Abstract
Background
Macrophages, osteoclasts, dendritic cells, and microglia are highly specialized cells that belong to the mononuclear phagocyte system. Functional and phenotypic heterogeneity within the mononuclear phagocyte system may reveal differentiation plasticity of a common progenitor, but developmental pathways leading to such diversity are still unclear.
Results
Mouse bone marrow cells were expanded in vitro in the presence of Flt3-ligand (FL), yielding high numbers of non-adherent cells exhibiting immature monocyte characteristics. Cells expanded for 6 days, 8 days, or 11 days (day 6-FL, day 8-FL, and day 11-FL cells, respectively) exhibited constitutive potential towards macrophage differentiation. In contrast, they showed time-dependent potential towards osteoclast, dendritic, and microglia differentiation that was detected in day 6-, day 8-, and day 11-FL cells, in response to M-CSF and receptor activator of NFκB ligand (RANKL), granulocyte-macrophage colony stimulating-factor (GM-CSF) and tumor necrosis factor-α (TNFα), and glial cell-conditioned medium (GCCM), respectively. Analysis of cell proliferation using the vital dye CFSE revealed homogenous growth in FL-stimulated cultures of bone marrow cells, demonstrating that changes in differential potential did not result from sequential outgrowth of specific precursors.
Conclusions
We propose that macrophages, osteoclasts, dendritic cells, and microglia may arise from expansion of common progenitors undergoing sequential differentiation commitment. This study also emphasizes differentiation plasticity within the mononuclear phagocyte system. Furthermore, selective massive cell production, as shown here, would greatly facilitate investigation of the clinical potential of dendritic cells and microglia.
Background
The mononuclear phagocyte system encompasses a widely distributed family of related cells exhibiting highly specialized functions such as macrophages, osteoclasts, dendritic cells, and microglia. Resident macrophages, found in most organs and connective tissues, serve as professional phagocytes, removing pathogens or apoptotic cells [1]. Microglia represents a unique category of mononuclear phagocytes distributed throughout the central nervous system (CNS) parenchyma in both white and grey matter [2]. Microglial cells share a number of immunological markers with other mononuclear phagocytes, yet they present a unique ramified morphology, which is best characterized by electron microscopy [3]. Besides their role as immune effectors of the CNS, microglial cells exert non-immunological functions, including production of neurotrophic factors and glutamate uptake [4,5]. Dendritic cells (DCs) are specialized in capturing antigens and initiating immune response through naive T-cell activation [6], and are also implicated in maintaining tolerance to self-antigen [7]. The diverse functions of DCs in immune regulation are dictated by the instructions they received during innate immune responses to different pathogens, but DC response may be also lineage-dependent as distinct myeloid and lymphoid DC lineages have been recently identified [8]. Osteoclasts are multinucleated, adherent, bone-resorbing cells found in the bone vicinity. They play an essential role in bone remodelling, as well as in regulating calcium homeostasis [9].
Functional and phenotypic heterogeneity within the mononuclear phagocyte system may reveal differentiation plasticity of a common progenitor, but the developmental pathways leading to macrophages, osteoclasts, DCs, or microglia are still unclear. Cell transfer experiments have established that alveolar macrophages in the lung, and Kupfer cells in the liver, derive from mature monocytes [10]. Late monocyte precursors have been shown to differentiate into osteoclasts in response to macrophage colony-stimulating factor (M-CSF) and receptor activator of NFκB ligand (RANKL) [9,11]. Similarly, DCs could be derived from circulating human monocytes following stimulation with GM-CSF and interleukin-4 [12], or from human CD34+ myeloid progenitors using GM-CSF and tumor necrosis factor-α (TNFα) [13]. Furthermore, bone marrow progenitors were recently identified through their ability to differentiate into DCs or osteoclasts, depending on whether RANKL was combined to granulocyte-macrophage colony stimulating-factor (GM-CSF) or M-CSF, respectively [14]. Whereas microglia has been well characterized in vivo and in vitro, little is known about microglial precursors, and attempts to induce in vitro microglial differentiation of mature or immature monocytes have been unsuccessful [3,15]. However, recent in vivo studies have shown that microglia and other CNS resident cells (macroglia and neurons) are continuously renewed from as yet uncharacterized progenitors originating from the adult bone marrow which are, themselves or their progeny, able to cross the blood-brain barrier and give rise to mature microglia [16,17]. Altogether, data from the literature plead for the myeloid origin of macrophages, osteoclasts, DCs, and microglia. However, a global approach proved necessary for delineating lineage relationships within the mononuclear phagocyte system. In particular, learning how cellular diversity is generated in this cellular system is of great importance regarding cellular or gene therapy protocols.
Here we describe ex vivo expansion of murine myeloid cells in response to Flt3 ligand (FL) and investigate their response to cytokines inducing differentiation towards macrophages (M-CSF), osteoclasts (M-CSF plus RANKL), dendritic cells (GM-CSF plus TNFα), or microglia (glial-cell conditioned medium: GCCM). Results support a model based on the sequential commitment of Flt3+ bone-marrow progenitors.
Results
FL expands a continuum of macrophage precursors from mouse bone marrow cells
FL exerts potent stimulatory effects on precursors of the monocyte/macrophage lineage, alone or in combination with M-CSF [10,18]. Furthermore, it was recently shown that FL stimulates generation of DC precursors [19,20] or macrophages [18] when used alone, and that of osteoclasts in combination with RANKL [21]. In our conditions, bone marrow cell proliferation was observed for at least 11 days in the presence of FL (Figure. 1A). FL was used at 5 ng/ml FL throughout the present study, as this dose was sufficient to initiate bone-marrow cell proliferation (Figure. 1B). More than 90% of non-adherent day 6 cells (day 6-FL cells) and day 8 cells (day 8-FL cells) were viable cells, but FL-stimulated cultures showed decreasing cell viability from day 8 of culture (not shown). Re-feeding day-8 cultures with 5 ng/ml FL resulted in only slightly increased viability of day-11 FL cells while it strongly stimulated cell proliferation (data not shown).
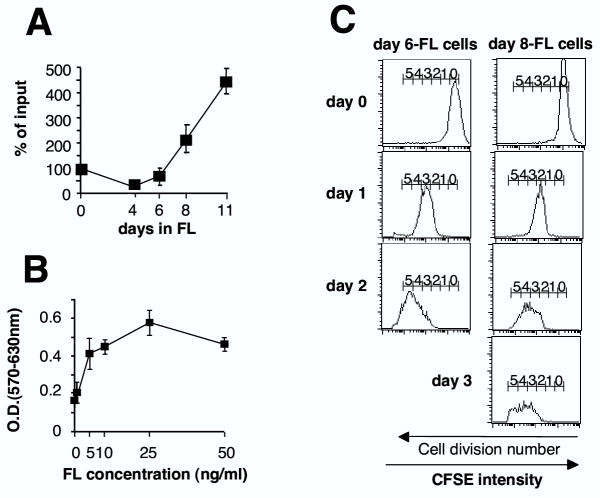
Figure 1.
FL promotes outgrowth of immature monocytic cells from mouse bone marrow in vitro (A) FL expanded non-adherent cells were counted after different culture durations. Data are expressed as % of initial cell number and represent mean ± SEM of five independent experiments (3 experiments for day 11). (B) FL-dose-dependent proliferation was assessed by using the MTT assay after 6 days of culture in 96-well plates. Data are mean ± SEM of three independent experiments. (C) Tracking division of day 6-FL cells (left panel) and day 8-FL cells (right panel). Cells were stained with CFSE then used for reseeding original culture flasks. Division number is indicated based on sequential halving of CFSE fluorescence. Data are representative of three independent experiments.
Next, we asked whether FL-stimulated growth between day 6 and day 11 could be accounted for by continuous expansion of a common progenitor cell population. Using CFSE labelling, we show that day 6-FL cells as well as day 8-FL cells actively and synchronously divided, consistent with rapid and homogeneous outgrowth of FL-cells (Fig. 1C). We conclude that the whole day 6-FL cell population generates day 8-FL cells that are precursors of day 11-FL cells.
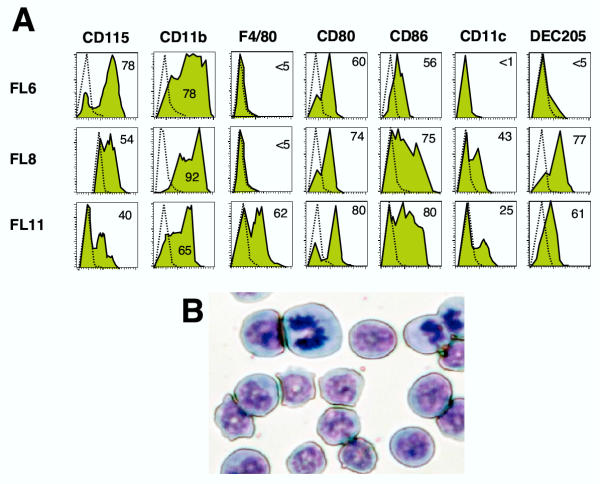
Day 6-FL cells express a homogeneous immunophenotype suggestive of maturing monocytic cells: CD11b+, CD115+ (M-CSF receptor) and F4/80-, a macrophage marker (Fig. 2A). Consistently, cells showed high nucleus/cytoplasm ratio, basophilic cytoplasm and bean- or ring-shaped nuclei (Fig. 2B). At later culture times, FL-stimulated cells maintained CD11b expression, but acquired expression of markers expressed on DCs, such as DEC 205 and CD11c, at day 8, and F4/80, at day 11 (Fig. 2A). Modulation of cell-surface marker expression suggested that FL-cells might develop various differentiation potentials along with culture duration.
Figure 2.
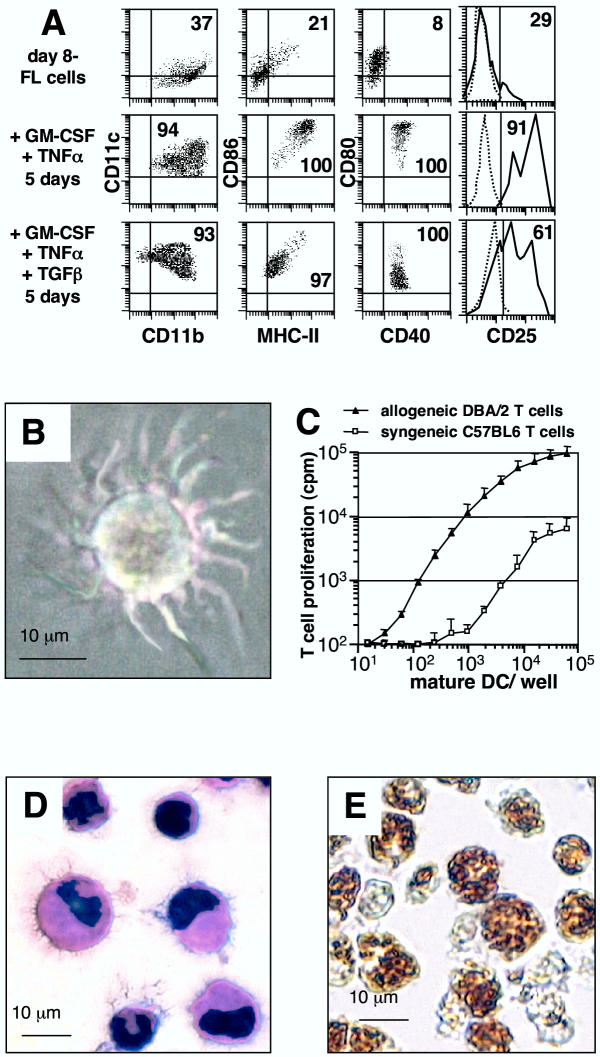
Characterization of FL cultivated cells. (A) Flow cytometry analyses of day 6-, day 8-, and day 11-FL cells. Filled histograms: expression levels of the markers indicated, dotted line: background staining with irrelevant antibodies. Results are representative of 3 to 5 independent experiments. Numbers represent the average percentage of positive cells. (B) May Grünwald Giemsa reagent staining of day 6-FL cells.
Macrophage potential of day 6-FL cells can be directed towards osteoclast differentiation
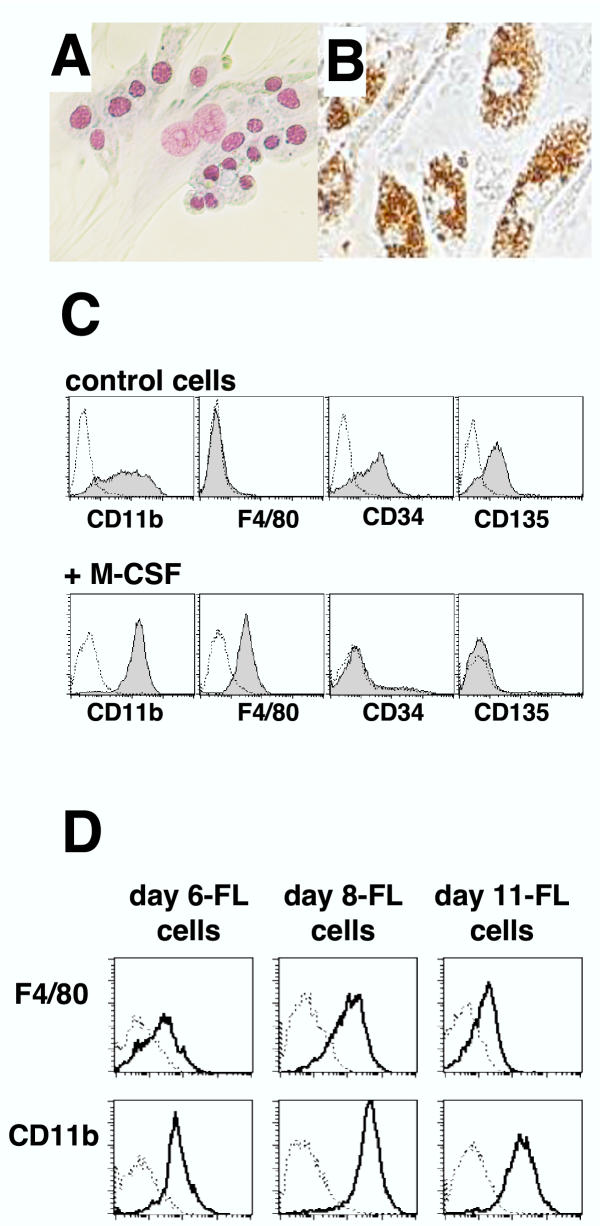
Stimulation of day 6-FL cells with M-CSF for 4 days resulted in limited proliferation, then differentiation into adherent (Fig. 3A), phagocytic (Fig. 3B) macrophage-like cells. Accordingly, morphology changes were accompanied by decreased CD34 and CD135 (Flt3) expression, but increased CD11b levels and induced F4/80 expression (Fig. 3C). Similarly, day 8-FL cells and day 11-FL cells differentiated to macrophages in response to M-CSF, as shown by expression of F4/80 and CD11b antigens (Fig. 3D), correlating with level of CD115 expression (Fig. 2A).
Figure 3.
Macrophage differentiation in response to M-CSF (A) Day 6-FL cells were stained with May Grünwald Giemsa reagent after 4 days of culture in M-CSF (500 U/ml). (B) Phagocytic activity of M-CSF-differentiated day-6 FL cells is shown by ingestion of brown latex beads. (C) Flow cytometry analysis of CD11b, F4/80, CD34 and CD135 expression on M-CSF-differentiated day 6-FL cells. (D) Flow cytometry analysis of CD11b and F4/80 expression on M-CSF-differentiated day 6-, day 8-, and day 11-FL cells.
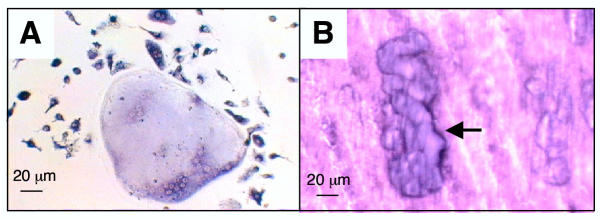
To determine whether FL-expanded precursors could be committed towards osteoclasts, a combination of M-CSF and RANKL was applied to day 6-FL cells, in the absence of FL. After 6 days of this treatment, large multinucleated cells were formed and all cells in the cultures were expressing tartrate-resistant acidic phosphatase (TRAP) activity, a classical osteoclast marker (Fig. 4A). Overall, when grown on dentin slices, cells formed typical resorption pits indicating that they were functional osteoclasts (Fig. 4B).
Figure 4.
Osteoclast differentiation of day 6-FL cells (A) Day 6-FL cells cultivated for 6 days in the presence of M-CSF and RANKL formed mixed population of large multinucleated cells together with mononucleated cells. Note that 100% of the cells are TRAP-positive, as indicated by their blue staining. (B) When grown on top of dentin slices, cells actively resorbed dentin as shown by arrows pointing to the toluidin blue stained resorption pits.
Macrophage potential of day 8-FL cells can be directed towards myeloid-related DC differentiation
Flt3+ myeloid progenitors purified from mouse bone marrow have been shown to generate both CD8α- and CD8α+ DCs in FL-supplemented cultures [19]. In preliminary experiments, we sought for culture conditions that could specifically generate myeloid-related DCs and found that a combination of GM-CSF and TNFα triggered a pure myeloid-related DC differentiation of day 8-FL cells. Differentiation occurred in the absence of [3H]-thymidine incorporation, but cell viability was greater than 90% (not shown), indicating massive differentiation of day 8-FL cells. Mature DC differentiation was assessed by phenotypic, morphological and functional analysis. CD11b expression was maintained, but cells acquired CD11c+, CD86high, MHCIIhigh, CD80high, CD40+, and CD25high expression (Fig. 5A), and were negative for CD8α, B220, IgM, CD3 and CD71 antigens (not shown). Consistent with mature DC immunophenotype, cells displayed a typical dendritic morphology (Fig. 5B) and elicited greatly enhanced allogeneic T-cell response compared to syngeneic stimulation (Fig. 5C), whereas they were unable to effectively phagocyte latex beads (data not shown).
Figure 5.
Day 8-FL cells can differentiate into immature or mature DCs depending on the presence of TGFβ (A) Flow cytometry analyses were performed on day 8-FL cells at day 0 and 5 after culture in the presence of 5 ng/ml GM-CSF plus 10 ng/ml TNF-α without or with 10 ng/ml TGF-β. Numbers are the percentages of total viable cells. Controls are positioned by quad limits and dotted histograms. Data are representative of 5 experiments. (B) Day 8-FL cells cultured for 5 days in the presence of GM-CSF+TNF-α showed mature DC morphology in culture. (C) Comparison of allo- and syngeneic stimulatory activities of day 8-FL-derived mature DCs generated from C57BL/6 mice after 5 days of culture in GM-CSF+TNF-α. Results are expressed as mean cpm ± SD from triplicate cultures and shown for one triplicate experiment representative of three. (D) GM-CSF+TNF-α+TGFβ- derived DCs showed immature morphology after May Grünwald Giemsa staining and (E) phagocytic activity.
Interestingly, addition of TGFβ directed DC differentiation towards immature immunophenotype (CD80low, CD86low, MHCIIlow; see Fig. 5A), morphology (shorter dendrites; see Fig. 5D), and function, as they could phagocyte latex beads (Fig. 5E). They were also potent allostimulatory cells (data not shown). These cells represent a double-negative CD4-/CD8- immature DC population but not typical Langerhans cells, as they do not contain any Birbeck granules (not shown).
Late commitment of macrophage precursors towards microglia differentiation
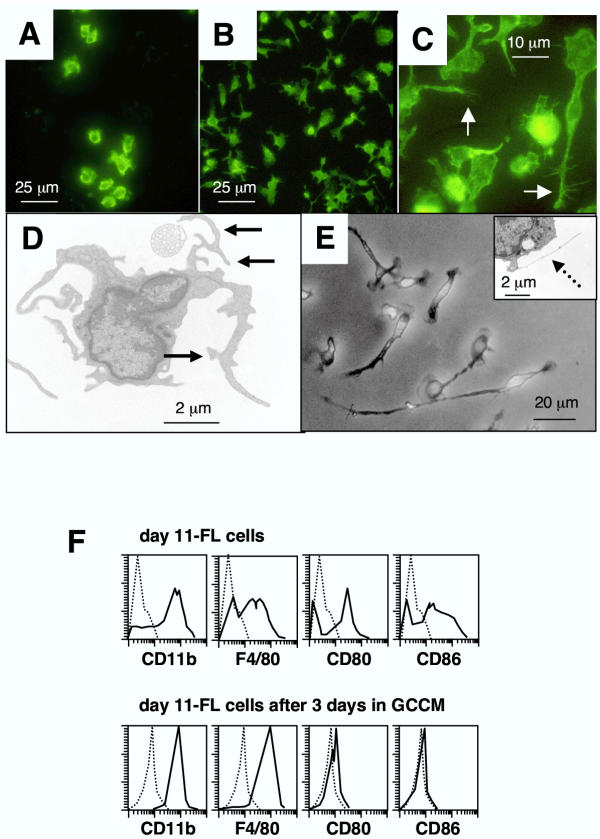
In order to evaluate microglia differentiation potential, day 11-FL cells were cultured for 3 or 6 days with or without 50% GCCM. Microglia cells share a number of immunological markers with other classes of mononuclear phagocytes but present a unique morphology characterized by the presence of multiple cellular processes and micro-spikes or spines [3]. GCCM-treated day 11-FL cells adhered to plastic, maintained CD11b expression (Fig. 6A, 6B) and 20% of cells developed a ramified morphology and bore actin-filled micro-spikes and pseudopodia (Fig. 6C). By performing electron microscopy on differentiated day 11-FL cells, we were able to ascertain the typical morphological features which characterize microglia in vitro[3]: thread-like structures of 0.05 nm diameter and cellular processes bearing numerous cell-surface spines of 0.1 nm diameter (Fig. 6D). After 6 days of GCCM treatment, process length reached more than 3 times the cell body diameter in 20% of cells (Fig. 6E), and more numerous thread-like structures were observed by electron microscopy (Fig. 6E, insert). As reported for cultured microglia [2,22], differentiated day 11-FL cells exhibited high cell surface expression of the macrophage markers CD115, CD11b and F4/80, and low or undetectable expression of CD80, CD86 (Fig. 6F) and MHC class II antigen (data not shown).
Figure 6.
Characterization of day 11-FL cells differentiated into microglial cells (A) CD11b staining of day 11-FL cells cultured for 3 days in presence of fresh media alone. (B) CD11b staining of day 11-FL cells cultured for 3 days in presence of 50% GCCM. (C) Actin-phalloidin staining of day-11 FL cells cultured for 3 days in presence of 50% GCCM. Note the presence of actin-filled spines and pseupodia (white arrow). (D) Electron microscopy photomicrograph of day 11-FL cells cultured for 3 days in presence of 50% GCCM. Differentiated cells exhibited cell processes bearing spines of 0.1 μm diameter (black arrows). (E) After 6 days in the presence of GCCM, cells exhibited longer processes expanding from the cell bodies as shown by phase-contrast microscopy. Insert shows typical thread-like structures of 0.05 μm diameter (dashed arrow). (F) FACS profiles compare immunophenotype of day 11-FL cells and adherent cells obtained after cultivating day 11-FL cells for 3 days in the presence of 50% GCCM.
Differentiation plasticity of FL-expanded macrophage precursors is time-dependent
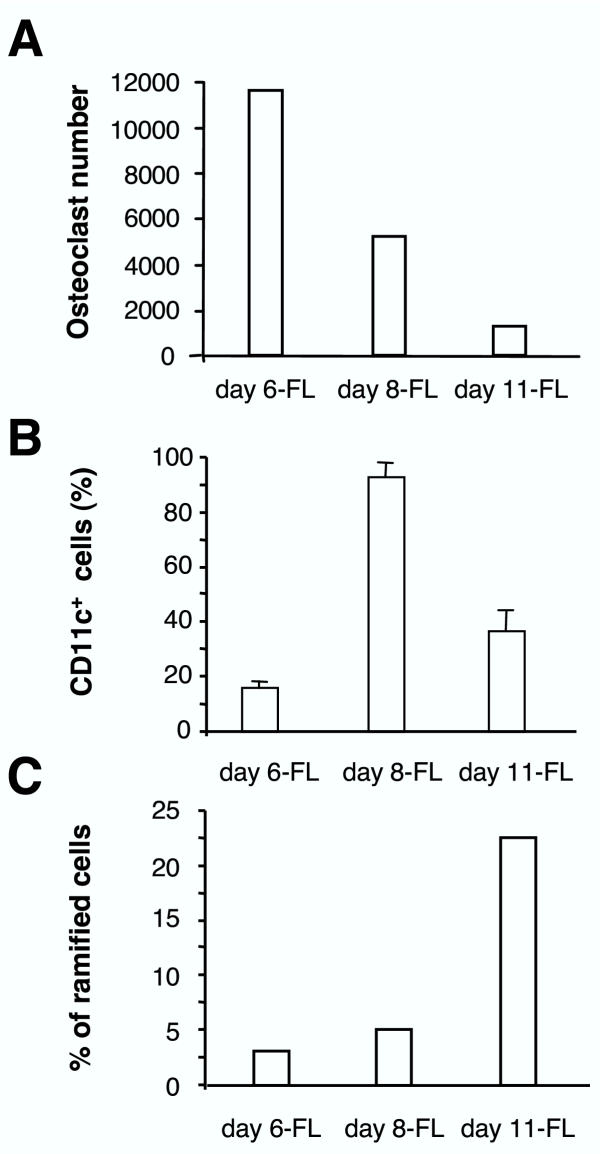
In experiments reported above, FL cells reproducibly differentiated into macrophages in response to M-CSF, whatever duration of the primary culture. Differentiation to osteoclasts, DCs, and microglia was obtained by cultivating day 6-, day 8- and day-11 FL cells in the presence of M-CSF and RANKL, GM-CSF and TNFα, or GCCM, respectively. Time-course studies showed that these conditions were optimal (Fig. 7). Indeed, osteoclast differentiation sharply decreased between day 6 and day 11 of primary cultures (Fig. 7A). Differentiation to mature DCs in response to GM-CSF and TNFα combination was maximal with day 8-FL cells, as shown by CD11c expression (Fig. 7B). Finally, percentage of typical microglial cells obtained in response to GCCM reached a peak using day 11-FL cells (Fig. 7C). These data clearly confirmed that differentiation ability of macrophage precursors was dependent on the duration of the primary cultures.
Figure 7.
Differentiation potential of FL cells is time dependent Day 6-, day 8-, and day 11-FL cells were differentiated to osteoclasts, DCs, and microglia using specific cytokine combinations. Percentage of differentiated cells was measured as specified below. (A) Cells were grown in presence of M-CSF and RANKL and the number of osteoclasts was counted 6 days later. Similar results have been consistently obtained in several experiments. (B) Cells were treated by GM-CSF and mature DC percentage was determined by flow cytometry determination of CD11c+ cells. Results are representative of 3 independent experiments. (C) Microglia differentiation was assessed by detection of ramified cells after cultivating the cells for 6 days in the presence of GCCM. This experiment is representative of 4 independent experiments.
Discussion
The culture system described here is to our knowledge the first to enable mass selective production of pure osteoclasts, pure CD8α- DCs, and pure macrophages, thus providing a unique tool to study primary cells from the mononuclear phagocyte system. Indeed, bone marrow from a single mouse (7–10 × 107 cells) currently yields 1–2 × 107 osteoclasts, 2–3 × 108 DCs, or 1–2 × 108 macrophages. In particular, this protocol will facilitate investigations on genetically modified cells obtained from transgenic or knockout mice, or following retrovirus-mediated cell transfer.
CFSE monitoring of cell division demonstrated that most day 6-FL cells are precursors of day 8-FL cells. Both cell types were shown to be macrophage precursors endowed with osteoclast or prominent DC differentiation potential, respectively. As both proliferation and cell mortality were limited or absent during the differentiation process (not shown), we conclude that osteoclast or DC differentiation of FL cells was not preceded by preferential expansion of distinct precursor sub-populations. Thus we propose that Flt3+ macrophage precursors commit sequentially to osteoclast and DC differentiation, possibly through time-dependent expression of intracellular factors. Consistent with this model, Miyamoto et al. have recently demonstrated that M-CSF and GM-CSF regulate differentiation of osteoclasts and DC from a common precursor whether they express c-Fos or not, respectively [14]. It remains to be shown whether FL-expanded osteoclast precursors are identical or not to those previously described by Arai et al. [11].
The protocol described in the present paper also represents a powerful tool for studying microglia differentiation control mechanisms. It now becomes possible to determine which soluble factors in GCCM induce microglia differentiation. In addition to macrophages and microglia, day 11-FL cells could also differentiate to DCs (30 to 40%), though less efficiently than day 8-FL cells (> 90%). It was recently suggested that native microglia are uncommitted precursors of DC and macrophages [23]. However, it is not clear whether this results from microglia plasticity or de-differentiation to an uncommitted precursor. In that respect, day 11-FL cells, that have no microglia characteristics, may represent a valuable model to delineate the relationship between DC and microglia pathways.
Facilitating in vitro studies on microglia differentiation may help to shed some light on several physiopathological processes. Indeed, microglia is the principal immunoeffector cell located in the CNS parenchyma, and its involvement in pathogenesis has been established for numerous neurological conditions including stroke, trauma and Alzheimer's disease [24]. Furthermore, bone marrow-derived progenitors committed to microglial differentiation should be ideally suited for CNS-targeted gene therapy since they may be able to migrate from blood to brain through an intact blood-brain barrier [16,17,25].
The unique DC properties of either eliciting immunity or ensuring self-tolerance [7], make them candidates for enhancing responses against foreign antigens in vaccination protocols [26] and against tumor antigens in cell immunotherapy [27], as well as making them targets for the treatment of autoimmunity and allograft rejection [6]. Whether mature or immature DCs should be targeted in these various fields is a matter of debate that could be solved with the two culture conditions described, with and without the use of TGFβ. These last years, substantial progress were made to characterize distinct subsets of DCs, based on differential phenotype and function, both in humans and mice. In mice, all DC subsets described so far express CD11c [28]. CD8α homodimer expression now appears to be more related to maturation state than lineage origin [8]. Flow cytometry analysis of spleen cells from mice treated with FL for 9 days reveals five pre-DC or DC subpopulations that occur in distinct microenvironments of the spleen [29]: two putative immature populations, CD11b+ Ly6C+ Gr-1+ (named A, B), and three CD11c+ DC populations distinguished by CD8α and CD11b expressions (named C, D, E). Although phenotypic characterization of these DCs does not exactly fit to the in vitro FL-derived DCs presented in this work, latter cells are closely related to spleen DCs of myeloid origin, referred to as population C by Pulendran et al.[29].
Plasmacytoid DCs, also called interferon producing cells (IPC) were recently characterized in humans and mice [30-33]. Both myeloid and plasmacytoid DCs were clearly expanded by FL in vivo, with or without GM-CSF mobilization [33]. Recently, Gilliet et al. demonstrated that plasmacytoid DC precursors are differentially regulated by FL and GM-CSF [20]. Though cellular phylogeny between Flt3+ progenitors and DCs become more and more clear, the relationships between Flt3+ bone-marrow progenitors (0.3% of total bone marrow cells), lymphoid progenitors and myeloid progenitors remains to be clarified. Concerning functional aspects, DC-dependent T-cell polarizing activity (Th1-, Th2-, Th3- or Tr-type) is not related to lineage origin, but rather depends on the stimuli used to activate these cells, probably due to their equipment and subsequent transduction of pathogen associated molecular pattern receptors, such as Toll receptor family [34].
Conclusions
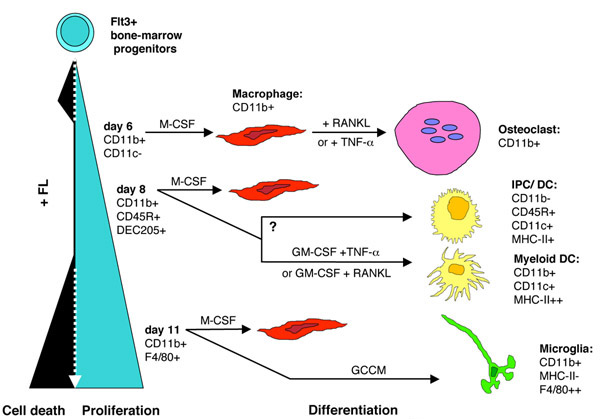
Based on the present study and data from the literature, we propose a model where early Flt3+ progenitors commit themselves to a sequential differentiation program proceeding through narrow windows; each time window corresponds to expression of a specific differentiation potential (Fig. 8). A first phase of selection (hatched line), likely through apoptotic death of Flt3- cells, would lead to Flt3+ cell expansion (plain line). Each time window may permit generation of different types of precursors able to differentiate into osteoclasts, then into DCs, and finally, for 25% of day 11-FL cells, into microglia cells. Moreover, FL cells can differentiate to macrophages in response to M-CSF whatever the duration of primary culture in FL.
Figure 8.
Model showing the sequential commitment of FL-expanded precursors to macrophages, osteoclasts, dendritic cells, and microglia GCCM: glial cell-conditioned medium; IPC: interferon producing cells.
Data obtained with transgenic FL-mice and FL deficient mice support a major role of FL in DC development [35]. In contrast, development of DC lineages is almost normal in lymphoid organs of mice deficient for GM-CSF or GM-CSF receptor [36]. It would be interesting to test whether DC differentiation from FL cultivated cells is dependent on the presence of GM-CSF, or whether it could be obtained in the presence of proinflammatory cytokines or bacteria-derived products, as recently shown [19]. Indeed, an attractive hypothesis would be that DCs represent the default cell type in the progeny of Flt3+ progenitors, unless appropriate microenvironment stimulation is added to differentiate progenitors to osteoclasts (M-CSF and RANKL), macrophages (M-CSF) or microglia (GCCM). Nevertheless, the picture emerging from our study is that diversity within the mononuclear phagocyte system is generated from a continuum of macrophage precursors whose differentiation potential might change as a function of relative ageing and cytokine environment. Whether this sequential commitment process is cell-autonomous or depends on paracrine/autocrine loops remains to be determined. However, in vivo experiments have clearly shown that tissue macrophages may differentiate into lymph-node DCs when exposed to a phagocytic stimuli [37]. Moreover, microglia could be induced to acquire specific DC or macrophage markers when exposed to GM-CSF or M-CSF, respectively [23]. Thus, mature macrophages, DCs, or microglia, may activate latent differentiation programs under appropriate stimuli. We have shown here that macrophage precursors show more limited differentiation potential, raising the attractive possibility that mature cells cells of the mononuclear phagocyte system are more capable of differentiation plasticity than their precursors.
Methods
FL-cell production and proliferation
Total bone marrow cells from C57BL/6 mice (IFFA-CREDO, France) were seeded at 5 × 105 cells/mL in Iscove's Modified Dulbecco's Medium (IMDM; Life Technologies) supplemented with 15% fetal calf serum (FCS; Dutscher) and 5 ng/mL human FL (R&D Systems). Indeed, preliminary experiments indicated that human FL was as efficient as murine FL in stimulating bone marrow cell proliferation (data not shown). Cultures were performed at 37°C, 5% CO2. After different durations of culture, as specified in the text, non-adherent cells were recovered following gentle flushing of the culture flasks, and therefore designated as FL-cells. For FL-dose response studies, cells were cultivated under the same conditions in 0.1 mL of culture medium per each culture well in 96-well plates. Proliferation was assessed after 6 days of culture using the MTT assay, which was performed as previously described [38].
For cell division studies, non-adherent cells were harvested from FL-stimulated cultures at day 6 or day 8, stained with carboxyfluorescein diacetate, succinimidyl ester (CFSE) as previously described [39], and cultured for durations as indicated. In order to ensure optimal growth, culture medium and flask were re-used for culturing CFSE labelled cells.
Macrophage differentiation in vitro
FL-cells were cultured for 4 days in the presence of 500 U/ml M-CSF. The source of M-CSF was supernatant from Sf9 insect cells expressing murine M-CSF from a baculovirus vector. One unit was defined as being able to stimulate one colony from 50,000 mouse bone marrow cells in standard agar cultures [40].
Osteoclast differentiation and characterization
Cells were seeded at 1.8 × 105 cells/well of 24-well plates in α-MEM medium (Life technologies) supplemented with 10% FCS (Hyclone), 1% glutamine, in the presence of M-CSF (500 U/mL) and human RANKL (3% of supernatant from RANKL cDNA-expressing yeast, corresponding to about 30 ng/ml of recombinant RANKL). TRAP activity in osteoclast cultures was identified by using the Leukocyte acid phosphatase kit (Sigma). For assessing resorption activity, cells were maintained on dentin slices for 14 days in a 10% CO2 incubator. Following cell removal, dentin slices were stained with toluidin blue to reveal resorption pits under microscope [41].
Dendritic cell differentiation and functional characterization
FL cells were seeded at 106/ml in RPMI 1640 supplemented with 10 mM Hepes, 2 mM L-glutamine, 40 μg/ml gentamycine, 10% FCS (Gibco-BRL), 5 × 10-5 M β-mercapto-ethanol (Sigma) and 5 ng/ml murine GM-CSF, 10 ng/ml murine TNF-α with or without 10 ng/ml human TGF-β (Peprotech). Additional 50% Percoll gradient permitted to enhance DC purity from 60–80% to >90%.
For assessing syngeneic or allogenic stimulation, cells were cultured in various numbers in presence of a constant number (105/well) of T cells purified from either C57BL/6 or DBA/2 mice. DNA synthesis was assessed after an 8-h pulse with 1 μCi [3H]TdR.
Microglia cell differentiation
Cells were washed once in PBS then cultured for 6 days at 5 × 105 cells/ml in 50% GCCM and 50% DMEM containing 10% FCS. To obtain GCCM, C57BL/6 mice were sacrificed on postnatal day 2, meninges were removed and mechanically dissociated cortices were cultivated for 10 days on polyornithine-coated dishes in DMEM supplemented with FCS 10% (Gibco Life Technologies). Culture supernatant was referred to as GCCM. Following culture in the presence of GCCM, adherent cells were either trypsinized and collected for FACS analysis, acetone-fixed for fluorescence studies or fixed with glutaraldehyde before electron microscopy analysis. For electron microscopy, cells were fixed directly in the culture flasks for 60 min. with glutaraldehyde in 0.1 M cacodylate buffer, postfixed in 0.1% osmium tetroxide, and dehydrated in graded ethanol solution. Gelatin capsules filled with araldite solution were put upside down over selected culture areas and polymerized at 60°C during 24 h before they were split from the culture flask. Ultrathin sections were contrasted with uranyl acetate and lead citrate. To visualize actin filaments, acetone-fixed cells were incubated with phalloidin-fluorescein (Sigma) for 50 min. at room temperature before washing in PBS.
FACS analysis
For immunofluorescence staining, 5 × 105 cells were incubated for 15 min. on ice with anti-CD16/32 monoclonal antibody (clone 24G2; Fc-Block reagent, Pharmingen), then FITC or phycoerythrin-conjugated antibodies were added for 40 min. incubation on ice. Cells were washed once in PBS before performing fluorescence analysis on a FACScan (Becton-Dickinson). Signal acquisition and result analysis were performed using Cell Quest software (Becton-Dickinson). Monoclonal antibodies used here were as follows: anti-CD4 (clone RM4-5), CD8α (5H10), B220 (RA3-6B2), F4/80 (clone CI:A3-1), and CD34 (clone MEC14.7) were from Caltag; anti-CD11b (clone M1/70), CD11c (clone HL3), CD40 (clone 3/23), CD80 (clone 16-10A1), CD86 (clone GL1), CD135 (clone A2F10.1), and MHC II (Iab; clone 25-9-17), were from Pharmingen; anti-CD25 (clone PC615.3) was from Coulter-Beckman. For staining with anti-CD115 (clone 2E-11, ref. [42]), cells were first incubated with goat IgG (Sigma) for 15 min. on ice, then 2E-11 antibody was added for a 40-min. incubation on ice. After washing in PBS, cells were resuspended in fluorescein conjugated F(ab')2 fragments of goat anti-rat immunoglobulins (Caltag) for a 40 min. incubation anice.
Authors' contributions
CSD, SA, PJ, and SN contributed equally to this work.
CSD conducted and performed experiments on DC potential, participated to FL cell immunophenotyping, and drafted corresponding parts of the manuscript; SA set up conditions for FL expansion of mouse bone marrow cells; PJ conducted experiments on osteoclastic potential and drafted the corresponding part of the manuscript; SN conducted experiments on microglia potential and drafted the corresponding part of the manuscript; MFG obtained the various FL cell populations studied here and performed CFSE experiments; CS carried out studies on macrophage differentiation potential; CD and OD carried out experiments on osteoclast differentiation and characterization; AR and MP participated in the study of DC differentiation; CD performed electron microscopy analyses; DH performed studies on Birbeck granules in DC differentiated cells; GRG was instrumental in providing anti-CD115 monoclonal antibody which greatly help to set up and standardize FL culture conditions; MFB and CRC are heads of groups working on microglia and DC, respectively; GM coordinated the study and participated to flow cytometry analyses.
Acknowledgments
Acknowledgements
Human RANKL cDNA was a generous gift of Osteopro, Denmark. Dentin slices were generous gifts from Dr T. Suda (Tokyo, Japan) and DrN. Takahashi (Nagano, Japan). This work was supported by grants from Centre National pour la Recherche Scientifique; INSERM; Association pour la Recherche sur le Cancer (grants 5753 and 5738); Ligue Nationale contre le Cancer (label "La Ligue" for GM's group) and Comité du Rhône de la Ligue; Région Rhone-Alpes (Programme Emergence); S.N. and O.D. were supported by grants from the Fondation pour la Recherche Médicale and the Ministère de l'Enseignement et de la Recherche. The authors wish to thank Larry R. Rohrschneider for critical review of the manuscript.
Contributor Information
Christine Servet-Delprat, Email: servet@cervi-lyon.inserm.fr.
Sylvie Arnaud, Email: sylvie.pecontal@free.fr.
Pierre Jurdic, Email: Pierre.Jurdic@ens-lyon.fr.
Serge Nataf, Email: nataf@lyon.inserm.fr.
Marie-France Grasset, Email: grasset@univ-lyon1.fr.
Caroline Soulas, Email: Caroline.Soulas@hcuge.ch.
Chantal Domenget, Email: cdomenge@ens-lyon.fr.
Olivier Destaing, Email: odestain@ens-lyon.fr.
Aymeric Rivollier, Email: rivollier@cervi-lyon.inserm.fr.
Magali Perret, Email: perret@cervi-lyon.inserm.fr.
Christiane Dumontel, Email: dumontel@lyon.inserm.fr.
Daniel Hanau, Email: daniel.hanau@efs-alsace.fr.
Gary L Gilmore, Email: ggilmore@wpahs.org.
Marie-Françoise Belin, Email: belin@lyon.inserm.fr.
Chantal Rabourdin-Combe, Email: rabourdin@cervi-lyon.inserm.fr.
Guy Mouchiroud, Email: gmouchir@biomserv.univ-lyon1.fr.
References
- Gordon S. The macrophage. Bioessays. 1995;17:977–986. doi: 10.1002/bies.950171111. [DOI] [PubMed] [Google Scholar]
- Perry VH. A revised view of the central nervous system microenvironment and major histocompatibility complex class II antigen presentation. J Neuroimmunol. 1998;90:113–121. doi: 10.1016/S0165-5728(98)00145-3. [DOI] [PubMed] [Google Scholar]
- Giulian D, Li J, Bartel S, Broker J, Li X, Kirkpatrick JB. Cell surface morphology identifies microglia as a distinct class of mononuclear phagocyte. J Neurosci. 1995;15:7712–7726. doi: 10.1523/JNEUROSCI.15-11-07712.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkabes S, DiCicco-Bloom EM, Black IB. Brain microglia/macrophages express neurotrophins that selectively regulate microglial proliferation and function. J Neurosci. 1996;16:2508–2521. doi: 10.1523/JNEUROSCI.16-08-02508.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M, Nakanishi H, Nabekura J, Akaike N. AMPA-kainate subtypes of glutamate receptor in rat cerebral microglia. J Neurosci. 2000;20:251–258. doi: 10.1523/JNEUROSCI.20-01-00251.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000;191:411–416. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traver D, Akashi K, Manz M, Merad M, Miyamoto T, Engleman EG, Weissman IL. Development of CD8alpha-positive dendritic cells from a common myeloid progenitor. Science. 2000;290:2152–2154. doi: 10.1126/science.290.5499.2152. [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- Kennedy DW, Abkowitz JL. Mature monocytic cells enter tissues and engraft. Proc Natl Acad Sci U S A. 1998;95:14944–14949. doi: 10.1073/pnas.95.25.14944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai F, Miyamoto T, Ohneda O, Inada T, Sudo T, Brasel K, Miyata T, Anderson TM, Suda T. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. J Exp Med. 1999;190:1741–1754. doi: 10.1084/jem.190.12.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caux C, Vanbervliet B, Massacrier C, Dezutter-Dambuyant B, de Saint-Vis B, Jacquet C, Yoneda K, Imamura S, Schmitt D, Banchereau J. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNF alpha. J Exp Med. 1996;184:695–706. doi: 10.1084/jem.184.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Ohneda O, Arai F, Iwamoto K, Okada S, Tagaji K, Anderson D, Suda T. Bifurcation of osteoclasts and dendritic cells from common progenitors. Blood. 2001;98:2544–2554. doi: 10.1182/blood.V98.8.2544. [DOI] [PubMed] [Google Scholar]
- Wilms H, Wollmer MA, Sievers J. In vitro-staining specificity of the antibody 5-D-4 for microglia but not for monocytes and macrophages indicates that microglia are a unique subgroup of the myelomonocytic lineage. J Neuroimmunol. 1999;98:89–95. doi: 10.1016/S0165-5728(99)00066-1. [DOI] [PubMed] [Google Scholar]
- Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–1782. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- Nicholls SE, Winter S, Mottram R, Miyan JA, Whetton AD. Flt3 ligand can promote survival and macrophage development without proliferation in myeloid progenitor cells. Exp Hematol. 1999;27:663–672. doi: 10.1016/S0301-472X(98)00072-1. [DOI] [PubMed] [Google Scholar]
- Brasel K, De Smedt T, Smith JL, Maliszewski CR. Generation of murine dendritic cells from flt3-ligand-supplemented bone marrow cultures. Blood. 2000;96:3029–3039. [PubMed] [Google Scholar]
- Gilliet M, Boonstra A, Paturel C, Antonenko A, Xu XL, Trinchieri G, O'Garra A, Liu YJ. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J Exp Med. 2002;195:953–958. doi: 10.1084/jem.20020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lean JM, Fuller K, Chambers TJ. FLT3 ligand can substitute for macrophage colony-stimulating factor in support of osteoclast differentiation and function. Blood. 2001;98:2707–2013. doi: 10.1182/blood.V98.9.2707. [DOI] [PubMed] [Google Scholar]
- Carson MJ, Reilly CR, Sutcliffe JG, Lo D. Mature microglia resemble immature antigen-presenting cells. Glia. 1998;22:72–85. doi: 10.1002/(SICI)1098-1136(199801)22:1<72::AID-GLIA7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Santambrogio L, Belyanskaya SL, Fischer FR, Cipriani B, Brosnan CF, Ricciardi-Catagnoli P, Stern LJ, Strominger JL, Riese R, et al. Developmental plasticity of CNS microglia. Proc Natl Acad Sci U SA. 2001;98:6295–6300. doi: 10.1073/pnas.111152498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia, a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Priller J, Flugel A, Wehner T, Boentert M, Haas CA, Prinz M, Fernandez-Klett F, Prass K, Bechmann I, de Boer AB, et al. Targeting of gene-modified hematopoietic cells to the central nervous system: use of green-fluorescent protein uncovers microglial engrafment. Nat Med. 2001;7:1356–1361. doi: 10.1038/nm1201-1356. [DOI] [PubMed] [Google Scholar]
- Su H, Messer R, Whitmire W, Fischer E, Portis JC, Caldwell HD. Vaccination against chlamydial genital tract infection after immunization with dendritic cells pulsed ex vivo with nonviable Chlamydiae. J Exp Med. 1998;188:809–818. doi: 10.1084/jem.188.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong L, Engleman EG. Dendritic cells in cancer immunotherapy. Annu Rev Immunol. 2000;18:245–273. doi: 10.1146/annurev.immunol.18.1.245. [DOI] [PubMed] [Google Scholar]
- Maraskovsky E, Brasel K, Teepe M, Roux ER, Lyman SD, Shortman K, McKenna HJ, et al. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med. 1996;184:1953–1970. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B, Lingappa J, Kennedy MK, Smith J, Teepe M, Rudensky A, Maliszewski CR, Maraskovski E. Developmental pathways of dendritic cells in vivo: distinct function, phenotype, and localization of dendritic cell subsets in FLT3 ligand-treated mice. J Immunol. 1997;159:2222–2231. [PubMed] [Google Scholar]
- Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, Colonna M, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- Nakano H, Yanagita M, Gunn MD. CD11c(+)B220(+)Gr-1(+) cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J Exp Med. 2001;194:1171–1178. doi: 10.1084/jem.194.8.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselin-Paturel C, Boonstra A, Dalod M, Durand I, Yessaad N, Dezutter-Dambuyant C, Vicari A, O'Garra A, Biron C, Briere F, Trinchieri G. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol. 2001;2:1144–1150. doi: 10.1038/ni736. [DOI] [PubMed] [Google Scholar]
- Bjorck P. Isolation and characterization of plasmacytoid dendritic cells from Flt3 ligand and granulocyte-macrophage colony-stimulating factor-treated mice. Blood. 2001;98:3520–3526. doi: 10.1182/blood.V98.13.3520. [DOI] [PubMed] [Google Scholar]
- Huang Q, Liu D, Majewski P, Schulte JC, Korn JM, Young RA, Lander ES, Hacohen N. The plasticity of dendritic cell responses to pathogens and their components. Science. 2001;294:870–875. doi: 10.1126/science.294.5543.870. [DOI] [PubMed] [Google Scholar]
- McKenna HJ. Role of hematopoietic factors/flt3 ligand in expansion and regulation of dendritic cells. Curr Opin Hematol. 2001;8:149–154. doi: 10.1097/00062752-200105000-00004. [DOI] [PubMed] [Google Scholar]
- Vremec D, Lieschke GJ, Dunn AR, Robb L, Metcalf D, Shortman K. The influence of granulocyte/macrophage colony-stimulating factor on dendritic cell levels in mouse lymphoid organs. Eur J Immunol. 1997;27:40–44. doi: 10.1002/eji.1830270107. [DOI] [PubMed] [Google Scholar]
- Randolph GJ, Inaba K, Robbiani DF, Steinman RM, Muller WA. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity. 1999;11:753–761. doi: 10.1016/s1074-7613(00)80149-1. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Fulcher DA, Lyons AB, Korn SL, Cook MC, Koleda C, Parish C, Fazekas de St Groth B, Basten A. The fate of self-reactive B-cells depends primarily on the degree of antigen receptor engagement and avaibility of T-cell help. J Exp Med. 1996;183:2313–2328. doi: 10.1084/jem.183.5.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourette RP, Arnaud S, Myles GM, Blanchet JP, Rohrschneider LR, Mouchiroud G. Mona, a novel hematopoietic-specific adaptor interacting with the macrophage colony-stimulating factor receptor, is implicated in monocyte/macrophage development. EMBO J. 1998;17:7273–7281. doi: 10.1093/emboj/17.24.7273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett TR, Dempster DW. A comparative study of disaggregated chick and rat osteoclasts in vitro: effects of calcitonin and prostaglandins. Endocrinology. 1987;120:602–608. doi: 10.1210/endo-120-2-602. [DOI] [PubMed] [Google Scholar]
- Shadduck RK, Waheed A, Mangan KF, Rosenfeld CS. Preparation of a monoclonal antibody directed against the receptor for murine colony-stimulating factor-1. Exp Hematol. 1993;21:515–520. [PubMed] [Google Scholar]