Abstract
Hepatocyte growth factor (HGF) and its receptor, Met, regulate a number of biological functions in epithelial and nonepithelial cells, such as survival, motility, proliferation, and tubular morphogenesis. The transcription factor NF-κB is activated in response to a wide variety of stimuli, including growth factors, and is involved in biological responses in part overlapping with those triggered by HGF. In this work we used the liver-derived MLP29 cell line to study the possible involvement of NF-κB in HGF/Met signaling. HGF stimulates NF-κB DNA binding and transcriptional activation via the canonical IκB phosphorylation-degradation cycle and via the extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase cascades. Phosphatidylinositol 3-kinase is not involved in Met-mediated NF-κB activation. Blockage of NF-κB activation in MLP29 cells by forced expression of the NF-κB super-repressor IκBα2A does not interfere with HGF-induced scatter but inhibits proliferation and tubulogenesis. Surprisingly, in the same cells NF-κB appears to be dispensable for the antiapoptotic function of HGF.
Hepatocyte growth factor (HGF) stimulates a wide variety of responses in epithelial cells. These include loss of cell-cell junctions and acquisition of motility (cell scatter), proliferation, survival, invasion of extracellular matrices, and tubular morphogenesis (16, 21). In vivo HGF has been implicated in angiogenesis (23), in organ regeneration (51), and in tumorigenesis (37). Gene targeting studies have revealed an essential role for HGF and its receptor, Met, in the development of liver, placenta, skeletal muscles, and specific sensory and motor nerves (13, 48, 49). All of these responses depend on the activation of an array of signaling pathways triggered by the Met receptor. This results in transcription of a subset of target genes, some of which are known (14, 24, 54, 69, 80) but most of which are still to be identified.
The transcription factor nuclear factor κB (NF-κB) was originally discovered for its role in controlling gene expression in the immune and inflammatory response (4). Subsequent work has shown that NF-κB is crucial in controlling apoptosis, proliferation, and differentiation in many cell types (57). NF-κB can be activated by a heterogeneous panel of stimuli, including cytokines, bacterial or viral products, and general stress factors (56). Most of the studies on NF-κB have been done using the prototypical NF-κB activators, such as tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), or bacterial lipopolysaccharide to stimulate target cells. More recently, NF-κB has been implicated in signaling downstream of a number of growth factor receptors, such as insulin, platelet-derived growth factor (PDGF) receptor, epidermal growth factor receptor (EGFR), nerve growth factor receptor (11, 31, 47, 63), and activated oncogenes (52).
In most unstimulated cells, NF-κB is a heterodimer of a p50 and a p65 subunit (also known as p65 RelA). NF-κB is retained in the cytoplasm by the IκB inhibitor proteins, which mask a nuclear localization signal on p65. Cell stimulation triggers a dual mechanism of NF-κB activation (64). The canonical mechanism involves serine phosphorylation of IκBα, followed by its ubiquitination and rapid proteasome-mediated degradation. Free NF-κB thus released can translocate into the nucleus and enhance transcription of target genes by binding to specific consensus sequences in their promoter region. Phosphorylation of IκBα is carried out by the multisubunit IκB kinase (IKK), which is in turn activated by the NF-κB-inducing kinase or by the mitogen-activated protein kinase MEKK1 (38). Once liberated from IκBα, the NF-κB complex is subject to a second level of regulation. This involves serine phosphorylation of p65 in the transactivation domain, by kinase(s) still to be identified (67, 76, 77). These modifications do not affect DNA binding but rather increase the transactivating potential of p65, possibly by modifying its interactions with proteins of the basal transcriptional machinery and/or with coactivators, such as the CREB-binding protein (CBP) and p300 (83). Several reports have shown that this regulation can be mediated by activation of mitogen-activated protein kinases (MAPKs) or of phosphatidylinositol 3-kinase (PI3K) and its target, the protein kinase Akt (36, 46, 55, 63, 71, 75).
Mice null for p65 share with HGF and Met knockouts a liver phenotype due to massive hepatocyte apoptosis in mid-gestation (7). This suggests that Met and NF-κB may be functionally linked in liver. We thus chose to use a liver-derived cell line which expresses physiological levels of Met, MLP29 (53) to study the effects of HGF stimulation on NF-κB. Compared to the cells most commonly used for this type of studies, the Madin-Darby canine kidney cells (MDCK) (40), MLP29 cells represent a better model since they respond to HGF with the whole array of its biological effects: scattering, survival, proliferation, and tubular morphogenesis (53). Conversely, most MDCK clones respond to HGF with scatter and tubulogenesis but not with proliferation (28).
We found that HGF stimulation enhances both NF-κB DNA binding and NF-κB-dependent transcriptional activity. The signaling mechanisms mediating these effects include the classical IκBα phosphorylation-degradation cycle, as well as the extracellular signal-regulated kinases 1 and 2 (ERK1/2) and p38 MAPK, but do not involve activation of the PI3K/Akt pathway. To test the effect of NF-κB inhibition on the biological responses to HGF, we generated MLP29 cells expressing high levels of the super-repressor IκBα-2A (IκBαSR) (22). Our results indicate that NF-κB activation contributes to HGF-mediated proliferation and tubulogenesis. Conversely, HGF-induced cell scatter and protection from apoptosis seem to occur independently from NF-κB.
MATERIALS AND METHODS
Cell culture and reagents.
The mouse liver cell line MLP29 was a gift of E. Medico (University of Turin, Turin, Italy). This clonal cell line was obtained by subcloning from a parent cell line as described in reference 53. Cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% fetal bovine serum (FBS) and penicillin-streptomycin. Cells with impaired NF-κB activation were generated by transfecting into MLP29 cells, using standard electroporation methods, the pRc-CMV-IκBα2A plasmid that codes for a mutant human IκBα which cannot be phosphorylated upon stimulation (IkBαSR, originally described in reference 79 and kindly provided by J. Downward, ICRF, London, United Kingdom). Stable transfectants were selected in medium containing 0.7 mg of G418 (Gibco-BRL)/ml. Positive clones were selected by Western blot analysis using an IκBα-specific rabbit polyclonal antibody (Clone C-21 from Santa Cruz). HGF was obtained from Sigma, and TNF-α was obtained from ICN.
Inhibitors of different signaling pathways were all dissolved in Me2SO. PD 098059 (Sigma), which inhibits the MAPK kinase MEK1, and SB 203580 (Sigma), a specific inhibitor of p38 MAPK, were used at 30 μM concentrations. The PI3K inhibitor wortmannin (Sigma) was used at a 100 nM concentration. The NF-κB inhibitor BAY-11-7082 (Alexis Biochemicals, San Diego, Calif.) (58) was used in a 2.5 to 10 μM range.
Western blot analysis.
Cells were seeded in six-well cell culture plates at a density of 1.5 × 105 cells/well. After a starving time of 12 to 24 h in medium with 0.1% FBS, cells were stimulated with the indicated reagents. HGF was used at a concentration of 40 ng/ml, and TNF-α was used at 100 ng/ml. Whole-cell lysates were prepared by scraping cells into hot lysis buffer (2.5% sodium dodecyl sulfate [SDS] in 125 mM Tris-HCl [pH 6.8]) after a washing step in ice-cold phosphate-buffered saline. Lysates were boiled for 5 min and subjected to three sonication steps for 1 min each time. Quantification of proteins was performed by using the Protein Assay Dye Reagents (Bio-Rad). Equal amounts of proteins were separated by SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Blocking was done in 10% bovine serum albumin in 1× Tris-buffered saline (TBS). Immunoblot analyses were performed with various specific primary antibodies, which were visualized with horseradish peroxidase-coupled goat anti-rabbit or anti-mouse immunoglobulins (Amersham), by using the enhanced chemiluminescence Western blotting system (Amersham). Between the various incubation periods, the blots were washed extensively with 1× TBST buffer (1× TBS with 0.1% Tween 20). For control of equal protein loading, the blots were stripped with 62.5 mM Tris-HCl (pH 6.8) containing 100 mM 2-mercaptoethanol and 2% SDS at 50°C and reprobed with antiactin antibody. Polyclonal antibodies against phospho-IκBα (Ser32), phospho-Akt (Ser473), and phospho-p38 MAPK were purchased from Cell Signaling Technology. The monoclonal antibodies against phospho-ERK1/2 (clone MAPK-YT) and β-actin (clone AC-74) were obtained from Sigma. Western blots shown are representative of results obtained in at least three separate experiments.
EMSA.
To prepare nuclear extracts, cells were washed with ice-cold phosphate-buffered saline and incubated on ice in 600 μl of buffer A (10 mM HEPES [pH 7.9], 10 mM KCl, 0.1 mM EDTA, 0.5% NP-40, 1 mM dithiothreitol and 1 mM phenylmethylsulfonyl fluoride). After 10 min, cells were scraped off and nuclei were separated by centrifugation at 3000 × g for 10 min. The supernatants (cytoplasmic extracts) were removed. Pellets were resuspended in 50 μl of buffer B (20 mM HEPES [pH 7.9], 400 mM KCl, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, and 2 μg each of leupeptin and aprotinin/ml) and incubated at 4°C for 20 min with vigorous mixing. The nuclear lysates were clarified by high-speed centrifugation. For the electrophoretic mobility shift assay (EMSA), 5 μg of the nuclear extract were preincubated for 10 min at room temperature in binding buffer (10 mM HEPES [pH 7.9], 50 mM KCl, 0.2 mM EDTA, 2.5 mM dithiothreitol, 10% glycerol, 0.5% NP-40) supplemented with 0.25 mg of poly(dI-dC)·poly(dI-dC) (Amersham)/ml. This mixture was subsequently incubated in a total volume of 20 μl at room temperature with [γ-32P]ATP-labeled oligonucleotide probe (Promega) corresponding to the human consensus NF-κB site. As a control for the quality of the nuclear extracts, EMSAs were also performed with an oligonucleotide probe with a specific binding site for the ubiquitously expressed transcription factor OCT-1 (Promega). DNA-protein complexes were resolved on a 4% nondenaturing polyacrylamide gel in 0.5× Tris-borate-EDTA, and the labeled complexes were visualized by autoradiography. Specificity of the DNA-protein complexes was determined by adding a 10-fold excess of unlabeled competitor consensus oligonucleotide or 2 μl of antibodies against the NF-κB subunits p65 and p50 (Santa Cruz) prior to the binding reaction with the labeled oligonucleotide probe.
Proliferation assay and cell death enzyme-linked immunosorbent assay (ELISA).
To determine proliferation, cells were plated at a concentration of 0.8 × 104 cells/3-cm dish. After 12 h in 5% serum, cells were starved for 24 h in medium with 0.1% FBS. Cells were then stimulated with 10 ng of HGF/ml in 0.1% FBS, or the medium was changed to 5% FBS. After 48 h, cells were trypsinized and counted in a Burker's hemocytometer chamber. Statistical analysis of the data was carried out using the Student t test.
To detect apoptosis, we used a quantitative sandwich-enzyme-immunoassay based on mouse monoclonal antibodies directed against histones and DNA (Cell Death Detection ELISAPLUS, catalog no. 1774425; Roche). One essential feature of apoptotic cells is the fragmentation of DNA, due to the activation of endonucleases. Actually not all the DNA is cleaved, because the nucleosome DNA is tightly complexed with histones and therefore is protected from the action of the enzyme. In this ELISA system, an antihistone antibody, which is coated to a 96-well plate, binds the histone-DNA complexes which are recognized by anti-DNA-peroxidase antibodies. This binding, which is quantifiable by a colorimetric reaction, represents a measure of the cells' apoptosis. Cells were treated according to the manufacturer's protocol. Briefly, 104 MLP29 cells were plated onto 96-well plates together with the indicated reagents (100 μM Etoposide, 10 ng of HGF/ml, and 100 ng of TNF-α/ml). After an incubation time of 16 to 20 h, cells were lysed and 20 μl of supernatant was transferred into 96-well plates together with antihistone and anti-DNA-peroxidase antibodies. DNA fragmentation was quantified by a colorimetric reaction, by measuring the absorbance at 405 nm.
Scatter and branching tubulogenesis assays.
For the scatter assay, cells were seeded at low density in a six-well plate and cultured for 1 to 2 days until they formed colonies. After a starvation time of 12 to 24 h in medium with 0.1% FBS, cells were stimulated overnight with or without 10 ng of HGF/ml. For the branching tubulogenesis assay, collagen solution (Vitrogen 100; Collagen Corp., Fremont, Calif.) was mixed with 10× DMEM and sterile water to a final collagen concentration of 2 mg/ml and neutralized with NaOH on ice. Collagen solution (0.4 ml) was allowed to gel at 37°C in a 24-well plate. Cells (5 × 103) were plated on top of the previously gelled collagen layer in a further 0.5 ml of collagen solution. Finally 0.5 ml of DMEM with 4% FBS was added. When indicated, the medium was supplemented with HGF (10 ng/ml) and with the NF-κB inhibitor BAY 11-7082 (2.5 to 5 μM). The medium was changed every 2 days, and the cultures were photographed at 5 to 7 days.
Transient transfection and luciferase reporter assay.
One day prior to transfection, cells were seeded in six-well cell culture plates at a density of 1.5 × 105 cells/well. Transient transfections were performed using Lipofectamine reagent (Life Technologies, Inc.) following the manufacturer's protocol, with 300 ng of κB-luciferase reporter plasmid, 3xκB-Luc (kindly provided by D. Besser, Rockefeller University, New York, N.Y.). Control transfections with 300 ng of the luciferase expression plasmid pGL3 Control (Promega) were performed to check the efficiency of transfection. Twelve hours after transfection, cells were starved in medium with 0.1% FBS for an additional 16 h and then stimulated with HGF (40 ng/ml) or TNF-α (100 ng/ml) for 8 h. Where indicated, prior to stimulation cells were preincubated for 1 h with specified inhibitors. Cells were harvested with 300 μl of Reporter Lysis Buffer (Promega), and the protein concentration was determined with Protein Assay Dye Reagents (Bio-Rad). Luciferase assays were performed on equal amount of protein (≈20 μg/sample). Luciferase activity was determined using the Luciferase Assay System (Promega) and the luminometer Junior (Berthold).
RESULTS
HGF increases NF-κB DNA binding and activates NF-κB-dependent transcription.
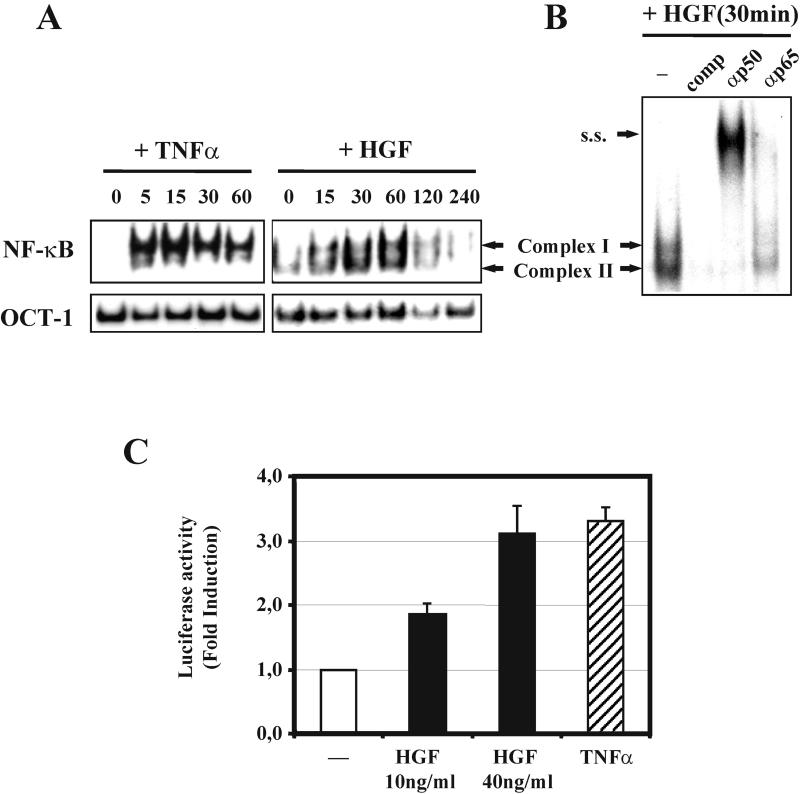
To assess whether HGF stimulation has any effect on NF-κB in epithelial cells, we first verified whether it modulates its DNA-binding activity. For this, we treated MLP29 cells with 40 ng of HGF/ml for different times and analyzed nuclear extracts by EMSA, using a radiolabeled DNA probe with a NF-κB consensus motif. While a basal level of low-molecular-weight NF-κB complex was already present in nonstimulated cells, 15 min after HGF stimulation both high- and low-molecular-weight complexes (Fig. 1A)became clearly detectable, reached a maximum at 30 to 60 min, and declined thereafter. Under similar conditions, stimulation with TNF-α (100 ng/ml) induced NF-κB DNA-binding activity more rapidly. High- and low-molecular-weight complexes were already present 5 min after stimulation and peaked at 15 min (Fig. 1A). Interestingly, while TNF-α induced predominantly the high-molecular-weight complex, HGF stimulation resulted in a pronounced induction also of the low-molecular-weight complex.
FIG. 1.
HGF enhances NF-κB DNA-binding and transcriptional activity. (A) Upper panel, MLP29 cells were stimulated with HGF (40 ng/ml) or TNF-α (100 ng/ml) for the indicated times (min). EMSAs were performed on nuclear extracts using a radiolabeled oligonucleotide probe containing a NF-κB binding site. Lower panel, to verify the quality of the nuclear extracts EMSAs were carried out in parallel with a probe specific for the constituive OCT-1 transcription factor. (B) To further characterize the NF-κB complex, nuclear extracts were preincubated with a 10-fold excess of unlabeled NF-κB oligonucleotide (comp, competitor) and with antibodies specific for the p50 or p65 subunit of NF-κB (αp50, αp65). High- and low-molecular-weight (Complex I and II) and supershifted (s.s.) complexes are indicated. Results in panels A and B are representative of experiments repeated at least three times. (C) MLP29 cells were transiently transfected with a 3xκB-Luc reporter plasmid and allowed to recover for 12 h. After an additional 16 h in medium with 0.1% serum, cells were stimulated for 8 h with HGF at the indicated concentrations or with TNF-α (100 ng/ml). Luciferase activity was determined on cell extracts normalized for protein concentration. Data are presented as fold luciferase induction, with nonstimulated control cells (—) normalized to 1. Results are the mean ± the standard error of the mean of the data obtained from at least three independent experiments.
The two types of NF-κB DNA complexes were further characterized by supershift analysis. Nuclear extracts were incubated with antibodies against the p50 or p65 subunits of NF-κB. This resulted, respectively, in the shift of both bands or in the abrogation of the higher-molecular-weight band (Fig. 1B). Thus, the high-molecular-weight NF-κB complex consists of a p65/p50 heterodimer, which is known to be transcriptionally active. The low-molecular-weight complex contains p50, presumably present as a p50/p50 homodimer or as a heterodimer with another NF-κB family member, such as p52. It should be noted that although low-molecular-weight complexes have been mostly described as transcriptionally silent, under some circumstances they may be capable of transcriptional activity (27).
Due to the complexity of its regulation, the increase in NF-κB DNA binding does not always correlate with the enhancement of NF-κB-dependent transcription. To determine whether HGF treatment induces NF-κB-mediated transcription, MLP29 cells were transiently transfected with a plasmid containing a promoter composed of three NF-κB binding sites linked to a luciferase reporter gene (3xκB-Luc). Stimulation with 40 ng of HGF/ml for 8 h caused a 3.1- ± 0.3-fold increase in luciferase activity (Fig. 1C). Lowering the HGF concentration to 10 ng/ml resulted in only a 1.8- ± 0.2-fold increase over the control, indicating that NF-κB transcriptional activity was induced by HGF in a dose-dependent manner.
In our system the increase in transcriptional NF-κB activity caused by TNF-α (100 ng/ml) was only slightly more pronounced than that caused by 40 ng of HGF/ml (Fig. 1C). The response to TNF-α did not change within a range of concentrations from 10 to 200 ng/ml.
Differential modulation of IκBα, ERK1/2, p38 MAPK, and Akt by HGF and TNF-α.
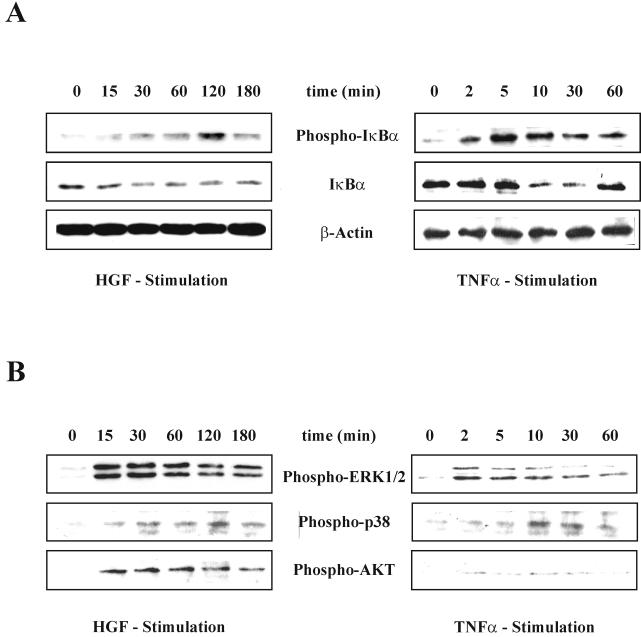
A crucial control point in NF-κB activation by TNF-α is the phosphorylation of IκBα at serines 32 and 36 followed by its ubiquitination and degradation, which result in the nuclear translocation of NF-κB. We wanted to investigate whether the activation of NF-κB by HGF involves the same mechanism. For this, we stimulated MLP29 cells with HGF (40 ng/ml) or TNF-α (100 ng/ml) for different times and monitored phosphorylation and degradation of IκBα by Western blotting, using specific polyclonal antibodies against the Ser32-phosporylated form of IκBα or against pan-IκBα.
As expected, TNF-α was very efficient at promoting phosphorylation of IκBα and subsequent degradation of the protein (Fig. 2A,right panel). Phosphorylation of IκBα reached its maximum as early as 5 min after stimulation. After 10 min, degradation of IκBα was nearly complete, and at 60 min, IκBα was already resynthesized, indicating NF-κB-dependent IκBα transcription. The fast kinetics of IκBα phosphorylation and degradation correlated well with those of induction of NF-κB DNA-binding activity by TNF-α (Fig. 1A). HGF stimulation resulted in a different pattern of modulation of IκBα (Fig. 2A, left panel). Phosphorylation of IκBα was seen only after 15 min and peaked as late as 2 h. Degradation of IκBα ensued, but the maximal loss of the protein never exceeded 40% of the total. In contrast to TNF-α stimulation, IκBα did not return to basal levels even after 3 h. The modest amount of IκBα phosphorylation and degradation seen in the first 60 min after HGF stimulation was probably responsible for the increase in NF-κB DNA-binding activity observed in the EMSA assay in the same time interval after HGF treatment (see Fig. 1A). The more pronounced phosphorylation of IκBα seen at 2 h appears to have no further effect on NF-κB DNA-binding activity.
FIG. 2.
Differential modulation of IκBα, ERK1/2, AKT, and p38 by HGF and TNF-α. MLP29 cells, starved in medium with 0.1% serum for 12 h, were stimulated for the indicated times either with HGF (40 ng/ml) or with TNF-α (100 ng/ml) (note that the time course for HGF and TNF-α stimulation is different). Phospho-IκBα and IκBα (A) and phospho-ERK 1/2, phospho-Akt, and phospho-p38 (B) were detected on Western blots of total proteins (15 μg/lane). The β-actin control confirming that an equal amount of protein was loaded in each lane applies to both panels A and B.
Recently other pathways besides IκBα degradation, such as the ERK1/2 and p38 MAPK cascades as well as PI3K/Akt, have been implicated in NF-κB transcriptional activation downstream of the TNF-α, IL-1, or PDGF receptors (36, 46, 63, 71, 75). To assess the effect of HGF and TNF-α on these three pathways in our cell model, we analyzed by Western blotting the activation of ERK1/2, Akt, and p38 following HGF and TNF-α stimulation (Fig. 2B). For this we used antibodies specific for the phosphorylated forms of these proteins. As expected, HGF activated all three pathways. Time course analysis revealed that both ERK1/2 and Akt were already fully phosphorylated after 15 min of HGF stimulation and that phosphorylation was sustained for at least 2 h. TNF-α resulted in a much less pronounced and more transient phosphorylation of ERK1/2, while phosphorylation of Akt was only barely detectable. P38 phosphorylation peaked at 30 min and was sustained for up to 3 h following HGF treatment. After TNF-α stimulation, maximal p38 phosphorylation was reached at 10 min, but it seemed to be more transient compared with that elicited by the HGF stimulus.
Inhibition of ERK1/2 or of p38 MAPK reduces HGF-induced NF-κB transcriptional activity without affecting IκBα phosphorylation.
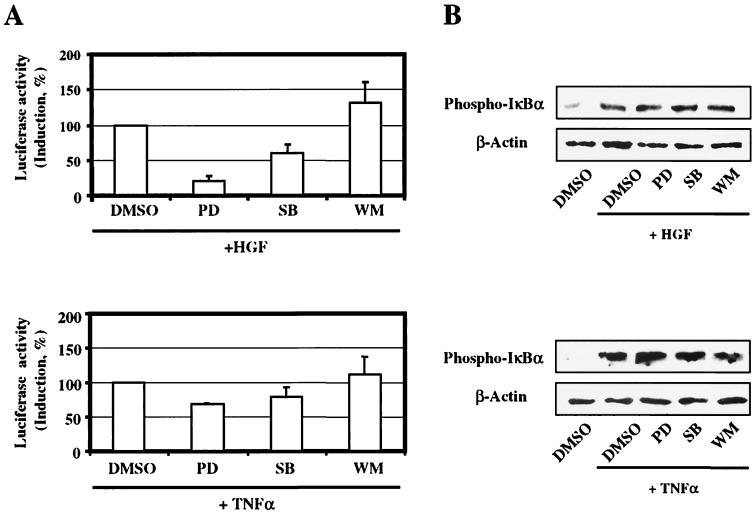
Pharmacological inhibitors, such as PD098059 (specific for MEK1), SB203580 (specific for p38 MAPK), and wortmannin (specific for PI3K) are commonly used to assess the contribution of these pathways to a biological response. The effect of the above inhibitors on HGF-induced NF-κB transcriptional activity was evaluated in a luciferase reporter assay. Cells transfected with the luciferase reporter plasmid 3xκB-Luc were preincubated for 1 h with the different inhibitors at the appropriate concentrations, and were stimulated with either HGF or TNF-α. Control assays on unstimulated cells showed that all three inhibitors had no effect on the basal level of luciferase activity (data not shown). HGF-induced NF-κB transcription was nearly abolished by PD098059 and was reduced to approximately 50% by SB203580 (Fig. 3A).Thus, both the ERK1/2 pathway and, to a lesser extent, the p38 MAPK pathway are involved in NF-κB-dependent transcriptional activation by HGF. In cells stimulated with TNF-α, the effect of the inhibitors was much less pronounced. The PI3K inhibitor wortmannin did not show an inhibitory effect on either HGF or TNF-α-induced NF-κB activation (Fig. 3A).
FIG. 3.
Effect of inhibitors on HGF and TNF-α-induced NF-κB transcriptional activity and IκBα phosphorylation. (A) Cells were transiently transfected with 3xκB-Luc reporter plasmid and incubated in rich medium for 12 h. Following 16 h in medium with 0.1% serum, cells were treated with the different inhibitors or with dimethyl sulfoxide (DMSO) for 1 h and then stimulated with HGF (40 ng/ml) or TNF-α (100 ng/ml) for 8 h prior to determination of luciferase activity. PD098059 (PD) and SB203580 (SB) were each used at a concentration of 30 μM; wortmannin (WM) was used at a concentration of 100 nM. Luciferase activity is expressed as percentage of induction, relative to that measured in cells stimulated with HGF or TNF-α, which was set to 100% (DMSO was without effect). Bars are the mean ± SEM of data collected from at least three independent experiments. (B) MLP29 cells were incubated for 12 h with medium containing 0.1% serum and pretreated either with PD098059 (PD), SB203580 (SB) (both used in a concentration of 30 μM), wortmannin (WM) (100 nM) or with DMSO alone for 1 h. Cells were then stimulated with HGF (40 ng/ml) for 1 h or with TNF-α (100 ng/ml) for 5 min, and IκBα phosphorylation was visualized by Western blotting.
To investigate whether the above pathways are involved in IκBα phosphorylation and degradation, we determined the level of phosphorylated IκBα in cells treated with the different inhibitors. Fig. 3B shows that neither HGF nor TNF-α induces IκBα phosphorylation via pathways sensitive to the inhibitors of ERK1/2, p38 MAPK or PI3K. This result was further confirmed by EMSAs demonstrating that NF-κB DNA-binding activity as well is not influenced by the use of the inhibitors (data not shown).
Taken together, our results suggest that HGF can modulate NF-κB-dependent transcription through mechanisms at least in part independent of IκBα degradation and nuclear translocation. These mechanisms involve the ERK1/2 and the p38 MAPK cascades. PI3K-dependent signaling appears to be dispensable for HGF-induced NF-κB activation.
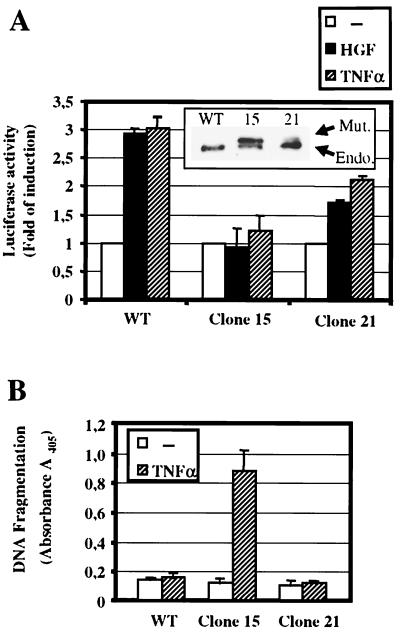
Stable expression of the specific NF-κB inhibitor IκBαSR abolishes NF-κB transcriptional activation by HGF and TNF-α and sensitizes MLP29 cells to TNF-α-induced cell death.
In order to further explore the role of NF-κB in HGF signaling, we wanted to determine whether NF-κB activity is required for the biological functions mediated by HGF. To answer this question we generated stable MLP29 clones expressing a mutant form of the human IκBα protein with serines 32 and 36 mutated into alanine (IkBα2A). This protein is no longer subject to phosphorylation and degradation and therefore acts as a potent and specific repressor of NF-κB activity (IkBα super-repressor, IkBαSR) (22, 79). A number of stable transfectants were selected by Western blot analysis. Positive clones were grouped according to high or low levels of IκBαSR expression. In high expressors, the level of IκBαSR was comparable to that of endogenous IκBα. In low expressors, the level of IκBαSR was lower (see representative clones of both groups in the inset of Fig. 4A).In clones of both groups activation of ERK1/2, p38 MAPK, and Akt upon HGF stimulation was similar to that in wild-type cells (not shown). Thus, expression of IκBαSR did not cause alterations in these signaling pathways. The inhibitory action of IκBαSR on NF-κB was verified by luciferase reporter assays with the two representative clones 15 (high-level expression of IκBαSR) and 21 (low-level expression of IκBαSR). In clone 15, TNF-α- and HGF-induced NF-κB transcriptional activity was nearly completely abolished, while in clone 21 it was approximately 50% reduced (Fig. 4A). The inhibitory activity of IκBαSR was further confirmed by assessing the ability of TNF-α to induce apoptosis in the transfected cells. It is known that while wild-type epithelial cells are mostly resistant to TNF-α-induced apoptosis, cells with impaired NF-κB activity become sensitive (8, 74, 81). We determined TNF-α-induced cell death by measuring histone-associated DNA fragments using an ELISA-based assay (see Materials and Methods). Fig. 4B shows that while wild-type and clone 21 cells were nearly unaffected by TNF-α, in clone 15 (expressing high levels of IκBαSR) DNA fragmentation was dramatically increased by TNF-α stimulation. This confirms that high levels of IκBαSR suppress NF-κB activation. On the other hand, the residual level of NF-κB present in clone 21 seems to be sufficient to counterbalance the proapoptotic effect of TNF-α stimulation.
FIG. 4.
Expression of IκBαSR inhibits HGF-induced NF-κB transcriptional activation and sensitizes MLP29 cells to TNF-α-induced cell death. (A) HGF (40 ng/ml)- or TNF-α (100 ng/ml)-induced NF-κB transcriptional activity was measured in wild-type MLP29 cells (WT) and in clones 15 and 21, using a luciferase reporter assay as described in Fig. 1C. Inset, Western blot of total protein extracts from clones 15 (high IκBαSR expressor) and 21 (low IκBαSR expressor), probed with anti-IκBα antibody. The human IκBαSR has a slightly lower mobility than the endogenous murine protein. Endo, endogenous murine IκBα; Mut, transfected human IκBαSR. (B) Wild-type MLP29 cells (WT), clone 15, and clone 21 were either treated with TNF-α (100 ng/ml) for 18 h or left untreated. Cells were lysed, and extracts were analyzed for DNA fragmentation using a photometric enzyme immunoassay, specific for cytoplasmic histone-associated-DNA fragments (Cell Death Detection ELISAPLUS kit; Roche). Absorbance was measured at 405 nm. Bars represent the mean ± standard error of the mean of data collected from three separate experiments.
NF-κB activation is dispensable for cell scattering but is required for HGF-induced proliferation.
Biological functions mediated by HGF in MLP29 cells include stimulation of cell motility (scatter), proliferation, survival, and tubulogenesis (53). These responses were evaluated in the cells expressing high or low levels of IκBαSR described above.
HGF is identical to scatter factor, a molecule which was independently identified for its ability to induce motility in epithelial cells (12; E. Gherardi and M. Stoker, Letter, Nature 346:228, 1990). MLP29 cells grow on plastic in tightly packed islands with cell-to-cell contacts mimicking the structure of an epithelial sheet. Addition of HGF to the medium causes loss of cell junctions and cell dispersal throughout the culture dish (scatter). The scatter response, which requires gene transcription and protein synthesis, is generally assessed by examining the culture dish after an overnight incubation with the growth factor (12; Gherardi and Stoker, letter).
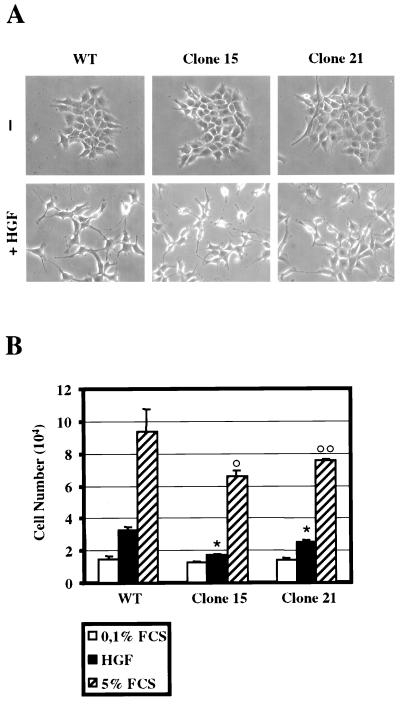
Control MLP29 cells and clones expressing high or low levels of IκBαSR (at least three independently isolated clones each) were seeded at low density and allowed to grow in 5% serum for 2 days, until they formed islets of closely packed cells. Cells were then starved for 24 h at 0.1% serum and stimulated overnight with 10 ng of HGF/ml. No differences could be detected in the scatter response of high- or low-level IκBαSR expressors with respect to wild-type MLP29 cells. Fig. 5Ashows the results obtained with representative clones 15 and 21.
FIG. 5.
NF-κB activation is dispensable for cell scattering but is required for HGF-induced proliferation. (A) Wild-type MLP29 cells (WT) and clones 15 and 21, expressing high and low levels of the IκBαSR, were kept in 5% serum until they formed tightly packed islands. Cells were then starved for 24 h in 0.1% serum and either left untreated (-) or treated with 10 ng/ml of HGF. Photographs were taken after an overnight incubation with the growth factor. (B) Wild-type MLP29 cells (WT) and clones 15 and 21 were seeded in 3-cm dishes (0.8 × 104 cells/dish) and grown for 12 h in 5% serum. Cells were starved in 0.1% serum for 24 h (cell numbers at the end of starvation time were similar for all cell types). Cells were then switched to 5% serum or to 0.1% serum supplemented with 10 ng of HGF/ml and incubated for an additional 48 h with one change of medium. After trypsinization, cells were counted in triplicate. Bars represent the mean of four independent experiments ± the standard error of the mean. ∗, P < 0.001, compared to WT treated with HGF; ○, P < 0.001, compared to WT treated with 5% serum; ○○, P < 0.05, compared to WT treated with 5% serum.
To test the effect of inhibition of NF-κB activation on the HGF-induced proliferative response, high- or low-level IκBαSR expressor clones (at least three independently isolated clones each) were plated at low density in 5% serum for 24 h. Cells were then starved in 0.1% serum for an additional 24 h and finally switched to growth medium (0.1% serum supplemented with 10 ng of HGF/ml, or 5% serum as a control). Proliferation was assessed by counting cells after 48 h of incubation in growth medium.
MLP29 cells responded to HGF stimulation by roughly doubling their number, while serum provided a more powerful growth stimulus (see also reference 53). The results obtained with representative clones 15 and 21 are shown in Fig. 5B. In clone 15 the proliferative response to HGF was almost completely abrogated. Conversely, clone 21 was still capable of responding to HGF, although at a reduced level compared to wild-type cells. Using higher concentrations of HGF (up to 40 ng/ml), proliferation was not rescued (data not shown). Interfering with NF-κB activation affected especially the proliferative response induced by HGF. In fact, when the growth stimulus was provided by serum, the inhibitory effect of the IκBαSR expression was much less pronounced (Fig. 5B).
Inhibition of NF-κB activation interferes with HGF-induced tubulogenesis.
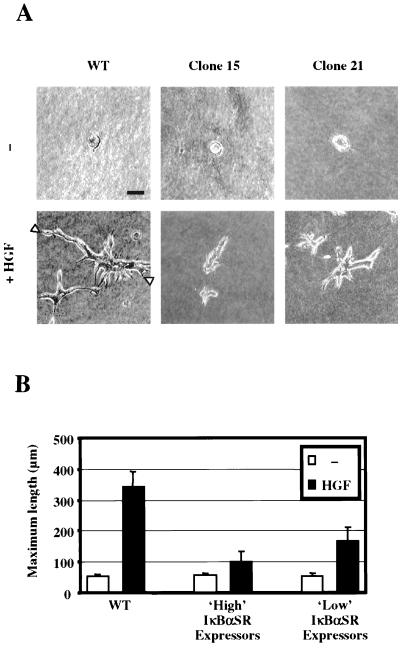
Next we tested the ability of IκBαSR-expressing clones to form tubules in collagen gels upon HGF stimulation. Tubulogenesis is a highly complex long-term process, specifically mediated by Met or by the other members of the Met family, Ron and Sea (53). MLP29 cells seeded in three-dimensional collagen gels grow to form spherical cysts. Within 1 week of HGF addition, the cysts undergo a morphogenetic process yielding tridimensional structures characterized by long branched tubules (53).
Wild-type MLP29 cells and at least three distinct clones expressing high and low levels of the IκBαSR protein (exemplified by clones 15 and 21) formed cysts equally well in collagen supplemented with 4% serum (Fig. 6A).Upon addition of HGF (10 ng/ml), wild-type MLP29 cells yielded the expected highly branched long tubular structures (Fig. 6A). On the other hand, high IκBαSR expressors produced at best short unbranched tubules (see Clone 15 in Fig. 6A). Tubulogenesis was also inhibited in low expressors, although to a lesser extent (clone 21 [Fig. 6A]). To quantitate this effect, the tubulogenesis assay was carried out (in triplicate) using high- and low-expressor clones (three distinct clones/type) and nontransfected cells as a control. The maximum length of 15 tubules/assay/clone was measured, without taking into account the number of branches. For cysts, we measured the diameter. The results of this analysis, shown in Fig. 6B, confirmed that inhibition of NF-κB activation heavily interferes with the morphogenetic process.
FIG. 6.
Expression of IκBαSR inhibits HGF-induced tubulogenesis. (A) Clones 15 and 21 were plated in collagen gels and either left untreated (-) or incubated with HGF (10 ng/ml). Branching tubules were observed 7 days later by phase-contrast light microscopy. Each clone was tested in three tubulogenesis assays with consistent results. The bar corresponds to 50 μm. (B) Tubulogenesis was quantified in three distinct clones/type (high and low expressors) by measuring the maximum length of whole tubules (distance between the two arrowheads in panel A; black columns) or the diameter of cysts (white columns). Experiments were carried out in triplicate, and the length of 15 tubular structures in each assay (or the diameter of 15 cysts) was measured for each clone. Each bar represents the mean ± the standard deviation.
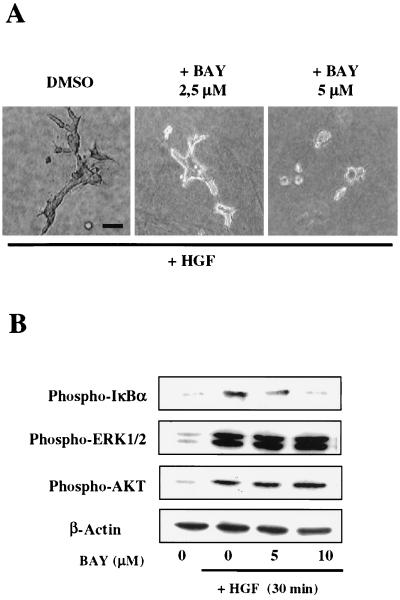
To further confirm these results, we treated wild-type MLP29 cells with BAY 11-7082, a known pharmacological inhibitor of IκBα phosphorylation (58). BAY 11-7082 was added together with HGF at the start of the tubulogenesis assay with MLP29 cells seeded in collagen gels. We used the compound at concentrations in the low micromolar range to avoid toxicity and to minimize aspecific effects. Fig. 7Ashows that BAY 11-7082 effectively inhibited tubule formation, in a dose-dependent way, confirming the requirement for NF-κB activation in HGF-dependent morphogenesis. To verify biochemically that the inhibitory effect of BAY 11-7082 was specific for IκBα phosphorylation, MLP29 cells were pretreated with 5 to 10 μM BAY 11-7082 for 1 h and stimulated with 40 ng of HGF/ml for 30 min. Fig. 7B shows that BAY 11-7082 was effective at reducing HGF-induced IκBα phosphorylation but did not interfere with ERK1/2 and Akt phosphorylation.
FIG. 7.
Effect of the NF-κB inhibitor BAY 11-7082 (BAY) on HGF-mediated tubulogenesis. (A) Wild-type MLP29 cells were plated in collagen gels. Twenty-four hours later, HGF (10 ng/ml) was added to the medium together with BAY 11-7082 at the indicated concentrations or with dimethyl sulfoxide (DMSO) alone. The medium was changed every 48 h. Pictures were taken after 1 week. Bar, 50 μm. The results are representative of three independent experiments. (B) Wild type MLP29 cells, starved in 0.1% serum for 24 h, were preincubated for 1 h with BAY 11-7082 at the indicated concentrations before HGF stimulation (40 ng/ml for 30 min). Phosphoproteins were detected on Western blots of total protein extracts (15 μg/lane). β-Actin was visualized to confirm that an equal amount of protein was loaded in each lane.
The antiapoptotic effect of HGF is independent of NF-κB activation.
Protection against apoptosis is a function that HGF shares with many other growth factors. We wanted to investigate whether this function was affected by the inhibition of NF-κB activation.
Inhibition of NF-κB activation confers sensitivity to TNF-α-induced cell death. Wild-type MLP29 cells and clones expressing low levels of IκBαSR did not show any increase in apoptosis following TNF-α treatment. Conversely, MLP29 clones expressing high levels of IκBαSR underwent massive DNA fragmentation (see Fig. 4B). HGF can protect hepatocytes from TNF-α-mediated cell damage and DNA fragmentation (68). To find out whether this protective effect requires NF-κB activation, we treated high-level IκBαSR expressors with HGF (at a concentration of 10 ng/ml) together with TNF-α and determined the extent of apoptosis as previously described. HGF caused a 70% reduction in DNA fragmentation (−69.5% ± 6.9% in clone 15) compared to cells treated with TNF-α alone.
HGF is also known to protect cells from apoptosis induced by DNA damaging agents, such as the topoisomerase inhibitor Etoposide (5). Control MLP29 cells and clone 15 were equally sensitive to micromolar concentrations of Etoposide, which caused about a 10-fold increase in apoptosis. As expected, in wild-type MLP29 cells addition of 10 ng of HGF/ml together with Etoposide resulted in a substantial suppression of DNA fragmentation relative to cells treated with Etoposide alone (−35.9% ± 3.4%). Interestingly, the extent of protection from cell death was essentially the same in clone 15 (−34.5% ± 8%). Similar results were obtained with two additional independently isolated high IκBαSR expressors.
These results indicate that HGF is capable of protecting cells against TNF-α- and Etoposide-mediated apoptosis in a NF-κB-independent way.
DISCUSSION
In this work we have identified NF-κB as an effector of Met signaling. HGF activates NF-κB through stimulation of its DNA binding activity and enhances its transcriptional activation. This occurs via the canonical IκBα phosphorylation-degradation cycle and via ERK1/2 and p38 MAPK stimulation. PI3K does not seem to be involved in NF-κB activation by HGF, at least in MLP29 liver cells. NF-κB activation downstream of Met seems to be essential for proliferation and tubular morphogenesis but not for scatter and for the antiapoptotic function of HGF.
Mechanisms of NF-κB activation by HGF.
HGF stimulation of MLP29 cells enhances NF-κB DNA binding at least in part through a mechanism involving phosphorylation-degradation of IκBα and translocation of NF-κB into the nucleus. How could Met, the HGF receptor, connect with the IKKs, which are responsible for IκBα phosphorylation? While the molecules linking the TNF-α and IL-1 receptors to the IKK multisubunit complex have been described in detail (3, 72), the proximal signaling component used by growth factor receptors to activate the IKKs is not yet understood. For the PDGF receptor it has been proposed that Akt itself may act as a bridge, since at least in fibroblasts, it seems to be responsible for phosphorylating IKKβ directly (63). However, this does not seem to be a general mechanism, since in the case of Met (this work) and of other receptors (25), inhibition of the PI3K/Akt pathway does not interfere with IκBα phosphorylation. Work done with the EGFR suggests two alternative ways in which tyrosine kinase receptors could be linked to the IKKs. Using transiently transfected cells it was shown that the EGFR engages the tumor necrosis factor receptor-interacting proteins RIP and NF-κB-inducing kinase to form a multisubunit complex, similar to but not identical with that of tumor necrosis factor receptor, which results in the phosphorylation of IKKs (31). In another cell system, the EGFR was shown to recruit and activate MEKK1 (itself an IKK kinase) via the Grb2 adapter (59). The latter mechanism could be hypothesized also for Met, which binds Grb2 at high affinity (60).
In our cell model, HGF/Met signaling promotes IκBα phosphorylation and degradation at a lower level than TNF-α. However, the two ligands have comparable effects on NF-κB-dependent transcription. IκBα phosphorylation and increased NF-κB DNA binding may therefore represent only an initial step of the HGF-mediated activation process, which can be reinforced by additional steps enhancing NF-κB transcriptional activity (67). Signaling pathways which positively modulate the NF-κB transcription potential independently from nuclear translocation have been described downstream of TNF-α, IL-1, and growth factor receptors. In particular, the ERK1/2 and p38 MAPK cascades were found to be involved in NF-κB activation by two groups working with nonepithelial cells (36, 75). Others found an involvement only of p38 MAPK (10, 43, 78), while ERK1/2 was reported to have no effect or a even negative effect on NF-κB activation (20). Our studies with specific inhibitors of these two pathways indicate that the ERK1/2 and, less extensively, also the p38 MAPK cascades are necessary for HGF-induced NF-κB transcriptional activation.
How these MAP kinases can modify the transcriptional activation of NF-κB remains to be defined. One possibility could be through phosphorylation of the NF-κB p65 subunit. Several reports indicate that phosphorylation of distinct serine residues in the p65 transactivation domain enhances the transcriptional potential of NF-κB (2, 71, 77, 83). This modification may facilitate interactions with proteins of the basal transcriptional apparatus, as well as with NF-κB coactivators, including CBP and p300 (83). It is unlikely that ERK1/2 and p38 phosphorylate p65 directly, for lack of the appropriate consensus sequence. On the other hand kinases such as casein kinase II, PKA, and PKC ζ, known to be capable of phosphorylating p65 in vivo (2, 77, 83), are not physiological substrates for ERK1/2 and p38 MAPK. Therefore, there may be a critical kinase still to be identified. Alternatively, it is possible that the MAPKs could enhance NF-κB-dependent gene expression by modulating the activity of the transcriptional machinery or of coactivator proteins. For example, p38 and ERK1 have been shown to phosphorylate, respectively, the TATA binding protein (1, 19) and CBP (1).
Another pathway which has been implicated in the enhancement of NF-κB-dependent transcription is PI3K/Akt. Recent studies have yielded conflicting results. An essential role for PI3K/Akt in enhancing the transcriptional activity of the NF-κB p65 subunit has been described downstream of TNF-α or Il-1 (46, 71). However, this pathway does not seem to be required in all cell types or for all stimuli (25, 45, 62). Our study provides clear evidence that in MLP29 cells PI3K activity is not essential for HGF-induced NF-κB activation. This is not due to a lack of activation of PI3K/Akt upon HGF stimulation, because we and others have shown that HGF is a potent inducer of this pathway (this work and references 17, 29, 40, and 73). On the other hand, given the virtual lack of response in Akt phosphorylation upon stimulation by TNF-α, it is less surprising that the inhibition of PI3K did not have any effect on TNF-α-mediated NF-κB activation.
Role of NF-κB in cell scatter, proliferation, and tubulogenesis.
HGF elicits in MLP29 cells the full array of its biological effects: scattering, survival, proliferation, and tubular morphogenesis (53). HGF-induced scattering was unaffected by the block of NF-κB activation obtained by expression of the IκBαSR. Scattering depends on PI3K activation in combination with a basal level of the ERK-MAPK pathway (40). The response of both pathways to HGF was unaffected in IκBαSR-expressing cells; thus, the lack of effect on scattering was expected.
Inhibition of NF-κB activation interfered with HGF-induced proliferation. NF-κB has been shown to contribute to cell cycle progression by inducing transcription of cyclin D1 in fibroblasts, myoblasts (30), and mammary carcinoma cells (33). Increased cyclin D1 levels have been observed also in response to HGF treatment (61). Our results suggest the hypothesis that NF-κB could be essential in order to mediate the HGF-dependent activation of cyclin D1 transcription. The lesser effect of the blockage in NF-κB activation on serum-induced proliferation suggests that the control of cyclin D1 transcription downstream of a more complex stimulus relies on multiple transcription factors.
Branching tubulogenesis is a complex process which is uniquely elicited by HGF in epithelial cells. It requires cell scatter, proliferation, and finally a phase during which cells become polarized to form the walls of tubular structures. In vitro, HGF-induced proliferation and tubulogenesis are not necessarily linked. In fact, in some cells, such as in the majority of MDCK clones, HGF is unable to elicit a proliferative response but acts as a powerful morphogen (28). In cells in which NF-κB activation is blocked (obtained either by IκBα-SR expression or by treatment with a pharmacological inhibitor of IκBα phosphorylation), HGF-induced tubulogenesis was abrogated. We think that it is unlikely that this inhibitory effect was due only to reduced proliferation. The tubulogenesis assay was carried out in 4% serum, a condition in which proliferation was only modestly reduced (even in high IκBα-SR expressors), while the defect in tubulogenesis was much more severe. Accordingly, with cells expressing high levels of IκBα-SR, formation of cysts in collagen was equal to that of wild-type MLP29 cells. Thus, the blocking of NF-κB activation seems to affect specifically the morphogenetic process.
A number of signaling pathways have been shown to be essential for tubulogenesis. Activation of the MAPK cascade and of PI3K are both required (40). Phosphorylation of the multiadaptor protein Gab-1 is essential (50). Binding of PLCγ to Gab-1 and recruitment and activation of the transcription factor Stat-3 to the Met receptor, both dispensable for cell scattering and growth, are also specifically required (15, 30). In this study we show that tubulogenesis was abrogated by a blocking of NF-κB activation, obtained either by IκBα-SR expression or by treatment with a pharmacological inhibitor of IκBα phosphorylation. We have thus identified the transcription factor NF-κB as another essential factor for HGF-stimulated growth of tubular structures. A link between NF-κB and tubulogenesis was suggested by previous studies of Shono et al. on TNF-α-mediated angiogenesis (70), a process with some similarities to tubulogenesis. According to this work, NF-κB promotes angiogenesis by inducing production of secondary angiogenic factors, such as IL-8. Another, more direct explanation for the NF-κB requirement may be related to the fact that tubulogenesis (which occurs in collagen gels) requires secretion of extracellular matrix proteases and cell polarization. NF-κB may be required for HGF-induced expression of cell adhesion molecules involved in cell polarization or for secretion of proteases, such as matrix metalloproteinase 9 (9, 42).
NF-κB is not essential for HGF-mediated protection from apoptosis.
HGF protects a broad range of diverse cell types from apoptosis induced by serum starvation, by various DNA-damaging agents, and also by death factors (17, 44, 68, 73). The antiapoptotic action of HGF is particularly important in liver cells. HGF protects hepatocytes in culture from Fas- and TNF-α-induced cell death (68, 73) and, in vivo, prevents endotoxin- or Fas-induced lethal hepatic failure (41). Furthermore, in mice null for HGF or Met the liver undergoes regression in mid-gestation, due to massive apoptosis of hepatocytes (48, 66). Although the precise mechanisms by which HGF promotes cell survival remain unclear, in most cell types there seems to be an involvement of the PI3K/Akt pathway, which results in the modulation of anti- or proapoptotic members of the Bcl-2 family, such as BAG-1, Bcl-xL, or Bad (5, 17, 68, 73).
NF-κB has been widely implicated in regulating the apoptotic program, especially for its antiapoptotic properties (6), more rarely as an inducer of cell death (39, 65). Similar to HGF and Met, the survival-promoting function of NF-κB is confirmed by the phenotype of the p65 knockout, which is characterized by embryonic lethality in mid-gestation due to massive apoptosis of the liver (7). Subsequently it was shown that NF-κB is essential to suppress TNF-α-induced cell death (8, 74). This function in counteracting the proapoptotic effect of TNF-α was confirmed in vivo by the generation of mice doubly deficient for p65 and TNF-α (26). In these animals the liver defect was rescued, implying that the role of NF-κB during liver development is that of protecting hepatocytes from TNF-α-mediated cytotoxicity. Recent reports have shown that protection against cell death by many growth factors, such as PDGF, NGF, IGF-I, and insulin, involves NF-κB activation (11, 32, 47, 63). Interestingly, in some cases Akt may link the action of growth factors and the activation of NF-κB in a common antiapoptotic route (32, 63).
In this work we confirmed the antiapoptotic effect of HGF in MLP29 hepatocyte precursor cells. TNF-α and Etoposide-induced apoptosis were both efficiently counteracted by HGF. Quite unexpectedly, however, we found that the survival effect of HGF is mostly NF-κB independent, as shown by the fact that protection occurred also in cells where NF-κB was blocked by the expression of the inhibitory protein IκBαSR. Our experiments do not exclude the possibility that in liver cells NF-κB may contribute to the antiapoptotic effect of HGF; however, this is certainly not a strict requirement, as shown by the nearly complete protection afforded by HGF in the absence of NF-κB activity. Further studies are needed to establish how the NF-κB-independent HGF-induced cell survival stimulus is implemented. It seems likely that the PI3K-Akt pathway, which has been shown to have a role in HGF-mediated protection from apoptosis in other cell types (82), may do so also in hepatocytes.
Our study and the data in the literature suggest a few final considerations on the role of NF-κB and HGF in protection of liver cells from apoptosis. There is evidence from in vivo studies that NF-κB is required in the liver to counteract TNF-α-mediated cytotoxicity under conditions when TNF-α production is likely be particularly high, such as embryonal development (7) or massive regeneration after a partial hepatectomy (34). In these circumstances there is also a requirement for concomitant antiapoptotic HGF/Met signaling (35, 66). In the simplest scenario to reconcile these data, NF-κB could lie downstream of Met in the execution of the HGF-induced antiapoptotic program. Our data show that this is not the case. In fact, in MLP29 cells in culture, HGF treatment can counteract the proapoptotic effect of TNF-α in the absence of NF-κB. Thus, in liver cells two distinct and independent antiapoptotic pathways must operate, one triggered by TNF-α and mediated by NF-κB, and another, triggered by HGF, which is NF-κB independent and probably PI3K mediated. The latter one, however, is not sufficient in vivo to sustain hepatocyte survival in p65−/− mice. The physiological level of the HGF ligand in the embryonal liver may be inadequate for this rescue.
TNF-α has been implicated as a causative agent in many disease states, such as septic shock or fulminant hepatic failure (18). Our data suggest that a treatment with high doses of HGF may be efficient in counteracting these pathological effects of TNF-α, independently of the activation state of NF-κB.
Acknowledgments
We thank Daniel Besser for helpful discussion and reagents.
This work was supported by funds from AIRC and CNR (Progetto Finalizzato Biotecnologie). The continuing support of the Compagnia di San Paolo and Fondazione CRT to C.P.'s laboratory is gratefully acknowledged.
Footnotes
This work is dedicated to the memory of Franco Tatò, who died on 7 July 2001.
REFERENCES
- 1.Ait-Si-Ait, S., D. Carlisi, S. Ramirez, L. C. Upegui-Gonzalez, A. Duquet, P. Robin, B. Rudkin, A. Harel-Bellan, and D. Trouche. 1999. Phosphorylation by p44 MAP kinase/ERK1 stimulates CBP histone acetyl transferase activity in vivo. Biochem. Biophys. Res. Commun. 262:157-162. [DOI] [PubMed]
- 2.Anrather, J., V. Csizmadia, M. P. Soares, and H. Winkler. 1999. Regulation of NF-κB RelA phosphorylation and transcriptional activity by p21ras and protein kinase Cξ in primary endothelial cells. J. Biol. Chem. 274:13594-13603. [DOI] [PubMed] [Google Scholar]
- 3.Ashkenazi, A., and V. M. Dixit. 1998. Death receptors: signaling and modulation. Science 281:1305-1308. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin, A. S., Jr. 1996. The NF-κB and IkB proteins: new discoveries and insights. Annu. Rev. Immunol. 14:649-681. [DOI] [PubMed] [Google Scholar]
- 5.Bardelli, A., P. Longati, D. Albero, S. Goruppi, C. Schneider, C. Ponzetto, and P. M. Comoglio. 1996. HGF receptor associates with the anti-apoptotic protein BAG-1 and prevents cell death. EMBO J. 15:6205-6212. [PMC free article] [PubMed] [Google Scholar]
- 6.Barkett, M., and T. Gilmore. 1999. Control of apoptosis by Rel/NF-κB transcription factors. Oncogene 18:6910-6924. [DOI] [PubMed] [Google Scholar]
- 7.Beg, A. A., W. C. Sha, R. T. Bronson, S. Ghosh, and D. Baltimore. 1995. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature 376:167-170. [DOI] [PubMed] [Google Scholar]
- 8.Beg, A. A., and D. Baltimore. 1996. An essential role for NF-κB in preventing TNFα induced cell death. Science 274:782-784. [DOI] [PubMed] [Google Scholar]
- 9.Bennett, J. H., M. J. Morgan, S. A. Whawell, P. Atkin, P. Roblin, J. Furness, and P. M. Speight. 2000. Metalloproteinase expression in normal and malignant oral keratinocytes: stimulation of MMP-2 and -9 by scatter factor. Eur. J. Oral Sci. 108:281-291. [DOI] [PubMed] [Google Scholar]
- 10.Bergman, M., L. Hart, M. Lindsay, P. J. Barnes, and R. Newton. 1998. IκBα degradation and nuclear factor-κB DNA binding are insufficient for interleukin-1β and tumor necrosis-α-induced κB-dependent transcription. J. Biol. Chem. 273:6607-6610. [DOI] [PubMed] [Google Scholar]
- 11.Bertrand, F., A. Atfi, A. Cadoret, G. L'Allemain, H. Robin, O. Lascols, J. Chapeau, and G. Cherqui. 1998. A role for nuclear factor κb in the antiapoptotic function of insulin. J. Biol. Chem. 273:2931-2938. [DOI] [PubMed] [Google Scholar]
- 12.Bhargava, M., A. Joseph, J. Knesel, R. Halaban, Y. Li, S. Pang, I. Goldberg, E. Setter, M. A. Donovan, R. Zarnegar, G. A. Michalopoulos, T. Nakamura, D. Faletto, and E. M. Rosen. 1992. Scatter factor and hepatocyte growth factor: activities, properties and mechanism. Cell Growth Differ. 3:11-20. [PubMed] [Google Scholar]
- 13.Birchmeier, C., and E. Gherardi. 1998. Developmental roles of HGF/SF and its receptor, the c-Met tyrosine kinase. Trends Cell Biol. 8:404-410. [DOI] [PubMed] [Google Scholar]
- 14.Boccaccio, C., G. Gaudino, G. Gambarotta, F. Galimi, and P. M. Comoglio. 1994. Hepatocyte growth factor (HGF) receptor expression is inducible and is part of the delayed-early response to HGF. J. Biol. Chem. 269:12846-12851. [PubMed] [Google Scholar]
- 15.Boccaccio, C., M. Andò, L. Tamagnone, A. Bardelli, P. Michieli, C. Battistini, and P. M. Comoglio. 1998. Induction of epithelial tubules by growth factor HGF depends on the STAT pathway. Nature 391:285-288. [DOI] [PubMed] [Google Scholar]
- 16.Boros, P., and C. M. Miller. 1995. Hepatocyte growth factor: a multifunctional cytokine. Lancet 345:293-295. [DOI] [PubMed] [Google Scholar]
- 17.Bowers, D. C., S. Fan, K. A. Walter, R. Abounader, J. A. Williams, E. M. Rosen, and J. Laterra. 2000. Scatter factor/hepatocyte growth factor protects against cytotoxic death in human glioblastoma via phosphatidylinositol 3-kinase- and AKT-dependent pathways. Cancer Res. 60:4277-4283. [PubMed] [Google Scholar]
- 18.Bradham, C. A., J. Plümpe, M. P. Manns, D. A. Brenner, and C. Trautwein. 1998. Mechanisms of hepatic toxicity. I. TNFα-induced liver injury. Am. J. Physiol. 275:G387-G392. [DOI] [PubMed]
- 19.Brent Carter, A., K. L. Knudtson, M. M. Monick, and G. W. Hunninghake. 1999. The p38 mitogen-activated protein kinase is required for NF-κB-dependent gene expression. J. Biol. Chem. 274:30858-30863. [DOI] [PubMed] [Google Scholar]
- 20.Brent Carter, A., and G. W. Hunninghake. 2000. A constitutive active MEK → ERK pathway negatively regulates NF-κB-dependent gene expression by modulating TATA-binding protein phosphorylation. J. Biol. Chem. 275:27858-27864. [DOI] [PubMed] [Google Scholar]
- 21.Brinkmann, V., H. Foroutan, H. Sachs, K. M. Weidner, and W. Birchmeier. 1995. Hepatocyte growth factor/scatter factor induces a variety of tissue specific morphogenic programs in epithelial cells. J. Cell Biol. 131:1573-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brockman, J. A., D. C. Scherer, T. A. McKinsey, S. M. Hall, X. Qi, W. Y. Lee, and D. W. Ballard. 1995. Coupling of a signal response domain in IkBα to multiple pathways for NF-κB activation. Mol. Cell. Biol. 15:2809-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bussolino, F., F. Di Renzo, M. Ziche, E. Bocchietto, M. Olivero, L. Naldini, G. Gaudino, L. Tamagnone, A. Coffer, and P. M. Comoglio. 1992. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J. Cell Biol. 119:629-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castagnino, P., M. V. Lorenzi, J. Yeh, D. Breckenridge, H. Sakata, B. Munz, S. Werner, and D. P. Bottaro. 2000. Neu differentiation factor/heregulin induction by hepatocyte and keratinocyte growth factors. Oncogene 19:640-648. [DOI] [PubMed] [Google Scholar]
- 25.Delhase, M., N. Li, and M. Karin. 2000. Kinase regulation in inflammatory response. Nature 406:367-368. [DOI] [PubMed] [Google Scholar]
- 26.Doi, T. S., M. W. Marino, T. Takahashi, T. Yoshida, T. Sakakura, L. J. Old, and Y. Obata. 1999. Absence of tumor necrosis factor rescues RelA-deficient mice from embryonic lethality. Proc. Natl. Acad. Sci. USA 96:2994-2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujita, T., G. P. Nolan, H. C. Liou, M. L. Scott, and D. Baltimore. 1993. The candidate proto-oncogene bcl-3 encodes a transcriptional coactivator that activates through NF-kappa B p50 homodimers. Genes Dev. 7:1354-1363. [DOI] [PubMed] [Google Scholar]
- 28.Gherardi, E., and M. Stoker. 1991. Hepatocyte growth factor-scatter factor: mitogen, motogen and met. Cancer Cells 3:227-232. [PubMed] [Google Scholar]
- 29.Gual, P., S. Giordano, T. A. Williams, S. Rocchi, E. Van Obberghen, and P. M. Comoglio. 2000. Sustained recruitment of phospholipase C-gamma to Gab-1 is required for HGF-induced branching tubulogenesis. Oncogene 19:1509-1518. [DOI] [PubMed] [Google Scholar]
- 30.Guttridge, D. C., C. Albanese, J. Y. Reuther, R. G. Pestell, and A. S. Baldwin, Jr. 1999. NF-κB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol. Cell. Biol. 19:5785-5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Habib, A. A., S. Chatterjee, S. K. Park, R. R. Ratan, S. Lefebvre, and T. Vartanian. 2001. The epidermal growth factor receptor engages receptor interacting protein and nuclear factor-kappa b (nf-kappa b)-inducing kinase to activate nf-kappa b. Identification of a novel receptor-tyrosine kinase signalosome. J. Biol. Chem. 276:8865-8874. [DOI] [PubMed] [Google Scholar]
- 32.Heck, S., F. Lezoualc'h, S. Egert, and C. Behl. 1999. Insulin-like growth factor-1-mediated neuroprotection against oxidative stress is associated with activation of nuclear factor κb. J. Biol. Chem. 274:9828-9835. [DOI] [PubMed] [Google Scholar]
- 33.Hinz, M., D. Krappmann, A. Eichten, A. Heder, C. Scheidereit, and M. Strauss. 1999. NF-κB function in growth control: regulation of cyclin expression and Go/G1-to-S-phase transition. Mol. Cell. Biol. 19:2690-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iimuro, Y., T. Nishiura, C. Hellerbrand, K. E. Behrns, R. Schoonhoven, J. W. Grisham, and D. A. Brenner. 1998. NFκB prevents apoptosis and liver dysfunction during liver regeneration. J. Clin. Investig. 101:802-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishiki, Y., H. Ohnishi, Y. Muto, K. Matsumoto, and T. Nakamura. 1992. Direct evidence that hepatocyte growth factor is a hepatotrophic factor for liver regeneration and has a potent antihepatitis effect in vivo. Hepatology 16:1227-1235. [PubMed] [Google Scholar]
- 36.Jefferies, C. A., and L. A. J. O'Neill. 2000. Rac1 regulates interleukin 1-induced nuclear factor κb activation in an inhibitory protein κbα-independent manner by enhancing the ability of the p65 subunit to transactivate gene expression. J. Biol. Chem. 275:3114-3120. [DOI] [PubMed] [Google Scholar]
- 37.Jiang, W., S. Hiscox, K. Matsumoto, and T. Nakamura. 1999. Hepatocyte growth factor/scatter factor, its molecular, cellular and clinical implications in cancer. Crit. Rev. Oncol.-Hematol. 29:209-248. [DOI] [PubMed] [Google Scholar]
- 38.Karin, M. 1999. The beginning of the end: IκB kinase (IKK) and NF-κB activation. J. Biol. Chem. 274:27339-27342. [DOI] [PubMed] [Google Scholar]
- 39.Kasibhatla, S., T. Brunner, L. Genestier, F. Echeverri, A. Mahboubi, and D. R. Green. 1998. DNA damaging agents induce expression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-κB and AP-1. Mol. Cell 1:543-551. [DOI] [PubMed] [Google Scholar]
- 40.Khwaja, A., K. Lehmann, B. M. Marte, and J. Downward. 1998. Phosphoinositide 3-kinase induces scattering and tubulogenesis in epithelial cells through a novel pathway. J. Biol. Chem. 273:18793-18801. [DOI] [PubMed] [Google Scholar]
- 41.Kosai, K., K. Matsumoto, S. Nagata, Y. Tsujimoto, and T. Nakamura. 1998. Abrogation of Fas-induced fulminant hepatic failure in mice by hepatocyte growth factor. Biochem. Biophys. Res. Commun. 244:683-690. [DOI] [PubMed] [Google Scholar]
- 42.Lee, P. P., J. J. Hwang, G. Murphy, and M. M. Ip. 2000. Functional significance of MMP-9 in tumor necrosis factor-induced proliferation and branching morphogenesis of mammary epithelial cells. Endocrinology 141:3764-3773. [DOI] [PubMed] [Google Scholar]
- 43.Liu, R. Y., C. Fan, G. Liu, N. E. Olashaw, and K. S. Zuckerman. 2000. Activation of p38 mitogen-activated protein kinase is required for tumor necrosis factor-α-supported proliferation of leukemia and lymphoma cell lines. J. Biol. Chem. 275:21086-21093. [DOI] [PubMed] [Google Scholar]
- 44.Liu, Y. 1999. Hepatocyte growth factor promotes renal epithelial cell survival by dual mechanisms. Am. J. Physiol. 277:F624-F633. [DOI] [PubMed]
- 45.Madge, L. A., and J. S. Pober. 2000. A phosphatidylinositol 3-kinase/Akt pathway, activated by tumor necrosis factor or interleukin-1, inhibits apoptosis but does not activate NFκB in human endothelial cells. J. Biol. Chem. 275:15458-15465. [DOI] [PubMed] [Google Scholar]
- 46.Madrid, L. V., C. V. Wang, D. C. Guttridge, A. J. G. Schottelius, A. S. Baldwin, Jr., and M. W. Mayo. 2000. Akt supresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-κB. Mol. Cell. Biol. 20:1626-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maggirwar, S. B., P. D. Sarmiere, S. Dewhurst, and R. S. Freeman. 1998. Nerve growth factor-dependent activation of NF-κB contributes to survival of sympathetic neurons. J. Neurosci. 18:10356-10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maina, F., F. Casagranda, E. Audero, A. Simeone, P. M. Comoglio, R. Klein, and C. Ponzetto. 1996. Uncoupling of Grb2 from the Met receptor in vivo reveals complex roles in muscle development. Cell 87:531-542. [DOI] [PubMed] [Google Scholar]
- 49.Maina, F., M. C. Hilton, C. Ponzetto, A. M. Davies, and R. Klein. 1997. Met receptor signaling is required for sensory nerve development and HGF promotes axonal growth and survival of sensory neurons. Genes Dev. 11:3341-3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maroun, C. R., M. Holgado-Madruga, I. Royal, M. A. Naujokas, T. M. Fournier, A. J. Wong, and M. Park. 1999. The Gab1 PH domain is required for localization of Gab1 at sites of cell-cell contact and epithelial morphogenesis from the Met receptor tyrosine kinase. Mol. Cell. Biol. 19:1784-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsumoto, K., and T. Nakamura. 1996. Emerging multipotent aspects of hepatocyte growth factor. J. Biochem. 119:591-600. [DOI] [PubMed] [Google Scholar]
- 52.Mayo, M. W., and A. S. Baldwin. 2000. The transcription factor NF-κB: control of oncogenesis and cancer therapy resistance. Biochem. Biophys. Acta 1470:M55-M62. [DOI] [PubMed]
- 53.Medico, E., A. M. Mongiovi, J. Huff, M. A. Jelinek, A. Follenzi, G. Gaudino, J. T. Parsons, and P. M. Comoglio. 1996. The tyrosine kinase receptors Ron and Sea control "scattering' and morphogenesis of liver progenitor cells in vitro. Mol. Biol. Cell 7:495-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Medico, E., G. Gambarotta, A. Gentile, P. M. Comoglio, and P. Soriano. 2001. A gene trap vector system for identifying transcriptionally responsive genes. Nat. Biotechnol. 19:579-582. [DOI] [PubMed] [Google Scholar]
- 55.Norris, J. L., and A. S. Baldwin, Jr. 1999. Oncogenic ras enhances NF-κB transcriptional activity through Raf-dependent and Raf-independent mitogen-activated protein kinase signaling pathways. J. Biol. Chem. 20:13841-13846. [DOI] [PubMed] [Google Scholar]
- 56.Pahl, H. L. 1999. Activators and target genes of Rel/NF-κB transcriptions factors. Oncogene 18:6853-6866. [DOI] [PubMed] [Google Scholar]
- 57.Perkins, N. D. 2000. The Rel/NF-κB family: friend and foe. Trends Biochem. Sci. 25:434-440. [DOI] [PubMed] [Google Scholar]
- 58.Pierce, J. W., R. Schoenleber, G. Jesmok, J. Best, S. A. Moore, T. Collins, and M. E. Gerritsen. 1997. Novel inhibitors of cytokine-induced IκBα phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J. Biol. Chem. 272:21096-21103. [DOI] [PubMed] [Google Scholar]
- 59.Pomerance, M., M. C. Multon, F. Parker, C. Venot, J. P. Blondeau, B. Tocqué, and F. Schweighoffer. 1998. Grb2 interaction with MEK-kinase 1 is involved in regulation of Jun-kinase activities in response to epidermal growth factor. J. Biol. Chem. 273:24301-24303. [DOI] [PubMed] [Google Scholar]
- 60.Ponzetto, C., A. Bardelli, Z. Zhen, F. Maina, P. dalla Zonca, S. Giordano, A. Graziani, G. Panayotou, and P. M. Comoglio. 1994. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell 77:261-271. [DOI] [PubMed] [Google Scholar]
- 61.Ponzetto, C., G. Pantè, C. Prunotto, A. Ieraci, and F. Maina. 2000. Met signaling mutants as tools for developmental studies. Int. J. Dev. Biol. 44:645-653. [PubMed] [Google Scholar]
- 62.Rauch, B. H., A. Weber, M. Braun, N. Zimmermann, and K. Schror. 2000. PDGF-induced Akt phosphorylation does not activate NF-κB in human vascular smooth muscle cells and fibroblasts. FEBS Lett. 481:3-7. [DOI] [PubMed] [Google Scholar]
- 63.Romashkova, J. A., and S. S. Makarov. 1999. NF-κB is a target of AKT in anti-apoptotic PDGF signalling. Nature 401:86-89. [DOI] [PubMed] [Google Scholar]
- 64.Rothwarf, D. M., and M. Karin. 1999. The NF-κB activation pathway: a paradigm in information transfer from membrane to nucleus. Science Signal Transduction Knowl. Environ. [Online.]. www.stke.org/cgi/content/full/OC_sigtrans;1999/5/re1. [DOI] [PubMed]
- 65.Ryan, K. M., M. K. Ernst, N. R. Rice, and K. H. Vousden. 2000. Role of NF-κB in p53-mediated programmed cell death. Nature 404:892-897. [DOI] [PubMed] [Google Scholar]
- 66.Schmidt, C., F. Bladt, S. Goedecke, V. Brinkmann, W. Zschiesche, M. Sharpe, E. Gherardi, and C. Birchmeier. 1995. Scatter factor/hepatocyte growth factor is essential for liver development. Nature 373:699-702. [DOI] [PubMed] [Google Scholar]
- 67.Schmitz, M. L., S. Bacher, and M. Kracht. 2001. IkB-independent control of NF-kB activity by modulatory phosphorylations. Trends Biochem. Sci. 26:186-190. [DOI] [PubMed] [Google Scholar]
- 68.Senaldi, G., C. L. Shaklee, B. Simon, C. G. Rowan, D. L. Lacey, and T. Hartung. 1998. Keratinocyte growth factor protects murine hepatocytes from tumor necrosis-induced apoptosis in vivo and in vivo. Hepatology 27:1584-1591. [DOI] [PubMed] [Google Scholar]
- 69.Seol, D. W., Q. Chen, and R. Zarnegar. 2000. Transcriptional activation of the hepatocyte growth factor receptor (c-met) gene by its ligand (hepatocyte growth factor) is mediated through AP-1. Oncogene 19:1132-1137. [DOI] [PubMed] [Google Scholar]
- 70.Shono, T., M. Ono, H. Izumi, S.-I. Jimi, K. Matsushima, T. Okamoto, K. Kohno, and M. Kuwano. 1996. Involvement of the transcription factor NF-κB in tubular morphogenesis of human microvascular endothelial cells by oxidative stress. Mol. Cell. Biol. 16:4231-4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sizemore, N., S. Leung, and G. R. Starck. 1999. Activation of phosphatidylinositol 3-kinase in response to interleukin-3 leads to phosphorylation and activation of the NF-κB p65/RelA subunit. Mol. Cell. Biol. 19:4798-4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stylianou, E., and J. Saklatvala. 1998. Interleukin-1. Int. J. Biochem. Cell. Biol. 30:1075-1079. [DOI] [PubMed] [Google Scholar]
- 73.Suzuki, A., M. Hayashida, H. Kawano, K. Sugimoto, T. Nakano, and K. Shiraki. 2000. Hepatocyte growth factor promotes cell survival from Fas-mediated cell death in hepatocellular carcinoma cells via Akt activation and Fas-death-inducing signaling complex suppression. Hepatology 32:796-802. [DOI] [PubMed] [Google Scholar]
- 74.Van Antwerp, D. J., S. J. Martin, T. Kafri, D. R. Green, and I. M. Verma. 1996. Suppression of TNFα induced apoptosis by NF-κB. Science 274:787-789. [DOI] [PubMed] [Google Scholar]
- 75.Vanden Berghe, W., S. Plaisance, E. Boone, K. De Bosscher, M. L. Schmitz, W. Fiers, and G. Haegeman. 1998. p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor-κb p65 transactivation by tumor necrosis factor. J. Biol. Chem. 273:3285-3290. [DOI] [PubMed] [Google Scholar]
- 76.Wang, D., and A. S. Baldwin. 1998. Activation of nuclear factor-kB-dependent transcription by tumor necrosis factor-α is mediated through phosphorylation of RelA/p65 on serine 529. J. Biol. Chem. 273:29411-29416. [DOI] [PubMed] [Google Scholar]
- 77.Wang, D., S. D. Westerheide, J. L. Hanson, and A. S. Baldwin, Jr. 2000. Tumor necrosis factor α-induced phosphorylation of RelA/p65 on Ser529 is controlled by casein kinase II. J. Biol. Chem. 275:32592-32597. [DOI] [PubMed] [Google Scholar]
- 78.Wesselborg, S., M. K. A. Bauer, M. Vogt, M. L. Schmitz, and K. Schulze-Osthoff. 1997. Activation of transcription factor NF-κB and p38 mitogen-activated protein kinase is mediated by distinct and separate stress effector pathways. J. Biol. Chem. 272:12422-12429. [DOI] [PubMed] [Google Scholar]
- 79.Whiteside, S. T., M. K. Ernst, O. Lebail, C. Laurent-Winter, N. Rice, and A. Israël. 1995. N- and T-terminal sequences control degradation of MAD3/IκBα in response to inducers of NF-κB activity. Mol. Cell. Biol. 15:5339-5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wojta, J., C. Kaun, J. M. Breuss, Y. Koshelnick, R. Beckmann, E. Hattey, M. Mildner, W. Weninger, T. Nakamura, E. Tschachler, and B. B. Binder. 1999. Hepatocyte growth factor increases expression of vascular endothelial growth factor and plasminogen activator inhibitor-1 in human keratinocytes and the vascular endothelial growth factor receptor flk-1 in human endothelial cells. Lab. Investig. 79:427-438. [PubMed] [Google Scholar]
- 81.Xiu, X., S. Bialik, B. E. Jones, Y. Iimuro, R. N. Kitsis, A. Srinivasan, D. A. Brenner, and M. J. Czaja. 1998. NF-κB inactivation converts a hepatocyte cell line TNFα response from proliferation to apoptosis. Am. J. Physiol. 275:C1058-C1066. [DOI] [PubMed]
- 82.Zhang, L., T. Himi, I. Morita, and S. Murota. 2000. Hepatocyte growth factor protects cultured rat cerebellar granule neurons from apoptosis via the phosphatidylinositol-3 kinase/Akt pathway. J. Neurosci. Res. 59:489-496. [DOI] [PubMed] [Google Scholar]
- 83.Zhong, H., R. E. Voll, and S. Ghosh. 1998. Phosphorylation of NF-κB p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol. Cell 1:661-671. [DOI] [PubMed] [Google Scholar]