Abstract
Cell cycle checkpoints are among the multiple mechanisms that eukaryotic cells possess to maintain genomic integrity and minimize tumorigenesis. Ionizing irradiation (IR) induces measurable arrests in the G1, S, and G2 phases of the mammalian cell cycle, and the ATM (ataxia telangiectasia mutated) protein plays a role in initiating checkpoint pathways in all three of these cell cycle phases. However, cells lacking ATM function exhibit both a defective G2 checkpoint and a prolonged G2 arrest after IR, suggesting the existence of different types of G2 arrest. Two molecularly distinct G2/M checkpoints were identified, and the critical importance of the choice of G2/M checkpoint assay was demonstrated. The first of these G2/M checkpoints occurs early after IR, is very transient, is ATM dependent and dose independent (between 1 and 10 Gy), and represents the failure of cells which had been in G2 at the time of irradiation to progress into mitosis. Cell cycle assays that can distinguish mitotic cells from G2 cells must be used to assess this arrest. In contrast, G2/M accumulation, typically assessed by propidium iodide staining, begins to be measurable only several hours after IR, is ATM independent, is dose dependent, and represents the accumulation of cells that had been in earlier phases of the cell cycle at the time of exposure to radiation. G2/M accumulation after IR is not affected by the early G2/M checkpoint and is enhanced in cells lacking the IR-induced S-phase checkpoint, such as those lacking Nbs1 or Brca1 function, because of a prolonged G2 arrest of cells that had been in S phase at the time of irradiation. Finally, neither the S-phase checkpoint nor the G2 checkpoints appear to affect survival following irradiation. Thus, two different G2 arrest mechanisms are present in mammalian cells, and the type of cell cycle checkpoint assay to be used in experimental investigation must be thoughtfully selected.
Genomic integrity is maintained by a complex network of checkpoints that are defined as control mechanisms enforcing dependency in the cell cycle. Within the cell cycle, both G1-S and G2-M phase transitions are under constant surveillance for the protection of cells from exogenous and endogenous DNA-damaging agents. These ordered dependencies are controlled by the regulation of certain gene products, mutations in which can result in altered stress responses, increased mutation rates, or genetic instability (11). Investigating the mechanisms of genetic regulation of cell cycle checkpoints thus contributes to an understanding of both cancer development and the responses of tumor cells to chemotherapy and radiotherapy.
Ionizing irradiation (IR) induces arrests in the G1, S, and G2 phases of the cell cycle. A number of gene products that control each of these checkpoints have been identified, including the ATM (ataxia telangiectasia mutated) protein kinase, which appears to be critical for initiation of each of these checkpoint pathways (12). The ATM gene is mutated in the autosomal recessive disease ataxia telangiectasia (AT), which is characterized by a pleiotropic phenotype including neuronal degeneration, oculocutaneous telangiectasias, immune dysfunction, and cancer predisposition (24). Substrates and target sites of the ATM kinase have been implicated in control of the G1 (p53, Chk2, and Mdm2) (1, 2, 7, 16, 17, 18, 19, 25), S-phase (Nbs1 and Chk2) (9, 15, 33), and G2 (Brca1 and hRad17) (3, 30) checkpoint pathways. Additional substrates of ATM involved in these cell cycle control pathways are likely to be identified.
Reports in the literature characterizing the defect in G2-to-M progression after IR in AT cells have presented apparent discrepancies. Some data demonstrate that AT cells fail to arrest in G2 after IR and continue to progress into mitosis (5, 20, 30, 32). On the other hand, other studies suggest that irradiated AT cells exhibit a prolonged G2 arrest after IR compared to cells from normal individuals (4, 23, 27). We investigated this apparent paradox and confirm that both phenotypic descriptions of cells lacking ATM activity are correct. We show that the conclusions drawn from assessment of the G2 checkpoint abnormality in AT cells are dependent on the cell cycle assay that is utilized and that two mechanistically distinct types of G2 cell cycle arrests occur after IR.
MATERIALS AND METHODS
Cell culture and irradiation.
Epstein-Barr virus-immortalized lymphoblastoid cell lines from healthy persons (GM0536; NIGMS Human Mutant Cell Repository, Camden, N.J.) and from persons who were homozygous for the ATM mutation (GM 1526) or the NBS1 mutation (NBS7078A) were cultured in RPMI 1640 supplemented with 15% fetal bovine serum. Simian virus 40 (SV40)-transformed human fibroblast cell lines from a normal donor (SVG; American Type Culture Collection, Manassas, Va.), AT patients (GM5849 and GM9607; NIGMS), and a Nijmegen breakage syndrome (NBS) patient (NBS1-LBI, generously provided by Malgorzata Zdzienicka from Leiden University, Netherlands) (14), human 293T cells, and tumor cell lines (human cervix cancer cell line HeLa, human neuroblastoma cell line SY5Y, and human breast cancer cell line HCC1937, all from the American Type Culture Collection) were all grown as monolayers in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum. All cell lines were grown at 37°C in a humidified atmosphere containing 5% CO2. Radiation from a 137Cs source was delivered at a rate of approximately 120 cGy/min.
Expression of Nbs1 constructs in NBS cells and BRCA1 constructs in Brca1 mutant cells.
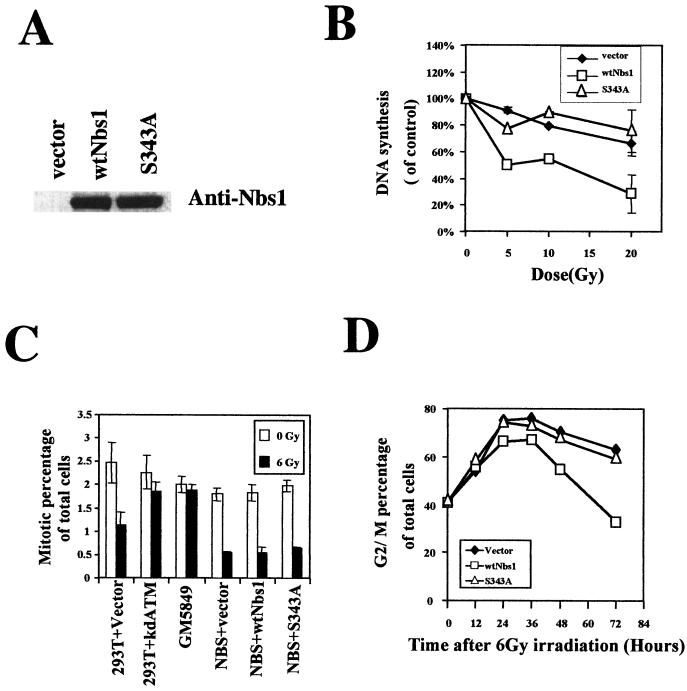
We generated NBS1-LBI cell lines that stably express wild-type Nbs1 or mutant Nbs1 (serine 343 to alanine) by retroviral infection. For assessment of Nbs1/p95 expression in the infectants, total cellular lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis in a 4 to 12% polyacrylamide precast gel (Invitrogen, Carlsbad, Calif.). Expressed Nbs1/p95 proteins were detected by Western blot analysis with an anti-Nbs1/p95 polyclonal antibody (Novus Biologicals Inc., Littleton, Colo.). Transfections of wild-type BRCA1 (generously provided by David Livingston) or mutant Brca1 (serine 1423 to alanine) into a Brca1-mutant cell line HCC1937, and transfections of wild-type ATM or kinase-dead ATM into 293T or HeLa cells were done transiently by using Lipofectamine (Life Technologies, Rockville, Md.). For clonogenic survival assays in HCC 1937 cells transfected with Brca1 constructs, 1 mg of Geneticin (G-418; Life Technologies) per ml was added to the medium 36 h after transfection.
Flow cytometric analysis. (i) PI staining.
Cells were harvested at various time points and fixed with 70% ethanol. Approximately 106 cells were incubated with 25 μg of propidium iodide (PI; Sigma, St. Louis, Mo.) per ml, and DNA content was determined with a FACSCalibur (Becton Dickinson, San Jose, Calif.). Data were plotted by using CellQuest software; 20,000 events were analyzed for each sample.
(ii) BrdUrd labeling and staining.
Cells were pulse-labeled with 30 μM 5-bromo-2"-deoxy-uridine (BrdUrd; Boehringer Mannheim, Mannheim, Germany) for 30 min and then irradiated. At various time points, cells were harvested and fixed in 70% methanol. Cells were then stained for both DNA content and BrdUrd incorporation by the acid denaturation-protease method by using fluorescein isothiocyanate (FITC)-conjugated anti-BrdUrd (Becton Dickinson) as described previously (6).
(iii) Immunofluorescent detection of phosphorylated histone H3.
Cells were harvested at various time points after IR and fixed in 70% ethanol at −20°C. After fixation, the cells were resuspended in 1 ml of 0.25% Triton X-100 in phosphate-buffered saline (PBS) and incubated on ice for 15 min. After centrifugation, the cell pellet was suspended in 100 μl of PBS containing 1% bovine serum albumin (BSA) and 0.75 μg of a polyclonal antibody that specific recognizes the phosphorylated form of histone H3 (Upstate Biotechnology, Lake Placid, N.Y.) and incubated for 3 h at room temperature. The cells were then rinsed with PBS containing 1% BSA and incubated with FITC-conjugated goat anti-rabbit immunoglobulin G antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.) diluted at a ratio of 1:30 in PBS containing 1% BSA. After a 30-min incubation at room temperature in the dark, the cells were stained with PI and cellular fluorescence was measured by a flow cytometer.
S-phase checkpoint assay.
Inhibition of DNA synthesis after irradiation was assessed as previously described (15, 20, 30). Briefly, cells were prelabeled with 10 nCi of [14C]thymidine (NEN Life Science Products, Inc., Boston, Mass.) for 24 h. Cells were irradiated and incubated for 30 min and pulse-labeled with 2.5 μCi of [3H]thymidine (NEN Life Science Products) per ml. Cells were harvested and fixed with 70% methanol. The amount of radioactivity was assayed in a liquid scintillation counter. The measure of DNA synthesis was derived from resulting ratios of 3H to 14C counts per minute, corrected for counts that resulted from channel crossover.
Clonogenic assays.
Cell lines were plated in triplicate at limiting dilutions into six-well plates, incubated for 24 h, and then exposed to a range of doses of IR (0 to 6 Gy) followed by incubation for 2 weeks. Prior to colony counting, cells were fixed in 95% methanol and stained with crystal violet. A population of more than 50 cells were counted as one surviving colony. The mean colony counts ± standard errors are presented in the figures.
RESULTS
ATM-dependent and -independent G2/M checkpoints.
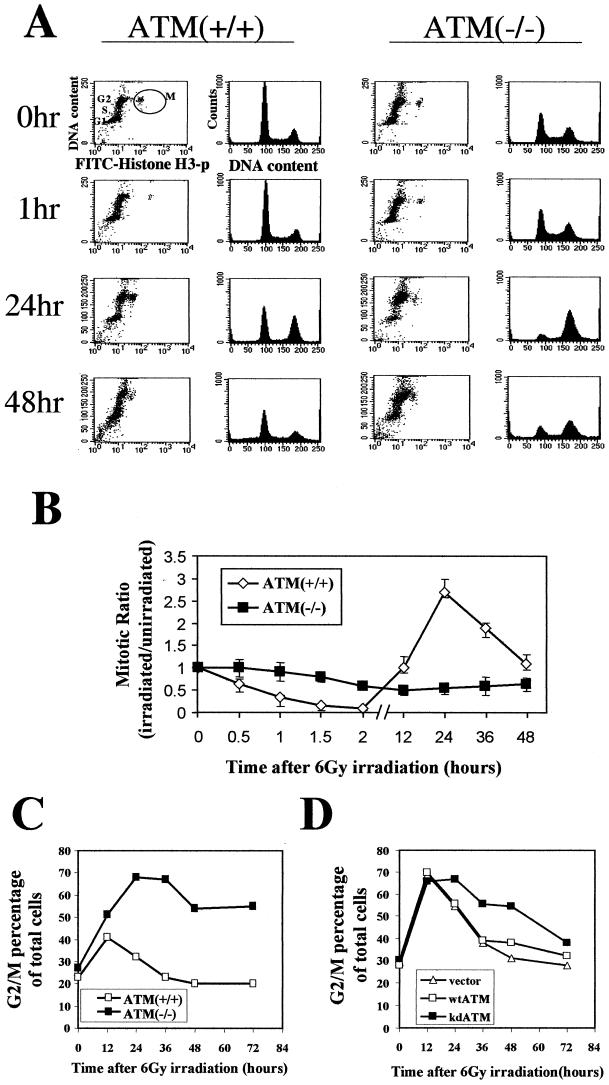
Cells from AT patients have previously been reported to exhibit both a defective G2 checkpoint (5, 20, 30, 32) and prolonged G2 accumulation (4, 23, 27) after exposure to ionizing radiation. In order to try to more fully understand this apparent inconsistency, we evaluated the G2 arrest in cells with normal or absent ATM function at various time points by two different assays. When a recently developed assay that uses flow cytometric assessment of histone H3 phosphorylation was used to distinguish mitotic cells from G2 cells (30), cells with wild-type ATM function were found to stop entering mitosis within the first hour after irradiation (Fig. 1A[first column] and B). By 12 h after irradiation, these cells release from G2 and begin to reenter mitosis (Fig. 1A [first column] and B). In fact, the cells appear to reenter mitosis somewhat synchronously and actually exhibit an increased number of cells in mitosis between 12 and 48 h after irradiation. In contrast, cells lacking ATM function continue to enter mitosis after irradiation (Fig. 1A [third column] and B), and the absolute percentage of mitotic AT cells does not vary very much over time after irradiation (Fig. 1B). These data are consistent with prior observations that ATM is required for an IR-induced G2/M checkpoint and demonstrate the rapid nature of its initiation and the transience of the arrest. Though the particular cells shown here for comparisons are not isogenic, we have previously demonstrated the critical dependency of this checkpoint assay on ATM kinase activity using isogenically comparable cells (30), and these data extend those previously published observations with a more extensive kinetic analysis.
FIG. 1.
Early G2/M checkpoint and late G2 accumulation. (A) Flow-cytometric profiles of cell cycle distribution before (0 h) and at the indicated time points after IR. Staining for DNA content (PI; y axis) and for histone H3 phosphorylation (FITC; x axis) is shown in the first and the third columns. M, mitotic cell population. DNA content (PI) is shown in the second and the fourth columns. (B) Ratio (irradiated/unirradiated) of mitotic cells (staining positive for phosphorylated histone H3) as a function of time after IR in HeLa [ATM(+/+)] and GM9607 [ATM(−/−)] cells. Error bars represent the variation around the averages of at least triplicate samples. (C) Percentage of total cells in G2/M by PI staining as a function of time after 6 Gy of IR in HeLa [ATM(+/+)] and GM9607 [ATM(−/−)] cells. (D) Percentage of total cells in G2/M (PI staining) as a function of time after 6 Gy of IR in HeLa cells transfected with vector alone (vector), wild-type ATM (wtATM), or kinase-dead ATM (kdATM). All G2/M percentages are representative of at least three experiments.
If the G2/M arrest is evaluated by assessing by the percentage of total cells with 4N DNA content by PI staining (representing cells in G2 plus M), rather than directly assessing the percentage of just mitotic cells, a very different set of conclusions is reached. Cells with normal ATM function show an increase in the number of cells with 4N DNA content within 12 h after irradiation (Fig. 1A [second column] and C). By 36 to 48 h after irradiation, the percentage of 4N-content cells with normal ATM function returns to baseline (Fig. 1A and C). In contrast, cells lacking ATM exhibit an exaggerated and markedly prolonged accumulation of cells with 4N DNA content after IR (Fig. 1A [fourth column] and C). Similar results were obtained when isogenically comparable cells were examined (Fig. 1D). Thus, not only is ATM required for the IR-induced accumulation of cells in G2/M, but a lack of ATM actually results in an enhancement of this accumulation. In addition, the G2 arrest evaluated by this yardstick occurs later and lasts longer than the arrest assessed by counting mitotic cells.
These observations support the concept that there are two separate perturbations of progression from G2 into mitosis after IR. One of these occurs very early after IR and is dependent on ATM and Brca1 (30). We define this as the early G2/M checkpoint. The other response after IR, which we refer to as G2 accumulation, is independent of ATM function and is actually enhanced by the lack of ATM. The explanation for the latter phenomenon is provided below.
Dose dependency of the two G2 checkpoints.
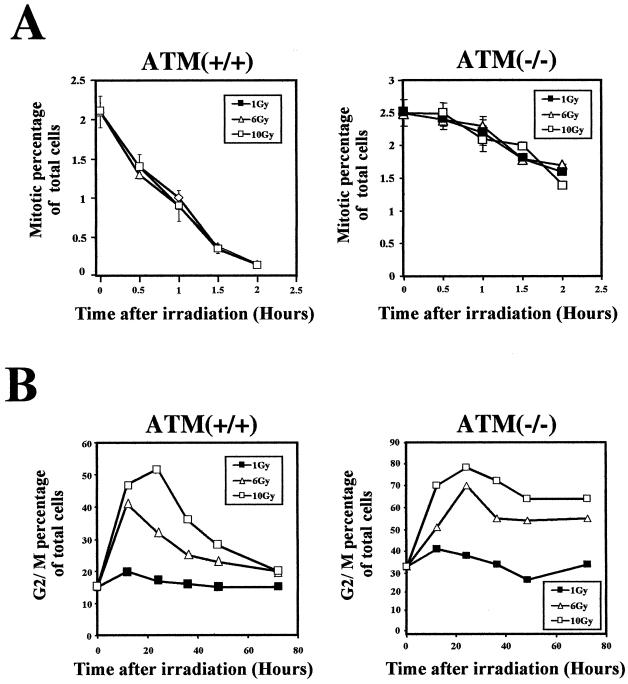
To further characterize these two distinct G2 responses, the IR dose dependencies of the early G2/M checkpoint and the G2 accumulation were assessed. The cessation of progression of cells from G2 into mitosis at early time points after irradiation was independent of the IR dose used over a range of 1 to 10 Gy (Fig. 2A).Though the amount of arrest was minimal in cells lacking ATM, these cells also exhibited no dose dependency in this dose range (Fig. 2A, right). Decreases in the arrest became apparent at doses less than 0.4 Gy (data not shown). In contrast, the amount of G2 accumulation over time as assessed by PI staining was dose dependent between 1 to 10 Gy (Fig. 2B). Though quantitative differences were predictably observed, this dose dependency was seen both in cells with ATM and in cells lacking ATM function. Though only these representative cell lines are shown here, several different cell lines with and without ATM function showed the same characteristics. The dose of IR also influenced the duration of the G2 accumulation, with higher doses leading to longer accumulation periods (Fig. 2B). Thus, dose dependency is another feature distinguishing the IR-induced early G2/M checkpoint and G2 accumulation.
FIG. 2.
Dose dependency of the two distinct G2 checkpoints. (A) Percentage of total cells in mitosis (staining positive for phosphorylated histone H3) at early time points after different doses of IR in HeLa [ATM(+/+)] and GM9607 [ATM(−/−)] cells. Error bars represent the variation around the averages of at least triplicate samples. (B) Dose dependency of G2/M accumulation (PI staining) at various times after IR in HeLa [ATM(+/+)] and GM9607 [ATM(−/−)] cells. All results for are representative of at least three experiments.
Links between S phase and G2 accumulation.
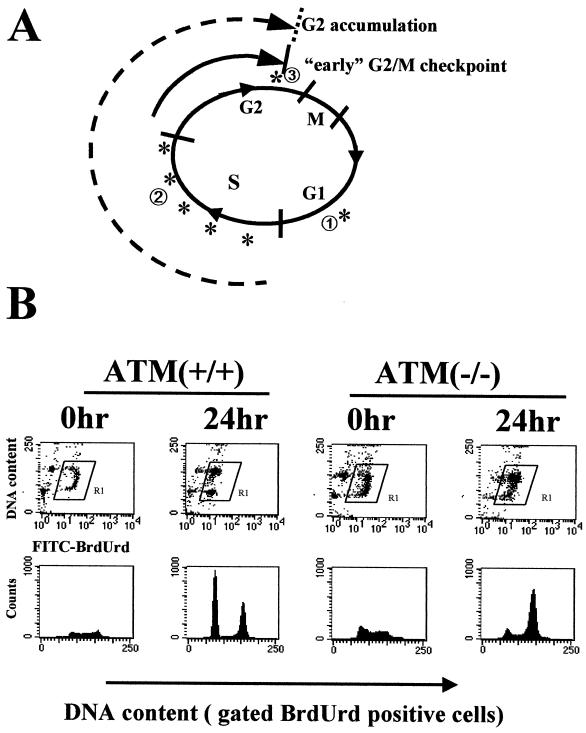
The differences noted in the time courses of the early G2/M checkpoint and the G2 accumulation have certain implications for mechanisms underlying the processes. Since the early G2/M checkpoint is measurable very shortly after the irradiation, this arrest must reflect the failure of cells that were in G2 at the time of the irradiation to progress into mitosis. In contrast, in order for the number of cells with 4N DNA content to increase above that seen prior to irradiation, cells from elsewhere in the cell cycle must be entering G2/M (Fig. 3A).In order to formally demonstrate that the cells that were accumulating in G2/M had been at an earlier stage of the cell cycle at the time of irradiation, cells were briefly pulse-labeled with the thymidine analog BrdUrd and then irradiated and harvested for flow-cytometric analysis at various times after irradiation. Uptake of the BrdUrd label identifies cells that were in S phase at the time of irradiation. Twenty-four hours after irradiation, the majority of the cells remaining in G2/M were positive for BrdUrd (Fig. 3B, top row, second column), demonstrating that the majority of cells remaining arrested were in S phase at the time of irradiation. It is noted that the BrdUrd-positive cells do escape G2 and progress back into the G1 phase of the cycle (Fig. 3B, bottom row, second column). Thus, the G2 accumulation does not appear to be a permanent arrest.
FIG. 3.
Most cells accumulating in G2/M after IR come from S phase. (A) Schematic representation of cell cycle progression after IR. Characterized checkpoints are labeled with asterisks. ①, G1 checkpoint, which is p53 dependent and occurs at restriction point; ②, ATM-dependent S-phase checkpoint, which occurs throughout S phase; ③, G2/M checkpoint, which is ATM dependent and occurs about 30 min prior to chromosome condensation. The early G2/M checkpoint measures the inhibition of cells that are in G2 at the time of IR from entering mitosis. G2/M accumulation, which is ATM independent, measures the accumulation of cells that were in S phase (or G1) at the time of irradiation. (B) HeLa [ATM(+/+)] and GM9607 [ATM(−/−)] cells were labeled with 30 μM BrdUrd for 30 min and then irradiated with 6 Gy. The top row shows flow-cytometric dot plots of BrdUrd labeling on the ordinate and DNA content on the abscissa before IR and 24 h after IR. The BrdUrd-positive cells (labeled R1) were gated and displayed as DNA content versus cell counts in the bottom row.
Examination of these cell cycle progression profiles in cells lacking ATM similarly demonstrated that the majority of cells accumulating in G2/M are BrdUrd positive and thus were in S phase at the time of irradiation (Fig. 3B, top row, fourth column). It is noted that some ATM-null cells irradiated during S phase survive and can make it back into G1, even though these cells lack the IR-induced S-phase checkpoint. One major difference between cells with and without ATM was that fewer BrdUrd-positive cells had released from G2/M and reentered G1 by 24 h in cells without ATM (Fig. 3B, bottom row, fourth column). This observation is consistent with the enhanced accumulation in G2/M seen in ATM-null cells and further demonstrates that a lack of release of irradiated S-phase cells contributes to this enhanced accumulation.
Defective S-phase checkpoints lead to prolonged G2 accumulation.
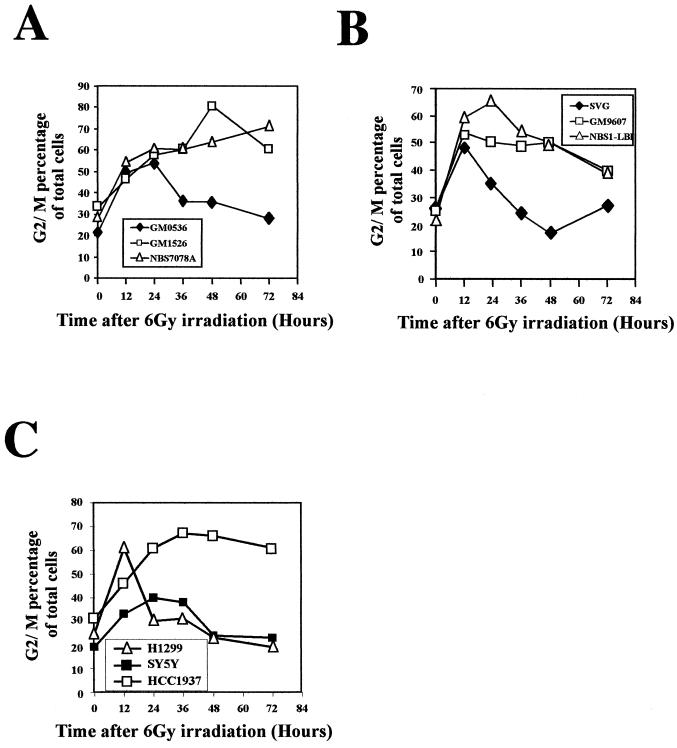
Cells lacking ATM function lack the IR-induced S-phase checkpoint (20, 24) and exhibit prolonged G2/M accumulation (Fig. 1 to 3). Since the majority of cells accumulating in G2/M were in S phase at the time of irradiation, we reasoned that perhaps it was the lack of an S-phase checkpoint that resulted in an enhanced G2/M accumulation. Thus, we investigated whether other cell types lacking an S-phase checkpoint also exhibited prolonged G2/M accumulation after IR. Nbs1 and Brca1 proteins are both required for the IR-induced S-phase checkpoint (15, 30). Similar to ATM-null cells, both fibroblasts and lymphoblasts lacking Nbs1 function exhibited the prolonged G2/M accumulation phenotype (Fig. 4A and B).Similarly, HCC1937 cells, which lack Brca1 function and lack an IR-induced S-phase checkpoint, exhibit an enhanced G2/M accumulation after IR (Fig. 4C). In contrast, cells defective in p53 function, either because of SV40 transformation (SVG) or lack of p53 expression (H1299), have an intact S-phase checkpoint, though they lack the IR-induced G1 checkpoint (13). In contrast to the cells defective in ATM, Nbs1, or Brca1 function, the p53-defective cells do not exhibit a prolonged G2/M accumulation (Fig. 4B and C). These data demonstrate a correlation between a defective S-phase checkpoint and an enhanced G2/M accumulation after IR.
FIG. 4.
Cell lines with defective S-phase checkpoint display prolonged G2/M accumulation after IR. (A) Quantitation of G2/M accumulation at various times after IR in normal (GM0536), AT (GM1526), and NBS (NBS7078A) lymphoblastoid cell lines. (B) Quantitation of G2/M accumulation in normal (SVG), AT (GM9607), and NBS (NBS1-LBI) SV40-transformed fibroblast cell lines. (C) Quantitation of G2/M accumulation at various times after IR in tumor cell lines with normal p53 function (SY5Y), lacking p53 (H1299), and lacking full-length Brca1 (HCC1937). All results are representative of at least three different experiments.
Since Nbs1 function is not required for the early IR-induced G2/M checkpoint (30), we can also conclude that prolonged G2/M accumulation does not correlate with the presence or absence of the early G2/M checkpoint. Since AT cells lack both the S-phase checkpoint and the early G2/M checkpoint, this conclusion could not have been reached by using ATM-null cells as a model. To extend these concepts beyond simple correlation, we generated isogenic cell lines for more formal evaluations of the links between these IR responses. Cells lacking Nbs1 function were stably complemented with either empty vector, wild-type Nbs1, or Nbs1 with serine 343 mutated to alanine. Complementation with wild-type Nbs1 restored the IR-induced S-phase checkpoint, while complementation with the S343A mutant, though it complements several of the defects in NBS cells (15, 33), failed to complement this particular defect (Fig. 5B).All of these NBS cells exhibit a normal early G2 checkpoint after IR (Fig. 5C). NBS cells complemented either with vector alone or with the S343A Nbs1 mutant exhibited the increased G2/M accumulation (Fig. 5D). In contrast, NBS cells complemented with wild-type Nbs1, which restores the IR-induced S-phase checkpoint, lost the enhanced G2/M accumulation phenotype (Fig. 5D). Thus, a more formal link between the S-phase checkpoint defect and enhanced G2/M accumulation after IR is established.
FIG. 5.
Prolonged G2 accumulation is correlated with the defective S-phase checkpoint but not the early G2/M checkpoint. NBS1-LBI cells that had been stably infected with empty vector, wild-type Nbs1, or S343A Nbs1 were used in the various checkpoint assays. (A) Expression of transduced Nbs1 proteins assessed by immunoblotting. (B) Replicative DNA synthesis assessed 30 min after various doses of IR in the NBS1-LBI cell lines. (C) Quantitation of early G2/M checkpoint data assessed by histone H3 phosphorylation in 293T cells transfected with empty vector or with kinase-dead ATM (293T+kdATM), an AT fibroblast cell line (GM5849), and the stable NBS cell lines. (D) Quantitation of percentage of total cells in G2/M as a function of time after 6 Gy of IR in the isogenic NBS cell lines. All results are representative of at least three different experiment.
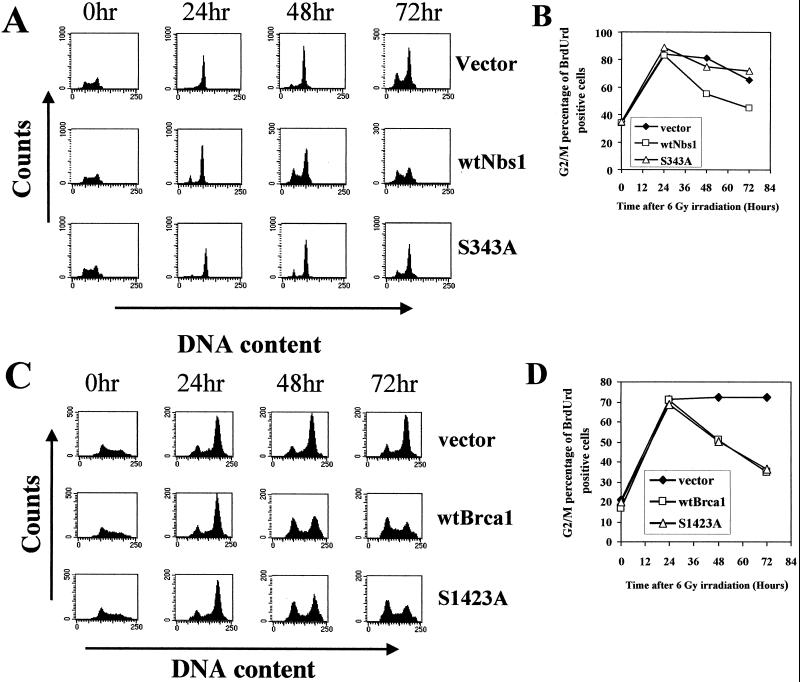
We had previously seen that the AT cells exhibiting the increased accumulation in G2/M were primarily cells that had been in S phase at the time of irradiation (Fig. 3B). We speculated that a failure to appropriately arrest in S phase after irradiation resulted in a prolonged arrest when the cells eventually got to G2. To directly establish this connection between lack of arrest of irradiated S-phase cells and enhanced G2/M accumulation linkage, we performed BrdUrd pulse-label experiments with these isogenically comparable cells. BrdUrd-positive NBS cells complemented with either vector alone or S343A mutant Nbs1 arrested longer in G2/M than NBS cells complemented with wild-type Nbs1 (Fig. 6).Thus, the enhanced G2/M accumulation appears to result primarily from cells in S phase that fail to appropriately transiently arrest in S phase in response to the irradiation.
FIG. 6.
Inappropriate replicative DNA synthesis after IR leads to prolonged G2/M accumulation. NBS1-LBI cells with stably introduced empty vector, wild-type Nbs1, or S343A Nbs1 were pulse-labeled with 30 μM BrdUrd 30 min before 6-Gy irradiation, and cells were harvested and analyzed at the indicated time points after IR. (A) The DNA content of cells that had taken up BrdUrd during the pulse period was examined at various times after IR. (B) Quantitation of the percentage of BrdUrd-positive NBS1-LBI cells in G2/M as a function of time after IR. All results are representative of at least three different experiments. (C) HCC1937 cells transfected with empty vector, wild-type Brca1, or S1423A Brca1 were pulse-labeled with BrdUrd, and evaluations were performed as for panel A. (D) Quantitation of BrdUrd-positive HCC1937 cells in G2/M as a function of time after IR. All results are representative of at least three different experiments.
NBS1-LBI cells, which exhibit prolonged G2/M accumulation after IR, lack the IR-induced S-phase checkpoint but have a normal early G2/M checkpoint. To further support the model linking the functionality of the S phase checkpoint to the magnitude of the G2/M accumulation, we also used cells with the converse phenotype (Table 1). HCC1937 cells, which lack Brca1 function and are defective in both the IR-induced S-phase and early G2/M checkpoints (30), exhibit the enhanced G2/M accumulation phenotype (Fig. 4D). Both the S-phase and G2 checkpoint defects are restored if we complement these cells with wild-type Brca1, but only the S-phase checkpoint is restored if we complement them with Brca1 containing serine 1423 mutated to alanine so that it cannot be phosphorylated (30). Thus, HCC1937 cells expressing S1423A Brca1 have a normal IR-induced S-phase checkpoint but lack the IR-induced early G2/M checkpoint. This is the only cell type we are aware of that has this particular phenotype. Using BrdUrd pulse-labeling and examining cell cycle progression of irradiated S-phase cells after IR, we found that HCC1937 cells complemented with either wild-type or S1423A Brca1 lose the enhanced G2/M accumulation phenotype (Fig. 6C and D). Since the cells complemented with S1423A Brca1 still lack the early IR-induced G2 arrest, this result convincingly demonstrates that the early IR-induced G2 checkpoint is mechanistically distinct from the G2 accumulation phenotype. Furthermore, this again demonstrates that cells irradiated during S phase that fail to exhibit an appropriate transient checkpoint will accumulate in G2/M for a prolonged period of time (Table 1).
TABLE 1.
Association of prolonged G2 accumulation with defective S-phase checkpointa
| Cell line and transfection | S-phase checkpoint | Early G2/M checkpoint | G2 accumulation | Radiation sensitivity |
|---|---|---|---|---|
| NBS1-LBI | ||||
| Vector | − | + | Prolonged | ↑ |
| Wild-type Nbs1 | + | + | + | + |
| S343A Nbs1 | − | + | Prolonged | + |
| HCC1937 | ||||
| Vector | − | − | Prolonged | ↑ |
| Wild-type Brca1 | + | + | + | + |
| S1423A Brca1 | + | − | + | + |
−, abnormal; +, normal; ↑, hypersensitive.
Neither G2/M checkpoint abnormality is linked to radiosensitivity.
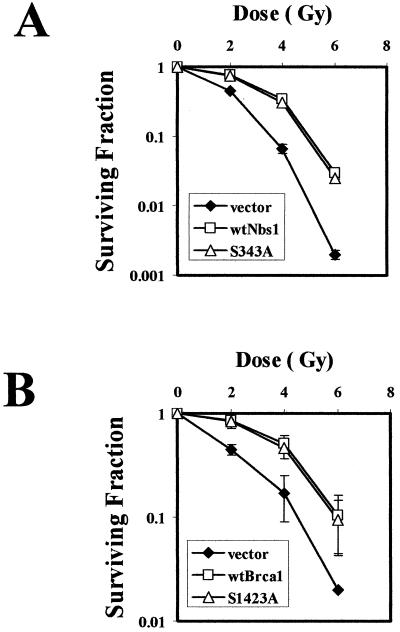
Saccharomyces cerevisiae cells with defective G2/M checkpoints are hypersensitive to DNA-damaging agents (29). Since we had generated cell lines selectively defective in one or the other of these IR-induced G2/M checkpoints, we investigated the potential impact of these checkpoints on radiation sensitivity. NBS cells complemented with Nbs1 protein mutated at serine 343 exhibit an abnormal S-phase checkpoint and prolonged G2 accumulation but have clonogenic survival similar to that of NBS cells complemented with wild-type Nbs1 (Fig. 7A).Thus, radiation sensitivity does not result simply from loss of the S-phase checkpoint or exaggerated G2 accumulation. Conversely, HCC1937 cells complemented with Brca1 mutated at serine 1423 are defective in the early G2/M checkpoint but exhibit clonogenic survival indistinguishable from that of cells complemented with wild-type Brca1 (Fig. 7B). Thus, within the limits of these assays, radiosensitivity can be totally dissociated from each of these checkpoint defects.
FIG. 7.
Radiation sensitivity and cell cycle checkpoint defects. (A) Expression of wild type Nbs1 (wtNbs1) or mutant Nbs1 (Serine 343 to alanine, S343A) rescues the hypersensitivity of NBS1-LBI cells in response to IR. (B) Expression of wild-type Brca1 (wtBrca1) or S1423A Brca1 rescues the radiation sensitivity of HCC1937 cells. Cells were exposed to 0 to 6 Gy of IR and incubated for 2 weeks prior to fixation, staining, and assessment of colony formation. The clonogenic survival assays were performed in triplicate. Standard errors are shown by error bars.
DISCUSSION
If the G2 checkpoint is assessed in the first few hours after irradiation, then cells that were in the G2 phase of the cell cycle during irradiation are being evaluated. In order to be able to determine whether cells are progressing from G2 into M, the assay used in this setting must be able to distinguish mitotic cells from G2 cells. This type of assessment reveals that AT cells fail to arrest in G2 after IR and continue to progress into mitosis (20, 30, 32). On the other hand, if cells are evaluated at later time points after irradiation, then the cells being evaluated would have been in S phase or even G1 at the time of irradiation. PI staining, which simply measures the DNA content of cells, is the most commonly used assessment of G2 arrest. In order for this assay to demonstrate an increase in the number of cells with 4N DNA content (representing G2 plus M), those cells must enter G2/M from elsewhere in the cycle. We demonstrate here that G2 arrest as measured by PI staining is distinct from the ATM-dependent IR-induced G2 checkpoint and reflects accumulation of cells that had been irradiated earlier in the cell cycle. In addition to different biochemical control mechanisms, these two pathways exhibit different kinetics and dose responses. Interestingly, if cells lack an IR-induced S-phase checkpoint (which is true of cells lacking ATM, Nbs1, or Brca1), then they exhibit a prolonged arrest when they get to G2 and show an enhanced G2 accumulation. Definitive support for these concepts was facilitated by complementation experiments using characterized mutants of these genes important in the S-phase checkpoint. These experiments explain the apparent paradox in the ATM literature about the nature of the G2 checkpoint defect in AT and provide insights into two different mechanisms that mammalian cells use to control cell cycle progression after DNA damage.
Checkpoints are cellular mechanisms that prevent or delay progression through the cell cycle when DNA is damaged or when crucial events have not been completed. In mammalian cells, loss of cell cycle checkpoints has been linked with genetic instability and cancer formation (11). It is conceivable, however, that cells could compensate for loss of a checkpoint in certain settings, perhaps through initiation of a later checkpoint. Our data confirm that radiosensitivity does not result from S-phase checkpoint defects (12) and also demonstrate directly that neither G2/M arrest abnormality alone confers radiosensitivity. Since it has also been previously shown that abrogation of the IR-induced G1 checkpoint does not confer radiosensitivity (26), it appears that abolition of single checkpoints in mammalian cells does not directly confer radiosensitivity. However, blockade of two checkpoints could act synergistically in creating genetic instability or decreasing cell viability. The observation that inhibition of the G2 checkpoint appears to more effectively sensitize cells to DNA damage if the cells also lack the G1 checkpoint (10, 21, 22, 28, 31) is consistent with this concept. In the experiments described here, the enhanced accumulation of irradiated cells in G2 if they failed to arrest first in S phase might reflect such a compensation and theoretically could give cells more time to detect and repair replicated damaged DNA. Such observations may have therapeutic implications because they would predict that blockade of a particular checkpoint pathway might have a more pronounced effect in a cell already lacking a particular checkpoint (as might be seen in a tumor cell) than in a normal cell which has retained compensatory checkpoints. Thus, targeting such pathways could have a beneficial therapeutic index.
The mechanism by which cells that were irradiated during S phase accumulate in G2 is clearly distinct from the mechanism that keeps irradiated G2 cells from entering mitosis. The latter arrest is transient, dose independent, ATM and Brca1 dependent, and independent of Nbs1. In contrast, the former accumulation occurs later, is dose dependent, and is ATM independent. Perhaps the hyperaccumulation of cells in G2 when the S-phase checkpoint is defective reflects engagement of a DNA replication checkpoint. It is reasonable to postulate that if cells fail to appropriately arrest DNA synthesis in the presence of DNA double-strand breaks, then they would enter G2 with DNA lesions that would be sensed as not fully or appropriately replicated. In S. cerevisiae, though there are clear distinctions between the pathways involved in DNA damage checkpoints and DNA replication checkpoints, mutations of some genes can alter both responses (8, 34). Building upon the assays and observations presented here, we can begin to address the molecular controls of the G2 accumulation and explore overlaps and distinctions with replication checkpoint pathways and DNA damage checkpoint pathways. Such insights could eventually lead to novel approaches to selective sensitization of tumors lacking particular checkpoint pathways.
Acknowledgments
We gratefully acknowledge the technical assistance of Diane Woods. We thank all members of the Kastan laboratory for helpful discussions, Malgorzata Zdzienicka for providing the NBS1-LBI cell line, and David Livingston for providing the wild-type BRCA1 cDNA.
This work was supported by grants from the National Institute of Health (CA71378 and CA21765) and by the American Lebanese Syrian Associated Charities of the St. Jude Children's Research Hospital.
REFERENCES
- 1.Ahn, J. Y., J. K. Schwarz, H. Piwnica-Worms, and C. E. Canman. 2000. Threonine 68 phosphorylation by ataxia telangiectasia mutated is required for efficient activation of Chk2 in response to ionizing radiation. Cancer Res. 60:5934-5936. [PubMed] [Google Scholar]
- 2.Banin, S., L. Moyal, S. Shieh, Y. Taya, C. W. Anderson, L. Chessa, N. I. Smorodinsky, C. Prives, Y. Reiss, Y. Shiloh, and Y. Ziv. 1998. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science 281:1674-1677. [DOI] [PubMed] [Google Scholar]
- 3.Bao, S., R. S. Tibbetts, K. M. Brumbaugh, Y. Fang, D. A. Richardson, A. Ali, S. M. Chen, R. T. Abraham, and X. F. Wang. 2001. ATR/ATM-mediated phosphorylation of human Rad17 is required for genotoxic stress responses. Nature 411:969-974. [DOI] [PubMed] [Google Scholar]
- 4.Beamish, H., and M. F. Lavin. 1994. Radiosensitivity in ataxia-telangiectasia: anomalies in radiation-induced cell cycle delay. Int. J. Radiat. Biol. 65:175-184. [DOI] [PubMed] [Google Scholar]
- 5.Beamish, H., R. Williams, P. Chen, and M. F. Lavin. 1996. Defect in multiple cell cycle checkpoints in ataxia-telangiectasia postirradiation. J. Biol. Chem. 271:20486-20493. [DOI] [PubMed] [Google Scholar]
- 6.Canman, C. E., and M. B. Kastan. 1998. Small contribution of G1 checkpoint control manipulation to modulation of p53-mediated apoptosis. Oncogene 16:957-966. [DOI] [PubMed] [Google Scholar]
- 7.Canman, C. E., D. S. Lim, K. A. Cimprich, Y. Taya, K. Tamai, K. Sakaguchi, E. Appella, M. B. Kastan, and J. D. Siliciano. 1998. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science 281:1677-1679. [DOI] [PubMed] [Google Scholar]
- 8.Enoch, T., A. M. Carr, and P. Nurse. 1992. Fission yeast genes involved in coupling mitosis to completion of DNA replication. Genes Dev. 6:2035-2046. [DOI] [PubMed] [Google Scholar]
- 9.Falck, J., N. Mailand, R. G. Syljuasen, J. Bartek, and J. Lukas. 2001. The ATM-Chk2-Cdc25A checkpoint pathway guards against radioresistant DNA synthesis. Nature 410:842-847. [DOI] [PubMed] [Google Scholar]
- 10.Flatt, P. M., and J. A. Pietenpol. 2000. Mechanisms of cell-cycle checkpoints: at the crossroads of carcinogenesis and drug discovery. Drug Metab. Rev. 32:283-305. [DOI] [PubMed] [Google Scholar]
- 11.Hartwell, L. H., and M. B. Kastan. 1994. Cell cycle control and cancer. Science 266:1821-1828. [DOI] [PubMed] [Google Scholar]
- 12.Kastan, M. B., and D. S. Lim. 2000. The many substrates and functions of ATM. Nat. Rev. Mol. Cell. Biol. 1:179-186. [DOI] [PubMed] [Google Scholar]
- 13.Kastan, M. B., O. Onyekwere, D. Sidransky, B. Vogelstein, and R. W. Craig. 1991. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 51:6304-6311. [PubMed] [Google Scholar]
- 14.Kraakman-van der Zwet, M., W. J. Overkamp, A. A. Friedl, B. Klein, G. W. Verhaegh, N. G. Jaspers, A. T. Midro, F. Eckardt-Schupp, P. H. Lohman, and M. Z. Zdzienicka. 1999. Immortalization and characterization of Nijmegen breakage syndrome fibroblasts. Mutat. Res. 434:17-27. [DOI] [PubMed] [Google Scholar]
- 15.Lim, D. S., S. T. Kim, B. Xu, R. S. Maser, J. Lin, J. H. Petrini, and M. B. Kastan. 2000. ATM phosphorylates p95/nbs1 in an S-phase checkpoint pathway. Nature 404:613-617. [DOI] [PubMed] [Google Scholar]
- 16.Matsuoka, S., M. Huang, and S. J. Elledge. 1998. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science 282:1893-1897. [DOI] [PubMed] [Google Scholar]
- 17.Matsuoka, S., G. Rotman, A. Ogawa, Y. Shiloh, K. Tamai, and S. Elledge. 2000. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc. Natl. Acad. Sci. USA 97:10389-10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maya, R., M. Balass, S. T. Kim, D. Shkedy, J. F. Leal, O. Shifman, M. Moas, T. Buschmann, Z. Ronai, Y. Shiloh, M. B. Kastan, E. Katzir, and M. Oren. 2001. ATM-dependent phosphorylation of Mdm2 on serine 395: role in p53 activation by DNA damage. Genes Dev. 15:1067-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melchionna, R., X. B. Chen, A. Blasina, and C. H. McGowan. 2000. Threonine 68 is required for radiation-induced phosphorylation and activation of Cds1. Nat. Cell Biol. 2:762-765. [DOI] [PubMed] [Google Scholar]
- 20.Morgan, S. E., C. Lovly, T. K. Pandita, Y. Shiloh, and M. B. Kastan. 1997. Fragments of ATM which have dominant-negative or complementing activity. Mol. Cell. Biol. 17:2020-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powell, S. N., J. S. DeFrank, P. Connell, M. Eogan, F. Preffer, D. Dombkowski, W. Tang, and S. Friend. 1995. Differential sensitivity of p53(−) and p53(+) cells to caffeine-induced radiosensitization and override of G2 delay. Cancer Res. 55:1643-1648. [PubMed] [Google Scholar]
- 22.Russell, K. J., L. W. Wiens, G. W. Demers, D. A. Galloway, S. E. Plon, and M. Groudine. 1995. Abrogation of the G2 checkpoint results in differential radiosensitization of G1 checkpoint-deficient and G1 checkpoint-competent cells. Cancer Res. 55:1639-1642. [PubMed] [Google Scholar]
- 23.Scott, D., A. R. Spreadborough, and S. A. Roberts. 1994. Radiation-induced G2 delay and spontaneous chromosome aberrations in ataxia-telangiectasia homozygotes and heterozygotes. Int. J. Radiat. Biol. 66:S157-S163. [PubMed]
- 24.Shiloh, Y. 1997. Ataxia-telangiectasia and the Nijmegen breakage syndrome: related disorders but genes apart. Annu. Rev. Genet. 31:635-662. [DOI] [PubMed] [Google Scholar]
- 25.Siliciano, J. D., C. E. Canman, Y. Taya, K. Sakaguchi, E. Appella, and M. B. Kastan. 1997. DNA damage induces phosphorylation of the amino terminus of p53. Genes Dev. 11:3471-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slichenmyer, W., W. Nelson, R. Slebos, and M. B. Kastan. 1993. Loss of a p53-associated G1 checkpoint does not decrease cell survival following DNA damage. Cancer Res. 53:4164-4168. [PubMed] [Google Scholar]
- 27.Tatsuka, M., O. Nikaido, K. Tatsumi, and H. Takebe. 1989. X-ray-induced G2 arrest in ataxia telangiectasia lymphoblastoid cells. Mutat. Res. 214:321-328. [DOI] [PubMed] [Google Scholar]
- 28.Wang, Q., S. Fan, A. Eastman, P. J. Worland, E. A. Sausville, and P. M. O'Connor. 1996. UCN-01: a potent abrogator of G2 checkpoint function in cancer cells with disrupted p53. J. Natl. Cancer Inst. 88:956-965. [DOI] [PubMed] [Google Scholar]
- 29.Weinert, T. 1988. The RAD9 gene controls the cell cycle response to DNA damage in Saccharomyces cerevisiae. Science 241:317-322. [DOI] [PubMed] [Google Scholar]
- 30.Xu, B., S. T. Kim, and M. B. Kastan. 2001. Involvement of Brca1 in S-phase and G2-phase checkpoints after ionizing irradiation. Mol. Cell. Biol. 21:3445-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao, S. L., A. J. Akhtar, K. A. McKenna, G. C. Bedi, D. Sidransky, M. Mabry, R. Ravi, M. I. Collector, R. J. Jones, S. J. Sharkis, E. J. Fuchs, and A. Bedi. 1996. Selective radiosensitization of p53-deficient cells by caffeine-mediated activation of p34cdc2 kinase. Nat. Med. 2:1140-1143. [DOI] [PubMed] [Google Scholar]
- 32.Zampetti-Bosseler, F., and D. Scott. 1981. Cell death, chromosome damage and mitotic delay in normal human, ataxia telangiectasia and retinoblastoma fibroblasts after X-irradiation. Int. J. Radiat. Biol. 39:547-558. [DOI] [PubMed] [Google Scholar]
- 33.Zhao, S., Y. C. Weng, S. S. Yuan, Y. T. Lin, H. C. Hsu, S. C. Lin, E. Gerbino, M. H. Song, M. Z. Zdzienicka, R. A. Gatti, J. W. Shay, Y. Ziv, Y. Shiloh, and E. Y. Lee. 2000. Functional link between ataxia-telangiectasia and Nijmegen breakage syndrome gene products. Nature 405:473-477. [DOI] [PubMed] [Google Scholar]
- 34.Zhou, B. B., and S. J. Elledge. 2000. The DNA damage response: putting checkpoints in perspective. Nature 408:433-439. [DOI] [PubMed] [Google Scholar]