Abstract
Axin2/Conductin/Axil and its ortholog Axin are negative regulators of the Wnt signaling pathway, which promote the phosphorylation and degradation of β-catenin. While Axin is expressed ubiquitously, Axin2 mRNA was seen in a restricted pattern during mouse embryogenesis and organogenesis. Because many sites of Axin2 expression overlapped with those of several Wnt genes, we tested whether Axin2 was induced by Wnt signaling. Endogenous Axin2 mRNA and protein expression could be rapidly induced by activation of the Wnt pathway, and Axin2 reporter constructs, containing a 5.6-kb DNA fragment including the promoter and first intron, were also induced. This genomic region contains eight Tcf/LEF consensus binding sites, five of which are located within longer, highly conserved noncoding sequences. The mutation or deletion of these Tcf/LEF sites greatly diminished induction by β-catenin, and mutation of the Tcf/LEF site T2 abolished protein binding in an electrophoretic mobility shift assay. These results strongly suggest that Axin2 is a direct target of the Wnt pathway, mediated through Tcf/LEF factors. The 5.6-kb genomic sequence was sufficient to direct the tissue-specific expression of d2EGFP in transgenic embryos, consistent with a role for the Tcf/LEF sites and surrounding conserved sequences in the in vivo expression pattern of Axin2. Our results suggest that Axin2 participates in a negative feedback loop, which could serve to limit the duration or intensity of a Wnt-initiated signal.
Axin is an important component of the canonical Wnt signal transduction pathway, which suppresses signaling activity in the absence of a Wnt ligand. Axin is believed to function by promoting the phosphorylation and consequent degradation of β-catenin, a key effector of the pathway (for a review, see references 2 and 18). The Axin gene (50) was identified as a consequence of a murine transgenic insertional mutation (30), which produced a new allele (AxinTg1) of a genetic locus originally named Fused and renamed Axin. Based on the phenotypic properties of mouse embryos mutant for several Axin alleles, it was hypothesized that Axin encoded a negative regulator of a step in embryonic axis formation (8, 30, 50). This hypothesis was confirmed by cloning of the Axin gene, together with studies in Xenopus embryos, which showed that Axin exerted its effects on axis formation by specifically blocking signaling through the Wnt/β-catenin pathway (50).
The mechanism by which Axin functions has begun to be elucidated by a variety of in vitro and in vivo studies, which suggest that it serves as a scaffold protein that binds directly many of the proteins involved in this signaling pathway (6, 9, 12-14, 17, 19, 20, 22, 28, 35, 36, 38). An Axin homolog, which has been variously called Axin2 (26), Conductin (1), or Axil (48), appears to be functionally similar to Axin, although it has not been characterized as extensively as has Axin. Components of the Wnt signal transduction pathway include receptors of the Frizzled family, the cytoplasmic protein Dishevelled (Dvl), the serine/threonine kinase GSK-3, β-catenin, APC, and LEF/Tcf transcription factors (3, 10, 27, 31). Axin contains several domains that mediate direct binding to APC, GSK-3, β-catenin, and Dvl, as well as to itself and to the Ser/Thr phosphatase PP2A (6, 9, 12-14, 17, 19, 20, 22, 28, 35, 36, 38). Although the domains of Axin critical for its activity vary somewhat depending on the functional assays employed, it appears that a central function of Axin is to promote the phosphorylation of β-catenin by GSK-3. According to prevailing models, in the absence of a Wnt signal, GSK-3 phosphorylates β-catenin, as well as APC and Axin, leading to the ubiquitination and degradation of cytosolic β-catenin. In the presence of a Wnt signal, GSK-3 activity is inhibited through an unknown mechanism involving Frizzled receptors as well as Dishevelled. This results in the hypophosphorylation of Axin, which lowers its affinity for β-catenin (15, 46) as well as its stability (47), thus releasing β-catenin from the degradation machinery. β-Catenin consequently accumulates and enters the nucleus, where it interacts with Tcf/LEF factors to regulate transcription of target genes (5, 32). The role of Axin in this signaling pathway predicts that it could function as a tumor suppressor gene, and indeed, mutations in human AXIN have been found to be associated with hepatocellular carcinoma (37).
Most signal transduction pathways contain negative feedback mechanisms, which serve to restrict the duration or spread of the signaling event following the initial stimulus (for a review, see reference (7). In this paper, we provide evidence that this role in the Wnt pathway can be fulfilled by Axin2/Conductin/Axil (which we will refer to as Axin2 henceforth). Like Axin, the murine and rat orthologs of Axin2 have been shown elsewhere to bind to APC, GSK-3, and β-catenin; to promote the phosphorylation of β-catenin by GSK-3; and to inhibit Wnt-induced axis formation in Xenopus embryos (1, 48). AXIN2 has been shown elsewhere to be a tumor suppressor, as mutations in the human gene are associated with colorectal carcinoma (25). Unlike Axin, which is expressed ubiquitously during mouse embryogenesis (50), Axin2 mRNA is expressed in a restricted pattern during embryogenesis and organogenesis (reference (1) and Fig. 1 herein). We noticed that many sites of Axin2 expression appeared to overlap with those of various members of the Wnt family, and thus, we hypothesized that the expression of Axin2 might be induced by Wnt signaling. We report here that endogenous Axin2 expression is induced in cultured cells or fetal tissue by activation of the Wnt pathway. Recent results by Lustig et al. (25a) reveal that Axin2 is also highly expressed in a number of murine and human tumors and tumor cell lines that are caused by β-catenin/Wnt signaling. We show here that a similar induction of Axin2 reporter constructs can be mediated by sequences in the promoter and first intron of the gene, which contain a series of conserved Tcf/LEF consensus binding sites. Analysis of Axin2/luciferase reporter constructs with mutation or deletion of these sites, as well as in vitro binding studies, strongly suggest that the Axin2 gene is a direct target of the Wnt pathway, mediated through Tcf/LEF factors. The same Axin2 genomic sequences that mediate Wnt induction in transfected cells are also sufficient to direct the characteristic Axin2 tissue-specific expression pattern in transgenic embryos.
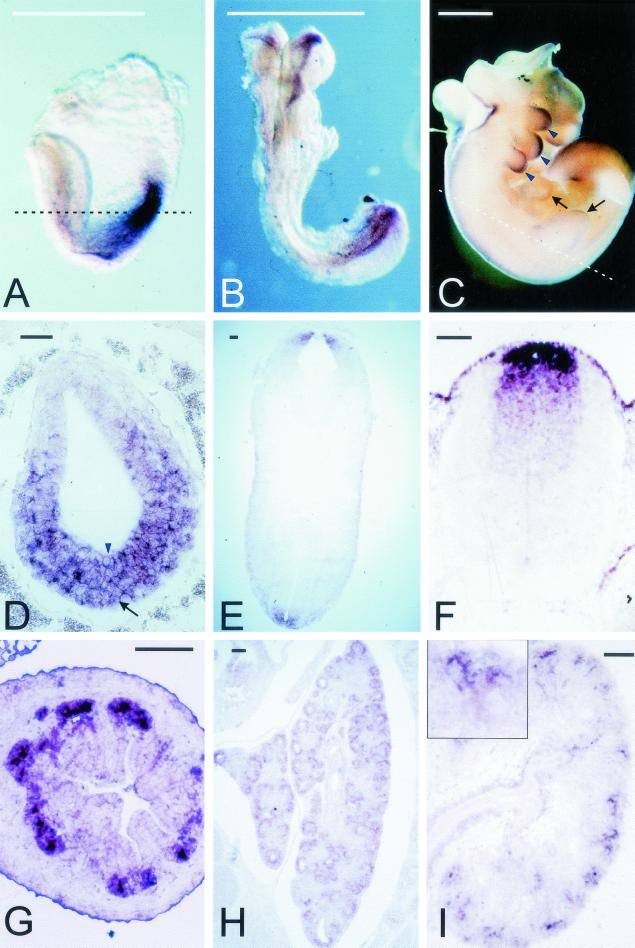
FIG.1.
Expression of Axin2 mRNA during mouse embryogenesis. (A to C) Whole-mount in situ hybridization. At E7.5 (A), Axin2 is expressed throughout the posterior region of the embryo; at E8.5 (B), expression is seen in the rostral and caudal ends of the neural ectoderm. At E9.5 (data not shown) and E10.5 (C), expression is seen along the entire length of the dorsal neural tube, as well as in the branchial arches (arrowheads) and limb buds (arrows). (D to F) In situ hybridization to transverse sections. At E7.5 (D), Axin2 is expressed in both the primitive streak mesoderm (arrow) and the posterior embryonic ectoderm (arrowhead). The approximate orientation of panel D is shown by the dotted line in panel A. At E10.5 (E) and E11.5 (F), strong expression is seen in the roof plate of the neural tube, with progressively weaker expression in a stripe of cells extending ventrally. The approximate orientation of panel E is shown by the dotted line in panel C. (G to I) Sections of developing organs from E14.5 embryos. In the lung (G), expression is seen throughout epithelial component. In the gut (H), Axin2 is expressed specifically in the epithelium at the base of the nascent villi, where the crypts will later form. In the kidney (I), expression is seen specifically in the ureteric buds; inset, higher magnification of branching ureteric bud tip. Bars, 1 mm (A to C) and 0.1 mm (D to I).
MATERIALS AND METHODS
Cell culture and transient transfection.
C57MG cells, which were stably transfected with a hemagglutinin-tagged Wnt1/5 chimera or lacZ gene in the vector pZNCX (16), were a gift of Jan Kitajewski. These cells were grown in Dulbecco's modified Eagle medium (Life Technologies) containing 10% fetal bovine serum (HyClone Laboratories Inc.) and 10μg of insulin per ml. 293T cells were cultured in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum in humidified 6% CO2. Cells were transfected using either a calcium phosphate mammalian cell transfection kit (5 Prime→3 Prime, Inc.) or Lipofectamine reagent (Life Technologies). The transient expression of d2EGFP in 293T cells was monitored using a Nikon Eclipse TE300 epifluorescence microscope.
In situ hybridization.
In situ hybridization was performed as described previously (45) with modifications. For whole-mount in situ hybridization, embryos were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) at 4°C overnight, washed 2× in PBT (PBS containing 0.1% Tween 20), and dehydrated through an ethanol series. The embryos were then treated with 10 μg of proteinase K per ml in PBT, washed 2× with PBT, and refixed with fresh 0.2% glutaraldehyde-4% paraformaldehyde in PBT. After being washed with PBT, they were subjected to prehybridization at 60°C for 4 h followed by hybridization with digoxigenin-labeled riboprobe at 60°C overnight. For in situ hybridization on sections, embryos were fixed in cold 4% paraformaldehyde in PBS, washed in PBS followed by saline, and dehydrated through an ethanol series. They were embedded in paraffin and sectioned at 8 μm. Riboprobes for hybridization were labeled with digoxigenin and detected with monoclonal antibody against digoxigenin conjugated with alkaline phosphatase (Roche), whose activity was detected with 5-bromo-4-chloro-3-indolylphosphate and nitroblue tetrazolium (Roche). The antisense and sense Axin2 riboprobes were made by linearizing a plasmid containing Axin2 cDNA (positions 1 to 2397) with NotI or SalI and transcribing with T3 or T7 RNA polymerase, respectively (digoxigenin labeling kit; Roche). For synthesis of the antisense riboprobe, Axin2 cDNA bp 1774 to 2787 in pBluescript II KS was linearized with SalI and transcribed T7 RNA polymerase. The sense probe was synthesized by linearizing the same plasmid with NotI and by transcription with T3 RNA polymerase.
Reverse transcription-PCR (RT-PCR) and Western blot analysis.
Total RNA was isolated using TRI reagent (Sigma) from C57MG cells stably transfected with either lacZ or Wnt1/5 expression vectors and 293T cells treated with either NaCl or LiCl for varying time periods. LiCl-treated 293T cells were lysed, and total cell lysate was used for Western blots as described previously (6). Axin2 and GSK-3β were detected with mouse anti-Conductin (Axin2) (kindly provided by Walter Birchmeier) and mouse anti-GSK-3β (Transduction Laboratories) antibodies. First-strand cDNA was synthesized using the Superscript Preamplification System (Life Technologies). The following primers were used for PCR: for Axin2, 5"-CTCCTTGGAGGCAAGAGC-3" and 5"-GGCCACGCAGCACCGCTG-3"; for Axin, 5"-TGCAGAGTCCCAAAATGAATG-3" and 5"-GAGCCTGTCCTTGTGTAC-3"; for β-catenin, 5"-ATGGCTACTCAAGCTGAC-3" and 5"-CAGCACTTTCAGCACTCTGC-3"; and for β-actin, 5"-AGGCCAACCGCGAAGATGACC-3" and 5"-GAAGTCCAGGGCGACGTAGCAC-3".
Preparation and analysis of rat embryonic endoderm.
The small intestine was removed from rat fetuses at day 14 postcoitum, and the endoderm was separated from the mesenchyme as described by Duluc et al. (4). One-millimeter endoderm fragments were cultured for 24 h on top of Wnt-1-expressing NIH 3T3 or lacZ-expressing cells (kindly provided by A. Kispert) as previously described (23). RNA was prepared using TRI reagent (Euromedex) and analyzed by RT-PCR for Axin2, cytokeratin 19, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) using the following primers. To amplify rat Axin2 mRNA (GenBank accession no. AF017757), the following primers were used: 5"-CAGGACCCACATCCTTCT-3" and 5"-ACGCGGAGGTGCACGCG G-3". The primers 5"-TTGAGATTGAGCTGCAGTCCCAGCT-3" and 5"-TTCCCAGGGGAGTCTCGCTGGTAGC-3" were used for cytokeratin 19, and 5"-ACCACAGTCCTGCCATCAC-3" and 5"-TCCACCACCCTGTTGCTGTA-3" were used for GAPDH. GAPDH transcript was used to standardize total RNA in the endoderm-NIH 3T3 cocultures, and the epithelium-specific cytokeratin 19 mRNA was used to monitor the endoderm contribution.
Isolation and sequence of mouse Axin2 cDNA and genomic sequences.
BLAST searches of dbEST using the mouse Axin amino acid sequence identified several human expressed sequence tags showing significant homology, but nonidentity, to mouse or human Axin. A cDNA clone from which one of these expressed sequence tags was derived was obtained and used as a probe to screen a mouse embryo cDNA library, and the full-length cDNA was isolated and sequenced. The deduced amino acid sequence was nearly identical to that of Conductin (1), with the exception of four differences: amino acid (aa) 474 Y(H), aa 484 P(S), aa 503 S(F), and aa 603 A(G) (Conductin residues in parentheses). Three more silent nucleotide differences were found (GenBank accession no. AF205889).
Isolation of Axin2 genomic sequences.
A mouse BAC library (RPCI-22M; Research Genetics) was screened with the 5" end of Axin2 cDNA (nucleotides 235 to 855 of GenBank accession no. AF073788) as a probe. Three positive BAC clones (86O5, 519M22, and 141P22) were identified and sequenced with primers near the 5" end of the mouse Axin2 cDNA sequence. Sequence analysis confirmed that these three BAC clones contained DNA upstream of the first exon of Axin2. Initially, we digested BAC 8605 DNA with HindIII and PvuII, cloned the products into the pCRII vector (Invitrogen), and screened them with the same Axin2 cDNA probe that was used for BAC library screening. Sequence analysis of a positive clone (containing a 1.5-kb insert and named pCRII-PIExon 2) showed that it contained only part of intron 1 and exon 2 of Axin2. Based on sequence information from this clone, we digested BAC 86O5 DNA with BglII and isolated a 5.6-kb clone, which contained the promoter, exon 1, and intron 1 sequence (see Fig. 3A). The entire region was sequenced.
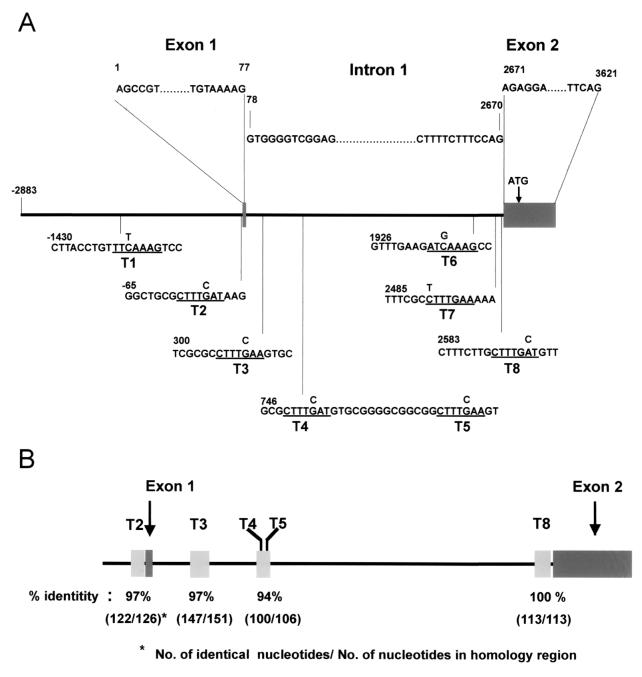
FIG. 3.
Axin2 promoter and intron 1 sequences contain potential Tcf/LEF binding sites within longer conserved DNA sequences. (A) A 5.6-kb DNA fragment containing the Axin2 promoter, exon 1, intron 1, and part of exon 2 was subcloned from a mouse BAC clone and sequenced. Exons are shown as gray boxes, and the sequence is numbered with bp 1 corresponding to the start of the cDNA sequence. Eight conserved Tcf/LEF binding sites (underlined and designated T1 to T8) were identified. The site-directed mutations introduced into each site (Fig. 5) are shown on top of the original sequence. (B) Conservation of five potential Tcf/LEF binding sites and surrounding sequences between human and mouse Axin2 promoter/intron 1 sequences. Sequence comparison between mouse and human BAC clones revealed strong conservation of sites T2, T3, T4, T5, and T8, as well as between 106 and 151 bp of the surrounding DNA sequence. The conserved sequences are as follows: T2, −128 to −3; T3, 262 to 412; T4 and T5, 701 to 806; and T8, 2534 to 2646.
Construction of plasmids.
The 5.6-kb DNA insert that contained the Axin2 promoter, exon 1, and intron 1 was cloned into BglII-digested pGL3-Basic vector (Promega), and this construct was named pGL3-Bgl(5.6). In order to add an endogeneous splice acceptor site from exon 2, DNA between two HindIII sites (one from bp 1727 in the intron [see Fig. 3A] and the other from a multiple cloning site in the vector) was replaced with the insert between the HindIII and AflII sites from pCRII-PIExon 2. All sites were filled in with Klenow DNA polymerase and joined by blunt-end ligation. During this cloning procedure, a HindIII site in intron 1 was removed as a result of a 4-bp insertion. This construct was designated Ax2-Luc. The 5.6-kb DNA fragment was excised from Ax2-Luc using KpnI and HindIII (the HindIII site was filled in with Klenow DNA polymerase) and cloned into the KpnI- and SmaI-digested pd2EGFP-1 vector (Clontech). This construct was named Ax2-d2EGFP. Ax2-XB was constructed by the deletion of the fragment between the XhoI and BglII sites, and Ax2-Int1 was made by the deletion of DNA between NruI and HindIII sites in Ax2-Luc. Deletion of promoter sequences, in the presence of the intron 1 deletion, was made by the removal (from Ax2-Int1) of DNA between MluI and NdeI for Ax2-Int1-848 and of MscI for Ax2-Int1-398.
Site-directed mutagenesis.
Site-directed mutations were introduced by standard PCR techniques using Pfu DNA polymerase (Stratagene). For example, to introduce a mutation in site T8 (see Fig. 3A), PCR was performed to the point mutation using Ax2-Luc as template, and the PCR product was cloned into the vector pCR-Blunt II-TOPO (Invitrogen). After the T8 point mutation was confirmed by sequencing, that DNA was used to introduce additional point mutations at T6 and T7, and the PCR product was cloned into pCR-Blunt II-TOPO. The fragment between XhoI and BglII sites in Ax2-Luc was replaced with the same region of DNA containing the three point mutations (M68 in Fig. 5). Site-directed mutations in other regions were introduced using similar methods.
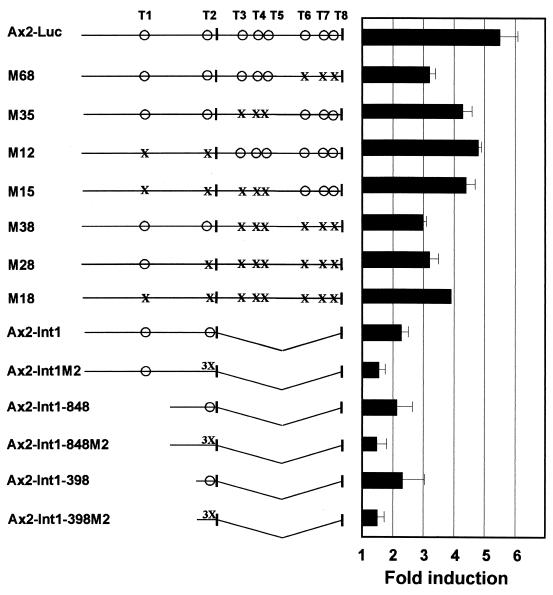
FIG. 5.
Mutation or deletion of Tcf/LEF binding sites in the Axin2 promoter/intron 1 leads to reduced induction by β-catenin. Axin2 promoter/intron 1 sequences were used to drive expression of luciferase reporter constructs. Vertical bars indicate exon 1 and part of exon 2. The lower six constructs shown lacked intron 1. Intact Tcf/LEF binding sites are indicated by ○, single nucleotide mutations (see Fig. 3A for specific mutations) are indicated by X, and a triple mutation in site T2 (from 5"-CTTTGAT-3" to 5"-CTTTCGC-3") is indicated by 3X. The fold induction of luciferase activity was measured after cotransfection with β-catenin and compared to the lacZ control, as described in the legend to Fig. 4. Error bars show standard deviations.
Luciferase assay.
The ratio between Axin2-driven fly luciferase and the constitutive expression of Renilla luciferase driven by the herpes simplex virus thymidine kinase promoter (pRL-TK; Promega) was measured using the dual-luciferase reporter assay system (Promega). To measure the effect of deletion or site-directed mutagenesis in Axin2 promoter-intron 1 sequences upon Wnt/β-catenin signaling, 293T cells were transiently cotransfected with the two luciferase reporter constructs and in some cases other plasmids such as β-catenin or Wnt1/5, Tcf, etc.
EMSA.
Since we used 293T cells for all luciferase assays for the responsiveness of the Axin2 promoter-intron to Wnt/β-catenin, 293T cells were lysed and crude nuclear extract was obtained for electrophoretic mobility shift assay (EMSA). Briefly, cells were lysed by a 10-min hypotonic treatment on ice in buffer A (10 mM HEPES [pH 7.9], 10 mM KCl, 1.5 mM MgCl2, 0.5 mM dithiothreitol [DTT], 0.5 mM phenylmethylsulfonyl fluoride [PMSF], 10 μg of leupeptin per ml, 10 μg of aprotinin per ml) followed by 15 strokes of Dounce homogenization. The extracts were then centrifuged at 4°C for 10 min at 10,000 × g. The pellet material was washed once with ice-cold buffer A and resuspended in 2 volumes of buffer C (20 mM HEPES [pH 7.9], 0.42 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, 25% glycerol, 0.5 mM PMSF, 10 μg of leupeptin per ml, 10 μg of aprotinin per ml). The suspension was frozen at −70°C for 10 min, thawed slowly, and incubated on ice for 15 min. The supernatant of a 15-min centrifugation of the suspension at 10,000 × g at 4°C represented the nuclear extract.
Wild-type T2 site (5"-CTGGAGCCGGCTGCGCTTTGATAAGGTCCTGGC-3" and 5"-GCCAGGACCTTATCAAAGCGCAGCCGGCTCCAG-3") and mutated T2 site (5"-CTGGAGCCGGCTGCGCTTTCGCAAGGTCCTGGC-3" and 5"-GCCAGGACCTTGCGAAAGCGCAGCCGGCTCCAG-3") oligonucleotides were end labeled with [γ-32P]ATP and T4 polynucleotide kinase and used as probes for EMSA (mutated sites and corresponding wild-type sequences in the Tcf binding site are underlined). The binding reaction mixture contained 50,000 cpm of 32P-labeled oligonucleotides and 10 μg of nuclear extract in 60 mM KCl-0.5 mM DTT-15 mM Tris (pH 7.5)-0.25 mg of bovine serum albumin-0.5 mM PMSF-10 μg of leupeptin per ml-10 μg of aprotinin per ml-50 ng of poly(dI-dC) per ml. It was incubated for 20 min at 25°C, and DNA-protein complexes were separated in 5% native acrylamide gels (in 0.5× Tris-buffered EDTA buffer) and visualized by autoradiography.
Generation and analysis of transgenic mice.
Ax2-d2EGFP plasmid was digested with AflII to remove vector sequences, and the 6.2-kb fragment was purified using the QIAquick gel extraction kit (Qiagen) for pronuclear injection. DNA concentration was determined by gel electrophoresis using standard markers. Prior to injection, DNA was diluted with injection buffer (5 mM Tris, 0.2 mM EDTA [pH 7.2]) and further purified with Ultrafree-MC centrifugal filtration units (Millipore). Pronuclear injection was performed according to a standard protocol (10a). DNA was injected into (B6 × CBA)F1 zygotes, and transgenic mice were bred either to the same F1 hybrid strain or to Swiss Webster mice. Tail DNA was isolated from the offspring and genotyped with two pairs of PCR primers and by Southern analysis. Two different primer pairs were used for genotyping: 5"-TCAGATTTCGCTTTTGAAAAAGCTG CGTCG-3" (from the Axin2 intron 1) and 5"-TGTGGTCGGGGTAGCGGCTG-3" (from d2EGFP), and 5"-CATCTGCACCACCGGCAAGC-3" and 5"-CTCCGGCGGGAAGCCATGGC-3" (both from d2EGFP). Transgenic founder mice or F1 transgenic progeny were mated with either F1 hybrid or Swiss Webster mice. Embryos were dissected in CO2 independent medium (Gibco BRL), transferred to PBS, and photographed using a Nikon SMZ 1500 fluorescence microscope.
Nucleotide sequence accession number. The sequence of the 5.6-kb clone was deposited in GenBank under accession no. AF343582.
RESULTS
During mouse embryogenesis, Axin2 mRNA is expressed in a restricted pattern that overlaps with sites of Wnt signaling.
To examine the expression of Axin2 during embryogenesis, in situ hybridization was performed on mouse embryos from E7.5 to E14.5. Unlike Axin mRNA, which is expressed ubiquitously throughout embryogenesis (50), Axin2 was expressed in a restricted pattern. At E7.5, Axin2 was expressed in the mesoderm of the primitive streak as well as in the overlying posterior embryonic ectoderm (Fig. 1A and D).At E8.5, expression was seen primarily in the head folds and the posterior neural tube (Fig. 1B). At E9.5 to 11.5, prominent expression was observed along the entire dorsal neural tube, as well as in the branchial arches and the limb buds (Fig. 1C and E). Expression in the neural tube was limited to the roof plate and a broader central region immediately ventral to the roof plate (Fig. 1E and F). At later stages, Axin2 expression was prominent in specific regions of the epithelial components of several developing organs, including the intervillus epithelium of the gut (Fig. 1G), the lung endoderm (Fig. 1H), the Wolffian duct (data not shown), and the tips of the ureteric bud in the kidney (Fig. 1I).
Interestingly, several sites of Axin2 expression overlap with sites where different Wnts are expressed during embryogenesis. For example, Wnt-1, Wnt-3, and Wnt-3a are expressed in the primitive streak and the dorsal neural tube in domains that overlap or are close to the region where Axin2 is expressed (24, 29). In the developing kidney, Wnt-11 is expressed in the tips of the ureteric bud (21), while Wnt-7b and Wnt-4 are expressed in nearby regions (21, 41). Wnt-2 and Wnt-7b are expressed in the developing lung endoderm (11). The intervillus epithelium of the gut undergoes reshaping during early postnatal development to form the crypts, in which cell proliferation is regulated by the β-catenin/LEF-Tcf pathway (for a review, see reference (40). The partially colocalized expression of Axin2 and Wnt genes or Wnt signaling components during embryogenesis led us to examine whether the transcription of Axin2 might be induced by Wnt signaling.
Expression of endogenous Axin2 mRNA is induced by Wnts.
We first examined the level of Axin2 mRNA in a mouse mammary gland cell line (C57MG) stably transfected with either Wnt1/5 (an oncogenic chimera of Wnts 1 and 5a, which induces high cytoplasmic levels of β-catenin [16]) or a control lacZ vector. RT-PCR analysis showed a strong induction of Axin2 mRNA in Wnt1/5-transfected cells, while the levels of Axin and β-catenin mRNA were unchanged (Fig. 2A).To examine the time course of induction, 293T cells were treated with LiCl, an inhibitor of GSK-3, and endogenous Axin2 mRNA and protein were assayed by RT-PCR (Fig. 2B) and Western blotting (Fig. 2C), respectively. Both Axin2 mRNA and protein showed a significant induction by 4 h and a stronger induction at 8 and 12 h of LiCl treatment, while treatment with cyclohexamide blocked the accumulation of Axin2 protein, demonstrating that it was due to de novo protein synthesis.
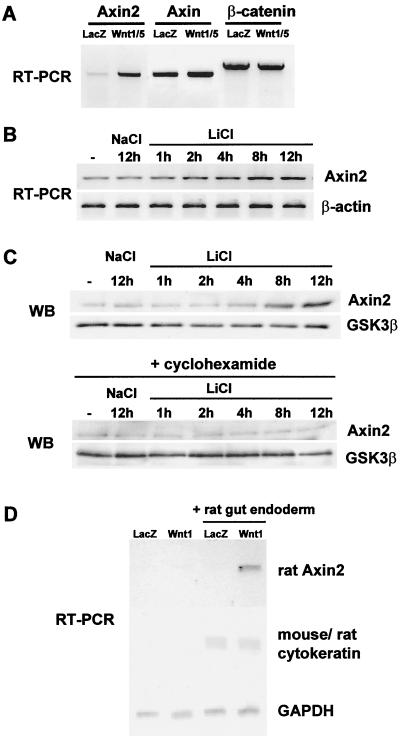
FIG. 2.
Induction of Axin2 mRNA by Wnts in cell culture and ex vivo tissue culture systems. (A) Induction of Axin2 mRNA in Wnt1/5-expressing C57MG cells. Total RNA was isolated from C57MG cells stably transfected with either lacZ or Wnt1/5 expression vectors, and RT-PCR was performed to measure the level of Axin2, Axin, and β-catenin mRNAs. (B and C) Induction of Axin2 mRNA and protein in LiCl-treated 293T cells. 293T cells were treated with 40 mM LiCl for different times, and RT-PCR and Western blot (WB) analysis were performed to measure the level of Axin2 mRNA and protein as well as control β-actin and GSK-3β. (D) Induction of Axin2 mRNA in embryonic gut endoderm cocultured with Wnt1-expressing NIH 3T3 cells. One-millimeter pieces of rat embryonic endoderm from E13 embryos were cocultured on either lacZ- or Wnt1-expressing NIH 3T3 cells for 24 h. Total RNA was isolated, and RT-PCR was performed with primer pairs specific for rat Axin2, as well as rat cytokeratin 19 (which cross-reacts with mouse cytokeratin 19) and GAPDH as controls.
It has recently been shown that expression of the rat Cdx1 homeobox gene is stimulated by Wnt signaling in the developing intestinal epithelium, by coculture of intestinal endoderm with Wnt-expressing cells (23). Since Axin2 was expressed in the embryonic mouse intestinal epithelium, we asked if rat Axin2 mRNA could be similarly induced in this ex vivo system. Control or Wnt-1-expressing NIH 3T3 cells were cocultured with endoderm isolated from the small intestine of 13-day rat embryos. After 24 h of coculture, total RNA was isolated. To monitor induction of Axin2 mRNA, semiquantitative RT-PCR was performed with rat Axin2-specific primers. As shown in Fig. 2D, the Axin2 mRNA level was clearly induced in the coculture with Wnt-expressing but not lacZ control cells. RT-PCR for cytokeratin 19, whose expression is specific to the gut epithelial cells, showed that there were equal endoderm RNA contributions in the cocultures, while GAPDH served as a control for the amount of RNA that was reverse transcribed.
Together, the induction by Wnt of the Axin2 mRNA level in C57MG cells and in ex vivo gut endoderm and the increased protein level in LiCl-treated 293T cells strongly suggested that Axin2 is a downstream target of Wnt signaling.
The Axin2 promoter and first intron contain conserved Tcf/LEF binding sites.
To investigate the mechanism by which Axin2 mRNA is induced by Wnts, we examined the Axin2 gene for the canonical Tcf/LEF binding sites that are known to mediate transcriptional regulation by β-catenin together with Tcf/LEF factors (32, 43). BAC clones containing the mouse Axin2 gene were identified, and a segment of 5,587 bp, including 2,883 bp of 5" flanking DNA, the first exon, the first intron, and part of exon 2 were sequenced. We identified eight core Tcf/LEF binding sites (5"-A/T A/T CAAAG-3") in this region, two in the 5" flanking DNA and six in the first intron (Fig. 3A).To ask if these sites are evolutionarily conserved, we compared the mouse Axin2 sequence to a human Axin2 genomic sequence (GenBank accession no. AC004805). The comparison revealed that five of the eight Tcf/LEF consensus sites identified in the mouse Axin2 promoter and first intron are identical in the human AXIN2 gene (Fig. 3B). Interestingly, sequences of approximately 100 to 150 bp surrounding sites were very highly conserved (Fig. 3B), and the spacing between these core Tcf/LEF sites was also very similar. For example, T8 and the surrounding 112 bp show a perfect match between human and mouse sequences, and sequences surrounding T2 and T3 are close to 100% conserved (Fig. 3B). The presence of core Tcf/LEF binding sites in promoter/intron 1 sequences of human and mouse Axin2, together with the induction of Axin2 mRNA by Wnts (Fig. 2), suggested that Axin2 expression is regulated via Tcf/LEF transcription factors. Furthermore, the remarkable evolutionary conservation of the surrounding (noncoding) regions strongly suggests that these sequences may play a role in gene regulation.
Axin2 promoter/intron 1 sequences mediate the response to β-catenin and Wnt signals.
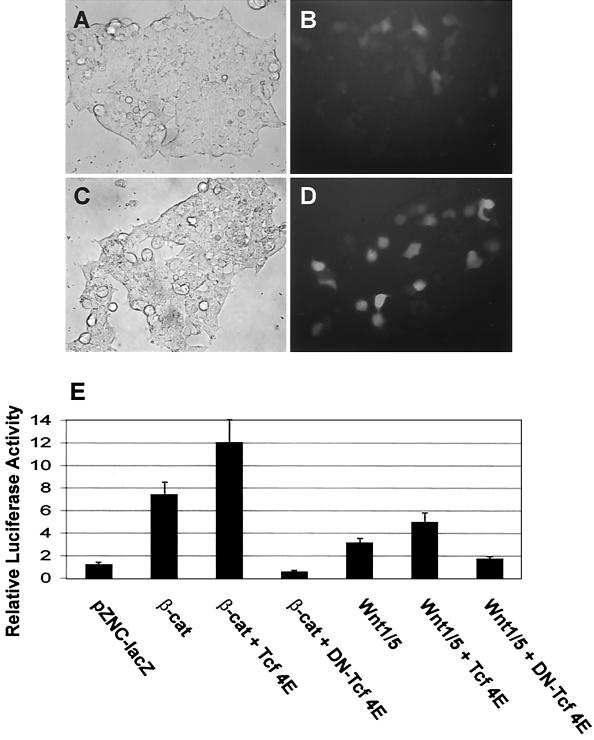
To test whether the Axin2 promoter/intron 1 sequences respond to β-catenin and Wnt signaling, the 5.6-kb DNA fragment was used to drive the expression of d2EGFP, a short-lived green fluorescent protein variant. The resulting construct, Ax2-d2EGFP, was first cotransfected into 293T cells with either control LacZ (Fig. 4A and B)or a β-catenin expression vector (Fig. 4C and D), and induction of d2EGFP was detected qualitatively using fluorescence microscopy. Cotransfection of β-catenin induced the expression of d2EGFP in the 293T cells.
FIG. 4.
The Axin2 promoter and intron 1 direct Wnt- or β-catenin-inducible expression of reporter genes. (A) Expression of d2EGFP under the Axin2 promoter/intron 1 is induced by β-catenin. Axin2 promoter/intron 1 sequence (−2883 to +2703) was cloned into the pd2EGFP-1 vector (Ax2-d2EGFP). Ax2-d2EGFP was cotransfected into 293T cells together with either control LacZ (A and B) or a wild-type hemagglutinin-tagged β-catenin expression plasmid (C and D), and transient expression of d2EGFP was monitored. Fluorescence microscopy shows induction of d2EGFP expression by β-catenin (D) compared to the lacZ control (B). (E) Axin2/luciferase activity is induced by Wnt1/5 or β-catenin and inhibited by dominant-negative Tcf-4E. The Axin2 promoter/intron 1 sequence (−2883 to +2703) was cloned into the vector pGL3-Basic to produce Ax2-Luc. Ax2-Luc and pRL-TK (Renilla luciferase under the thymidine kinase [TK] promoter) were cotransfected into 293T cells together with the indicated plasmids, and the ratio of Ax2-driven fruit fly luciferase to TK-driven constitutively expressed Renilla luciferase activity was measured. Cotransfection of either β-catenin or Wnt1/5 increased the ratio, indicating induction. Addition of Tcf increased the degree of induction, while the addition of dominant-negative Tcf-4E (DN) blocked the induction by β-catenin or Wnt1/5. The error bars indicate standard deviations.
To measure quantitatively the responsiveness of the Axin2 promoter/intron 1 to either β-catenin or Wnt, the 5.6-kb DNA fragment was cloned into a luciferase expression vector (to produce Ax2-Luc) and luciferase activity was measured after transfection into 293T cells (Fig. 4E). Cotransfection of β-catenin or Wnt1/5 induced Ax2-Luc activity by about six- or threefold, respectively, compared to control LacZ-cotransfected cells. To determine whether the activation of Ax2-Luc is mediated by Tcf, we cotransfected either wild-type Tcf-4E or the dominant- negative mutant DN-Tcf-4E (42). We found that Tcf-4E further enhanced luciferase expression, while DN-Tcf-4E inhibited the induction of luciferase activity by either β-catenin or Wnt1/5 (Fig. 4E). These data suggest that Tcf/LEF factors mediate the activation of the Axin2 promoter/intron 1 by β-catenin and Wnt1/5.
Tcf/LEF sites are required for full induction of Axin2 by β-catenin.
To test whether the Tcf/LEF consensus sites are responsible for the induction of Axin2 by Wnt and β-catenin, single point mutations (Fig. 3A) were introduced into each of the eight Tcf/LEF sites, in various combinations (Fig. 5). Introduction of a single point mutation into each of the eight Tcf/LEF sites, or into different subsets of the Tcf/LEF sites, consistently reduced but did not eliminate the responsiveness to β-catenin. Mutation of sites T6, T7, and T8 in the first intron (M68) had the greatest effect, causing a 42% reduction in the degree of induction by β-catenin. Mutation of sites T3, T4, and T5 in the intron had a smaller effect (M35), while mutation of T1 and T2 in the 5" flanking DNA had little effect when the other six sites were intact (M12). Deletion of the entire intron 1 (Ax2-Int1) reduced the degree of induction from 5.5-fold with the intact Axin2 sequences down to 1.9-fold (Ax2-Int1), confirming that intron sequences are important for the response to β-catenin but showing that promoter sequences also contribute to the response.
Truncation of 5" flanking sequences to −398 (removing site T1) had no effect on induction, showing that site T2 is sufficient to mediate twofold induction (Fig. 5). Because of the concern that single point mutations may not fully eliminate Tcf/LEF binding, we next introduced a triple mutation into site T2, in three constructs lacking intron 1. In all three cases, this caused a major reduction in the degree of induction (Fig. 5).
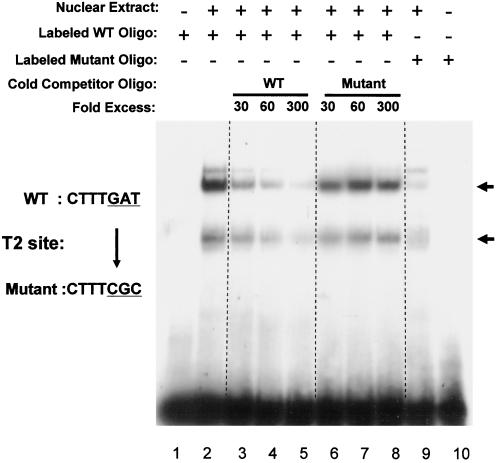
To obtain further evidence that the consensus site T2 is a bona fide Tcf/LEF binding site, nuclear extracts from 293T cells were analyzed in EMSAs using oligonucleotides containing the wild-type or mutated T2 site. The wild-type oligonucleotide showed two major shifted bands, possibly shifted by Tcf (lower band) and the Tcf-β-catenin complex (upper band), while an oligonucleotide with a triple mutation in the Tcf/LEF consensus site (the same mutation used in the reporter assays) showed greatly reduced binding of these proteins (Fig. 6). Furthermore, while wild-type unlabeled competitor oligonucleotide eliminated most of the binding, mutant competitor oligonucleotide had little if any effect on binding to the wild-type oligonucleotide.
FIG. 6.
Mutation of a potential Tcf/LEF binding site abolishes shifted DNA-protein complexes in an EMSA. Duplex oligonucleotides containing wild-type (WT) or mutated T2 sites (from 5"-CTTTGAT-3" to 5"-CTTTCGC-3") were incubated with 293T cell nuclear extract (lanes 2 to 9), and DNA-protein complexes were separated in 5% native polyacrylamide gels. No shifted band was detected without nuclear extract (lanes 1 and 10). Mutation of site T2 caused a clear reduction in the shifted bands (compare lanes 2 and 9). An increasing amount of wild-type cold oligonucleotide efficiently eliminates the shifted bands while mutated oligonucleotide does not (compare lanes 3 to 5 with lanes 6 to 8).
Overall, the analysis of mutations in reporter assays, the demonstration that dominant-negative Tcf-4E inhibits β-catenin-induced expression (Fig. 4E), and the EMSA data strongly suggest that Axin2 transcription is controlled by Tcf/LEF factors.
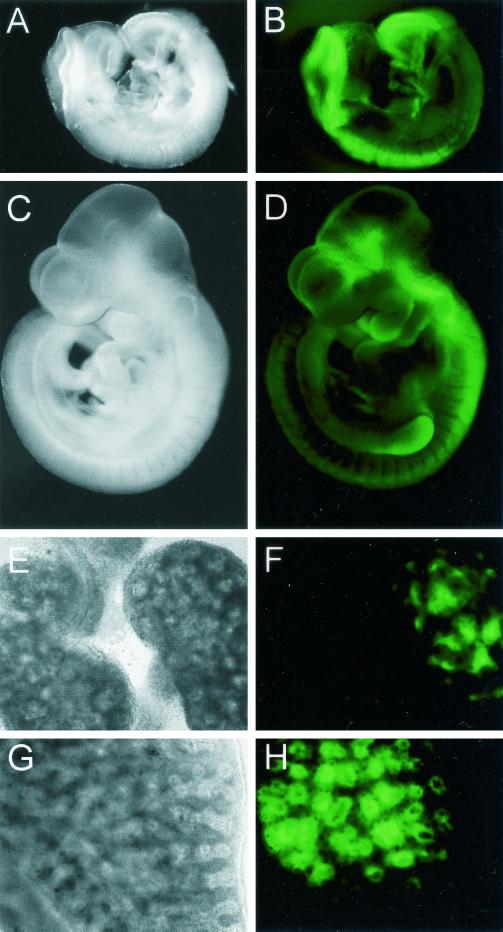
Expression of d2EGFP driven by the Axin2 promoter/intron 1 in transgenic mice mimics endogenous Axin2 expression.
We next tested whether the Axin2 promoter/intron 1 sequences that mediate Wnt/β-catenin induction in cell lines are also sufficient to direct expression in transgenic mice, in the pattern characteristic of endogenous Axin2. To monitor dynamic changes of Axin2 expression during embryonic development, we used d2EGFP, a form of green fluorescent protein with reduced stability, as a reporter for transcriptional activity. Three independent lines of Ax2/d2EGFP transgenic mice were obtained, and the embryos from each line showed a similar pattern of d2EGFP expression. As shown in Fig. 7,d2EGFP was strongly expressed in the dorsal aspect of the head folds and the posterior neural tube at E8.5 (Fig. 7A and B), which is similar to the distribution of endogenous Axin2 mRNA at this stage (Fig. 1B). At E9.5 to 10.5, Axin2 expression is most prominent in the dorsal neural tube, the branchial arches, and the limb buds (Fig. 1C), and d2EGFP was expressed in all of these regions (Fig. 7C and D). At later stages, Axin2 is expressed in several developing organs including the lung endoderm and the ureteric bud tips in the kidney (Fig. 1) (reference (1) and data not shown). d2EGFP showed a similar pattern of expression in the fetal kidney (Fig. 7E and F) and lung (Fig. 7G and H). Overall, the expression pattern of d2EGFP in transgenic mice indicates that the 5.6-kb Axin2 DNA fragment that we tested includes most if not all of the regulatory sequences required for proper Axin2 expression in vivo.
FIG. 7.
Expression of Ax2/d2EGFP in transgenic embryos and developing organs is similar to the endogenous expression of Axin2. (A and C) Dark field; (E and G) bright field; (B, D, F, and H) d2EGFP fluorescence. (A and B) At E8.5, d2EGFP driven by the Axin2 promoter/intron 1 is strongly expressed in the head folds, tail bud region, and dorsal neural tube. (C and D) At E10.5, d2EGFP is expressed along the full length of the dorsal neural tube, as well as in the branchial arches and limb buds and in regions of the brain. Endogenous Axin2 is also expressed in the brain at a similar stage, although the sites have not been well characterized (Zhang and Costantini, unpublished). (E and F) Expression of d2EGFP in the ureteric bud tips of a transgenic E14.5 kidney (right) but not in a wild-type control kidney (left). (G and H) Expression of d2EGFP in E14.5 transgenic lung epithelium.
DISCUSSION
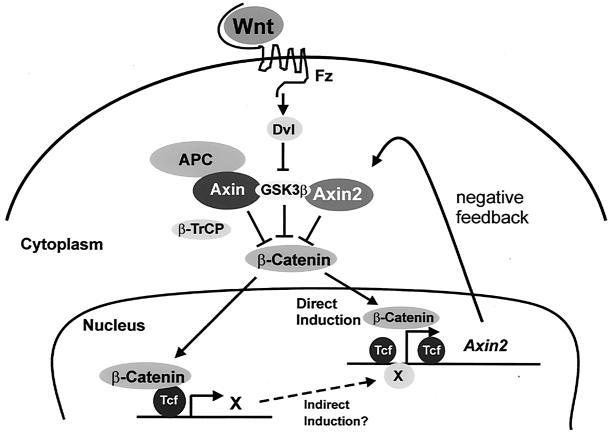
Both Axin and its homolog Axin2/Conductin/Axil are believed to act as scaffold proteins, which bind several components of the canonical Wnt signal transduction pathway and promote the phosphorylation of β-catenin by GSK-3 and its consequent degradation. Thus, both Axin and Axin2 appear to serve as negative regulators of the signaling pathway, and consistent with this role, both have been shown to act as tumor suppressors in humans. Axin, which is expressed ubiquitously, is believed to act as a constitutive modulator of the Wnt pathway and is thus a key component of the mechanism that prevents spontaneous signal transduction in the absence of a Wnt signal. Here, we show that Axin2 plays a complementary role, being transcriptionally induced following the reception of a Wnt/β-catenin signal. This property of Axin2 may create a negative feedback loop to silence the signaling pathway following transduction of the Wnt signal (Fig. 8).
FIG. 8.
A model for the role of Axin2 in Wnt signal transduction. Upon transduction of a Wnt signal, transcription of the Axin2 gene is induced via the β-catenin/Tcf pathway. Our point mutation analysis, as well as data from cotransfection of DN-Tcf, suggests that Axin2 expression is controlled by Tcf/LEF factors. In addition to direct induction, Axin2 may be further induced by an indirect mechanism, in which Tcf enhances the expression of unknown transcription factor(s) X, which in turn enhances Axin2 expression through a mechanism not requiring the Tcf/LEF binding sites. Thus, Axin2 may provide a negative feedback loop for the down regulation of β-catenin to normal levels after a Wnt signal.
The evidence that first led us to test this hypothesis was the apparent overlap between several sites of Axin2 and Wnt gene expression during embryogenesis and organogenesis. For example, the expression of Axin2 in the primitive streak and the dorsal neural tube (Fig. 1A to F) was reminiscent of the expression patterns of Wnt-1, Wnt-3, and Wnt-3a (24, 29). Another notable site of Axin2 expression was the intervillus epithelium of the fetal gut, a region that gives rise to the crypts in which the gut stem cells arise and proliferate. The proliferation of these stem cells is known to be regulated by the Wnt/β-catenin/Tcf4 pathway, and mutations in APC or β-catenin (2, 31), as well as Axin2 itself (25), predispose the cells to oncogenic transformation leading to colon cancer. Therefore, the strong expression of Axin2 in these cells might be explained if the Axin2 gene were itself a target of this signaling pathway. It is also important to point out that the overlap between Axin2 and Wnt expression is only partial: there are many sites where members of the Wnt pathway (including those believed to activate the canonical Wnt pathway) are expressed where Axin2 is apparently not expressed. Conversely, it is not clear that every site of Axin2 expression corresponds to a site where the Wnt/β-catenin/Tcf pathway is active. Indeed, in the adult mouse as well as the adult human, Axin2 mRNA is found in most if not all tissues (T. Zhang and F. Costantini, unpublished data), and it seems unlikely that all of these adult expression sites are maintained by Wnt signaling. Therefore, the Wnt pathway appears to be only one of perhaps several mechanisms by which transcription of Axin2 can be activated and/or maintained.
In the mouse mammary gland cell line C57MG, we found that the level of endogenous Axin2 mRNA was strongly induced following expression of the chimeric Wnt1/5. Similarly, in rat fetal gut endoderm cocultured with cells expressing Wnt-1, the endogenous rat Axin2 mRNA was strongly induced. These experiments clearly showed that the expression of Axin2 can be induced by Wnts both in cell lines and in fetal tissue. Further support for this conclusion comes from recent findings of Lustig et al. (25a), who have observed that expression of Conductin (Axin2) is highly elevated in several tumors and tumor cell lines that are induced by β-catenin/Wnt signaling.
While these findings did not distinguish between direct and indirect induction, several observations strongly argue for a direct effect. First, the time required for induction of Axin2 expression following treatment of 293T cells with LiCl (Fig. 2B and C) is within the range observed for several other Wnt/β-catenin target genes whose promoters contain Tcf/LEF sites (2 to 8 h [J. Willert and R. Nusse, personal communication]). Second, when fused to a luciferase reporter, a 5.6-kb mouse Axin2 DNA fragment including all eight Tcf/LEF sites was sufficient to mediate Wnt- or β-catenin-inducible expression of luciferase in 293T cells. This indicates that the induction of Axin2 is mediated at the transcriptional level. Furthermore, this induction could be largely inhibited by cotransfection of a dominant-negative mutant form of Tcf-4E, showing that the transcriptional activation is mediated by Tcf/LEF factors. Third, introduction of a single point mutation into each of the eight Tcf/LEF sites, in various combinations, reduced the degree of induction by β-catenin, confirming that it is mediated, at least in part, through a subset of the Tcf/LEF consensus sites. While the failure of these mutations to eliminate entirely the induction by β-catenin suggests that part of the induction might be indirect, it is also possible that the single point mutations were insufficient to eliminate the function of the Tcf/LEF motifs. For this reason, we examined the effects of a triple mutation in site T2 in the Axin2 promoter using reporter and protein binding assays. While a 398-bp promoter fragment including site T2 mediated 2.3-fold induction of the reporter, mutation of site T2 eliminated most of the induction. Fourth, in an EMSA, the ability of an oligonucleotide including site T2 to bind proteins from 293T cell extracts was largely eliminated by the same mutation. Together, these results strongly suggest that at least some of the consensus Tcf/LEF sites in the promoter and first intron of the Axin2 gene are responsible for direct transcriptional regulation by Tcf/LEF factors.
The ability of the Axin2 promoter and first intron to direct tissue-specific expression of a d2EGFP reporter was tested in transgenic mice, and the transgene was found to largely recapitulate the expression of endogenous Axin2 during embryogenesis and organogenesis. This suggests that the Tcf/LEF sites and the surrounding conserved sequences that we have identified play a role in the in vivo expression pattern of Axin2, although the role of specific sequences for tissue-specific expression remains to be tested. The transgenic mice that we have produced will also be useful for studies of the regulation of Axin2, for example, crossing the mice with those with mutations in various Wnt pathway components.
The most important implication of our studies is that Axin2 appears to participate in a negative feedback loop, which could serve to limit the duration or intensity of a Wnt-initiated signal. Such negative feedback loops are critical for the precise control of signaling during development and have been identified in many of the well-characterized signaling pathways (7). There are several other examples of mechanisms that could provide additional negative feedback loops in the Wnt pathway. First, it has been shown that Tcf1 is a target gene for Tcf4 in epithelial cells and that the most abundant Tcf1 isoforms lack a β-catenin interaction domain, so that Tcf1 might serve as a feedback repressor of β-catenin/Tcf4 target genes (33). Second, β-TrCP, which targets the ubiquitination and degradation of β-catenin, is itself induced (through a posttranscriptional mechanism) by β-catenin/Tcf signaling, causing accelerated degradation of β- catenin (39). Third, the protein naked cuticle, which is induced by wingless in Drosophila melanogaster, acts directly through Dsh to limit wingless activity (34, 44, 49). Therefore, the induction of Axin2 appears to be one of several mechanisms for feedback regulation of β-catenin upon activation of the Wnt pathway.
Acknowledgments
We thank Zaiqi Wu for excellent technical assistance, Jan Kitajewski for comments on the manuscript, and Walter Birchmeier for the anti-Conduction (Axin2) antibody.
This work was supported by grants from the NIH (to F.C.), the Association pour la Recherche sur le Cancer (to J.-N.F.), and the Korean Ministry of Science and Technology (00-J-LF-01-B-78, to C.-K.J.). Claire Domon received a fellowship of the Ligue Nationale contre le Cancer.
REFERENCES
- 1.Behrens, J., B. A. Jerchow, M. Wurtele, J. Grimm, C. Asbrand, R. Wirtz, M. Kuhl, D. Wedlich, and W. Birchmeier. 1998. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science 280:596-599. [DOI] [PubMed] [Google Scholar]
- 2.Bienz, M., and H. Clevers. 2000. Linking colorectal cancer to Wnt signaling. Cell 103:311-320. [DOI] [PubMed] [Google Scholar]
- 3.Cadigan, K. M., and R. Nusse. 1997. Wnt signaling: a common theme in animal development. Genes Dev. 11:3286-3305. [DOI] [PubMed] [Google Scholar]
- 4.Duluc, I., J. N. Freund, C. Leberquier, and M. Kedinger. 1994. Fetal endoderm primarily holds the temporal and positional information required for mammalian intestinal development. J. Cell Biol. 126:211-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eastman, Q., and R. Grosschedl. 1999. Regulation of LEF-1/TCF transcription factors by Wnt and other signals. Curr. Opin. Cell Biol. 11:233-240. [DOI] [PubMed] [Google Scholar]
- 6.Fagotto, F., E. Jho, L. Zeng, T. Kurth, T. Joos, C. Kaufmann, and F. Costantini. 1999. Domains of axin involved in protein-protein interactions, wnt pathway inhibition, and intracellular localization. J. Cell Biol. 145:741-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman, M. 2000. Feedback control of intercellular signalling in development. Nature 408:313-319. [DOI] [PubMed] [Google Scholar]
- 8.Gluecksohn-Schoenheimer, S. 1949. The effects of a lethal mutation responsible for duplications and twinning in mouse embryos. J. Exp. Zool. 110:47-76. [DOI] [PubMed] [Google Scholar]
- 9.Hart, M. J., R. de los Santos, I. N. Albert, B. Rubinfeld, and P. Polakis. 1998. Downregulation of beta-catenin by human Axin and its association with the APC tumor suppressor, beta-catenin and GSK3 beta. Curr. Biol. 8:573-581. [DOI] [PubMed] [Google Scholar]
- 10.Hlsken, J., and J. Behrens. 2000. The wnt signalling pathway. J. Cell Sci. 113:3545-3546. [DOI] [PubMed] [Google Scholar]
- 10a.Hogan, B. R. Beddington, F. Costantini, and E. Lacy. 1994. Manipulating the mouse embryo: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 11.Hogan, B. L., J. Grindley, S. Bellusci, N. R. Dunn, H. Emoto, and N. Itoh. 1997. Branching morphogenesis of the lung: new models for a classical problem. Cold Spring Harbor Symp. Quant. Biol. 62:249-256. [PubMed] [Google Scholar]
- 12.Hsu, W., L. Zeng, and F. Costantini. 1999. Identification of a domain of axin that binds to the serine/threonine protein phosphatase 2A and a self-binding domain. J. Biol. Chem. 274:3439-3445. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda, S., S. Kishida, H. Yamamoto, H. Murai, S. Koyama, and A. Kikuchi. 1998. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta- catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO J. 17:1371-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Itoh, K., V. E. Krupnik, and S. Y. Sokol. 1998. Axis determination in Xenopus involves biochemical interactions of axin, glycogen synthase kinase 3 and beta-catenin. Curr. Biol. 8:591-594. [DOI] [PubMed] [Google Scholar]
- 15.Jho, E., S. Lomvardas, and F. Costantini. 1999. A GSK3beta phosphorylation site in axin modulates interaction with beta-catenin and Tcf-mediated gene expression. Biochem. Biophys. Res. Commun. 266:28-35. [DOI] [PubMed] [Google Scholar]
- 16.Julius, M. A., S. D. Rai, and J. Kitajewski. 1999. Chimeric Wnt proteins define the amino-terminus of Wnt-1 as a transformation-specific determinant. Oncogene 18:149-156. [DOI] [PubMed] [Google Scholar]
- 17.Julius, M. A., B. Schelbert, W. Hsu, E. Fitzpatrick, E. Jho, F. Fagotto, F. Costantini, and J. Kitajewski. 2000. Domains of axin and disheveled required for interaction and function in wnt signaling. Biochem. Biophys. Res. Commun. 276:1162-1169. [DOI] [PubMed] [Google Scholar]
- 18.Kikuchi, A. 1999. Roles of Axin in the Wnt signalling pathway. Cell. Signal. 11:777-788. [DOI] [PubMed] [Google Scholar]
- 19.Kishida, S., H. Yamamoto, S. Hino, S. Ikeda, M. Kishida, and A. Kikuchi. 1999. DIX domains of Dvl and Axin are necessary for protein interactions and their ability to regulate β-catenin stability. Mol. Cell. Biol. 19:4414-4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kishida, S., H. Yamamoto, S. Ikeda, M. Kishida, I. Sakamoto, S. Koyama, and A. Kikuchi. 1998. Axin, a negative regulator of the wnt signaling pathway, directly interacts with adenomatous polyposis coli and regulates the stabilization of beta-catenin. J. Biol. Chem. 273:10823-10826. [DOI] [PubMed] [Google Scholar]
- 21.Kispert, A., S. Vainio, L. Shen, D. H. Rowitch, and A. P. McMahon. 1996. Proteoglycans are required for maintenance of Wnt-11 expression in the ureter tips. Development 122:3627-3637. [DOI] [PubMed] [Google Scholar]
- 22.Li, L., H. Yuan, C. D. Weaver, J. Mao, G. H. Farr III, D. J. Sussman, J. Jonkers, D. Kimelman, and D. Wu. 1999. Axin and Frat1 interact with dvl and GSK, bridging Dvl to GSK in Wnt-mediated regulation of LEF-1. EMBO J. 18:4233-4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lickert, H., C. Domon, G. Huls, C. Wehrle, I. Duluc, H. Clevers, B. I. Meyer, J. N. Freund, and R. Kemler. 2000. Wnt/(beta)-catenin signaling regulates the expression of the homeobox gene Cdx1 in embryonic intestine. Development 127:3805-3813. [DOI] [PubMed] [Google Scholar]
- 24.Liu, P., M. Wakamiya, M. J. Shea, U. Albrecht, R. R. Behringer, and A. Bradley. 1999. Requirement for Wnt3 in vertebrate axis formation. Nat. Genet. 22:361-365. [DOI] [PubMed] [Google Scholar]
- 25.Liu, W., X. Dong, M. Mai, R. S. Seelan, K. Taniguchi, K. K. Krishnadath, K. C. Halling, J. M. Cunningham, C. Qian, E. Christensen, P. C. Roche, D. I. Smith, and S. N. Thibodeau. 2000. Mutations in AXIN2 cause colorectal cancer with defective mismatch repair by activating beta- catenin/TCF signalling. Nat. Genet. 26:146-147. [DOI] [PubMed] [Google Scholar]
- 25a.Lustig, B., B. Jerchow, M. Sachs, S. Weiler, T. Pietsch, U. Karsten, M. van de Wetering, H. Clevers, P. M. Schlag, W. Birchmeier, and J. Behrens. 2001. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol. Cell. Biol. 22:1184-1193. [DOI] [PMC free article] [PubMed]
- 26.Mai, M., C. Qian, A. Yokomizo, D. I. Smith, and W. Liu. 1999. Cloning of the human homolog of conductin (AXIN2), a gene mapping to chromosome 17q23-q24. Genomics 55:341-344. [DOI] [PubMed] [Google Scholar]
- 27.Miller, J. R., and R. T. Moon. 1996. Signal transduction through β-catenin and specification of cell fate during embryogenesis. Genes Dev. 10:2527-2539. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura, T., F. Hamada, T. Ishidate, K. Anai, K. Kawahara, K. Toyoshima, and T. Akiyama. 1998. Axin, an inhibitor of the Wnt signalling pathway, interacts with beta-catenin, GSK-3beta and APC and reduces the beta-catenin level. Genes Cells 3:395-403. [DOI] [PubMed] [Google Scholar]
- 29.Parr, B. A., M. J. Shea, G. Vassileva, and A. P. McMahon. 1993. Mouse Wnt genes exhibit discrete domains of expression in the early embryonic CNS and limb buds. Development 119:247-261. [DOI] [PubMed] [Google Scholar]
- 30.Perry, W. L. I., T. J. Vasicek, J. J. Lee, J. M. Rossi, L. Zeng, T. Zhang, S. M. Tilghman, and F. Costantini. 1995. Phenotypic and molecular analysis of a transgenic insertional allele of the mouse Fused locus. Genetics 141:321-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polakis, P. 2000. Wnt signaling and cancer. Genes Dev. 14:1837-1851. [PubMed] [Google Scholar]
- 32.Roose, J., and H. Clevers. 1999. TCF transcription factors: molecular switches in carcinogenesis. Biochim. Biophys. Acta 1424:M23-M37. [DOI] [PubMed] [Google Scholar]
- 33.Roose, J., G. Huls, M. van Beest, P. Moerer, K. van der Horn, R. Goldschmeding, T. Logtenberg, and H. Clevers. 1999. Synergy between tumor suppressor APC and the beta-catenin-Tcf4 target Tcf1. Science 285:1923-1926. [DOI] [PubMed] [Google Scholar]
- 34.Rousset, R., J. A. Mack, K. A. Wharton, Jr., J. D. Axelrod, K. M. Cadigan, M. P. Fish, R. Nusse, and M. P. Scott. 2001. Naked cuticle targets dishevelled to antagonize Wnt signal transduction. Genes Dev. 15:658-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakanaka, C., J. B. Weiss, and L. T. Williams. 1998. Bridging of beta-catenin and glycogen synthase kinase-3beta by Axin and inhibition of beta-catenin-mediated transcription. Proc. Natl. Acad. Sci. USA 95:3020-3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakanaka, C., and L. T. Williams. 1999. Functional domains of axin. Importance of the C terminus as an oligomerization domain. J. Biol. Chem. 274:14090-14093. [DOI] [PubMed] [Google Scholar]
- 37.Satoh, S., Y. Daigo, Y. Furukawa, T. Kato, N. Miwa, T. Nishiwaki, T. Kawasoe, H. Ishiguro, M. Fujita, T. Tokino, Y. Sasaki, S. Imaoka, M. Murata, T. Shimano, Y. Yamaoka, and Y. Nakamura. 2000. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat. Genet. 24:245-250. [DOI] [PubMed] [Google Scholar]
- 38.Smalley, M. J., E. Sara, H. Paterson, S. Naylor, D. Cook, H. Jayatilake, L. G. Fryer, L. Hutchinson, M. J. Fry, and T. C. Dale. 1999. Interaction of Axin and Dvl-2 proteins regulates Dvl-2-stimulated TCF-dependent transcription. EMBO J. 18:2823-2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spiegelman, V. S., T. J. Slaga, M. Pagano, T. Minamoto, Z. Ronai, and S. Y. Fuchs. 2000. Wnt/beta-catenin signaling induces the expression and activity of betaTrCP ubiquitin ligase receptor. Mol. Cell 5:877-882. [DOI] [PubMed] [Google Scholar]
- 40.Stappenbeck, T. S., M. H. Wong, J. R. Saam, I. U. Mysorekar, and J. I. Gordon. 1998. Notes from some crypt watchers: regulation of renewal in the mouse intestinal epithelium. Curr. Opin. Cell Biol. 10:702-709. [DOI] [PubMed] [Google Scholar]
- 41.Stark, K., S. Vainio, G. Vassileva, and A. P. McMahon. 1994. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 372:679-683. [DOI] [PubMed] [Google Scholar]
- 42.Tetsu, O., and F. McCormick. 1999. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398:422-426. [DOI] [PubMed] [Google Scholar]
- 43.van de Wetering, M., R. Cavallo, D. Dooijes, M. van Beest, J. van Es, J. Loureiro, A. Ypma, D. Hursh, T. Jones, A. Bejsovec, M. Peifer, M. Mortin, and H. Clevers. 1997. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 88:789-799. [DOI] [PubMed] [Google Scholar]
- 44.Wharton, K. A., Jr., G. Zimmermann, R. Rousset, and M. P. Scott. 2001. Vertebrate proteins related to Drosophila Naked Cuticle bind Dishevelled and antagonize Wnt signaling. Dev. Biol. 234:93-106. [DOI] [PubMed] [Google Scholar]
- 45.Wilkinson, D. G. 1992. Whole mount in situ hybridization of vertebrate embryos, p. 75-83. In D. G. Wilkinson (ed.), In situ hybridization: a practical approach. IRL Press, Oxford, United Kingdom.
- 46.Willert, K., S. Shibamoto, and R. Nusse. 1999. Wnt-induced dephosphorylation of axin releases beta-catenin from the axin complex. Genes Dev. 13:1768-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamamoto, H., S. Kishida, M. Kishida, S. Ikeda, S. Takada, and A. Kikuchi. 1999. Phosphorylation of Axin, a Wnt signal negative regulator, by glycogen synthase kinase-3beta regulates its stability. J. Biol. Chem. 274:10681-10684. [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto, H., S. Kishida, T. Uochi, S. Ikeda, S. Koyama, M. Asashima, and A. Kikuchi. 1998. Axil, a member of the Axin family, interacts with both glycogen synthase kinase 3β and β-catenin and inhibits axis formation of Xenopus embryos. Mol. Cell. Biol. 18:2867-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yan, D., J. B. Wallingford, T. Q. Sun, A. M. Nelson, C. Sakanaka, C. Reinhard, R. M. Harland, W. J. Fantl, and L. T. Williams. 2001. Cell autonomous regulation of multiple Dishevelled-dependent pathways by mammalian Nkd. Proc. Natl. Acad. Sci. USA 98:3802-3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeng, L., F. Fagotto, T. Zhang, W. Hsu, T. J. Vasicek, W. L. I. Perry, J. J. Lee, S. M. Tilghman, B. M. Gumbiner, and F. Costantini. 1997. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell 90:181-192. [DOI] [PubMed] [Google Scholar]