Abstract
Objective
To revise and expand the 1996 Osteoporosis Society of Canada clinical practice guidelines for the management of osteoporosis, incorporating recent advances in diagnosis, prevention and management of osteoporosis, and to identify and assess the evidence supporting the recommendations.
Options
All aspects of osteoporosis care and its fracture complications — including classification, diagnosis, management and methods for screening, as well as prevention and reducing fracture risk — were reviewed, revised as required and expressed as a set of recommendations.
Outcomes
Strategies for identifying and evaluating those at high risk; the use of bone mineral density and biochemical markers in diagnosis and assessing response to management; recommendations regarding nutrition and physical activity; and the selection of pharmacologic therapy for the prevention and management of osteoporosis in men and women and for osteoporosis resulting from glucocorticoid treatment.
Evidence
All recommendations were developed using a justifiable and reproducible process involving an explicit method for the evaluation and citation of supporting evidence.
Values
All recommendations were reviewed by members of the Scientific Advisory Council of the Osteoporosis Society of Canada, an expert steering committee and others, including family physicians, dietitians, therapists and representatives of various medical specialties involved in osteoporosis care (geriatric medicine, rheumatology, endocrinology, obstetrics and gynecology, nephrology, radiology) as well as methodologists from across Canada.
Benefits, harm and costs
Earlier diagnosis and prevention of fractures should decrease the medical, social and economic burdens of this disease.
Recommendations
This document outlines detailed recommendations pertaining to all aspects of osteoporosis. Strategies for identifying those at increased risk (i.e., those with at least one major or 2 minor risk factors) and screening with central dual-energy x-ray absorptiometry at age 65 years are recommended. Bisphosphonates and raloxifene are first-line therapies in the prevention and treatment of postmenopausal osteoporosis. Estrogen and progestin/progesterone is a first-line therapy in the prevention and a second-line therapy in the treatment of postmenopausal osteoporosis. Nasal calcitonin is a second-line therapy in the treatment of postmenopausal osteoporosis. Although not yet approved for use in Canada, hPTH(1-34) is expected to be a first-line treatment for postmenopausal women with severe osteoporosis. Ipriflavone, vitamin K and fluoride are not recommended. Bisphosphonates are the first-line therapy for the prevention and treatment of osteoporosis in patients requiring prolonged glucocorticoid therapy and for men with osteoporosis. Nasal or parenteral calcitonin is a first-line treatment for pain associated with acute vertebral fractures. Impact-type exercise and age-appropriate calcium and vitamin D intake are recommended for the prevention of osteoporosis. Validation: All recommendations were graded according to the strength of the evidence; where the evidence was insufficient and recommendations were based on consensus opinion alone, this is indicated. These guidelines are viewed as a work in progress and will be updated periodically in response to advances in this field.
Osteoporosis is a major public health problem in Canada (and worldwide) and its prevalence is increasing. In Canada, approximately 1 in 4 women and 1 in 8 men have osteoporosis.1 Because some 25% of the population will be over 65 years of age by 2041, the incidence of osteoporosis is expected to rise steeply over the next few decades.2 The public health and clinical importance of osteoporosis lies in the fractures associated with the disease. According to conservative estimates, a 50-year-old Caucasian woman has a remaining lifetime risk of 40% for hip, vertebra or wrist fractures.3
This morbidity burden has considerable medical, social and financial implications. Many vertebral fractures are occult and asymptomatic; however, an increased mortality rate is associated with them, as for hip fractures.4,5,6 Mortality rate is 20% higher on average within 1 year of a hip fracture.7 Put another way, for women, the 1-in-6 lifetime risk of hip fracture is greater than the 1-in-9 risk of developing breast cancer, and the death rate associated with hip fracture is higher.8,9 Moreover, 50% of women who sustain a hip fracture do not return to their previous functional state and become dependent on others for daily activities. About 20% require long-term care.7
The greatest direct expenditures associated with osteoporosis arise from treatment of fractures and their sequelae. Although difficult to assess accurately, these costs are substantial. According to estimates,10 in 1993 the total acute care cost for osteoporosis (admission to hospital, outpatient care and drug therapy) was over Can$1.3 billion. Over the past decade, these costs have increased and in the United States have risen to Can$17–20 billion a year. These burgeoning costs may outstrip the resources designated to deal with osteoporotic fractures (i.e., orthopedic surgeons, operating room time and space, rehabilitation programs, drug budgets).
Although osteoporotic fractures are an important cause of morbidity, disability and mortality, they are preventable. With this in mind, the Scientific Advisory Council (SAC) of the Osteoporosis Society of Canada (OSC) set itself the task of updating and expanding the 1996 consensus statements1,11 into evidence-based guidelines.
Methods
Process
In 1999, in consultation with its SAC, the OSC created a Guidelines Steering Committee and identified the following areas related to osteoporosis for review: risk factors, diagnosis, nutrition, physical activity, drug therapies and alternative or complementary therapies. The task of the steering committee, which was made up of members of the SAC, was to direct the organization of the guidelines. Sixty-five stakeholders were recruited to participate in the process; they included additional members of the SAC, family physicians, dietitians, therapists and representatives of the various medical specialties involved in osteoporosis care (geriatric medicine, rheumatology, endocrinology, obstetrics and gynecology, nephrology and radiology), and methodologists from across Canada. These stakeholders were divided into section committees, each comprising 4–9 members and a chair. Each section committee was to review the literature and develop recommendations in one of the identified areas.
The section committees identified key questions within their review area to be addressed in the guidelines. A decision was made to focus on management of primary osteoporosis. However, although no formal review of the literature was undertaken regarding risk factors for, or management of, secondary osteoporosis, the committees chose to review certain papers regarded as pivotal in this area — in particular, trials evaluating glucocorticoid-induced osteoporosis. In addition, the search for risk factors focused on risk factors for fragility fracture, the most important clinical outcome of osteoporosis. Therefore, no formal review of the literature was undertaken regarding risk factors for low bone mineral density (BMD).
Under the direction of the steering committee, the section committees carried out an extensive literature search for articles relevant to each of the key questions. Searches for both review and original articles were carried out in the following databases: Medline, Embase, HealthStar, Cancerlit, Cinahl, Grateful Med, Toxline, Psychinfo and the Cochrane Collaboration. All review articles were scanned for additional original papers. Each database was searched as far back as records existed and forward to May 2000. In addition, some singularly important and pivotal studies published after our cut-off date were selected and addressed in these guidelines. All abstracts retrieved were reviewed by the chair and one other member of the appropriate section committee to determine their applicability to each question. If an abstract or title was deemed applicable, the full article was obtained, numbered and distributed to 2 or 3 committee members for review.
A total of 89,804 abstracts were retrieved; from these, 6941 full articles were obtained for review. Two or 3 reviewers independently reviewed each article using a standardized form. Each article was assigned a level of evidence based on the question addressed and the design of the study (Table 1).12 If the reviewers did not achieve consensus, the article was reviewed again. If there was still no consensus, members of the steering committee were asked to review the article and make a decision.
Table 1
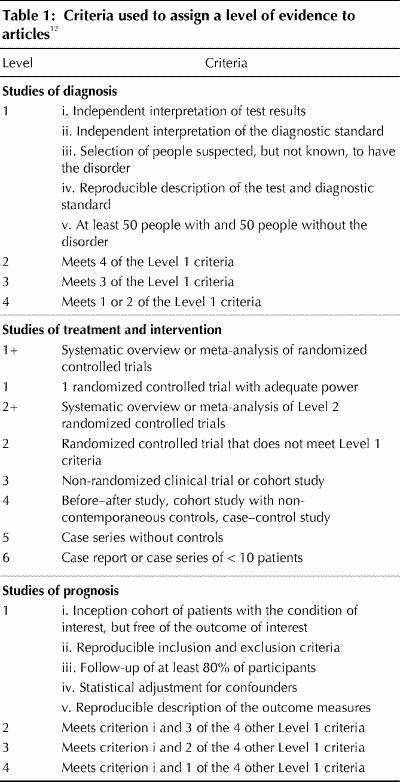
The principles used for developing these guidelines, assigning levels of evidence to the relevant articles and making and grading recommendations were drawn from the guidelines literature.13,14
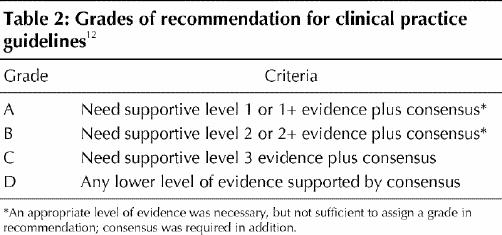
Once all key articles had been reviewed and assigned a level of evidence, each section committee reviewed the data and developed recommendations. Recommendations were graded according to the system used to grade recommendations for diabetes,12 which incorporates both level of evidence and expert consensus (Table 2). Recommendations were assigned a grade of D when they were based only on committee consensus in the absence of clear supporting evidence or when evidence was weak. Before a final grade was assigned, all recommendations were reviewed by the steering committee, which included several methodologists who were neither directly involved in the initial assessment of evidence nor with the grading of the recommendations. If appropriate, the assigned level of evidence or grade of recommendation was modified on the basis of this final assessment.
Table 2
Definitions
Osteoporosis was defined at a 1993 consensus conference as “a systemic skeletal disease characterized by low bone mass and micro-architectural deterioration of bone tissue with a resultant increase in fragility and risk of fracture.”15 Recently a United States National Institutes of Health consensus conference modified this definition as follows: “a skeletal disorder characterized by compromised bone strength predisposing a person to an increased risk of fracture. Bone strength reflects the integration of 2 main features: bone density and bone quality.”16 Probably the only clinically applicable index of bone quality at present is a patient's history of a fragility fracture. In the absence of methods of measuring bone quality, the diagnosis of osteoporosis tends to be made on the basis of low bone density. (Note: The World Health Organization (WHO)17 defines fragility fracture as “a fracture caused by injury that would be insufficient to fracture normal bone: the result of reduced compressive and/or torsional strength of bone.” Clinically, a fragility fracture may be defined as one that occurs as a result of minimal trauma, such as a fall from a standing height or less, or no identifiable trauma.)
In interpreting BMD results, the OSC decided to adopt the widely used WHO18,19 study group's definitions, which are based on a comparison of a patient's BMD with the mean for a normal young adult population of the same sex and race. The patient is assigned a “T-score,” which is the number of standard deviations above or below the mean BMD for normal young adults as follows:
1. Normal BMD is defined as a T-score between +2.5 and –1.0 (i.e., the patient's BMD is between 2.5 standard deviations (SDs) above the young adult mean and one SD below the young adult mean).
2. Osteopenia (low BMD) is associated with a T-score between –1.0 and –2.5, inclusive. Osteopenia is also a term used by radiologists to indicate that the bones on a plain x-ray film appear to be of decreased mineral content.
3. Osteoporosis is defined as a T-score lower than –2.5.
The WHO study group added a 4th category “severe osteoporosis” to describe patients whose T-score is below –2.5 and who also have suffered a fragility fracture. The recommendations concerning risk factors in this document should make the importance of fracture history in assessing a patient for osteoporosis very clear.
The term “efficacious” is used in reference to evidence from a randomized controlled trial (RCT); the term “effective” refers to evidence from a nonexperimental observational study. “Perimenopause” describes the several years of change before and during the first year beyond final menstrual flow. “Menopause” refers to one or more years following the final menstrual flow. There has been a change from previous terminology about therapy with estrogen and progestin or progesterone for postmenopausal women. Approximately 10 years ago, the OSC adopted the term “ovarian hormone therapy” (OHT) to reflect its awareness that the hormonal changes during the menopause transition and menopause are entirely normal. Although the SAC maintains this position, to aid in understanding by those who use these guidelines, it was decided to use the terms “estrogen and progestin/progesterone therapy” and the abbreviation for hormone replacement therapy, “HRT.”
Finally, a recommendation that a specific therapy be used as “first-line” therapy for osteoporosis relies on Level 1 evidence for prevention of fragility fracture (mainly vertebral fracture), but this may be modified by other extenuating circumstances (e.g., unfavorable risk–benefit profile). “Second-line” therapy is the term used when adequate evidence exists for preventing loss of BMD, but inadequate data are available regarding fracture prevention or there are problems with the study or its interpretation.
Identifying those at high risk
The OSC recommends that all postmenopausal women and men over 50 years of age be assessed for the presence of risk factors for osteoporosis. The selected key risk factors should aid physicians in identifying those who require further assessment and investigation to determine whether medical intervention is needed to reduce their risk of osteoporotic (fragility) fracture. The main areas of concern are wrist, humerus, ribs, vertebral body, pelvis and hip. When a patient is identified as having a high risk for fracture, a discussion regarding treatment is recommended. Clinical judgment and the patient's preference, as well as evidence-based clinical trial data, will determine if, when and what treatment is initiated.
Selection of risk factors for clinical use
Many factors other than a low BMD have been suggested as predictors of risk of future fracture. In elderly women with no history of hip fracture, such variables as bone density, calcium intake, maternal history and even hair colour were related to the incidence of hip fracture during 4 years of follow-up.20 Important predictive factors were bone density in combination with age, fracture history, various drug treatments, weight loss and physical fitness. A review of 94 cohort studies and 76 case–control studies revealed about 80 factors considered to be related to future fracture risk.21 However, when classified according to their strength of association with fracture, only 15% had relative risk ratios greater than 2. Most were associations with primary disorders such as hyperparathyroidism or with treatments such as glucocorticoid therapy. The remaining important factors included low body weight, physical inactivity and aging.
The presence of a key risk factor should alert the physician to the need for further assessment and possibly active intervention, such as pharmacologic therapy, to prevent fracture. BMD is the best quantifiable predictor of osteoporotic fracture, and low BMD and other major risk factors combine to further increase a person's risk of fracture. Therefore, BMD should be measured in a postmenopausal woman or a man over the age of 50 with 1 of the other major risk factors for fracture.
Risk factors for osteoporotic fracture should not be considered to be independent of one another; they are additive and must be considered in the context of baseline age and sex-related risk of fracture. For example, a 55 year old with low BMD is at significantly less risk than a 75 year old with the same low BMD. A person with low BMD and a prior fragility fracture is at considerably more risk than another person with the same low BMD and no fracture.
Osteoporotic fractures occur most commonly in men and women over 65 years of age, and medical interventions have only been demonstrated to be effective in preventing fractures in populations with an average age over 65 years. However, most currently approved therapies for osteoporosis prevent or reverse bone loss when initiated at or soon after the age of 50 years. Therefore, it seems prudent to begin the identification of people at high risk for osteoporosis in their 50s, if they are willing to accept a treatment.
Four key risk factors for fracture
After reviewing the literature and considering the effect of potential confounders, we identified 4 key factors as predictors of fracture related to osteoporosis: low BMD, prior fragility fracture, age and family history of osteoporosis. Other factors that are commonly cited — weight < 57 kg, weight loss since age 25, high caffeine intake and low calcium intake — were not found to be consistent independent predictors of fracture risk, after taking into consideration age and/or BMD.
Bone mineral density
The relation between BMD and fracture risk has been calculated in a large number of studies. A meta-analysis by Marshall and colleagues22 of some of the earlier studies probably still represents the best estimate. BMD is clearly the most readily quantifiable predictor of fracture risk for those who have not yet suffered a fragility fracture. For each standard deviation of BMD below a baseline level (either mean peak bone mass or mean for the reference population of the person's age and sex), the fracture risk approximately doubles. This risk should always be viewed in the context of the person's age. A 25 year old with a low BMD (e.g., a T-score of –2.5) has a very low 10-year risk of fracture that is not appreciably greater than that of a 25 year old with a high BMD. However, a person with the same BMD at age 65 has a much higher 10-year risk of fracture.
What are the risk factors for low BMD? Or, for practical purposes, who should be selected for BMD measurements? This is a question with major economic implications. What criteria should be used to select people for BMD measurements?
Risk factors for osteoporosis are summarized in Table 3. A BMD measurement is recommended for those with at least one major or 2 minor risk factors (Figure 1; Table 3). Several attempts have been made to develop decision tools to aid physicians in selecting patients for BMD testing23,24,25 using a variety of combinations of risk factors, including age, prior fractures, estrogen use, rheumatoid arthritis, smoking, low body weight and family history of osteoporotic fracture.
Table 3
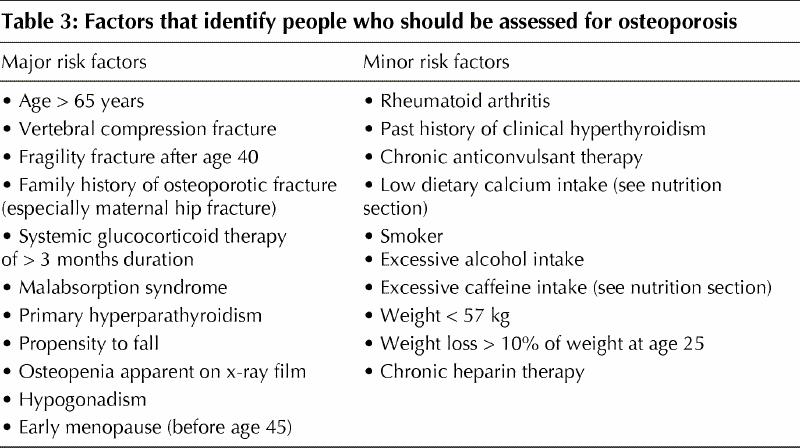
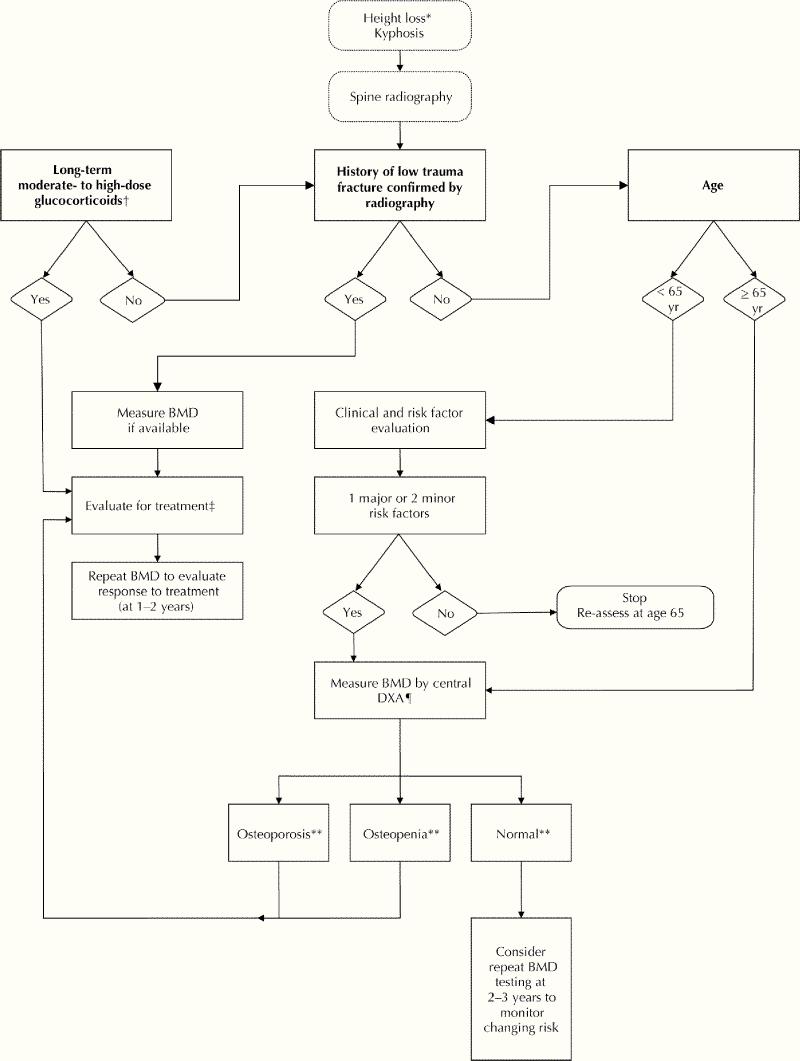
Fig. 1: Who should be tested for osteoporosis? (Note: *4 cm historical height loss; 2 cm prospective height loss [Grade D]. †Low to moderate: 2.5–7.5 mg prednisone/day; moderate to high: > 7.5 mg prednisone/day. ‡See Fig. 2. ¶Central DXA = spine and hip. **As defined by the World Health Organization.)
None of these decision tools is without problems and, if applied to the general population of postmenopausal women over the age of 50, will result in a significant number being selected for BMD measurement.26 However, all of these decision tools seem to identify at least 90% of women over 65 years of age as candidates for BMD measurement. The National Osteoporosis Foundation guidelines25 suggest it is also cost-effective to measure bone density in all women over age 65, but this recommendation was based on the assumption that patients would receive low-cost estrogen–progesterone therapy.
It is abundantly clear from epidemiology studies that age is a major risk factor for fracture. Because low BMD is also a major risk factor for fracture and BMD decreases with age, there must also be an age at which it is worthwhile to begin using BMD as a screening tool. The OSC has taken the position that BMD testing is appropriate for targeted case-finding among people under age 65 and for all women age 65 and older because of the high risk of osteoporosis and fracture after that age.
Prior fragility fracture
A prior fragility fracture places a person at increased risk for another one.20,27,28,29,30 The increased risk is 1.5- to 9.5-fold depending on age at assessment, number of prior fractures and the site of the incident fracture.27,28,30,31,32,33,34
Vertebral fractures have been best studied in this regard. The presence of a vertebral fracture increases the risk of a second vertebral fracture at least 4-fold.35,36 A study of the placebo group in a recent major clinical trial37 showed that 20% of those who experienced a vertebral fracture during the period of observation had a second vertebral fracture within 1 year. Vertebral fractures are also indicators of increased risk of fragility fractures at other sites, such as the hip.38 In a clinical trial of risedronate,38 the combination of a vertebral fracture and low bone density was associated with a doubling of the 3-year risk of hip fracture (from 3% to 6%) in women over the age of 70. Similarly, wrist fractures predict vertebral and hip fractures.30 Patients with a hip fracture are at increased risk of a second hip fracture. Pooling the results from all studies (women and men) and for all fracture sites, the risk of subsequent fracture among those with a prior fracture at any site is 2.2 times that of people without a prior fragility fracture (95% confidence interval [CI] 1.9–2.6).30
Age
Age is clearly a major contributor to fracture risk.20,26,34,39 As summarized in a recent review by Kanis and others,40 the 10-year probability of experiencing a fracture of forearm, humerus, spine or hip increases as much as 8-fold between ages 45 and 85 for women and 5-fold for men (Table 4).
Table 4

Family history of osteoporotic fracture
This factor has been best studied with respect to hip fracture. The Study of Osteoporotic Fractures,20 for example, identified a maternal history of hip fracture as a key risk factor for hip fracture in a population of elderly women. A history of hip fracture in a maternal grandmother also carries an increased risk of hip fracture.41
Although most studies have focused on the index person's mother or other female family members, genetic influence on risk of osteoporosis is multifactorial, and one should not ignore a history of osteoporotic fracture in first- or second-degree male relatives. The emphasis on the presence of osteoporotic fractures in patients' female relatives in epidemiology studies probably reflects the belief that osteoporosis is mostly a disease of women. It is now clear that osteoporosis is common in men; therefore, although the recommendations focus on hip fractures in a patient's mother or grandmother, other family members should be included during assessment of genetic contribution to osteoporosis risk.
Genetic influence on osteoporosis and BMD is extremely important; it has been estimated that heredity accounts for 50–80% of the variability in BMD.42 Genetic influences on bone have been the subject of major scientific investigations, and a number of genes have been associated with osteoporosis. However, these discoveries have not yet resulted in a clinical application in the diagnosis and treatment of osteoporosis at the practitioner level; thus, we have chosen not to review the genetics of osteoporosis in this document, beyond emphasizing the importance of a family history of osteoporosis.
Fewer studies have considered risk factors for osteoporotic fractures in men, but, as in women, age, low BMD and prior fragility fractures increase this risk. Although we do not list family history of fracture as a risk factor for men, it should not be ignored. We identified 3 studies,43,44,45 of osteoporotic fracture in men that provided Level 1 evidence for osteoporosis risk factors, but 2 of these44,45 did not focus on family history of fragility fracture.
Other major risk factors
Falls
Because fractures are frequently associated with falls, a history of falls or factors that increase the risk of falling should be included in an assessment of risk. Risk factors for falling include those associated with general frailty, such as reduced muscle strength (inability to rise from a chair without assistance), impaired balance and low body mass.20 Reduced visual acuity also increases risk of falling.20 A prospective study46 of elderly, ambulatory women identified 3 factors that were significantly predictive of risk for subsequent hip fracture and were independent of proximal femur BMD: a slower gait, difficulty in performing a heel-to-toe walk and reduced visual acuity. In a subsequent study47 in the same group of women, DXA, ultrasound, gait speed and age were equally effective in identifying women at high risk of fracture. Combination of the various predictors increased sensitivity, but not to a level that would be useful for population screening. It should be noted that falls cause fractures irrespective of whether a patient has osteoporosis, but a person who has osteoporosis is at even greater risk of fracture if he or she also has a propensity to fall.
Glucocorticoid use
Systemic glucocorticoid therapy lasting more than 2–3 months for any disorder is a major risk factor for bone loss and fracture, particularly among postmenopausal women and men over age 50.48 Most reviews and guidelines focus on a daily dose of prednisone of ≥ 7.5 mg (or equivalent) as the threshold for assessment and clinical intervention to prevent or treat glucocorticoid-induced osteoprosis.48 Two major groups of high-risk patients can be identified.
· Patients whose physician is planning to prescribe ≥7.5 mg prednisone daily for more than 3 months or has already done so should be assessed for initiation of a bone-sparing therapy (see Figure 1).
· Patients who have received glucocorticoid therapy for more than 3 months at a dose < 7.5 mg prednisone daily should be assessed for risk of osteoporosis and should at least have BMD measured, as doses slightly higher than 2.5 mg/day over a prolonged period are associated with increased fracture risk.
A retrospective cohort study49 of data derived from the United Kingdom's General Practice Research Database, compared 244 235 patients receiving prednisone with 244 235 patients matched for age, sex and type of office practice; doses between 2.5 mg/day and 7.5 mg/day were associated with an increased risk of fracture. Regardless of whether the prednisone or the disease for which the prednisone was given caused the increased risk of fracture, the lesson from this large case–control study is that patients receiving more than 2.5 mg of prednisone daily should be viewed as being at increased risk and further assessment should be carried out (at least BMD measurement).
Other conditions
A variety of clinical conditions are associated with bone loss and secondary osteoporosis, and clinicians should consider the individual patient's risk for osteoporosis. Such conditions that are likely to be encountered by a family physician include hypogonadism, early menopause (before age 45), chronic heparin therapy, malabsorption syndromes, rheumatoid arthritis and a past history of clinical hyperthyroidism. The risk factors listed in Table 3 should be used to assess people with these conditions for risk of developing osteoporosis or for the presence of osteoporosis. The identification of these people is predicated on the fact that a proven therapeutic intervention is available.
Summary statements
1. Four key factors — low bone mineral density (BMD),22 prior fragility fracture,27,28,30,31,32,33,34 age20,26,34,41 and family history of osteoporosis20,41 — stand out as predictors of fracture related to osteoporosis [Level 1].
2. Low BMD should be considered a major risk factor, but those who have suffered a vertebral fracture or other osteoporotic fracture should be considered to have osteoporosis even if their BMD is not in the range associated with osteoporosis50 [Level 1].
3. Glucocorticoid therapy is a major risk factor for osteoporosis and fracture if it is continued beyond 3 months48 even if the dose is slightly higher than 2.5 mg of prednisone daily49 [Level 2].
Recommendations
1. The major risk factors listed in Table 3 are most predictive of osteoporosis in postmenopausal women, but where applicable, are also relevant to the assessment of men over 50 years of age. These risk factors have a cumulative effect such that, for example, if a person has a low BMD in addition to a fragility fracture or is over 65 and has a BMD in the range associated with osteoporosis, he or she should be considered to be at high risk for fracture and a candidate for therapy [Grade A].
2. People receiving Ž 7.5 mg of prednisone daily for more than 3 months should be assessed for initiation of a bone-sparing therapy [Grade A].
3. People receiving more than 2.5 mg of prednisone daily should be regarded as being at increased risk of fragility fracture and require further assessment (at least BMD measurement) [Grade B].
4. People with other conditions or medications known to be associated with osteoporosis should be assessed for other risk factors. Those with low bone density or a prior fragility fracture are candidates for therapeutic intervention [Grade D].
The diagnosis of osteoporosis
Historically, osteoporosis was diagnosed late in the course of the disease when bone had become weakened to the point of fracturing. By virtue of the WHO study group definition of osteoporosis,17 diagnosis now depends on measurement of BMD. The WHO classification is based on risk of fracture, but the available evidence and, therefore, the classification was developed for use in postmenopausal Caucasian women. We were careful not to take a position on gender and racial matching. There is still debate over the reference group to be used to derive T-scores in men. The measured BMD is compared with the mean BMD in young adults of the same sex and race.
Fracture recognition
Established osteoporosis may still be recognized on radiographs of the spine. However, because some two-thirds of spinal fractures are not diagnosed clinically, one cannot rely on radiographs obtained to investigate back pain. Although there is some debate over what constitutes a vertebral fracture, deformity — the most widely used criterion — is derived from measurements of the vertical height of a vertebra at its anterior margin, centre (or mid-position) and posterior margin on lateral spine radiographs. If these measurements differ from each other or from the same measurements in the supra- or sub-adjacent vertebrae by 20% or more, the vertebra is considered to have a fracture deformity if congenital, developmental, degenerative or other causes of such deformities are excluded.33 Level 1 evidence shows that the presence of one such prevalent fracture implies a risk of further fracturing that is equal to the risk associated with a BMD of one standard deviation below the mean peak density. Better recognition and measurement of vertebral deformities presents a major opportunity for increased early recognition of osteoporosis.
Bone measurement
In general, there is a paucity of good prospective trials of diagnostic technology for measuring bone, compared with trials of interventions. Most reported investigations are either cross-sectional studies (Level 2) or comparisons of 2 or more technologies in populations that are usually predominantly Caucasian postmenopausal women. Data for men and people of other races are few.
The techniques for measuring bone may be divided into those that measure the central skeleton (spine, proximal femur, whole skeleton, etc.) and those that measure some part of the peripheral skeleton. Measurement of the central skeleton is most widely carried out using dual-energy x-ray absorptiometry (DXA). There is Level 1 evidence that DXA bone measurement (with consideration of age) is the most effective way to estimate fracture risk in postmenopausal Caucasian women.22,41
Density measurement in the peripheral skeleton by quantitative ultrasound (QUS) is a widely reported technique. Large-scale, prospective, evidence-based studies51,52 of the efficacy of calcaneal QUS measurements were carried out in 2 groups of women, one aged ≥65 and one aged ≥75 years. Meta-analysis of these studies53 indicated a relative risk per standard deviation (RR/SD) of 1.6 (95% CI 1.4–1.8) for hip fracture, whereas direct hip measurement yielded a stronger prediction: RR/SD of 2.4. Although prediction of fracture risk at other sites (wrist and spine) on the basis of calcaneal ultrasound was about the same as direct measurement at these sites,52 it seems that BMD of the hip is preferred for predicting its fracture risk.
Before calcaneal ultrasonometry can be considered as a replacement for central DXA, large prospective studies must be undertaken to demonstrate that it is at least as good as DXA for fracture prediction in perimenopausal and postmenopausal women and that treatment based on calcaneal ultrasound results is at least as efficacious. Although there is Level 1 evidence that QUS provides measurements of bone density that can be used to estimate risk with power similar to DXA, all studies have been carried out in elderly populations.54,55
There are at least 6 commercial quantitative ultrasound devices designed to measure bone “quality” of the calcaneus. Crossover studies have shown that there is good correlation between the 6 different devices for both the speed of sound (SOS) and broadband ultrasound attenuation (BUA) parameters; the correlation coefficients were significant at 0.73–0.93 for SOS and 0.71–0.92 for BUA. However, the results from the various ultrasound devices were not interchangeable.52 To compare the results from different ultrasound devices, standardization equations must be developed through crossover studies as was done to compare Hologic, LUNAR and Norland central DXA measurements.54,55
Monitoring response to treatment of osteoporosis by ultrasonic measurements of the calcaneus as a surrogate for direct measurement of the lumbar spine and femoral neck or total hip has not proved useful. Correlations between changes in BUA, SOS and mathematical combinations of the 2, so-called “stiffness” and mineral changes in the central regions were either not significant or were too small to be clinically helpful.56 This lack of association may be a function of at least 2 factors. The precision error of calcaneal ultrasonometry may not be sufficiently low to disclose mineral changes in the calcaneus over relevant intervals such as 1–3 years following treatment. For example, with a stiffness precision error of 2.3%, a positive or negative change of 6.4% must be achieved for it to be considered significant at the 95% confidence level. Also, the calcaneus may respond differently to treatment than the lumbar spine and femur. Other techniques for measuring peripheral bone density — peripheral quantitative tomography (pQCT), calcaneal and radial DXA, radiographic absorptiometry, etc. — have been found to discriminate between those with and those without prevalent fractures in postmenopausal Caucasian women. However, the studies do not provide Level 1 evidence. In men of all races and in non-Caucasian postmenopausal women, it is likely that the same relation between QUS and fracture exists, but the data are too few to make this statement with confidence. Data suggest that combining bone measurement with other means of risk estimation or combining permutations of bone measurement methods can improve risk estimation, but consensus on this approach has yet to emerge in the literature.
Most experience in estimating fracture risk has been gained from axial (central) DXA measurements of BMD. However, DXA equipment for spine and femur BMD measurement is not readily accessible in remote areas or where population densities are low. In such cases, less expensive, portable alternatives such as ultrasound, radiogrammetry, radiographic absorptiometry and single-photon absorptiometry (SPA) are available, but the relation between reduced BMD at an appendicular bone site and increased fracture risk is less well known for these techniques.
SPA measurements of radius BMD predict future fragility fracture in both men and women.57 When a large population of older white women was followed after baseline measurements of axial and appendicular BMD, BMD at peripheral sites was found to be predictive of future fracture risk.58 The relative risk of future hip fracture per population standard deviation reduction in BMD was the same for the mid-radius (RR 1.7), the distal radius (RR 1.8) and the spine (RR 1.7). In this same study, the relative risk was found to be greater when measurements were made at the calcaneus (RR 2.3) or the hip (RR 3.0). In another study,59 the odds ratio for risk of vertebral deformity was similar when measured using metacarpal radiographic absorptiometry, spine DXA, radius SPA, calcaneus DXA or calcaneus ultrasound. Odds ratios were 1.4–1.9 per standard deviation reduction after accounting for age, and all measurements provided useful information regarding the probability of vertebral deformity.
The propagation of ultrasound through bone depends on bone mass, bone structure and bone material properties. BUA is a measure of the variation in ultrasound attenuation with the frequency of the incident sound wave. SOS in bone can be measured by observing the time required for ultrasound to travel a given distance. Prospective studies have shown that, in older women, both BUA and SOS predict the occurrence of fracture with a strength similar to that of DXA.60,61
Radiogrammetry is the geometric measurement of bone dimensions on high-resolution radiographs. The recent introduction of computer-controlled analysis of digital x-ray images has improved the precision of radiogrammetry, making it comparable to that obtained with DXA and suggesting a possible diagnostic role for such measurements where DXA is not available. Radiogrammetric results correlate with both axial and appendicular DXA results.62 Radiogrammetry also yields similar cross-sectional information about BMD and fracture risk to that obtained using SPA and quantitated computed tomography.63 No data are available relating the results of computer-controlled radiogrammetry to estimation of fracture risk.
BMD measured by radiographic absorptiometry of the phalanges correlates with BMD of the distal forearm and BMD of the lumbar spine and proximal femur.64
During treatment for osteoporosis, changes in axial and appendicular BMD are not strongly related to changes in fracture risk.65 Only a fraction of the decrease in fracture risk produced by anti-resorptive therapy can be accounted for by the small increase observed in BMD.
Precision and serial measurements
Evaluating changes in BMD over time can determine the rate of bone loss (differentiating “fast losers” from “slow losers”) and confirm a positive response to treatment. However, the average rate of bone loss in postmenopausal women is 0.5–2% per year and most treatments lead to an increase in BMD of 1–6% over 3 years. Given these relatively small changes, only a very precise test will detect short-term changes. A clear understanding of the interpretation of serial measurements and the statistical principles surrounding their interpretation is necessary to determine whether a change is clinically meaningful and to avoid mistaking random fluctuations for real changes. In turn, this understanding will help in determining the time interval required between measurements to allow for accurate assessment of response to treatment or progression of disease.
Human factors (in both operator and patient) rather than instrumentation are usually the major source of variation. A quality assurance program to monitor the performance of both operator and equipment will ensure optimum testing and appropriate procedures.66,67,68
Techniques have been described for comparing results from different machines and vendors. Although DXA results from different devices are highly correlated, methods are too inexact to apply to individual patients and are still best suited for group comparisons, such as in clinical trials.54,55 Results from DXA scanners from the same vendor and of identical design can show significant calibration differences. Even after cross-calibration, the precision error between different machines is greater than the error obtained when a single machine is used.69 Thus, the same device should be used for baseline and follow-up measurements.
There is some debate over the method for expressing changes in measurements and their interpretation. A change can be reported as the absolute difference in bone density measurements (g/cm2 for DXA) or as a relative change (%), which is seen most frequently. Evidence indicates that error in absolute measurements is as great (if not greater) in the elderly and osteoporotic patients as in young, normal patients and that the absolute difference between measurements expressed in g/cm2 be used to determine significance rather than the difference in relative changes expressed in percentage.70 Measurement precision is affected by clinical setting, patient population, site of measurement and device design. When young patients with normal BMD are studied in a research setting, the short-term variability in lumbar spine BMD measured by DXA is about 1%. In an older population with a high prevalence of disease and underlying osteoporosis, this number can be as high as 1.7%.71 Long-term variability is greater (2–3%) and that number is more important in clinical care. Variability in the femoral neck is higher (up to 3.2%) than that of the total hip region (up to 2.5%).72 It is not sufficient to accept vendor-supplied estimates of precision, as these are usually derived under optimal conditions and typically underestimate the error encountered in the clinical setting. Each BMD laboratory should determine its own measurement precision for each site commonly assessed in a typical clinical population and use this as the basis for interpreting change. Standardized methods for calculating precision are well described73,74 and should be familiar to the BMD laboratory.
BMD and fracture risk in men
There are insufficient data on the relation between BMD and fracture risk in men. A few prospective studies75 suggest that men fracture at a higher BMD than women; others76,77 suggest that the BMD–fracture risk relationship is similar for men and women. Data from prospective large-scale trials are needed to understand the BMD–fracture risk relationship in men. The risk of fracture depends not only on BMD, but also on other factors such as the likelihood of falls and bone size and geometry. Bone size is greater in men than women even after adjusting for height and weight.78 The pattern of age-related bone loss is also different in men. Endocortical thinning increases with age in women, but not in men,79 which also affects bone strength. The relation between BMD and fracture risk may also differ in men because bone size creates an artifact that affects areal BMD (areal BMD is bone mineral content divided by bone area and corresponds to what is measured by current DXA machines), and DXA overestimates BMD in men relative to women. As a result, areal BMD provided by current DXA machines may be of advantage in evaluating fracture risk in men as the larger bone may have a greater biomechanical advantage compared with the smaller bone size in women
As the lifetime risk of a fragility fracture after age 50 in men is approximately 13%,75 this risk is best estimated by using a male-reference database. This is currently being done across Canada. Based on male reference data, if BMD is measured at hip, spine and radius by DXA and the lowest measure used to make the evaluation using the criterion of a T-score below –2.5, approximately 19% of the male population over the age of 50 years has been found to have osteoporosis.75
There are even fewer data on the BMD–fracture risk relationship in the non-Caucasian population. However, it is becoming apparent that men are as prone to fracture as women at a given BMD.80,81 Asian Americans have been found to have a lower BMD than Caucasians but also have a lower hip fracture rate.82 However, correcting for differences in skeletal size, their apparent BMD is actually higher than white women, which is consistent with the observed lower hip fracture rate. The appropriate cut-off points for diagnosis have not yet been established due to insufficient data.
Figures 1 and 2 outline who should be tested and treated. Significant height loss, kyphosis, personal history of fragility fracture after age 40, long-term use of glucocorticoids, clinical risk factors and age over 65 (see Table 3) should all be considered as potential triggers for ordering a BMD measurement, spinal radiography or both. A non-traumatic vertebral height reduction of 20–25% should be considered as a vertebral fracture.33
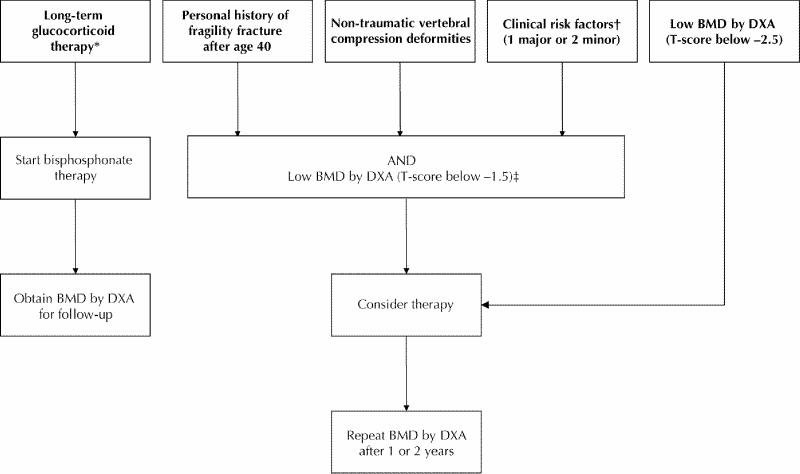
Fig. 2: Who should undergo a fracture risk assessment and be treated for osteoporosis? (Note: *≥7.5 mg prednisone for more than 3 months. †See Table 3. ‡We have arbitrarily chosen T-score below –1.5; non-traumatic vertebral compression deformities [Grade A]117; personal history of fragility fracture after age 40 [Grade D]; clinical risk factors [Grade D].)
The following laboratory tests are recommended in all patients with osteoporosis to exclude secondary causes: complete blood count, serum calcium, total alkaline phosphatase, serum creatinine and serum protein electrophoresis. These laboratory tests are discussed in further detail in the OSC's 1996 clinical practice guidelines for the diagnosis and management of osteoporosis.11 Clinical suspicion of other secondary causes will determine the need for further investigation.
Summary statements
4. Dual-energy x-ray absorptiometry (DXA) is the most widely investigated tool for estimating fracture risk in women and is the single best tool for assessing risk22,80 [Level 1]. There are sufficient and consistent data to support the use of central DXA in case finding.
5. Screening of all postmenopausal women or all men over age 50 is not justified according to available data. However, measuring bone density in men and women after the age of 65, recognizing that after this age fracture risk increases, is justifiable25 [Level 3].
6. All bone density measurement techniques predict the risk of all low-trauma fractures22,40,41,51,52 [Level 1].
7. The best predictor of relative risk of fracture at the proximal femur is measurement of bone density at that site22,53 [Level 1].
8. Clinical evaluation combined with BMD assessment out-performs any single method of risk-assessment; age, BMD and prevalent fracture(s) are the best risk indicators20,21,26,30,39 [Level 1].
9. The most accurate indicator of BMD is the actual measurement of BMD. BMD is not well predicted by “osteopenia” on skeletal radiographs or by risk factors for low BMD21,26 [Level 1]. Although current decision tools are useful in highlighting the risk factors for low BMD, they are not meant to replace BMD measurement. The decision to measure BMD should be based on age-related risk, the presence of other risk factors for fracture and consultation with the patient [consensus]. BMD should be measured only if it will affect management decisions.
10. Because fractures of the spine and hip are the most clinically important low-trauma fractures resulting from osteoporosis and because DXA provides the best measurements of bone at the spine and hip reflecting fracture risk, DXA is the optimum technology at present for use in risk assessment22,40,41,53 [Level 1].
11. DXA can be used to assess sites that are responsive to therapy83,84,85,86 [Level 1].
12. Justification for the clinical use of DXA assumes a clear understanding of its application, the need for quality assurance and careful determination of BMD with sufficient precision to provide clear indications of the least significant change67,69,70,71,72,73,74 [Level 4].
13. Calcaneal quantitative ultrasonometry (QUS) appears to be effective in estimating risk of fracture in postmenopausal women over 65 years of age52,59,60,61 [Level 1]. Evidence for the use of QUS in men and younger women is limited. QUS data appear to be machine specific to a greater degree than data from DXA machines.52,59,60,61
14. Calcaneal QUS is not sufficiently precise for follow-up at clinically relevant intervals56 [Level 1].
15. Other bone measurements (radiogrammetry, radiographic absorptiometry, quantitative ultrasonometry, etc.) may have particular application in risk assessment (but not follow-up) in situations where geography and population size limit access to DXA. However, there is no Level 1 evidence for their widespread use [consensus].
16. Uncertainty about the definition of a vertebral fracture and marked variation in observer performance in this context contribute to much of the variation in findings especially in cross-sectional studies33 [consensus].
17. Consistency in measuring, recognizing and reporting vertebral fractures presents an opportunity in osteoporotic fracture-risk assessment [consensus].
18 Evidence for the use of bone measurement in men and in non-Caucasian women is meager. Existing data do not contradict the inferences already made [consensus].
Recommendations
5. Targeted case-finding strategies for those at increased risk (at least one major or 2 minor risk factors) are recommended, and BMD measurement with central DXA at age 65 is recommended [Grade A].
6. Central (hip and spine) DXA remains the most accurate tool for evaluating BMD in clinical settings. Access to BMD measurement should not be limited by decision tools based on clinical risk factors [Grade A].
7. Patients should be monitored using central (total hip and spine) DXA in clinical settings 1–2 years after initiating therapy [Grade A].
8. Quantitative ultrasonometry may be considered for diagnosis of osteoporosis, but not for follow-up at this time [Grade C].
9. A height loss of > 2 cm in a year or historical height loss of > 4 cm should be followed by thoracolumbar spine radiography to determine the presence of vertebral fractures [Grade D].
Role of biochemical markers of bone turnover
Remodeling is a normal, natural process that maintains skeletal strength, enables repair of microfractures and is essential for calcium homeostasis. During the remodeling process, osteoblasts synthesize a number of cytokines, peptides and growth factors that are released into the circulation. Their concentration thus reflects the rate of bone formation. Bone formation markers include serum osteocalcin, bone-specific alkaline phosphatase and procollagen I carboxyterminal propeptide (PICP).
Osteoclasts produce bone degradation products that are also released into the circulation and are eventually cleared via the kidney. These include collagen cross-linking peptides and pyridinolines, which can be measured in the blood or urine and enable estimation of bone resorption rate. Bone resorption markers include urinary hydroxyproline, urinary pyridinoline (PYR), urinary deoxypyridinoline (D-PYR) as well as collagen Type I cross-linked N telopeptide (NTX) and collagen Type I cross-linked C telopeptide (CTX).
Markers of bone formation and resorption are of value in estimating bone turnover rates. These biochemical markers may be used to identify fast bone losers.87 Numerous cross-sectional studies88,89 have shown that bone turnover rates as evaluated by markers increase at menopause and remain elevated. Bone turnover rate in postmenopausal women correlates negatively with BMD.90
Most of the prospective studies evaluating the relationship between bone turnover and rates of bone loss have been short-term and have been limited by the precision error of the densitometer.91,92,93,94,95 The utility of bone markers to identify fast bone losers was prospectively evaluated in a large cohort of healthy postmenopausal women over 4 years.87 Higher levels of bone formation and resorption markers were significantly associated with faster and possibly greater BMD loss.
In population studies, it appears that markers of bone resorption may be useful predictors of fracture risk and bone loss. Elevated bone resorption markers may be associated with an increased fracture risk in elderly women96,97 although the data are not uniform. The association of markers of bone resportion with hip fracture risk is independent of BMD, but a low BMD combined with high bone resorption biomarker doubled the risk associated with either of these factors alone.96 However, the predictive value of biomarkers in assessing individual patients has not yet been confirmed.91 Biomarker measurements are also currently limited by their high variability within individuals.97
Biomarkers may be of value in predicting and monitoring response to potent antiresorptive therapy in clinical trials. Normalization of bone formation and resorption markers following antiresorptive therapy has been prospectively observed.92,98,99 Reduction in biochemical markers appears to be correlated with a decrease in vertebral fracture incidence99 in some studies, but is not necessarily always predictive of response to therapies.
A weak inverse correlation between BMD and NTX has been observed in men.100 Other studies have shown resorptive markers to be poorly correlated with BMD. Thus the situation in men is less clear and more large-scale prospective trials are required.
Summary statements
19. Bone turnover markers appear to be of value in the assessment of fracture risk in elderly postmenopausal women in population studies96 [Level 2]. Additional studies with fracture endpoints are needed to confirm the usefulness of these markers in individual patients. Bone turnover markers may have a future role in the clinical management of osteoporosis.
20. In population studies, the combination of low BMD and high bone turnover markers may provide a superior indication of fracture risk to either BMD or bone turnover markers alone96 [Level 2].
Recommendations
10. Bone turnover markers should not yet be used for routine clinical management. Additional studies are needed to confirm their use in individual patients. However, with refinement of assay technology and better understanding of biological variability, we believe they will become a useful adjunct for risk assessment and management [Grade B].
Prevention and treatment of osteoporosis
Pharmacologic interventions
Because osteoporosis is a multifactorial condition, its prevention and management are complex. From prevention to treatment of established disease, the goal is to intervene as early as possible to ensure retention of bone mass and to preserve structural integrity of the skeleton, thus preventing fragility fractures.
The results of large prospective RCTs, carried out over the last 10 years, have helped guide our therapeutic options, which include non-pharmacologic approaches that should be recommended for all patients. Currently available drug therapies are all anti-resorptive and focus on decreasing bone turnover. They have been shown to reduce fracture risk for some, although not necessarily all, fragility fractures. Newer therapies aimed at increased bone formation are being studied and are about to be released. It is difficult to assess the relative anti-fracture efficacy of the various therapies, as they have not been compared directly in trials.
Bisphosphonates
Several anti-resorptive agents have been used successfully in the treatment of postmenopausal osteoporosis. However, recent trials of the bisphosphonates consistently provide the best evidence of efficacy in preventing both vertebral and non-vertebral fractures. Bisphosphonates are stable analogues of naturally occurring pyrophosphate. They contain 2 phosphonate groups attached to a single carbon atom to give a P-C-P structure. This structure renders them chemically stable and is responsible for the strong affinity of the bisphosphonates for bone.101
Bisphosphonates inhibit bone resorption through their effects on osteoclasts.102 They interfere with osteoclast recruitment, differentiation and action as well as enhancing osteoclast apoptosis.102 Bisphosphonates can be classified into 2 groups based on their mode of action102: those that most closely resemble pyrophosphate (such as clodronate and etidronate) can be incorporated into cytotoxic adenosine triphosphate (ATP) analogues; the more potent nitrogen-containing bisphosphonates (alendronate and risedronate) induce apoptosis in osteoclasts by interfering with protein prenylation through their effects on the mevalonate pathway and, therefore, the intracellular trafficking of key regulatory proteins. These 2 mechanisms of action may help explain some of the pharmacologic differences between the 2 classes of bisphosphonates.
Currently the bisphosphonates approved for the treatment of osteoporosis in Canada are etidronate, alendronate and risedronate. Although all bisphosphonates, these drugs vary considerably in potency, their ability to inhibit bone resorption, toxicity and dosing regimens. Oral absorption of bisphosphonates is poor, at only 1–5%, even when the medication is taken on an empty stomach. The plasma half-life is 1 hour with 40–80% clearance by the kidneys. The remaining drug is taken up by the bone where it has a long half-life. The most common side effect of bisphosphonates is gastrointestinal upset, which is often dose-related.
Etidronate: Etidronate was the first bisphosphonate to show a benefit in the treatment of osteoporosis.103,104,105,106,107,108,109,110,111,112,113 It is generally well tolerated; reports of gastrointestinal upset are few, diarrhea being the most common complaint. When administered continuously for long periods, etidronate can cause impaired mineralization of bone with results similar to osteomalacia. As a result, etidronate is given in an intermittent fashion, typically 400 mg/day for 2 weeks every 3 months.
Two RCTs111,113 examined the anti-fracture efficacy of cyclical etidronate in postmenopausal women with prevalent vertebral fractures. In both, etidronate produced significant increases in lumbar spine BMD with variable reductions in vertebral fracture rates. These studies indicate that etidronate has some effect in preventing new vertebral fractures in postmenopausal women with severe osteoporosis. There is no evidence of a beneficial effect of etidronate on risk of hip or non-vertebral fracture.
Alendronate: Alendronate is a nitrogen-containing bisphosphonate, which is given continuously at a dose of 5 mg/day for the prevention of osteoporosis and 10 mg/day for the treatment of established osteoporosis. Recently, a weekly dose of alendronate (70 mg) was shown to have an effect on BMD that was comparable to that of a 10-mg daily dose regimen.114 Alendronate is generally well tolerated, although rare cases of esophagitis have been reported.115
Alendronate has been studied extensively for the treatment of osteoporosis.84,85,86,114,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132 In an initial 3-year study, alendronate significantly reduced the incidence of new fractures.85 Its efficacy has since been examined in two large populations of postmenopausal women, one with and one without pre-existing vertebral fractures.117 In the group with vertebral fractures, treatment with alendronate reduced the incidence of vertebral, hip and wrist fractures by about 50% over 3 years; the risk of multiple vertebral fractures was reduced by 90%. This was the first RCT to show hip fracture benefits in calcium- and vitamin D-replete osteoporotic women. In a post-hoc analysis,133 a reduction in the rate of clinical vertebral fractures was demonstrated as early as 1 year into the study.
The anti-fracture efficacy of alendronate has also been examined in postmenopausal women with no prior vertebral fractures.118 Alendronate increased BMD at all measured sites and significantly reduced (36%) the clinical vertebral fracture rate among women with initial T-scores below –2.5. The Fosamax International Trial Study Group (FOSIT)127 demonstrated a reduction in non-vertebral fracture incidence within 1 year in postmenopausal women with a T-score below –2.0. Alendronate prevents bone loss in normal postmenopausal women but anti-fracture efficacy in this context has not been demonstrated.
In summary, alendronate is beneficial in the prevention of vertebral, hip and non-vertebral fractures in postmenopausal women. It consistently increases bone mass at all measured sites. Alendronate has been used in patients who were also taking estrogen or raloxifene and had an additive effect in increasing BMD; however an additional anti-fracture benefit has not been demonstrated.124
Risedronate: Risedronate is generally well tolerated, with occasional reports of headache and diarrhea as side effects. Many studies have demonstrated risedronate efficacy, using both daily and once-weekly treatment regimens.38,83,134,135,136,137,138 Recently, 2 large, 3-year, multicentre RCTs136,137 evaluated the efficacy of risedronate in the treatment of postmenopausal osteoporosis. After 3 years of treatment at 5 mg/day, risedronate reduced the incidence of vertebral fractures by 41–49% and non-vertebral fractures by 39–33%. In a preplanned analysis, treatment with risedronate at 5 mg/day was shown to reduce the incidence of vertebral fractures within the first year of therapy by 61–65%. No significant differences in adverse events were seen between the risedronate and placebo groups.
In a large RCT38 designed to determine the efficacy of risedronate in the prevention of hip fractures, the drug was shown to reduce hip fracture rates in those with low femoral neck BMD by 40%. Among the latter women, risedronate reduced hip fracture by 60% in those with prior vertebral fracture. Risedronate did not significantly reduce the risk of hip fracture among elderly women selected primarily on the basis of risk factors other than low BMD.
In conclusion, risedronate at 5 mg/day, given over 3 years, is well tolerated and reduces the incidence of both vertebral and non-vertebral fractures in women with established postmenopausal osteoporosis. Furthermore, these studies were the first to show a significant reduction in the incidence of vertebral fractures (clinical and subclinical fractures) within 1 year of therapy.
A comprehensive evaluation of the evidence to date for the efficacy of these bisphosphonates is outlined in Hodsman et al.139
Combination therapy: Cyclic etidronate has been used in combination with estrogen therapy in postmenopausal women.140,141 In a randomized study,141 at the end of 4 years, combination therapy produced a greater increase in BMD than either estrogen or etidronate alone; patients on estrogen or etidronate alone had lesser increases in spine and hip BMD.
The combined effect of alendronate and estrogen in postmenopausal women was studied in women who had been receiving estrogen replacement therapy for at least 1 year.124 They were randomly assigned to receive either 10 mg/day of alendronate or placebo. After 12 months, the patients taking alendronate in addition to estrogen showed significantly greater increases in BMD of the lumbar spine and trochanter; however, no conclusions about fracture rate reduction could be drawn. The results of this trial were supported by a 2-year trial of postmenopausal women who were randomly chosen to be treated with placebo, 10 mg/day of alendronate, conjugated estrogen or both treatments.121 Lumbar spine BMD in the placebo group remained stable over the 2 years. The alendronate and conjugated estrogen groups had similar gains in BMD, whereas the group given both treatments had a significantly greater gain than either of the single-treatment groups. These results suggest that, in those initiating therapy, the combination of alendronate and estrogen is more effective than either treatment alone. Although increases in BMD have been demonstrated with combination therapies, no direct evidence of fracture rate reduction has been shown.
Bisphosphonate treatment in men: There is no RCT evidence of benefit from treatment with etidronate. Alendronate has been studied in the treatment of osteoporosis in men and has been shown to increase BMD significantly,142 while reducing vertebral fractures. One large study of risedronate in men on glucocorticoid therapy demonstrated a significant decrease in vertebral fractures after 1 year.143
Bisphosphonates and glucocorticoid-induced osteoporosis: Studies of glucocorticoid-induced osteoporosis are directed at 2 groups: those starting preventive therapy at the time of glucocorticoid initiation and those on chronic long-term glucocorticoid therapy who require treatment for osteoporosis. There is ample evidence that etidronate therapy maintains BMD in patients taking glucocorticoids.144,145,146,147,148,149,150,151,152,153,154,155,156 Etidronate on initiation of glucocorticoid therapy has resulted in a slight increase in lumbar spine BMD, compared with bone loss with placebo.144,145,147,149,151 One study144 suggested that etidronate might be of benefit in preventing vertebral fractures. Two-year RCTs146,149 of etidronate in patients on long-term glucocorticoids demonstrated increases in BMD. These results suggest that etidronate is beneficial in the prevention and treatment of glucocorticoid-induced bone loss and may reduce the risk of fractures in glucocorticoid-treated postmenopausal women.
Alendronate has been studied in glucocorticoid-treated patients157,158,159 and in those with Cushing's syndrome.160 Statistically significant benefit has been shown in the spine, trochanter and femoral neck at doses of 5 and 10 mg/day. Alendronate benefitted all groups, including men, premenopausal and postmenopausal women; in postmenopausal women who were on HRT, alendronate therapy provided added benefit.158 Alendronate was effective in both the prevention and treatment of glucocorticoid-induced osteoporosis and reduced vertebral fracture risk.159
Risedronate has been studied in both the prevention and treatment of glucocorticoid-induced osteoporosis,161,162,163 and significant differences in lumbar spine and hip BMD have been observed compared with placebo. Analysis of pooled data from these studies revealed a significant reduction in the incidence of vertebral fractures among those taking 5 mg of risedronate daily.163
The newer nitrogen-containing bisphosphonates — alendronate and risedronate — should be considered first-line therapy for postmenopausal women with established osteoporosis who are at high risk for fracture. There is good evidence that they prevent both vertebral and non-vertebral fractures, including hip fractures. Bisphosphonates are the only therapy shown to be efficacious in reducing vertebral fracture in glucocorticoid-induced osteoporosis.
Bisphosphonates, particularly the more potent alendronate and risedronate, are effective in reducing risk of fracture in high-risk patients, with benefits seen as early as the first year of therapy.
Summary statements
21.In postmenopausal women with osteoporosis,
a. alendronate85,117,118,127,133 and risedronate38,136,137 are efficacious in preventing vertebral and non-vertebral fractures [Level 1]
b. alendronate117 and risedronate38 prevent hip fractures in postmenopausal women with severe osteoporosis [Level 1]
c. alendronate84,85,86,114,117,118,119,120,122,123,125,127,128,130,131,132,133 and risedronate38,83,136,137,138 increase BMD at spine and hip [Level 1]
d. etidronate is efficacious in preventing vertebral fractures111,113 [Level 2]
e. etidronate increases BMD at the spine and maintains BMD at the femoral neck111,113 [Level 1].
22. In early postmenopausal women at risk of developing osteoporosis, alendronate,123,125 risedronate135 and etidronate103,107,108,109 are efficacious in increasing or maintaining BMD at the spine and femoral neck [Level 1].
23. In men with osteoporosis,
a. alendronate is efficacious in preventing vertebral fractures142 [Level 1]
b. alendronate142 [Level 1] and etidronate164 [Level 3] increase BMD at the spine; alendronate142 increases femoral neck BMD [Level 1] and etidronate164 maintains it [Level 3].
24. For glucocorticoid-induced osteoporosis,
a. in postmenopausal women, alendronate, etidronate and risedronate are efficacious in preventing vertebral fractures144,156,158,161,162,163 [Level 1]
b. in men, risedronate143 is efficacious in preventing vertebral fractures [Level 2]
c. alendronate,158,159 etidronate144,156 and risedronate161,163 increase BMD at the spine and maintain or increase BMD at the hip [Level 1].
Recommendations
11. Bisphosphonates are a first-line preventive therapy in postmenopausal women with low bone density: alendronate [Grade A]; etidronate [Grade A]; risedronate [approved in Canada for prevention, but data thus far only published in abstract form].
12. Bisphosphonates are a first-line treatment for postmenopausal women with osteoporosis, especially those with pre-existing vertebral fractures: alendronate [Grade A]; risedronate [Grade A]; etidronate [Grade B].
13. Bisphosphonates are the first-line therapy for the prevention of glucocorticoid-induced osteoporosis: alendronate [Grade A]; risedronate [Grade A]; etidronate [Grade A].
14. Bisphosphonates are the first-line therapy for the treatment of glucocorticoid-induced osteoporosis in patients requiring prolonged glucocorticoid therapy: alendronate [Grade A]; risedronate [Grade A]; etidronate [Grade B].
15. Bisphosphonates are the first-line treatment for men with low bone mass or osteoporosis: alendronate [Grade A]; etidronate [Grade B].
16. In premenopausal women with osteopenia or osteoporosis, the use of bisphosphonates has not been examined and is not yet recommended in the absence of an identified secondary cause of osteoporosis. However, in certain circumstances, they may be considered. In the absence of evidence of safety of these drugs in pregnancy, contraception would be prudent and treatment should be stopped in the event of pregnancy [Grade D].
Calcitonin
Calcitonin is a naturally occurring peptide hormone. Although its precise physiologic role in adult health is not well understood, at pharmacologic dose levels calcitonin inhibits osteoclast activity and, thus, acts as an anti-resorptive agent.
Because it is a polypeptide, calcitonin cannot be taken by mouth and was initially given by injection.165,166 This route of administration was associated with a high rate of side effects, which limited its use as a long-term osteoporosis treatment. A nasal spray vehicle that allows calcitonin to pass through the nasal mucosa was found to cause fewer side effects.167
Because fish forms of calcitonin are more potent in humans than the human form, recombinant salmon calcitonin has become the standard chemical form of the drug.165,166,167
Calcitonin treatment of postmenopausal women with osteoporosis: We found 25 reports of RCTs of calcitonin in postmenopausal women with osteoporosis.116,119,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191 Most used salmon calcitonin delivered by nasal spray. Results based on surrogate endpoint parameters of bone biochemical markers or bone densitometry were generally consistent across studies: calcitonin treatment produced modest, but reproducible, reductions in bone resorption (5–20% greater than placebo) and increases in BMD (1–8% greater than placebo) over 1–5 years.
Only one study — Prevent Recurrence of Osteoporotic Fractures (PROOF) Study168 — had sufficient power and was designed to detect a change in fracture rates. In that investigation, a daily dose of 200 IU of nasal salmon calcitonin significantly reduced vertebral fractures by 33–36%. Although this study was a prospective RCT, its results are classified as Level 2 evidence because of concerns about the absence of a dose response (no significant fracture reduction with the daily dose of 400 IU) and a high drop-out rate. The study was not powered to detect a reduction in non-vertebral fractures.
Several other studies,172,174,175 produced data showing reduced vertebral fracture rates in calcitonin-treated groups, but either the nature of the studies or the data analysis did not meet the criteria for a Level 1 RCT.
Calcitonin in the prevention of postmenopausal osteoporosis: Most calcitonin studies do not provide sufficient information to determine how the study population would fall into current diagnostic categories. As no studies were found that definitively addressed osteoporosis prevention in postmenopausal women, calcitonin cannot be recommended for use in this setting.
Calcitonin use in premenopausal women: One RCT191 investigated calcitonin efficacy in premenopausal women. No benefit was found, but the dose of nasal salmon calcitonin was less than the accepted effective dose. Thus although evidence is absent, calcitonin may be considered a treatment option in premenopausal women because of its safety profile and the lack of therapeutic alternatives for this group.
Calcitonin and glucocorticoid-induced osteoporosis: Calcitonin has been studied for both prevention and treatment of glucocorticoid-induced osteoporosis. Four reports used nasal salmon calcitonin; 3 others investigated injectable calcitonin.192,193,194,195,196,197,198 In prevention studies, calcitonin reduced bone loss caused by glucocorticoids but did not lead to a net gain in BMD.193,194,198 In osteoporotic patients or those on long-term glucocorticoids, calcitonin produced a net gain in BMD.192,195,196,197 No data on fractures are available for either group. Therefore, although injectable or nasal calcitonin may be used in the prevention or treatment of glucocorticoid-induced osteoporosis, it is not a drug of first choice, as fracture-outcome data are available for other drugs.
Calcitonin in vertebral fracture pain: Four RCTs199,200,201,202 have shown that calcitonin reduces the pain associated with acute vertebral fractures. Both injectable (2 studies) and nasal salmon calcitonin (2 studies) have been investigated. Patients were studied 3–14 days following fracture. Within 3 days, pain was significantly less in the calcitonin-treated group than in the placebo group; in 7–10 days, these patients showed marked improvement; and benefit was maintained for 28 days (the limits of the longest study). The daily dose of injected calcitonin was 100 IU, whereas 200 IU/day was given in the nasal delivery studies. A head-to-head comparison has shown the equivalence of these doses.203 There are no substantial data on pain relief in other types of fractures or in chronic vertebral fractures.
Side effects: The only absolute contraindication to the use of nasal or injectable salmon calcitonin is known hypersensitivity to calcitonin or the drug vehicle.165,166,167 In animal tests, calcitonin caused lower birthweight when given during pregnancy and reduced milk production when given during lactation.165,166,167 In the absence of human data, calcitonin should be avoided in pregnancy and breastfeeding.
Anaphylaxis and other severe allergic reactions have been reported, but they are rare for both formulations. Skin testing using a diluted sample can be performed before administering the full dosage, although this is not standard clinical practice for the nasal formulation.165,166,167
Up to 30% of nasal salmon calcitonin users will experience nasal irritation over a 5-year period. Minor nosebleeds (< 15%), assorted nose symptoms (< 15%) and nasal ulceration (< 5%) also occur.167 Most of these side effects are mild or moderate and do not lead to drug discontinuation. Serious side effects are rare (< 1%).167
Adverse effects are more frequent with injectable calcitonin than nasal. The most common are nausea or vomiting (< 40%), flushing (< 35%) and skin rash at the injection site (< 10%).165,166 Although not serious, these manifestations can lead to discontinuation. Serious side effects are rare (< 1%). 165,166
Antibodies to calcitonin develop in people treated with either formulation in a dose-related manner. However, they do not appear to influence drug efficacy or to be related to side effects and do not need to be monitored.165,166,167,168
Summary statements
25. Nasal calcitonin is efficacious in preventing vertebral fractures in postmenopausal women with severe osteoporosis168 [Level 2]. BMD at the hip and the spine is maintained or minimally increased116,119,168,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191 [Level 1]. Nasal calcitonin has not been shown to be efficacious in preventing non-vertebral fractures168 [Level 2].
26. In those recently started on glucocorticoid therapy, calcitonin slows bone loss at all sites and prevents loss at some sites193,194,198 [Level 2].
27. In those with established glucocorticoid-induced osteoporosis, calcitonin maintains or increases BMD192,195,196,197 [Level 2].
28. Calcitonin is efficacious in reducing the pain associated with acute vertebral fractures199,200,201,202 [Level 1].
Recommendations
17. Nasal calcitonin is a second-line treatment for postmenopausal women with osteoporosis [Grade B].
18. Due to its safety profile, nasal calcitonin can be considered for use in nonpregnant premenopausal women with osteoporosis [Grade D].
19. Nasal calcitonin can be considered for use in men with osteoporosis [Grade D].
20. Nasal or parenteral calcitonin is a first-line treatment for pain associated with acute vertebral fractures [Grade A].
Hormone replacement therapy for postmenopausal women
Hormone replacement therapy (HRT) and ovarian hormone therapy (OHT) are terms that the OSC has used synonymously. Postmenopausal women are not hormonally deficient, as low estrogen and progesterone levels are the norm; therefore “replacement” is not an appropriate term. However, to conform with current international usage, the OSC adopted “HRT” as the acronym for combined estrogen and progestin/progesterone therapy.
One of the most common uses for HRT (or estrogen or progesterone alone) is to treat hot flushes and night sweats (vasomotor symptoms) occurring as a result of reduced levels of estrogen and progesterone. All doses, delivery methods and kinds of HRT are efficacious in reducing vasomotor symptoms.204
The accelerated phase of bone loss that begins with irregular flow in perimenopause205 continues for 4–5 years and sometimes up to 10 years after menopause.206 HRT in postmenopausal women is efficacious in halting this bone loss and increasing BMD at all measured sites.
The average age for menopause (defined by 1 year without flow) is about 51 years. Women who experience an early (before age 40) or relatively early (before age 45) menopause are at increased risk for osteoporosis.207 For this reason, HRT is important in women whose menopause occurs before age 45.
Although HRT has been used for over 60 years to treat osteoporosis and, until recently, has been the primary treatment, the clinical trial evidence for its efficacy has been suboptimal. The first bisphosphonate trials were published in the 1990s; however, until the last decade, the designs of osteoporosis therapy trials have been cohort, case–control or epidemiology studies in postmenopausal women who asked for or whose physicians prescribed HRT. Women who reported taking HRT were also those who were adherent to therapy. We now know that studies with such designs are predisposed to healthy-cohort and compliance biases that make therapy appear more effective than it actually is.208
Until recently, only a single, small, 1-year randomized double-blind placebo-controlled trial209 of transdermal estrogen has shown vertebral fracture prevention, although there are some methodologic problems with this study. There have been no RCTs designed to show hip fracture prevention. An ongoing, large prospective randomized double-blind placebo-controlled therapy trial (Women's Health Initiative)210 in the United States was terminated early because of an unfavourable risk–benefit ratio with estrogen–progesterone combination therapy (Premarin and Provera); there was a significant increase in relative risk for coronary artery disease (hazard ratio [HR] 1.29; 95% nominal CI 1.02–1.63), invasive breast cancer (HR 1.26; CI 1.00–1.59), stroke (HR 1.41; CI 1.07–1.85) and venous thromboembolism (HR 2.11; CI 1.58–2.82) although the absolute risk, while still significant, was small. On the positive side, it was finally demonstrated that a continuous estrogen–progesterone regimen significantly decreases the risk of fractures at all sites including the hip (HR 0.66; CI 0.45–0.98) and significantly decreases colorectal cancer (HR 0.63; CI 0.43–0.92). Only the combined estrogen–progesterone arm of the study has been discontinued. The estrogen-only arm210 is still being followed and will yield additional information.
Important risks with estrogen and progestin/progesterone therapy include venous thromboembolism210,211 and cancers of the breast and endometrium.212,213,214,215,216 In current users this therapy, if taken for more than 5 years following menopause, increases the risk for breast cancer. Irregular vaginal bleeding as well as the risk of endometrial cancer is increased with the use of estrogen without progestin/progesterone or with insufficient doses of progestin/progesterone. Absolute risk of pulmonary embolism per 10,000 person-years attributable to HRT increased by 8 events and risk of all venous thromboembolic disease increased by 18 events.210
Summary statements
29. In postmenopausal women with osteoporosis, HRT is efficacious in preventing clinical vertebral fractures209,210 and in preventing non-vertebral fractures, including hip fractures210 [Level 1].
30. In postmenopausal women, HRT is efficacious in increasing BMD at all sites88,217,218,219,220 [Level 1].
31. In current users, HRT taken for more than 5 years after menopause increases the risk of invasive breast cancer by 26%, the risk of coronary heart disease by 29% and the risk of stroke by 41%210 [Level 1].
32. The use of estrogen without progestin/progesterone increases irregular vaginal bleeding and the risk of endometrial cancer210,212,213,214,215,216 [Level 1].
33. HRT increases the risk of venous thromboembolism from 16 with placebo to 34 with HRT per 10,000 person-years over 5 years210 [Level 1].
34. HRT is efficacious in the treatment of vasomotor symptoms204 [Level 1].
Recommendations
21. HRT is a first-line preventive therapy in postmenopausal women with low bone density. However, when used only for the prevention of postmenopausal osteoporosis, the risks of HRT may outweigh the benefits [Grade A].
22. HRT is a first-line preventive therapy for women who experience menopause before age 45 [Grade D].
23. HRT is a second-line treatment for postmenopausal women with osteoporosis [Grade B]. With prolonged use of HRT taken only for the treatment of postmenopausal osteoporosis, the substantial risks of cardiovascular disease, stroke and invasive breast cancer may lead to an unfavorable risk–benefit ratio.
Selective estrogen-receptor modulators
Selective estrogen-receptor modulators (SERMs) are nonhormonal agents that bind to estrogen receptors with an affinity equivalent to that of estradiol, but they have estrogen agonist effects in some tissues and antagonist effects in others. The structure of any ligand is an important factor in determining the conformational changes that occur in the estrogen receptor when the ligand binds to it. Each ligand seems to produce a different final shape in the estrogen receptor and this shape determines interactions with protein cofactors and DNA response elements that ultimately translate into tissue-specific estrogen agonist or antagonist effects.221
Raloxifene is the only SERM that has been approved for the prevention and treatment of osteoporosis. It is taken as a single tablet (60 mg/day) without regard to meals, calcium and vitamin D supplements or time of day. Raloxifene has estrogen-agonistic effects on bone and lipid metabolism and estrogen antagonistic effects in the breast and uterus.
Skeletal effects: A large RCT, the Multiple Outcomes of Raloxifene Evaluation (MORE),35 examined the anti-fracture efficacy of raloxifene in late postmenopausal women with osteoporosis (T-score below –2.5 at lumbar spine or femoral neck). Raloxifene significantly reduced the incidence of new vertebral fracture in those with (30% reduction) and without (50% reduction) prior vertebral fracture. Furthermore, raloxifene significantly reduced the incidence of 2 or more new vertebral fractures in both groups. However, the risk of non-vertebral fracture was not significantly reduced. Compared with placebo, raloxifene significantly increased BMD at the lumbar spine and femoral neck and significantly reduced the bone turnover markers.
In a post-hoc analysis222 involving a small proportion of the study population, raloxifene was found to decrease the risk of new clinical vertebral fractures at 1 year by 68% compared with placebo. Moreover data from the 4th year of the MORE trial suggest a sustained vertebral anti-fracture efficacy.223
Extra-skeletal effects: Compared with placebo, raloxifene treatment for 2 years resulted in significant reductions in total and low-density lipoprotein (LDL) cholesterol.224 There were no significant differences in high-density lipoprotein (HDL) cholesterol and triglyceride levels. Four-year results from the MORE trial showed similar effects on lipids.225 Raloxifene therapy for 4 years did not significantly affect the overall risk of cardiovascular events in the total population, but did significantly reduce the risk of such events among women at high risk and among those with established cardiovascular disease. In contrast to HRT,226 there was no evidence that raloxifene caused an early increase in risk of cardiovascular events although there were too few events during the first year to draw definitive conclusions. Adequately powered randomized prospective trials with cardiovascular events as predefined outcomes are needed before raloxifene is used for the prevention of such events.
Raloxifene significantly reduced (84%) the incidence of estrogen-receptor-positive invasive breast cancer after 4 years in postmenopausal women with osteoporosis who were at low risk of breast cancer.227 Additional observation confirms this protective effect and indicates that 93 women would need to be treated with raloxifene for 4 years to prevent one case of invasive breast cancer.227 Again, a prospective RCT in women at high risk of breast cancer is needed before raloxifene is used for the prevention of breast cancer. The compound has not been studied in women with a history of breast cancer, nor in menstruating women.
Side effects: Raloxifene appears to be generally safe and well tolerated. Although patients taking raloxifene experienced an increase in hot flashes and leg cramps compared with placebo,228,229 these symptoms were usually mild to moderate and did not cause women to discontinue the drug. There was no association between leg cramps and the risk of venous thromboembolism. In contrast to estrogen and tamoxifen, raloxifene did not cause more vaginal bleeding or endometrial cancer than placebo.228,229,230,231
Venous thromboembolism is a serious side effect associated with raloxifene, although it is reported infrequently: 1.44 and 3.32 events per 1000 person-years for placebo and raloxifene at 60 mg/day, respectively.227 The magnitude of the relative risk is similar to that observed with both HRT210,211 and tamoxifen.232 Raloxifene is contraindicated in patients with past history of venous thromboembolism. It would be prudent to stop this medication 3 days before any prolonged immobilization.
Raloxifene is a first-line therapy in postmenopausal women for the prevention and treatment of osteoporosis. If additional studies confirm the positive extraskeletal effects, raloxifene could improve the overall benefits of a therapeutic intervention in postmenopausal women with low short-term risk of fracture.
Summary statements
35. Raloxifene is efficacious in preventing vertebral fractures in postmenopausal women with osteoporosis35,223 [Level 1]. It increases BMD at the spine and femoral neck35,223 [Level 1]. Raloxifene has not yet been shown to be efficacious in preventing non-vertebral fractures35 [Level 2].
36. In postmenopausal women with osteoporosis, raloxifene decreases the incidence of estrogen-receptor-positive invasive breast cancer227,228 [Level 1]. However, it is not yet recommended for the prevention or treatment of breast cancer.
37. Raloxifene does not increase the risk of endometrial hyperplasia or endometrial cancer228,230,231 [Level 1].
38. Raloxifene increases the risk of venous thromboembolism from 1.44 to 3.32 events per 1000 person-years227 [Level 1].
39. Raloxifene has no beneficial effect on vasomotor symptoms and may increase their incidence228,229 [Level 1].
Recommendations
24. Raloxifene is a first-line therapy in the prevention of further bone loss in postmenopausal women with low bone density [Grade A].
25. Raloxifene is a first-line treatment for postmenopausal women with osteoporosis [Grade A].
Alternative or adjunct therapies
Alternative therapies are those that are not currently an integral part of conventional medicine.233 At this time, vitamin K and ipriflavone are the only alternative therapies for which there are sufficient data on BMD and fracture outcomes to warrant inclusion in clinical guidelines for osteoporosis.
Ipriflavone — a synthetic phytoestrogen: Phytoestrogens are weak estrogen-like chemicals produced by plants; they have estrogen agonist and antagonist effects. There are 3 major groups of naturally occurring phytoestrogens: the isoflavones (found principally in soybeans and other legumes), the lignans (found principally in flax seed, fruits and vegetables) and the coumestans (found in bean sprouts and fodder crops). Epidemiologic studies suggest that populations with high phytoestrogen intakes (such as Asians living in Asia) have lower rates of hip fracture than North Americans.234 However, direct evidence for a protective effect of natural phytoestrogens in humans is extremely sparse.
There is considerably more data on the synthetic phytoestrogen, ipriflavone.235,236,237,238,239,240,241,242,243,244,245,246,247,248,249 Trials of ipriflavone are difficult to compare because of differences in BMD measurement techniques and sites measured. Interpretation of these studies is also limited by the fact that RCTs of ipriflavone have not consistently ensured adequate intake of calcium and vitamin D in either the treatment or placebo arms. Further, data on the long-term effects of ipriflavone on other estrogen-sensitive tissues (breast and uterus) are lacking, and the largest study to date247 suggests that ipriflavone use was associated with significant lymphopenia in 29 of the 237 treated women. Only one study247 reported fracture outcomes. Although this study did not demonstrate any difference in the occurence of vertebral fractures among women taking ipriflavone compared with women taking placebo, only a small number of women had vertebral fractures during the 36-month follow-up. Larger studies are needed to determine whether ipriflavone protects against vertebral fractures.
Summary statements
40. Due to differences in techniques for measuring BMD and sites measured, trials of ipriflavone for the prevention of bone loss and fractures in postmenopausal women are difficult to compare.235,236,237,238,239,240,241,242,243,244,245,246,247,248,249
41. Ipriflavone (200 mg, 3 times daily) is efficacious in maintaining BMD in the spine in postmenopausal women235,239 [Level 1].
42. Ipriflavone is not efficacious in preventing fractures in postmenopausal women with osteoporosis247 [Level 2].
43. Ipriflavone has not been studied in men or premenopausal women.
Recommendations
26. Ipriflavone may be considered as a second-line preventive therapy in postmenopausal women [Grade B].
27. Ipriflavone is not recommended for treatment of postmenopausal women with osteoporosis [Grade B].
28. Because there is inconclusive evidence regarding the long-term safety of ipriflavone, patients taking it should be monitored closely [Grade B],
29. Ipriflavone is not recommended for use in men or premenopausal women [Grade D].
Vitamin K: Two types of vitamin K occur naturally: vitamin K1, which is found in plants (such as lettuce) and vitamin K2, which is found in meat, cheese and fermented products. Vitamin K is important in the function of bone proteins. Circulating levels of vitamin K are lower in patients with hip fractures compared with controls and observational studies suggest that high levels of dietary vitamin K are associated with lower risk of hip fracture.250,251 These findings have led to the development of RCTs examining the effects of vitamin K treatment on BMD or fracture.252,253,254,255,256 The studies are limited by the fact that RCTs of vitamin K (typically menatetrone, 45 mg/day) did not examine calcium or vitamin D intake in either the treatment or placebo arms.
Summary statements
44. Vitamin K is not efficacious in preventing bone loss associated with medication-induced ovarian failure252[Level 2].
45. Vitamin K may be efficacious in slowing bone loss in postmenopausal women with osteoporosis, but has not been shown to be superior to calcium and vitamin D255,256 [Level 1].
46. Vitamin K may be efficacious in the treatment of postmenopausal women with severe osteoporosis, but has not been shown to be superior to calcium and vitamin D254 [Level 2].
47. Vitamin K has not been studied in men or premenopausal women.
Recommendations
30. Vitamin K is not currently recommended for the prevention of postmenopausal osteoporosis [Grade B].
31. Vitamin K is not currently recommended for the treatment of postmenopausal women with osteoporosis [Grade B].
32. Vitamin K is not recommended for use in men or premenopausal women [Grade D].
Fluoride
Sodium fluoride is a potent stimulator of bone formation. It was initially investigated as a therapy for osteoporosis in 1964257 and gained popularity through the 1970s and 1980s.258 It was the first agent to be reported as capable of increasing axial BMD in patients with osteoporosis259 — mainly in uncontrolled studies. In 1989, a consensus report260 expressed cautious optimism about the efficacy of fluoride therapy, but recognized the high incidence of side effects, particularly with some formulations.
The 1990s marked the introduction of RCTs into osteoporosis research and the use of precise vertebral fracture morphometry. However, fluoride compounds have not been adequately investigated using modern, evidence-based standards; almost all of the studies have been small and have had limited power. Furthermore, the clinical profile of fluoride treatment varies greatly with different pharmacologic compounds and formulations in terms of bioavailability and side effects. Thus, the studies that do exist are not, for the most part, comparable.
Fluoride in the treatment of postmenopausal women: Five RCTs examined fluoride therapy and the prevention of vertebral fractures in postmenopausal women.261,262,263,264,265 They varied in duration (from 2 to 4 years) and used different pharmacologic preparations of fluoride (plain NaF, enteric-coated NaF, Na-monofluorophosphate and slow-release fluoride) and different fluoride doses and are, thus, not comparable. However, no study demonstrated a significant reduction in vertebral fractures, despite consistent and significant increases in spinal BMD of as much as 6–8% a year. One small randomized study263 of therapy with slow-release fluoride claimed to show a reduction in vertebral fractures, but quoted the data only as grouped fracture rates and did not indicate a significant reduction in the number of women with newly fractured vertebrae. With fluoride therapy, even a major increase in BMD cannot be considered as a surrogate marker for fracture prevention. Sodium fluoride therapy has not been shown to be effective in preventing fractures in postmenopausal osteoporosis, and there have been no studies in premenopausal women.
Fluoride therapy in men: In one small RCT,266 60 men with a mean age of 52 years and a mean lumbar spine T-score of –2.74 were divided equally into treatment and control groups. The treatment group received 114 mg of Na-monofluorophosphate (15 mg fluoride ion) daily in cycles of 3 months of treatment and 1 month without fluoride. After 36 months the number of patients with vertebral fractures was reduced by 75% (12 patients experienced vertebral fractures in the control group; 4 in the treatment group). Among those in the treatment group, 10 patients experienced adverse effects. This single RCT demonstrating an effect on fractures in men stands in contrast to the negative results for women. It is not likely that the effects of fluoride would be different in men and women, nor is there any direct evidence for this. Thus, it must be concluded that anti-fracture efficacy of fluoride therapy for osteoporosis has not yet been demonstrated.
Fluoride and glucocorticoid-induced osteoporosis: Four RCTs of fluoride therapy in glucocorticoid-induced osteoporosis267,268,269,270 demonstrated 2- to 10-fold increases in spinal BMD over 1–2 years of fluoride treatment, but were too small to show a significant anti-fracture effect.
Toxicity: The toxic effects of fluoride are dose-related and the prevalence of adverse effects differs with different pharmacologic preparations. In 5 of the studies mentioned above,261,262,264,265,271 patients showed significant gastrointestinal toxicity (gastric pain and nausea) and skeletal toxicity (lower extremity pain, and stress fractures). Toxicity was particularly associated with plain fluoride and monofluorophosphate264,265; both these formulations can cause gastrointestinal as well as skeletal side effects. Far fewer gastrointestinal side effects were associated with enteric-coated preparations262 and even fewer with the slow-release fluoride preparation.263
Summary statements
48. Fluoride preparations have not been shown to reduce vertebral or non-vertebral fractures in postmenopausal women with osteoporosis261,262,264,265 despite consistent and sustained increases in spinal BMD.261,262,263,264,265 Fluoride preparations maintain or marginally increase BMD at the femoral neck262,263,264,265 [Level 1].
Recommendations
33. Fluoride is not recommended for treatment of postmenopausal women with osteoporosis [Grade A].
34. Fluoride is not recommended for use in premenopausal women or in men [Grade D].
Parathyroid hormone
Parathyroid hormone (PTH) was reported as a clinical treatment for osteoporosis in 1980,272 but its commercial development was delayed until the advent of central DXA densitometry, which allowed rapid assessment of the hormone's efficacy in increasing bone mass. The synthetic N-terminal fragment, hPTH(1-34), has been used almost exclusively in published reports, culminating in the pharmaceutical trials of teriparatide rhPTH(1-34). At the time of writing, teriparatide was expected to receive regulatory approval in the United States to be followed shortly in other countries including Canada. Another PTH hormone containing the amino-acid sequence rhPTH(1-84) is currently undergoing phase III evaluation.
PTH in the treatment of postmenopausal osteoporosis: The pivotal RCT of teriparatide273 evaluated its efficacy in reducing vertebral and non-vertebral fractures in 1637 postmenopausal women with at least one vertebral fracture at enrolment. This trial was terminated prematurely at a median period of 21 months because of the occurrence of osteosarcomas in a long-term toxicology study in rats treated with large doses of teriparatide from infancy to senescence (see below).
Fracture reduction depended on the type of fracture analysed. For new vertebral fractures, the relative risk was approximately 0.35 compared with placebo. The risk of new vertebral fractures (radiographic deformities) for women with moderate to severe vertebral fractures was reduced by up to 90%. For non-vertebral fractures, the relative risk was 0.47 with no evidence that either dose (20 or 40 mg/day injected subcutaneously) was more effective.273 Compared with placebo treatment, teriparatide resulted in dose-dependent increases in BMD at both the lumbar spine (10–14%) and total hip or femoral neck (3–4%).273 Although, other small RCTs of hPTH(1-34) have not been powered to evaluate anti-fracture efficacy, similar and consistent increases in spine and hip BMD were observed over periods of 1 –3 years of therapy.274,275,276
PTH in male osteoporosis: There are few data from which to evaluate the effects of PTH in male osteoporosis. In a small uncontrolled cohort study of 8 men with severe osteoporosis, Slovik and colleagues277 reported a large gain in lumbar spine BMD (measured by quantitated computed tomography) with no significant change in forearm BMD following 12 months of PTH(1-34) treatment. In a small RCT278 lasting 18 months, PTH(1-34) resulted in a 13.5% increase in lumbar spine BMD among 10 men with severe osteoporosis compared with a control group of 13 men treated only with placebo injections together with calcium and vitamin D. BMD was measured by DXA. Preliminary data have also been presented on the use of teriparatide in an RCT conducted in 437 men as part of its regulatory trials.279 Dose-dependent increases in BMD of 6–9% measured by DXA in the lumbar spine and 2–3% in the femoral neck were observed over 12 months; insignificant changes were observed in the placebo-treated patients. In the teriparatide trial, the increase in lumbar spine BMD mirrored the changes seen in a larger trial in postmenopausal women.273 These studies were of 18 months duration or less and were not powered to detect anti-fracture efficacy; however, the comparable increases in BMD in men and postmenopausal women leads us to expect similar anti-fracture efficacy.
PTH and glucocorticoid-induced osteoporosis: To date, the only study of PTH in secondary osteoporosis is a 12-month RCT in 51 postmenopausal women with glucocorticoid-induced osteoporosis.280 All women had been on chronic estrogen therapy; nearly a third had vertebral fractures at baseline and were receiving clinically significant doses of prednisone for an average of 12–15 years before enrolment. Compared with the control group on estrogen therapy, treatment with PTH(1-34) resulted in a significant (11.1%) gain in BMD in the lumbar spine and an insignificant average gain of 2.9% in the femoral neck. The trial cohort was followed for an additional 12 months while they continued estrogen therapy and further small increments in BMD were observed in the group previously treated with PTH(1-34).281 Despite the apparent high risk of incident fractures in this trial cohort, very few vertebral or clinical fractures were observed; in any event, the trial was too small to detect anti-fracture efficacy for PTH.
Side effects during PTH therapy have been relatively scarce. Pain and induration at the injection sites were likely due to the vehicle used to reconstitute the peptide274,275 and were not seen with teriparatide.273 Nausea, headaches, dizziness and leg cramps were observed infrequently as dose-dependent side effects during the teriparatide trials.273 Not surprisingly the pharmacologic properties of PTH resulted in occasional episodes of hypercalcemia or hypercalciuria during the teriparatide trials, which were obviated by either cessation of concurrent calcium supplementation or minor dose reductions.273 To date the toxicology data from teriparatide, documenting late-onset osteosarcomas in rats treated with large doses of rhPTH(1-34) from infancy to senescence, has not been seen in human studies. Currently, the consensus is that limited exposure (1–2 years) to PTH therapy in older people with osteoporosis does not expose this population to the risk of osteosarcoma or any other neoplasm.
Summary statements
49. hPTH(1-34) is efficacious in preventing both vertebral and non-vertebral fractures in postmenopausal women with severe osteoporosis.273 hPTH(1-34) increases BMD at all skeletal sites with the exception of the radius273 [Level 1].
50. In men with severe osteoporosis, hPTH(1-34) increases BMD at the spine277,278,279 [Level 2].
51. In postmenopausal women with glucocorticoid-induced osteoporosis, hPTH(1-34) increases BMD at the spine280 [Level 2].
Recommendations
35. Although hPTH(1-34) is not yet approved for use in Canada, it is expected to become a first-line treatment for postmenopausal women with severe osteoporosis [Grade A].
36. hPTH(1-34) is also expected to become a recommended treatment for men and people with severe osteoporosis who are receiving prolonged glucocorticoid therapy [Grade D].
Non-pharmacologic interventions
Nutrition
The nutrition section committee's mandate was to determine whether calcium, vitamin D or selected nutritional variables could be used in osteoporosis prevention and treatment (Figure 3). The questions addressed concerned the effect of the intake of nutrients and other food components on subsequent attainment of peak bone mass, as well as prevention of bone loss and fractures. The initial scan of the literature revealed 16 058 abstracts from which 996 studies were reviewed. The resulting evidence-based database included 56 studies on vitamin D, calcium or both, and 26 on other nutrients and food-related components.

Fig. 3: Optimal treatment for osteoporosis in postmenopausal women. (Note: *Mainly vertebral fracture. Only alendronate and risedronate and recently continuous estrogen-progesterone have been shown to decrease hip fracture risk.)
The nutrient intake recommendations have been evaluated with respect to the effect of the nutrient on bone health; other functions of the nutrients have not been examined. If an essential nutrient had no apparent effect on bone, it is recommended that no additional intake of nutrient is needed, recognizing that bone is a complex tissue that would require the presence of all essential nutrients for synthesis and maintenance. As data on dietary levels needed for bone growth of infants and children are lacking, the recommendations apply only to adults unless stated otherwise. Intake recommendations represent dietary goals for an individual. The recommended values are the lowest or most consistently reported effective amounts that were tested, plus background levels of the nutrient. Thus, recommendations are for the total dietary intake.
Summary statements
Calcium and vitamin D
52. Adequate calcium and vitamin D through diet or supplements are essential for the prevention of osteoporosis and, taken together, are essential adjuncts to preventative therapy107,108,109,123,125,230,282 [Level 1].
53. Calcium and vitamin D should not be used as the sole treatment of osteoporosis; however, calcium and vitamin D through diet or supplements are essential adjuncts to osteoporosis treatment35,38,85,106,113,117,118,136,137,283,284,285 [Level 1].
54. The recommended calcium intake from all sources (where “all sources” means total diet and supplement) is as follows:
a. prepubertal children (ages 4–8 years) — 800 mg/ day286,287,288,289 [Level 1]
b. adolescents (ages 9–18 years) — 1300 mg/ day287,290,291,292 [Level 1]
c. premenopausal women — 1000 mg/day293,294,295 [Level 1]
d. men after adolescence and until the age of 50 years — 1000 mg/day296,297 [Level 3]
e. menopausal women — 1500 mg/day282,283,284,285,298,299,300,301,302,303,304,305 [Level 1]
f. men over the age of 50 years — 1500 mg /day285,296,297 [Level 1]
g. women 18 years and over who are pregnant or lactating — same as nonpregnant adult, i.e., 1000 mg/day306,307,308,309 [Level 1].
55. Vitamin D3 (cholecalciferol) is preferred over vitamin D2 (ergocalciferol)310 [Level 2].
56. For Canadians, sun exposure does not appear to be sufficient to replace ingested forms of vitamin D311 [Level 3].
57. The recommended vitamin D intakes from all sources (where “all sources” means total diet and supplement) are as follows:
a. men and women under 50 years — 400 IU (10 μg)/day311,312,313 [Level 4]
b. men and women > 50 years — 800 IU (20 μg)/ day282,283,284,285,314 [Level 1].
Macronutrients — protein, fatty acids, dietary fibre
58. Increasing protein intake among those who have inadequate dietary protein has a positive effect on the risk of hip fracture in men and women315,316 [Level 3].
59. There is no good-quality evidence to support or refute the benefits of essential fatty acids or dietary fibre on BMD or fracture risk.
Diet-related lifestyle factors — caffeine, salt
60. Heavy caffeine ingestion (> 4 cups coffee/day) is significantly associated with hip fracture in men and women317,318 [Level 2].
61. The effects of sodium on BMD are equivocal; however, in studies in which sodium intake is measured properly, there is a significant negative effect for women319 [Level 3] and men320 [Level 5] when daily intake exceeds 2100 mg (90 mmol).
Other micronutrients
62. In both men and women who have normal digestion, providing additional dietary magnesium has no significant effect on the risk of hip fracture296,321,322,323 [Level 3].
63. In men and menopausal women, providing additional dietary copper has no significant effect on the risk of hip fracture296,324 [Level 3].
64. There is no significant association between fracture risk and zinc intake in men325 [Level 3] and additional dietary zinc intake has no significant effect on BMD in women322 [Level 5].
65. There is no good-quality evidence to support or refute the benefits of iron on BMD or fracture risk; however, in women over 39 years, high intake of iron (> 30 mg/day) may be associated with an increased risk of hip fracture326 [Level 4].
66. Few studies have adequately addressed dietary phosphorus. In the normal range of daily intake, assessed without consideration of phosphate additives in processed foods, there does not appear to be any significant relation between phosphorus intake and hip fractures in men325 [Level 3] or BMD in women320 [Level 5].
67. There is no good-quality evidence to support or refute the effect of providing dietary silica, boron or strontium, or additional manganese, on BMD or fracture risk.
Recommendations
37. The following daily intake levels are recommended for calcium:
a. prepubertal children (ages 4–8 years) — 800 mg/day [Grade B]
b. adolescents (ages 9–18 years) — 1300 mg/day [Grade B]
c. women (ages 19–50 years) — 1000 mg/day [Grade A]
d. women over 50 years — 1500 mg/day [Grade A]
e. pregnant or lactating women (≥ 18 years) — 1000 mg/day [Grade A]
f. men (ages 19–50 years) — 1000 mg/day [Grade C]
g. men over 50 years — 1500 mg/day [Grade C].
38. The following daily intake levels are recommended for Vitamin D3:
a. women (ages 19–50 years) — 400 IU (10 μg)/day [Grade D]
b. women over 50 years — 800 IU (20 μg)/day [Grade A]
c. pregnant or lactating women (≥ 18 years) — 400 IU (10 μg)/day [Grade D]
d. men (ages 19–50 years) — 400 IU (10 μg)/day [Grade D]
e. men over 50 years — 800 IU (20 μg)/day [Grade A].
Vitamin D3 is specified as it shows greater potency than Vitamin D2; therefore more of the latter may be required to meet these recommendations.
39. Maintaining adequate protein intake is important [Grade C].
40. Excess caffeine (> 4 cups coffee/day) should be avoided [Grade B].
41. Excess dietary sodium (> 2100 mg/day or > 90 mmol/day) should be avoided as it reduces BMD in adult men and women [Grade C].
42. No evidence exists to recommend additional intakes of the following nutrients for the prevention or treatment of osteoporosis: magnesium, copper, zinc, phosphorus, manganese, iron, essential fatty acids [Grade D].
Physical activity and falls prevention
Physical activity will benefit skeletal structure and strength; and the detrimental effects of immobilization are well known. Physical activity varies in type, frequency, duration, intensity and age of onset. It affects different parts of the skeleton differently, according to the pattern of stress produced. An additional complication is that overactivity, by affecting hormonal status, especially in premenopausal women, and perhaps because of associated undernutrition, can be detrimental to the skeleton.
Sports are the most extreme form of physical activity normally undertaken, but by their nature are not amenable to RCTs. They also fall mainly into the 2 categories of physical activity — aerobic or impact type (jogging, field and racquet sports, gymnastics) and endurance and strength type (weightlifting, body building, swimming, cycling and use of static exercise machines) — and so can offer insight into the type of physical activity most likely to be valuable.
Physical activity and BMD
Children, before and during puberty: The question of greatest importance is probably whether a permanent change in the skeleton can be induced by physical activity, such that it will bring benefit throughout the rest of life. Clearly the time of growth would represent the best chance of achieving this. In children, interpretation of BMD changes is difficult, as the usual method for measuring BMD (by DXA) is size sensitive; the density of small bones tends to be underestimated and that of large bones overestimated. Thus it is important to match control and study groups for stage of growth and puberty and take into account any effect of the physical activity on growth, which could occur, for example, through a delay in puberty.
An RCT large enough and long enough to provide a definite answer to our question is not available and likely never will be. We must piece together the answer as best we can from the available evidence.
Two RCTs, one in boys and one in girls aged 9–12 years have shown that an exercise program of 7 months' duration, entailing jumping, will produce changes in BMD and some measures of skeletal size. In girls, the impact was greater for those entering puberty than for younger children327,328; however, benefit is not confined to the time of puberty, but also occurs at younger ages.329,330,331 Most of the sports that children participate in are impact types, such as baseball, basketball and soccer, and are associated with improved BMD. Gymnastics is particularly effective. Non-impact exercises, such as swimming and resistance strength training are of little benefit.332,333
Young adults after puberty: Benefits from impact-type exercises are seen in young adults after puberty,334,335,336,337 with the best results in those who have exercised throughout childhood.338 Running produces variable results in both men (see below) and women depending on nutrition and hormone changes. This effect in young women is reviewed by Khan and colleagues.339
Weight training in young adulthood also gives inconsistent results.340,341 Young male olympic weight lifters had greater BMD, although potential use of anabolic steroids in such competitors has been reported.342,343
Older adults — men, premenopausal and postmenopausal women: Case–control studies344,345,346,347,348 have shown varying degrees of BMD increase in men who participate in sports. However, many of these studies included adults who had been active in sports since childhood.349,350,351 In a study of adult male tennis players, BMD was found to be 15% greater at the lumbar spine and 11% at the proximal femur.349 For long-distance running, benefit appears to occur among those who run up to 15–20 miles a week; longer distances, for whatever reason, result in little benefit or actual reduction in bone density.352,353,354 Most intervention studies of men are case–control and not randomized. There is a great need for large-scale randomized long-term trials.
A meta-analysis355 of 8 RCTs (6–36 months duration) in premenopausal women (16–44 years old) reviewed whether impact exercise versus non-impact exercise reduced age-related bone loss. Impact exercises included high-impact aerobics, running and jump training. Non-impact exercises included stretching, resistance training and weight-lifting. The studies were limited by small sample sizes and high dropout rates. Bone loss in the lumbar spine was 1.5% lower in the group participating in impact exercises (95% CI 0.6%–2.4%) and 1.2% lower in those in the non-impact exercise group (95% CI 0.7%–1.7%). One study in female college students found that running (impact) and weight-training (non-impact) were equally effective in reducing bone loss.356 Overall, studies with high compliance had a greater impact on maintaining or improving BMD.
Studies in postmenopausal women similarly tend to be small and short term, although there are many more RCTs. As these studies involve trying to change an activity pattern, compliance becomes an issue, although under study conditions it tends to be relatively high (50–100%). Most investigators have studied the impact of physical activity in those who have chosen to participate fully compared with lower compliers and a control group. Therefore, the studies explore efficacy rather than effectiveness and do not carry out intention-to-treat analyses.
Brisk walking, dancing and jumping appear to slow or prevent bone loss in postmenopausal women, although the results are not entirely consistent.300,357,358,359,360,361,362,363,364,365,366 Physical activities designed to improve strength and endurance or the strength of specific muscles that act on the bone in question (mostly weight training or the use of stationary equipment) produce inconsistent results.367,368,369,370,371,372,373,374 The potential benefits of physical activity in synergy with HRT are unclear, as results are inconsistent.363,375,376
Several meta-analyses have been conducted on the effect of physical activity on bone loss in postmenopausal women. Wolff and co-workers377 concluded that physical activity prevented or reversed almost 1% of bone loss per year in both the lumbar spine and femoral neck. Several other meta-analyses355,378 have also found a greater benefit of physical activity, particularly impact exercise, at the spine. BMD at the hip may also benefit from impact exercise but the effect of non-impact exercises on hip BMD remains unproven.355
Physical activity and fracture prevention: Case–control studies379,380 of older adults with hip fractures have shown that these people had lower activity levels through adult life. A large prospective, observational study381 found faster rates of BMD loss from the hip in those most inactive (bed or chair bound). A prospective study382 of 9012 men over 7 years found fewer fragility fractures in men who did more weight-bearing activity. Intense activity (defined as activity beyond walking) was associated with a reduction in hip fracture occurrence in the most active group (HR 0.38; 95% CI 0.16–0.91) in a 21-year cohort study.383
There are no long-term prospective RCTs of physical activity exploring fracture outcomes.
Physical activity and falls prevention: In adults over the age of 65 years living independently, physical activity has been shown to reduce falls.384 Physical activity included individually tailored programs of progressive muscle strengthening, balance retraining exercises and a walking plan which reduced the number of people sustaining falls and the number of people with fall-related injuries over 1 year (RR 0.80, 95% CI 0.66–0.98). A reduced rate of falls was also found in those who continued the activity for a second year.385,386,387
Tai chi has also been shown to reduce falls.388 One of the limitations of this study was that when “falls” were redefined to discount minor events, such as stumbling, the study results were no longer statistically significant.
Group-delivered exercise programs that have not been individually prescribed appear to be not as effective in reducing falls, and further study is needed in this area.
Other programs to reduce falls: Home hazard assessment and modification prescribed by an occupational therapist for older adults with a history of falling have been shown to reduce the risk of falling both inside and outside the home (RR 0.64, 95% CI 0.49–0.84).389 Those without a history of falls did not receive benefit from this program.
Withdrawal of psychotropic medication is also effective in reducing falls among the elderly living in the community.386
Educational preventive home visits (evaluation of medical, functional, psychosocial and environmental factors followed by recommendations) have not been found to be effective in reducing falls in community-dwelling elderly.390
Multi-faceted programs in community-dwelling elderly people are effective in reducing falls (pooled RR 0.79, 95% CI 0.67–0.94) in those with a history of falling or known risk factors for falls.384,391,392 Also, Tinetti and colleagues393 showed a reduction in the number of falls using a multifactorial intervention (adjusted incidence rate ratio 0.69, 95% CI 0.52–0.90). Such interventions include screening of health and environment risk factors, assessment of physical activity and home hazards and modification and withdrawal of psychotropic medications. These programs have only been found to be positive in North America, which may be due to differences in health care systems and differences in the types of multifactorial and multidisciplinary interventions.
Summary statements
68. Children who exercise habitually have stronger bones than those who do not329,331,338,394 [Level 3].
69. Exercising throughout puberty may be particularly efficacious in producing a stronger skeleton327,328 [Level 1].
70. Impact exercises lead to an improvement in BMD in both boys and girls327,328 [Level 1].
71. Impact exercises and sports that include them as a component are more efficacious at all ages than strength, endurance or non-weight-bearing activities332,333,359 [Level 4].
72. Physical activity in men, particularly of the impact type, is associated with greater BMD344,345,346,347,348 [Level 4].
73. In premenopausal women, both impact and non-impact exercise prevent bone loss in the lumbar spine, with impact exercise somewhat more beneficial355,356 [Level 2+].
74. In postmenopausal women, impact exercise may reduce the rate of bone loss or lead to some bone gain, at least in the short term. Response to non-impact or endurance exercises is lower and more inconsistent300,357,358,359,360,364,365,367,368,371,372,373 [Level 1].
75. In both men and women, excessive physical activity, such as that associated with long-distance running, can be detrimental352,353,354 [Level 4].
76. A higher level of activity throughout middle life is associated with a reduced risk of hip fracture in old age [consensus].
77. Exercise programs that are individually tailored and include muscle strengthening, balance training and walking over 1 year are effective in reducing falls384,385,386,387 [Level 1+] and injuries384 [Level 2+]. General group-delivered exercise programs have not been shown to be effective in reducing falls.
78. Multifactorial programs that combine interventions are effective in reducing falls in both unselected people and those with a history of falling or with known risk factors for falls384,391,392,393 [Level 1+].
Recommendations
43. Children, particularly those entering and passing through puberty, should be encouraged to participate in impact exercises or sports (mainly field and court sports) [Grade B].
44. Throughout life, both men and women should be encouraged to participate in exercise, particularly in weight-bearing exercises, which include impact as a component [Grade C for men; Grade B for pre- and menopausal women].
45. For older men and women at risk of falling or who have fallen, tailored programs that are based on individual assessment, contain exercises to improve strength and balance and, where necessary, are multidisciplinary in nature should be made available [Grade A].
Conclusion
These clinical practice guidelines are intended to provide family practitioners with the current best evidence from clinical research to help them make health care decisions about osteoporosis. For each section in this document, we have followed the steps necessary to develop recommendations based on evidence-based medicine: defining a question, gathering and summarizing the evidence and making a judgment on that evidence. As in many other fields of medicine, the evidence in the literature on osteoporosis is rapidly growing and we expect these guidelines to be a work in progress that will need to be updated to integrate new evidence.
Health care decisions should, as far as possible, be evidence-based and adapted to patient needs to ensure appropriate resource utilization, good adherence to therapy and optimal outcomes. That is what makes medicine an art as well as a science.
Acknowledgments
We gratefully acknowledge the contributions of the staff at the OSC, especially Joyce Gordon, president and CEO; Sylvia Kowal, director of marketing, programs and communications and Cathy Loveys, program coordinator. In addition, we gratefully acknowledge the contributions of Linda Huestis, Rick Palidwor, Mary Bowyer, Jessie McGowan and Cathy Cameron. Finally, we thank Diane Adams and Julie Parrot for their database and administrative assistance.
Footnotes
Lists of the members of the Scientific Advisory Council, the Guidelines Steering Committee and the section committees appear at the end of the article.
Endorsing organizations
Canadian Association on Gerontology
Canadian Society of Endocrinology and Metabolism
Canadian Society for Exercise Physiology
Canadian Orthopaedic Association
Dietitians of Canada
This article has been peer reviewed.
Contributors: Section committees, overseen by Dr. Jacques P. Brown and Dr. Robert G. Josse, researched and developed the guidelines and the Scientific Advisory Council reviewed and approved them. Members of the Scientific Advisory Council, the Guidelines Steering Committee and the section committees appear at the end of this article.
CMAJ supplement included with this issue:
2002 Clinical Practice Guidelines for the Diagnosis and Management of Osteoporosis in Canada
These guidelines were developed under the auspices of the Scientific Advisory Council of the Osteoporosis Society of Canada. The process was facilitated by funding from Eli Lilly Canada, Inc., Merck Frosst Canada, Inc., Novartis Pharmaceuticals Canada, Inc., Procter and Gamble Pharmaceuticals, Aventis Pharma Inc. and Wyeth-Ayerst Canada, Inc. None of the funding sources had a role in the collection, analysis or interpretation of the data or in the decision to publish this report.
Competing interests: Drs. J. Brown, Josse, Bogoch, Jolly, Kaiser, Karaplis, Kendler, Khan, Murray, Ste-Marie and Yuen have been consultants for various pharmaceutical companies. Drs. Josse, Bogoch, Jolly, Kendler, Leslie, Ste-Marie and Yuen have received research funds from various pharmaceutical companies. Drs. J. Brown, Josse, Bogoch, T. Brown, Derzko, Jolly, Kaiser, Karaplis, Kendler, Khan, Kvern, Leslie, Morrish, Murray, Ste-Marie and Yuen have received speaker fees or educational grants or both from various pharmaceutical companies. Drs. Josse, Derzko, Kaiser, Karaplis, Kendler, Khan, Kvern, Leslie, Murray, Ste-Marie and Yuen have received travel assistance from various pharmaceutical companies. No competing interests were declared by the other members of the Scientific Advisory Council.
Correspondence to: Dr. Jacques P. Brown, Centre de recherche du CHUL, Room S-784, 2705, boul. Laurier, Ste-Foy QC G1V 4G2; fax: 418-654-2142; email: jacques.brown@crchul.ulaval.ca
Reprint requests: Osteoporosis Society of Canada, 33 Laird Dr., Toronto ON M4G 3S9; fax: 416-696-2673; email: osc@osteoporosis.ca
References
- 1.Hanley DA, Josse RG. Prevention and management of osteoporosis: consensus statements from the Scientific Advisory Board of the Osteoporosis Society of Canada: 1. Introduction. CMAJ 1996;155:921-3. [PMC free article] [PubMed]
- 2.Papadimitropoulos EA, Coyte PC, Josse RG, Greenwood CE. Current and projected rates of hip fracture in Canada. CMAJ 1997;157:1357-63. [PMC free article] [PubMed]
- 3.Melton LJ III, Chrischilles EA, Cooper C, Lane AW, Riggs BL. Perspective: how many women have osteoporosis? J Bone Miner Res 1992;7:1005-10. [DOI] [PubMed]
- 4.Cauley JA, Thompson DE, Ensrud KE, Scott JC, Black DM. Risk of mortality following clinical fractures. Osteoporos Int 2000;11:556-61. [DOI] [PubMed]
- 5.Cooper C, Atkinson EJ, Jacobsen SJ, O'Fallon WM, Melton LJ III. Population-based study of survival after osteoporotic fractures. Am J Epidemiol 1993;137:1001-5. [DOI] [PubMed]
- 6.Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet 1999;353:878-82. [DOI] [PubMed]
- 7.Chrischilles EA, Butler CD, Davis CS, Wallace RB. A model of lifetime osteoporosis impact. Arch Intern Med 1991;151:2026-32. [PubMed]
- 8.Cummings SR, Black DM, Rubin SM. Lifetime risks of hip, colles', or vertebral fracture and coronary heart disease among white postmenopausal women. Arch Intern Med 1989;149:2445-8. [PubMed]
- 9.Melton LJ III. Who has osteoporosis? A conflict between clinical and public health perspectives. J Bone Miner Res 2000;15:2309-14. [DOI] [PubMed]
- 10.Goeree ROB, Pettitt DB, Cuddy L, Ferraz M, Adachi J. An assessment of the burden of illness due to osteoporosis in Canada. J Soc Obstet Gynaecol Can 1996;18(suppl July):15-24.
- 11.Osteoporosis Society of Canada. Clinical practice guidelines for the diagnosis and management of osteoporosis. CMAJ 1996;155:1113-33. [PMC free article] [PubMed]
- 12.Meltzer S, Leiter L, Daneman D, Gerstein H, Lau D, Ludwig S, et al. 1998 Clinical practice guidelines for the management of diabetes in Canada. CMAJ 1998;159(8 suppl):S1-29. [PMC free article] [PubMed]
- 13.Carruthers SG, Larochelle P, Haynes RB, Petrasovits A, Schiffrin E. Report of the Canadian Hypertension Society Consensus Conference: 1. Introduction. CMAJ 1993;149:289-93. [PMC free article] [PubMed]
- 14.Greenhalgh T. Assessing the methodological quality of published papers. BMJ 1997;315:305-8. [DOI] [PMC free article] [PubMed]
- 15.Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med 1993;94:646-50. [DOI] [PubMed]
- 16.Osteoporosis prevention, diagnosis and therapy. NIH consensus statements 2000;17(1):1-45. [http://consensus.nih.gov/cons/111/111_intro.htm] [PubMed]
- 17.Guidelines for preclinical evaluation and clinical trials in osteoporosis. Geneva: WHO; 1998:59.
- 18.Kanis JA, Melton LJ III, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res 1994;9:1137-41. [DOI] [PubMed]
- 19.Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: report of a WHO Study Group. Geneva: WHO; 1994. Tech. rep. series. [PubMed]
- 20.Cummings SR, Nevitt MC, Browner WS, Stone K, Fox KM, Ensrud KE, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med 1995;332:767-73. [DOI] [PubMed]
- 21.Geusens P, Hochberg MC, van der Voort DJ, Pols H, Van der Klift M, Siris E, et al. Performance of risk indices for identifying low bone density in postmenopausal women. Mayo Clin Proc 2002;77:629-37. [DOI] [PubMed]
- 22.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 1996;312:1254-9. [DOI] [PMC free article] [PubMed]
- 23.Ungar WJ, Josse R, Lee S, Ryan N, Adachi R, Hanley D, et al. The Canadian SCORE questionnaire: optimizing the use of technology for low bone density assessment. Simple calculated osteoporosis risk estimate. J Clin Densitom 2000;3:269-80. [DOI] [PubMed]
- 24.Cadarette SM, Jaglal SB, Kreiger N, McIsaac WJ, Darlington GA, Tu JV. Development and validation of the osteoporosis risk assessment instrument to facilitate selection of women for bone densitometry. CMAJ 2000;162:1289-94. [PMC free article] [PubMed]
- 25.National Osteoporosis Foundation. Osteoporosis: review of the evidence for prevention, diagnosis, and treatment and cost-effectiveness analysis. Osteoporos Int 1998;8(suppl 4):S7-80. [PubMed]
- 26.Cadarette SM, Jaglal SB, Murray TM, McIsaac WJ, Joseph L, Brown JP. Evaluation of decision rules for referring women for bone densitometry by dual-energy X-ray absorptiometry. JAMA 2001;286:57-63. [DOI] [PubMed]
- 27.Wasnich RD, Davis JW, Ross PD. Spine fracture risk is predicted by non-spine fractures. Osteoporos Int 1994;4:1-5. [DOI] [PubMed]
- 28.Davis JW, Grove JS, Wasnich RD, Ross PD. Spatial relationships between prevalent and incident spine fractures. Bone 1999;24:261-4. [DOI] [PubMed]
- 29.Ismail AA, Cockerill W, Cooper C, Finn JD, Abendroth K, Parisi G, et al. Prevalent vertebral deformity predicts incident hip though not distal forearm fracture: results from the European prospective osteoporosis. Osteoporos Int 2001;12:85-90. [DOI] [PubMed]
- 30.Klotzbuecher CM, Ross PD, Landsman P, Abbott TAI, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res 2000;15:721-39. [DOI] [PubMed]
- 31.Ross PD, Davis JW, Epstein RS, Wasnich RD. Pre-existing fractures and bone mass predict vertebral fracture incidence in women. Ann Intern Med 1991;114:919-23. [DOI] [PubMed]
- 32.Tromp AM, Smit JH, Deeg DJH, Bouter LM, Lips P. Predictors for falls and fractures in the longitudinal aging study Amsterdam. J Bone Miner Res 1998;13:1932-9. [DOI] [PubMed]
- 33.Black DM, Palermo L, Nevitt MC, Genant HK, Christensen L, Cummings SR. Defining incident vertebral deformity: a prospective comparison of several approaches. The Study of Osteoporotic Fractures Research Group. J Bone Miner Res 1999;14:90-101. [DOI] [PubMed]
- 34.Fox KM, Cummings SR, Williams E, Stone K, Study of Osteoporotic Fractures. Femoral neck and intertrochanteric fractures have different risk factors, a prospective study. Osteoporos Int 2000;11:1018-23. [DOI] [PubMed]
- 35.Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: Results from a 3-year randomized clinical trial. JAMA 1999;282:637-45. [DOI] [PubMed]
- 36.Black DM, Arden NK, Palarmo L, Pearson J, Cummings SR. Prevalent vertebral deformities predict hip fractures and new vertebral deformities but not wrist fractures. J Bone Miner Res 1999;14:821-8. [DOI] [PubMed]
- 37.Lindsay R, Silverman SL, Cooper C, Hanley DA, Barton I, Broy SB. Risk of new vertebral fracture in the year following a fracture. JAMA 2001; 285(3): 320-3. [DOI] [PubMed]
- 38.McClung MR, Geusens P, Miller PD, Zippel H, Bensen W, Roux C, et al. Effects of risedronate on the risk of hip fracture in elderly women. N Engl J Med 2001;344:333-40. [DOI] [PubMed]
- 39.Cummings SR, Black DM, Nevitt MC, Browner WS, Cauley JA, Genant HK, et al. Appendicular bone density and age predict hip fracture in women. JAMA 1990;263:665-8. [PubMed]
- 40.Kanis JA, Johnell O, Oden A, Dawson A, De Laet C, Jonsson B. Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos Int 2001;12:989-95. [DOI] [PubMed]
- 41.Torgerson DJ, Campbell MK, Thomas RE, Reid DM. Prediction of perimenopausal fractures by bone mineral density and other risk factors. J Bone Miner Res 1996;11:293-7. [DOI] [PubMed]
- 42.Patel MS, Rubin LA, Cole DEC. Genetic determinants of osteoporosis. In Hendreson JE, Goltzman D (editors). The osteoporosis primer. Cambridge: Cambridge University Press; 2000:131-46.
- 43.Nyquist F, Gardsell P, Sernbo I, Jeppsson JO, Johnell O. Assessment of sex hormones and bone mineral density in relation to occurrence of fracture in men: a prospective population-based study. Bone 1998;22:147-51. [DOI] [PubMed]
- 44.Mussolino ME, Looker AC, Madans JH, Langlois JA, Orwoll ES. Risk factors for hip fracture in white men: the NHANES I Epidemiologic Follow-up Study. J Bone Miner Res 1998;13:918-24. [DOI] [PubMed]
- 45.Nguyen TV, Eisman JA, Kelly PJ, Sambrook PN. Risk factors for osteoporotic fractures in elderly men. Am J Epidemiol 1996;144:255-63. [DOI] [PubMed]
- 46.Dargent-Molina P, Favier F, Grandjean H, Baudoin C, Schott AM, Hausherr E, et al. Fall-related factors and risk of hip fracture: the EPIDOS prospective study [published erratum appears in Lancet 1996;348:416]. Lancet 1996;348:145-9. [DOI] [PubMed]
- 47.Dargent-Molina P, Schott AM, Hans D, Favier F, Grandjean H, Baudoin C, et al. Separate and combined value of bone mass and gait speed measurements in screening for hip fracture risk: results from the EPIDOS study. Osteoporos Int 1999;9:188-92. [DOI] [PubMed]
- 48.Adachi JD, Olszynski WP, Hanley DA, Hodsman AB, Kendler DL, Siminoski KG. Management of corticosteroid-induced osteoporosis. Semin Arthritis Rheum 2000;29:228-51. [DOI] [PubMed]
- 49.Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Use of oral corticosteroids and risk of fractures. J Bone Miner Res 2000;15:993-1000. [DOI] [PubMed]
- 50.Melton LJ III, Atkinson EJ, Cooper C, O'Fallon WM, Riggs BL. Vertebral fractures predict subsequent fractures. Osteoporos Int 1999;10:214-21. [DOI] [PubMed]
- 51.Faulkner K, Abbott TA, Furman WD, Panish J, Siris E, Miller P. Fracture risk assessment in NORA is comparable across peripheral sites. J Bone Miner Res 2001;16(suppl 1):S144.
- 52.Njeh CF, Hans D, Li J, Fan B, Fuerst T, He YQ, et al. Comparison of six calcaneal quantitative ultrasound devices: precision and hip fractures. Osteoporos Int 2000;11:1051-62. [DOI] [PubMed]
- 53.Woodhouse A, Black DM. BMD at various sites for the prediction of hip fractures: a meta analysis. J Bone Miner Res 2000;15(suppl 1):S145.
- 54.Genant HK, Grampp S, Gluer CC, Faulkner KG, Jergas M, Engelke K, et al. Universal standardisation for dual X-ray absorptiometry: patient and phantom cross-calibration results. J Bone Miner Res 1994;9:1503-14. [DOI] [PubMed]
- 55.Hui SL, Gao S, Zhou XH, Johnston CC, Lu Y, Gluer CC, et al. Universal standardization of bone density measurements: a method with optimal properties for calibration among several instruments. J Bone Miner Res 1997;12:1463-70. [DOI] [PubMed]
- 56.Rosenthall L, Caminis J, Tenehouse A. Calcaneal ultrasonometry: response to treatment in comparison with dual x-ray absorptiometry measurements of the lumbar spine and femur. Calcif Tissue Int 1999;64:200-4. [DOI] [PubMed]
- 57.Gardsell P, Johnell O, Nilsson BE. The predictive value of forearm bone mineral content measurements in men. Bone 1990;11:229-32. [DOI] [PubMed]
- 58.Cummings SR, Black DM, Nevitt MC, Browner W, Cauley J, Ensrud KE, et al. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet 1993;341:72-5. [DOI] [PubMed]
- 59.Ross P, Huang C, Davis J, Imose K, Yates J, Vogel J, et al. Predicting vertebral deformity using bone densitometry at various skeletal sites and calcaneus ultrasound. Bone 1995;16:325-32. [DOI] [PubMed]
- 60.Bauer DC, Gluer CC, Cauley JA, Vogt TM, Ensrud KE, Genant HK, et al. Broadband ultrasound attenuation predicts fractures strongly and independently of densitometry in older women. A prospective study. Arch Intern Med 1997;157:629-34. [PubMed]
- 61.Hans D, Dargent-Molina P, Schott AM, Sebert JL, Cormier C, Kotzki PO, et al. Ultrasonographic heel measurements to predict hip fracture in elderly women: the EPIDOS prospective study. Lancet 1996;348:511-4. [DOI] [PubMed]
- 62.Adami S, Zamberlan N, Gatti D, Zanfisi C, Braga V, Broggini M, et al. Computed radiographic absorptiometry and morphometry in the assessment of postmenopausal bone loss. Osteoporos Int 1996;6:8-13. [DOI] [PubMed]
- 63.Wishart JM, Horowitz M, Bochner M, Need AG, Nordin BEC. Relationships between metacarpal morphometry, forearm and vertebral bone density and fractures in postmenopausal women. Br J Radiol 1993;66:435-40. [DOI] [PubMed]
- 64.Ravn P, Overgaard K, Huang C, Ross PD, Green D, McClung M, et al. Comparison of bone densitometry of the phalanges, distal forearm and axial skeleton in early postmenopausal women participating in the EPIC study. Osteoporos Int 1996;6:308-13. [DOI] [PubMed]
- 65.Riggs BL, Melton LJ III. Bone turnover matters: the raloxifene treatment paradox of dramatic decreases in vertebral fractures without commensurate increases in bone density. J Bone Miner Res 2002;17:11-4. [DOI] [PubMed]
- 66.Gluer CC, Wu CY, Genant HK. Broadband ultrasound attenuation signals depend on trabecular orientation: an in-vitro study. Osteoporos Int 1993;3:185-91. [DOI] [PubMed]
- 67.Faulkner KG, McClung MR. Quality control of DXA instruments in multicenter trials. Osteoporos Int 1995;5:218-27. [DOI] [PubMed]
- 68.Blunt BA. DXA technologists: educate yourselves. Radiol Technol 1998;70:223-4. [PubMed]
- 69.Kolta S, Ravaud P, Fechtenbaum J, Dougados M, Roux C. Follow-up of individual patients on two DXA scanners of the same manufacturer. Osteoporos Int 2000;11:709-13. [DOI] [PubMed]
- 70.Ravaud P, Reny JL, Giraudeau B, Porcher R, Dougados M, Roux C. Individual smallest detectable difference in bone mineral density measurements. J Bone Miner Res 1999;14:1449-56. [DOI] [PubMed]
- 71.Sievanen H, Oja P, Vuori I. Precision of dual energy x-ray absortiometry in determining bone mineral density and content of various skeletal sites. J Nucl Med 1992;33:1137-42. [PubMed]
- 72.Wahner HW, Looker A, Dunn WL, Walters LC, Hauser MF, Novak C. Quality control of bone densitometry in a national health survey (NHANES III) using three mobile examination centers. J Bone Miner Res 1994;9:951-60. [DOI] [PubMed]
- 73.Gluer CC, Blake G, Blunt BA, Jergas M, Genant HK. Accurate assessment of precision errors: how to measure the reproducibility of bone densitometry techniques. Osteoporos Int 1995;5:262-70. [DOI] [PubMed]
- 74.Gluer C. Monitoring skeletal changes by radiological techniques. J Bone Miner Res 1999;14:1952-62. [DOI] [PubMed]
- 75.Melton LJ III, Atkinson EJ, O'Connor MK, O'Fallon WM, Riggs BL. Bone density and fracture risk in men. J Bone Miner Res 1998;13:1915-23. [DOI] [PubMed]
- 76.de Laet CE, Van Hout BA, Burger H, Weel AE, Hofman A, Pols HAP. Hip fracture prediction in elderly men and women: validation in the Rotterdam study. J Bone Miner Res 1998;13:1587-93. [DOI] [PubMed]
- 77.Cheng S, Suominen H, Sakari-Rantala R, Laukkanen P, Avikainen V, Heikkinen E. Calcaneal bone mineral density predicts fracture occurrence: a five-year follow-up study in elderly people. J Bone Miner Res 1997;12:1075-82. [DOI] [PubMed]
- 78.Gilsanz V, Boechat MI, Gilsanz R, Loro ML, Roe TF, Goodman WG. Gender differences in vertebral sizes in adults: biomechanical implications. Radiology 1994;190:678-82. [DOI] [PubMed]
- 79.Seeman E. From density to structure: growing up and growing old on the surfaces of bone. J Bone Miner Res 1997;12:509-21. [DOI] [PubMed]
- 80.Kanis JA, Gluer CC. An update on the diagnosis and assessment of osteoporosis with densitometry. Osteoporos Int 2000; 11:192-202. [DOI] [PubMed]
- 81.Van der Klift M, de Laet CE, McCloskey EV, Hofman A, Pols HA. The incidence of vertebral fractures in men and women: the Rotterdam study. J Bone Miner Res 2002;17:1051-6. [DOI] [PubMed]
- 82.Looker AC, Orwoll ES, Johnston CC Jr, Lindsay RL, Wahner HW, Dunn WL, et al. Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res 1997;12:1761-8. [DOI] [PubMed]
- 83.Clemmesen B, Ravn P, Zegels B, Taquet AN, Christiansen C, Reginster JY. A 2-year phase II study with 1-year of follow-up of risedronate (NE-58095) in postmenopausal osteoporosis. Osteoporos Int 1997;7:488-95. [DOI] [PubMed]
- 84.Chesnut CH III, McClung M, Ensrud KE, Bell N, Genant H, Harris S. Alendronate treatment of the postmenopausal osteoporotic woman: effect of multiple dosages on bone mass and bone remodeling. Am J Med 1995;99:144-52. [DOI] [PubMed]
- 85.Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, Bell NH, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. N Engl J Med 1995;333:1437-43. [DOI] [PubMed]
- 86.Devogelaer JP, Broll H, Correa-Rotter R, Cumming DC, De Deuxchaisnes DC, Geusens P, et al. Oral alendronate induces progressive increases in bone mass of the spine, hip, and total body over three years in postmenopausal women with osteoporosis. Bone 1996;18:141-50. [DOI] [PubMed]
- 87.Garnero P, Sornay-Rendu E, Duboeuf F, Delmas PD. Markers of bone turnover predict postmenopausal forearm bone loss over 4 years: the OFELY study. J Bone Miner Res 1999;14:1614-21. [DOI] [PubMed]
- 88.Chesnut CH III, Bell NH, Clark GS, Drinkwater BL, English SC, Johnston CC Jr, et al. Hormone replacement therapy in postmenopausal women: urinary N-telopeptide of type I collagen monitors therapeutic effect and predicts response of bone mineral density. Am J Med 1997;102:29-37. [DOI] [PubMed]
- 89.Garnero P, Sornay-Rendu E, Chapuy MC, Delmas PD. Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res 1996;11:337-49. [DOI] [PubMed]
- 90.Garnero P, Dargent-Molina P, Hans D, Schott AM, Bréart G, Meunier PJ, et al. Do markers of bone resorption add to bone mineral density and ultrasonographic heel measurement for the prediction of hip fracture in elderly women? Osteoporos Int 1998;8:563-9. [DOI] [PubMed]
- 91.Rogers A, Hannon RA, Eastell R. Biochemical markers as predictors of rates of bone loss after menopause. J Bone Miner Res 2000;15:1398-404. [DOI] [PubMed]
- 92.Rosen CJ, Chesnut CH III, Mallinak NJS. The predictive value of biochemical markers of bone turnover for bone mineral density in early postmenopausal women treated with hormone replacement or calcium Supplementation. J Clin Endocrinol Metab 1997;82:1904-10. [DOI] [PubMed]
- 93.Keen RW, Nguyen T, Sobnack R, Perry LA, Thompson PW, Spector TD. Can biochemical markers predict bone loss at the hip and spine?: a 4-year prospective study of 141 early postmenopausal women. Osteoporos Int 1996;6:399-406. [DOI] [PubMed]
- 94.Szulc P, Arlot M, Chapuy MC, Duboeuf F, Meunier PJ, Delmas PD. Serum undercarboxylated osteocalcin correlates with hip bone mineral density in elderly women. J Bone Miner Res 1994;9:1591-5. [DOI] [PubMed]
- 95.Eastell R, Robins SP, Colwell T, Assiri AMA, Riggs BL, Russell RGG. Evaluation of bone turnover in type I osteoporosis using biochemical markers specific for both bone formation and bone resorption. Osteoporos Int 1993;3:255-60. [DOI] [PubMed]
- 96.Garnero P, Hausherr E, Chapuy MC, Marcelli C, Grandjean H, Muller C, et al. Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res 1996;11:1531-8. [DOI] [PubMed]
- 97.Looker AC, Bauer DC, Chesnut CH III, Gundberg M, Hochberg MC, Klee G, et al. Clinical use of biochemical markers of bone remodeling: current status and future directions. Osteoporos Int 2000;11:467-80. [DOI] [PubMed]
- 98.Kress BC, Mizrahi IA, Armour KW, Marcus R, Emkey RD, Santora AC. Use of bone alkaline phosphatase to monitor alendronate therapy in individual postmenopausal osteoporotic women. Clin Chem 1999;45:1009-17. [PubMed]
- 99.Eastell R, Barton I, Hannon RA, Garnero P, Chines A, Pack S, et al. Antifracture efficacy of risedronate: prediction by change in bone resorption markers. J Bone Miner Res 2001;16 suppl:S163.
- 100.Khosla S, Melton LJ III, Atkinson EJ, O'Fallon WM, Klee GG, Riggs BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab 1998;83:2266-74. [DOI] [PubMed]
- 101.Fleisch HA. Bisphosphonates: preclinical aspects and use in osteoporosis. Ann Med 1997;29:55-62. [DOI] [PubMed]
- 102.Russell RG, Rogers MJ. Bisphosphonates: from the laboratory to the clinic and back again. Bone 1999;25:97-106. [DOI] [PubMed]
- 103.Evans RA, Somers NM, Dunstan CR, Royle H, Kos S. The effect of low-dose cyclical etidronate and calcium on bone mass in early postmenopausal women. Osteoporos Int 1993;3:71-5. [DOI] [PubMed]
- 104.Guanabens N, Farrerons J, Perez-Edo L, Monegal A, Renau A, Carbonell J. Cyclical etidronate versus sodium fluoride in established postmenopausal osteoporosis: a randomized 3-year trial. Bone 2000;27:123-8. [DOI] [PubMed]
- 105.Gurlek A, Bayraktar M, Gedik O. Comparison of calcitriol treatment with etidronate-calcitriol and calcitonin-calcitriol combinations in Turkish women with postmenopausal osteoporosis: a prospective study. Calcif Tissue Int 1997;61:39-43. [DOI] [PubMed]
- 106.Harris ST, Watts NB, Jackson RD, Genant HK, Wasnich RD, Ross P. Four-year study of intermittent cyclic etidronate treatment of postmenopausal osteoporosis: three years of blinded therapy followed by one year of open therapy. Am J Med 1993;95:557-67. [DOI] [PubMed]
- 107.Heath DA, Bullivant BG, Boiven C, Balena R. The effects of cyclical etidronate on early postmenopausal bone loss: An open, randomized controlled study. J Clin Densitometry 2000;3:27-33. [DOI] [PubMed]
- 108.Herd RJ, Balena R, Blake GM, Ryan PJ, Fogelman I. The prevention of early postmenopausal bone loss by cyclical etidronate therapy: a 2-year, double-blind, placebo-controlled study. Am J Med 1997;103:92-9. [DOI] [PubMed]
- 109.Meunier PJ, Confavreux E, Tupinon I, Hardouin C, Delmas PD, Balena R. Prevention of early postmenopausal bone loss with cyclical etidronate therapy (a double-blind placebo-controlled study and 1-year follow-up). J Clin Endocrinol Metab 1997;82:2784-91. [DOI] [PubMed]
- 110.Mukherjee T, Barad D, Turk R, Freeman R. A randomized, placebo-controlled study on the effect of cyclic intermittent etidronate therapy on the bone mineral density changes associated with six months of gonadotropin-releasing hormone agonist treatment. Am J Obstet Gynecol 1996;175:105-9. [DOI] [PubMed]
- 111.Storm T, Thamsborg G, Steiniche T, Genant HK, Sorensen OH. Effect of intermittent cyclical etidronate therapy on bone mass and fracture rate in women postmenopausal osteoporosis. N Engl J Med 1990;322:1265-71. [DOI] [PubMed]
- 112.Surrey ES, Voigt B, Fournet N, Judd HL. Prolonged gonadotropin-releasing hormone agonist treatment of symptomatic endometriosis: the role of cyclic sodium etidronate and low-dose norethindrone "add-back" therapy. Fertil Steril 1995;63:747-55. [PubMed]
- 113.Watts NB, Harris ST, Genant HK, Wasnich RD, Miller PD, Jackson RD, et al. Intermittent cyclical etidronate treatment of postmenopausal osteoporosis. N Engl J Med 1990;323:73-9. [DOI] [PubMed]
- 114.Schnitzer T, Bone HG, Crepaldi G, Adami S, McClung M, Kiel D, et al. Therapeutic equivalence of alendronate 70 mg once-weekly and alendronate 10 mg daily in the treatment of osteoporosis. Aging (Milano) 2000;12:1-12. [PubMed]
- 115.Liberman UI, Hirsch LJ. Esophagitis and alendronate. N Engl J Med 1996;335:1069-70. [DOI] [PubMed]
- 116.Adami S, Passeri M, Ortolani S, Broggini M, Carratelli L, Caruso I, et al. Effects of oral alendronate and intranasal salmon calcitonin on bone mass and biochemical markers of bone turnover in postmenopausal women with osteoporosis. Bone 1995;17:383-90. [DOI] [PubMed]
- 117.Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996;348:1535-41. [DOI] [PubMed]
- 118.Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor EL, Musliner TA, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 1998;280:2077-82. [DOI] [PubMed]
- 119.Downs RW Jr, Bell NH, Ettinger MP, Walsh BW, Favus MJ, Mako B, et al. Comparison of alendronate and intranasal calcitonin for treatment of osteoporosis in postmenopausal women. J Clin Endocrinol Metab 2000;85:1783-8. [DOI] [PubMed]
- 120.Gonnelli S, Cepollaro C, Pondrelli C, Martini S, Montagnani A, Monaco R, et al. Bone turnover and the response to alendronate treatment in postmenopausal osteoporosis. Calcif Tissue Int 1999;65:359-64. [DOI] [PubMed]
- 121.Bone HG, Greenspan SL, McKeever C, Bell N, Davidson M, Downs RW, et al. Alendronate and estrogen effects in postmenopausal women with low bone mineral density. Alendronate/Estrogen Study Group. J Clin Endocrinol Metab 2000;85:720-6. [DOI] [PubMed]
- 122.Greenspan SL, Parker RA, Ferguson L, Rosen HN, Maitland-Ramsey L, Karpf DB. Early changes in biochemical markers of bone turnover predict the long-term response to alendronate therapy in representative elderly women: a randomized clinical trial. J Bone Miner Res 1998;13:1431-8. [DOI] [PubMed]
- 123.Hosking D, Chilvers CED, Christiansen C, Ravn P, Wasnich R, Ross P, et al. Prevention of bone loss with alendronate in postmenopausal women under 60 years of age. N Engl J Med 1998;338:485-92. [DOI] [PubMed]
- 124.Lindsay R, Cosman F, Lobo RA, Walsh BW, Harris ST, Reagan JE, et al. Addition of alendronate to ongoing hormone replacement therapy in the treatment of osteoporosis: a randomized, controlled clinical trial. J Clin Endocrinol Metab 1999;84:3076-81. [DOI] [PubMed]
- 125.McClung M, Clemmesen B, Daifotis A. Alendronate prevents postmenopausal bone loss in women without osteoporosis. A double-blind, randomized, controlled trial. Ann Intern Med 1998;128:253-61. [DOI] [PubMed]
- 126.Pivonello R, Faggiano A, Di Somma C, Klain M, Filippella M, Salvatore M, et al. Effect of a short-term treatment with alendronate on bone density and bone markers in patients with central diabetes insipidus. J Clin Endocrinol Metab 1999;84:2349-52. [DOI] [PubMed]
- 127.Pols HAP, Felsenberg D, Hanley DA, Stepan J, Munoz-Torres M, Wilkin TJ, et al. A multinational, placebo-controlled, randomized study of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: Results of the FOSIT Study. Osteoporos Int 1999;9:461-8. [DOI] [PubMed]
- 128.Ravn P, Bidstrup M, Wasnich RD, Davis JW, McClung MR, Balske A, et al. Alendronate and estrogen-progestin in the long-term prevention of bone loss: Four-year results from the early postmenopausal intervention cohort study: A randomized, controlled trial. Ann Intern Med 1999;131:935-42. [DOI] [PubMed]
- 129.Schneider PF, Fischer M, Allolio B, Felsenberg D, Schroder U, Semler J, et al. Alendronate increases bone density and bone strength at the distal radius in postmenopausal women. J Bone Miner Res 1999;14:1387-93. [DOI] [PubMed]
- 130.Shiraki M, Kushida K, Fukunaga M, Kishimoto H, Kaneda K, Minaguchi H, et al. A placebo-controlled, single-blind study to determine the appropriate alendronate dosage in postmenopausal Japanese patients with osteoporosis. Endocr J 1998;45:191-201. [DOI] [PubMed]
- 131.Shiraki M, Kushida K, Fukunaga M, Kishimoto H, Taga M, Nakamura T, et al. A double-masked multicenter comparative study between alendronate and alfacalcidol in Japanese patients with osteoporosis. Osteoporos Int 1999;10:183-92. [DOI] [PubMed]
- 132.Tucci JR, Tonino RP, Emkey RD, Peverly CA, Kher U, Santora AC. Effect of three years of oral alendronate treatment in postmenopausal women with osteoporosis. Am J Med 1996;101:488-501. [DOI] [PubMed]
- 133.Black DM, Thompson DE, Bauer DC, Ensrud K, Musliner T, Hochberg MC. Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial (FIT) Research Group. J Clin Endocrinol Metab 2000;85:118-24. [DOI] [PubMed]
- 134.Delmas PD, Balena R, Confravreux E, Hardouin C, Hardy P, Bremond A. Bisphosphonate risedronate prevents bone loss in women with artificial menopause due to chemotherapy of breast cancer: a double-blind, placebo-controlled study. J Clin Oncol 1997;15:955-62. [DOI] [PubMed]
- 135.Fogelman I, Ribot C, Smith R, Ethgen D, Sod E, Reginster JY. Risedronate reverses bone loss in postmenopausal women with low bone mass: results from a multinational, double-blind, placebo-controlled trial. BMD-MN Study Group. J Clin Endocrinol Metab 2000;85:1895-900. [DOI] [PubMed]
- 136.Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA 1999;282:1344-52. [DOI] [PubMed]
- 137.Reginster JY, Minne HW, Sorensen OH, Hooper M, Roux C, Brandi ML, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Osteoporos Int 2000; 11:83-91. [DOI] [PubMed]
- 138.Brown JP, Kendler DL, McClung MR, Emkey RD, Adachi JD, Bolognese MA, et al. The efficacy and tolerability of risedronate once a week for the treatment of postmenopausal osteoporosis. Calcif Tissue Int 2002;71:103-11. [DOI] [PubMed]
- 139.Hodsman AB, Hanley DA, Josse R. Do bisphosphonates reduce the risk of osteoporotic fractures? An evaluation of the evidence to date. CMAJ 2002;166:1426-30. [PMC free article] [PubMed]
- 140.Wimalawansa SJ. Combined therapy with estrogen and etidronate has an additive effect on bone mineral density in the hip and vertebrae: four-year randomized study. Am J Med 1995;99:36-42. [DOI] [PubMed]
- 141.Wimalawansa SJ. A four-year randomized controlled trial of hormone replacement and bisphosphonate, alone or in combination, in women with postmenopausal osteoporosis. Am J Med 1998;104:219-26. [DOI] [PubMed]
- 142.Orwoll E, Ettinger M, Weiss S, Miller P, Kendler D, Graham J, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med 2000;343: 604-10. [DOI] [PubMed]
- 143.Reid DM, Adami S, Devogelaer JP, Chines AA. Risedronate increases bone density and reduces vertebral fracture risk within one year in men on corticosteroid therapy. Calcif Tissue Int 2001;69:242-7. [DOI] [PubMed]
- 144.Adachi JD, Bensen WG, Brown J, Hanley D, Hodsman A, Josse R, et al. Intermittent etidronate therapy to prevent corticosteroid-induced osteoporosis. N Engl J Med 1997;337:382-7. [DOI] [PubMed]
- 145.Cortet B, Hachulla E, Barton I, Bonvoisin B, Roux C. Evaluation of the efficacy of etidronate therapy in preventing glucocorticoid-induced bone loss in patients with inflammatory rheumatic diseases: A randomized study. Rev Rhum Engl Ed 1999;66:214-9. [PubMed]
- 146.Geusens P, Dequeker J, Vanhoof J, Stalmans R, Boonen S, Joly J, et al. Cyclical etidronate increases bone density in the spine and hip of postmenopausal women receiving long term corticosteroid treatment. A double blind, randomised placebo controlled study. Ann Rheum Dis 1998;57:724-7. [DOI] [PMC free article] [PubMed]
- 147.Jenkins EA, Walker-Bone KE, Wood A, McCrae FC, Cooper C, Cawley MID. The prevention of corticosteroid-induced bone loss with intermittent cyclical etidronate. Scand J Rheumatol 1999;28:152-6. [DOI] [PubMed]
- 148.Lems WF, Jacobs JW, Bijlsma JW, van Veen GJ, Houben HH, Haanen HC, et al. Is addition of sodium fluoride to cyclical etidronate beneficial in the treatment of corticosteroid induced osteoporosis? Ann Rheum Dis 1997;56:357-63. [DOI] [PMC free article] [PubMed]
- 149.Mulder H, Struys A. Intermittent cyclical etidronate in the prevention of corticosteroid-induced bone loss. Br J Rheumatol 1994;33:348-50. [DOI] [PubMed]
- 150.Pitt P, Li F, Todd P, Webber D, Pack S, Moniz C. A double blind placebo controlled study to determine the effects of intermittent cyclical etidronate on bone mineral density in patients on long-term oral corticosteroid treatment. Thorax 1998;53:351-6. [DOI] [PMC free article] [PubMed]
- 151.Roux C, Oriente P, Laan R, Hughes RA, Ittner J, Goemaere S, et al. Randomized trial of effect of cyclical etidronate in the prevention of corticosteroid-induced bone loss. Ciblos Study Group. J Clin Endocrinol Metab 1998;83:1128-33. [DOI] [PubMed]
- 152.Skingle SJ, Crisp AJ. Increased bone density in patients on steroids with etidronate. Lancet 1994;344:543-4. [DOI] [PubMed]
- 153.Struys A, Snelder AA, Mulder H. Cyclical etidronate reverses bone loss of the spine and proximal femur in patients with established corticosteroid-induced osteoporosis. Am J Med 1995;99:235-42. [DOI] [PubMed]
- 154.Wolfhagen FH, van Buuren HR, den Ouden JW, Hop WC, van Leeuwen JP, Schalm SW, et al. Cyclical etidronate in the prevention of bone loss in corticosteroid-treated primary biliary cirrhosis. A prospective, controlled pilot study. J Hepatol 1997;26:325-30. [DOI] [PubMed]
- 155.Worth H, Stammen D, Keck E. Therapy of steroid-induced bone loss in adult asthmatics with calcium, vitamin D, and a diphosphonate. Am J Resp Crit Care Med 1994;150:394-7. [DOI] [PubMed]
- 156.Brown JP, Olszynski W, Hodsman A, Bensen W, Tenenhouse A, Anastassiades T, et al. The positive effect of etidronate therapy is maintained after the drug is terminated in patients who are using corticosteroids. J Clin Densitometry 2001; 4(4):363-71. [DOI] [PubMed]
- 157.Gonnelli S, Rottoli P, Cepollaro C, Pondrelli C, Cappiello V, Vagliasindi M, et al. Prevention of corticosteroid-induced osteoporosis with alendronate in sarcoid patients. Calcif Tissue Int 1997;61:382-5. [DOI] [PubMed]
- 158.Saag KG, Emkey R, Schnitzer TJ, Brown JP, Hawkins F, Goemaere S, et al. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. Glucocorticoid-Induced Osteoporosis Intervention Study Group. N Engl J Med 1998;339:292-9. [DOI] [PubMed]
- 159.Adachi JD, Saag KG, Delmas PD, Liberman UA, Emkey RD, Seeman E. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum 2001;44:202-11. [DOI] [PubMed]
- 160.Di Somma C, Colao A, Pivonello R, Klain M, Faggiano A, Tripodi FS, et al. Effectiveness of chronic treatment with alendronate in the osteoporosis of Cushing's disease. Clin Endocrinol 1998;48:655-62. [DOI] [PubMed]
- 161.Cohen S, Levy RM, Keller M, Boling E, Emkey RD, Greenwald M, et al. Risedronate therapy prevents corticosteroid-induced bone loss: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum 1999;42:2309-18. [DOI] [PubMed]
- 162.Reid DM, Hughes RA, Laan RF, Sacco-Gibson NA, Wenderoth DH, Adami S. Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: a randomized trial. European Cortciosteroid-Induced Osteoporosis Treatment Study. J Bone Miner Res 2000;15: 1006-13. [DOI] [PubMed]
- 163.Wallach S, Cohen S, Reid DM, Hughes RA, Hosking DJ, Laan RF. Effects of risedronate treatment on bone density and vertebral fracture in patients on corticosteroid therapy. Calcif Tissue Int 2000;67:277-85. [DOI] [PubMed]
- 164.Anderson FH, Francis RM, Bishop JC, Rawlings DJ. Effect of intermittent cyclical disodium etidronate therapy on bone mineral density in men with vertebral fractures. Age Ageing 1997;26:359-65. [DOI] [PubMed]
- 165.Repchinsky C (editor). Compendium of pharmaceuticals and specialties. 36. Ottawa: Canadian Pharmacists Association; 2001:236-7.
- 166.Repchinsky C (editor). Compendium of pharmaceuticals and specialties. 36. Ottawa: Canadian Pharmacists Association; 2001:244-5.
- 167.Repchinsky C (editor). Compendium of pharmaceuticals and specialties. 36. Ottawa: Canadian Pharmacists Association 2001:924-6.
- 168.Chesnut CH III, Silverman S, Andriano K, Genant HK, Gimona A, Harris S. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the Prevent Recurrence of Osteoporotic Fractures Study. Am J Med 2000;109:267-76. [DOI] [PubMed]
- 169.Gonnelli S, Cepollaro C, Pondrelli C, Martini S, Rossi S, Gennari C. Ultrasound parameters in osteoporotic patients treated with salmon calcitonin: a longitudinal study. Osteoporos Int 1996;6:303-7. [DOI] [PubMed]
- 170.Reginster JY, Meurmans L, Deroisy R, Jupsin I, Biquet I, Albert A, et al. A 5-year controlled randomized study of prevention of postmenopausal trabecular bone loss with nasal salmon calcitonin and calcium. Eur J Clin Invest 1994; 24:565-9. [DOI] [PubMed]
- 171.Kapetanos G, Symeonides PP, Dimitriou C, Karakatsanis K, Potoupnis M. A double blind study of intranasal calcitonin for established postmenopausal osteoporosis. Acta Orthop Scand Suppl 1997;275:108-11. [DOI] [PubMed]
- 172.Reginster JY, Deroisy R, Lecart MP, Sarlet N, Zegels B, Jupsin I, et al. A double-blind, placebo-controlled, dose-finding trial of intermittent nasal salmon calcitonin for prevention of postmenopausal lumbar spine bone loss. Am J Med 1995;98:452-8. [DOI] [PubMed]
- 173.Overgaard K, Riis BJ, Christiansen C, Hansen MA. Effect of salcatonin given intranasally on early postmenopausal bone loss. BMJ 1989;299:477-9. [DOI] [PMC free article] [PubMed]
- 174.Overgaard K, Hansen MA, Jensen SB, Christiansen C. Effect of salcatonin given intranasally on bone mass and fracture rates in established osteoporosis: a dose-response study. BMJ 1992;305:556-61. [DOI] [PMC free article] [PubMed]
- 175.Rico H, Hernandez ER, Revilla M, Gomez-Castresana F. Salmon calcitonin reduces vertebral fracture rate in postmenopausal crush fracture syndrome. Bone Miner 1992;16:131-8. [DOI] [PubMed]
- 176.Thamsborg G, Jensen JE, Kollerup G, Hauge EM, Melsen F, Sorensen OH. Effect of nasal salmon calcitonin on bone remodeling and bone mass in postmenopausal osteoporosis. Bone 1996;18:207-12. [DOI] [PubMed]
- 177.Gennari C, Agnusdei D, Montagnani M, Gonnelli S, Civitelli R. An effective regimen of intranasal salmon calcitonin in early postmenopausal bone loss. Calcif Tissue Int 1992;50:381-3. [DOI] [PubMed]
- 178.Thamsborg G, Storm TL, Sykulski R, Brinch E, Nielsen HK, Sorensen OH. Effect of different doses of nasal salmon calcitonin on bone mass. Calcif Tissue Int 1991;48:302-7. [DOI] [PubMed]
- 179.Rico H, Revilla M, Hernandez ER, Villa LF, Alvarez de Buergo M. Total and regional bone mineral content and fracture rate in postmenopausal osteoporosis treated with salmon calcitonin: a prospective study. Calcif Tissue Int 1995;56:181-5. [DOI] [PubMed]
- 180.Overgaard K. Effect of intranasal salmon calcitonin therapy on bone mass and bone turnover in early postmenopausal women: a dose-response study. Calcif Tissue Int 1994;55:82-6. [DOI] [PubMed]
- 181.Lyritis GP, Magiasis B, Tsakalakos N. Prevention of bone loss in early nonsurgical and nonosteoporotic high turnover patients with salmon calcitonin: the role of biochemical bone markers in monitoring high turnover patients under calcitonin treatment. Calcif Tissue Int 1995;56:38-41. [DOI] [PubMed]
- 182.Ellerington MC, Hillard TC, Whitcroft SI, Marsh MS, Lees B, Banks LM, et al. Intranasal salmon calcitonin for the prevention and treatment of postmenopausal osteoporosis. Calcif Tissue Int 1996;59:6-11. [DOI] [PubMed]
- 183.Overgaard K, Riis BJ, Christiansen C, Podenphant J, Johansen JS. Nasal calcitonin for treatment of established osteoporosis. Clin Endocrinol 1989;30: 435-42. [DOI] [PubMed]
- 184.Reginster JY, Denis D, Deroisy R, Lecart MP, de Longueville M, Zegels B, et al. Long-term (3 years) prevention of trabecular postmenopausal bone loss with low-dose intermittent nasal salmon calcitonin. J Bone Miner Res 1994;9: 69-73. [DOI] [PubMed]
- 185.Reginster JY, Denis D, Albert A, Deroisy R, Lecart MP, Fontaine MA, et al. 1-Year controlled randomised trial of prevention of early postmenopausal bone loss by intranasal calcitonin. Lancet 1987;2:1481-3. [DOI] [PubMed]
- 186.Fioretti P, Gambacciani M, Taponeco F, Melis GB, Capelli N, Spinetti A. Effects of continuous and cyclic nasal calcitonin administration in ovariectomized women. Maturitas 1992;15:225-32. [DOI] [PubMed]
- 187.Grigoriou O, Papoulias I, Vitoratos N, Papadias C, Konidaris S, Antoniou G, et al. Effects of nasal administration of calcitonin in oophorectomized women: 2-year controlled double-blind study. Maturitas 1997;28:147-51. [DOI] [PubMed]
- 188.Mango D, Ricci S, Manna P, Natili G, Dell'Acqua S. Preventive treatment of cortical bone loss with salmon nasal calcitonin in early postmenopausal women. Minerva Endocrinol 1993;18:115-21. [PubMed]
- 189.Flicker L, Hopper JL, Larkins RG, Lichtenstein M, Buirski G, Wark JD. Nandrolone decanoate and intranasal calcitonin as therapy in established osteoporosis. Osteoporos Int 1997;7:29-35. [DOI] [PubMed]
- 190.Hizmetli S, Elden H, Kaptanoglu E, Nacitarhan V, Kocagil S. The effect of different doses of calcitonin on bone mineral density and fracture risk in postmenopausal osteoporosis. Int J Clin Pract 1998;52:453-5. [PubMed]
- 191.Arnala I, Saastamoinen J, Alhava EM. Salmon calcitonin in the prevention of bone loss at perimenopause. Bone 1996;18:629-32. [DOI] [PubMed]
- 192.Luengo M, Picado C, Del Rio L, Guanabens N, Montserrat JM, Setoain J. Treatment of steroid-induced osteopenia with calcitonin in corticosteroid-dependent asthma. A one-year follow-up study. Am Rev Respir Dis 1990;142: 104-7. [DOI] [PubMed]
- 193.Adachi JD, Bensen WG, Bell MJ, Bianchi FA, Cividino AA, Craig GL, et al. Salmon calcitonin nasal spray in the prevention of corticosteroid-induced osteoporosis. Br J Rheumatol 1997;36:255-9. [DOI] [PubMed]
- 194.Healey JH, Paget SA, Williams-Russo P, Szatrowski TP, Schneider R, Spiera H, et al. A randomized controlled trial of salmon calcitonin to prevent bone loss in corticosteroid-treated temporal arteritis and polymyalgia rheumatica. Calcif Tissue Int 1996;58:73-80. [DOI] [PubMed]
- 195.Ringe JD, Welzel D. Salmon calcitonin in the therapy of corticoid-induced osteoporosis. Eur J Clin Pharmacol 1987;33:35-9. [DOI] [PubMed]
- 196.Kotaniemi A, Piirainen H, Paimela L, Leirisalo-Repo M, Uoti-Reilama K, Lahdentausta P, et al. Is continuous intranasal salmon calcitonin effective in treating axial bone loss in patients with active rheumatoid arthritis receiving low dose glucocorticoid therapy? J Rheumatol 1996;23:1875-9. [PubMed]
- 197.Luengo M, Pons F, Martinez de Osaba MJ, Picado C. Prevention of further bone mass loss by nasal calcitonin in patients on long term glucocorticoid therapy for asthma: a two year follow up study. Thorax 1994;49:1099-102. [DOI] [PMC free article] [PubMed]
- 198.Grotz WH, Rump LC, Niessen A, Schmidt-Gayk H, Reichelt A, Kirste G, et al. Treatment of osteopenia and osteoporosis after kidney transplantation. Transplantation 1998;66:1004-8. [DOI] [PubMed]
- 199.Lyritis GP, Paspati I, Karachalios T, Ioakimidis D, Skarantavos G, Lyritis PG. Pain relief from nasal salmon calcitonin in osteoporotic vertebral crush fractures. A double blind, placebo-controlled clinical study. Acta Orthop Scand Suppl 1997;68:112-4. [DOI] [PubMed]
- 200.Lyritis GP, Tsakalakos N, Magiasis B, Karachalios T, Yiatzides A, Tsekoura M. Analgesic effect of salmon calcitonin in osteoporotic vertebral fractures: a double-blind placebo-controlled clinical study. Calcif Tissue Int 1991;49:369-72. [DOI] [PubMed]
- 201.Pun KK, Chan LW. Analgesic effect of intranasal salmon calcitonin in the treatment of osteoporotic vertebral fractures. Clin Ther 1989;11:205-9. [PubMed]
- 202.Lauro R, Palmier G. Effects of calcitonin on pain related to recent osteoporotic vertebral fractures: a single-blind controlled clinical study against ipriflavone. Acta Toxicol Ther 1993;14:73-83.
- 203.Combe B, Cohen C, Aubin F. Equivalence of nasal spray and subcutaneous formulations of salmon calcitonin. Calcif Tissue Int 1997;61:10-5. [DOI] [PubMed]
- 204.MacLennan A, Lester S, Moore V. Oral estrogen replacement therapy versus placebo for hot flushes: a systematic review. Climacteric 2001;4:58-74. [PubMed]
- 205.Prior JC. Perimenopause: The complex endocrinology of the menopausal transition. Endocr Rev 1998;19:397-428. [DOI] [PubMed]
- 206.Okano H, Mizunama H, Soda M, Kagami I, Miyamoto S, Ohsawa M, et al. The long term effect of menopause on postmenopausal bone loss in Japanese women: results from a prospective study. J Bone Miner Res 1998;13:303-9. [DOI] [PubMed]
- 207.Cummings SR, Kelsey JL, Nevitt MC, O'Dowd KJ. Epidemiology of osteoporosis and osteoporotic fractures. Epidemiol Rev 1985;7:178-208. [DOI] [PubMed]
- 208.Petitti DB. Hormone replacement therapy and heart disease prevention. Experimentation trumps observation [editorial]. JAMA 1998;280:650-2. [DOI] [PubMed]
- 209.Lufkin EG, Wahner HW, O'Fallon WM, Hodgson SF, Kotowicz MA, Lane AW, et al. Treatment of postmenopausal osteoporosis with transdermal estrogen. Ann Intern Med 1992;117:1-9. [DOI] [PubMed]
- 210.Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative Randomized Controlled Trial. JAMA 2002;288:321-33. [DOI] [PubMed]
- 211.Grady D, Wenger NK, Herrington D, Khan S, Furberg C, Hunninghake D, et al. Postmenopausal hormone therapy increases risk for venous thromboembolic disease: the heart and estrogen/progestin replacement study. Ann Intern Med 2000;132:689-96 [DOI] [PubMed]
- 212.Persson I, Weiderpass E, Bergkvist L, Bergstrom R, Schairer C. Risks of breast and endometrial cancer after estrogen and estrogen-progestin replacement. Cancer Causes Control 1999;10:253-60. [DOI] [PubMed]
- 213.Persson I, Adami HO, Bergkvist L, Lindgren A, Pettersson B, Hoover R, et al. Risk of endometrial cancer after treatment with oestrogens alone or in conjunction with progestogens: results of a prospective study. BMJ 1989;298: 147-51. [DOI] [PMC free article] [PubMed]
- 214.Paganini-Hill A, Ross RK, Henderson BE. Endometrial cancer and patterns of use of oestrogen replacement therapy: a cohort study. Br J Cancer 1989;59:445-7. [DOI] [PMC free article] [PubMed]
- 215.Persson IR, Adami HO, Eklund G, Johansson ED, Lindberg BS, Lindgren A. The risk of endometrial neoplasia and treatment with estrogens and estrogen-progestogen combinations. First results of a cohort study after one to four completed years of observation. Acta Obstet Gynecol Scand 1986;65:211-7. [DOI] [PubMed]
- 216.Gambrell RD Jr. Hormones in the etiology and prevention of breast and endometrial cancer. South Med J 1984;77:1509-15. [DOI] [PubMed]
- 217.Cooper C, Stakkestad JA, Radowicki S, Hardy P, Pilate C, Dain MP, et al. Matrix delivery transdermal 17beta-estradiol for the prevention of bone loss in postmenopausal women. Osteoporos Int 1999;9:358-66. [DOI] [PubMed]
- 218.The Writing Group for the PEPI Trial. Effects of hormone therapy on bone mineral density: results from the postmenopausal estrogen/progestin interventions (PEPI) trial. JAMA 1996;276:1389-96. [PubMed]
- 219.Recker RR, Davies KM, Dowd RM, Heaney RP. The effect of low-dose continuous estrogen and progesterone therapy with calcium and vitamin D on bone in elderly women. A randomized, controlled trial. Ann Intern Med 1999;130:897-904. [DOI] [PubMed]
- 220.Weiss SR, Ellman H, Dolker M, For the transdermal estradiol investigator group. A randomized controlled trial of four doses of transdermal estradiol for preventing postmenopausal bone loss. Obstet Gynecol 1999;94:330-6. [DOI] [PubMed]
- 221.Grese TA, Sluka JP, Bryant HU, Cullinan GJ, Glasebrook AL, Jones CD. Molecular determinants of tissue selectivity in estrogen receptor modulators. Proc Natl Acad Sci 1997;94:14105-10. [DOI] [PMC free article] [PubMed]
- 222.Maricic M, Adachi JD, Sarkar S, Wu W, Wong M, Harper KD. Early effects of raloxifene on clinical vertebral fractures at 12 months in postmenopausal women with osteoporosis. Arch Intern Med 2002;162:1140-3. [DOI] [PubMed]
- 223.Eastell R, Adachi J, Harper K, Sarkar S, Delmas PD, Ensrud K. The effects of raloxifene on incident vertebral fractures in postmenopausal women with osteoporosis: 4-year results from the MORE trial [abstract]. J Bone Miner Res 2000;15(suppl 229):F418.
- 224.de Valk-de Roo GW, Stehouwer CDA, Meijer P, Mijatovic V, Kluft C, Kenemans P, et al. Both raloxifene and estrogen reduce major cardiovascular risk factors in heathy postmenopausal women. Arterioscler Thromb Vasc Biol 1999;19:2993-3000. [DOI] [PubMed]
- 225.Barrett-Connor E, Grady D, Sashegyi A, Anderson PW, Cox A, Hoszowski K, et al. Raloxifene and cardiovascular events in osteoporotic postmenopausal women. The MORE investigators. JAMA 2002;287:847-57. [DOI] [PubMed]
- 226.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs BL, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA 1998;280:605-13. [DOI] [PubMed]
- 227.Cauley J, Norton L, Lippman M, Eckert S, Krueger K, Purdie D, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results form the MORE trial. Breast Cancer Res Treat 2001;65:125-34. [DOI] [PubMed]
- 228.Cummings SR, Eckert S, Krueger KA, Grady D, Powles TJ, Cauley JA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: Results from the MORE randomized trial. JAMA 1999;281:2189-97. [DOI] [PubMed]
- 229.Davies GC, Huster WJ, Lu Y, Plouffe L, Lakshmanan M. Adverse events reported by postmenopausal women in controlled trials with raloxifene. Obstet Gynecol 1999;93:558-65. [DOI] [PubMed]
- 230.Delmas PD, Bjarnason NH, Mitlak BH, Ravoux AC, Shah AS, Huster WJ, et al. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med 1997;337:1641-7. [DOI] [PubMed]
- 231.Walsh BW, Kuller LH, Wild RA, Paul S, Farmer M, Lawrence JB, et al. Effects of raloxifene on serum lipids and coagulation factors in healthy postmenopausal women. JAMA 1998;279:1445-51. [DOI] [PubMed]
- 232.Fisher B, Costantino JP, Wickherham DL, Redmond CK, Kavanah M, Cronin WM. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998;90:1371-88. [DOI] [PubMed]
- 233.What is complementary and alternative medicine (CAM)? [Note 1]. Bethesda: National Center for Complementary and Alternative Medicine. http://www.nccam.nih.gov/health/whatiscam/#sup1 (viewed 24 September 2002).
- 234.Scheiber MD, Rebar RW. Isoflavones and postmenopausal bone health: a viable alternative to estrogen therapy? Menopause 1999;6:233-41. [DOI] [PubMed]
- 235.Gambacciani M, Ciaponi M, Cappagli B, Piaggesi L, Genazzani AR. Effects of combined low dose of the isoflavone derivative ipriflavone and estrogen replacement on bone mineral density and metabolism in postmenopausal women. Maturitas 1997;28:75-81. [DOI] [PubMed]
- 236.Valente M, Bufalino L, Castiglione GN, D'Angelo R, Mancuso A, Galoppi P, et al. Effects of 1-year treatment with ipriflavone on bone in postmenopausal women with low bone mass. Calcif Tissue Int 1994;54:377-80. [DOI] [PubMed]
- 237.Gambacciani M, Spinetti A, Cappagli B, Taponeco F, Felipetto R, Parrini D, et al. Effects of ipriflavone administration on bone mass and metabolism in ovariectomized women. J Endocrinol Invest 1993;16:333-7. [DOI] [PubMed]
- 238.Adami S, Bufalino L, Cervetti R, Di Marco C, Di Munno O, Fantasia L, et al. Ipriflavone prevents radial bone loss in postmenopausal women with low bone mass over 2 years. Osteoporos Int 1997;7:119-25. [DOI] [PubMed]
- 239.Kovacs AB. Efficacy of ipriflavone in the prevention and treatment of postmenopausal osteoporosis. Agents Actions 1994;41:86-7. [DOI] [PubMed]
- 240.Ushiroyama T, Okamura S, Ikeda A, Ueki M. Efficacy of ipriflavone and 1a vitamin D therapy for the cessation of vertebral bone loss. Int J Gynaecol Obstet 1995;48:283-8. [DOI] [PubMed]
- 241.Agnusdei D, Zacchei F, Bigazzi S, Cepollaro C, Nardi P, Montagnani M, et al. Metabolic and clinical effects of ipriflavone in established post-menopausal osteoporosis. Drugs Exp Clin Res 1989;15:97-104. [PubMed]
- 242.Nozaki M, Hashimoto K, Inoue Y, Ogata R, Okuma A, Nakano H. Treatment of bone loss in oophorectomized women with a combination of ipriflavone and conjugated equine estrogen. Int J Gynaecol Obstet 1998;62:69-75. [DOI] [PubMed]
- 243.Agnusdei D, Gennari C, Bufalino L. Prevention of early postmenopausal bone loss using low doses of conjugated estrogens and the non-hormonal, bone-active drug ipriflavone. Osteoporos Int 1995;5:462-6. [DOI] [PubMed]
- 244.Cecchettin M, Bellometti S, Cremonesi G, Solimeno LP, Torri G. Metabolic and bone effects after administration of ipriflavone and salmon calcitonin in postmenopausal osteoporosis. Biomed Pharmacother 1995;49:465-8. [DOI] [PubMed]
- 245.Ohta H, Komukai S, Makita K, Masuzawa T, Nozawa S. Effects of 1-year ipriflavone treatment on lumbar bone mineral density and bone metabolic markers in postmenopausal women with low bone mass. Horm Res 1999;51: 178-83. [DOI] [PubMed]
- 246.Katase K, Kato T, Hirai Y, Hasumi K, Chen JT. Effects of ipriflavone on bone loss following a bilateral ovariectomy and menopause: a randomized placebo-controlled study. Calcif Tissue Int 2001;69:73-7. [DOI] [PubMed]
- 247.Alexandersen P, Toussaint A, Christiansen C, Devogelaer JP, Roux C, Fechtenbaum J, et al. Ipriflavone in the treatment of postmenopausal osteoporosis: a randomized controlled trail. JAMA 2001;285:1482-8. [DOI] [PubMed]
- 248.Gennari C, Agnusdei D, Crepaldi G, Isaia G, Mazzuoli G, Ortolani S, et al. Effect of ipriflavone-a synthetic deriviative of natural isoflavones-on bone mass loss in the early years after menopause. Menopause 1998;5:9-15. [PubMed]
- 249.Agnusdei D, Crepaldi G, Isaia G, Mazzuoli G, Ortolani S, Passeri M, et al. A double blind, placebo-controlled trial of ipriflavone for prevention of postmenopausal spinal bone loss. Calcif Tissue Int 1997;61:142-7. [DOI] [PubMed]
- 250.Feskanich D, Weber P, Willett WC, Rockett H, Booth SL, Colditz GA. Vitamin K intake and hip fractures in women: a prospective study. Am J Clin Nutr 1999;69:74-9. [DOI] [PubMed]
- 251.Hart JP, Catterall A, Dodds RA, Klenerman L, Shearer MJ, Bitensky L, et al. Circulating vitamin K1 levels in fractured neck of femur. Lancet 1984;2:283. [DOI] [PubMed]
- 252.Somekawa Y, Chigughi M, Harada M, Ishibashi T. Use of vitamin K2 (Menatetrenone) and 1,25-dihydroxyvitamin D3 in the prevention of bone loss induced by leuprolide. J Clin Endocrinol Metab 1999;84:2700-4. [DOI] [PubMed]
- 253.Iwamoto I, Kosha S, Noguchi SI, Murakami M, Fujino T, Douchi T, et al. A longitudinal study of the effect of vitamin K-2 on bone mineral density in postmenopausal women a comparative study with vitamin D-3 and estrogen-progestin therapy. Maturitas 1999;31:161-4. [DOI] [PubMed]
- 254.Shiraki M, Shiraki Y, Aoki C, Miura M. Vitamin K-2 (menatetrenone) effectively prevents fractures and sustains lumbar bone mineral density in osteoporosis. J Bone Miner Res 2000;15:515-21. [DOI] [PubMed]
- 255.Iwamoto J, Takeda T, Ichimura S. Effect of menatetrenone on bone mineral density and incidence of vertebral fractures in postmenopausal women with osteoporosis: a comparison with the effect of etidronate. J Orthop Sci 2001;6:487-92. [DOI] [PubMed]
- 256.Iwamoto J, Takeda T, Ichimura S. Effect of combined administration of vitamin D3 and vitamin K2 on bone mineral density of the lumbar spine in postmenopausal women with osteoporosis. J Orthop Sci 2000;5:546-51. [DOI] [PubMed]
- 257.Rich C, Ensinck J, Ivanovich P. The effects of sodium fluoride on calcium metabolism of subjects with metabolic bone diseases. J Clin Invest 1964;43:545-56. [DOI] [PMC free article] [PubMed]
- 258.Jowsey J, Riggs BL, Kelly PJ, Hoffman DL. Effect of combined therapy with sodium fluoride, vitamin D and calcium in osteoporosis. J Lab Clin Med 1971; 78:994-5. [PubMed]
- 259.Harrison JE, McNeill KG, Sturtridge WC, Bayley TA, Murray TM, Williams C, et al. Three-year changes in bone mineral mass of postmenopausal osteoporotic patients based on neutron activation analysis of the central third of the skeleton. J Clin Endocrinol Metab 1981;52:751-8. [DOI] [PubMed]
- 260.Heaney RP, Baylink DJ, Johnston CC Jr, Melton LJ III, Meunier PJ, Murray TM, et al. Fluoride therapy for the vertebral crush fracture syndrome. A status report. Ann Intern Med 1989;111:678-80. [DOI] [PubMed]
- 261.Gambacciani M, Spinetti A, Taponeco F, Piaggesi L, Cappagli B, Ciaponi M, et al. Treatment of postmenopausal vertebral osteopenia with monofluorophospate: a long-term calcium-controlled study. Osteoporos Int 1995;5:467-71. [DOI] [PubMed]
- 262.Meunier PJ, Sebert JL, Reginster JY, Briancon D, Appelboom T, Netter P, et al. Fluoride salts are no better at preventing new vertebral fractures than calcium-vitamin D in postmenopausal osteoporosis: the FAVO Study. Osteoporos Int 1998;8:4-12. [DOI] [PubMed]
- 263.Pak CY, Sakhaee K, Adams-Huet B, Piziak V, Peterson RD, Poindexter JR. Treatment of postmenopausal osteoporosis with slow-release sodium fluoride. Final report of a randomized controlled trial. Ann Intern Med 1995;123:401-8. [DOI] [PubMed]
- 264.Reginster JY, Meurmans L, Zegels B, Rovati LC, Minne HW, Giacovelli G, et al. The effect of sodium monofluorophosphate plus calcium on vertebral fracture rate in postmenopausal women with moderate osteoporosis: a randomized, controlled trial. Ann Intern Med 1998;129:1-8. [DOI] [PubMed]
- 265.Riggs BL, Hodgson SF, O'Fallon WM, Chao EY, Wahner HW, Muhs JM, et al. Effect of fluoride treatment on the fracture rate in postmenopausal women with osteoporosis. N Engl J Med 1990;322:802-9. [DOI] [PubMed]
- 266.Ringe JD, Dorst A, Kipshoven C, Rovati LC, Setnikar I. Avoidance of vertebral fractures in men with idiopathic osteoporosis by a three year therapy with calcium and low-dose intermittent monofluorophosphate. Osteoporos Int 1998;8:47-52. [DOI] [PubMed]
- 267.Lippuner K, Haller B, Casez JP, Montandon A, Jaeger P. Effect of disodium monofluorophosphate, calcium and vitamin D Supplementation on bone mineral density in patients chronically treated with glucocorticosteroids: a prospective, randomized, double-blind study. Miner Electrolyte Metab 1996;22:207-13. [PubMed]
- 268.Guaydier-Souquieres G, Kotzki PO, Sabatier JP, Basse-Cathalinat B, Loeb G. In corticosteroid-treated respiratory diseases, monofluorophosphate increases lumbar bone density: a double-masked randomized study. Osteoporos Int 1996;6:171-7. [DOI] [PubMed]
- 269.Rizzoli R, Chevalley T, Slosman DO, Bonjour JP. Sodium monofluorophosphate increases vertebral bone mineral density in patients with corticosteroid-induced osteoporosis. Osteoporos Int 1995;5:39-46. [DOI] [PubMed]
- 270.Lems WF, Jacobs WG, Bijlsma JW, Croone A, Haanen HC, Houben HH, et al. Effect of sodium fluoride on the prevention of corticosteroid-induced osteoporosis. Osteoporos Int 1997;7:575-82. [DOI] [PubMed]
- 271.Hedlund LR, Gallagher JC. Increased incidence of hip fracture in osteoporotic women treated with sodium fluoride. J Bone Miner Res 1989;4:223-5. [DOI] [PubMed]
- 272.Reeve J, Meunier PJ, Parsons JA, Bernat M, Bijvoet OL, Courpron P, et al. Anabolic effect of human parathyroid hormone fragment on trabecular bone in involutional osteoporosis: a multicentre trial. Br Med J 1980;280:1340-4. [DOI] [PMC free article] [PubMed]
- 273.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001; 344:1434-41. [DOI] [PubMed]
- 274.Hodsman AB, Fraher LJ, Watson PH, Ostbye T, Stitt LW, Adachi JD, et al. A randomized controlled trial to compare the efficacy of cyclical parathyroid hormone versus cyclical parathyroid hormone and sequential calcitonin to improve bone mass in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 1997;82:620-8. [DOI] [PubMed]
- 275.Lindsay R, Nieves J, Formica C, Henneman E, Woelfert L, Shen V, et al. Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet 1997;350:550-5. [DOI] [PubMed]
- 276.Fujita T, Inoue T, Morii H, Morita R, Norimatsu H, Orimo H, et al. Effect of an intermittent weekly dose of human parathyroid hormone (1-34) on osteoporosis: a randomized double-masked prospective study using three dose levels. Osteoporos Int 1999;9:296-306. [DOI] [PubMed]
- 277.Slovik DM, Rosenthal DI, Doppelt SH, Potts JT Jr, Daly MA, Campbell JA, et al. Restoration of spinal bone in osteoporotic men by treatment with human parathyroid hormone (1-34) and 1,25-dihydroxyvitamin D. J Bone Miner Res 1986;1:377-81. [DOI] [PubMed]
- 278.Kurland ES, Cosman F, McMahon DJ, Rosen CJ, Lindsay R, Bilezikian JP. Parathyroid hormone as a therapy for idiopathic osteoporosis in men: effects of bone mineral density and bone markers. J Clin Endocrinol Metab 2000; 85:3069-70. [DOI] [PubMed]
- 279.Orwoll E, Scheele WH, Calancy AD, Adami S, Syveren U, Diez-Perez A. Recombinant human parathyroid hormone (1-34) therapy reduces the incidence of moderate/severe vertebral fractures in men with low bone density. J Bone Miner Res 2001;16(suppl):S162.
- 280.Lane NE, Sanchez S, Modin GW, Genant HK, Pierini E, Arnaud CD. Parathyroid hormone treatment can reverse corticosteroid-induced osteoporosis. Results of a randomized controlled clinical trial. J Clin Invest 1998;102:1627-33. [DOI] [PMC free article] [PubMed]
- 281.Lane NE, Sanchez S, Modin GW, Genant HK, Pierini E, Arnaud CD. Bone mass continues to increase at the hip after parathyroid hormone treatment is discontinued in glucocorticoid-induced osteoporosis: Results of a randomized controlled clinical trial. J Bone Miner Res 2000;15:944-51. [DOI] [PubMed]
- 282.Baeksgaard L, Andersen KP, Hyldstrup L. Calcium and vitamin D Supplementation increases spinal BMD in healthy, postmenopausal women. Osteoporos Int 1998;8:255-60. [DOI] [PubMed]
- 283.Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med 1992;327:1637-42. [DOI] [PubMed]
- 284.Chapuy MC, Arlot ME, Delmas PD, Meunier PJ. Effect of calcium and cholecalciferol treatment for three years on hip fractures in elderly women. BMJ 1994;308:1081-2. [DOI] [PMC free article] [PubMed]
- 285.Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D Supplementation on bone density in men and women 65 years of age or older. N Engl J Med 1997;337:670-6. [DOI] [PubMed]
- 286.Bonjour JP, Carrie AL, Ferrari S, Clavien H, Slosman D, Theintz G, et al. Calcium-enriched foods and bone mass growth in prepubertal girls: a randomized, double-blind, placebo-controlled trial. J Clin Invest 1997;99:1287-94. [DOI] [PMC free article] [PubMed]
- 287.Johnston CC Jr, Miller JZ, Slemenda CW, Reister TK, Hui S, Christian JC, et al. Calcium Supplementation and increases in bone mineral density in children. N Engl J Med 1992;327:82-7. [DOI] [PubMed]
- 288.Lee WT, Leung SS, Wang SH, Xu YC, Zeng WP, Lau J, et al. Double-blind, controlled calcium Supplementation and bone mineral accretion in children accustomed to a low-calcium diet. Am J Clin Nutr 1994;60:744-50. [DOI] [PubMed]
- 289.Lee WT, Leung SS, Leung DM, Tsang HS, Lau J, Cheng JC. A randomized double-blind controlled calcium Supplementation trial, and bone and height acquisition in children. Br J Nutr 1995;74:125-39. [DOI] [PubMed]
- 290.Lloyd T, Andon MB, Rollings N, Martel JK, Landis JR, Demers LM, et al. Calcium Supplementation and bone mineral density in adolescent girls. JAMA 1993;270:841-4. [PubMed]
- 291.Lloyd T, Martel JK, Rollings N, Andon MB, Kulin H, Demers LM, et al. The effect of calcium Supplementation and tanner stage on bone density, content and area in teenage women. Osteoporos Int 1996;6:286-3. [DOI] [PubMed]
- 292.Chan GM, Hoffman K, McMurry M. Effects of dairy products on bone and body composition in pubertal girls. J Pediatr 1995;126:551-6. [DOI] [PubMed]
- 293.Baran D, Sorensen A, Grimes J, Lew R, Karellas A, Johnson B, et al. Dietary modification with dairy products for preventing vertebral bone loss in premenopausal women: a three-year prospective study. J Clin Endocrinol Metab 1990;70:264-70. [DOI] [PubMed]
- 294.Freudenheim JL, Johnson NE, Smith EL. Relationships between usual nutrient intake and bone-mineral content of women 35-65 years of age: Longitudinal and cross-sectional analysis. Am J Clin Nutr 1986;44:863-76. [DOI] [PubMed]
- 295.Rico H, Revilla M, Villa LF, Alvarez de Buergo M, Arribas I. Longitudinal study of the effect of calcium pidolate on bone mass in eugonadal women. Calcif Tissue Int 1994;54:477-80. [DOI] [PubMed]
- 296.Holbrook TL, Barrett-Connor EL, Wingard DL. Dietary calcium and risk of hip fracture: 14-year prospective population study. Lancet 1988;2:1046-9. [DOI] [PubMed]
- 297.Lau E, Donnan S, Barker DJP, Cooper C. Physical activity and calcium intake in fracture of the proximal femur in Hong Kong. BMJ 1988;297:1441-3. [DOI] [PMC free article] [PubMed]
- 298.Chevalley T, Rizzoli R, Nydegger V, Slosman D, Rapin CH, Michel JP, et al. Effects of calcium Supplements on femoral bone mineral density and vertebral fracture rate in vitamin-D-replete elderly patients. Osteoporos Int 1994;4:245-52. [DOI] [PubMed]
- 299.Dawson-Hughes B, Dallal GE, Krall EA, Sadowski L, Sahyoun N, Tannenbaum S. A controlled trial of the effect of calcium Supplementation on bone density in postmenopausal women. N Engl J Med 1990;323:878-83. [DOI] [PubMed]
- 300.Prince R, Devine A, Dick I, Criddle A, Kerr D, Kent N, et al. The effects of calcium Supplementation (milk powder or tablets) and exercise on bone density in postmenopausal women. J Bone Miner Res 1995;10:1068-75. [DOI] [PubMed]
- 301.Reid IR, Ames RW, Evans MC, Gamble GD, Sharpe SJ. Effect of calcium Supplementation on bone loss in postmenopausal women [published erratum appears in N Engl J Med 1993 Oct 21;329(17):1281]. N Engl J Med 1993;328:460-4. [DOI] [PubMed]
- 302.Reid IR, Ames RW, Evans MC, Sharpe SJ, Gamble GD. Determinants of the rate of bone loss in normal postmenopausal women. J Clin Endocrinol Metab 1994;79:950-4. [DOI] [PubMed]
- 303.Reid IR, Ames RW, Evans MC, Gamble GD, Sharpe SJ. Long-term effects of calcium Supplementation on bone loss and fractures in postmenopausal women: a randomized controlled trial. Am J Med 1995;98:331-5. [DOI] [PubMed]
- 304.Riggs BL, O'Fallon WM, Muhs J, O'Connor MK, Kumar R, Melton LJ III. Long-term effects of calcium Supplementation on serum parathyroid hormone level, bone turnover, and bone loss in elderly women. J Bone Miner Res 1998;13:168-74. [DOI] [PubMed]
- 305.Recker RR, Hinders S, Davies KM, Heaney RP, Stegman MR, Lappe JM, et al. Correcting calcium nutritional deficiency prevents spine fractures in elderly women. J Bone Miner Res 1996;11:1961-6. [DOI] [PubMed]
- 306.Chan GM, McMurry M, Westover K, Engelbert-Fenton K, Thomas MR. Effects of increased dietary calcium intake upon the calcium and bone mineral status of lactating adolescent and adult women. Am J Clin Nutr 1987;46:319-23. [DOI] [PubMed]
- 307.Cross NA, Hillman LS, Allen SH, Krause GF. Changes in bone mineral density and markers of bone remodeling during lactation and postweaning in women consuming high amounts of calcium. J Bone Miner Res 1995;10:1312-20. [DOI] [PubMed]
- 308.Kalkwarf HJ, Specker BL, Bianchi DC, Ranz J, Ho M. The effect of calcium Supplementation on bone density during lactation and after weaning. N Engl J Med 1997;337:523-8. [DOI] [PubMed]
- 309.Prentice A, Jarjou LM, Cole TJ, Stirling DM, Dibba B, Fairweather-Tait S. Calcium requirements of lactating Gambian mothers: effects of a calcium Supplement on breast-milk calcium concentration, maternal bone mineral content, and urinary calcium excretion. Am J Clin Nutr 1995;62:58-67. [DOI] [PubMed]
- 310.Trang HM, Cole DE, Rubin LA, Pierratos A, Siu S, Vieth R. Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nutr 1998;68:854-8. [DOI] [PubMed]
- 311.Vieth R, Cole DE, Hawker GA, Trang HM, Rubin LA. Wintertime vitamin D insufficiency is common in young Canadian women, and their vitamin D intake does not prevent it. Eur J Clin Nutr 2001;55:1091-7. [DOI] [PubMed]
- 312.Ala-Houhala M, Koskinen T, Koskinen M, Visakorpi JK. Double blind study on the need for vitamin D Supplementation in prepubertal children. Acta Paediatr Scand 1988;77:89-93. [DOI] [PubMed]
- 313.Komulainen M, Tuppurainen MT, Kroger H, Heikkinen AM, Puntila E, Alhava E, et al. Vitamin D and HRT: no benefit additional to that of HRT alone in prevention of bone loss in early postmenopausal women. A 2.5-year randomized placebo-controlled study. Osteoporos Int 1997;7:126-32. [DOI] [PubMed]
- 314.Dawson-Hughes B, Dallal GE, Krall EA, Harris S, Sokoll LJ, Falconer G. Effect of vitamin D Supplementation on wintertime and overall bone loss in healthy postmenopausal women. Ann Intern Med 1991;115:505-12. [DOI] [PubMed]
- 315.Tylavsky FA, Anderson JJB. Dietary factors in bone health of elderly lactoovovegetarians and omnivorous women. Am J Clin Nutr 1988;48(3 suppl):842-9. [DOI] [PubMed]
- 316.Munger RG, Cerhan JR, Chiu BC. Prospective study of dietary protein intake and risk of hip fracture in postmenopausal women. Am J Clin Nutr 1999;69:147-52. [DOI] [PubMed]
- 317.Hernandez-Avila M, Colditz GA, Stampfer MJ, Rosner B, Speizer FE, Willett WC. Caffeine, moderate alcohol intake and risk of fractures of the hip and forearm in middle-aged women. Am J Clin Nutr 1991;54:157-63. [DOI] [PubMed]
- 318.Kiel DP, Felson DT, Hannan MT, Anderson JJ, Wilson PW. Caffeine and the risk of hip fracture: the Framingham Study. Am J Epidemiol 1990;132:675-84. [DOI] [PubMed]
- 319.Devine A, Criddle RA, Dick IM, Kerr DA, Prince RL. A longitudinal study of the effect of sodium and calcium intakes on regional bone density in postmenopausal women. Am J Clin Nutr 1995;62:740-5. [DOI] [PubMed]
- 320.Yano K, Heilbrun LK, Wasnich RD, Hankin JH, Vogel JM. The relationship between diet and bone mineral content of multiple skeletal sites in elderly Japanese-American men and women living in Hawaii. Am J Clin Nutr 1985;42:877-88. [DOI] [PubMed]
- 321.Angus RM, Sambrook PN, Pocock NA, Eisman JA. Dietary intake and bone mineral density. Bone Miner 1988;4:265-77. [PubMed]
- 322.Earnshaw SA, Worley A, Hosking DJ. Current diet does not relate to bone mineral density after the menopause. The Nottingham Early Postmenopausal Intervention Cohort (EPIC) Study Group. Br J Nutr 1997;78:65-72. [DOI] [PubMed]
- 323.New SA, Robins SP, Campbell MK, Martin JC, Garton MJ, Bolton-Smith C, et al. Dietary influences on bone mass and bone metabolism: Further evidence of a positive link between fruit and vegetable consumption and bone health? Am J Clin Nutr 2000;71:142-51. [DOI] [PubMed]
- 324.Conlan D, Korula R, Tallentire D. Serum copper levels in elderly patients with femoral-neck fractures. Age Ageing 1990;19:212-4. [DOI] [PubMed]
- 325.Elmstahl S, Gullberg B, Janzon L, Johnell O, Elmstahl B. Increased incidence of fractures in middle-aged and elderly men with low intakes of phosphorus and zinc. Osteoporos Int 1998;8:333-40. [DOI] [PubMed]
- 326.Michaelsson K, Holmberg L, Mallmin H, Sorensen S, Wolk A, Bergstrom R, et al. Diet and hip fracture risk: A case-control study. Int J Epidemiol 1995;24:771-82. [DOI] [PubMed]
- 327.Petit MA, McKay HA, MacKelvie KJ, Heinonen A, Khan KM, Beck TJ. A randomized school-based jumping intervention confers site and maturity-specific benefits on bone structural properties in girls: a hip structural analysis study. J Bone Miner Res 2002;17:363-72. [DOI] [PubMed]
- 328.MacKelvie KJ, McKay HA, Petit MA, Moran O, Khan KM. Bone mineral responses to a 7-month randomized controlled, school-based jumping intervention in 121 prepubertal boys: associations with ethnicity and body mass index. J Bone Miner Res 2002;17:834-44. [DOI] [PubMed]
- 329.Bradney M, Pearce G, Naughton G, Sullivan C, Bass S, Beck T, et al. Moderate exercise during growth in prepubertal boys: changes in bone mass, size, volumetric density, and bone strength: a controlled prospective study. J Bone Miner Res 1998;13:1814-21. [DOI] [PubMed]
- 330.Morris FL, Naughton GA, Gibbs JL, Carlson JS, Wark JD. Prospective ten-month exercise intervention in premenarcheal girls: Positive effects on bone and lean mass. J Bone Miner Res 1997;12:1453-62. [DOI] [PubMed]
- 331.Slemenda CW, Miller JZ, Hui SL, Reister TK, Johnston CC Jr. Role of physical activity in the development of skeletal mass in children. J Bone Miner Res 1996;6:1227-33. [DOI] [PubMed]
- 332.Cassell C, Benedict M, Specker B. Bone mineral density in elite 7- to 9-yr-old female gymnasts and swimmers. Med Sci Sports Exerc 1996;28:1243-6. [DOI] [PubMed]
- 333.Courteix D, Lespessailles E, Peres SL, Obert P, Germain P, Benhamou CL. Effect of physical training on bone mineral density in prepubertal girls: a comparative study between impact-loading and non-impact-loading sports. Osteoporos Int 1998;8:152-8. [DOI] [PubMed]
- 334.Duppe H, Gardsell P, Johnell O, Ornstein E. Bone mineral density in female junior, senior and former football players. Osteoporos Int 1996;6:437-41. [DOI] [PubMed]
- 335.Nordstrom P, Pettersson U, Lorentzon R. Type of physical activity, muscle strength, and pubertal stage as determinants of bone mineral density and bone area in adolescent boys. J Bone Miner Res 1998;13:1141-8. [DOI] [PubMed]
- 336.Slemenda CW, Johnston CC Jr. High intensity activities in young women: site specific bone mass effects among female figure skaters. Bone Miner 1993;20:125-32. [DOI] [PubMed]
- 337.Taaffe DR, Snow-Harter C, Connolly DA, Robinson TL, Brown MD, Marcus R. Differential effects of swimming versus weight-bearing activity on bone mineral status of eumenorrheic athletes. J Bone Miner Res 1995;10:586-93. [DOI] [PubMed]
- 338.Cooper C, Cawley M, Bhalla A, Egger P, Ring F, Morton L, et al. Childhood growth, physical activity, and peak bone mass in women. J Bone Miner Res 1995;10:940-7. [DOI] [PubMed]
- 339.Khan KM, Liu-Ambrose T, Sran MM, Ashe MC, Donaldson MG, Wark JD. New criteria for female athlete triad syndrome? As osteoporosis is rare, should osteopenia be among the criteria for defining the female athlete triad syndrome? Br J Sports Med 2002;36:10-3. [DOI] [PMC free article] [PubMed]
- 340.Heinonen A, Sievanen H, Kannus P, Oja P, Vuori I. Effects of unilateral strength training and detraining on bone mineral mass and estimated mechanical characteristics of the upper limb bones in young women. J Bone Miner Res 1996;11:490-501. [DOI] [PubMed]
- 341.Chilibeck PD, Calder A, Sale DG, Webber CE. Twenty weeks of weight training increases lean tissue mass but not bone mineral mass or density in healthy, active young women. Can J Physiol Pharmacol 1996;74:1180-5. [PubMed]
- 342.Conroy BP, Kraemer WJ, Maresh CM, Fleck SJ, Stone MH, Fry AC, et al. Bone mineral density in elite junior Olympic weightlifters. Med Sci Sport Exerc 1993;25:1103-9. [PubMed]
- 343.Karlsson MK, Johnell O, Obrant KJ. Bone mineral density in weight lifters. Calcif Tissue Int 1993;52:212-5. [DOI] [PubMed]
- 344.Dinc H, Savci G, Demirci A, Sadikoglu MY, Tuncel E, Yavuz H. Quantitative computed tomography for measuring bone mineral density in athletes. Calcif Tissue Int 1996;58:398-401. [DOI] [PubMed]
- 345.Mayoux-Benhamou MA, Leyge JF, Roux C, Revel M. Cross-sectional study of weight-bearing activity on proximal femur bone mineral density. Calcif Tissue Int 1999;64:179-83. [DOI] [PubMed]
- 346.Orwoll ES, Ferar J, Oviatt SK, McClung MR, Huntington K. The relationship of swimming exercise to bone mass in men and women. Arch Intern Med 1989;149:2197-200 [PubMed]
- 347.Pettersson U, Nordstrom P, Lorentzon R. A comparison of bone mineral density and muscle strength in young male adults with different exercise level. Calcif Tissue Int 1999;64:490-8. [DOI] [PubMed]
- 348.Smith R, Rutherford OM. Spine and total body bone mineral density and serum testosterone levels in male athletes. Eur J Appl Physiol Occup Physiol 1993;67:330-4. [DOI] [PubMed]
- 349.Calbet JAL, Moysi JS, Dorado C, Rodriguez LP. Bone mineral content and density in professional tennis players. Calcif Tissue Int 1998;62:491-6. [DOI] [PubMed]
- 350.Kannus P, Haapasalo H, Sievanen H, Oja P, Vuori I. The site-specific effects of long-term unilateral activity on bone mineral density and content. Bone 1994;15:279-84. [DOI] [PubMed]
- 351.Kontulainen S, Kannus P, Haapasalo H, Heinonen A, Sievanen H, Oja P, et al. Changes in bone mineral content with decreased training in competitive young adult tennis players and controls: A prospective 4-yr follow-up. Med Sci Sports Exerc 1999;31:646-52. [DOI] [PubMed]
- 352.Bilanin JE, Blanchard MS, Russek-Cohen E. Lower vertebral bone density in male long distance runners. Med Sci Sports Exerc 1989;21:66-70. [DOI] [PubMed]
- 353.Hetland ML, Haarbo J, Christiansen C. Low bone mass and high bone turnover in male long distance runners. J Clin Endocrinol Metab 1993;77:770-5. [DOI] [PubMed]
- 354.MacDougall JD, Webber CE, Martin J, Ormerod S, Chesley A, Younglai EV, et al. Relationship among running mileage, bone density, and serum testosterone in male runners. J Appl Physiol 1992;73:1165-70. [DOI] [PubMed]
- 355.Wallace BA, Cumming RG. Systematic review of randomized trials of the effect of exercise on bone mass in pre- and postmenopausal women. Calcif Tissue Int 2000;67:10-8. [DOI] [PubMed]
- 356.Snow-Harter C, Bouxsein ML, Lewis BT, Carter DR, Marcus R. Effects of resistance and endurance exercise on bone mineral status of young women: a randomized exercise intervention trial. J Bone Miner Res 1992;7:761-9. [DOI] [PubMed]
- 357.Bassey EJ, Ramsdale SJ. Weight-bearing exercise and ground reaction forces: a 12-month randomized controlled trial of effects on bone mineral density in healthy postmenopausal women. Bone 1995;16:469-76. [DOI] [PubMed]
- 358.Bravo G, Gauthier P, Roy PM, Payette H, Gaulin P, Harvey M, et al. Impact of a 12-month exercise program on the physical and psychological health of osteopenic women. J Am Geriatr Soc 1996;44:756-62. [DOI] [PubMed]
- 359.Brooke-Wavell K., Jones PRM, Hardman AE. Brisk walking reduces calcaneal bone loss in post-menopausal women. Clin Sci (Lond) 1997;92:75-80. [DOI] [PubMed]
- 360.Ebrahim S, Thompson PW, Baskaran V, Evans K. Randomized placebo-controlled trial of brisk walking in the prevention of postmenopausal osteoporosis. Age Ageing 1997;26:253-60. [DOI] [PubMed]
- 361.Grove KA, Londeree BR. Bone density in postmenopausal women: high impact vs low impact exercise. Med Sci Sports Exerc 1992;24:1190-4. [PubMed]
- 362.Heinonen A, Oja P, Sievanen H, Pasanen M, Vuori I. Effect of two training regimens on bone mineral density in healthy perimenopausal women: a randomized controlled trial. J Bone Miner Res 1998;13:483-90. [DOI] [PubMed]
- 363.Kohrt WM, Snead DB, Slatopolsky E, Birge SJ Jr. Additive effects of weight-bearing exercise and estrogen on bone mineral density in older women. J Bone Miner Res 1995;10:1303-11. [DOI] [PubMed]
- 364.Martin D, Notelovitz M. Effects of aerobic training on bone mineral density of postmenopausal women. J Bone Miner Res 1993;8:931-6. [DOI] [PubMed]
- 365.McCartney N, Hicks AL, Martin J, Webber CE. Long-term resistance training in the elderly: effects on dynamic strength, exercise capacity, muscle, and bone. J Gerontol A Biol Sci Med Sci 1995;50:B97-104. [DOI] [PubMed]
- 366.Thompson JL, Gylfadottir UK, Moynihan S, Jensen CD, Butterfield GE. Effects of diet and exercise on energy expenditure in postmenopausal women. Am J Clin Nutr 1997;66:867-73. [DOI] [PubMed]
- 367.Kerr D, Morton A, Dick I, Prince R. Exercise effects on bone mass in postmenopausal women are site-specific and load-dependent. J Bone Miner Res 1996;11:218-25. [DOI] [PubMed]
- 368.McCartney N, Hicks AL, Martin J, Webber CE. A longitudinal trial of weight training in the elderly: continued improvements in year 2. J Gerontol A Biol Sci Med Sci 1996;51:B425-33. [DOI] [PubMed]
- 369.Nelson M, Fiatarone M, Morganti C, Trice I, Greenberg R, Evans W. Effects of High-Intensity Strength Training on Multiple Risk Factors for Osteoporotic Fractures — a randomised controlled trial. JAMA 1994;272:1909-14. [DOI] [PubMed]
- 370.Nichols JF, Nelson KP, Peterson KK. Bone mineral density responses to high-intensity strength training in active older women. J Aging Phys Activity 1995;3:26-38.
- 371.Revel M, Mayoux-Benhamou MA, Rabourdin JP, Bagheri F, Roux C. One-year psoas training can prevent lumbar bone loss in postmenopausal women: a randomized controlled trial. Calcif Tissue Int 1993;53:307-11. [DOI] [PubMed]
- 372.Rhodes EC, Martin AD, Taunton JE, Donnelly M, Warren J, Elliot J. Effects of one year of resistance training on the relation between muscular strength and bone density in elderly women. Br J Sports Med 2000;34:18-22. [DOI] [PMC free article] [PubMed]
- 373.Sinaki M, Wahner HW, Offord KP, Hodgson SF. Efficacy of nonloading exercises in prevention of vertebral bone loss in postmenopausal women: a controlled trial. Mayo Clin Proc 1989;64:762-9. [DOI] [PubMed]
- 374.Smidt GL, Lin SY, O'Dwyer KD, Blanpied PR. The effect of high-intensity trunk exercise on bone mineral density of postmenopausal women. Spine 1992;17:280-5. [DOI] [PubMed]
- 375.Heikkinen J, Kurttila-Matero E, Kyllonen E, Vuori J, Takala T, Vaananen HK. Moderate exercise does not enhance the positive effect of estrogen on bone mineral density in postmenopausal women. Calcif Tissue Int 1991;49(suppl):S83-4. [DOI] [PubMed]
- 376.Notelovitz M, Martin D, Tesar R, Khan FY, Probart C, Fields C, et al. Estrogen therapy and variable-resistance weight training increase bone mineral in surgically menopausal women. J Bone Miner Res 1991;6:583-90. [DOI] [PubMed]
- 377.Wolff I, Van Croonenborg JJ, Kemper HCG, Kostense PJ, Twisk JWR. The effect of exercise training programs on bone mass: A meta-analysis of published controlled trials in pre- and postmenopausal women. Osteoporos Int 1999;9:1-12. [DOI] [PubMed]
- 378.Berard A, Bravo G, Gauthier P. Meta-analysis of the effectiveness of physical activity for the prevention of bone loss in postmenopausal women. Osteoporos Int 1997;7:331-7. [DOI] [PubMed]
- 379.Boyce WJ, Vessey MP. Habitual physical inertia and other factors in relation to risk of fracture of the proximal femur. Age Ageing 1988;17:319-27. [DOI] [PubMed]
- 380.Cooper C, Wickham C, Coggon D. Sedentary work in middle life and fracture of the proximal femur. Br J Ind Med 1990;47:69-70. [DOI] [PMC free article] [PubMed]
- 381.Hannan MT, Felson DT, Dawson-Hughes B, Tucker KL, Cupples LA, Wilson PWF, et al. Risk factors for longitudinal bone loss in elderly men and women: The Framingham Osteoporosis Study. J Bone Miner Res 2000;15:710-20. [DOI] [PubMed]
- 382.Joakimsen RM, Fonnebo V, Magnus JH, Stormer J, Tollan A, Sogaard AJ. The Tromso Study: physical activity and the incidence of fractures in a middle-aged population. J Bone Miner Res 1998;13:1149-57. [DOI] [PubMed]
- 383.Kujala UM, Kaprio J, Kannus P, Sarna S, Koskenvuo M. Physical activity and osteoporotic hip fracture risk in men. Arch Intern Med 2000;160:705-8. [DOI] [PubMed]
- 384.Gillespie LD, Gillespie WJ, Cumming R, Lamb SE, Rowe BH. Interventions to reduce the incidence of falling in the elderly. Cochrane Database Syst Rev 1999;(1).
- 385.Campbell AJ, Robertson MC, Gardner MM, Norton RN, Tilyard MW, Buchner DM. Randomised controlled trial of a general practice programme of home based exercise to prevent falls in elderly women. BMJ 1997;315: 1065-9. [DOI] [PMC free article] [PubMed]
- 386.Campbell AJ, Robertson MC, Gardner MM, Norton RN, Buchner DM. Psychotropic medication withdrawal and a home-based exercise program to prevent falls: a randomized, controlled trial. J Am Geriatr Soc 1999;47:850-3. [DOI] [PubMed]
- 387.Roberston MC, Devlin N, Gardner MM, Campbell AJ. Effectiveness and economic evaluation of a nurse delivered home exercise program to prevent falls. 1: Randomized controlled trial. BMJ 2001;322:697-701. [DOI] [PMC free article] [PubMed]
- 388.Wolf SL, Barnhart HX, Kutner NG, McNeely E, Coogler C, Xu T. Reducing frailty and falls in older persons: an investigation of Tai Chi and computerized balance training. Atlanta FICSIT Group. Frailty and Injuries: Cooperative Studies of Intervention Techniques. J Am Geriatr Soc 1996;44:489-97. [DOI] [PubMed]
- 389.Cumming RG, Thomas M, Szonyi G, Salkeld G, O'Neill E, Westbury C, et al. Home visits by an occupational therapist for assessment and modification of environmental hazards: a randomized trial of falls prevention. J Am Geriatr Soc 1999;47:1397-402. [DOI] [PubMed]
- 390.Van Haastregt JCM, Diederiks JPM, Van Rossum E, De Witte LP, Crebolder HFJM. Effects of preventive home visits to elderly people living in the community: Systematic review. BMJ 2000;320:754-8. [DOI] [PMC free article] [PubMed]
- 391.Close J, Ellis M, Hooper R, Glucksman E, Jackson S, Swift C. Prevention of falls in the elderly trial (PROFET): a randomised controlled trial. Lancet 1999;353:93-7. [DOI] [PubMed]
- 392.Van Haastregt JC, Diederiks JP, van Rossum E, de Witte LP, Voorhoeve PM, Crebolder HF. Effects of a programme of multifactorial home visits on falls and mobility impairments in elderly people at risk: a randomised controlled trial. BMJ 2000;321:994-8. [DOI] [PMC free article] [PubMed]
- 393.Tinetti ME, Baker DI, McAvay G, Claus EB, Garrett P, Gottschalk M, et al. A multifactorial intervention to reduce the risk of falling among elderly people living in the community. N Engl J Med 1994;331:821-7. [DOI] [PubMed]
- 394.Bailey DA, McKay HA, Mirwald RL, Crocker PRE, Faulkner RA. A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: The University of Saskatchewan Bone Mineral Accrual Study. J Bone Miner Res 1999;14:1672-9. [DOI] [PubMed]


