Abstract
A 3-day-old male alpaca cria was presented for lack of vigor and failure to urinate since birth. Based on the history, laboratory data, ultrasonographs, surgical findings, and postmortem examination, the cria was diagnosed with bilateral renal agenesis and hypoplastic bladder, a congenital condition rarely seen in veterinary medicine.
Résumé
Agénésie rénale bilatérale chez un alpaga cria. Un alpaga cria mâle, âgé de 3 jours, a été présenté pour manque d’énergie et absence de miction depuis la naissance. En tenant compte de l’histoire, des données de laboratoire, des échographies, des trouvailles chirurgicale et de l’examen postmortem, un diagnostic d’agénésie rénale bilatérale et d’hypoplasie de la vessie a été posé. Il s’agit d’une condition congénitale rarement rencontrée en médecine vétérinaire.
(Traduit par Docteur André Blouin)
Case description
A 3-day-old, male alpaca cria was presented to the North Carolina State University Veterinary Teaching Hospital because of an observed decrease in voluntary nursing and because no evidence of urination had been detected. The cria had been delivered in breech and cush position and weighed 6.73 kg. The 7-year-old dam had no prior history of dystocia and previously had successfully raised 3 crias. The cria passed meconium after administration of 3, 5 mL enemas. Colostrum from the dam (180 mL) was bottle fed shortly after birth. At 2 d of age, the cria was nursing normally, and tetanus antitoxin/ toxoid and selenium were given. Later that day, the cria stopped nursing and the owner bottle fed 60 to 120 mL of goat’s milk.
Upon presentation, the cria was bright, alert, and responsive. Abnormalities on physical examination included mild crepitus upon manipulation of each tarsus, mild discomfort on abdominal palpation, and no wetness of the fiber around the prepuce. Laboratory studies consisted of doing a complete blood (cell) count (CBC) and serum biochemical analysis. Abnormalities on CBC included evidence of inflammation and suspected infection. Marked leukocytosis of 39 900 × 109/L (reference range 8000 to 23 800 × 109/L) with elevated white cell parameters were measured; specifically neutrophils were 30 723 × 109/L (range 2502 to 13 411 × 109/L), bands were 2394 × 109/L (range 0 to 91 × 109/L), and monocytes were 4788 × 109/L (range 0 to 1462 × 109/L). Serum biochemical analysis revealed abnormal values for glucose of 14.5 mmol/L (range 5.7 to 9.0 mmol/L), urea nitrogen of 46.4 mmol/L (range 4.3 to 11.1 mmol/L), creatinine of 1997 μmol/L (range 106 to 256 μmol/L), phosphorus of 5.8 mmol/L (range 1.5 to 3.2 mmol/L), potassium of 8.6 mmol/L (range 3.6 to 6.2 mmol/L), and gamma glutamyl transferase (GGT) of 212.0 U/L (range 4.3 to 11.1 U/L). Serum total protein and albumin were decreased at 45 g/L (range 48 to 70 g/L) and 27 g/L (range 31 to 52 g/L), respectively. Reference ranges for hematological parameters have been published by Fowler (1,2).
Abdominal ultrasonographs were taken. The right kidney and the bladder were not identified. There was a hyperechoic, 12.8-mm mass containing suspected mineral that caused acoustic shadowing in the region of the left kidney. Normal architecture of the left kidney was not seen. A mild peritoneal effusion was noted. The changes were thought to be consistent with congenital aplasia, hypoplasia/dysplasia, or both, of the kidneys. The inability to see the bladder was considered a consequence of it not being distended with urine, not being present, or being displaced. However, in most patients, even an empty bladder can be identified.
An exploratory ventral midline celiotomy under general anesthesia was elected for definitive diagnosis and prognosis. Three percent isoflurane, delivered by an anesthetic mask, was used to induce the cria. Maintenance anesthesia was provided by 2% isoflurane. The cria was placed in dorsal recumbency and prepared for surgery. A 15-cm midline incision extending caudally from approximately 5-cm cranial to the umbilicus was made. The peritoneal cavity was thoroughly inspected; each adrenal gland was visualized, but neither kidney was present. There was no definitive evidence of a bladder. After confirming that the urinary system had not developed, the cria was euthanized while under general anesthesia.
Gross examination of the organs at necropsy revealed a small (5 × 1 cm), flat, sac-like organ associated with the umbilical arteries that was possibly a hypoplastic bladder. Projecting from the caudal end of the structure were paired tubules measuring approximately 1 mm in diameter (Figure 1). The external urethra was markedly stenotic, yet patent upon catheterization. There was no recognizable connection of the urethra with the suspected bladder remnant. The genital tract was present, but hypoplastic. The lungs were described as heavy, wet, and did not float in formalin.
Figure 1.
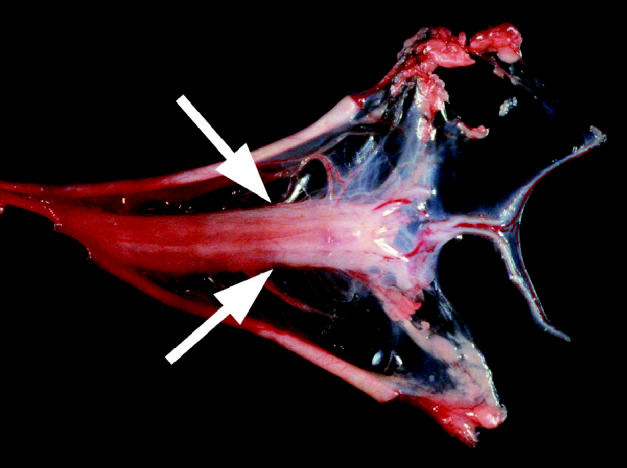
Gross examination of the organs at necropsy revealed a small (5 × 1 cm), flat, sac-like organ associated with the umbilical arteries that was possibly a hypoplastic bladder. (Arrows) projecting from the caudal end of the structure were paired tubules measuring approximately 1 mm in diameter. The external urethra was markedly stenotic yet patent upon catheterization. There was no recognizable connection of the urethra to the suspected bladder remenant.
Histopathological examination of the lungs showed congested parenchyma. Alveolar and bronchiolar spaces were filled with lacy, fibrillar, eosinophilic proteinaceous material. Microscopic evaluation of the urinary tract revealed that the sac-like structure, consistent with the hypoplastic bladder, contained multiple layers of irregularly arranged smooth muscle bundles and luminal transitional epithelium with 2 blind-ended ureters projecting from it.
Discussion
Congenital anomalies in the alpaca have not been described frequently or thoroughly understood by veterinarians and alpaca owners. Paul-Murphy (3) presented a comprehensive review of congenital conditions in llamas, along with their diagnosis and a comparison with conditions in other species. In llamas, renal agenesis is usually unilateral and diagnosed by palpation per rectum or at necropsy (3). For purposes of this discussion, agenesis refers to the complete absence of an organ and its associated primordium. Aplasia refers to the absence of an organ due to failure of development of the primordium. Hypoplasia refers to incomplete development or underdevelopment of an organ with decreased numbers of cells (4).
Renal agenesis is rare in small animals and is usually diagnosed as an extraneous f inding on necropsy or exploratory surgery (5). The anomaly is usually unilateral and is often associated with other abnormalities (6). It has been suggested that renal agenesis is hereditary in domestic species, but this has not been confirmed (7). Bilateral agenesis is lethal; in cases of unilateral agenesis, the opposite kidney undergoes compensatory hypertrophy (8). Definitive diagnosis of renal agenesis without doing surgery requires use of computed tomography (CT), magnetic resonance imaging (MRI), excretory urograms, or abdominal ultrasonographs. In this patient, the structure suspected of representing a remnant of the left kidney on ultrasonography was not described on necropsy or histopathologic examination and may have been fibrous tissue in the same region. Bilateral renal agenesis has not, to the authors’ knowledge, been described in equids, ruminants, or camelids.
Embryologically, the kidneys develop from the intermediate mesoderm of the dorsal body walls, giving rise to 3 successive nephroic structures: the pronephros, the mesonephros, and the metanephros. The mesonephric duct evaginates to form the metanephric diverticulum (ureteral buds), which grows and forms the renal pelvis (6,9). The metanephric tissue is functional but not required in the fetus to excrete urine, since, the placenta is capable of removing all waste produced by the fetus (6).
Renal agenesis in the dog is caused by a degeneration of the ureteral bud. Without the ureteral bud, the metanephros is not induced to form the kidney (8). The mesonephros and metanephros are closely related to development of other urogenital organs, therefore disruptions in development can be associated with other urogenital congenital abnormalities (6,10). In this case, there was no recognizable metanephric tissue; therefore, renal agenesis resulted. However, the presence of blind-ended ureters and an underdeveloped bladder and genital tract is consistent with hypoplasia of these structures caused by failure of the ureteral buds to invade into absent metanephric tissue and develop into renal pelvises, collecting tubules, and papillary ducts.
Recently, an animal model has been developed to study renal agenesis. The mouse model is a highly inbred strain (FUBI-failure of ureteric bud invasion) with high spontaneous occurrence of bilateral renal agenesis. Genetic analysis of the model has revealed 2 or more genes associated with the anomaly, mapped to the modifier loci, fubi1, on chromosome 2 (11).
Renal agenesis has been studied in humans, but further studies are required to clarify pathogenic interactions between genetic and environmental factors (12). A 36-week gestational-age male was stillborn with bilateral renal agenesis and a 47, XXY karyotype, as well as features of Potter facies (13). Potter facies, also known as the oligohydramnios sequence, presents as facial and limb anomalies, and pulmonary hypoplasia, most likely due to compression of the fetus by the uterus because of absence of amniotic fluid. Absence of amniotic fluid is a direct sequela of bilateral renal aplasia because without a fetal renal system there is a lack of fetal urine, a major component of amniotic fluid (4). The pulmonary hypoplasia is severe, causing pneumomediastinum and pneumothorax due to the effects of normal negative pressure over-distending the hypoplastic lungs during resuscitation efforts at birth (14).
Oligohydramnios may explain the pathologic findings in the pulmonary system in this case. The lungs did not float in formalin and significant protein was observed on histopathologic examination, indicating that they may have been hypoplastic. However, pulmonary lesions were not severe and the cria was able to oxygenate for the first 3 d of life.
Prenatal ultrasonography can effectively detect human terminal fetal bilateral uropathies, but it is not sufficiently sensitive to detect infants that will have impaired renal function. However, fetal serum β2-microglobulin has been described as a marker for renal function and predicts postnatal serum creatinine for uropathies (15). Fetal serum creatinine cannot be used, because it crosses the placenta and is cleared by the mother (16). Fetal serum β2-microglobulin is a light chain class 1 major histocompatibility antigen that is completely filtered by the fetal glomeruli and resorbed and catabolized by the tubular cells (17). Low glomerular filtration with renal impairment will decrease β2-microglobulin catabolism, thereby increasing serum levels (18,19).
The clinical signs and findings in this cria were consistent with bilateral renal agenesis and bladder hypoplasia. Renal azotemia and hyperglycemia were due to inability of the body to filter blood, remove waste, and maintain homeostasis. Mild hypoproteinemia was probably due to decreased nutritional intake. The cause of the elevated white blood cell count is unknown.
To the authors’ knowledge, this is the first report of bilateral renal agenesis in an alpaca. Because of the small alpaca population in North America, occurrences of bilateral renal agenesis are likely rare. Further study of the condition is required to elucidate environmental and genetic risk factors for renal agenesis. Early detection of renal malformation may become more practical with use of an assay for serum β2-microglobulin. CVJ
Footnotes
Dr. Poulsen’s current address is Department of Medical Sciences, School of Veterinary Medicine, University of Wisconsin-Madison, 2015 Linden Drive, Madison, Wisconsin 53706, USA.
References
- 1.Fowler ME. Medicine and Surgery of South American Camelids. 2nd ed. Ames: Iowa State Univ Pr., 1998:364–376.
- 2.Fowler ME, Zinkl, JG Reference ranges for hematologic and serum biochemical values in llamas. Am J Vet Res. 1989;12:2049–53. [PubMed] [Google Scholar]
- 3.Paul-Murphy J. Obstetrics, neonatal care, and congenital conditions. Vet Clin North Am Food Anim Pract. 1989;5:183–202. doi: 10.1016/s0749-0720(15)31009-4. [DOI] [PubMed] [Google Scholar]
- 4.Robbins SL, Cotran RS. Pathologic Basics of Disease. 7th ed. Philadelphia: Elsevier Saunders, 2005:472.
- 5.Diez-Prieto I, Garcia-Rodriguez MB, Rios-Granja MA, Cano-Rabano MJ, Gonzalo-Orden JM, Perez-Garcia CC. Diagnosis of renal agenesis in a beagle. J Small Anim Pract. 2001;42:599–602. doi: 10.1111/j.1748-5827.2001.tb06036.x. [DOI] [PubMed] [Google Scholar]
- 6.Finco DR. Congenital, inherited, and familial renal diseases. In: Finco DR, Osborne CA, eds. Canine and Feline Nephrology and Urology. Baltimore: Williams & Wilkins, 1995:471–483.
- 7.DiBartola SP. Familial renal disease in dogs and cats. In: Ettinger SJ, Feldman, EC eds. Textbook of Veterinary Internal Medicine. Diseases of the Dog and Cat. 4th ed. vol 2. Philidelphia: WB Saunders, 1995:1796–1801.
- 8.Noden DM, De Lahunta A. Derivatives of intermediate mesoderm. In: The Embryology of the Domestic Animal: Developmental Mechanisms and Malformations. Baltimore: Williams and Wilkins, 1985:312–321.
- 9.Brownie CF, Tess MW, Prasad RD. Bilateral renal agenesis in two litters of Shetland sheepdogs. Vet Hum Toxicol. 1988;30:483–485. [PubMed] [Google Scholar]
- 10.Agut A, Fernandez Del Palacio MJ, Laredo FG, Murciano J, Bayon A, Soler M. Unilateral renal agenesis associated with additional congenital abnormalities of the urinary tract in a Pekingese bitch. J Small Anim Pract. 2002;43:32–35. doi: 10.1111/j.1748-5827.2002.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 11.Kamba T, Higashi S, Kamoto T, et al. Failure of ureteric bud invasion, a new model of renal agenesis in mice. Am J Pathol. 2001;159:2347–2352. doi: 10.1016/S0002-9440(10)63084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bianca S, Ingegnosi C, Ettore G. Reproductive risk factors in unilateral and bilateral renal agenesis. Congenit Anom (Kyoto) 2003;43:79–80. doi: 10.1111/j.1741-4520.2003.tb01030.x. [DOI] [PubMed] [Google Scholar]
- 13.Barroeta JE, Stopyra GA. 47, XXY with associated bilateral renal agenesis. Arch Pathol Lab Med. 2004;128:44–45. doi: 10.5858/2004-128-e44-XWABRA. [DOI] [PubMed] [Google Scholar]
- 14.Siegel MJ, Herman TE. Special imaging casebook. J Perinatol. 2000;20:397–398. doi: 10.1038/sj.jp.7200222. [DOI] [PubMed] [Google Scholar]
- 15.Berry SM, Lecolier B, Smith RS, et al. Predictive value of serum β2-microglobulin for neonatal renal function. Lancet. 1995;345:1277–1278. doi: 10.1016/s0140-6736(95)90928-1. [DOI] [PubMed] [Google Scholar]
- 16.Dommergues M, Muller F, Ngo S, et al. Fetal serum β2- microglobulin predicts postnatal renal function in bilateral uropathies. Kidney Int. 2000;58:312–316. doi: 10.1046/j.1523-1755.2000.00167.x. [DOI] [PubMed] [Google Scholar]
- 17.Revillard JP, Vincent C. Structure and metabolism of beta 2-microglobulin. Contrib Nephrol. 1988;62:44–53. doi: 10.1159/000415474. [DOI] [PubMed] [Google Scholar]
- 18.Nolte S, Mueller B, Pringsheim W. Pediatric Nephrol. 1991;5:473–577. doi: 10.1007/BF00856641. [DOI] [PubMed] [Google Scholar]
- 19.Schardun GHS, Statius van Eps LW. β2-microglobulin: Its significance in the evaluation of renal function. Kidney Int. 1987;32:635–641. doi: 10.1038/ki.1987.255. [DOI] [PubMed] [Google Scholar]


