Abstract
Substantial data implicate the commensal flora as triggers for the initiation of enteric inflammation or inflammatory disease relapse. We have shown that enteric epithelia under metabolic stress respond to nonpathogenic bacteria by increases in epithelial paracellular permeability and bacterial translocation. Here we assessed the structural basis of these findings. Confluent filter-grown monolayers of the human colonic T84 epithelial cell line were treated with 0.1 mM dinitrophenol (which uncouples oxidative phosphorylation) and noninvasive, nonpathogenic Escherichia coli (strain HB101, 106 CFU) with or without pretreatment with various pharmacological agents. At 24 h later, apoptosis, tight-junction protein expression, transepithelial resistance (TER; a marker of paracellular permeability), and bacterial internalization and translocation were assessed. Treatment with stabilizers of microtubules (i.e., colchicine), microfilaments (i.e., jasplakinolide) and clathrin-coated pit endocytosis (i.e., phenylarsine oxide) all failed to block DNP+E. coli HB101-induced reductions in TER but effectively prevented bacterial internalization and translocation. Neither the TER defect nor the enhanced bacterial translocations were a consequence of increased apoptosis. These data show that epithelial paracellular and transcellular (i.e., bacterial internalization) permeation pathways are controlled by different mechanisms. Thus, epithelia under metabolic stress increase their endocytotic activity that can result in a microtubule-, microfilament-dependent internalization and transcytosis of bacteria. We speculate that similar events in vivo would allow excess unprocessed antigen and bacteria into the mucosa and could evoke an inflammatory response by, for example, the activation of resident or recruited immune cells.
In many respects the human interaction with bacteria is one of conflict. A voluminous literature illustrates how the pathogen affects host target cells and the host response to destroy or eradicate the pathogen (23). In contrast, the human colon is home to an immense and complex microflora of commensal bacteria. Indeed, the presence of a normal microbiota is a requirement for mucosal immune cell education and normal gut physiology, such as short-chain fatty acid synthesis as an energy source for the colonocyte (33, 35). However, evidence now indicates that commensal bacteria and their products may trigger or exacerbate chronic idiopathic intestinal inflammatory diseases, such as Crohn's disease (28). For instance, virtually every rodent model of colitis, whether spontaneous or chemically induced, is significantly less severe if the animals are raised in germfree conditions (16, 38). Similarly, bowel rest via surgically diverting the fecal stream and antibiotic treatment can lead to symptomatic relief in some patients with Crohn's disease (26, 37).
A common feature of gut inflammation is increased epithelial permeability, both paracellular (i.e., the pathway between adjacent cells) and transcellular (i.e., material crosses apical and basolateral plasmalemmas) permeability (40, 42). Whether a decrease in barrier function is a cause or effect of intestinal inflammation remains a point of debate. Nevertheless, increased access of bacteria and their products to the mucosa and submucosa and the resident immune cells therein would be expected to elicit immune activity with potentially proinflammatory consequences. Maintenance of the epithelial barrier is an energy-dependent process, and we, and others, have noted swollen and irregular mitochondria in the epithelium of resected tissues from some patients with inflammatory bowel disease (IBD) (12, 30, 41). Thus, we hypothesized that deranged energy metabolism in the epithelium that could be evoked by a variety of insults, including infection (13, 18, 20), ischemia (47), or medications (45), could result in decreased barrier function and enhanced bacterial translocation. By using dinitrophenol (DNP), a classic uncoupler of oxidative phosphorylation, we have shown that model epithelia under metabolic stress do indeed respond to nonpathogenic, noninvasive Escherichia coli strain HB101 by a decrease in transepithelial resistance, increased bacterial internalization and translocation, and increased interleukin-8 synthesis (30).
In the present study we assessed the mechanism(s) of DNP+E. coli HB101-evoked decreases in paracellular permeability and increased bacterial translocation, focusing on delineating the structural basis underlying these events. The data show that epithelial paracellular permeability and bacterial translocation are controlled by different mechanisms and that viable bacteria cross the epithelium predominantly via a transcellular route that is dependent on a functional enterocytic cytoskeleton. These data indicate that when stressed (i.e., when the energy balance is perturbed) enteric epithelial cells have the potential to actively endocytose bystander commensal bacteria, which proceed to cross the epithelial layer.
MATERIALS AND METHODS
Cell lines and bacteria.
The human colon cancer-derived crypt-like T84 epithelial cell line was maintained and cultured in a 1:1 (vol/vol) mixture of Dulbecco-Vogt modified Eagle medium and Ham F-12 supplemented with 10% fetal bovine serum as described previously (29, 30). E. coli strain HB101 was grown and cultured in Luria-Bertani (LB) broth. Using negative staining and electron microscopy (24), we compared E. coli HB101 to the known pathogen enterohemorrhagic E. coli (O157:H7 strain Cl-56), with only the latter bearing obvious pili and sex pili (Fig. 1A). Bacteria were grown to mid-log growth phase, pelleted, and resuspended in antibiotic-free T84 medium, and ∼106 CFU/ml was added to the apical surface of T84 monolayers (to mimic in vivo exposure) grown on transwell supports or plastic culture plates. DNP was added apically and simultaneously with the E. coli, and all drug treatments were added as a 1-h pretreatment into the apical compartment of the culture wells (drugs were not removed from the culture well by replacing with fresh medium). Epithelial monolayers with or without bacteria (with or without pharmacological agents) were coincubated in antibiotic-free medium at 37°C in 5% CO2 for up to 24 h.
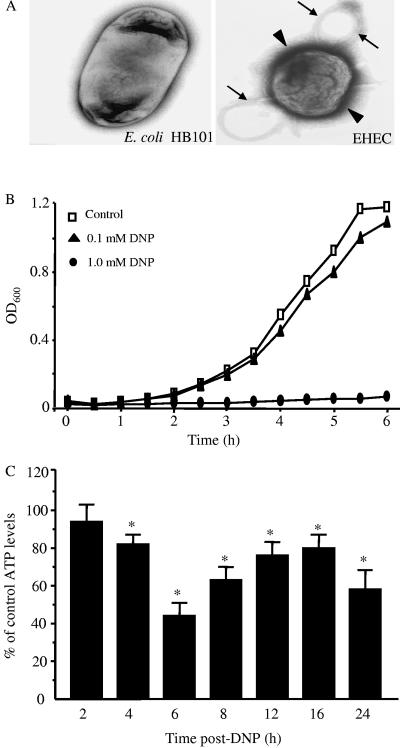
FIG. 1.
Low-dose DNP does not affect E. coli HB101 growth but does reduce epithelial ATP content. (A) Comparison of E. coli HB101 to enterohemorrhagic E. coli, where only the latter was found to have abundant pili (arrowhead) and sex pili (arrows). (B) Representative bacterial growth curve (one experiment of three) showing that 0.1 mM DNP does not affect E. coli growth. (C) The same dose of DNP results in a significant decrease in T84 cell ATP levels by 4 h posttreatment (n= three to five experiments/time point with two to three epithelial preparations/experiment; ATP was normalized to time-matched naive controls; ✽, P < 0.05 compared to control as determined by ANOVA, followed by pairwise Student t test comparisons).
Reagents and antibodies.
The following drugs were purchased from Sigma Chemical Co., St. Louis, MO: 2,4-dinitrophenol (DNP; inhibits oxidative phosphorylation; 0.1 mM) (30), benzyloxycarbonyl-Val-Ala-Asp fluoromethylketone (Z-VAD-fmk; pan-caspase inhibitor; 10 to 100 μM) (8), chloramphenicol (blocks bacterial protein synthesis; 20 μM), colchicine (inhibits tubulin polymerization; 0.1 μM), cytochalasin D (inhibits actin polymerization; 0.2 μM) (6), gentamicin, methyl-β-cyclodextrin (MβCD; depletes plasma membrane cholesterol; 3 mM) (21), phenylarsine oxide (PAO; inhibits clathrin-coated pit formation; 1 μM) (21), and staurosporine (10 μM). Jasplakinolide (stabilizes filamentous [F]-actin; 0.05 and 0.1 μM) was from Molecular Probes, Eugene, OR (10).
Rabbit polyclonal anti-caspase-3 antibodies and anti-cleaved caspase-3 antibodies were from Cell Signaling Technology, Beverly, MA. Rabbit polyclonal antibodies against human claudin-1, claudin-2, occludin, zona occludens-1 (ZO-1),and mouse monoclonal anti-claudin-4 were all purchased from Zymed Laboratories, Inc., San Francisco, CA.
Epithelial ATP levels, mitochondria enzymes and viability.
ATP levels in naive and DNP (0.1 mM) treated epithelial monolayers were assessed according to the protocol of Crouch et al. (9), and the levels of cytochrome c oxidase were assessed by an established methodology (36). Epithelial viability was assessed by using exclusion of the vital dye trypan blue, measurement of released lactate dehydrogenase (19), and Western blotting for intact and cleaved caspase-3 (seebelow).
Epithelial barrier function. (i) TER.
T84 cells were seeded at 106 cells/ml onto semipermeable filters supports (Costar, Inc., Cambridge, MA) and grown to confluence as defined by transepithelial resistance (TER) of ≥600 Ω/cm2 and after 5 to 7 days of culture were typically ≥1,000 Ω/cm2, as measured with a voltmeter and matched electrodes (Millicell-ERS; Millipore, Bedford, MA). TER, an accepted index of paracellular permeability (27), was recorded before and 24 h after treatment and is expressed as the percentage of pretreatment values (30).
(ii) HRP flux.
Flux assays were conducted as described in detail elsewhere (3). Briefly, 24 h after DNP+E. coli HB101 treatment, horseradish peroxidase (HRP; 10 μmol/liter) was added to the apical side of filter-grown T84 cell monolayers, and samples (200 μl) were collected from the basal compartment of the culture well 2 h later. Intact HRP was determined by kinetic enzymatic assay and is presented as the percentage HRP recovered relative to the initial concentration added to the apical compartment of the culture well.
(iii) Bacterial internalization assay.
Semiconfluent monolayers of T84 cells were grown in six-well plates and inoculated with E. coli HB101 with or without DNP and incubated for 24 h at 37°C. A sample of bacterial culture was collected, and the number of bacteria was determined by using the method Miles and Misra (22). Epithelia were washed extensively and treated with gentamicin (200 μg/ml) for 1 h and then rinsed with sterile phosphate-buffered saline (three times), lysed with cold Triton X-100 (10 min at 4°C), and plated onto LB agar. The number of internalized, viable bacteria was determined and is presented as a percentage of the total number of bacteria after the 24-h growth period (in contrast to TER measurement, bacterial internalization was assessed in ∼80% confluent monolayers, since we reasoned that the exposure of lateral epithelial cell membranes would allow maximum opportunity for bacterial internalization and hence a better baseline against which to judge the effects of the drug treatments).
(iv) Bacterial translocation.
T84 cells were grown to confluence on 3.0-μm-pore-size filters, transferred to antibiotic-free medium, and inoculated apically with E. coli HB101 with or without DNP. Aliquots were collected from the basolateral compartment 24 h later, and the presence of bacteria was determined by growth of colonies on LB agar plates.
Immunoblotting for caspase-3 and TJ proteins.
Cells were pelleted and rinsed in cold PBS and then lysed in 300 μl of ice-cold lysis buffer (20 mM Tris [pH 7.5], 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM NaVO3, and complete protease inhibitor cocktail [Roche, Indianapolis, IN]) for 30 min, with vortexing at 10-min intervals. Subsequently, for the analyses of tight-junction (TJ) proteins, samples were sonicated (15 pulses, 2 to 3 s/pulse). Lysates were clarified by centrifugation (13,000 rpm for 10 min at 4°C), protein concentrations determined by using the Bio-Rad/Bradford microplate assay (Bio-Rad, Hercules, CA), and samples were stored at −70°C. Sodium dodecyl sulfate (SDS) loading buffer (2% SDS, 50 mM Tris-HCl, 100 mM dithiothreitol, 1% bromophenol blue, 5% glycerol) was added to each sample (40 μg of protein), which were heated to 90 to 100°C for 5 min and then loaded into SDS-10% polyacrylamide gel electrophoresis minigels (Bio-Rad). Separated proteins were electroblotted to polyvinylidene difluoride membranes (VWR, Mississauga, Ontario, Canada) and blocked in 5% nonfat Carnation powdered milk-TBST for 1 h. The primary antibodies and concentrations used were as follows: anti-β-actin (1:500), anti-ZO-1 (1:200), anti-occludin (1:500), anti-claudin-1 (1:200), anti-claudin-2 (1:200), anti-claudin-4 (1:200), and anti-caspase-3 (1:1,000). Blots were washed and incubated with secondary antibody-HRP conjugates for 1 h (goat anti-rabbit or rabbit anti-mouse [both at 1:2,000; Santa Cruz Biotechnology]) and then washed extensively and immunoreactive proteins visualized by using enhanced chemiluminescence (ECL; Amersham Pharmacia, Piscataway, NJ) and exposure of the membrane to Kodak XB-1 film (Eastman Kodak Company, Rochester, NY). Equal protein loading was assessed by total protein staining with Coomassie brilliant blue and subsequently (based on pilot studies) stripping and reprobing the gel for claudin-4 expression (the expression of this TJ protein was not affected by any of the experimental treatments). Densitometry was performed on digitized images by using software provided by the National Institutes of Health, Bethesda, MD.
Statistical analyses.
TER and HRP flux data were compared by analysis of variance (ANOVA), followed by post hoc pairwise statistical comparisons. Bacterial translocation data were compared by using the chi-square test with Yates' correction. A level of statistical significant difference was set at P ≤ 0.05. The data are presented as the means ± the standard error and the number (n) of epithelial preparations.
RESULTS
We have shown that exposure of confluent T84 cell monolayers to DNP (0.1 mM) or E. coli HB101 (106 CFU/ml) alone for ∼24 h had no or negligible effects on TER (30, 32), whereas epithelia exposed to DNP+E. coli HB101 displayed a significant drop in TER (30). In addition, with E. coli inoculation only, bacteria were cultured from medium retrieved from the basal compartment of the culture well 24 h after inoculation in ∼30% of epithelial preparations, whereas E. coli traversed every monolayer (i.e., 100%) concomitantly treated with DNP (30). The drop in TER and enhanced bacterial translocation observed when confluent T84 cell monolayers were exposed to DNP+E. coli HB101 is the focus of this investigation, and thus the majority of studies involve four treatment groups: naive controls, cells receiving pharmacological agent treatment only, DNP plus E. coli HB101 (DNP+E. coli HB101; i.e., the positive control), and DNP+E. coli HB101+pharmacological agent (i.e., test condition). The ability of all of the pharmacological agents alone to affect bacterial growth and TER was tested. None of the pharmacological agents (at the specified doses) affected growth of the bacteria and, likewise, with the exception of cytochalasin D, none of the drugs evoked a consistent or appreciable drop in TER. Since there is limited E. coli translocation across naive T84 cell monolayers (30), we did not explore the effect of the various pharmacological treatments on E. coli transcytosis in the absence of DNP. Indeed, it is unlikely that the drugs would promote E. coli transcytosis since in the presence of DNP they actually block the enhanced transcellular passage of E. coli HB101 (see below).
Figure 1A shows that the E. coli HB101 used in the present study was morphologically distinct from enterohemorrhagic E. coli, a bacterium known to directly reduce TER (31), by the absence of abundant pili. Figure 1B confirms that the low dose of DNP used (i.e., 0.1 mM) did not adversely affect the growth of the E. coli HB101. However, the same dose of DNP did effectively reduce epithelial ATP levels (Fig. 1C), and measurement of mitochondria-associated cytochrome c oxidase at 24 h posttreatment confirmed perturbation of mitochondrial function: control = 0.69 ± 0.04 versus DNP (0.1 mM) = 0.5 ± 0.01 nM/min/mg of protein (n = 3; P < 0.05). These data corroborate our early findings of DNP-induced mitochondrial dysfunction based on the 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) assay (30).
Changes in pH or nutrient depletion in the medium could explain the altered epithelial function, but this proved not to be the case. Thus, pH values were not significantly different when the culture medium from E. coli HB101 or DNP+E. coli HB101 for 24 h were compared: all ranged from 7.0 to 7.2. Increasing the fetal bovine serum concentration to 20% (vol/vol)did not ablate the effect of DNP+E coli HB101, and lowering serum levels to 0.5% in the presence of E. coli HB101 only did not significantly reduce TER values (n = 3).
Increased epithelial permeability and bacterial translocation in DNP+E. coli HB101-treated T84 cells is not due to apoptosis.
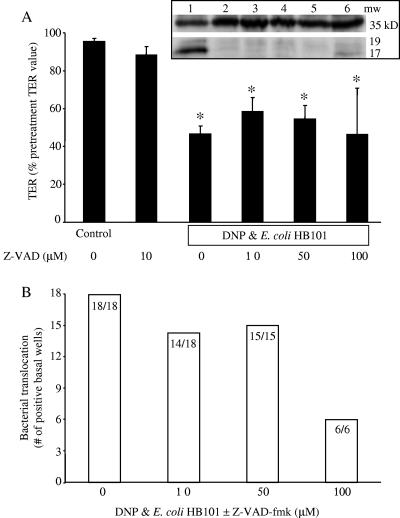
The increase in epithelial permeability (i.e., the drop in TER), and bacterial translocation could be a consequence of increased T84 cell death in the presence of the DNP+E. coli HB101. Trypan blue exclusion revealed no obvious differences in T84 cell viability between the treatments (data not shown). Similarly, DNP+E. coli HB101 treatment did not result in increased lactate dehydrogenase release into the culture medium: control = 127 ± 20 U/liter, DNP only = 133 ± 21 U/liter, E. coli only = 156 ± 23 U/liter, and DNP+E.coli HB101 = 135 ± 21 U/liter (n = 6). Western blotting for intact and cleaved caspase-3 revealed no evidence of increased apoptosis in the DNP+E. coli HB101-treated epithelia compared to the other groups (n = 3; Fig. 2 [inset]) and use of the pan-caspase inhibitor, Z-VAD-fmk (10 to 100 μM), affected neither the DNP+E. coli HB101-induced drop in TER nor the increase in bacterial translocation (Fig. 2) (Z-VAD-fmk alone [10 to 100 μM] affected neither TER nor E. coli translocation).
FIG. 2.
Increased apoptosis does not account for the drop in TER or the increased bacterial translocation induced by exposure to DNP and E. coli HB101. (A) Cotreatment with the pan-caspase inhibitor, Z-VAD-fmk (Z-VAD), did not prevent the drop in TER (presented as a percentage of the pretreatment value for each individual monolayer, i.e., TER was measured before and after treatment on all monolayers) observed 24 h after exposure to DNP (0.1 mM) and E. coli HB101 (106 CFU) (mean ± the standard error of the mean [SEM]; ✽, P < 0.05 compared to control; n = 6 to 18 monolayers from six separate experiments; starting TER range = 1,200 to 3,000 Ω/cm2). The inset shows a representative immunoblot (n = 3) showing no enhanced caspase-3 cleavage in the DNP+E. coli HB101-treated T84 cells and that Z-VAD-fmk was active since it blocked staurosporine-induced caspase-3 cleavage from the 39-kDa form (upper band) to the 19- and 17-kDa fragments (lower bands). Lanes: 1, staurosporine (10 μM); 2, staurosporine (10 μM) plus Z-VAD-fmk (100 μM); 3, naive; 4, DNP+E. coli HB101; 5, DNP+E. coli HB101+Z-VAD-fmk; 6, staurosporine (1 μM). (B) The bacterial translocation across T84 cell monolayers is not prevented by Z-VAD-fmk exposure (numbers inside bars indicate number of T84 cell monolayers positive for bacterial translocation compared to the total number of epithelial preparations observed).
DNP and E. coli HB101-induced drop in TER is not abrogated by microtubule or microfilament stabilization.
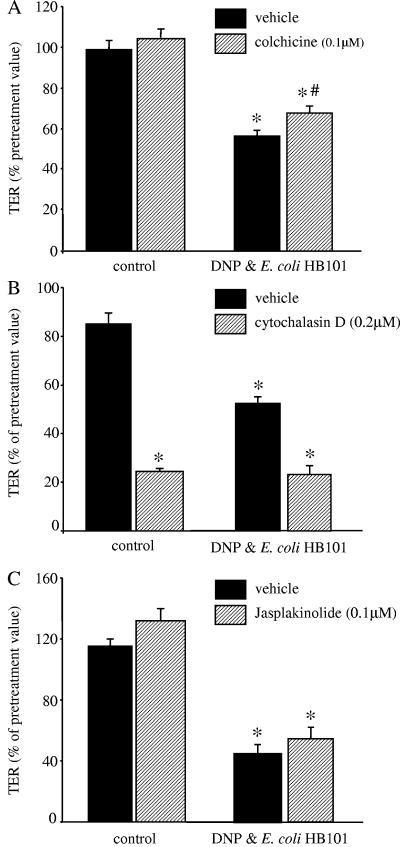
Enterocytic paracellular permeability is largely controlled by the intercellular TJs that are connected to the cytoskeleton. Interference with normal assembly and disassembly of microtubules with colchicine resulted in a small recovery of TER (∼10 to 15%); others have shown that microtubule architecture can contribute to paracellular permeability (2), whereas the agents that prevent actin rearrangements, cytochalasin D and jasplakinolide, did not prevent the drop in TER evoked by 24 h of culture with DNP+E. coli HB101 (Fig. 3). None of the drugs affected the growth of E. coli HB101 (n = two to three growth curves; data not shown).
FIG. 3.
Blockade of cytoskeleton changes in the enterocyte has little impact on DNP and E. coli HB101-induced reductions in TER. (A) Interference with microtubule assembly and rearrangement with colchicine results in a small but statistically significant inhibition of the DNP (0.1 mM)+E. coli (106 CFU)-induced decrease in TER at 24 h posttreatment (✽, P < 0.05 compared to control; #, P < 0.05 compared to vehicle only; n = 12 monolayers from four experiments). (B and C) In contrast, the microfilament stabilizers, cytochalasin D or jasplakinolide, did not prevent the drop in TER; in fact, cytochalasin D alone significantly reduced TER across control epithelia (mean ± the SEM; n = 4 to 12 monolayers from three to four separate experiments; starting TER range = 620 to 3,000 Ω/cm2; ✽, P < 0.05 compared to control).
DNP and E. coli HB101-induced drop in TER is associated with reduced occludin and zona occludens (ZO)-1 but not claudin expression.
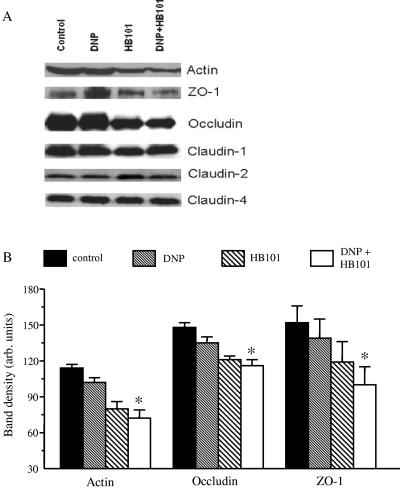
Reduced TER implies increased “openness” of the TJs or altered TJ structure. Analysis of protein extracts 12-h post-DNP+E. coli HB101 revealed no consistent alteration in TJ protein expression, despite the small but statistically significant reduction in TER previously observed at this time point (30). There was a tendency for ZO-1 levels to be slightly reduced in the cotreated epithelia, but this was not a statistically significant change (data not shown). However, Fig. 4A shows that DNP or E. coli HB101 alone had variable effects on the expression of epithelial actin, the TJ protein, occludin, and the TJ-associated protein, ZO-1, but again these failed to reach statistical significance compared to expression levels in control naive T84 cell monolayers. However, the expression of all three proteins was consistently and statistically significantly reduced in T84 cells treated 24 h previously with DNP+E. coli HB101 (Fig. 4; n = 4 to 6), although not strikingly different from the cells treated with E. coli only. Analysis of the same protein extracts or stripping and reprobing of the previous blots showed no consistent changes in the levels of the TJ proteins claudin-1, -2, or -4 (Fig. 4; n = 2 to 5). Coomassie brilliant blue staining of the gels indicated equal protein loading (data not shown).
FIG. 4.
Exposure to E. coli HB101 with or without DNP results in altered expression of TJ proteins. (A) Representative immunoblots show the reduced expression of TJ proteins 24 h after culture with DNP (0.1 mM) and E. coli HB101 (106 CFU/ml). Expression of claudin-1, -2, and -4 was not consistently altered (n = 2 to 5). Equal amounts of protein were loaded, and the blots were reprobed for claudin-4 (whose expression was unaltered by any treatment), and total protein staining of the gels (not shown). (B) Densitometric analysis (arbitrary units) reveals that although exposure to DNP or E. coli alone can reduce the expression of specific TJ proteins, such reductions were only statistically significant for actin (n = 4), occludin (n = 6), and ZO-1 (n = 6) when the epithelial were exposed to DNP and E. coli (✽, P < 0.05 compared to control naive T84 cell preparations as determined by ANOVA).
Interference with the enterocyte cytoskeleton reduces E. coli HB101 internalization and epithelial translocation.
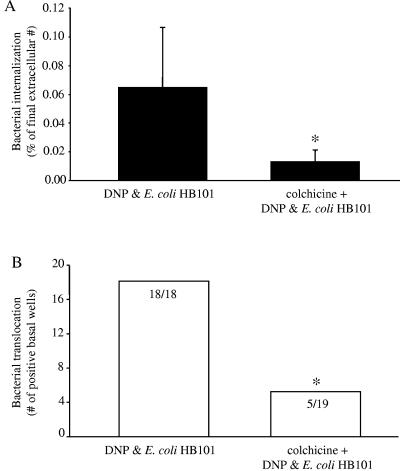
Colchicine cotreatment resulted in lower levels of intracellular E. coli compared to those of cells treated with DNP+E. coli HB101 only (Fig. 5A). The level of extracellular bacteria was not different between the two conditions. Corroborating these data, bacterial translocation into the basal compartment of the culture well occurred in all of the epithelial monolayers treated with DNP+E. coli HB101 but in only ∼25% (i.e., levels seen with E. coli HB101 treatment only) of the epithelial preparations cotreated with colchicine (Fig. 5B). Moreover, the transepithelial flux of HRP was significantly reduced by colchicine (Table 1), suggesting that increased bacterial translocation could be due to increased nonspecific endocytosis in the concomitantly stressed (i.e., DNP-treated) epithelia. Many pathogenic bacteria affect epithelial signaling pathways, and so we cannot unequivocally exclude the possibility that a specific bacterium-induced change in an epithelial signaling pathway leads to the enhanced bacterial uptake and translocation.
FIG. 5.
Interference with microtubule assembly reduces both E. coli HB101 internalization and translocation in DNP-cotreated epithelia. (A) Colchicine (0.1 μM) treatment of T84 cell monolayers exposed to DNP (0.1 mM) and E. coli (106 CFU) significantly reduces bacterial internalization (mean ± the SEM; n = 4 to 12 monolayers from three to four separate experiments; ✽, P < 0.05 compared to DNP+E. coli as determined by a Student t test). (B) The reduced internalization of the E. coli is accompanied by reduced translocation into the basal compartment of the culture well (numbers inside the bars indicate the number of T84 cell monolayers positive for bacterial translocation compared to total number of epithelial preparations observed; ✽, P < 0.05 compared to DNP and E. coli as determined by a chi-squared test).
TABLE 1.
Enhanced transepithelial flux of HRP is blocked by microtubule and microfilament stabilizationa
| Conditions | n | % HPR (10−2) (mean ± SEM) |
|---|---|---|
| Control | 12 | 0.21 ± 0.06 |
| Control + colchicine | 3 | 0.05 ± 0.01* |
| DNP + E. coli | 12 | 0.46 ± 0.08* |
| Colchicine + DNP + E. coli | 9 | 0.33 ± 0.07 |
| Jasplakinolide + DNP + E. coli | 3 | 0.17 ± 0.04 |
The mean % HPR recovered from basal culture wells relative to the apical concentration is given. n, Number of monolayers from one to four experiments. *, P < 0.05 compared to control. DNP was used at 0.1 mM, E. coli HB101 was used at 106 CFU inocula, and colchicine and jasplakinolide were used at 0.1 μM.
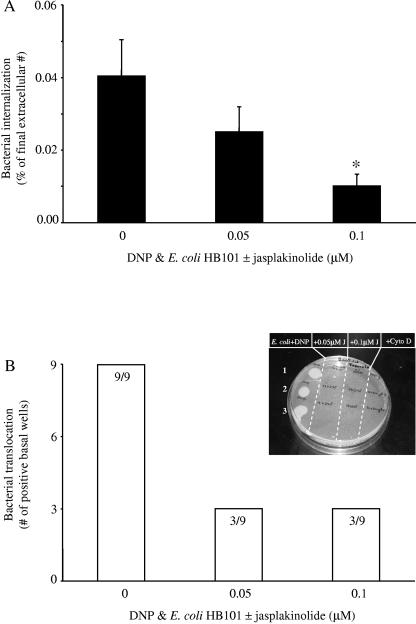
Comparable observations were made with agents that either stabilize or prevent microfilament rearrangements. Figure 6 shows that cotreatment with jasplakinolide significantly reduced E. coli HB101 internalization and that translocation was reduced to ∼33%. Also, HRP fluxes were reduced in DNP+E. coli+jasplakinolide treatment epithelial monolayers compared to those exposed to DNP+E. coli for 24 h (similar observations were made with cytochalasin D [data not shown]).
FIG. 6.
Interference with microfilament assembly reduces both E. coli HB101 internalization and translocation in DNP-cotreated epithelia. (A) Jasplakinolide treatment of T84 cell monolayers exposed to DNP (0.1 mM) and E. coli (106 CFU) significantly reduces bacterial internalization (mean ± the SEM; n = 4 to 12 monolayers from three to four separate experiments; ✽, P < 0.05 compared to DNP and E. coli only [i.e., zero jasplakinolide]). (B) The reduced internalization of the E. coli is accompanied by reduced translocation into the basal compartment of the culture well (numbers inside the bars indicate the number of T84 cell monolayers positive for bacterial translocation compared to the total number of epithelial preparations observed). The inset shows a representative plate showing the lack of bacterial colony growth from medium obtained from the basolateral culture well of epithelia treated with jasplakinolide (J) or cytochalasin D (Cyto D; 0.2 μΜ). Each spot is from a separate epithelial monolayer.
Pharmacological blockade of endocytotic pathways reduces E. coli HB101 internalization and epithelial translocation.
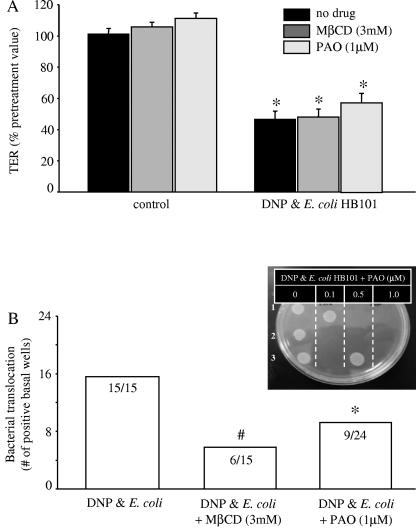
The findings that metabolic stress in the context of nonpathogenic bacteria resulted in enhanced bacterial internalization led us to consider that blocking endocytosis would reduce bacterial internalization and subsequent translocation. Using MβCD and PAO to reduce membrane cholesterol levels and clathrin-coated pit formation, respectively, two related processes (34, 46), we observed no statistically significant amelioration of the DNP+E. coli HB101-induced drop in TER (Fig. 7A). In contrast, cotreatment with either drug resulted in inhibition of bacterial internalization (MβCD reduced internalization by 85% compared to that in T84 cells treated with DNP+E. coli only (n = six monolayers), and PAO reduced bacterial internalization by ∼45% [n = nine monolayers]). Either pharmacological agent reduced translocation to ∼40% compared to 100% in DNP+E. coli HB101-treated epithelial monolayers (Fig. 7B). Neither MβCD nor PAO affected bacterial growth (n = two to three growth curves), and bacterial counts from aliquots of culture medium taken from the apical side of the epithelial monolayers 24 h after culture with DNP+E. coli HB101 with or without MβCD or PAO were not significantly different (data not shown).
FIG. 7.
Pharmacological blockade of endocytosis reduces E. coli HB101 translocation but not reduced TER in DNP-cotreated epithelia. (A) Neither MβCD (which depletes membrane cholesterol) nor PAO (which inhibits clathrin-coated pit formation) prevents the DNP (0.1 mM)- and E. coli HB101 (106 CFU)-induced decrease in TER at 24 h posttreatment (mean ± the SEM; n = 6 to 24 monolayers from three to eight separate experiments; starting TER range = 720 to 2,400 Ω/cm2; ✽, P < 0.05 compared to control). (B) However, the increased bacterial translocation was reduced by both MβCD and PAO (numbers inside bars indicate the number of T84 cell monolayers positive for bacterial translocation compared to total number of epithelial preparations observed; #, P = 0.1; ✽, P < 0.05 compared to DNP and E. coli as determined by chi-squared test). The inset presents a representative plate showing the lack of bacterial colony growth from medium obtained from the basolateral culture well of epithelia treated with increasing doses of PAO. Each spot is from a separate epithelial monolayer.
Inhibition of bacterial protein synthesis ameliorates reduced TER.
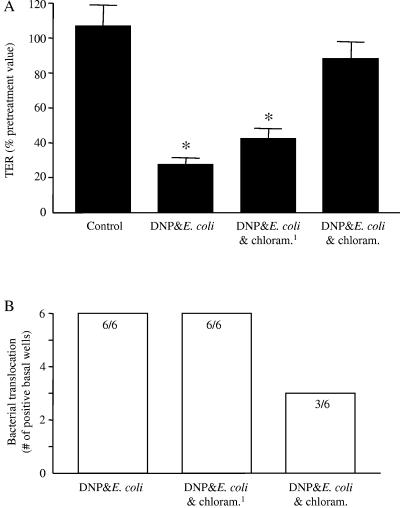
In our earlier study (30), we showed that viable bacteria were a requirement to observe the DNP+E. coli-induced drop in TER. Expanding on this finding, bacterial protein synthesis was inhibited with chloramphenicol (20 μg/ml). Two experimental protocols were used. The drop in TER that occurs 24 h after treatment with DNP+E. coli HB101 did not occur when chloramphenicol was present in the culture medium, clearly implicating bacterial protein synthesis as a requirement for the epithelial paracellular permeability defect (Fig. 8A). In contrast to the complete inhibition of the TER defect, chloramphenicol treatment was less effective in preventing the enhanced bacterial translocation (Fig. 8B). Also, ANOVA analysis of HRP flux across chloramphenicol-treated epithelia revealed that this rate was not significantly different from epithelia treated with DNP+E. coli HB101 only, but it was also not significantly different from the flux rates across control, noninfected epithelia: control = 0.045 ± 0.02, DNP+E. coli HB101 = 0.101 ± 0.036, DNP+E. coli HB101+chloramphenicol = 0.064 ± 0.03% of apical HRP (n = 6; mean ± the standard deviation; the control and DNP+E. coli HB101-treated groups are not statistically different). In a final series of experiments, E. coli HB101 cells were treated with chloramphenicol for 24 h and then rinsed and added to confluent T84 cell monolayers along with DNP (i.e., no chloramphenicol present during the experiment) for 24 h. Inhibition of bacterial protein synthesis prior to the addition of DNP had no sustained effect and prevented neither the DNP+E. coli-induced drop in TER nor the increased bacterial translocation (Fig. 8).
FIG. 8.
Bacterial protein synthesis is important for the reduced TER caused by DNP and E. coli. (A) Chloramphenicol (chloram.; 20 μg/ml) inhibition of bacterial protein synthesis prevents the increase in paracellular permeability indicated by reduced TER 24 h exposure to DNP (0.1 mM) and E. coli HB101 (106 CFU) (mean ± the SEM; n = nine monolayers from three experiments; ✽, P < 0.05 compared to control; starting TER range = 910 to 2,080 Ω/cm2). (B) The treatment described for panel A did not ameliorate the increased bacterial translocation (numbers inside bars indicate the number of T84 cell monolayers positive for bacterial translocation compared to the total number of epithelial preparations observed). Pretreatment of bacteria with chloramphenicol (“chloram.1”) for 24 h prior to use in coculture experiments did not affect the subsequent DNP+E. coli-induced loss of epithelial barrier function.
DISCUSSION
The interaction of enteric bacterial pathogens and their toxins or products with enterocytes is yielding intriguing insights into the microbial pathogenesis of disease (17). By comparison, the interplay of commensal microflora with the gut epithelium has been, until recently, largely overlooked, presumably because of the perceived lack of involvement of these bacteria in disease, which is an outdated notion, particularly in the context of idiopathic gut disease (11). We have shown that gut-derived epithelia exposed to DNP and a noninvasive, nonpathogenic E. coli displayed decreased barrier function: both paracellular and transcellular permeability were increased (30). The present study assessed this barrier defect and reveals differences in the regulation of transcellular and paracellular permeability. In addition, and complementing studies of paracellular permeability (25, 32, 39), our data underscore the importance of appropriate control of epithelial transcellular permeability to health and disease. In fact, transcellular passage was the predominant route by which viable bacteria crossed an intact epithelial monolayer in this model system.
Generally, bacterial pathogens that navigate the intestinal epithelial barrier do so either via host microfilament or microtubule-dependent transcytosis (5, 6, 48) or by rearrangement of the epithelial TJs (4). Decreased barrier function occurs in patients with Crohn's disease and increased bacterial adherence of the normal microflora is observed on gut tissues from these patients (11, 42, 49). Previous studies have shown that nonpathogenic bacteria can increase gut paracellular permeability (14, 15). These findings highlight the disease-promoting potential of the commensal microflora, particularly in the context of another perturbation in gut function (e.g., reduced integrity of the intestinal barrier). The simplest explanation for the decrease in epithelial barrier function elicited by DNP+E. coli HB101 exposure is decreased epithelial cell viability. There is evidence to support and refute small increases in epithelial cell death as a factor contributing to reduced barrier function induced by proinflammatory cytokines (1, 7, 51). However, analyses did not identify apoptosis, or necrosis, as the cause of the increased epithelial permeability evoked by DNP+E. coli HB101 in our model system.
Decreased TER is indicative of altered TJ structure or function. Our previous study showed focal derangements of epithelial perijunctional F-actin in DNP+E. coli HB101-treated T84 monolayers (30). Corroborating this finding, immunoblot analyses now reveal decreased expressions of actin, ZO-1, and occludin that were statistically significant in DNP+E. coli HB101-treated epithelia at 24 h posttreatment. Diminished expression of these TJ components was observed to a lesser degree in epithelia exposed to E. coli HB101 or DNP alone, and yet neither agent elicited a significant drop in TER (30, 32). We have shown that 12 h post-DNP+E. coli HB101 treatment there can be a 20 to 30% drop in TER (30); however, this is not associated with a consistent reduction in expression of any of the TJ proteins examined. These findings illustrate the complexity of regulation of epithelial paracellular permeability and imply redundancy within the structure of the TJ, such that reduced expression of any individual TJ protein need not necessarily cause massive impairment of the paracellular pathway. The data indicate that for commensal bacteria to evoke a significant decrease in epithelial barrier function requires an additional perturbation in the enterocyte. The cumulative effect of reduced actin, ZO-1, and occludin in epithelial monolayers exposed to DNP+E. coli HB101 likely accounts for the consistent 50 to 60% drop in TER. Pharmacological agents used to prevent rearrangements of the enterocyte cytoskeleton or block endocytosis did not ameliorate the decreased TER induced by DNP+E. coli HB101. These findings suggest a direct effect on the production of TJ proteins or structure of the TJ and not interference with the cytoskeleton, as has been described for EPEC infection (32), or enhanced TJ protein internalization (21). A subsequent challenge will be defining the intracellular signaling pathways that are evoked or perturbed and lead to the altered TJ expression.
In contrast to TER, the increase in bacterial translocation was significantly reduced by treatment with colchicine, jasplakinolide, or cytochalasin D, clearly indicating that like their pathogenic “cousins,” the increased internalization and translocation of commensal bacteria across concomitantly stressed epithelia is dependent on an intact and functional enterocyte cytoskeleton (microtubules and microfilaments). Rapid movement of large intact protein antigens across the epithelium has been demonstrated in intestinal tissues from patients with IBD (40). Use of pharmacological agents that interfere with the host cytoskeleton indicated that the transcellular pathway is a significant, if not the predominant, route by which the E. coli traversed the epithelium in this model system. This postulate is substantiated by the demonstration that blockade of endocytosis with two different, but complementary, pharmacological agents (i.e., with MβCD or PAO) (34, 46) significantly reduced E. coli translocation across concomitantly stressed epithelia. E.coli strain HB101 is a noninvasive organism, and this coupled to the enhanced HRP flux across DNP+E. coli-treated monolayers suggests active and nonspecific uptake by the enterocyte. However, the increased transcellular and paracellular permeability is dependent on viable bacteria (30), an observation confirmed here with bacteria in which protein synthesis was inhibited by chloramphenicol. Thus, the alteration in epithelial barrier function is the summation of input from the bacteria and active epithelial involvement. The full appreciation of this relationship will require the unraveling of the mobilization of enterocytic signaling pathways that govern epithelial paracellular and transcellular permeability and how commensal organisms can affect these pathways in metabolically stressed and/or hypoxic epithelia.
We have shown that a model enteric epithelial cell line under metabolic stress and exposed to noninvasive, nonpathogenic E. coli becomes responsive to the bacteria with a resultant reduction in barrier function; should similar events occur in vivo they could have a significant impact on gut function (28, 43). Thus, we speculate that a defect in mitochondria in gut epithelia could lead to excess amounts of lumen-derived antigenic material and microbes entering the mucosa. The subsequent activation of immune cells, such as macrophages, would result in the production of proinflammatory mediators that could feedback onto the epithelium and other cells, enhancing the barrier defect and causing overt inflammation. In support of this hypothesis we have shown that macrophages can modulate epithelial function (50) and that tumor necrosis factor alpha can increase HRP endocytosis in ileal tissue from patients with Crohn's disease (44), whereas others have also implicated tumor necrosis factor alpha in the epithelial barrier defect observed in IBD (51).
In conclusion, the present study shows that a defect in epithelial energy balance (mimicked here by DNP treatment) results in non-noxious, nonpathogenic bacteria being internalized along with concomitant increases in epithelial transcellular and paracellular permeability (30). The data presented here highlight the divergence in control of paracellular and transcellular permeability pathways, with the latter route being a major portal for commensal bacteria to cross the epithelial mucosal barrier. As such, we contend that awareness of epithelial transcytosis should feature more prominently in analyses of gut homeostasis and pathophysiological reactions.
Acknowledgments
This study was supported by a group grant from the Crohn's and Colitis Foundation of Canada (CCFC). A.N. was supported by a Premier's Research Excellence Award to D.M.M., and O.S. and D.P. were supported by CCFC/Canadian Association of Gastroenterology summer studentships. P.M.S. holds a Canada Research Chair in Gastrointestinal Disease.
We thank S. Raha and M. Tarnopolsky (McMaster University) for technical assistance with the measurement of mitochondrial enzymes.
Editor: V. J. DiRita
REFERENCES
- 1.Abreu, M. T., A. A. Pallidino, E. T. Arnold, R. S. Kwon, and J. A. McRoberts. 2000. Modulation of barrier function during Fas-mediated apoptosis in human intestinal epithelial cells. Gastroenterology 119:1524-1536. [DOI] [PubMed] [Google Scholar]
- 2.Banan, A. A., A. Farhadi, J. Z. Fields, L. J. Zhang, M. Shaikh, and A. Keshavarzian. 2003. The delta-isoform of protein kinase C causes inducible nitric-oxide synthase and nitric oxide up-regulation: key mechanism for oxidant-induced carbonylation, nitration, and disassembly of the microtubule cytoskeleton and hyperpermeability of barrier of intestinal epithelia. J. Pharmacol. Exp. Ther. 305:482-494. [DOI] [PubMed] [Google Scholar]
- 3.Berin, M. C., P.-C. Yang, L. Ciok, S. Waserman, and M. H. Perdue. 1999. Role of IL-4 in macromolecular transport across intestinal epithelium. Am. J. Physiol. Cell Physiol. 276:C1046-C1052. [DOI] [PubMed] [Google Scholar]
- 4.Berkes, J., V. K. Viswanathan, S. D. Savkovic, and G. Hecht. 2003. Intestinal epithelial responses to enteric pathogens: effects on the tight junction, barrier, ion transport, and inflammation. Gut 52:439-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biswas, D., K. Itoh, K., and C. Sasakawa. 2003. Role of microfilaments and microtubules in the invasion of INT-407 cells by Campylobacter jejuni. Microbiol. Immunol. 47:469-473. [DOI] [PubMed] [Google Scholar]
- 6.Boudeau, J., A.-L. Glasser, E. Masseret, B. Joly, and A. Darfeuille-Michaud. 1999. Invasive ability of an Escherichia coli strain isolated from the ileal mucosa of a patient with Crohn's disease. Infect. Immun. 67:4499-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruewer, M., A. Luegering, T. Kucharzik, C. A. Parkos, J. L. Madara, A. M. Hopkins, and A. Nusrat. 2003. Pro-inflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J. Immunol. 171:6164-6172. [DOI] [PubMed] [Google Scholar]
- 8.Ching, J. C., N. L. Jones, P. J. Ceponis, M. A. Karmali, and P. M. Sherman. 2002. Escherichia coli Shiga-like toxins induce apoptosis and cleavage of poly(ADP-ribose) polymerase via in vitro activation of caspases. Infect. Immun. 70:4669-4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crouch, S. P. M., R. Kozlowski, K. J. Slater, and J. Fletcher. 1993. The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J. Immunol. Methods 160:81-88. [DOI] [PubMed] [Google Scholar]
- 10.Dalby-Payne, J. R., E. V. O'Loughlin, and P. Gunning. 2003. Polarization of specific tropomyosin isoforms in gastrointestinal epithelial cells and their impact on CFTR at the apical surface. Mol. Biol. Cell 14:4365-4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darfeuille-Michaud, A., J. Boudeau, P. Bulois, C. Neut, A. L. Glasser, N. Barnich, M. A. Bringer, A. Swidsinski, L. Beaugerie, and J. F. Colombel. 2004. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn's disease. Gastroenterology 127:412-421. [DOI] [PubMed] [Google Scholar]
- 12.Delpre, G., I. Avidor, R. Steinherz, U. Kadish, and M. Ben-Bassat. 1989. Ultrastructural abnormalities in endoscopically and histologically normal and involved colon in ulcerative colitis. Am. J. Gastroenterol. 84:1038-1046. [PubMed] [Google Scholar]
- 13.Dickman, K. G., S. J. Hempson, J. Anderson, S. Lippe, L. Zhao, R. Burakoff, and R. D. Shaw. 2000. Rotavirus alters paracellular permeability and energy metabolism in caco-2 cells. Am. J. Physiol. Gastrointest. Liver Physiol. 279:G757-G766. [DOI] [PubMed] [Google Scholar]
- 14.El Asmar, R., P. Panigrahi, P. Bamford, I. Berti, T. Not, G. V. Coppa, C. Catassi, and A. Fasano. 2002. Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology 123:1607-1615. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Lafuente, A., M. Antolin, F. Guarner, E. Crespo, and J.-R. Malagelada. 2001. Modulation of colonic barrier function by the composition of the commensal flora in the rat. Gut 48:503-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Lafuente, A., M. Antolin, F. Guarner, E. Crespo, E. Salasm, P. Forcada, M. Laguarda, J. Gavalda, J. A. Baena, J. Vilaseca, and J.-R. Malagelada. 1997. Incrimination of anaerobic bacteria in the induction of experimental colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 272:G10-G15. [DOI] [PubMed] [Google Scholar]
- 17.Goosney, D. L., M. de Grado, and B. B. Finlay. 1999. Putting E. coli on a pedestal: a unique system to study signal transduction and the actin cytoskeleton. Trends Cell Biol. 9:11-14. [DOI] [PubMed] [Google Scholar]
- 18.He, D., S. J. Hagen, C. Pothoulakis, M. Chen, N. D. Medina, M. Warny, and J. T. LaMont. 2000. Clostridium difficile toxin A causes early damage to mitochondria in cultured cells. Gastroenterology 119:139-150. [DOI] [PubMed] [Google Scholar]
- 19.Howe, K. L., A. Wang, M. M. Hunter, B. A. Stanton, and D. M. McKay. 2004. TGFβ down-regulation of the CFTR: a means to limit epithelial chloride secretion. Exp. Cell Res. 298:473-484. [DOI] [PubMed] [Google Scholar]
- 20.Humphrey, C. D., D. M. Montag, and F. E. Pittman. 1986. Morphologic observations of experimental Campylobacter jejuni infection in the hamster intestinal tract. Am. J. Pathol. 122:152-159. [PMC free article] [PubMed] [Google Scholar]
- 21.Ivanov, A. I., A. Nusrat, and C. A. Parkos. 2004. Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol. Biol. Cell 15:176-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones, N., M. H. Perdue, P. M. Sherman, and D. M. McKay. 2002. Bacterial interactions with host epithelium in vitro. Methods Mol. Biol. 188:383-400. [DOI] [PubMed] [Google Scholar]
- 23.Kaper, J. B., J. P. Nataro, and H. L. Mobley. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2:123-140. [DOI] [PubMed] [Google Scholar]
- 24.Kim, S.-H., and Y.-H. Kim. 2004. Escherichia coli O157:H7 adherence to HEp-2 cells is implicated with curli expression and outer membrane integrity. J. Vet. Sci. 5:119-124. [PubMed] [Google Scholar]
- 25.Kinugasa, T., T. Sakaguchi, X. Gu, and H.-C. Reinecker. 2000. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology 118:1001-1011. [DOI] [PubMed] [Google Scholar]
- 26.Linskens, R. K., X. W. Huijsdens, P. H. Savelkoul, C. M. Vandenbroucke-Grauls, and S. G. Meuwissen. 2001. The bacterial flora in inflammatory bowel disease: current insights in pathogenesis and the influence of antibiotics and probiotics. Scand. J. Gastroenterol. 234(Suppl.):29-40. [DOI] [PubMed] [Google Scholar]
- 27.Madara, J. L., and J. Stafford. 1989. Interferon-γ directly affects barrier function of cultured intestinal epithelial monolayers. J. Clin. Investig. 83:724-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKay, D. M. 1999. Intestinal inflammation and the gut microflora. Can. J. Gastroenterol. 13:509-516. [DOI] [PubMed] [Google Scholar]
- 29.McKay, D. M., and P. K. Singh. 1997. Superantigen-activation of immune cells evokes epithelial (T84) transport and barrier abnormalities via interferon-γ and tumour necrosis factor-α: inhibition of increased permeability, but not diminished secretory responses by transforming growth factor β2. J. Immunol. 159:2382-2390. [PubMed] [Google Scholar]
- 30.Nazli, A., P.-C. Yang, J. Jury, K. L. Howe, J. L. Watson, J. D. Söderholm, P. M. Sherman, M. H. Perdue, and D. M. McKay. 2004. Epithelia under metabolic stress perceive commensal bacteria as a threat. Am. J. Pathol. 164:947-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Philpott, D. J., D. M. McKay, W. Mak, M. H. Perdue, and P. M. Sherman. 1998. Signal transduction pathways involved in enterohemorrhagic Escherichia coli-induced alterations in T84 epithelial permeability. Infect. Immun. 66:1680-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Philpott, D. J., D. M. McKay, P. M. Sherman, and M. H. Perdue, M. H. 1996. Infection of T84 cells with enteropathogenic Escherichia coli alters barrier and transport functions. Am. J. Physiol. Gastrointest. Liver Physiol. 270:G634-G645. [DOI] [PubMed] [Google Scholar]
- 33.Rakoff-Nahoum, S., J. Paglino, F. Eslami-Varzaneh, S. Edberg, and R. Medzhitov. 2004. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell 118:229-241. [DOI] [PubMed] [Google Scholar]
- 34.Rodal, S. K., G. Skretting, O. Garred, F. Vilhardt, B. van Deurs, and K. Sandvig. 1999. Extraction of cholesterol with methyl-β-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol. Biol. Cell 10:961-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rook, G. A. W., and J. L. Stannford. 1998. Give us this day our daily germs. Immunol. Today 19:113-116. [DOI] [PubMed] [Google Scholar]
- 36.Rustin. P., D. Chretien, T. Bourgeron, B. Gerard, A. Rotig, J. M. Saudubray, and A. Munnich. 1994. Biochemical and molecular investigations in respiratory chain deficiencies. Clin. Chim. Acta 228:35-51. [DOI] [PubMed] [Google Scholar]
- 37.Rutgeerts, P., K. Goboes, M. Peeters, M. Hiele, F. Penninckx, R. Aerts, R. Kerremans, and G. Vantrappen. 1991. Effect of faecal stream diversion on recurrence of Crohn's disease in the neoterminal ileum. Lancet 338:771-774. [DOI] [PubMed] [Google Scholar]
- 38.Sadlack, B., H. Marz, H. Schorle, A. Schimpl, A. C. Feller, and I. Horak. 1993. Ulcerative colitis like disease in mice with a disrupted interleukin-2 gene. Cell 75:253-261. [DOI] [PubMed] [Google Scholar]
- 39.Schmitz. H., C. Barmeyer, M. Fromm, N. Runkel, H.-D. Foss, C. J. Bentzel, E.-O. Riecken, and J.-D. Schulzke. 1999. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology 116:301-309. [DOI] [PubMed] [Google Scholar]
- 40.Schurmann, G., M. Bruwer, A. Klotz, K. W. Schmid, N. Senninger, and K. P. Zimmer. 1999. Transepithelial transport processes at the intestinal mucosa in inflammatory bowel disease. Int. J. Colorectal Dis. 14:41-46. [DOI] [PubMed] [Google Scholar]
- 41.Söderholm, J. D., G. Olaison, K. H. Peterson, L. E. Franzen, T. Lindmark, M. Wiren, C. Tagesson, and R. Sjodahl. 2002. Augmented increase in tight junction permeability by luminal stimuli in the non-inflamed ileum of Crohn's disease. Gut 50:307-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Söderholm, J. D., K. Holmgren-Peterson, G. Olaison, L. E. Franzen, B. Westrom, K.-E. Magnusson, and R. Sjodahl. 1999. Epithelial permeability to proteins in the noninflamed ileum of Crohn's disease. Gastroenterology 117:65-72. [DOI] [PubMed] [Google Scholar]
- 43.Söderholm, J. D., and M. H. Perdue. 2001. Stress and the gastrointestinal tract. II. Stress and intestinal barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 280:G7-G13. [DOI] [PubMed] [Google Scholar]
- 44.Söderholm, J. D., C. Streuker, P.-C. Yang, C. Paterson, P. K. Singh, D. M. McKay, P. M. Sherman, K. Croitoru, and M. H. Perdue. 2004. Increased epithelial uptake of protein antigens in the ileum of Crohn's disease mediated by TNFα. Gut 53:1813-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Somasundaram, S., G. Sigthorsson, R. J. Simpson, J. Watts, M. Jacob, I. A. Tavares, S. Rafi, A. Roseth, R. Foster, A. B. Price, J. M. Wrigglesworth, and I. Bjarnason. 2000. Uncoupling of intestinal mitochondrial oxidative phosphorylation and inhibition of cyclooxygenase are required for the development of NSAID-enteropathy in the rat. Aliment. Pharm. Ther. 14:639-650. [DOI] [PubMed] [Google Scholar]
- 46.Subtil, A., I. Gaidarov, K. Kobylarz, M. A. Lampson, J. H. Keen, and T. E. McGraw. 1999. Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc. Natl. Acad. Sci. USA 96:6775-6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor, D. E., S. P. Kantrow, and C. A. Piantadosi. 1998. Mitochondrial respiration after sepsis and prolonged hypoxia. Am. J. Physiol. Respir. Physiol. 275:L139-L144. [DOI] [PubMed] [Google Scholar]
- 48.Yoshida, S., and C. Sasakawa. 2003. Exploiting host microtubule dynamics: a new aspect of bacterial invasion. Trends Microbiol. 11:139-143. [DOI] [PubMed] [Google Scholar]
- 49.Zamora, S. A., R. J. Hilsden, J. B. Meddings, J. D. Butzner, R. B. Scott, and L. R. Sutherland. 1999. Intestinal permeability before and after ibuprofen in families of children with Crohn's disease. Can. J. Gastroenterol. 13:31-36. [DOI] [PubMed] [Google Scholar]
- 50.Zareie, M., P. K. Singh, J. Irvine, P. M. Sherman, D. M. McKay, and M. H. Perdue. 2001. Monocyte/macrophage activation by normal bacteria and bacterial products: implications for epithelial pathophysiology in Crohn's disease. Am. J. Pathol. 158:1101-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeissig, S., C. Bojarski, N. Buergel, J. Mankertz, M. Zeitz, M. Fromm, and J.-D. Schulzke. 2004. Down-regulation of epithelial apoptosis and barrier repair in active Crohn's disease by tumour necrosis factor-α antibody treatment. Gut 53:1295-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]