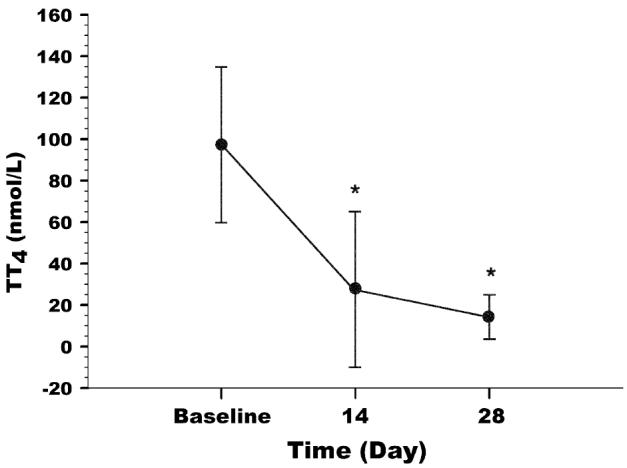
Mean and standard deviation (s) of serum total thyroxin (TT4) concentrations at baseline and after transdermal administration of methimazole. * Value significantly different from baseline (P < 0.0001).
Four owners reported a nonhomogeneous texture of the transdermal gel, resulting in a combination of clear liquid and thick paste. Most owners were satisfied with the ease of administration of the topical gel.
Discussion
The main goal of using a transdermal formulation is to avoid oral daily administration of methimazole in uncooperative cats. Another advantage of transdermal administration is in avoiding hepatic first-pass effect, such that a lower dose of methimazole can be used to obtain an equivalent plasma concentration and clinical effect, with a reduced risk of side effects (12). The skin, an efficient initial barrier to the external environment, is also a large and dynamic component of the body that can be used as an ideal site for drug administration and drug transport to the systemic circulation (12,13). Several factors can influence transdermal drug absorption in animals, such as the presence of fur and sweat glands, skin thickness, blood perfusion, skin temperature, hydration, and integrity of the stratum corneum (12). Metabolism of drugs can also occur in the epidermis, thus reducing systemic absorption (12). Pleurolecithin organogel, a lipophilic substance, offers the advantage of penetrating the statum corneum and, thus, promoting drug diffusion to deeper skin layers (13).
In this study, transdermal methimazole was effective in establishing a euthyroid state in all of the hyperthyroid cats that completed the study, a result that is comparable with the results of 2 previous studies (14,15). In contrast to oral methimazole, where up to 15% of patients showed gastrointestinal side effects (4), no cat in this study presented such adverse effects. Thrombocytopenia is reported to occur in less than 5% of cats treated with oral methimazole and the pathophysiologic mechanism remains unknown (4,16–18). Despite its transdermal application, methimazole caused thrombocytopenia in 1 cat, suggesting that hematological follow-ups in patients treated with transdermal methimazole should be carried out (1).
The increase in BUN and serum creatinine in 1 cat was attributed to the presence of underlying renal failure that was masked by hyperthyroidism but became apparent following the return to a euthyroid state (16).
Although, except for 1 patient, no cutaneous reactions were noted at the site of application of the methimazole gel during the length of the study, a long-term follow-up would be necessary to ensure that no significant modifications of the epidermal barrier occurs that could alter product absorption.
Minor technical complications were observed with the transdermal gel. One third of owners reported a nonhomogeneous texture of the product, caused by drug precipitation, which most likely resulted in variable concentrations of methimazole from one dose to the next. The product eff icacy did not appear to be affected, although high concentrations of methimazole could contribute to short- and long-term side effects (4). The owner of the cat withdrawn from the study because of a cutaneous reaction and thrombocytopenia did not report any change in the texture of the transdermal methimazole. Owners were advised to mix the gel and transfer it to a new syringe if precipitation was observed. Given the elevated cost and the poor stability of transdermal methimazole, only 2 clients chose to continue with long-term topical therapy.
Methimazole pharmacokinetics were not performed in this study, as it was difficult to validate an appropriate methodology to assay methimazole concentrations. Pharmacokinetic studies need to be performed on hyperthyroid cats receiving topical methimazole in order to adequately determine serum level variability and bioavailability, as opposed to recent findings reported in normal cats (10). Long-term stability studies, beyond 14 d, should also be performed on the transdermal gel formulation to ensure constant serum concentrations are maintained.
Methimazole administered as a transdermal gel appears to be a safe and effective option for the treatment of hyperthyroid cats. However, this product should be reserved for short-term treatment, treatment of cats with gastrointestinal signs related to oral methimazole administration, or use in cats in which oral treatment is not feasible because of its cost, its stability, and the lack of pharmacokinetic studies of it in hyperthyroid cats.
Acknowledgment
The authors thank Mr. Maxime Moreau for the statistical analyses. CVJ
Footnotes
Funding: Fond de Santé Animale — CHUV, Faculté de Médecine Vétérinaire, Université de Montréal.
References
- 1.Mooney CT, Thoday KL. CVT Update: Medical treatment of hyperthyroidism in cats. In: Bonagura JD, ed. Kirk’s Current Veterinary Therapy XIII. Philadelphia: Saunders, 2000:333–337.
- 2.Peterson ME. Hyperthyroidism. In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine, 5th ed. Philadelphia: Saunders, 2000:1400–1417.
- 3.Peterson ME, Graves TK, Cavanagh I. Serum thyroid hormone concentrations fluctuate in cats with hyperthyroidism. J Vet Intern Med. 1987;1:142–146. doi: 10.1111/j.1939-1676.1987.tb02002.x. [DOI] [PubMed] [Google Scholar]
- 4.Feldman EC, Nelson RW. Canine and Feline Endocrinology and Reproduction, 3rd ed. Philadelphia: Saunders, 2004:152–215.
- 5.Peterson ME, Hurvitz AI, Leib MS, et al. Propylthiouracil- associated hemolytic anemia, thrombocytopenia and antinuclear antibodies in cats with hyperthyroidism. J Am Vet Med Assoc. 1984;18:86. [PubMed] [Google Scholar]
- 6.Trepanier LA, Peterson ME. Pharmacokinetics of methimazole in normal cats and cats with hyperthyroidism. Res Vet Sc. 1991;50:69–74. doi: 10.1016/0034-5288(91)90055-s. [DOI] [PubMed] [Google Scholar]
- 7.Trepanier LA, Peterson ME, Aucoin DP. Pharmacokinetics of intavenous and oral methimazole following single- and multiple dose administration in normal cats. Vet Pharmacol Ther. 1991;14:367–373. doi: 10.1111/j.1365-2885.1991.tb00850.x. [DOI] [PubMed] [Google Scholar]
- 8.Peterson ME. Comparison of the disposition of carbimazole and methimazole in clinically normal cats. Res Vet Sc. 1993;54:351–355. doi: 10.1016/0034-5288(93)90134-2. [DOI] [PubMed] [Google Scholar]
- 9.Graves TK. Complications of treatment and concurrent illness associated with hyperthyroidism in cats. In: Bonagura JD, ed. Kirk’s Current Veterinary Therapy XII. Philadelphia: WB Saunders, 1995:369–372.
- 10.Hoffman SB, Yoder AR, Trepanier LA. Bioavailability of transdermal methimazole in a pluronic lecithin organogel (PLO) in healthy cats. J Vet Pharmacol Ther. 2002;25:189–193. doi: 10.1046/j.1365-2885.2002.00405.x. [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann G, Marks SL, Taboada J, Hosgood GL, Wolfsheimer KJ. Topical methimazole treatment of cats with hyperthyroidism [Abstract], Proc 19th Am Coll Vet Int Med, Denver, Colorado 2001:112.
- 12.Riviere JE, Papich MG. Potential and problems of developing transdermal patches for veterinary applications. Adv Drug Deliv Rev. 2001;50:175–203. doi: 10.1016/s0169-409x(01)00157-0. [DOI] [PubMed] [Google Scholar]
- 13.Magnusson BM, Walters KA, Roberts MS. Veterinary drug delivery: potential for skin penetration enhancement. Adv Drug Deliv Rev. 2001;50:205–227. doi: 10.1016/s0169-409x(01)00158-2. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman G, Marks SL, Taboada J, Hosgood GL, Wolfsheimer KJ. Transdermal methimazole treatment in cats with hyperthyroidism. J Feline Med Surg. 2003;5:77–82. doi: 10.1016/S1098-612X(02)00095-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sartor LL, Trepanier LA, Kroll MM, Rodan I, Challoner L. Efficacy and safety of transdermal methimazole in the treatment of cats with hyperthyroidism. J Vet Intern Med. 2004;18:65–655. doi: 10.1892/0891-6640(2004)18<651:easotm>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Becker TJ, Graves TK, Kruger JM, Braselton WE, Nachreiner RF. Effects of methimazole on renal function in cats with hyperthyroidism. J Am Anim Hosp Assoc. 2000;36:215–23. doi: 10.5326/15473317-36-3-215. [DOI] [PubMed] [Google Scholar]
- 17.Retsios E. Pharm Profile: Methimazole. Compend Contin Educ Pract Vet. 2001;23:36–41. [Google Scholar]
- 18.Peterson ME, Kintzer PP, Hurvitz AI. Methimazole treatment of 262 cats with hyperthyroidism. J Vet Intern Med. 1988;2:150–157. doi: 10.1111/j.1939-1676.1988.tb02812.x. [DOI] [PubMed] [Google Scholar]


