Abstract
Activation of Wnt signaling through β-catenin/TCF complexes is a key event in the development of various tumors, in particular colorectal and liver tumors. Wnt signaling is controlled by the negative regulator conductin/axin2/axil, which induces degradation of β-catenin by functional interaction with the tumor suppressor APC and the serine/threonine kinase GSK3β. Here we show that conductin is upregulated in human tumors that are induced by β-catenin/Wnt signaling, i.e., high levels of conductin protein and mRNA were found in colorectal and liver tumors but not in the corresponding normal tissues. In various other tumor types, conductin levels did not differ between tumor and normal tissue. Upregulation of conductin was also observed in the APC-deficient intestinal tumors of Min mice. Inhibition of Wnt signaling by a dominant-negative mutant of TCF downregulated conductin but not the related protein, axin, in DLD1 colorectal tumor cells. Conversely, activation of Wnt signaling by Wnt-1 or dishevelled increased conductin levels in MDA MB 231 and Neuro2A cells, respectively. In time course experiments, stabilization of β-catenin preceded the upregulation of conductin by Wnt-1. These results demonstrate that conductin is a target of the Wnt signaling pathway. Upregulation of conductin may constitute a negative feedback loop that controls Wnt signaling activity.
The Wnt signaling pathway is involved in a variety of developmental processes and in tumor formation, in particular of colorectal and liver tumors (2, 8, 33). A hallmark of Wnt signaling is the stabilization of cytoplasmic β-catenin followed by its association with TCF transcription factors, which leads to the transcription of Wnt target genes (5, 18, 30, 46). The cytoplasmic component conductin (also named axin2 or axil) functions as a negative regulator of Wnt signaling by inducing degradation of β-catenin (3, 29, 40, 49). Biochemically, conductin acts as a scaffold for the assembly of a multiprotein complex which includes the tumor suppressor APC and the serine/threonine kinase GSK3β. In this complex, β-catenin is phosphorylated by GSK3β, which leads to its ubiquitination and degradation in proteasomes (1, 21, 49). Conductin induces downregulation of β-catenin when transiently overexpressed in colon carcinoma cells and inhibits Wnt-induced as well as endogenous axis formation in early Xenopus embryos (3, 14, 51).
Conductin is related to axin, with which it shares 45% amino acid identity (3, 51). Conductin and axin have similar biochemical and cell biological properties but may differ in their in vivo functions. While axin is homogenously distributed in the mouse embryo (51), conductin is more selectively expressed in specific tissues (3; B. Jerchow and W. Birchmeier, unpublished data). Axin has been identified as the product of the fused gene locus in the mouse. The fused mutations lead to defects in embryonal body axis formation (47, 51).
During embryonal development, Wnt signaling stabilizes β-catenin by blocking the activity of the conductin/axin-based β-catenin degradation complex. This pathway involves the activation of several intermediary components by Wnts, including the cytoplasmic protein dishevelled, which interacts with conductin/axin (22, 27, 28, 36). Due to truncating mutations of APC or point mutations in the phosphorylation sites of β-catenin, various tumor types show constitutive stabilization of β-catenin and permanent activation of TCF/β-catenin-driven gene transcription (6, 31, 33). Specifically, the APC gene is mutated in the inherited disease familial adenomatous polyposis (FAP), which leads to formation of multiple colorectal adenomas and carcinomas and in addition accounts for about 80% of sporadic colorectal carcinomas (33). β-Catenin is mutated in about 5% of colorectal carcinomas (31, 38) and in up to 50% of hepatoblastomas and hepatocellular carcinomas (23, 25, 44), as well as in a variety of other tumors (2). Alterations of axin and conductin/axin2 have also been described previously for hepatocellular carcinomas and for a fraction of unstable microsatellite colorectal tumors, respectively (29, 39).
Several lines of evidence suggest an essential role for β-catenin/Wnt signaling in tumorigenesis. Accumulation of β-catenin in the cytoplasm and nucleus was demonstrated previously on tissue sections of colorectal and liver tumors, although regional differences within a given tumor exist (16, 20, 24, 25). Furthermore, several target genes of β-catenin/TCF complexes with a possible function in tumorigenesis, such as c-myc, cyclin D1, and matrilysin, have been described elsewhere (9, 15, 45). Moreover, transgenic expression of stabilized versions of β-catenin in mice induces tumor formation (13), and Min (multiple intestinal neoplasia) mice, which carry a germ line mutation of APC, develop spontaneous tumors in the small intestine that show stabilization of β-catenin (32, 43).
In the study reported in this paper, we have determined the expression pattern of conductin in a variety of human tumors and normal tissue. We found that conductin is upregulated in colorectal adenomas and carcinomas and in liver tumors, in comparison to the corresponding normal tissues. Biochemical manipulation of Wnt signaling in cell lines and genetic analysis of Min mice indicate that conductin itself is a target of Wnt signaling. Our data suggest that conductin is part of a negative feedback loop which serves to restrain Wnt signaling activity by limiting the amounts of β-catenin. As upregulation of conductin appears to be an early event in β-catenin/Wnt-induced tumorigenesis, conductin might be a useful marker for early tumor diagnosis.
MATERIALS AND METHODS
Generation of the anticonductin monoclonal antibody C/G7.
Female NMRI mice were immunized intraperitoneally with 75 μg of a recombinant fragment of mouse conductin (amino acids 2 to 396) (3) in phosphate-buffered saline (PBS) with Freund's adjuvant. Eight weeks later, the mice were boosted intraperitoneally with 75 μg of the conductin fragment in PBS without adjuvant. Spleen cells from one mouse were harvested 4 days after the boost and fused with X63-Ag8.653 myeloma cells by employing standard techniques, except that azaserine was used in the selection medium. Growth medium was RPMI 1640 supplemented initially with 15 and then 10% fetal calf serum (FCS; Biochrom, Berlin, Germany) and finally with 0.5% hybridoma serum (Pandar, Heidelberg, Germany). Initial hybridoma growth and cloning were supported by peritoneal feeder cells from NMRI mice. Hybridoma supernatants were tested in an enzyme-linked immunosorbent assay with recombinant conductin used as a coating at 5 μg/ml. Bovine thyroglobulin served as a negative control, and affinity-purified rabbit polyclonal antiserum against conductin (3) was used as a positive control. One hybridoma clone specific for conductin was selected (C/G7; immunoglobulin G1 κ chain), recloned, and frozen. Immunofluorescence tests and Western blotting with conductin- or axin-transfected Neuro2A cells showed that C/G7 is specific for conductin and does not cross-react with axin. Large-scale production of C/G7 hybridoma supernatant was carried out by growing hybridoma in dialysis tubes in a special gyration apparatus (Kretzschmar, Berlin, Germany).
Cell culture, Western blotting, and transfections.
Cells were cultured in Dulbecco modified Eagle medium (DMEM) containing 10% FCS and streptomycin-penicillin. The colon, lung, breast, bladder, pancreas, and prostate carcinoma and the hepatoblastoma cell lines used in Fig. 1 have been described previously (7, 10, 25). The melanoma cell lines SK-MEL3 and RPMI 7951 were obtained from the American Type Culture Collection, and Juso, Parl, IGR-3, and Strömer were obtained from J. P. Johnson, University of Munich.
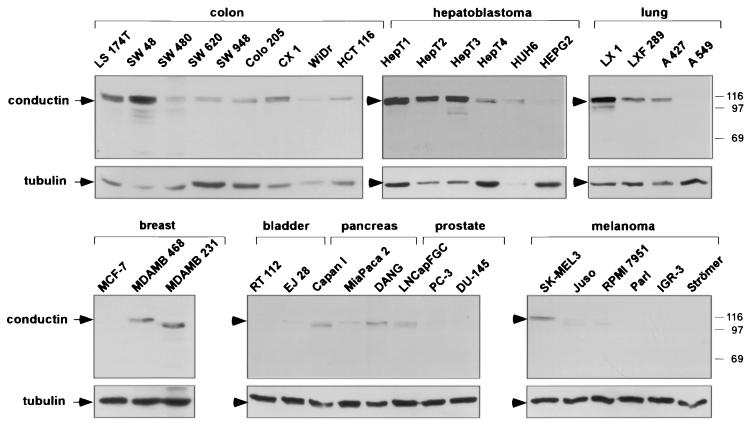
FIG. 1.
Western blot analysis of conductin in human tumor cell lines. Western blotting was performed using the C/G7 antibody on protein extracts from the indicated tumor cell lines. Equal amounts of protein were loaded per lane. Conductin was detected as a double band around 100 kDa in most cell lines. In some cell lines, either the upper or the lower band is seen. The same blots were also probed with antitubulin antibodies to demonstrate similar protein loading. Note that the Western blots of conductin in the lower panels (breast, bladder, pancreas, prostate, and melanoma) were exposed five times longer than those in the upper panels (colon, hepatoblastoma, and lung). Numbers at right are molecular masses in kilodaltons.
For Western blot analysis of conductin in human tumor cell lines, 3 × 106 cells were cultured overnight on 10-cm-diameter dishes and extracted with 500 μl of lysis buffer (L-CAM assay buffer containing 1% Triton X-100 and 1 mM phenylmethylsulfonyl fluoride [4]). For analysis of human tumors, normal and tumor samples were snap-frozen and stored at −80°C. Cryostat sections (10 μm) were extracted in lysis buffer on ice for 15 min, sonicated for 10 s, and centrifuged for 15 min at 16,000 × g. Equal amounts of extracted protein as determined by the Bradford protein assay (Bio-Rad) were separated by sodium dodecyl sulfate (SDS)-8% polyacrylamide gel electrophoresis and blotted on Immobilon membranes (Amersham). Proteins were detected using mouse C/G7 antibody against conductin (concentrated hybridoma supernatant at 1:100 dilution), rabbit antiaxin (1:200 dilution; gift from K. Willert, Stanford University), mouse anti-cytokeratin 19 (1:100; Santa Cruz), rat antitubulin (1:1,000; Serotec, Kidlington, United Kingdom), and mouse anti-β-catenin (1:400; Transduction Laboratories) antibodies. After incubation with peroxidase-coupled secondary antibodies, blots were developed using enhanced chemiluminescence reagent (NEN) and exposed to X-ray films (Kodak).
The T-REx system (Invitrogen, Carlsbad, Calif.) was used according to the manufacturer's instructions to generate DLD1 colorectal tumor cells expressing tetracycline-inducible dominant-negative TCF. In short, 107 cells were transfected by electroporation with 20 μg of FspI-linearized pcDNA6TR. Cells were grown in RPMI supplemented with 10% FCS, antibiotics, and blasticidin (10 μg/ml). After 3 weeks of selection, blasticidin-resistant colonies were expanded and transfected with pcDNA4TO-luciferase. One clone (TR7-DLD1) that showed the strongest induction was chosen and subsequently transfected with 20 μg of PvuI-linearized ΔNTCF1 or ΔNTCF4 expression plasmids coding for TCF proteins that lack the β-catenin binding domain (26). After selection on Zeocin (500 μg/ml), resistant colonies were tested for dominant-negative TCF induction by immunohistochemical staining after addition of doxycycline. The clones with the strongest induction (named ΔNTCF1-DLD1 and ΔNTCF4-DLD1) were used in the experiments. A luciferase reporter assay (Top/Fop assay) (26, 30) was used to confirm that dominant-negative TCF induction resulted in abrogation of β-catenin/TCF-driven transcription. For Western blots, 5 × 105 TR7-DLD1, ΔNTCF1-DLD1, or ΔNTCF4-DLD1 cells were plated overnight on 10-cm-diameter dishes, induced with 1 μg of doxycycline (Sigma-Aldrich Fine Chemicals, St. Louis, Mo.) per ml for 12 h, and extracted in 300 μl of lysis buffer.
To obtain conditioned media, confluent Rat2/Wnt-1 and Rat2/MV7 cells were cultured overnight in serum-free DMEM (12). For stimulation of MDA MB 231 breast carcinoma cells, 3 × 105 cells were cultured overnight in six-well dishes, serum starved in DMEM for 16 h, and incubated with conditioned media. At the indicated time points, cells were lysed for 20 min on ice either in 100 μl of hypotonic buffer (20 mM Tris-Cl [pH 7.5], 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride) to obtain the cytosolic fractions for analysis of β-catenin (12) or in L-CAM assay buffer containing Triton X-100 for analysis of conductin. In the time course experiment shown in Fig. 5D, both conductin and β-catenin were detected in the cytosolic extracts.
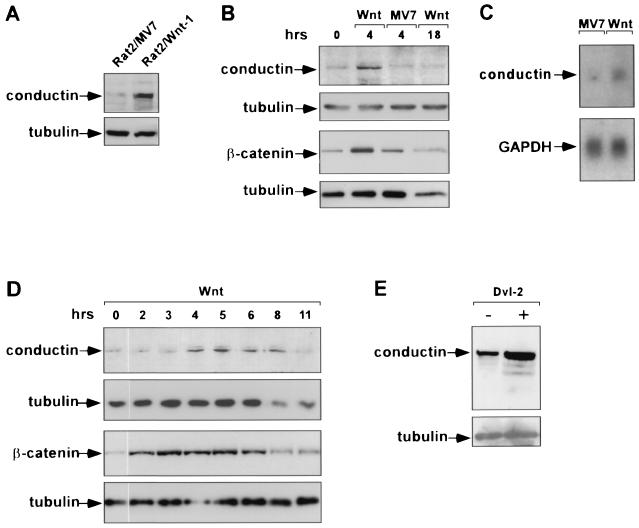
FIG. 5.
Upregulation of conductin by Wnt signaling. (A) Expression of conductin in Rat2/Wnt-1 cells and Rat2/MV7 control cells demonstrated by Western blotting using the C/G7 antibody. Note increased levels of conductin in the Wnt-1-expressing cells. (B) Upregulation of conductin in MDA MB 231 breast carcinoma cells by Wnt-1. Cells were incubated with media conditioned by Rat2/Wnt-1 (Wnt) or Rat2/MV7 cells (MV7) for 4 and 18 h as indicated above the lanes. Conductin levels were determined by Western blotting as described for panel A. Western blotting for β-catenin was performed on cytosolic extracts prepared from parallel cultures. Note that both conductin and β-catenin levels increase after 4 h of stimulation by Wnt-1-conditioned medium and decrease to baseline after 18 h. Control conditioned medium had no effect. (C) Increase in conductin mRNA after treatment of MDA MB 231 cells with Wnt-1-conditioned medium for 4 h as determined by Northern blotting. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (D) Time course of upregulation of conductin and β-catenin by Wnt-1 in MDA MB 231 cells as determined by Western blotting. In this experiment, both conductin and β-catenin were detected from the same cytosolic extracts. Note that β-catenin peaks between 2 and 6 h while conductin peaks between 4 and 8 h after stimulation with Wnt-1. Tubulin was probed to demonstrate protein loading. (E) Upregulation of conductin after transient expression of dishevelled-2 (Dvl-2) in Neuro2A cells. Conductin and tubulin were detected by Western blotting.
For transient transfections, 106 Neuro2A cells were cultured overnight on 10-cm-diameter dishes and transfected with a mixture of 20 μg of a mouse dishevelled-2 expression plasmid (Dvl-2 [28]), 2.5 μg of pCMVCD20, and 2.5 μg of pSGCD20. An expression plasmid for vav-2 instead of Dvl-2 was used as a negative control. Cells were trypsinized after 24 h and incubated with anti-CD20 antibody (Dianova), and the transfected cells were captured with magnetic beads (Miltenyi) coated with anti-mouse immunoglobulin G. Eluted cells were extracted in lysis buffer and processed for Western blots as described above.
Northern blots and cancer profiling arrays.
TR7-DLD1 or ΔNTCF1-DLD1 (4.5 × 106) cells were plated on three 150-mm-diameter plates in DMEM-10% FCS. For induction of ΔNTCF1, the cells were treated the next day for 14 h with 1 μg of doxycycline/ml. MDA MB 231 cells were treated with Wnt-1-conditioned medium or control medium for 4 h. Total RNA was isolated with Trizol reagent (Invitrogen), and poly(A)+ RNA was isolated with the Oligotex mRNA Midi kit (Qiagen Inc., Valencia, Calif.) according to the manufacturer's protocol. Two micrograms of poly(A)+ RNA per lane was electrophoresed on formaldehyde agarose gels (37) followed by blotting on Hybond-XL membranes (Amersham Pharmacia Biotech Inc., Piscataway, N.J.) by capillary transfer. Hybridization of the blots was carried out overnight at 65°C with 32P-labeled cDNA probes derived from human conductin (Image clone IMAGp998I141906) and axin (Image clone IMAGp998B12254) or rat glyceraldehyde-3-phosphate dehydrogenase in 5× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7])-5× Denhardt's solution-0.5% SDS containing 100 μg of sheared and denatured salmon testis DNA/ml. The filters were washed at 65°C for 20 min each with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.1% SDS, 0.5× SSC-0.5% SDS, and 0.1× SSC-0.1% SDS, followed by exposure to X-ray films.
The cancer profiling array membrane (PT3578-1; Clontech Laboratories, Inc., Palo Alto, Calif.) was hybridized with the cDNA probes described above according to the manufacturer's protocol. In brief, the membrane was prehybridized for 30 min with 10 μl of ExpressHyb solution (Clontech) at 65°C. The labeled cDNA probes were mixed with 30 μg of Cot-1 DNA (Roche Molecular Biochemicals, Indianapolis, Ind.), 150 μg of sheared salmon testis DNA, and 50 μl of 20× SSC in a total volume of 200 μl. The mixture was heated at 95°C for 5 min, incubated at 65°C for 30 min, and mixed with 5 ml of fresh ExpressHyb solution. The filter was hybridized overnight at 65°C and washed four times at 65°C for 20 min with 2× SSC-0.5% SDS, once with 0.2× SSC-0.5% SDS, and finally for 5 min at room temperature with 2× SSC. For consecutive hybridizations, filters were stripped in boiling 0.5% SDS solution. For normalization, the filter was finally hybridized with a human ubiquitin probe (Clontech). The filter was exposed to X-ray films for documentation. For quantification, the filter was scanned on an FLA-3000 image reader (Fuji Photo Film Co. Ltd.) and the spots were analyzed with AIDA 2.3.1 software (Raytest USA Inc., Wilmington, Del.).
Mouse experiments.
C57BL/6 male Min (Apc+/Min) mice were obtained commercially (The Jackson Laboratory, Bar Harbor, Maine). Conductin+/lacZ mice were generated from heterozygous embryonic stem cells derived from the E14 embryonic stem cell line that were injected into C57BL/6 blastocysts (described elsewhere [Jerchow and Birchmeier, unpublished]). Heterozygous Min mice and conductin+/lacZ mice were crossed to obtain double heterozygous mice. For intestinal analysis, mice were sacrificed at 6 to 8 months. The intestines were collected, flushed with PBS, opened longitudinally, and mounted on Whatman paper. Either tumors were dissected for cryosectioning and subsequent β-galactosidase staining, or whole-mount staining of the intestine was performed. β-Galactosidase staining was carried out as described elsewhere (17). Embedding in paraffin was performed according to the manufacturer's protocol (Paraplast; Sherwood Medical). Stained tissue was cut at 10 μm and counterstained with nuclear fast red. In situ hybridizations were performed as described elsewhere on paraffin sections of mouse intestine using antisense probes specific for conductin and TCF4 (19).
RESULTS
Upregulation of conductin in human tumors.
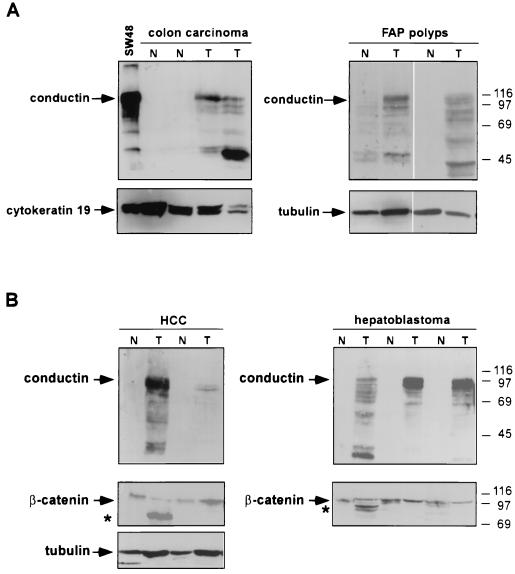
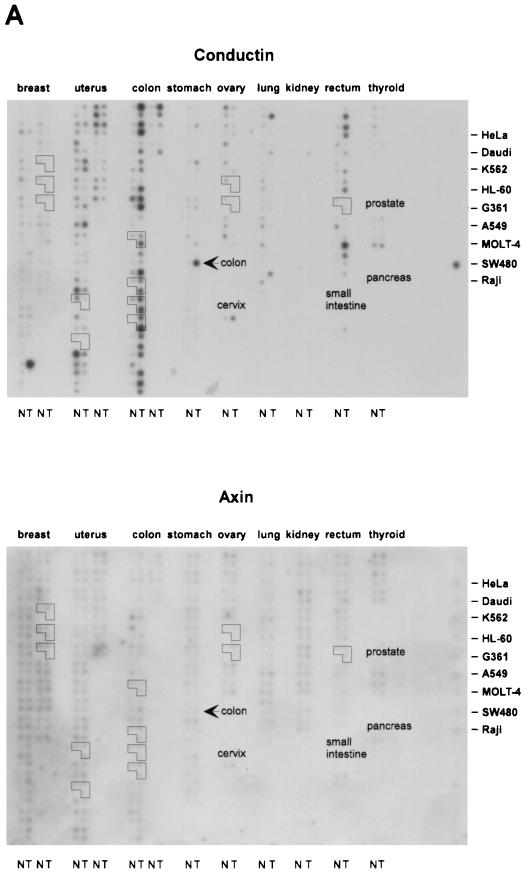
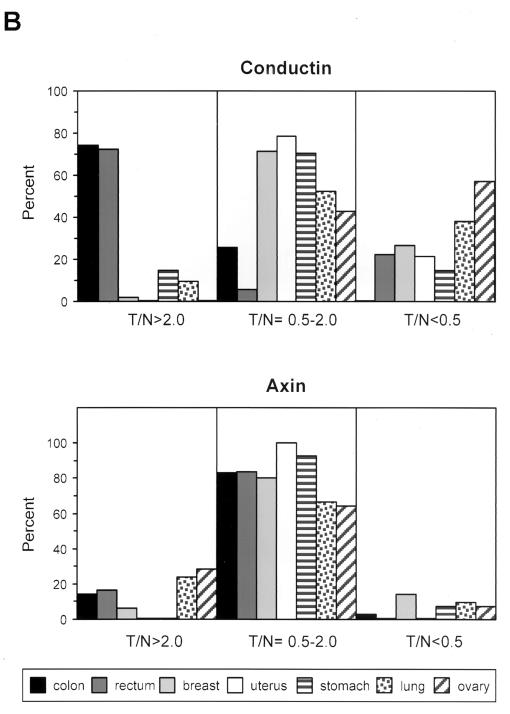
We have studied the expression of conductin in a variety of tumor cell lines from different tissues by Western blotting with a novel monoclonal antibody, C/G7. High amounts of conductin were readily detected in the majority of colon carcinoma cells, in all hepatoblastoma cells, and in some lung carcinoma cells. In contrast, most breast, bladder, pancreas, and prostate carcinoma and melanoma cells expressed low levels of conductin, which could be detected only after overexposure of the blots (Fig. 1).To analyze conductin expression in human tumors, we performed Western blotting on colon carcinoma tissue samples and on corresponding normal colon mucosa from the same patients. Conductin was present in high amounts in the tumors but was barely detectable in the normal tissue (Fig. 2A).We also analyzed adenomas from FAP patients and again found upregulation of conductin in the tumors compared to the normal mucosa (Fig. 2A). Upregulation of conductin was also seen in sporadic colon adenomas (data not shown). Similarly, conductin levels were markedly increased in liver tumors (hepatocellular carcinomas and hepatoblastomas) compared to those in normal liver tissue (Fig. 2B). Thus, the conductin protein appears to be expressed in large amounts in tumors that show activated β-catenin/Wnt signaling. These results prompted us to analyze conductin mRNA levels in a larger number of different normal and tumor tissues. We made use of a cancer profiling array (Clontech), which contains normalized cDNA from 241 tumor and corresponding normal tissues from individual patients. After hybridization of this array with a human conductin cDNA probe, we found the strongest signals in samples of uterus, colon, and rectum (Fig. 3A). Again, a significant upregulation of conductin compared to normal tissue was observed in colon and rectal tumors. Quantification of the results showed a more than twofold increase in conductin mRNA levels in about 70% of the examined colon and rectal carcinomas (range, 2.2- to 14.4-fold [Fig. 3B]). Conductin mRNA expression was also elevated, albeit to a lesser extend, in most of the remaining colorectal tumor samples. However, in the majority of the other tumor types, no significant differences between normal and tumor tissue were observed, with the exception of 60 and 40% of uterine and lung carcinomas, respectively, which showed a more than twofold decrease in conductin levels. In contrast to the differential expression pattern of conductin, axin was equally expressed in the various tissue samples, and no differences between normal and tumor tissues were seen (Fig. 3).
FIG. 2.
Western blot analysis of conductin in human tumors and normal tissues. Snap-frozen tissues were extracted and processed for Western blotting as described in Materials and Methods. (A) Western blots of normal (N) and corresponding tumor (T) tissue from sporadic colon carcinomas and from adenomatous polyps of FAP patients. The C/G7 antibody detects the major conductin bands at 100 kDa as well as lower-molecular-weight degradation products in the tumor tissues. Cytokeratin 19 and tubulin were also probed to show equal loading. (B) Western blots of normal (N) and tumor (T) tissue from hepatocellular carcinomas (HCC) and hepatoblastoma. Detection of β-catenin on the same samples is also shown. β-Catenin species with reduced size (∗) possibly result from deletions of the regulatory N terminus of the protein (see reference 25). The tumor extractions were performed with detergent-containing buffer. Thus, the β-catenin signal does not necessarily reflect the cytosolic signaling form alone but may include the cadherin-bound fraction. Tubulin was probed to show equal protein loading in the HCC samples; for the hepatoblastoma samples, equal protein loading was verified by Ponceau S staining. Numbers at right are molecular masses in kilodaltons.
FIG. 3.
Expression analysis of conductin and axin in human tumors and normal tissues. (A) 32P-labeled cDNA probes of conductin and axin were hybridized on a cancer profiling array (Clontech) containing cDNA from 241 human tumor (T) and corresponding normal (N) tissues from individual patients. The outlined groups of dots represent normal, tumor, and metastatic tissues from the same patient. The position of cDNAs from different tumor cell lines is indicated on the right. Note that SW480, which is a colorectal cancer cell line, is strongly positive for conductin. (B) Quantification of the results shown in panel A for a subset of tumor types by PhosphorImager analysis. Results were normalized to the hybridization signals of a ubiquitin probe (see Materials and Methods). The percentage of cases showing tumor/normal tissue expression ratios (T/N) of >2.0, 0.5 to 2.0, and <0.5 is given for each tumor type. Expression changes in the metastases outlined in panel A are not included in the quantification.
Control of conductin expression by Wnt signaling.
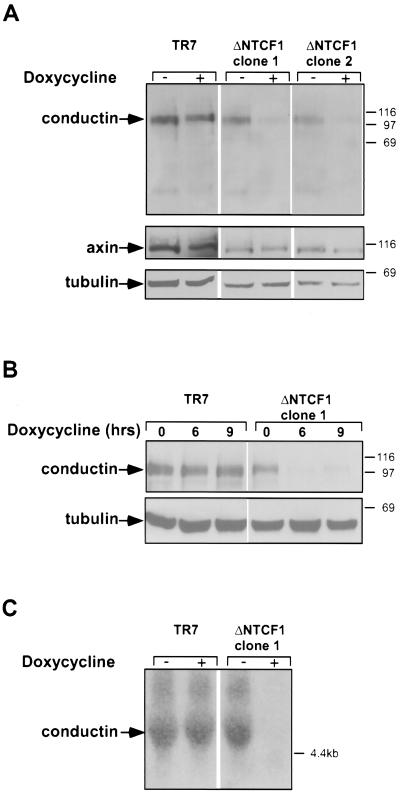
Colon carcinomas and liver tumors show active Wnt signaling because of mutation of APC or β-catenin (25, 33, 44). In those lung carcinoma cell lines that expressed high levels of conductin, we detected nuclear staining of β-catenin, indicative of the activation of Wnt signaling (data not shown). We therefore speculated that conductin is a target of Wnt signaling, which is upregulated in response to TCF/β-catenin activity in tumor cells. To analyze this, we used transfectants of the colon carcinoma cell line DLD1 that express dominant-negative mutants of TCF1 or TCF4 in a tetracycline-inducible manner (M. van de Wetering and H. Clevers, unpublished data). In the parental DLD1 cell line, the APC gene is mutated and the cells show activated TCF/β-catenin-dependent transcription (31). Induction of dominant-negative TCF1 in the DLD1 transfectants by addition of the tetracycline analog doxycycline resulted in a marked reduction in the protein and mRNA levels of conductin (Fig. 4). This effect was already apparent after 6 h of doxycycline treatment (Fig. 4B) and was also observed with dominant-negative TCF4 (data not shown). In contrast to the regulation seen with conductin, axin expression was not significantly altered by dominant-negative TCFs (Fig. 4A).
FIG.4.
Downregulation of conductin by dominant-negative TCF. (A) Downregulation of conductin upon expression of dominant-negative TCF1 in DLD1 colon carcinoma cells. The parental DLD1 cell clone (TR7), containing the Tet repressor, and two cell clones containing tetracycline-inducible dominant-negative TCF1 (ΔNTCF1 clones 1 and 2) were treated with doxycycline as indicated for 12 h. Conductin, axin, and tubulin (as a loading control) were detected by Western blotting on extracts of these cells. (B) Time course of downregulation of conductin by dominant-negative TCF determined by Western blotting. (C) Northern blot analysis of conductin mRNA in TR7 and ΔNTCF1 clone 1 after treatment with doxycycline for 14 h.
We also examined whether activation of the Wnt pathway by Wnt-1 leads to changes in conductin expression (Fig. 5).We found that conductin levels were higher in Rat2/Wnt-1 cells, which secrete Wnt-1 (12), than in control Rat2/MV7 cells, which lack Wnt-1 expression (Fig. 5A). Furthermore, incubation of MDA MB 231 breast carcinoma cells with Wnt-1-conditioned medium for 4 h significantly increased the levels of conductin (Fig. 5B). In accordance with previous reports, β-catenin levels were also increased after Wnt-1 stimulation (12). Both proteins declined to normal levels after 18 h. Control conditioned medium had no effect (Fig. 5B). We could also detect an upregulation of conductin mRNA in MDA MB 231 cells after stimulation with Wnt-1 (Fig. 5C). In a more extended time course experiment, we found that the increase in β-catenin protein preceded that of conductin by about 1 to 2 h, i.e., the peak of β-catenin was detected between 2 and 6 h, while that of conductin was seen between 4 and 8 h following exposure of cells to Wnt-1 (Fig. 5D). Transfection of the upstream activator of Wnt signaling, dishevelled-2 (28), into Neuro2A cells resulted in a significant upregulation of conductin (Fig. 5E). Together, the results shown in Fig. 4 and 5 indicate that conductin is a target of Wnt signaling that is transcriptionally activated by TCF/β-catenin complexes.
In vivo regulation of conductin by APC.
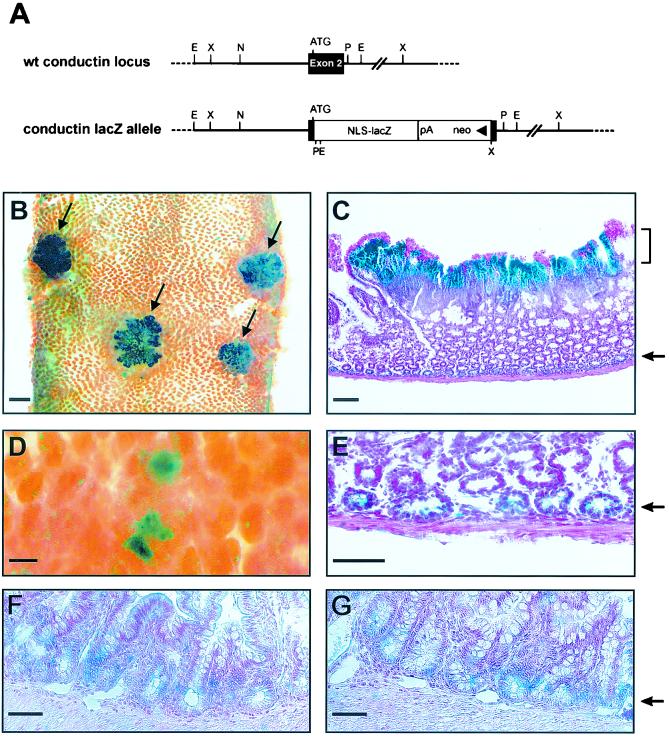
To examine β-catenin/Wnt-induced conductin expression in vivo, we crossed heterozygous conductin+/lacZ knockout mice carrying a lacZ gene in the conductin gene locus with Min mice which harbor a germ line mutation in the APC gene and develop spontaneous tumors of the small intestine (43). In the conductin+/lacZ mice, the lacZ gene is under the control of the conductin promoter, and β-galactosidase staining faithfully reflects the conductin expression pattern during embryonal development (Fig. 6A and B)(Jerchow and Birchmeier, unpublished). After tumors had appeared in the Min × conductin+/lacZ intercrosses, we performed whole-mount stainings of the small intestine for β-galactosidase activity. We found strong staining in the tumors, which appeared as polyps surmounting the intestinal villus compartment (Fig. 6B, arrows), indicating that the conductin gene promoter was activated. In sections of these tumors, the staining was mainly confined to the luminal aspect of the adenomatous tissue and declined in a gradient towards the basal normal mucosa (Fig. 6C). A similar gradient of expression was also observed by in situ hybridization for conductin mRNA (data not shown). Staining for β-galactosidase was heterogenous within the adenoma and particularly strong in areas which showed β-catenin reactivity in the nucleus (data not shown). In addition to the strong staining of the tumors, we observed faint but clearly distinguishable signals in crypt-like structures adjacent to the muscularis layer (Fig. 6C and E, arrows). β-Galactosidase activity was also detected in individual spots that correspond to small, early-stage adenomatous lesions (Fig. 6D). To compare expression of conductin and TCF4 in the intestine, we performed in situ hybridization experiments. Expression of both TCF4 and conductin was confined to the basal crypt epithelium (Fig. 6F and G, arrow). TCF4 expression was also detected in the polyps of Min mice (data not shown). The results demonstrate that conductin expression is activated in experimental tumors that result from loss of APC and, further, suggest a role for conductin in negative feedback regulation of Wnt signaling.
FIG. 6.
Upregulation of conductin expression in Min tumors. Min mice were crossed with heterozygous conductin+/lacZ mice. Expression of lacZ and thus activation of the conductin promoter were detected by β-galactosidase staining of the small intestine. (A) Schematic representation of the conductin wild-type (wt) locus and the targeted allele in the vicinity of exon 2. In the conductin lacZ allele, a lacZ gene containing a nuclear localization signal (NLS) was introduced in frame to the endogenous conductin start codon by the homologous recombination technique, thereby replacing most of exon 2. (B) Prominent whole-mount β-galactosidase staining of adenomas (arrows) in the intestine. Note that the intestine was opened longitudinally to expose the luminal site. Bar, 1 mm. (C) Paraffin section of a tumor showing strong β-galactosidase staining in the adenoma (bracket) and light staining of crypt epithelium adjacent to the muscularis layer (arrow). Counterstaining was performed with nuclear fast red. Bar, 100 μm. (D) Whole-mount β-galactosidase staining of early dysplastic lesions. Bar, 100 μm. (E) Magnification of the crypt epithelium layer in panel C to reveal β-galactosidase staining in the epithelial cells. Bar, 50 μm. (F) In situ hybridization for TCF4 mRNA on tissue sections of mouse intestinal epithelium. Bar, 50 μm. (G) In situ hybridization of conductin mRNA on tissue sections of mouse intestinal epithelium. Bar, 50 μm. Note similar staining patterns for TCF4 and conductin in the basal crypt epithelium in panels F and G.
DISCUSSION
Conductin is a major negative regulator of Wnt signal transduction by virtue of its ability to promote degradation of β-catenin. Our experiments suggest that upregulation of conductin constitutes a novel mechanism of negative feedback regulation in the Wnt pathway. This hypothesis is based on the following findings: (i) tumors that show activation of the Wnt signaling pathway have increased conductin protein and mRNA levels compared to those of the corresponding normal tissues, (ii) conductin expression in colorectal tumor cells can be blocked by expression of dominant-negative TCF, and (iii) conductin expression is upregulated following activation of the Wnt pathway through either Wnt-1 or dishevelled in cell culture or after loss of APC in Min mice.
There is increasing evidence that Wnt signaling is subject to negative feedback regulation at various levels. For instance, the Wnt orthologue wingless in Drosophila melanogaster induces expression of the cytoplasmic protein naked cuticle, which in turn blocks dishevelled and restrains further signaling (35, 48, 50). The F-box protein βTrCP, a ubiquitin ligase receptor involved in targeting β-catenin to proteasomes, is also activated by Wnt/β-catenin signaling (42). Moreover, certain isoforms of TCF transcription factors which lack the β-catenin binding domain are upregulated by β-catenin signaling and act as dominant-negative repressors of Wnt-dependent transcription (34). While naked cuticle and TCF isoforms are direct target genes of the Wnt pathway, upregulation of βTrCP appears to occur at the posttranscriptional level. We now may add conductin to the list of negative feedback inhibitors of Wnt signaling. Conductin appears to be upregulated by transcriptional activation of the conductin promoter, since we have observed changes in conductin mRNA levels upon interference with Wnt signaling by dominant-negative TCF (Fig. 4C) or after activation of the pathway by Wnt-1 (Fig. 5C). Moreover, activation of conductin promoter activity was directly visualized in the tumors of Min/conductin+/lacZ intercrosses (Fig. 6). In addition, TCF4 and conductin are coexpressed in the crypt epithelium and in the polyps, which suggests that TCF4 could be an activator of conductin gene transcription. Indeed, in a recent study, TCF consensus binding sites were detected in the conductin/axin2 promoter and promoter activity was increased by expression of β-catenin (23a).
As would be expected for a target of the Wnt pathway, the upregulation of conductin in response to Wnt-1 follows that of β-catenin (Fig. 5D). The apparent decrease of β-catenin levels at later time points after Wnt stimulation (Fig. 5D) may be a direct consequence of the upregulation of conductin. Elevated levels of conductin will increase the number of β-catenin degradation complexes, which will enhance the capacity of the β-catenin degradation machinery. This mechanism allows spatial and temporal fine tuning of Wnt signaling during embryogenesis. It may also counteract fluctuations of β-catenin levels that arise from changes in cadherin-mediated cell-cell adhesion during morphogenesis.
While expression of conductin is clearly regulated by Wnt signaling, expression of the related protein axin appears to be constitutive. Axin levels are similar in normal and tumor tissues and are not significantly changed by dominant-negative TCF in colorectal tumor cells. This indicates that axin is not involved in the feedback mechanisms proposed here for conductin. During embryonal development of the mouse, axin shows a widespread distribution, which contrasts with the more restricted expression pattern of conductin (3, 23a, 51; Jerchow and Birchmeier, unpublished). It thus appears that axin is a constitutively expressed component of the β-catenin degradation complex that is essential for the maintenance of its basal activity. In contrast, conductin is an inducible component that is specifically upregulated in response to increased β-catenin levels. The reason for this differential regulation is not known, but it is possible that axin has additional cellular roles apart from the canonical Wnt pathway that require the maintenance of a constant expression level of the protein. For instance, axin was recently shown to control Smad signaling in the transforming growth factor β pathway (11).
The consequences of feedback regulation of Wnt signaling for tumor development are not clear. Although overexpression of conductin can downregulate β-catenin in human tumor cell lines (3), the conductin levels that we observed in tumor tissues are apparently not sufficient to eliminate β-catenin completely and to prevent tumor formation. However, it is of interest that the accumulation and nuclear localization of β-catenin, as seen on tissue sections of colorectal tumors, are not homogenous but confined to specific areas of tumor dedifferentiation (16, 24). Conductin might thus be involved in this fine regulation of β-catenin in tumors, and it will be of interest to carefully correlate β-catenin and conductin expression patterns with histological differentiation in tumor tissues. In the Min mice, we observed the most prominent upregulation of conductin at the top of the adenomas (Fig. 5C). Recently, a top-down sequence of colorectal tumorigenesis has been proposed based on the finding that APC mutations occur in dysplastic cells at the top but not at the bases of small human colorectal adenomas (41). The expression pattern that we observed for conductin is compatible with such a model.
In our large-scale analysis of conductin expression in tumors, we found significant upregulation of the conductin mRNA in those carcinomas that are known to be induced by Wnt/β-catenin signaling, i.e., in colorectal and liver tumors. Hence, conductin expression seems to be closely correlated with tumorigenesis induced by the Wnt pathway. Moreover, induction of conductin appeared to be an early event during tumorigenesis, as conductin was upregulated already in adenomatous polyps of FAP patients as well as in early neoplastic lesions in Min mice. Conductin may therefore be an early marker for colorectal tumors. Interestingly, in a significant fraction of other tumor types, most prominently in ovarian cancers, conductin was downregulated compared to the normal tissue. Further studies are needed to analyze whether transcriptional downregulation of conductin leads to the stabilization of β-catenin and is of relevance for tumor development in these particular tissues.
Acknowledgments
We thank T. Kirchner for performing β-catenin staining on intestinal tumors of Min mice and for helpful discussions, L. Sorokin for critical reading of the manuscript, A. Brown for the Rat2/Wnt-1 and Rat2/CV7 cells, K. Willert for the antiaxin antibodies, and J. Sussman for the dishevelled-2 expression plasmid. We also thank S. Goletz for help in the preparation of the C/G7 antibody; N. Sochnikova and J. Hülsken for help with the in situ hybridizations; K. Feller, A. Koberling, and I. Wendler for excellent technical assistance; and A. Döbler for secretarial support.
This work was supported by a grant from the Deutsche Forschungsgemeinschaft to J.B. (SFB 366).
REFERENCES
- 1.Aberle, H., A. Bauer, J. Stappert, A. Kispert, and R. Kemler. 1997. Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 16:3797-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behrens, J. 2000. Control of beta-catenin signaling in tumor development. Ann. N. Y. Acad. Sci. 910:21-33. [DOI] [PubMed] [Google Scholar]
- 3.Behrens, J., B. A. Jerchow, M. Wurtele, J. Grimm, C. Asbrand, R. Wirtz, M. Kuhl, D. Wedlich, and W. Birchmeier. 1998. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science 280:596-599. [DOI] [PubMed] [Google Scholar]
- 4.Behrens, J., M. M. Mareel, F. M. Van Roy, and W. Birchmeier. 1989. Dissecting tumor cell invasion: epithelial cells acquire invasive properties after the loss of uvomorulin-mediated cell-cell adhesion. J. Cell Biol. 108:2435-2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behrens, J., J. P. von Kries, M. Kuhl, L. Bruhn, D. Wedlich, R. Grosschedl, and W. Birchmeier. 1996. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature 382:638-642. [DOI] [PubMed] [Google Scholar]
- 6.Bienz, M., and H. Clevers. 2000. Linking colorectal cancer to Wnt signaling. Cell 103:311-320. [DOI] [PubMed] [Google Scholar]
- 7.Brinkmann, V., H. Foroutan, M. Sachs, K. M. Weidner, and W. Birchmeier. 1995. Hepatocyte growth factor/scatter factor induces a variety of tissue-specific morphogenic programs in epithelial cells. J. Cell Biol. 131:1573-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cadigan, K. M., and R. Nusse. 1997. Wnt signaling: a common theme in animal development. Genes Dev. 11:3286-3305. [DOI] [PubMed] [Google Scholar]
- 9.Crawford, H. C., B. M. Fingleton, L. A. Rudolph-Owen, K. J. Goss, B. Rubinfeld, P. Polakis, and L. M. Matrisian. 1999. The metalloproteinase matrilysin is a target of beta-catenin transactivation in intestinal tumors. Oncogene 18:2883-2891. [DOI] [PubMed] [Google Scholar]
- 10.Frixen, U. H., J. Behrens, M. Sachs, G. Eberle, B. Voss, A. Warda, D. Lochner, and W. Birchmeier. 1991. E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J. Cell Biol. 113:173-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furuhashi, M., K. Yagi, H. Yamamoto, Y. Furukawa, S. Shimada, Y. Nakamura, A. Kikuchi, K. Miyazono, and M. Kato. 2001. Axin facilitates Smad3 activation in the transforming growth factor beta signaling pathway. Mol. Cell. Biol. 21:5132-5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giarre, M., M. V. Semenov, and A. M. Brown. 1998. Wnt signaling stabilizes the dual-function protein beta-catenin in diverse cell types. Ann. N. Y. Acad. Sci. 857:43-55. [DOI] [PubMed] [Google Scholar]
- 13.Harada, N., Y. Tamai, T. Ishikawa, B. Sauer, K. Takaku, M. Oshima, and M. M. Taketo. 1999. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 18:5931-5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hart, M. J., R. de los Santos, I. N. Albert, B. Rubinfeld, and P. Polakis. 1998. Downregulation of beta-catenin by human Axin and its association with the APC tumor suppressor, beta-catenin and GSK3 beta. Curr. Biol. 8:573-581. [DOI] [PubMed] [Google Scholar]
- 15.He, T. C., A. B. Sparks, C. Rago, H. Hermeking, L. Zawel, L. T. da Costa, P. J. Morin, B. Vogelstein, and K. W. Kinzler. 1998. Identification of c-MYC as a target of the APC pathway. Science 281:1509-1512. [DOI] [PubMed] [Google Scholar]
- 16.Herter, P., C. Kuhnen, K. M. Muller, A. Wittinghofer, and O. Muller. 1999. Intracellular distribution of beta-catenin in colorectal adenomas, carcinomas and Peutz-Jeghers polyps. J. Cancer Res. Clin. Oncol. 125:297-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hogan, B., R. Beddington, F. Costantini, and E. Lacey. 1994. Manipulating the mouse embryo. Cold Spring Harbor Laboratory Press, Plainview, N.Y.
- 18.Huber, O., R. Korn, J. McLaughlin, M. Ohsugi, B. G. Herrmann, and R. Kemler. 1996. Nuclear localization of beta-catenin by interaction with transcription factor LEF-1. Mech. Dev. 59:3-10. [DOI] [PubMed] [Google Scholar]
- 19.Huelsken, J., R. Vogel, V. Brinkmann, B. Erdmann, C. Birchmeier, and W. Birchmeier. 2000. Requirement for beta-catenin in anterior-posterior axis formation in mice. J. Cell Biol. 148:567-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hugh, T. J., S. A. Dillon, G. O'Dowd, B. Getty, M. Pignatelli, G. J. Poston, and A. R. Kinsella. 1999. Beta-catenin expression in primary and metastatic colorectal carcinoma. Int. J. Cancer 82:504-511. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda, S., S. Kishida, H. Yamamoto, H. Murai, S. Koyama, and A. Kikuchi. 1998. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO J. 17:1371-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Itoh, K., A. Antipova, M. J. Ratcliffe, and S. Sokol. 2000. Interaction of Dishevelled and Xenopus Axin-related protein is required for Wnt signal transduction. Mol. Cell. Biol. 20:2228-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeng, Y. M., M. Z. Wu, T. L. Mao, M. H. Chang, and H. C. Hsu. 2000. Somatic mutations of beta-catenin play a crucial role in the tumorigenesis of sporadic hepatoblastoma. Cancer Lett. 152:45-51. [DOI] [PubMed] [Google Scholar]
- 23a.Jho, E.-h., T. Zhang, C. Domon, C.-K. Joo, J.-N. Freund, and F. Costantini. 2002. Wnt/β-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol. Cell. Biol. 22:1172-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirchner, T., and T. Brabletz. 2000. Patterning and nuclear beta-catenin expression in the colonic adenoma-carcinoma sequence. Analogies with embryonic gastrulation. Am. J. Pathol. 157:1113-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koch, A., D. Denkhaus, S. Albrecht, I. Leuschner, D. von Schweinitz, and T. Pietsch. 1999. Childhood hepatoblastomas frequently carry a mutated degradation targeting box of the beta-catenin gene. Cancer Res. 59:269-273. [PubMed] [Google Scholar]
- 26.Korinek, V., N. Barker, P. J. Morin, D. van Wichen, R. de Weger, K. W. Kinzler, B. Vogelstein, and H. Clevers. 1997. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science 275:1784-1787. [DOI] [PubMed] [Google Scholar]
- 27.Li, L., H. Yuan, C. D. Weaver, J. Mao, G. H. Farr III, D. J. Sussman, J. Jonkers, D. Kimelman, and D. Wu. 1999. Axin and Frat1 interact with dvl and GSK, bridging Dvl to GSK in Wnt-mediated regulation of LEF-1. EMBO J. 18:4233-4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li, L., H. Yuan, W. Xie, J. Mao, A. M. Caruso, A. McMahon, D. J. Sussman, and D. Wu. 1999. Dishevelled proteins lead to two signaling pathways. Regulation of LEF-1 and c-Jun N-terminal kinase in mammalian cells. J. Biol. Chem. 274:129-134. [DOI] [PubMed] [Google Scholar]
- 29.Liu, W., X. Dong, M. Mai, R. S. Seelan, K. Taniguchi, K. K. Krishnadath, K. C. Halling, J. M. Cunningham, L. A. Boardman, C. Qian, E. Christensen, S. S. Schmidt, P. C. Roche, D. I. Smith, and S. N. Thibodeau. 2000. Mutations in AXIN2 cause colorectal cancer with defective mismatch repair by activating beta-catenin/TCF signalling. Nat. Genet. 26:146-147. [DOI] [PubMed] [Google Scholar]
- 30.Molenaar, M., M. van de Wetering, M. Oosterwegel, J. Peterson-Maduro, S. Godsave, V. Korinek, J. Roose, O. Destree, and H. Clevers. 1996. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell 86:391-399. [DOI] [PubMed] [Google Scholar]
- 31.Morin, P. J., A. B. Sparks, V. Korinek, N. Barker, H. Clevers, B. Vogelstein, and K. W. Kinzler. 1997. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 275:1787-1790. [DOI] [PubMed] [Google Scholar]
- 32.Paulsen, J. E., I. L. Steffensen, E. M. Loberg, T. Husoy, E. Namork, and J. Alexander. 2001. Qualitative and quantitative relationship between dysplastic aberrant crypt foci and tumorigenesis in the Min/+ mouse colon. Cancer Res. 61:5010-5015. [PubMed] [Google Scholar]
- 33.Polakis, P. 2000. Wnt signaling and cancer. Genes Dev. 14:1837-1851. [PubMed] [Google Scholar]
- 34.Roose, J., G. Huls, M. van Beest, P. Moerer, K. van der Horn, R. Goldschmeding, T. Logtenberg, and H. Clevers. 1999. Synergy between tumor suppressor APC and the beta-catenin-Tcf4 target Tcf1. Science 285:1923-1926. [DOI] [PubMed] [Google Scholar]
- 35.Rousset, R., J. A. Mack, K. A. Wharton, Jr., J. D. Axelrod, K. M. Cadigan, M. P. Fish, R. Nusse, and M. P. Scott. 2001. Naked cuticle targets dishevelled to antagonize Wnt signal transduction. Genes Dev. 15:658-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salic, A., E. Lee, L. Mayer, and M. W. Kirschner. 2000. Control of beta-catenin stability: reconstitution of the cytoplasmic steps of the wnt pathway in Xenopus egg extracts. Mol. Cell 5:523-532. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Plainview, N.Y.
- 38.Samowitz, W. S., M. D. Powers, L. N. Spirio, F. Nollet, F. van Roy, and M. L. Slattery. 1999. Beta-catenin mutations are more frequent in small colorectal adenomas than in larger adenomas and invasive carcinomas. Cancer Res. 59:1442-1444. [PubMed] [Google Scholar]
- 39.Satoh, S., Y. Daigo, Y. Furukawa, T. Kato, N. Miwa, T. Nishiwaki, T. Kawasoe, H. Ishiguro, M. Fujita, T. Tokino, Y. Sasaki, S. Imaoka, M. Murata, T. Shimano, Y. Yamaoka, and Y. Nakamura. 2000. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat. Genet. 24:245-250. [DOI] [PubMed] [Google Scholar]
- 40.Seidensticker, M. J., and J. Behrens. 2000. Biochemical interactions in the wnt pathway. Biochim. Biophys. Acta 1495:168-182. [DOI] [PubMed] [Google Scholar]
- 41.Shih, I. M., T. L. Wang, G. Traverso, K. Romans, S. R. Hamilton, S. Ben-Sasson, K. W. Kinzler, and B. Vogelstein. 2001. Top-down morphogenesis of colorectal tumors. Proc. Natl. Acad. Sci. USA 98:2640-2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spiegelman, V. S., T. J. Slaga, M. Pagano, T. Minamoto, Z. Ronai, and S. Y. Fuchs. 2000. Wnt/beta-catenin signaling induces the expression and activity of betaTrCP ubiquitin ligase receptor. Mol. Cell 5:877-882. [DOI] [PubMed] [Google Scholar]
- 43.Su, L. K., K. W. Kinzler, B. Vogelstein, A. C. Preisinger, A. R. Moser, C. Luongo, K. A. Gould, and W. F. Dove. 1992. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science 256:668-670. [DOI] [PubMed] [Google Scholar]
- 44.Terris, B., P. Pineau, L. Bregeaud, D. Valla, J. Belghiti, P. Tiollais, C. Degott, and A. Dejean. 1999. Close correlation between beta-catenin gene alterations and nuclear accumulation of the protein in human hepatocellular carcinomas. Oncogene 18:6583-6588. [DOI] [PubMed] [Google Scholar]
- 45.Tetsu, O., and F. McCormick. 1999. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398:422-426. [DOI] [PubMed] [Google Scholar]
- 46.van de Wetering, M., R. Cavallo, D. Dooijes, M. van Beest, J. van Es, J. Loureiro, A. Ypma, D. Hursh, T. Jones, A. Bejsovec, M. Peifer, M. Mortin, and H. Clevers. 1997. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 88:789-799. [DOI] [PubMed] [Google Scholar]
- 47.Vasicek, T. J., L. Zeng, X. J. Guan, T. Zhang, F. Costantini, and S. M. Tilghman. 1997. Two dominant mutations in the mouse fused gene are the result of transposon insertions. Genetics 147:777-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wharton, K. A., Jr., G. Zimmermann, R. Rousset, and M. P. Scott. 2001. Vertebrate proteins related to Drosophila Naked Cuticle bind Dishevelled and antagonize Wnt signaling. Dev. Biol. 234:93-106. [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto, H., S. Kishida, T. Uochi, S. Ikeda, S. Koyama, M. Asashima, and A. Kikuchi. 1998. Axil, a member of the Axin family, interacts with both glycogen synthase kinase 3β and β-catenin and inhibits axis formation of Xenopus embryos. Mol. Cell. Biol. 18:2867-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yan, D., J. B. Wallingford, T. Q. Sun, A. M. Nelson, C. Sakanaka, C. Reinhard, R. M. Harland, W. J. Fantl, and L. T. Williams. 2001. Cell autonomous regulation of multiple Dishevelled-dependent pathways by mammalian Nkd. Proc. Natl. Acad. Sci. USA 98:3802-3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeng, L., F. Fagotto, T. Zhang, W. Hsu, T. J. Vasicek, W. L. Perry III, J. J. Lee, S. M. Tilghman, B. M. Gumbiner, and F. Costantini. 1997. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell 90:181-192. [DOI] [PubMed] [Google Scholar]