Abstract
Grb10 is a member of the Grb7 family of adapter proteins lacking intrinsic enzymatic function and encodes functional domains including a pleckstrin homology (PH) domain and an SH2 domain. The role of different Grb10 splice variants in signal transduction of growth factors like insulin or insulin-like growth factor has been described as inhibitory or stimulatory depending on the presence of a functional PH and/or SH2 domain. Performing a yeast two-hybrid screen with the c-kit cytoplasmic tail fused to LexA as a bait and a mouse embryo cDNA library as prey, we found that the Grb10 SH2 domain interacted with the c-kit receptor tyrosine kinase. In the course of SCF-mediated activation of c-kit, Grb10 is recruited to the c-kit receptor in an SH2 domain- and phosphotyrosine-dependent but PH domain-independent manner. We found that Akt and Grb10 form a constitutive complex, suggesting a role for Grb10 in the translocation of Akt to the cell membrane. Indeed, coexpression studies revealed that Grb10 and c-kit activate Akt in a synergistic manner. This dose-dependent effect of Grb10 is wortmannin sensitive and was also seen at a lower level in cells in which c-kit was not expressed. Expression of a Grb10 mutant lacking the SH2 domain as well as a mutant lacking the PH domain did not influence Akt activity. Grb10-induced Akt activation was observed without increased phosphatidylinositol 3-kinase (PI3-kinase) activity, suggesting that Grb10 is a positive regulator of Akt downstream of PI3-kinase. Significantly, deficient activation of Akt by a constitutively activated c-kit mutant lacking the binding site for PI3-kinase (c-kitD814V/Y719F) could be fully compensated by overexpression of Grb10. In Ba/F3 cells, the incapacity of c-kitD814V/Y719F to induce interleukin-3 (IL-3)-independent growth could be rescued by overexpression of Grb10. In contrast, expression of the SH2 deletion mutant of Grb10 together with c-kitD814V/Y719F did not render Ba/F3 cells independent of IL-3. In summary, we provide evidence that Grb10 is part of the c-kit signaling pathway and that the expression level of Grb10 critically influences Akt activity. We propose a model in which Grb10 acts as a coactivator for Akt by virtue of its ability to form a complex with Akt and its SH2 domain-dependent translocation to the cell membrane.
The Grb10 superfamily of adaptor proteins consists so far of four members: Grb7, Grb10, Grb14, and Mig-10. Structural common features of this family are an N-terminal proline-rich putative SH3 domain binding region, pleckstrin homology (PH) domain, a BPS domain, and (exept for Mig-10) a C-terminal SH2 domain. Grb10 (mGrb10α) was originally identified as a binding partner of the epidermal growth factor receptor (EGFR) (39) and of the Ret receptor tyrosine kinase (40). After this initial characterization, several splice variants of Grb10 were isolated by yeast two-hybrid screens using the insulin receptor (IR) and the insulin-like growth factor receptor (IGF-R) as a bait (for reviews, see references 17 and 31). Despite the clear involvement of Grb10 in pathways activated by IR and IGF-R, there is still some controversy about whether its effect is inhibitory or stimulatory. A negative effect of Grb10 on IR signaling (27) as well as on IGF-R signaling (33, 47) has been reported. In contrast, Wang et al. have demonstrated that Grb10 plays a positive role in the transmission of mitogenic signals from the platelet-derived growth factor BB (PDGF-BB), IGF-R, and IR (50). The observed effects are differentially dependent on the Grb10 SH2, the BPS, the PH, and the proline-rich domain.
In addition to the association of Grb10 with different growth factor receptors at the cell membrane, there are intracellular ligands for Grb10. Grb10 interacts with MEK1 and the mitochondria-associated Raf pool (35, 36). Indeed, sequence homology analysis revealed an N-terminal Ras-associating domain with the ability to bind small GTPases of the Ras superfamily (52). Other ligands of Grb10 include Nedd4, a ubiquitin protein ligase (32). We previously showed the association of endogenous Grb10 with BCR-Abl in a phosphotyrosine-dependent fashion (5). Tyrosine phosphorylation of Grb10 has also been reported as a result of its interaction with Tec (29) and Src (26).
Grb10 has been suggested as a downstream target in the phosphatidylinositol 3-kinase (PI3-K) signaling pathway (19). Although no direct effect of Grb10 on PI3-K or protein kinase B (PKB)/Akt has been observed, overexpression of a Grb10 isoform (hGrb10zeta) has been reported to negatively influence the insulin-stimulated activity of glycogen synthase in primary rat hepatocytes (34), which normally is regulated by PI-3K/Akt.
So far, three human isoforms of Akt have been found, PKBα/Akt1, PKBβ/Akt2, and PKBγ/Akt3, which are expressed differentially at both the mRNA and protein levels (2, 3, 6, 10, 13, 14, 23). Akt family proteins consist of a central kinase domain with specificity for serine or threonine residues in substrate proteins. Activation of Akt is regulated primarily in a PI3-K-dependent manner and involves direct binding of PI3-K-generated phospholipids to the Akt PH domain (21) and Akt translocation to the cell membrane, where the close proximity to other regulatory kinases leads to phosphorylation and activation of Akt (4, 16, 43, 51). Activated Akt then detaches from the plasma membrane and translocates to mitochondria and the nucleus, where it is thought to phosphorylate its substrates (4, 30). Substrates of Akt containing the consensus site RXRXX(S/T) include Bad (18), caspase-9 (12), and transcription factors of the forkhead family (7, 11). These findings have provided important insights into the mechanism of Akt-mediated survival. In addition, a recent report links Akt-mediated P21Cip1/WAF1 phosphorylation in Her2/neu-overexpressing breast cancers to the deregulation of cell proliferation in cancer cells (53). A novel negative regulator at the plasma membrane, CTMP, has also recently been identified (28).
Akt activity is induced by a variety of ligand-activated receptor tyrosine kinases including EGF-R, PDGF-R, and c-kit (8, 22, 37). The mechanism in c-kit-induced Akt activity involves association of the p85 subunit of PI3-K with c-kit and the subsequent activation of PI3-K, leading to activation of Akt. Tyrosine 719 in its phosphorylated state within the c-kit cytoplasmic tail is the critical residue in mediating the interaction with the C-terminal SH2 domain of p85 (8, 44). The crucial role of the PI3-K/Akt signaling pathway in c-kit-mediated signaling has become evident in knock-in mice expressing the c-kitY719F mutation encoding defects in spermatogenesis and oogenesis (9, 25).
Our interest in Grb10 was initiated by the identification of Grb10 as a c-kit binding adaptor performing a yeast two-hybrid screen. The consequent finding that Akt associates with Grb10 and that Grb10 is recruited to the cell membrane in the process of stem cell factor (SCF)-mediated c-kit activation suggested a novel role for Grb10 in c-kit-mediated activation of Akt. Moreover, we provide evidence that the expression level of the adaptor protein Grb10 might critically influence Akt activity.
MATERIALS AND METHODS
Reagents and antibodies.
Recombinant SCF was purchased from R&D Systems GmbH, Wiesbaden, Germany. Polyclonal anti-Akt and anti-c-kit antibodies were obtained from Santa Cruz Biotechnology Inc., Heidelberg, Germany. Anti-Grb10 antibodies were purchased from Santa Cruz (A18/K20, sc) and Upstate Biotechnology, Lake Placid, N.Y. The anti-PI3-K antibody was obtained from Upstate Biotechnology. The monoclonal antibodies against epitoped-tagged fusion proteins were from Boehringer/Roche, Mannheim, Germany (HA-Tag, clone 12CA5) and from Stratagene (FLAG-Tag, clone M2). The phospho-specific Akt antibodies (Ser473 and Thr308) and the phospho-specific p44/42 mitogen-activated protein kinase (MAPK) (Thr202/Tyr204) antibody (clone E10) and the p44/42 MAPK antibody were obtained from New England Biolabs (NEB) GmbH (Schwalbach/Taunus, Germany). Antiphosphotyrosine antibodies were purchased from Upstate Biotechnology (4G10) and Pharmingen, Hamburg, Germany (PY20). Primers were obtained from a commercial source (MWG-Biotech GmbH, Ebersberg, Germany).
Yeast two-hybrid screen.
The yeast two-hybrid screening method has been described previously (5, 15). Here, the cytoplasmic tail of murine c-kit (amino acids [aa] 544 to 975) was fused to LexA sequences in the yeast expression vector BTM116. A mouse embryo cDNA library was used as bait (Clontech, Heidelberg, Germany).
GST fusion construct and GST binding studies.
The glutathione S-transferase (GST)-Grb10 SH2 construct and the procedure for pull-down assays have been described previously (5). Briefly, lysates from nonstimulated and SCF-stimulated Mo7e and HMC-1 cells were incubated with the GST fusion construct or with GST alone. Bound fractions of recombinant proteins collected by using gluthathione-beads were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed for presence of c-kit.
A purified GST fusion protein of full-length Akt was obtained from a commercial source (Upstate Biotechnology). After preaclearing the lysates from COS cells expressing FLAG-tagged Grb10 constructs with glutathione beads, 2 μg of GST-Akt or only GST bound to gluthathione beads was incubated at 4°C in equal volumes of lysates for 1 h. The beads were washed, and bound fractions were subjected to SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes. Bound Grb10 protein was visualized by Western blotting using an anti-FLAG antibody.
Expression constructs.
The murine Grb10 cDNA (pRK5 Grb10) was kindly provided by Renato Baserga, Kimmel Cancer Institute, Philadelphia, Pa. To obtain an epitope-tagged Grb10, the Grb10 cDNA was cloned into pCMVTag from Stratagene (N-terminal FLAG). The Grb10ΔSH2 (Grb10ΔEcoRI-end) and Grb10ΔPH (Grb10ΔPstI) mutants were created using appropriate internal restriction sites to delete the corresponding region (EcoRI and PstI, respectively). The HA-Grb10-Akt fusion constructs were created by insertion of the respective PCR-Grb10 constructs or regions upstream of the Akt wild-type (WT) cDNA. For mutation of the SH2 domain of Grb10 (SH2M), the arginine within the FLLR motif of Grb10 was changed to leucine by site-directed mutagenesis using appropriate primers. The HA-tagged PKB/Akt constructs (pCMV6 HA-Akt, pCMV6 HA Aktkin-) were a generous gift from Thomas Franke, Department of Pharmacology, Columbia University, New York, N.Y. The EYFP-c-kit fusion construct (SS-EYFP-kit) will be described elsewhere (T. Jahn, P. Seipel, S. Coutiho, S. Urschel, K. Schwarz, C. Miething, H. Serve, C. Peschel, and J. Duyster, submitted for publication). Briefly, using PCR-based site-directed mutagenesis (Stratagene, Heidelberg, Germany), an EcoRI site was inserted directly downstream of the signal sequence of the murine c-kit cDNA using the following primer: 5" CTG CTC CGT GGC CAG ACA GAA TTC GCC ACG TCT CAG CCA TCT GC 3". The EYFP cDNA was equipped with a linker of 3 glycines and flanking EcoRI sites and inserted into the mutated c-kit cDNA using the EcoRI site. The mutant c-kit constructs were obtained by PCR-based site-directed mutagenesis with primers containing the indicated amino acid changes at the nucleotide level. All mutations were verified by sequencing or restriction analysis.
Cell culture and transfection methods.
Mo7e cells were cultured in RPMI with 10% fetal calf serum (FCS) and human interleukin-3 (hIL-3), and K562 cells and HMC-1 cells were cultured in RPMI with 10% FCS. COS7 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FCS. COS7 cells were transfected using Gene Porter (GTS Inc., Biozol GmbH, Eching, Germany) as recommended by the manufacturer. For immunocprecipitation experiments, transfections were carried out in 60-mm-diameter plates. For Western blot analysis of lysates (Akt activation), 35-mm plates were used. Ba/F3 cells were cultured in RPMI containing 10% FCS and murine IL-3 (mIL-3). Ba/F3 cells stably expressing c-kit and Grb10 constructs were obtained by electroporation and subsequent selection with Zeocin (Invitrogen, Groningen, The Netherlands) and G418 (Sigma, Taufkirchen, Germany).
SCF stimulation.
Cells were starved for at least 6 h in DMEM or RPMI lacking FCS and stimulated with 500 ng of SCF per ml for 4 min.
Wortmannin/LY294002 treatment of cells.
Cells were incubated for 30 min in DMEM lacking FCS containing wortmannin (300 nM; Sigma) or LY294002 (10μM, Sigma) prior to SCF stimulation. Untreated cells were incubated in media containing the same concentration of dimethyl sulfoxide.
Cell proliferation assay.
A total of 3 × 104 Ba/F3 cells (expressing c-kit and Grb10 constructs as indicated) per well were plated into 96-well plates in 200 μl of medium lacking IL-3. After the indicated period, the viable cells in each well were assayed to their ability to transform MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] (CellTiter 96; Promega, Mannheim, Germany) into a purple formazan. Then 40 μl of a 10-mg/ml MTS solution was added to each well. After an incubation period of 4 h at 37°C, the reaction was stopped by adding 50 μl of 10% SDS and the absorbance of the samples was measured in an enzyme-linked immunosorbent assay reader at 490 nm.
Immunoprecipitations and immunoblotting.
Cells were harvested in cold phosphate-buffered saline PBS containing 1 mM sodium vanadate, pelleted, and lysed in lysis buffer containing 10 mM Tris-HCl (pH 7.4), 5 mM EDTA, 130 mM NaCl, and 1% Triton. Proteinase inhibitor cocktail tablets (Complete) were added as specified by the manufacturer (Boehringer/Roche). After clarification by centrifugation and preclearing with protein A-Sepharose, antibody-protein complexes were brought down with 30 μl of protein A-Sepharose (Amersham/Pharmacia Biotech AG, Freiburg, Germany). In an immunoprecipitation experiment, 2 μg of antibody was used per immunoprecipitation. Lysates and bound fractions of precipitates were subjected to SDS-PAGE, and blotting was performed on PVDF membranes (Immobilon-P; Millipore GmbH, Eschborn, Germany). Immunodetection of phosphotyrosine was done using a mixture of the antiphosphotyrosine antibodies 4G10 and PY20. To examine the activation of PKB/Akt, lysates were analysed with an anti-phospho-Akt antibody. After being stripped, the same membrane was probed with an anti-Akt antibody to verify the presence of equal amounts of Akt on the blot. Blots were developed using SuperSignal chemoluminescent substrates from Pierce Chemical Company (KMF GmbH, St. Augustin, Germany).
Akt kinase assay.
The nonradioactive assay was performed as recommended by the manufacturer (NEB GmbH). Briefly, transfected COS1 cells were lysed and an anti-Akt immunoprecipitation was performed. The kinase reaction was carried out in the presence of ATP and GSK-3 substrate. Supernatants of this reaction were separated by SDS-PAGE, and the phosphorylated substrate was visualized by Western blotting using an anti-pGSK-3α/β antibody (Ser21/9).
PI3-K assay.
Anti-PI3-K immunoprecipitates (antibody from Upstate Biotechnology) were assayed for 10 min at 37°C in the presence of 20 μg of PI (Sigma) in 10 mM Tris-HCl (pH 7.4)-150 mM NaCl-15 mM MgCl2-5 mM EDTA-30 μCi of [γ-33P]ATP (Amersham) together with 60 μM cold ATP (total volume, 80 μl). The reaction mixtures were acidified, and the reactions were stopped by adding 20 μl of 6 N HCl. Then 160 μl of CHCl3-methanol (1:1) was added to the samples to extract the lipids. Samples were briefly vortexed and centrifuged, and equal volumes of radioactive lipids from the organic phase were spotted on thin-layer chromatography plates (Merck) and separated by chromatography in CHCl3-methanol-H2O-NH4OH (60:47:11.3:2). The plates were dried, and radiolabeled lipids were visualized by autoradiography (BIOMAX; Kodak).
RESULTS
Grb10 binds activated c-kit via its SH2 domain.
To identify new intermediates of the c-kit signaling pathway, we performed a yeast two-hybrid screen using the c-kit cytoplasmic tail fused to LexA as a bait and a mouse embryo cDNA library as prey. LexA-mediated dimerization of the c-kit cytoplasmic tail allows the auto-tyrosine phosphorylation of c-kit in yeast and hence the identification of SH2 domain-mediated interactions. Among proteins already known to bind c-kit (e.g., PI3-K), Grb10 was identified as a specific binding partner for c-kit. Sequence analysis revealed that two of the identified clones represented slightly differing lengths of the murine Grb10 C terminus encoding part of the BPS domain and the complete SH2 domain (aa 488 to 620 and aa 472 to 620 [data not shown]).
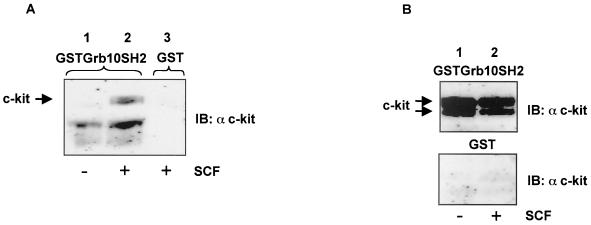
A GST fusion construct containing the Grb10 SH2 domain bound the membrane-associated mature form of c-kit from SCF-stimulated Mo7e cells in a phosphotyrosine-dependent manner (Fig. 1A).HMC-1 cells express a constitutively active form of c-kit. Using lysates from these cells, both the precursor and the mature membrane-bound form of c-kit were bound by GST-Grb10 SH2 in an SCF-independent manner. This indicates that the tyrosine(s) responsible for binding the Grb10 SH2 domain is constitutively phosphorylated in both forms of this c-kit mutant (Fig. 1B; see also Fig. 2B, lower panel).
FIG. 1.
Binding of GST-Grb10 SH2 to c-kit. (A) Bound fractions of GST-Grb10SH2 incubated with lysates from Mo7e cells without (lane 1) and with (lane 2) SCF stimulation and GST alone incubated with a lysate from SCF-stimulated Mo7e cells (lane 3) were separated by SDS-PAGE and immunoblotted (IB) with an anti c-kit antibody. (B) Bound fractions of GST-Grb10SH2 (upper panel) and GST alone (lower panel) incubated with lysates from HMC-1 cells without (lane 1) and with (lane 2) SCF stimulation were separated by SDS-PAGE and immunoblotted with an anti c-kit antibody.
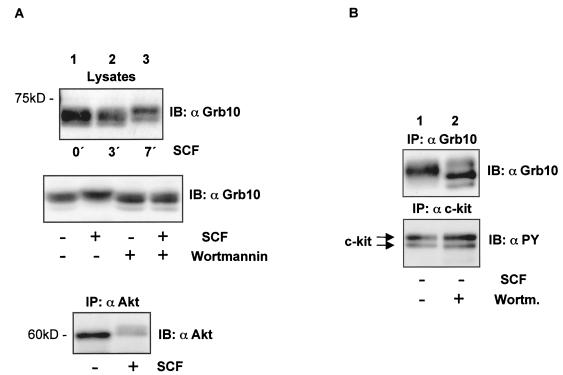
FIG. 2.
The migration pattern of Grb10 is SCF and wortmannin sensitive. (A) (Top panel) Lysates from Mo7e cells treated with SCF for the times indicated were separated by SDS-PAGE and immunoblotted (IB) with an anti-Grb10 antibody. (Middle panel) Lysates from nonstimulated and SCF-stimulated Mo7e cells treated with wortmannin as indicated were separated by SDS-PAGE and immunoblotted with an anti-Grb10 antibody. (Bottom panel) An anti-Akt immunoprecipitate (IP) from nonstimulated and SCF-stimulated Mo7e cells was separated by SDS-PAGE and immunoblotted with an anti-Akt antibody. (B) An anti-Grb10 immunoprecipitate (upper panel) and an anti-c-kit immunoprecipitate (lower panel) from nonstimulated HMC-1 cells treated with wortmannin as indicated were separated by SDS-PAGE and immunoblotted with an anti-Grb10 and an anti-PY antibody, respectively.
A commercially available polyclonal antibody raised against the Grb10 SH2 domain detected multiple splice variants in lysates from Mo7e cells (Fig. 2A,top panel). We noticed a mobility shift resulting in delayed migration of Grb10 isoforms from cells stimulated with SCF (Fig. 2A, top panel). Phosphorylation on serine/threonine residues is a likely reason for this result since we were unable to detect tyrosine phosphorylation of Grb10 as analyzed by probing Grb10 immunoprecipitates from unstimulated and SCF-stimulated Mo7e cells. To exclude the possibility that the antibody recognizing the SH2 domain of Grb10 would not precipitate the tyrosine-phosphorylated fraction of Grb10, we repeated the analysis with epitope-tagged Grb10 and again detected no tyrosine phosphorylation of Grb10 (data not shown).
Phosphorylation of Grb10 in a wortmannin-dependent fashion had been reported previously (19). SCF-mediated activation of c-kit results in the activation of the wortmannin-sensitive PI3-K/Akt pathway (8) (Fig. 2A, bottom panel). Therefore, we tested whether inhibition of c-kit-mediated PI3-K activation by wortmannin would affect the modification of Grb10. Indeed, incubation of Mo7e cells in wortmannin-containing media prior to SCF stimulation abolished the change in the migration pattern of Grb10 (Fig. 2A, middle panel). This result shows that the modification of Grb10 is inhibited by blocking the PI3-K pathway and is in line with the hypothesis of phosphorylation of Grb10 on serine and/or threonine residues. HMC-1 cells express a constitutively activated mutant of c-kit. Inhibition of PI3-K in HMC-1 cells by wortmannin resulted in an inverse electrophoretic shift in the form of accelerated migration of Grb10 (Fig. 2B, upper panel), indicating that Grb10 is constitutively modified in these cells as a result of constitutive activation of PI3-K. Wortmannin did not influence the tyrosine phosphorylation of either form (precursor and membrane-bound mature form) of c-kit (Fig. 2B, lower panel).
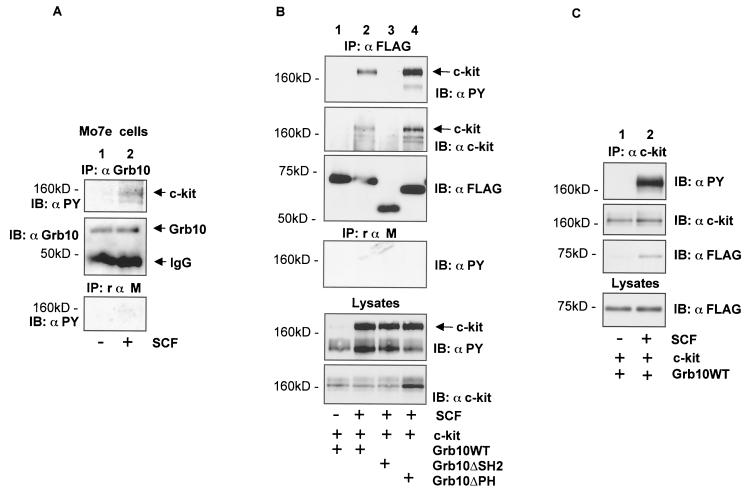
We investigated, whether endogenous Grb10 and c-kit form a complex in Mo7e cells (Fig. 3A).We could demonstrate that tyrosine-phosphorylated c-kit was coprecipitated with Grb10 from SCF-stimulated cells (Fig. 3A, top panel). Thus, endogenous Grb10 is recruited to the activated c-kit receptor in Mo7e cells.
FIG. 3.
SCF-induced complex formation between c-kit and Grb10. (A) An anti-Grb10 immunoprecipitation (IP) (top two panels) and a control immunoprecipitation (bottom panel) using equivalent amounts of nonspecific rabbit anti-mouse IgG were simultaneously performed using lysates from nonstimulated and SCF-stimulated Mo7e cells, and the products were separated by SDS-PAGE, and immunoblotted (IB) with the antibodies indicated. (B) COS1 cells (60-mm plates) transiently expressing SS-EYFP-kit (4 μg) and various FLAG epitope-tagged Grb10 constructs (4 μg) as indicated were treated with SCF or left untreated. An anti-FLAG immunoprecipitation and a control immunoprecipitation using equivalent amounts of nonspecific rabbit anti-mouse IgG (rαm) were simultaneously performed. Cell lysates were separated by SDS-PAGE and immunoblotted with the antibodies indicated (top four panels). Lysates from the same cells were separated by SDS-PAGE and immunoblotted with anti-PY and anti-cKit antibodies (bottom two panels). (C) An anti-c-kit immunoprecipitation was performed using lysates from nonstimulated and SCF-stimulated COS1 cells transiently expressing SS-EYFP-kit (3 μg) and FLAG epitope-tagged Grb10WT (3 μg), separated by SDS-PAGE, and immunoblotted with antibodies as indicated (top three panels). Lysates from the same cells were separated by SDS-PAGE and immunoblotted with the antibody indicated (bottom panel).
To further characterize the complex formation between c-kit and Grb10, we used a COS1 cell-based transient-expression system. In agreement with the data obtained with GST fusions and endogenous proteins, complex formation between Grb10WT and c-kit was dependent on stimulation of c-kit with SCF as analyzed in coimmunoprecipitation experiments when Grb10 was immunoprecipitated (Fig. 3B, lanes 1 and 2) and when c-kit was immunoprecipitated (Fig. 3C). A Grb10 deletion mutant lacking a functional SH2 domain (Grb10ΔSH2) failed to bind activated c-kit, demonstrating that an intact Grb10 SH2 domain is required for the interaction of c-kit and Grb10 (Fig. 3B, lane 3). Deletion of the PH domain of Grb10 (Grb10ΔPH) did not reduce complex formation with c-kit (Fig. 3B, lane 4).
Constitutive association of Grb10 and Akt is independent of the Grb10 SH2 and PH domain.
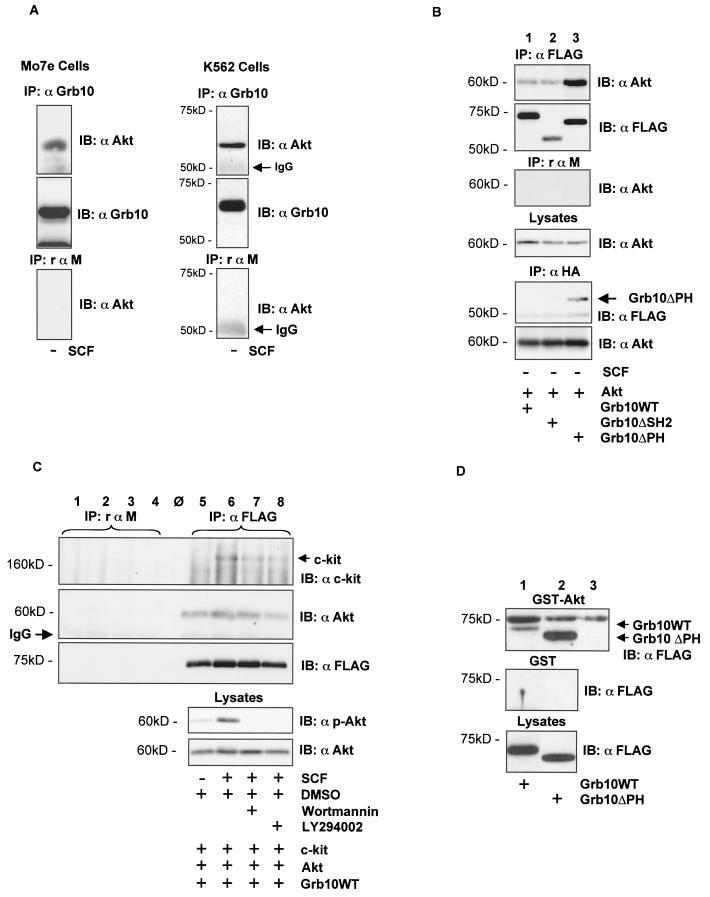
The modification of Grb10 downstream of PI3-K/Akt and its translocation to the c-kit receptor following SCF stimulation prompted us to test whether there is a complex between Grb10 and Akt. In both cell lines, Mo7e and K562, we were able to detect a complex between endogenous Grb10 and Akt in a coimmunoprecipitation experiment (Fig. 4A).
FIG. 4.
Complex formation of Grb10 and Akt. (A) Products of an anti-Grb10 immunoprecipitation (IP) using lysates from Mo7e cells (left panel) and from K562 cells (right panel) were separated by SDS-PAGE and immunoblotted (IB) with the antibodies indicated (top two panels). A control immunoprecipitation (bottom panels) using nonspecific rαm IgG was simultaneously performed, and the product was immunoblotted with α-Akt. (B) Lysates from nonstimulated COS1 cells transiently expressing HA epitope-tagged WT Akt (4 μg) and various FLAG epitope-tagged Grb10 constructs (4 μg) were subjected to an anti-FLAG immunoprecipitation (top two panels) and an anti-HA immunoprecipitation (bottom two panels). A control immunoprecipitation with nonspecific rabbit rαm IgG was performed (third panel from top). Immunoprecipitates were separated by SDS-PAGE and immunoblotted with the antibodies indicated. Lysates of the same cells were separated by SDS-PAGE and immunoblotted with an anti-Akt antibody to confirm equal expression levels of Akt (fourth panel from top). (C) SCF-induced association between c-kit and Grb10 and constitutive association between Grb10 and Akt are independent of Akt activity. An anti-FLAG immunoprecipitation (lanes 5 to 8) and a control immunoprecipitation (lanes 1 to 4) using nonspecific rαm IgG were performed using lysates from COS1 cells transfected with SS-EYFP-kitWT (4 μg), WT Akt (2 μg), and FLAG-tagged Grb10WT (4 μg). The cells were treated with SCF and PI3-K inhibitors prior to lysis as indicated. Immunoprecipitates were separated by SDS-PAGE and immunoblotted with the antibodies as indicated (top three panels). Lysates of the same cells were separated by SDS-PAGE and immunoblotted with a phosphospecific anti-Akt antibody to confirm the activation status of Akt and with an anti-Akt antibody to confirm equal expression levels of Akt. (D) GST-Akt binds to Grb10 and Grb10ΔPH. After preaclearing the lysates from COS1 cells expressing FLAG-tagged Grb10WT (lane 1) and Grb10ΔPH (lane 2) with glutathione beads, 2 μg of GST-Akt or only GST bound to glutathione beads was incubated at 4°C in equal volumes of lysates for 1 h. The beads were washed, and bound fractions were subjected to SDS-PAGE and transferred to PVDF membranes. Bound Grb10 protein was visualized by Western blotting using an anti-FLAG antibody. Lane 3 contains equal amounts of the GST-Akt protein to control cross-reactivity of the anti-FLAG antibody with GST-Akt fragments. This figure shows the results of two separate experiments.
Next we studied the association of Grb10 and Akt using COS1 cells expressing HA-tagged WT Akt and FLAG-tagged Grb10 mutants (Fig. 4B). Complex formation was independent of the Grb10 SH2 domain (Fig. 4B, lane 2) and was even enhanced when WT Akt was coexpressed with the Grb10 mutant lacking the PH domain (lane 3). Enhanced complex formation between Akt and Grb10ΔPH was also evident, performing the reverse procedure immunoprecipitating Akt (Anti-HA immunoprecipitation) and detecting the tagged Grb10 proteins with an anti-FLAG antibody (Fig. 3B, lower two panels, lane 3). Coprecipitating Grb10WT and Grb10ΔSH2 were not detected, most probably because of the limited sensitivity of this antibody (Fig. 3B, bottom two panels, lane 1 and 2).
In addition, we performed GST binding studies (Fig. 4D). Equal amounts of GST-Akt (top panel) and GST control were incubated with lysates from COS1 cells overexpressing FLAG-tagged Grb10WT (lane1) and Grb10ΔPH (lane2). As a control, GST-Akt was run on the same gel (lane 3) to control cross-reacting bands due to GST-Akt fragments running at about the same size as Grb10. Grb10WT and Grb10ΔPH were precipitated by GST-Akt (arrows). Although this figure shows two separate experiments, it again seems that Grb10ΔPH is able to form a more stable complex with Akt than Grb10WT.
Thus, complex formation of Grb10 and Akt is independent of the Grb10 SH2 domain and Grb10PH domain and most probably involves the N-terminal region of Grb10.
Association of Grb10 with c-kit and Akt is independent of the activation status of the PI3-K pathway.
We tested the influence of the PI3-K inhibitors wortmannin and LY294002 on complex formation between Grb10 and c-kit and between Grb10 and Akt. When Grb10WT was immunoprecipitated from unstimulated and SCF-stimulated cells and from SCF-stimulated cells pretreated with either wortmannin or LY2940002, the association between Grb10 and Akt was not influenced by PI3-K inhibitors (Fig. 4C, second panel, lanes 5 to 8, control immunoprecipitation with rabbit anti-mouse immunoglobulin G (IgG) from the same lysates on lanes 1 to 4). Similarly, SCF-dependent association of Grb10 with c-kit was not abolished by treatment of the cells with PI3-K inhibitors (Fig. 4C, top panel, lanes 5 to 8). Akt activity as determined by immunoblotting lysates from these cells with a phoshospecific Akt antibody was strongly induced on SCF stimulation and completely inhibited in SCF-stimulated cells pretreated with either wortmannin or LY294002. Thus, association of Grb10 with c-kit and Akt is independent of the activation status of the PI3-K pathway. This finding is in line with the previous result of a constitutive complex between Grb10 and Akt in unstimulated Mo7e cells and in K562 cells (where PI3-K/Akt is constitutively activated by BCR-Abl [45]).
Overexpression of Grb10 induces Akt activity.
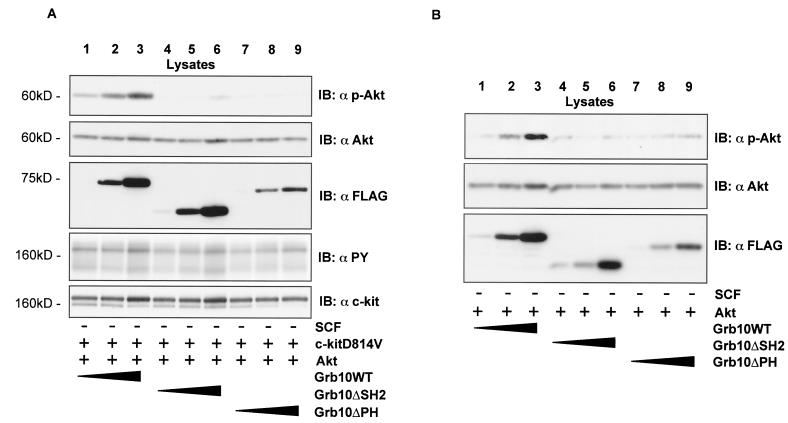
The close association between Grb10 and Akt, combined with the ability of Grb10 to translocate to the c-kit receptor following SCF stimulation, allowed us to speculate that Grb10 might be involved in the translocation of Akt to the cell membrane and its subsequent activation. Therefore we tested whether Grb10 could influence c-kit-mediated Akt activity. c-kitD814V is a constitutively activated mutant of c-kit which is frequently seen in human mastocytosis (24, 41, 49). In cells expressing c-kit (D814V), overexpression of Grb10 led to a dose-dependent increase of Akt activity (Fig. 5A,lanes 1 to 3). Translocation of Grb10 to the activated c-kit receptor seems to be involved in this effect since expression of Grb10ΔSH2 did not influence Akt activity (lanes 4 to 6). Interestingly, expression of Grb10ΔPH also failed to induce more Akt activity. Therefore the expression level of Grb10 critically influences Akt activity and requires intact Grb10 SH2 and PH domains.
FIG. 5.
(A) Coexpression of constitutively activated c-kit and Grb10 reveals SH2 and PH domain-dependent synergism in Akt activation in a concentration-dependent manner. (B) Grb10 alone enhances Akt activity in a concentration-dependent manner. (C) The Grb10 effect on Akt activity is wortmannin dependent. Lysates from COS1 cells (35-mm plates) transiently expressing SS-EYFP-kit D814V (1.5 μg [A]), HA-Akt (1.5 μg [A] and 3 μg [B and C]) and FLAG-Grb10 constructs (increasing amounts of 1, 2, and 3 μg [A and B] and 3 μg [C]) as indicated and treated with inhibitors (C) as indicated were separated by SDS-PAGE and immunoblotted (IB) with the antibodies indicated. DMSO, dimethyl sulfoxide.
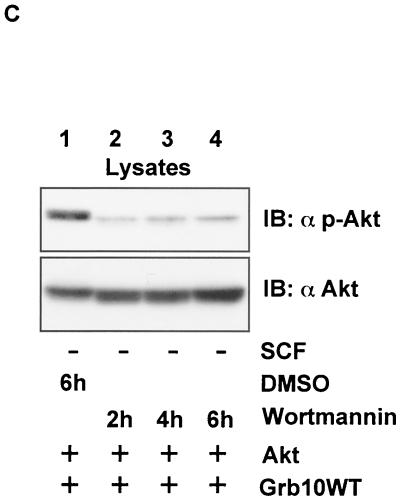
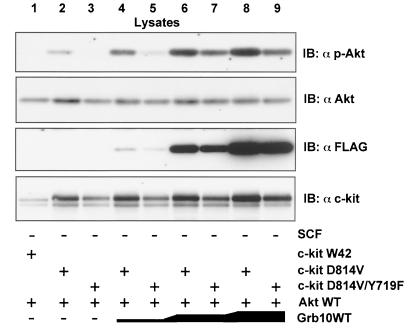
In the following experiment, c-kit expression was omitted to examine if overexpression of Grb10 alone is sufficient to induce Akt activity. As shown in Fig. 5B (lanes 1 to 3), overexpression of Grb10 alone induced Akt activity in a dose-dependent manner. Again, both the SH2 and PH domains of Grb10 seemed to be critically involved in this effect since Grb10ΔSH2 (lanes 4 to 6) and Grb10ΔPH (lanes 7 to 9) had no or significantly less effect on the activity of Akt. Wortmannin treatment completely inhibited Akt phosphorylation induced by Grb10 (Fig. 5C). Therefore, Grb10-mediated Akt activation is dependent on the wortmannin-sensitive PI3-K pathway. To verify that the increase of Akt autophosphorylation in fact represents an increase in Akt kinase activity, we performed immunoprecipitation kinase assays (Fig. 6,top panel). Akt kinase activity was greatly enhanced in cells overexpressing constitutively activated c-kit and Grb10 WT (Fig. 6A, lane 1) whereas expression of Grb10ΔPH (lane 2) and Grb10ΔSH2 (lane 3), together with activated c-kit, did not increase Akt kinase activity significantly. Akt kinase activity strongly correlated with phosphorylation of Akt both at Ser473 (second panel) and at Thr308 (third panel). To analyze the specificity of the Grb10 effect for the Akt pathway, phosphorylation of p44/42 MAPK was tested and found to be not affected by expression of Grb10WT or the Grb10 mutant constructs (Fig. 6A, fifth panel). Functionality of this antibody was demonstrated by serum stimulation of starved COS1 cells (Fig. 6B).
FIG. 6.
(A) Grb10 induces Akt kinase activity. An Akt kinase assay was performed using COS1 cells expressing c-kit D814V (2 μg), HA-Akt (2 μg) or FLAG-Grb10 constructs (4 μg) as indicated. Aliquots of the kinase reaction mixture were separated by SDS-PAGE, and blots were analyzed with an pGSK3α/β antibody (top panel). This assay was performed twice, and similar results were obtained. Lysates from the same cells were separated by SDS-PAGE, and Western blot analyses (IB) were performed using the antibodies indicated. (B) To demonstrate the functionality of the phosphospecific MAPK antibody, COS1 cells were either serum starved for 4 h or grown normally in media containing 10% FCS. Lysates from the cells were separated by SDS-PAGE and immunoblotted with the phosphospecific MAPK antibody (upper panel) or the anti-MAPK antibody (lower level).
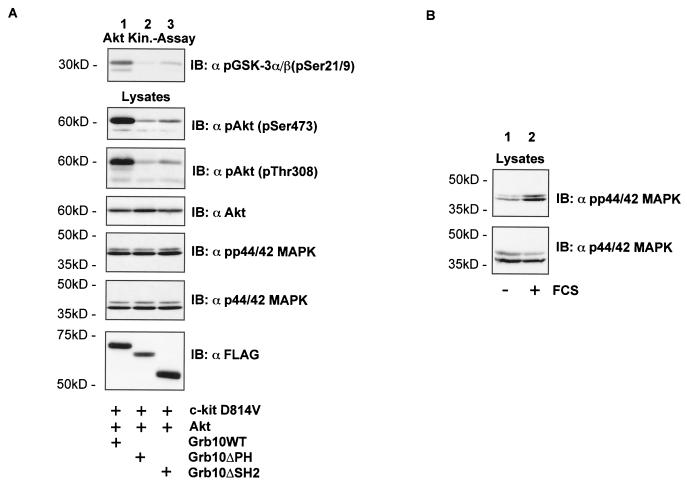
Grb10-induced Akt activation could be due to increased PI3-K activity. Therefore, PI3-K activity levels were determined from cells expressing c-kit D814V, Akt, and different Grb10 constructs (Fig. 7A).No significant changes in PI3-K activity were observed in cells expressing either vector control (Fig. 7A, top panel, lane 1), Grb10WT (lane 2), or the Grb10 mutants (lanes 3 and 4). Lysates from the same cells were immunoblotted for activated Akt (second panel) and Akt (third panel). In a second experiment, expression of the activated c-kit receptor was ommitted (Fig. 7B) and PI3-K activities from cells expressing either vector control (lane 2), Grb10WT (lane 3), or Grb10 mutants (lanes 4 and 5) were tested and shown to be comparable. As a negative control, cells expressing the vector control were incubated for 1 h with 300 nM wortmannin (lane 1). As expected, PI3-K activity was greatly reduced by preincubation with wortmannin.
FIG. 7.
(A) Grb10 induces Akt activity without affecting PI3-K activity. A PI3-K assay was performed using COS1 cells expressing c-kit D814V (3 μg), HA-Akt (3 μg), and FLAG-Grb10 constructs or FLAG vector control (6 μg) as indicated. Equal volumes of lipids from the organic phase were spotted on thin-layer chromatography plates and separated by chromatography. The plates were dried, and radiolabeled lipids were visualized by autoradiography (top panel). Lysates from the same cells were separated by SDS-PAGE, and Western blots analyses (IB) were performed using the antibodies indicated. Three independent experiments of the PI3-K assay showed similar results. (B) PI3-K activity levels (upper panel) were determined from COS1 cells expressing vector control (lanes 1 and 2) either treated for 1 h with wortmannin (300 nM) (lane 1) or untreated (lane 2) and from COS1 cells expressing Grb10 constructs as indicated (lanes 3 to 5). Lysates of the same cells were separated by SDS-PAGE, and Western blot analyses were performed using an anti-FLAG antibody to verify expression of Grb10 constructs (lower panel).
These results show that overexpression of Grb10 leads to activation of Akt without affecting PI3-K activity. Using phosphospecific antibodies for the related kinases PKCζ and p70S6K, we were unable to detect changes in the activation status of these AGC family members (data not shown). Therefore, the effect of Grb10 seems to be specific for the Akt signaling pathway and is located downstream of PI3-K.
A Grb10-Akt hybrid protein is constitutively activated.
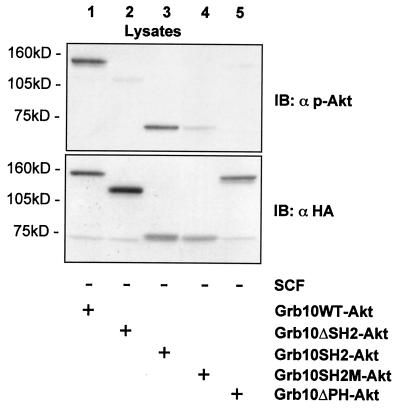
To further demonstrate the relevance of the Grb10-Akt complex for Akt activation, we created a variety of HA-tagged Grb10-Akt hybrid proteins. If Grb10 association with Akt is critical for Akt activation, such hybrid constructs should show enhanced Akt activation when expressed in cells. As shown in Fig. 8(lane 1), a fusion protein of Grb10WT and Akt showed strong phosphorylation on serine 473, indicating Akt activation. In line with a crucial role for the SH2 domain of Grb10, a chimera of Grb10ΔSH2 and Akt showed no or significantly reduced activation of the fused Akt (lane 2). The Grb10SH2 domain alone was sufficient to mediate the activation in such hybrid proteins (lane 3). Since part of the BPS domain is present in the Grb10SH2-Akt hybrid protein which could contribute to the activation, we introduced a mutation into the Grb10SH2 domain that rendered it nonfunctional (Grb10SH2M-Akt). Akt fused to the mutated nonfunctional SH2 domain of Grb10 was significantly less phosphorylated (lane 4). In agreement with the results from coexpression of Akt and Grb10ΔPH, the fusion of Grb10ΔPH with Akt also showed significantly reduced phosphorylation (lane 5). Expression levels of all constructs were verified by immunoblotting the lysates with an anti-HA antibody (Fig. 8, lower panel).
FIG. 8.
Fusion constructs of WT Akt with various Grb10 fragments suggest a crucial role of the Grb10 SH2 and PH domains in the activation of Akt. Fusion constructs of HA-tagged WT Akt and various Grb10 constructs were created (see Materials and Methods for details) and transiently expressed in COS1 cells (5 μg). Lysates were separated by SDS-PAGE and immunoblotted (IB) with the antibodies indicated.
Grb10 overexpression is able to rescue a constitutively activated c-kit mutant deficient in PI3-K activation.
Phosphorylation of tyrosine 719 (Y719) within the c-kit cytoplasmic tail triggers the association of the C-terminal SH2 domain of the p85 subunit of PI3-K following SCF-induced activation of c-kit. Integrity of this site is a prerequisite for c-kit-induced PI3-K activity resulting in PKB/Akt activation (44). We verified that c-kitY719F is still able to recruit Grb10 (data not shown). We created a double mutant of c-kitD814V/Y719F, which, unlike the c-kitD814V single mutant (49), is unable to induce IL-3-independent growth of Ba/F3 cells due to reduced activation of PI3-K/Akt (see Fig. 10A). This pathway is critical for IL-3-mediated survival (46). In Fig. 9we show that Akt activation by c-kitD814V/Y719F was significantly reduced compared to that of c-kitD814V (Fig. 9, top panel, compare lanes 2 and 3) but could be restored by overexpression of Grb10 (Fig. 9, lanes 3, 5, 7, and 9).
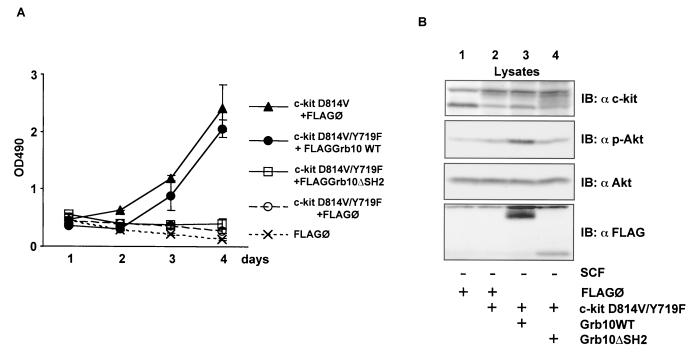
FIG. 10.
Grb10 expression overrides incapacity of c-kitD814V/Y719F to induce IL-3-independent growth of Ba/F3 cells. (A) Ba/F3 cells stably expressing c-kit and Grb10 constructs as indicated were plated in media without IL-3, in 96-well plates at 3 × 104 cells per well. After the indicated period, the viable cells in each well were assayed by their ability to transform MTS into a purple formazan. The absorbance of the samples was measured in an ELISA reader at 490 nm (OD490). Values for day 5 are not shown, because cells at high density were already overgrown (c-kit D814V+FLAG/O, c-kit D814V/Y719F+FLAGGrb10 WT). (B) Lysates from Ba/F3 cells assayed in panel A were analyzed by Western blotting (IB) for expression of c-kit (top panel, anti-c-kit) and Grb10 constructs (bottom panel, anti-FLAG). Activation of endogenous Akt was analyzed by probing the lysates with a phosphospecific Akt antibody (second panel), and levels of endogenous Akt were determined with an anti-Akt antibody (third panel).
FIG. 9.
Grb10 rescues impaired Akt activation by c-kitD814V/Y719F. Lysates from COS1 cells transiently expressing various SS-EYFP-kit (2 μg), HA-Akt (3 μg), and FLAG-Grb10 constructs (increasing amounts of 1, 2, and 3 μg) as indicated and treated with SCF or left untreated were separated by SDS-PAGE and immunoblotted (IB) with the antibodies indicated.
To verify the biological significance of the effect of Grb10 on Akt activity, we applied our findings to a cell culture model of c-kit-mediated induction of IL-3-independent growth. Ba/F3 cells with stable expression of the constitutive active c-kitD814V mutant showed IL-3-independent growth (Fig. 10A).Cells expressing the c-kitD814V/Y719F mutant or cells expressing only vector control (FLAG/Ø) were unable to grow without IL-3. However, IL-3-independent growth could be restored in cells expressing c-kitD814V/Y719F when Grb10 was coexpressed. The growth of these cells was comparable to that of the cells expressing c-kitD814V alone. Ba/F3 cells with expression of Grb10 alone did not grow continuously without IL-3. However, preliminary results suggest increased survival of Grb10-expressing Ba/F3 cells after IL-3 withdrawal (data not shown). According to the data obtained in the transient-expression system showing lack of a positive effect on Akt activation by Grb10ΔSH2, cells expressing c-kitD814V/Y719F and Grb10ΔSH2 failed to grow without IL-3. Akt activation in Ba/F3 cells expressing c-kitD814V/Y719F and Grb10WT was significantly enhanced compared to that in cells with expression of c-kitD814V/Y719F alone or c-kitD814V/Y719F together with Grb10ΔSH2 (Fig. 10B, second panel, compare lane 3 with lanes 2 and 4).
DISCUSSION
Our interest in Grb10 was initiated by its identification as a new intermediate of the c-kit signaling pathway. We show that Grb10 is recruited to the c-kit receptor in a phosphotyrosine/SH2 domain-mediated fashion. The SH2 domain is sufficient to mediate this interaction since GST-Grb10SH2 bound to activated c-kit from Mo7e cells as well as from HMC-1 cells. Since the SH2 deletion mutant of Grb10 that we created also lacks part of the recently identified BPS domain, we cannot completely rule out the possibility that the association of c-kit is influenced by this region. Although PH domains have been reported to mediate or influence membrane association, translocation of Grb10 to the c-kit receptor in the course of SCF stimulation is independent of a functional Grb10 PH domain.
Our observation of a wortmannin-sensitive mobility shift of Grb10 by activated c-kit is compatible with the hypothesis that Grb10 undergoes phosphorylation on serine/threonine residues as a result of c-kit-mediated activation of PI3-K. Other groups have reported a similar observation in IR-mediated signaling (19), and a close family member of Grb10, Grb14, also has been shown to undergo serine phosphorylation in the course of fibroblast growth factor activation (42).
The recruitment of Grb10 to the RTK c-kit raises the question of whether Grb10 becomes phosphorylated on tyrosine residues by c-kit. As mentioned, we were unable to detect tyrosine phosphorylation of Grb10 from stimulated cells by using two different antiphosphotyrosine antibodies. However, tyrosine phosphorylation of Grb10 was reported as an effect of the nonreceptor tyrosine kinases Tec (29) and Src family kinases after treatment of cells with pervanadate (26).
We found that Grb10 forms a constitutive complex with Akt. This complex was identified in COS1 cells overexpressing Grb10 and Akt as epitope-tagged constructs as well as in Mo7e and K562 cells with endogenous proteins. This association most probably involves the N-terminal region of Grb10, since both Grb10ΔSH2 and Grb10ΔPH were able to form this complex. Interestingly, the complex between Grb10ΔPH and Akt seemed to be more stable than the complex between Grb10WT and Akt. It might therefore be speculated that the Grb10PH domain plays a role in the dissociation of this complex.
The striking stimulatory effect of Grb10 overexpression on Akt activation in a dose-dependent manner may represent the functional correlation of our finding of complex formation between Grb10 and Akt. The observation that increased Akt activity levels were seen without increased PI3-K activity levels suggests that Grb10 is a positive regulator of the Akt pathway downstream of PI3-K. Our data support the hypothesis that Grb10 acts as an adaptor involved in the relocalization of Akt to the cell membrane, which results in activation of Akt. That activation of Akt was seen with and (to a lesser extent and at a lower level) without the expression of activated c-kit could be explained by the promiscuous nature of the Grb10 SH2 domain, which causes Grb10 membrane association with tyrosine-phosphorylated membrane-bound proteins even in the absence of activated c-kit. In agreement with our findings, Wang et al. have observed a positive growth-stimulatory effect of overexpressed Grb10 in fibroblasts (50). Increased activation of Akt by Grb10 could be responsible for these findings.
It is possible that overexpression of Grb10 influences other Akt-related signaling pathways. We did not observe any significant changes in phosphorylation of MAPK or the Akt-related kinases PKCζ or p70S6K (Fig. 6 and data not shown). Therefore, the stimulatory effect of Grb10 so far seems to be specific for the Akt signaling pathway.
Grb10 association with an activated membrane-bound receptor is a critical factor for activation of Akt, since Grb10ΔSH2 failed to enhance Akt activity although this Grb10 mutant retained the ability to bind Akt. We hypothesize that Akt bound to Grb10ΔSH2 does not become translocated to the cell membrane and therefore fails to become activated. Accordingly, fusion of Akt to full-length Grb10 as well as to the Grb10 SH2 domain results in activation of the Akt hybrid protein. In line with these findings of a positive regulatory role of Grb10, Wang et al. have reported a dominant negative effect of the microinjected Grb10 SH2 domain on PDGF-BB and insulin-induced DNA synthesis (50). Importantly, v-Akt has been shown to be constitutively activated as a result of its constitutive localization at the cell membrane by the viral gag sequence (1). The observation that wortmannin was able to block the activation of Akt by Grb10, together with the result that PI3-K activity levels are not influenced by Grb10, clearly shows that the effect of Grb10 is located downstream of PI3-K and is in line with a role for Grb10 as an adaptor transporting Akt to the cell membrane.
The Grb10ΔPH mutant failed to significantly influence Akt activity despite its ability to form a complex with Akt and to become recruited to the c-kit receptor. Impaired release of Akt from Grb10ΔPH due to a more stable complex in the coimmunoprecipitation studies as well as in the GST binding experiments might serve as an explanation for this finding. The release of Akt from the complex with Grb10 might be necessary for subsequent complete activation and translocation of Akt. Interestingly, we were unable to detect a complex between activated Akt and Grb10 by using an antibody against phosphorylated serine 473 of Akt (unpublished data), suggesting that Akt bound to Grb10 might not be activated. This could be explained by the possible dissociation of the complex following activation of Akt. Additionally, Akt association with activated c-kit could be detected only when a kinase-defective mutant of Akt was used instead of WT Akt, indicating that the release of Akt requires kinase activity (unpublished data). However, the observation that the chimeric construct of Grb10ΔPH fused to Akt was less activated than was the fusion construct with Grb10WT suggests that the Grb10 PH domain might influence Akt activity in a currently undefined way.
We show that concomitant expression of Grb10 and a c-kit mutant defective in PI3-K activation (c-kitD814V/Y719F) was able to overcome deficient Akt activation of this c-kit mutant in COS1 cells. Using a cell culture model of c-kit-induced IL-3-independent growth in Ba/F3 cells, we show that Grb10 expression is able to rescue the inability of c-kitD814V/Y719F to induce IL-3-independent growth by enhancing Akt activity in these cells. These data are in agreement with a critical role for Grb10 as an adaptor linking Akt to PI3-K. The dose-dependent effect of Grb10 on the activation of Akt, together with its ability to rescue an incomplete oncogene like c-kitD814V/Y719F, highlights the importance of a well-controlled expression of Grb10 in cells. Interestingly, a contribution to the malignancy of carcinoma cells has been reported for another member of the Grb10 family: aberrant elevated expression of Grb7 significantly enhances the invasive potential of esophageal carcinoma cells (48).
Our group has previously identified Grb10 as an interaction partner of BCR-Abl (5). It is an interesting speculation that recruitment of Grb10 to BCR-Abl has a similar effect on Akt activation, since Akt activation is required for BCR-Abl-mediated leukemogenesis (45). Indeed, we show that in the CML cell line K562, a constitutive association between Grb10 and Akt is also present.
The existence of (so far) three splice variants with eventually differential ability to form a complex with Akt might reflect a posttranscriptional way of controlling the biological effect of Grb10 in normal cells. Interestingly, hGrb10γ has the ability to form tetramers with other Grb10 isoforms in a head-to-tail fashion in vivo. This oligomerization interferes with the ability of Grb10 to bind activated receptors (20). Thus, selective accumulation of hGrb10γ could antagonize and regulate Grb10 function in cells. The proposed costimulatory role of Grb10 in activation of Akt could lead to constitutively elevated basal Akt activation levels in cells where Grb10 is amplified. However, it is important to note that the Grb10 version we used is the murine Grb10α with functional PH and SH2 domains. With regard to the variety of splice variants, it might be difficult to predict a single predominant effect of changes in the expression pattern of Grb10. Thus, careful evaluation of the differential effects of Grb10 isoforms and their tissue-specific expression pattern is necessary to understand the possible implications of aberrant expression of Grb10. It is important to note that the deletion affecting the PH domain in the human splice variant Grb10α (former Grb-IR) is almost identical to that in the Grb10ΔPH mutant we created (deletion of aa −7 to +39 and of aa −8 to +44, respectively, counting from the first amino acid of the PH domain). Grb-IR/Grb10α lacks a functional PH domain, and its role in IR signaling has been described as inhibitory (27). In contrast, the effect of the human Grb10 homologue with an intact PH domain (Grb-IR-SV) has been described as positive stimulatory (38, 50). Based on our observations and on these previous reports, we would like to forward the hypothesis that alternative splicing of Grb10 selectively affecting the functionality of the PH domain might serve as a novel alternative means of regulating Akt activity in cells.
Acknowledgments
This work was supported by a grant to J.D. from the Deutsche José-Carreras-Stiftung and the Mildred-Scheel-Stiftung and by SFB grant 456 to J.D. and C.P. T.J. is supported by a fellowship from the Deutsche José-Carreras-Stiftung.
REFERENCES
- 1.Ahmed, N. N., T. F. Franke, A. Bellacosa, K. Datta, M. E. Gonzalez-Portal, T. Taguchi, J. R. Testa, and P. N. Tsichlis. 1993. The proteins encoded by c-akt and v-akt differ in post-translational modification, subcellular localization and oncogenic potential. Oncogene 8:1957-1963. [PubMed] [Google Scholar]
- 2.Altomare, D. A., K. Guo, J. Q. Cheng, G. Sonoda, K. Walsh, and J. R. Testa. 1995. Cloning, chromosomal localization and expression analysis of the mouse Akt2 oncogene. Oncogene 11:1055-1060. [PubMed] [Google Scholar]
- 3.Altomare, D. A., G. E. Lyons, Y. Mitsuuchi, J. Q. Cheng, and J. R. Testa. 1998. Akt2 mRNA is highly expressed in embryonic brown fat and the AKT2 kinase is activated by insulin. Oncogene 16:2407-2411. [DOI] [PubMed] [Google Scholar]
- 4.Andjelkovic, M., D. R. Alessi, R. Meier, A. Fernandez, N. J. Lamb, M. Frech, P. Cron, P. Cohen, J. M. Lucocq, and B. A. Hemmings. 1997. Role of translocation in the activation and function of protein kinase B. J. Biol. Chem. 272:31515-31524. [DOI] [PubMed] [Google Scholar]
- 5.Bai, R. Y., T. Jahn, S. Schrem, G. Munzert, K. M. Weidner, J. Y. Wang, and J. Duyster. 1998. The SH2-containing adapter protein GRB10 interacts with BCR-ABL. Oncogene 17:941-948. [DOI] [PubMed] [Google Scholar]
- 6.Bellacosa, A., T. F. Franke, M. E. Gonzalez-Portal, K. Datta, T. Taguchi, J. Gardner, J. Q. Cheng, J. R. Testa, and P. N. Tsichlis. 1993. Structure, expression and chromosomal mapping of c-akt: relationship to v-akt and its implications. Oncogene 8:745-754. [PubMed] [Google Scholar]
- 7.Biggs, W. H., III, J. Meisenhelder, T. Hunter, W. K. Cavenee, and K. C. Arden. 1999. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc. Natl. Acad. Sci. USA 96:7421-7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blume-Jensen, P., R. Janknecht, and T. Hunter. 1998. The kit receptor promotes cell survival via activation of PI 3-kinase and subsequent Akt-mediated phosphorylation of Bad on Ser136. Curr. Biol. 8:779-782. [DOI] [PubMed] [Google Scholar]
- 9.Blume-Jensen, P., G. Jiang, R. Hyman, K. F. Lee, S. O'Gorman, and T. Hunter. 2000. Kit/stem cell factor receptor-induced activation of phosphatidylinositol 3′-kinase is essential for male fertility. Nat. Genet. 24:157-162. [DOI] [PubMed] [Google Scholar]
- 10.Brodbeck, D., P. Cron, and B. A. Hemmings. 1999. A human protein kinase Bgamma with regulatory phosphorylation sites in the activation loop and in the C-terminal hydrophobic domain. J. Biol. Chem. 274:9133-9136. [DOI] [PubMed] [Google Scholar]
- 11.Brunet, A., A. Bonni, M. J. Zigmond, M. Z. Lin, P. Juo, L. S. Hu, M. J. Anderson, K. C. Arden, J. Blenis, and M. E. Greenberg. 1999. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96:857-868. [DOI] [PubMed] [Google Scholar]
- 12.Cardone, M. H., N. Roy, H. R. Stennicke, G. S. Salvesen, T. F. Franke, E. Stanbridge, S. Frisch, and J. C. Reed. 1998. Regulation of cell death protease caspase-9 by phosphorylation. Science 282:1318-1321. [DOI] [PubMed] [Google Scholar]
- 13.Cheng, J. Q., B. Ruggeri, W. M. Klein, G. Sonoda, D. A. Altomare, D. K. Watson, and J. R. Testa. 1996. Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc. Natl. Acad. Sci. USA 93:3636-3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coffer, P. J., and J. R. Woodgett. 1991. Molecular cloning and characterisation of a novel putative protein-serine kinase related to the cAMP-dependent and protein kinase C families. Eur. J. Biochem. 201:475-481. (Erratum, 205:1217, 1992.) [DOI] [PubMed] [Google Scholar]
- 15.Coutinho, S., T. Jahn, M. Lewitzky, S. Feller, P. Hutzler, C. Peschel, and J. Duyster. 2000. Characterization of Grb4, an adapter protein interacting with Bcr-Abl. Blood 96:618-624. [PubMed] [Google Scholar]
- 16.Currie, R. A., K. S. Walker, A. Gray, M. Deak, A. Casamayor, C. P. Downes, P. Cohen, D. R. Alessi, and J. Lucocq. 1999. Role of phosphatidylinositol 3,4,5-trisphosphate in regulating the activity and localization of 3-phosphoinositide-dependent protein kinase-1. Biochem. J. 337:575-583. [PMC free article] [PubMed] [Google Scholar]
- 17.Daly, R. J. 1998. The Grb7 family of signalling proteins. Cell. Signalling 10:613-618. [DOI] [PubMed] [Google Scholar]
- 18.Datta, S. R., H. Dudek, X. Tao, S. Masters, H. Fu, Y. Gotoh, and M. E. Greenberg. 1997. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91:231-241. [DOI] [PubMed] [Google Scholar]
- 19.Dong, L. Q., H. Du, S. G. Porter, L. F. Kolakowski, Jr., A. V. Lee, L. J. Mandarino, J. Fan, D. Yee, and F. Liu. 1997. Cloning, chromosome localization, expression, and characterization of an Src homology 2 and pleckstrin homology domain-containing insulin receptor binding protein hGrb10gamma. J. Biol. Chem. 272:29104-29112. (Erratum, 273:4288, 1998.) [DOI] [PubMed] [Google Scholar]
- 20.Dong, L. Q., S. Porter, D. Hu, and F. Liu. 1998. Inhibition of hGrb10 binding to the insulin receptor by functional domain-mediated oligomerization. J. Biol. Chem. 273:17720-17725. [DOI] [PubMed] [Google Scholar]
- 21.Franke, T. F., D. R. Kaplan, L. C. Cantley, and A. Toker. 1997. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science 275:665-668. [DOI] [PubMed] [Google Scholar]
- 22.Franke, T. F., S. I. Yang, T. O. Chan, K. Datta, A. Kazlauskas, D. K. Morrison, D. R. Kaplan, and P. N. Tsichlis. 1995. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 81:727-736. [DOI] [PubMed] [Google Scholar]
- 23.Jones, P. F., T. Jakubowicz, F. J. Pitossi, F. Maurer, and B. A. Hemmings. 1991. Molecular cloning and identification of a serine/threonine protein kinase of the second-messenger subfamily. Proc. Natl. Acad. Sci. USA 88:4171-4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanakura, Y., T. Furitsu, T. Tsujimura, J. H. Butterfield, L. K. Ashman, H. Ikeda, H. Kitayama, Y. Kanayama, Y. Matsuzawa, and Y. Kitamura. 1994. Activating mutations of the c-kit proto-oncogene in a human mast cell leukemia cell line. Leukemia 8(Suppl. 1):S18-S22. [PubMed] [Google Scholar]
- 25.Kissel, H., I. Timokhina, M. P. Hardy, G. Rothschild, Y. Tajima, V. Soares, M. Angeles, S. R. Whitlow, K. Manova, and P. Besmer. 2000. Point mutation in kit receptor tyrosine kinase reveals essential roles for kit signaling in spermatogenesis and oogenesis without affecting other kit responses. EMBO J. 19:1312-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langlais, P., L. Q. Dong, D. Hu, and F. Liu. 2000. Identification of Grb10 as a direct substrate for members of the Src tyrosine kinase family. Oncogene 19:2895-2903. [DOI] [PubMed] [Google Scholar]
- 27.Liu, F., and R. A. Roth. 1995. Grb-IR: a SH2-domain-containing protein that binds to the insulin receptor and inhibits its function. Proc. Natl. Acad. Sci. USA 92:10287-10291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maira, S. M., I. Galetic, D. P. Brazil, S. Kaech, E. Ingley, M. Thelen, and B. A. Hemmings. 2001. Carboxyl-terminal modulator protein (CTMP), a negative regulator of PKB/Akt and v-Akt at the plasma membrane. Science 294:374-380. [DOI] [PubMed] [Google Scholar]
- 29.Mano, H., K. Ohya, A. Miyazato, Y. Yamashita, W. Ogawa, J. Inazawa, U. Ikeda, K. Shimada, K. Hatake, M. Kasuga, K. Ozawa, and S. Kajigaya. 1998. Grb10/GrbIR as an in vivo substrate of Tec tyrosine kinase. Genes Cells 3:431-441. [DOI] [PubMed] [Google Scholar]
- 30.Meier, R., D. R. Alessi, P. Cron, M. Andjelkovic, and B. A. Hemmings. 1997. Mitogenic activation, phosphorylation, and nuclear translocation of protein kinase Bbeta. J. Biol. Chem. 272:30491-30497. [DOI] [PubMed] [Google Scholar]
- 31.Morrione, A. 2000. Grb10 proteins in insulin-like growth factor and insulin receptor signaling. Int. J. Mol. Med. 5:151-154. [DOI] [PubMed] [Google Scholar]
- 32.Morrione, A., P. Plant, B. Valentinis, O. Staub, S. Kumar, D. Rotin, and R. Baserga. 1999. mGrb10 interacts with Nedd4. J. Biol. Chem. 274:24094-24099. [DOI] [PubMed] [Google Scholar]
- 33.Morrione, A., B. Valentinis, M. Resnicoff, S. Xu, and R. Baserga. 1997. The role of mGrb10alpha in insulin-like growth factor I-mediated growth. J. Biol. Chem. 272:26382-26387. [DOI] [PubMed] [Google Scholar]
- 34.Mounier, C., and B. Posner. 2001. Specific inhibition by hGrb10zeta of insulin-induced glycogen sythase activation: evidence for a novel signaling pathway. Mol. Cell. Endocrinol. 173:15-27. [DOI] [PubMed] [Google Scholar]
- 35.Nantel, A., M. Huber, and D. Y. Thomas. 1999. Localization of endogenous Grb10 to the mitochondria and its interaction with the mitochondrial-associated Raf-1 pool. J. Biol. Chem. 274:35719-35724. [DOI] [PubMed] [Google Scholar]
- 36.Nantel, A., K. Mohammad-Ali, J. Sherk, B. I. Posner, and D. Y. Thomas. 1998. Interaction of the Grb10 adapter protein with the Raf1 and MEK1 kinases. J. Biol. Chem. 273:10475-10484. [DOI] [PubMed] [Google Scholar]
- 37.Okano, J., I. Gaslightwala, M. J. Birnbaum, A. K. Rustgi, and H. Nakagawa. 2000. Akt/protein kinase B isoforms are differentially regulated by epidermal growth factor stimulation. J. Biol. Chem. 275:30934-30942. [DOI] [PubMed] [Google Scholar]
- 38.O'Neill, T. J., D. W. Rose, T. S. Pillay, K. Hotta, J. M. Olefsky, and T. A. Gustafson. 1996. Interaction of a GRB-IR splice variant (a human GRB10 homolog) with the insulin and insulin-like growth factor I receptors. Evidence for a role in mitogenic signaling. J. Biol. Chem. 271:22506-22513. [DOI] [PubMed] [Google Scholar]
- 39.Ooi, J., V. Yajnik, D. Immanuel, M. Gordon, J. J. Moskow, A. M. Buchberg, and B. Margolis. 1995. The cloning of Grb10 reveals a new family of SH2 domain proteins. Oncogene 10:1621-1630. [PubMed] [Google Scholar]
- 40.Pandey, A., H. Duan, P. P. Di Fiore, and V. M. Dixit. 1995. The Ret receptor protein tyrosine kinase associates with the SH2-containing adapter protein Grb10. J. Biol. Chem. 270:21461-21463. [DOI] [PubMed] [Google Scholar]
- 41.Piao, X., R. Paulson, P. van der Geer, T. Pawson, and A. Bernstein. 1996. Oncogenic mutation in the Kit receptor tyrosine kinase alters substrate specificity and induces degradation of the protein tyrosine phosphatase SHP-1. Proc. Natl. Acad. Sci. USA 93:14665-14669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reilly, J. F., G. Mickey, and P. A. Maher. 2000. Association of fibroblast growth factor receptor 1 with the adaptor protein Grb14. Characterization of a new receptor binding partner. J. Biol. Chem. 275:7771-7778. [DOI] [PubMed] [Google Scholar]
- 43.Sable, C. L., N. Filippa, C. Filloux, B. A. Hemmings, and E. Van Obberghen. 1998. Involvement of the pleckstrin homology domain in the insulin-stimulated activation of protein kinase B. J. Biol. Chem. 273:29600-29606. [DOI] [PubMed] [Google Scholar]
- 44.Serve, H., N. S. Yee, G. Stella, L. Sepp-Lorenzino, J. C. Tan, and P. Besmer. 1995. Differential roles of PI3-kinase and Kit tyrosine 821 in Kit receptor-mediated proliferation, survival and cell adhesion in mast cells. EMBO J. 14:473-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skorski, T., A. Bellacosa, M. Nieborowska-Skorska, M. Majewski, R. Martinez, J. K. Choi, R. Trotta, P. Wlodarski, D. Perrotti, T. O. Chan, M. A. Wasik, P. N. Tsichlis, and B. Calabretta. 1997. Transformation of hematopoietic cells by BCR/ABL requires activation of a PI-3k/Akt-dependent pathway. EMBO J. 16:6151-6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Songyang, Z., D. Baltimore, L. C. Cantley, D. R. Kaplan, and T. F. Franke. 1997. Interleukin 3-dependent survival by the Akt protein kinase. Proc. Natl. Acad. Sci. USA 94:11345-11350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stein, E. G., T. A. Gustafson, and S. R. Hubbard. 2001. The BPS domain of Grb10 inhibits the catalytic activity of the insulin and IGF1 receptors. FEBS Lett. 493:106-111. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka, S., K. Sugimachi, H. Kawaguchi, H. Saeki, S. Ohno, and J. R. Wands. 2000. Grb7 signal transduction protein mediates metastatic progression of esophageal carcinoma. J. Cell. Physiol. 183:411-415. [DOI] [PubMed] [Google Scholar]
- 49.Tsujimura, T., K. Hashimoto, H. Kitayama, H. Ikeda, H. Sugahara, I. Matsumura, T. Kaisho, N. Terada, Y. Kitamura, and Y. Kanakura. 1999. Activating mutation in the catalytic domain of c-kit elicits hematopoietic transformation by receptor self-association not at the ligand-induced dimerization site. Blood 93:1319-1329. [PubMed] [Google Scholar]
- 50.Wang, J., H. Dai, N. Yousaf, M. Moussaif, Y. Deng, A. Boufelliga, O. R. Swamy, M. E. Leone, and H. Riedel. 1999. Grb10, a positive, stimulatory signaling adapter in platelet-derived growth factor BB-, insulin-like growth factor I-, and insulin-mediated mitogenesis. Mol. Cell. Biol. 19:6217-6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wijkander, J., L. S. Holst, T. Rahn, S. Resjo, I. Castan, V. Manganiello, P. Belfrage, and E. Degerman. 1997. Regulation of protein kinase B in rat adipocytes by insulin, vanadate, and peroxovanadate. Membrane translocation in response to peroxovanadate. J. Biol. Chem. 272:21520-21526. [DOI] [PubMed] [Google Scholar]
- 52.Wojcik, J., J. A. Girault, G. Labesse, J. Chomilier, J. P. Mornon, and I. Callebaut. 1999. Sequence analysis identifies a ras-associating (RA)-like domain in the N-termini of band 4.1/JEF domains and in the Grb7/10/14 adapter family. Biochem. Biophys. Res. Commun. 259:113-120. [DOI] [PubMed] [Google Scholar]
- 53.Zhou, B. P., Y. Liao, W. Xia, B. Spohn, M. H. Lee, and M. C. Hung. 2001. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat. Cell Biol. 3:245-252. [DOI] [PubMed] [Google Scholar]