Abstract
Leprosy is an infectious, neurodegenerative disease of humans caused by Mycobacterium leprae. Despite effective control programs, the incidence of leprosy remains stubbornly high, suggesting that transmission may be more common than expected. The rationale of this work was to use bioinformatics and comparative genomics to identify potentially antigenic proteins for diagnostic purposes. This approach defined three classes of proteins: those restricted to M. leprae (class I), those present in M. leprae with orthologues in other organisms besides mycobacteria (class II), and exported or surface-exposed proteins (class III). Twelve genes (two class I, four class II, and six class III proteins) were cloned in Escherichia coli, and their protein products were purified. Six of these proteins were detected in cell extracts of M. leprae by immunoblotting. The immunogenicity of each recombinant protein was then investigated in leprosy patients by measuring the reactivity of circulating antibody and gamma interferon (IFN-γ) responses in T-cell restimulation assays. Several class II and class III proteins were recognized by circulating antibodies. Importantly, most class II proteins elicited IFN-γ responses that were significantly stronger than those produced by previously identified antigens. Among them, two class II proteins, ML0308 and ML2498, showed marked humoral and cellular immunogenicity, therefore providing promising candidates for the diagnosis of both tuberculoid and lepromatous forms of leprosy.
Mycobacterium leprae, the etiologic agent of leprosy, has undergone a reductive evolutionary process through adaptation to its intracellular parasitic lifestyle. Its genome contains 1,614 genes coding for proteins and 1,133 pseudogenes, the largest number described to date for a bacterium (12). The genes encoding all the biosynthetic pathways have been retained, whereas those coding for many catabolic pathways and the respiratory chains and several genes involved in DNA replication, recombination, and repair are missing or seriously impaired (12). Nevertheless, this minimal gene set is sufficient for M. leprae to colonize macrophages and Schwann cells and to cause serious damage to the human peripheral nervous system (27).
Leprosy remains a public health problem in spite of the global coverage of multidrug therapy (MDT) promoted by the World Health Organization (WHO) and the dramatic decrease in prevalence. However, the number of new cases of leprosy has remained obstinately constant in the last decade (30). Leprosy develops after an estimated incubation period of 2 to 10 years and shows a complex spectrum of clinical forms (23). Tuberculoid leprosy (TT) and lepromatous leprosy (LL) are polar and stable forms of leprosy. TT patients are characterized by strong local cell-mediated immunity and a bacterial index (BI) of zero in the lesions. In contrast, LL patients display M. leprae-specific systemic T-cell anergy, a high BI, and a strong humoral response. Between the LL and TT poles, three unstable interpolar forms of leprosy coexist, i.e., borderline tuberculoid, borderline borderline, and borderline lepromatous forms (BT, BB, and BL, respectively) (23). They are characterized by a gradual degradation of cell-mediated immunity in parallel with an increase in the BI and the humoral response from the interpolar BT toward the interpolar BL leprosy type. BT, BB, and BL leprosy patients may experience a type I reaction, or reversal reaction, which is marked by an amelioration of the cellular immune response that could lead to nerve damage. Some BL leprosy patients develop a type 2 reaction, or erythema nodosum leprosum, a painful, immunologically mediated inflammatory condition. Both the reversal reaction and erythema nodosum leprosum can occur before, during, and after MDT (5, 20).
In countries where leprosy is endemic, leprosy diagnosis is mainly based on clinical symptoms and occasionally verified by light microscopy. The availability of a diagnostic method has long been desired to detect infection and the presence of multiplying M. leprae before clinical signs become apparent. The discovery and characterization of phenolic glycolipid 1 (PGL-1) provided hope for the development of a specific test for leprosy, and much effort was devoted to detecting circulating antibodies as a test for infection (8, 9). Unfortunately, anti-PGL-1 immunoglobulin M (IgM) antibodies are abundant in LL patients and scarce or absent in TT patients. Additionally, PGL-1 itself is an unusually stable molecule, often persisting in patients who have completed MDT. Mitsuda introduced an intradermal skin test, in which a cell-mediated immune response to lepromin, a complex mixture of proteins derived from extracts of heat-killed M. leprae, is assessed (20). Because of the anergy that characterizes LL patients, these individuals are lepromin negative, therefore reducing the utility of this test. Nevertheless, tests based on protein or peptide antigens still offer great promise for identifying recently infected persons or TT cases and other borderline patients, provided that their cell-mediated immunity remains vigorous. A potentially confounding factor, however, is previous immunization with related cross-reacting antigens such as those present in Mycobacterium bovis BCG or environmental mycobacteria.
Two new approaches have been used to develop improved skin test antigens. Firstly, armadillo-derived M. leprae cells have been fractionated, and the proteins associated with the membrane, cell wall, and cytoplasm have been purified and immunologically characterized (21). This first generation of antigens, which comprised the most abundant M. leprae proteins (i.e., major membrane protein I [MMP-I], MMP-II, antigen 85-B [Ag85B], elongation factor Tu [EF-Tu], and GroES), encountered serious problems of cross-reactivity with their counterparts in pathogenic as well as environmental mycobacteria (3, 10, 31). Peptide antigens representing potentially M. leprae-specific epitopes were used to evaluate responses in leprosy patients and healthy individuals from areas where leprosy is not endemic. Although initial findings were promising, immunological responses to these peptides were of poor specificity and sensitivity (13).
With the availability of the complete genome sequences of M. leprae (12), the principal members of the M. tuberculosis complex (11, 15), and four environmental mycobacteria, M. avium, M. smegmatis, M. marinum, and the emerging pathogen M. ulcerans, we are now ideally positioned to adopt a more rational route to antigen discovery. Using genomics and bioinformatics, sets of potential antigens can be defined that are restricted to M. leprae, including secreted or surface-exposed proteins, and screened in silico for potential T-cell epitopes. After validation, these new antigens could serve as the basis of a diagnostic test capable of revealing infection before clinical signs become apparent or of detecting early stages of leprosy.
MATERIALS AND METHODS
Bioinformatic methods.
Potential coding sequences were predicted and analyzed as previously described, using Artemis for annotation (12, 24, 26). We conducted a search for genes that fulfilled one of the following criteria: (i) present in M. leprae but absent from all other genomes, (ii) present in M. leprae but absent from all other mycobacterial genomes, and (iii) coding for secreted and/or exported and surface-exposed proteins with restricted distribution. To this end, the genome of M. leprae was compared with the genomes of M. tuberculosis (http://genolist.pasteur.fr/TubercuList/), M. bovis (http://genolist.pasteur.fr/BoviList/), M. avium, M. smegmatis (both available athttp://www.tigr.org/tdb/mdb/mdbinprogress.html), M. marinum (http://www.sanger.ac.uk/Projects/M_marinum), and M. ulcerans (http://www.pasteur.fr/recherche/unites/Lgmb/mycogenomics.html).
Each protein sequence of M. leprae served as a query sequence against the proteomic data in public databases, using FASTA and BLASTP. The resulting proteins were classified into three classes according to the above conditions and are described in Table 1.
TABLE 1.
Potential antigenic targets selected in silico
| Class | Accession no. | Functional categorya | Function | Mr |
|---|---|---|---|---|
| I | ML0008 | 8 | Unknown | 13,722.5 |
| ML0126 | 8 | Unknown | 30,831.8 | |
| ML0678 | 8 | Unknown | 19,767.5 | |
| ML0757 | 8 | Unknown | 24,909.2 | |
| ML0957 | 8 | Unknown | 13,939.6 | |
| ML1057 | 8 | Unknown | 12,207.9 | |
| ML1420 | 8 | Unknown | 13,138.9 | |
| ML1603 | 8 | Unknown | 9,376.0 | |
| ML1829 | 8 | Unknown | 13,334.3 | |
| ML1915 | 8 | Unknown | 12,433.0 | |
| ML1979 | 8 | Unknown | 14,233.5 | |
| ML2244 | 8 | Unknown | 12,552.1 | |
| ML2249 | 8 | Unknown | 11,200.5 | |
| ML2252 | 8 | Unknown | 8,720.8 | |
| ML2264 | 8 | Unknown | 22,683.5 | |
| ML2283 | 8 | Unknown | 11,758.5 | |
| ML2567 | 8 | Unknown | 17,051.2 | |
| II | ML0308 | 10 | Conserved hypothetical protein | 25,899.1 |
| ML0333 | 10 | Conserved hypothetical protein | 26,453.1 | |
| ML0336 | 3 | ABC transporter ATP-binding protein | 30,185.6 | |
| ML0394 | 7 | Methyltransferase | 19,170.1 | |
| ML0397 | 3 | Transporter | 63,963.7 | |
| ML0398 | 3 | d-Ribose binding protein | 36,939.9 | |
| ML0447 | 10 | Conserved hypothetical protein | 17,104.5 | |
| ML0458 | 7 | Oxidoreductase | 32,874.6 | |
| ML0578 | 7 | Phosphoenolpyruvate carboxylase | 102,515 | |
| ML1419 | 9 | Regulatory protein | 60,968.8 | |
| ML1553 | 2 | Prolyl tRNA synthetase | 53,584.9 | |
| ML2177 | 7 | Uridine phosphorylase | 35,081.0 | |
| ML2341 | 9 | Transcriptional regulatory protein | 78,379.4 | |
| ML2346 | 10 | Conserved hypothetical protein | 33,938.9 | |
| ML2498 | 1 | Enoyl-CoA hydratase | 23,119.4 | |
| ML2649 | 7 | Oligosaccharide deacetylase | 29,553.7 | |
| III | ML0376 | 3 | Membrane protein | 36,916.8 |
| ML0410 | 6 | PE3 (PE family protein) | 10,951.4 | |
| ML0466 | 3 | Secreted protein | 32,914.4 | |
| ML0568 | 3 | Secreted protein | 18,361.6 | |
| ML0842 | 7 | Cysteine desulfurase | 44,537.5 | |
| ML1053 | 6 | PE5 (PE family) | 10,117.0 | |
| ML1055 | 3 | EsxK1 (ESAT-6 family) | 11,298.6 | |
| ML1056 | 3 | EsxL1 (ESAT-6 family) | 10,260.2 | |
| ML1182 | 6 | PPE9 (PPE family) | 43,119.6 | |
| ML1795 | 0 | HSP 18 | 16,706.9 | |
| ML1828 | 6 | PPE12 (PPE family) | 59,192.3 | |
| ML2088 | 7 | Putative cytochrome P450 | 47,154.3 | |
| ML2242 | 10 | Conserved hypothetical protein | 25,730.2 | |
| ML2531 | 3 | EsxH (ESAT-6 family) | 10,392.6 | |
| ML2532 | 3 | EsxG (ESAT-6 family) | 10,191.3 | |
| ML2534 | 6 | PE10 (PE family) | 10,243.6 | |
| ML2667 | 3 | Membrane transport protein | 65,080.5 |
Functional categories: 0, virulence, detoxification, adaptation; 1, lipid metabolism; 2, information pathways; 3, cell wall and cell processes; 6, PE/PPE; 7, intermediate metabolism and respiration; 8, unknown; 9, regulatory proteins; 10, conserved hypothetical.
Cloning, overexpression, and purification of recombinant M. leprae proteins.
A group of 12 coding sequences coding for two proteins from class I, four proteins from class II, and six proteins from class III were selected and cloned using the Gateway in vitro recombination method (Invitrogen) (2). Each target gene was amplified by PCR from genomic clones selected from Lorist6- and pYUB18-based cosmid libraries (Table 2). PCR products were first subcloned into the shuttle vector pDONR201, and the resulting products (entry clones) were mixed with the expression vector pDEST17 (destination vector). The reaction products were transformed into Escherichia coli DH5α cells. After screening, DNAs of selected recombinant clones were prepared with QIAfilter (QIAGEN Inc., Valencia, CA) and sequenced on an ABI 3700 DNA analyzer. The E. coli strain BL21(DE3) was used for protein expression (Table 2). Cells were grown in 1 liter of 2YT medium supplemented with ampicillin (100 μg/ml) at 20°C or at 37°C. The cells were then harvested by centrifugation, and cell pellets were washed once with protein extraction buffer containing 100 mM sodium phosphate buffer, 150 mM NaCl, pH 7, before centrifugation and resuspension in 50 ml of protein extraction buffer supplemented with a cocktail of protease inhibitors (Complete; Roche Diagnostics GmbH, Mannheim, Germany). The cells were lysed by sonication (Branson Sonifier 250). Lysates were centrifuged at 18,000 × g (Eppendorf centrifuge 5810R) for 30 min at 4°C, and the pellets were resuspended in 30 ml of 6 M guanidine hydrochloride, 100 mM sodium phosphate, 300 mM NaCl, pH 8. The whole suspension, previously clarified by centrifugation, was loaded into a 2-ml Ni-nitrilotriacetic acid-agarose superflow column using a fast-performance liquid chromatography device at a flow rate of 0.5 ml/min. Protein elution was monitored at 280 nm. The column was washed successively with buffer containing 8 M urea, 100 mM sodium phosphate, and 300 mM NaCl set at different pHs (8.0, 6.4, and 5.9) at a flow rate of 1 ml/min to remove nonspecifically bound proteins. All proteins were eluted with 8 M urea, 100 mM sodium phosphate, 300 mM NaCl, pH 4.15. The eluted proteins (30 to 60 ml) were dialyzed against 100 mM ammonium carbonate buffer, pH 7. Insoluble proteins were recovered by centrifugation (18,000 × g, 4°C, 30 min), and solubilized proteins were concentrated by lyophilization. Protein concentrations were determined by the Bradford method (Bio-Rad, Munich, Germany). The Limulus amebocyte lysate test (BioWhittaker, Verviers, Belgium) was used to ensure that samples contained no detectable lipopolysaccharide. The following recombinant proteins of M. leprae were used as controls (Yonsei University, Korea): the 35-kDa MMP-I, the 22-kDa MMP-II, the 34.8-kDa Ag85B, the 43.6-kDa EF-Tu, and GroES, the 10-kDa heat shock protein.
TABLE 2.
Genomic clones, expression vectors, protein expression of M. leprae recombinant proteins, and antiserum titers
| Accession no. | Classa | Functional category (function)b | Genomic clone (cosmid) | Mr | Mr with His tag | Protein expression conditions (strain/yield [mg liter−1]/ temp [°C]) | Antiserum titerc |
|---|---|---|---|---|---|---|---|
| ML0757 | I | 8 (unknown) | MLCY024 | 24,909 | 27,542 | BL21/3/20-37 | 1/3,200 |
| ML1057 | I | 8 (unknown) | B1912 | 12,207 | 14,840 | BL21/2/20-37 | 1/2,500 |
| ML0308 | II | 10 (conserved hypothetical) | MLCY174 | 25,899 | 28,532 | BL21-GC+/5/20 | 1/3,200 |
| ML1553 | II | 2 (prolyl tRNA synthetase) | B0596 | 53,584 | 56,267 | BL21/3/37-20 | 1/5,000 |
| ML2177 | II | 7 (uridine phosphorylase) | L0611 | 35,081 | 37,714 | BL21/3/20-37 | 1/2,500 |
| ML2498 | II | 1 (enoyl-CoA hydratase) | MLCY609 | 23,119 | 25,752 | BL21/4/37 | 1/5,000 |
| ML0410 | III | 6 (PE family) | B1620 | 10,951 | 13,584 | BL21/6/20-37 | 1/5,000 |
| ML1053 | III | 6 (PE family) | B1912 | 10,117 | 12,750 | BL21/4/37 | 1/10,000 |
| ML1055 | III | 3 (ESAT-6 family) | MLCY258 | 11,298 | 13,931 | BL21/5/37 | 1/10,000 |
| ML1056 | III | 3 (ESAT-6 family) | B1912 | 10,260 | 12,893 | BL21/7/37 | 1/2,500 |
| ML1182 | III | 6 (PPE family) | B1701 | 43,119 | 45,752 | BL21-GC+/3/20 | 1/1,600 |
| ML2534 | III | 6 (PE family) | MLCY693 | 10,243 | 12,876 | BL21-pLys/3/20 | 1/5,000 |
I, restricted to M. leprae; II, has orthologues in other organisms besides mycobacteria; III, surface-exposed or exported proteins of limited distribution.
Functional categories: 1, lipid metabolism; 2, information pathways; 3, cell wall and cell processes; 6, PE/PPE; 7, intermediate metabolism and respiration; 8,unknown; 10, conserved hypothetical.
Antiserum titers from rabbits immunized intradermally with 250 μg recombinant protein.
Immunodetection of recombinant proteins in cellular extracts of M. leprae.
Polyclonal antibodies for immunodetection were generated in rabbits by intradermally injecting 250 μg of the recombinant proteins emulsified in 1 ml of phosphate-buffered saline (PBS)-incomplete Freund's adjuvant (1:1). Immunization was repeated four times. The sera were titrated by an indirect enzyme-linked immunosorbent assay (ELISA), with the recombinant proteins (10 μg/ml) as the coating layer. Lyophilized cellular extracts of M. leprae, kindly provided by John Spencer (Colorado State University) (22), were resuspended in 1 M urea and 5× Laemmli sample buffer in order to obtain a final protein concentration of 2 mg/ml. Thirty micrograms of cytosolic, membrane, and cell wall fractions was electrophoresed in a 13% or a 10 to 20% sodium dodecyl sulfate (SDS)-polyacrylamide gel. The proteins were electrotransferred onto Hybond-C membranes (Amersham Biosciences, Arlington Heights, IL), which were immediately blocked overnight with 0.5% bovine serum albumin (BSA) in Tris-buffered saline (TBS; 50 mM Tris-HCl, 150 mM NaCl, pH 7.5) at 4°C. The membranes were washed three times with TBS, soaked at room temperature for 1 h in 0.5% BSA-TBS, pH 7.5, and then incubated either with immune serum or with preimmune serum as a control. The membranes were washed three times with TBS and were incubated for 1 h with horseradish peroxidase-conjugated anti-rabbit IgG (Amersham Biosciences). Chemiluminescence detection using an ECL+ kit (Amersham Biosciences) was visualized with an LAS 3000 image reader (Fujifilm). Independent experiments were performed at least three times.
Study population.
The population used to screen for protein immunogenicity, as measured by gamma interferon (IFN-γ) release, was composed of 25 former leprosy patients. They were diagnosed clinically, bacteriologically, and histologically from 1986 to 2003 at the Korean Institute of Leprosy Research, Euiwang City, Republic of Korea. Six BT, two BB, eight BL, and nine LL patients comprised this group. Seven were BI positive at the time of sampling, and 18 were BI negative; 12 patients were positive for anti-PGL-1 antibodies, while 13 were negative (9). At the time of the experiment, 11 patients were being treated with MDT, 5 were being treated with dapsone, and 9 had received no treatment. All subjects gave their informed consent prior to venipuncture. The appropriate local ethics committees granted approval for this study.
Whole blood assay.
IFN-γ measurements in whole blood restimulation assays were performed as described previously (17, 28, 29). Briefly, 5 ml of blood was taken by venipuncture from each patient and immediately mixed with heparin at a concentration of 20 U/ml. The blood was diluted 10-fold with sterile RPMI 1640 tissue culture medium containing 100 U/ml penicillin, 100 mg/ml streptomycin, and 2 mM l-glutamine. Proteins (10 μg/ml), concanavalin A, phytohemagglutinin (5 to 10 μg/ml), M. leprae soluble antigens (MLSA), or medium alone was added to the wells of 96-well round-bottomed tissue culture plates in a volume of 20 μl, to which 180 μl of diluted blood was added within 2 to 4 hours of blood collection. Tests were carried out in triplicate. After 5 days of incubation in a 37°C incubator in the presence of 5% CO2, supernatants were frozen at −20°C until analysis. The supernatants were subsequently tested for IFN-γ in an ELISA using BD OptEIA set human IFN-γ (BD Biosciences, San Diego, Calif.). Cytokine production was calculated for triplicate wells after the subtraction of nonspecific cytokine production in nonstimulated cultures.
Human serum samples.
Sera from 42 anonymous leprosy patients were selected from the leprosy serum collection of the Department of Microbiology, Yonsei University, and were manipulated according to standard procedures. The patients were classified clinically as 3 BT, 1 BB, 24 BL, and 14 LL cases and were all BI positive (range, 1.8 to 5.0), and their sera contained antibodies to PGL-1 in all but 5 cases. All patients were being treated with MDT at the time of sampling. Sera from 21 healthy, BCG-vaccinated Korean controls were used for control purposes, and these contained no antibodies to PGL-1. The internal review boards at Yonsei University College of Medicine approved the use of these serum samples for our purposes. Sera were screened for the presence of antibodies to the recombinant M. leprae proteins ML0308, ML1553, ML2177, ML2498, ML0410, ML1053, ML1055, and ML1056 as described previously (25). Briefly, 100 μl of recombinant antigen (10 μg/ml in 0.1 M carbonate-bicarbonate buffer, pH 9.6) was used to coat the wells of 96-well flat-bottomed ELISA plates (Nunc) at 4°C overnight. The plates were washed with PBS, 0.05% Tween 80, pH 7.5 (PBS/T), four times, and the wells were blocked with PBS-1% BSA in PBS/T, pH 7.5. The serum samples were diluted 1:200 in 5% goat serum (Invitrogen, Carlsbad, CA) in PBS/T, pH 7.5, and 100 μl of this dilution was added to the wells. Following 2 h of incubation at room temperature, the plates were washed four times as described above, and 100 μl of horseradish peroxidase-conjugated goat anti-human IgG (Calbiochem, San Diego, California) was added at a dilution of 1:10,000 in 5% goat serum in PBS/T, pH 7.5. After 2 h of incubation at room temperature and washing, 100 μl of o-phenylenediamine (Sigma, St. Louis, Missouri) was added. After 15 min, the reaction was stopped with 100 μl of 0.5 M H2SO4, and the absorbance at 490 nm was read. Sera were tested in duplicate. To normalize the background, we used the mean absorbance of the control wells without antigens.
RESULTS
Definition of new protein antigens in silico.
Comparative genomics was used to identify potential protein antigens that could ultimately serve as the basis for an immunodiagnostic test for leprosy. To optimize the potential specificity of our candidates, we selected proteins with limited distribution in other bacterial genomes. At the start of this project, 136 genes were known to be restricted to the M. leprae genome (class I) (12). However, since some of them were likely to correspond to pseudogenes or gene fragments, a reanalysis of class I genes was performed with more stringent criteria, such as the codon usage, GC content, and positional base preference routines contained in Artemis (24). This selection reduced our class I candidates to a set of 17 proteins that were likely to be strictly M. leprae specific. Class II genes have orthologues or paralogues in species other than mycobacteria, and several of them are predicted to encode enzymes. Of the 29 class II genes, 16 were retained for further analysis, including those for several conserved hypothetical proteins, uridine phosphorylase, and an enoyl-coenzyme A (CoA) hydratase. Class III contains 17 proteins that are likely to be exported or surface exposed, with several of them being members of the PE and PPE (derived from the motifs Pro-Glu [PE] and Pro-Pro-Glu [PPE], respectively) or ESAT-6 (6-kDa early secreted antigenic target) protein family, first described for M. tuberculosis (11) but now known to be present in many mycobacteria. Six class III proteins with limited distribution are absent from M. tuberculosis but present in Mycobacterium avium subsp. paratuberculosis (ML0376, ML0466, ML0842, ML1795, ML2242, and ML2667). The complete list of the selected class I, II, and III candidate antigens is presented in Table 1.
Cloning, overexpression, and purification of recombinant M. leprae proteins.
Candidate genes were subcloned into pDEST17 vectors, thereby introducing a His-tag motif, MSYYHHHHHHLESTSLYKKAGS, at the N terminus to simplify protein purification. All of the recombinant proteins were insoluble, regardless of the growth conditions and bacterial host strains. Expression yields could be improved by modifying E. coli growth conditions in some instances (Table 2). The expression of ML1182 was only possible in strain BL21 CodonPlus (DE-3)-RP (Stratagene). The ML1553, ML2177, and ML2498 genes, which encode the prolyl-tRNA synthetase, uridine phosphorylase, and enoyl-CoA hydratase enzymes, respectively, were cloned into pBAD vectors to achieve tighter regulation of protein expression, but nevertheless, the expression products remained insoluble. All recombinant proteins were purified by affinity chromatography under denaturing conditions.
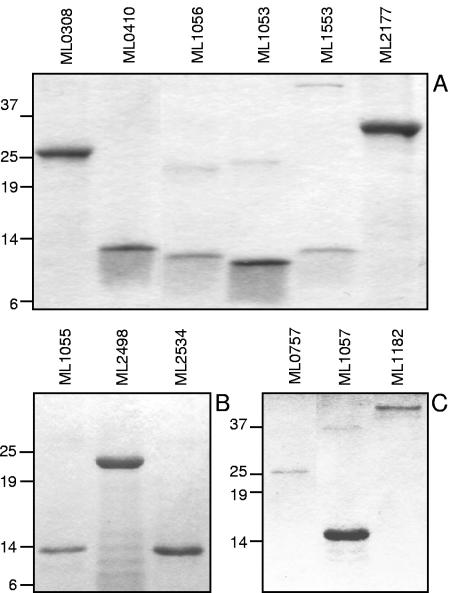
Figure 1 shows the electrophoretic profiles of the resulting purified recombinant proteins. Some candidates, such as ML1053, a PE protein, formed multimeric bands resistant to SDS, dithiothreitol, and boiling. However, contamination with other proteins was excluded, as electrospray ionization mass spectrometry analysis confirmed the homogeneity of the preparation and the expected molecular weight (data not shown). ML1553, ML1182, and ML2498 showed a tendency to degrade. The homogeneity of the protein samples was verified by Western blot analysis using anti-His-tag polyclonal antibodies (data not shown).
FIG. 1.
Electrophoretic profiles of recombinant proteins. Electrophoresis was done on 13% (A) or 10 to 20% gradient (B and C) SDS-polyacrylamide gels.
Immunolocalization of native M. leprae proteins.
Before undertaking immunological studies, we examined whether our candidate antigens were immunogenic in animal models. When rabbits were immunized with the recombinant proteins, immune sera with potent reactivities were generated, as measured by antibody titers in an ELISA (Table 2).
These polyclonal antibodies were then used to probe Western blots of cell extracts of armadillo-derived M. leprae to determine if our candidate antigens are expressed in the bacilli and to investigate their subcellular localization. Figure 2 shows that the class I protein ML0757 (Mr, 24,909) was detected in both the cytosolic and cell wall fractions. Conversely, neither the recombinant ML0757 protein nor the M. leprae fractions reacted with preimmune serum (Fig. 2A). The class II hypothetical protein ML0308 was located in all three subcellular fractions, with the expected molecular weight (Mr, 25,899) (Fig. 2B), and appeared to be more abundant in the membrane fraction of M. leprae. The putative prolyl tRNA synthetase (ML1553; Mr, 53,584) was detected mainly in the membrane fraction, although a weak band with a similar mass was also observed in the cytosolic fraction (Fig. 2C). ML2498, a putative enoyl-CoA hydratase (Mr, 23,119), was located in the membrane fraction (Fig. 2D). A class III protein member, ML0410 (Mr, 10,951), belonging to the PE family was detected in the membrane fraction (Fig. 2E), while another PE protein, ML1053, was detected in both the cytosolic and membrane fractions (data not shown). Control blots for ML0308, ML1553, ML2498, ML1053, and ML0410 with preimmune sera were all negative (data not shown). ML1057 (class I), ML2177 (class II), ML1182, ML2534, ML1055, and ML1056 (all class III) could not be detected by immunoblotting with immune sera.
FIG. 2.
Subcellular localization of native proteins in armadillo-derived M. leprae. (A) ML0757; (B) ML0308; (C) ML1553; (D) ML2498; (E) ML0410. Fractions of M. leprae were separated by electrophoresis and blotted onto Hybond-C membranes, and immunodetection was performed with rabbit antisera. rProt, purified recombinant protein; CYT, cytoplasmic fraction; MB, membrane fraction; CW, cell wall fraction.
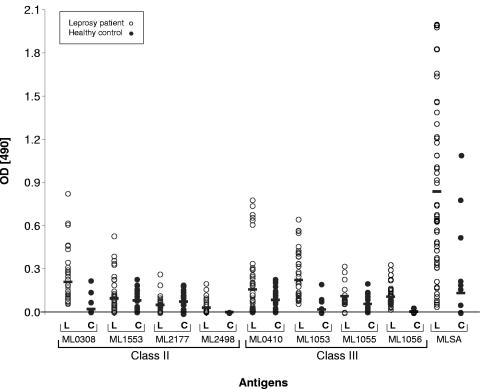
Circulating antibodies against M. leprae proteins.
Since all 12 candidate antigens were immunogenic in animal models and since 6 of 12 proteins were detectable in cell extracts of M. leprae, we then examined if immune responses to any of these antigens were measurable in infected humans. We first investigated whether our candidates generated antibody responses in leprosy patients by testing the reactivities of 42 serum samples to the recombinant proteins in an ELISA. In control experiments, sera from 21 healthy Korean individuals were tested for reactivity. As shown in Fig. 3, MLSA was strongly recognized by patient sera, with a mean optical density (±standard deviation) of 0.83 ± 0.6, whereas the mean value for healthy controls was only 0.13 ± 0.3. The ML0308, ML1553, ML2177, ML2498, ML0410, ML1053, ML1055, and ML1056 proteins were also recognized by circulating antibodies, although to a lesser extent than MLSA. The other proteins did not elicit any signals (data not shown). Among the reactive antigens, the class II protein ML0308 showed the best signal, with a mean optical density of 0.21 ± 0.16 for patient sera compared to a value of 0.02 ± 0.06 for control sera, followed by the class III proteins ML0410 and ML1053 (mean values of 0.16 ± 0.20 and 0.27 ± 0.19, respectively, for patient sera, and 0.08 ± 0.08 and 0.02 ± 0.012, respectively, for healthy control sera). However, the ML1055, ML1553, and ML2177 proteins also reacted to a similar extent with sera from healthy controls, indicating that they may share cross-reacting epitopes with proteins from other organisms.
FIG. 3.
Antibody responses against candidate M. leprae antigens. Sera from 42 individuals with leprosy and 21 healthy controls were tested in duplicate, and the mean absorbance for control wells without antigen was subtracted from that for sample wells before analysis. Open symbols correspond to sera from leprosy patients (L), and solid symbols correspond to sera from healthy controls (H). Horizontal bars indicate the resulting mean values for each M. leprae recombinant protein. OD, optical density.
IFN-γ responses in leprosy patients and healthy controls.
We then examined if a group of Korean leprosy patients who had been treated and cured showed IFN-γ responses to our candidate antigens. For this purpose, T-cell restimulation assays were performed on whole blood samples. Potent and comparable IFN-γ production levels were generated in response to stimulation with phytohemagglutinin and concanavalin A in all patients (data not shown) and in response to stimulation with MLSA in all but 4 of 25 patients (Fig. 4).
FIG. 4.
IFN-γ responses to candidate M. leprae antigens in former leprosy patients. Diluted whole blood samples from 25 former leprosy patients were stimulated for 5 days in vitro, and their IFN-γ production was measured as mean values, in pg ml−1, of triplicate wells after subtraction of the nonspecific cytokine production level in unstimulated cultures. Horizontal bars indicate the resulting mean values for each antigen.
In the first experiment, the IFN-γ responses of lymphocytes to the candidate proteins ML0308, ML1053, ML1055, and ML1056 were compared to the responses to M. leprae antigens with established immunogenicity, such as MMP-I, MMP-II, antigen 85-B, EF-Tu, and GroES, in eight former leprosy patients. Two sets of recombinant proteins, namely, ML1553, ML2177, ML2498, and ML0410 and ML0757, ML1057, and ML1182, were tested in two independent experiments, with each including 10 former leprosy patients. Figure 4 shows that of the 12 candidate antigens, proteins ML0757, ML1057 (both class I), and ML1055 (class III) did not elicit significant IFN-γ production in leprosy patients, whereas the other candidates from class II and class III did, especially ML2177, ML2498, and the PE proteins ML0410 and ML1053. In particular, these last candidates showed stronger IFN-γ responses than the potent antigen Ag85B, illustrating their potential use for leprosy diagnosis. We were unable to establish a clear correlation between the IFN-γ response and the type of leprosy, although cells from BT leprosy patients were slightly more productive, possibly as a result of the patients having received MDT. In control experiments performed with lymphocytes from healthy individuals from France, much lower or insignificant levels of IFN-γ were detected after incubation with each of the recombinant M. leprae proteins (data not shown).
DISCUSSION
Since the leprosy elimination program started in 1991, the global prevalence of the disease has dropped dramatically (∼90%). Some 112 of the 125 countries where leprosy is endemic have reached the WHO's proposed goal, namely, a prevalence of <1 per 10,000 inhabitants. Nowadays, the number of new cases detected yearly, at >500,000, is greater than that predicted by WHO, despite the massive use of MDT, and this may be a consequence of the relatively long incubation period of the disease. Many factors concerning leprosy transmission remain poorly understood. With the aim of developing a specific immunological test for the diagnosis of leprosy before the clinical signs become apparent, we have applied comparative genomics and bioinformatics to define a set of 50 potential antigens. Some of these appear to be restricted to M. leprae, while others have a wider distribution and include proteins that are likely to be secreted or surface exposed.
Here we have produced and characterized the first 12 proteins from our antigen discovery project and report the identification of 6 of them in subcellular compartments of M. leprae by immunodetection. Our inability to detect the remaining six proteins may indicate that they are of low abundance or are unstable in extracts of armadillo-derived M. leprae, despite the inclusion of protease inhibitors. Other workers have reported similar discrepancies (21). Recently, a proteomic analysis of soluble/cytosolic and membrane fractions of M. leprae led to the identification of 147 protein spots, but none of these corresponded to proteins in this study (22). Our work thus enriches our knowledge of the M. leprae proteome. Class I proteins are restricted to the leprosy bacillus, but their existence is hypothetical since they show no sequence similarity to known proteins. Furthermore, because of their small size, there is also a risk that some of them may correspond to pseudogene products. Further in silico refinement using more stringent criteria led us to focus on 16 of the 136 candidates (Table 1). Two class I proteins, ML0757 and ML1057 (Table 2), were analyzed in this work, and ML0757 was detected in cell extracts of the bacillus, where it was found in the cell wall fraction. Both ML0757 and ML1057 induced weak IFN-γ responses, thus indicating that the proteins are present during infection. These results confirmed the bioinformatic predictions and encourage us to examine more class I proteins in future work.
Class II proteins, some of which have putative functions but no known counterparts in mycobacteria, yielded the best results in the immunological analysis. While most of the M. leprae genes have orthologues in M. tuberculosis, some genes show stronger similarity to their eukaryotic equivalents and are in all probability the result of horizontal gene transfer events (7, 12, 14). Examples of this may be found in the ML1553 gene, encoding a eukaryotic-like prolyl tRNA synthetase, and the ML2177 gene, coding for a uridine phosphorylase that resembles the mouse enzyme. In contrast, the ML2498 gene codes for an enoyl-CoA hydratase that is typically prokaryotic, and the ML0308 gene encodes a conserved hypothetical protein with orthologues in archaebacteria (http://genolist.pasteur.fr/Leproma/). All four of these proteins are antigenic in humans and recognized by T cells and/or antibodies (Fig. 3 and 4).
Class III proteins are likely to be secreted or present in the cell envelope, and some of them belong to the PE, PPE, and ESAT-6 protein families that are confined to mycobacteria (1, 4, 6, 11). Several members of these protein families from M. tuberculosis are known to be immunodominant antigens that are strongly recognized by T cells, a highly desirable property for the development of an immunodiagnostic test. The best studied of these is the ESAT-6 protein, a potent T-cell antigen, which is used as part of a diagnostic test for tuberculosis in humans and cattle (18, 19, 32). Initial work by Ottenhoff et al. (16) confirmed the immunodominance of the M. leprae ESAT-6 orthologue, ML0049, but showed that problems of potential cross-reactivity arise in individuals likely to have been infected or exposed to M. tuberculosis. The sequence of the M. leprae orthologue of CFP-10 has 40% identity (60% homology) at the protein level with M. tuberculosis CFP-10 and may also be subject to cross-recognition. For this reason, we have concentrated on other, more divergent members of the ESAT-6 family of M. leprae, namely, ML1055 and ML1056, together with one PPE protein (ML1182) and three PE proteins (ML0410, ML1053, and ML2534). Nevertheless, the ESAT-6 protein ML1055 (EsxK1), but not ML1056 (EsxL1), appears to cross-react with sera from healthy individuals (Fig. 3).
While the ESAT-6 family proteins stimulated low levels of IFN-γ production by lymphocytes, the PE proteins ML0410 and ML1053 seemed to be far more immunogenic. These antigens, together with the four from class II, represent promising candidates for further testing in order to appraise their true diagnostic potential. One of the shortcomings of the present antigen discovery program was the use of blood from MDT-treated leprosy patients rather than from untreated patients, who are more likely to be encountered in the field and for whom a better diagnostic test is required. In the next phase of this work, which will be limited to the best protein candidates, both alone and in combination, we will perform more extensive controls using whole blood assays of both healthy and diseased individuals from areas where leprosy is endemic and will also include subjects suffering from tuberculosis to determine possible cross-reactivity. Only in this way will we able to assess the specificity and sensitivity of these antigens and to establish their usefulness for developing a specific immunological test for the early diagnosis of leprosy. Early detection will result in the prompt initiation of multidrug therapy, thereby increasing the possibility that both the prevalence and the incidence of leprosy can be further reduced.
Acknowledgments
We are much indebted to Nathalie Berger, Jean-Michel Betton, and the structural genomics platform of the Institut Pasteur Génopole for providing the expression constructs. We thank John Spencer for providing the subcellular fractions of M. leprae and the Leonard Wood Memorial Center, Cebu, The Philippines, and the Korean Institute of Leprosy Research, Anyang, Republic of Korea, for providing anonymous serum samples for the study.
This work received the financial support of the Institut Pasteur, the Association Française Raoul Follereau, the Génopole Programme, and the National Institutes of Health, National Institute of Allergy and Infectious Diseases (grant RO1-AI47197-01A1 and contract NO1-AI25469).
Editor: J. L. Flynn
REFERENCES
- 1.Banu, S., N. Honore, B. Saint-Joanis, D. Philpott, M. C. Prevost, and S. T. Cole. 2002. Are the PE-PGRS proteins of Mycobacterium tuberculosis variable surface antigens? Mol. Microbiol. 44:9-19. [DOI] [PubMed] [Google Scholar]
- 2.Betton, J. M. 2004. High throughput cloning and expression strategies for protein production. Biochimie 86:601-605. [DOI] [PubMed] [Google Scholar]
- 3.Brahmbhatt, S., R. Hussain, S. Zafar, G. Dawood, T. H. Ottenhoff, J. W. Drijfhout, G. Bothamley, S. Smith, F. V. Lopez, and H. M. Dockrell. 2002. Human T cell responses to peptides of the Mycobacterium leprae 45-kD serine-rich antigen. Clin. Exp. Immunol. 128:140-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brennan, M. J., G. Delogu, Y. Chen, S. Bardarov, J. Kriakov, M. Alavi, and W. R. Jacobs, Jr. 2001. Evidence that mycobacterial PE_PGRS proteins are cell surface constituents that influence interactions with other cells. Infect. Immun. 69:7326-7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Britton, W. J., and D. N. Lockwood. 2004. Leprosy. Lancet 363:1209-1219. [DOI] [PubMed] [Google Scholar]
- 6.Brodin, P., I. Rosenkrands, P. Andersen, S. T. Cole, and R. Brosch. 2004. ESAT-6 proteins: protective antigens and virulence factors? Trends Microbiol. 12:500-508. [DOI] [PubMed] [Google Scholar]
- 7.Brosch, R., S. V. Gordon, K. Eiglmeier, T. Garnier, and S. T. Cole. 2000. Comparative genomics of the leprosy and tubercle bacilli. Res. Microbiol. 151:135-142. [DOI] [PubMed] [Google Scholar]
- 8.Buhrer-Sekula, S., M. G. Cunha, N. T. Foss, L. Oskam, W. R. Faber, and P. R. Klatser. 2001. Dipstick assay to identify leprosy patients who have an increased risk of relapse. Trop. Med. Int. Health 6:317-323. [DOI] [PubMed] [Google Scholar]
- 9.Cho, S. N., S. H. Kim, R. V. Cellona, G. P. Chan, T. T. Fajardo, G. P. Walsh, and J. D. Kim. 1992. Prevalence of IgM antibodies to phenolic glycolipid I among household contacts and controls in Korea and the Philippines. Lepr. Rev. 63:12-20. [DOI] [PubMed] [Google Scholar]
- 10.Chua-Intra, B., J. Ivanyi, A. Hills, J. Thole, C. Moreno, and H. M. Vordermeier. 1998. Predominant recognition of species-specific determinants of the GroES homologues from Mycobacterium leprae and M. tuberculosis. Immunology 93:64-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, B. G. Barrell, et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 12.Cole, S. T., K. Eiglmeier, J. Parkhill, K. D. James, N. R. Thomson, P. R. Wheeler, N. Honore, T. Garnier, C. Churcher, D. Harris, K. Mungall, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. M. Davies, K. Devlin, S. Duthoy, T. Feltwell, A. Fraser, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, C. Lacroix, J. Maclean, S. Moule, L. Murphy, K. Oliver, M. A. Quail, M. A. Rajandream, K. M. Rutherford, S. Rutter, K. Seeger, S. Simon, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, K. Taylor, S. Whitehead, J. R. Woodward, and B. G. Barrell. 2001. Massive gene decay in the leprosy bacillus. Nature 409:1007-1011. [DOI] [PubMed] [Google Scholar]
- 13.Dockrell, H. M., S. Brahmbhatt, B. D. Robertson, S. Britton, U. Fruth, N. Gebre, M. Hunegnaw, R. Hussain, R. Manandhar, L. Murillo, M. C. Pessolani, P. Roche, J. L. Salgado, E. Sampaio, F. Shahid, J. E. Thole, and D. B. Young. 2000. A postgenomic approach to identification of Mycobacterium leprae-specific peptides as T-cell reagents. Infect. Immun. 68:5846-5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eiglmeier, K., J. Parkhill, N. Honore, T. Garnier, F. Tekaia, A. Telenti, P. Klatser, K. D. James, N. R. Thomson, P. R. Wheeler, C. Churcher, D. Harris, K. Mungall, B. G. Barrell, and S. T. Cole.2001. The decaying genome of Mycobacterium leprae. Lepr. Rev. 72:387-398. [PubMed] [Google Scholar]
- 15.Garnier, T., K. Eiglmeier, J. C. Camus, N. Medina, H. Mansoor, M. Pryor, S. Duthoy, S. Grondin, C. Lacroix, C. Monsempe, S. Simon, B. Harris, R. Atkin, J. Doggett, R. Mayes, L. Keating, P. R. Wheeler, J. Parkhill, B. G. Barrell, S. T. Cole, S. V. Gordon, and R. G. Hewinson. 2003. The complete genome sequence of Mycobacterium bovis. Proc. Natl. Acad. Sci. USA 100:7877-7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geluk, A., K. E. van Meijgaarden, K. L. Franken, Y. W. Subronto, B. Wieles, S. M. Arend, E. P. Sampaio, T. de Boer, W. R. Faber, B. Naafs, and T. H. Ottenhoff. 2002. Identification and characterization of the ESAT-6 homologue of Mycobacterium leprae and T-cell cross-reactivity with Mycobacterium tuberculosis. Infect. Immun. 70:2544-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hussain, R., A. Kaleem, F. Shahid, M. Dojki, B. Jamil, H. Mehmood, G. Dawood, and H. M. Dockrell. 2002. Cytokine profiles using whole-blood assays can discriminate between tuberculosis patients and healthy endemic controls in a BCG-vaccinated population. J. Immunol. Methods 264:95-108. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, P. D., R. L. Stuart, M. L. Grayson, D. Olden, A. Clancy, P. Ravn, P. Andersen, W. J. Britton, and J. S. Rothel. 1999. Tuberculin-purified protein derivative-, MPT-64-, and ESAT-6-stimulated gamma interferon responses in medical students before and after Mycobacterium bovis BCG vaccination and in patients with tuberculosis. Clin. Diagn. Lab. Immunol. 6:934-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lalvani, A., A. A. Pathan, H. McShane, R. J. Wilkinson, M. Latif, C. P. Conlon, G. Pasvol, and A. V. Hill. 2001. Rapid detection of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Am. J. Respir. Crit. Care Med. 163:824-828. [DOI] [PubMed] [Google Scholar]
- 20.Languillon, J. 1999. Précis de léprologie, p. 45-62. In J. Languillon, P. Bourrel, P. Saint-André, G. Discamps, and G. Baquillon (ed.), Leprosy, 1st ed. Pré Presse-Pastel Créations, Lavaur, France.
- 21.Marques, M. A., S. Chitale, P. J. Brennan, and M. C. Pessolani. 1998. Mapping and identification of the major cell wall-associated components of Mycobacterium leprae. Infect. Immun. 66:2625-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marques, M. A., B. J. Espinosa, E. K. Xavier da Silveira, M. C. Pessolani, A. Chapeaurouge, J. Perales, K. M. Dobos, J. T. Belisle, J. S. Spencer, and P. J. Brennan. 2004. Continued proteomic analysis of Mycobacterium leprae subcellular fractions. Proteomics 4:2942-2953. [DOI] [PubMed] [Google Scholar]
- 23.Ridley, D. S., and W. H. Jopling. 1966. Classification of leprosy according to immunity. A five-group system. Int. J. Lepr. Other Mycobact. Dis. 34:255-273. [PubMed] [Google Scholar]
- 24.Rutherford, K., J. Parkhill, J. Crook, T. Horsnell, P. Rice, M. A. Rajandream, and B. Barrell. 2000. Artemis: sequence visualization and annotation. Bioinformatics 16:944-945. [DOI] [PubMed] [Google Scholar]
- 25.Spencer, J. S., H. J. Kim, A. M. Marques, M. Gonzalez-Juarerro, M. C. Lima, V. D. Vissa, R. W. Truman, M. L. Gennaro, S. N. Cho, S. T. Cole, and P. J. Brennan. 2004. Comparative analysis of B- and T-cell epitopes of Mycobacterium leprae and Mycobacterium tuberculosis culture filtrate protein 10. Infect. Immun. 72:3161-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tekaia, F., S. V. Gordon, T. Garnier, R. Brosch, B. G. Barrell, and S. T. Cole. 1999. Analysis of the proteome of Mycobacterium tuberculosis in silico. Tuber. Lung Dis. 79:329-342. [DOI] [PubMed] [Google Scholar]
- 27.Vissa, V. D., and P. J. Brennan. 2004. The genome of Mycobacterium leprae: a minimal mycobacterial gene set. Genome Biol. 2:reviews1023.1-1023.8. [Online.] http://genomebiology.com/2001/2/8/reviews/1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weir, R. E., P. J. Brennan, C. R. Butlin, and H. M. Dockrell. 1999. Use of a whole blood assay to evaluate in vitro T cell responses to new leprosy skin test antigens in leprosy patients and healthy subjects. Clin. Exp. Immunol. 116:263-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weir, R. E., A. R. Morgan, W. J. Britton, C. R. Butlin, and H. M. Dockrell. 1994. Development of a whole blood assay to measure T cell responses to leprosy: a new tool for immuno-epidemiological field studies of leprosy immunity. J. Immunol. Methods 176:93-101. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization. 2004. The global leprosy situation, p. 8-11. In WHO leprosy elimination project: status report 2003. World Health Organization, Geneva, Switzerland.
- 31.Winter, N., J. A. Triccas, B. Rivoire, M. C. Pessolani, K. Eiglmeier, E. M. Lim, S. W. Hunter, P. J. Brennan, and W. J. Britton. 1995. Characterization of the gene encoding the immunodominant 35 kDa protein of Mycobacterium leprae. Mol. Microbiol. 16:865-876. [DOI] [PubMed] [Google Scholar]
- 32.Wood, P. R., and S. L. Jones. 2001. BOVIGAM: an in vitro cellular diagnostic test for bovine tuberculosis. Tuberculosis (Edinburgh) 81:147-155. [DOI] [PubMed] [Google Scholar]