Abstract
The characterization of protective antigens is essential for the development of an effective, subunit-based vaccine against paratuberculosis. Surface-exposed and secreted antigens, present abundantly in mycobacterial culture filtrate (CF), are among the well-known protective antigens of Mycobacterium tuberculosis and Mycobacterium bovis. Culture filtrate, prepared from Mycobacterium avium subsp. paratuberculosis ATCC 19698 grown as a surface pellicle on synthetic Sauton medium, was strongly and early recognized in experimentally infected B6 bg/bg beige mice and cattle, as indicated by elevated spleen cell gamma interferon (IFN-γ) secretion and lymphoproliferative responses of peripheral blood mononuclear cells, respectively. Strong proliferative and ex vivo IFN-γ responses against antigen 85 (Ag85) complex (a major protein component from M. bovis BCG culture filtrate) could be detected in cattle as early as 10 weeks after oral M. avium subsp. paratuberculosis infection. Synthetic peptides from the Ag85A and Ag85B components of this complex were strongly recognized, whereas T-cell responses were weaker against peptides from the Ag85C protein. A promiscuous T-cell epitope spanning amino acids 145 to 162 of Ag85B (identical sequence in M. bovis and M. avium subsp. paratuberculosis) was identified in experimentally infected cattle. Finally, young calves, born from cows with confirmed paratuberculosis, demonstrated proliferative responses to purified, recombinant Ag85A and Ag85B from M. avium subsp. paratuberculosis. These results indicate that the M. avium subsp. paratuberculosis Ag85 homologues are immunodominant T-cell antigens that are recognized early in experimental and natural infection of cattle.
On the basis of DNA-DNA hybridization and numerical taxonomy analysis, the mycobacterial species Mycobacterium avium is subdivided into three subspecies, M. avium subsp. avium, M. avium subsp. paratuberculosis, and M. avium subsp. silvaticum, which share extensive sequence identity (8). Nevertheless, these subspecies can be differentiated from each other on the basis of host range, mycobactin dependence, and the presence of specific insertion elements (17). M. avium subsp. paratuberculosis causes Johne's disease, a severe gastroenteritis in ruminants, with a significant impact on the agricultural economy, particularly the dairy industry (17). In the Belgian cattle population, paratuberculosis prevalence was determined by a serological survey, conducted from December 1997 to March 1998. This approach resulted in an estimated true herd prevalence of M. avium subsp. paratuberculosis infection of 6% (6). Dairy cattle usually start fecal shedding at 2 years of age and develop clinical symptoms around 4 years of age. Infection with M. avium subsp. paratuberculosis is commonly acquired early in life via the fecal-oral route through the ingestion of contaminated colostrum, milk, water, or feed (46) and possibly through intrauterine transmission (42). M. avium subsp. paratuberculosis is extremely robust, and bacteria were reported to survive up to 250 days in water and feces and on pastures (27).
Cell-mediated immune responses seem to control the initial infection for a sustained period of time, and clinical symptoms only appear in cows after a number of years, often after the first or second calving, possibly because of enhanced intracellular multiplication of M. avium subsp. paratuberculosis organisms caused by alterations in the hormonal milieu (15). Decreased cell-mediated responses are likely related to a loss of antigen-specific CD4+ T cells, which is most prominent in the ileum lesions from symptomatic animals (24). Also, Khalifeh et al. demonstrated that transforming growth factor β and interleukin-10 (IL-10) mRNA levels are higher in cows that have progressed to the clinical stage of the disease, compared to subclinically infected or healthy cows (23). It is not clear for the moment, whether this reflects a shift from a Th1- to a Th2-biased immune response or rather the development of a regulatory T-cell circuit (10). Vaccines consisting of whole killed or attenuated live M. avium subsp. paratuberculosis bacilli can provide partial protection by delaying fecal shedding and reducing the number of clinically affected animals, but they do not protect against infection. In the context of bovine tuberculosis (M. bovis) control and eradication programs, it is worth mentioning that animals immunized with these paratuberculosis vaccines develop positive reactions in the tuberculin skin test (the reference bovine tuberculosis detection method), and therefore paratuberculosis vaccination is subject to approval by the veterinary services. Moreover, because of the impact on trading of living animals, farmers are reluctant to use paratuberculosis vaccination (25). It is clear that the development of an efficient paratuberculosis subunit vaccine, which would not interfere with tuberculosis detection, would offer a solution.
The precise M. avium subsp. paratuberculosis antigens that induce a protective immune response are poorly defined. Secreted and surface-exposed cell wall proteins are major antigens recognized by the protective immune response against M. tuberculosis and M. bovis, and immunization with whole-culture filtrate, a rich source of extracellular proteins, can protect mice and guinea pigs to some extent against subsequent challenge with the tubercle bacillus (1, 39). A major fraction of the secreted proteins in culture filtrates of M. tuberculosis and M. bovis BCG is represented by the antigen 85 (Ag85) complex, a 30- to 32-kDa family of three proteins (Ag85A, Ag85B, and Ag85C) (51) that all possess an enzymatic mycolyl transferase activity, required for the biogenesis of cord factor (trehalose-dimycolate) (5), and that are encoded by three paralogous genes (fbpA, fbpB, and fbpC) located in distinct regions of the bacterial genome (9). Healthy individuals infected with M. tuberculosis or Mycobacterium leprae (28) and BCG-vaccinated mice (19) show strong T-cell proliferative and IFN-γ responses against Ag85A, and both Ag85A and Ag85B are promising candidates for future tuberculosis vaccines (3, 20, 22, 33). Members of the Ag85 family are found in all mycobacteria, and sequence comparison indicates that the Ag85 gene family arose by duplication of an ancestral gene, before the emergence of the actually known mycobacterial species (9).
The genes encoding the three Ag85 components from M. avium subsp. paratuberculosis have been sequenced, and at the protein level, a 99% sequence identity with M. avium was found, with a single amino acid residue difference for each protein (13): serine/proline in position 155 of the mature Ag85A protein, serine/asparagine in position 120 of the mature Ag85B protein, and isoleucine/threonine in position 284 of the mature Ag85C protein in M. avium subsp. paratuberculosis and M. avium, respectively. In comparison to the mature protein sequences of M. bovis (which differ from those of M. tuberculosis by only one residue in position 100 of the mature Ag85B protein), the M. avium subsp. paratuberculosis 85A protein sequence (map 0216) shares 82% identity, the M. avium subsp. paratuberculosis 85B protein sequence (map 1609c) shares 86% identity, and the M. avium subsp. paratuberculosis 85C protein sequence (map 3531c) shares 87% identity (13). Recently, it has been suggested that the Ag85B component together with GroES could be associated with the heat resistance of M. avium subsp. paratuberculosis (45).
Little is known so far about the immune recognition of culture filtrate antigens and of Ag85 homologs, in particular, during M. avium subsp. paratuberculosis infection. In experimentally infected American bison (Bison bison) inoculated with M. avium subsp. paratuberculosis organisms, it was shown that Ag85 was a major antigen produced during infection that could be detected in the serum by Western blot analysis with Ag85-specific monoclonal antibodies (MAbs) (30). However, for serodiagnosis of paratuberculosis, the sensitivity of an Ag85-based enzyme-linked immunosorbent assay was clearly lower than that of a 35-kDa-based assay (43). Here, we report on culture filtrate- and Ag85-specific T-cell responses in mice and cattle experimentally infected with M. avium subsp. paratuberculosis ATCC 19698 through the intravenous and oral route, respectively. Our results indicate that Ag85 homologs are immunodominant T-cell antigens in M. avium subsp. paratuberculosis infection, inducing strong proliferative and IFN-γ responses. Their vaccine potential for bovine paratuberculosis remains to be determined.
MATERIALS AND METHODS
Bacteria and antigens.
M avium subsp. paratuberculosis ATCC 19698 was purchased from the American Tissue Culture Collection and grown on solid Löwenstein-Jensen medium. Subsequently, cultures were maintained in liquid 7H9 medium supplemented with 10% oleic acid-albumin-dextrose-catalase and mycobactin J (Allied Laboratories Inc. and Synbiotics Europe, respectively) (2 μg/ml), and (after an intermediate 6-month passage on solid potato soaked in synthetic, protein-free Sauton medium supplemented with mycobactin J) bacteria were grown as a surface pellicle on Sauton medium. M. avium subsp. paratuberculosis was grown for 4 weeks at 39°C, culture filtrate (CF) was separated from the bacteria, and CF proteins were precipitated by 80% saturated ammonium sulfate. Precipitate was extensively dialyzed against phosphate-buffered saline. Bacteria were stored as concentrated pellets in Sauton medium with 20% glycerol at −70°C. The number of bacteria was determined by plating on mycobactin J-supplemented Middlebrook 7H11 medium and visual counting of the CFU after 8 weeks of incubation at 39°C. Enumeration of M. avium subsp. paratuberculosis organisms in spleen from infected mice was performed by plating serial dilutions of organ homogenate on the same 7H11 medium. M. bovis BCG and M. tuberculosis H37Rv culture filtrates were prepared from 2-week-old cultures on synthetic Sauton medium as described previously. Native 30- to 32-kDa Ag85 was purified from BCG CF using sequential chromatography on phenyl-Sepharose, DEAE Sephacel, and Sephadex G75 (11). Bovine purified protein derivative (PPD-B) from M. bovis Vallée and avian purified protein derivative (PPD-A) from M. avium (strain 42) were kindly given by J. Nyabenda from the WIV-Pasteur Institute, Brussels, Belgium.
SDS-PAGE.
Ammonium sulfate-concentrated CF from M. avium subsp. paratuberculosis was analyzed on 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels stained with silver nitrate. Ag85 homologues were visualized by Western blot analysis, using Ag85-specific MAb TD17.4 (21).
Preparation of genomic DNA from M. avium subsp. paratuberculosis.
Genomic DNA of M. avium subsp. paratuberculosis ATCC 19698 was prepared as described previously by Tanghe et al. for Mycobacterium ulcerans (47). Briefly, bacteria were lysed with lysozyme, pronase, and SDS. After phenol-chloroform-isoamyl alcohol extraction and RNase A treatment, DNA was precipitated with ethanol, and the pellet was resuspended in Tris-EDTA buffer. DNA was analyzed by agarose gel electrophoresis, and the purity was evaluated by spectrophotometry. DNA was kept at −20°C until use.
Cloning and purification of recombinant mature Ag85A and Ag85B from M. avium subsp. paratuberculosis.
The fbpA and fbpB genes encoding the mature Ag85A (304 residues) and Ag85B (290 residues) proteins were amplified by PCR from M. avium subsp. paratuberculosis genomic DNA using primers derived from sequences of M. avium (36, 37). The primers used were 5′-CGGGATCCGCGTTCTCGCGCCCCGGTCTGCCGGTGGA-3′ (forward 85A), 5′-CCCAAGCTTGGGTTAGGTGCCCTGGCCGTTCCCGGC-3′ (reverse 85A), 5′-CCCAAGCTTGGGTTTTCGCGTCCGGGCCTGCC-5′ (forward 85B), and 5′-CCCAAGCTTGGGTTATCCGCCGCCGCCCGGGGA-3′ (reverse 85B). These primers were designed with BamHI and HindIII sites for Ag85A cloning in pQE-80L (QIAGEN) vector and with only the HindIII site for Ag85B cloning in this vector due to the presence of an internal BamHI site in the fbpB gene. Genes were amplified without the mycobacterial signal sequence. fbpA was obtained by PCR (Expand Polymerase; Roche) with 30 cycles of amplification (10 cycles at 94°C for 20 s, and 67°C for 30 s, 72°C for 1 min; 5 cycles at 94°C for 40 s, 67°C for 30 s, and 72°C for 1.25 min; 5 cycles at 94°C for 1 min, 67°C for 30 s, and 72°C for 1.5 min; 10 cycles at 94°C for 1.20 min, 67°C for 30 s, and 72°C for 2.15 min). The same protocol was used for fbpB amplification except that the hybridization temperature was 68°C. Amplified fragments were purified by agarose gel separation followed by purification using a QIAkit PCR kit (QIAGEN). Purified genes were ligated into a pQE-80L (QIAGEN) expression vector predigested with BamHI/HindIII for 85A cloning and by HindIII for 85B cloning (T4 DNA ligase; Roche). Ligations were electroporated into Escherichia coli DH5α cells, and positive clones were screened on LB-ampicillin medium and confirmed by restriction enzyme digestion. The integrity of cloned sequences was checked by sequence analysis. Plasmids containing either the 85A or 85B sequence were transformed into Top-10F′ E. coli (Invitrogen) for expression. Recombinant proteins were purified by affinity chromatography on an immobilized nickel-nitrilotriacetic acid column, according to standard procedures. As a result of the cloning procedures, recombinant Ag85A and Ag85B proteins were both 317 amino acid (aa) residues long, including the six NH2-terminal histidines.
Peptide synthesis.
Peptides spanning the entire sequence of the mature BCG Ag85A sequence (295 aa) were synthesized as 20-mer peptides overlapping by 10 residues, with the exception of the 19-mer spanning aa 35 to 53 and the carboxy-terminal peptide spanning aa 275 to 295. Peptides spanning the entire mature BCG Ag85B sequence (285 aa) were synthesized as 18-mer peptides overlapping by nine residues, with the exception of a 21-mer peptide spanning aa 240 to 260 and the carboxy-terminal 15-mer peptide spanning residues 271 to 285. Peptides spanning the entire mature BCG Ag85C sequence (294 aa) were synthesized as 20-mer peptides overlapping by 10 residues, with the exception of two 18-mer peptides (aa 31 to 50 and 41 to 60, residues 33 and 34 lacking in Ag85C) and the carboxy-terminal 14-mer peptide (aa 281 to 294) (14).
Mice.
C57BL/6OlaHsd-bg (B6 bg/bg) mice (age 8 to 12 weeks) were bred at the animal facilities of the WIV-Pasteur Institute from breeding couples originally obtained from Harlan (The Netherlands).
Cattle.
All animals used in this study were from the Friesian-Holstein breed and originated from herds officially free of bovine tuberculosis. Five 2- to 3-week-old calves originated from paratuberculosis-free herds. Two calves born from cows suffering from clinical paratuberculosis (as confirmed by Pourquier serology and by M. avium subsp. paratuberculosis-positive fecal and postmortem organ cultures) were monitored for 47 weeks.
Experimental infection of mice and cattle.
Mice were infected intravenously in a lateral tail vein with 3 × 105 (peptide mapping) or 106 CFU of M. avium subsp. paratuberculosis ATCC 19698, grown in Middlebrook 7H9 medium supplemented with oleic acid-albumin-dextrose-catalase and mycobactin J. Five 2- to 3-week-old calves (animals 6074, 6075, 7782, 7783, and 7785) were infected by the oral route with 10 mg (108 CFU) of M. avium subsp. paratuberculosis (ATCC 19698) cells per day for 10 consecutive days. These animals were subclinically infected during the 2-year follow-up period and excreted M. avium subsp. paratuberculosis sporadically in their feces. They did not show any seroconversion against either protoplasmic or lipoarabinamannan antigens and did not present any clinical signs. The evolution of ex vivo IFN-γ production in response to PPD-A and PPD-B in these animals will be reported in another paper (unpublished data). Animals were tuberculin tested 125 weeks postinfection, and the reactions were read 72 h later: with PPD-A, one animal (7785) scored positive, two animals scored doubtful (6074 and 7782), and two animals scored negative (6075 and 7783). The five animals scored negative in skin tests with PPD-B.
Mouse spleen cell cytokine production.
At indicated time points, infected mice were killed by cervical dislocation; spleens were removed aseptically and homogenized by gentle disruption in a loosely fitting Dounce homogenizer. Spleens from three to six mice per group were analyzed individually or pooled, as indicated. Spleen cells were washed and resuspended at 4 × 106 white blood cells/ml in RPMI 1640 medium supplemented with 10% fetal calf serum, penicillin, streptomycin, 5 × 10−5 M 2-mercaptoethanol, and indomethacin (1 μg/ml; Sigma). Cells were cultured in a humidified CO2 incubator in round-bottom 96-well microplates. A volume of 180 μl of cells was added to 20 μl of the respective antigens (19). PPD-B, PPD-A, and CF were used at final concentrations of 25 μg/ml (each); synthetic peptides at were used at a concentration of 10 μg/ml (each); and native Ag85 from BCG and recombinant E. coli-derived Ag85A and 85B proteins from M. avium subsp. paratuberculosis were used at a concentration of 5 μg/ml (each). Culture supernatants from at least three wells were collected and pooled after 24 h (IL-2) and 72 h (IFN-γ) and stored at −20° until testing.
Proliferation assays in infected cattle.
Blood was collected on heparin by venipuncture and stored for 24 h at room temperature in order to decrease background proliferation of unstimulated cells. Proliferative responses were analyzed using a whole-blood assay in 10% autologous plasma. Briefly, heparinized blood was diluted 1:10 in RPMI 1640 medium supplemented with 5 × 10−5 M 2-mercaptoethanol. A volume of 180 μl of cells was mixed with 20 μl of antigen in 96-well round-bottom microwell plates, and cultures were incubated in a humidified CO2 incubator for 7 days. After 6 days, cells were pulsed overnight with tritiated thymidine (0.4 μCi/well) and collected on a Titertek Cell Harvester. Radioactivity recovered on the filters was counted in a Betaplate Liquid Scintillation Counter, and results were expressed as mean cpm ± standard deviation (SD) of triplicate cultures.
Cultured (in vitro) IFN-γ production in infected cattle.
Heparinized blood was stored for 24 h at room temperature as described for proliferation assays. It was then centrifuged for 10 min at 1,500 rpm, and plasma was replaced by the same volume of RPMI 1640 medium supplemented with 10% fetal calf serum, penicillin, streptomycin, and 5 × 10−5 M 2-mercaptoethanol in order to reduce background IFN-γ levels in unstimulated cells. Leukocytes were counted in a Coulter Counter and whole-blood-cell cultures were adjusted to 106 white blood cells/ml in complete RPMI 1640 medium. A volume of 180 μl of cells was mixed with 20 μl of antigen (concentrations as above) in 96-well round-bottom microwell plates, and cultures were incubated in a humidified CO2 incubator. After 6 days of culture, supernatants from at least three wells were pooled for each antigen and stored at −20° until testing.
Bovine ex vivo IFN-γ production.
Tests were performed as previously described (49). Heparinized blood was collected from the jugular vein, and 1-ml aliquots were incubated without antigen or with purified Ag85 from M. bovis BCG at a final concentration of 5 μg/ml. Cells were incubated for 18 h at 37°C in a humidified 5% CO2 incubator in 1-ml tubes. After centrifugation, plasma supernatants were collected and stored at −20°C until testing.
Mouse IL-2 assay.
IL-2 activity was quantified using a bioassay, measuring the uptake of tritiated thymidine by the murine IL-2-dependent CTLL-2 cell line as described previously (19). Each sample was tested in duplicate. IL-2 levels are expressed as mean cpm. In this assay, a standard IL-2 preparation (21) of 600 pg/ml yielded ±15,000 cpm, and the assay detection limit was 30 pg/ml. IL-2 values were considered positive when they were at least twofold higher than control cpm values.
Mouse IFN-γ assay.
IFN-γ activity was quantified in a sandwich enzyme-linked immunosorbent assay using coating antibody R4-6A2 and biotinylated detection antibody XMG1.2 (both from Pharmingen). The detection limit was around 10 pg/ml.
Bovine IFN-γ assay.
Bovine IFN-γ was determined using a Bovine IFN-γ EASIA kit (catalogue no. KBC1232; BioSource Europe S.A., Nivelles, Belgium). For the day 6 in vitro IFN-γ culture supernatants, optical density values were converted to pg/ml, using a standard curve of recombinant bovine IFN-γ (Serotec) at an initial concentration of 20 ng/ml.
RESULTS
Preparation of culture filtrate from M. avium subsp. paratuberculosis ATCC 19698 grown as a surface pellicle on synthetic Sauton medium.
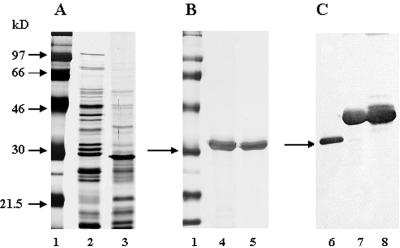
M. avium subsp. paratuberculosis ATCC 19698 was adapted to grow as a surface pellicle on synthetic, protein-free Sauton medium supplemented with mycobactin J. Comparison of the 4-week-old M. avium subsp. paratuberculosis CF with the protein profile of a 2-week-old M. tuberculosis H37Rv CF by SDS-PAGE indicated that in the region of the 30- to 32-kDa mycolyl transferase (Ag85), one protein of approximately 30 kDa was strongly expressed in the M. avium subsp. paratuberculosis CF. Trypsin digestion and matrix-assisted laser desorption ionization-time of flight analysis demonstrated that this band corresponded to the Ag85B homolog of M. avium subsp. paratuberculosis (I. Georis and R. Wattiez, personal communication). In M. tuberculosis CF, three protein bands can be detected in this region, corresponding to Ag85B, Ag85A, and Ag85C (18) (Fig. 1A). Recombinant His-tagged Ag85A (map 0216) and Ag85B (map 1609c) were purified by affinity chromatography on a nickel-nitrilotriacetic acid column and visualized by silver staining (Fig. 1B) and by Western blot analysis using the Ag85-specific MAb TD 17-4 (Fig. 1C).
FIG. 1.
(A) Comparative SDS-PAGE (12%) of culture filtrates from M. avium subsp. paratuberculosis ATCC 19698 (lane 3) and M. tuberculosis H37Rv (lane 2) grown as surface pellicles on synthetic Sauton medium. Proteins were visualized by silver nitrate staining. Molecular weight markers are shown in lane 1. (B) Silver nitrate staining of purified, recombinant E. coli-derived antigen 85A (lane 4) and Ag85B (lane 5) from M. avium subsp. paratuberculosis separated by 12% SDS-PAGE. (C) Western blot analysis of CF from M. avium subsp. paratuberculosis (lane 6) and of recombinant Ag85A (lane 7) and Ag85B (lane 8) from M. avium subsp. paratuberculosis using Ag85-specific MAb TD 17-4. The arrows indicate the position of the 30-kDa molecular weight marker (A and B) and of the native Ag85B protein in M. avium subsp. paratuberculosis CF (C).
Immune response against culture filtrate from M. avium subsp. paratuberculosis ATCC 19698 in mutant B6 bg/bg mice infected intravenously with M. avium subsp. paratuberculosis.
Although the mouse is not a target species for Johne's disease, the low cost, the availability of genetically defined inbred strains, and a range of immunological tools make this animal species a very attractive model for pilot studies. Inbred mice with a C57BL/6 background are more susceptible to M. avium subsp. paratuberculosis than inbred CBA mice (7). We therefore decided to perform an experimental infection in mutant B6 bg/bg beige mice. The mutant beige strain is deficient in NK cell activity and displays impaired chemokine response and neutrophil recruitment upon M. avium infection (2, 16). Beige mice have also been reported to be more susceptible to M. avium subsp. paratuberculosis infection (44). As shown in Table 1, spleen cells from beige mice infected intravenously with 106 CFU of M. avium subsp. paratuberculosis produced significant levels of IFN-γ in response to culture filtrate from M. avium subsp. paratuberculosis and from M. bovis BCG, to PPD from M. avium, and to native Ag85 purified from M. bovis BCG CF. IFN-γ levels in response to bovine PPD were lower than in response to PPD-A, although the difference was not statistically significant. More importantly, strong IFN-γ responses were detected following stimulation with recombinant E. coli-derived Ag85A and Ag85B proteins from M. avium subsp. paratuberculosis. IFN-γ responses could be detected in spleen cell cultures as early as 5 weeks postinfection (p.i.), and these responses further increased at week 10 p.i. Spleen cell IFN-γ responses of naive B6 bg/bg mice were very low following stimulation with purified Ag85. Bacterial infection persisted in the spleen during this period: 6.04 ± 0.12 log10 CFU (n = 6) at week 5 and 5.49 ± 0.11 log10 CFU (n = 4) at week 10. Intravenous injection with a similar dose of M. bovis BCG resulted in a rapid decline in spleen CFU counts between month 1 and month 2 (4.84 ± 0.22 [n = 3] versus 3.47 ± 0.49 [n = 4]), confirming that M. avium subsp. paratuberculosis displays a certain virulence in beige mice.
TABLE 1.
IFN-γ production in spleen cell cultures from B6 bg/bg mice infected intravenously with 1 × 106 CFU of M. avium subsp. paratuberculosis ATCC 19698
| Antigena | Amt of IFN-γ (pg/ml) in cultureb
|
||
|---|---|---|---|
| Uninfected (3) | 5 weeks p.i. (6) | 10 weeks p.i. (4) | |
| Medium | 78 ± 21 | 56 ± 54 | 41 ± 29 |
| CF M. avium subsp. paratuberculosis | 85 ± 42 | 7,703 ± 2,354 | 17,292 ± 10,136 |
| CF M. bovis BCG | 71 ± 25 | 8,479 ± 1,834 | 18,381 ± 8,037 |
| PPD-A | 164 ± 34 | 7,594 ± 2,214 | 16,760 ± 8,437 |
| PPD-B | 155 ± 16 | 4,757 ± 889 | 10,946 ± 2,892 |
| Ag85 (BCG) | 99 ± 41 | 5,465 ± 1,662 | 10,417 ± 2,481 |
| rAg85Aptb | 151 ± 18 | 6,510 ± 1,785 | 13,937 ± 7,462 |
| rAg85Bptb | 126 ± 49 | ND | 7,490 ± 4,731 |
C57BL/6 bg/bg mice, uninfected or infected with M. avium subsp. paratuberculosis were either unstimulated (medium) or stimulated as follows: CF and PPD, 25 μg/ml; Ag85, 5 μg/ml. rAg85Aptb and rAg85Bptb are recombinant antigens from M. avium subsp. paratuberculosis.
Data represent mean IFN-γ (pg/ml) levels/group ± SD of mice tested individually; the number of animals tested/group is given in parenthesis. ND, not determined.
Homologues of the Ag85 complex in CF from M. avium subsp. paratuberculosis are immunodominant T-cell antigens in experimentally infected mice.
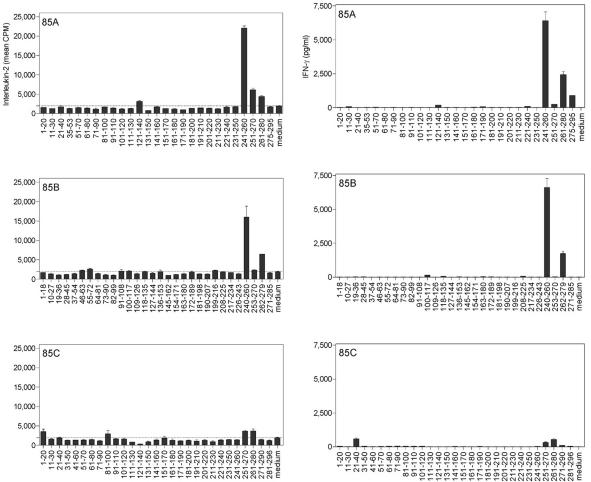
In view of the high sequence homology reported for the M. avium subsp. paratuberculosis and M. bovis BCG Ag85 proteins, we sought to characterize the H-2b-restricted, cross-reactive T-cell epitopes of Ag85 in M. avium subsp. paratuberculosis-infected mice, using synthetic overlapping peptides spanning the entire mature sequence of the three Ag85 components from M. bovis BCG. As shown in Fig. 2, spleen cells of B6 bg/bg mice infected intravenously with 3 × 105 CFU of M. avium subsp. paratuberculosis showed strong IL-2 (Fig. 2, left panels) and IFN-γ (Fig. 2, right panels) responses against certain peptides of Ag85A and Ag85B from BCG. These peptides were the same as those we have previously identified in H-2b haplotype B6 mice vaccinated with M. bovis BCG, infected with M. tuberculosis, or vaccinated with DNA encoding the M. tuberculosis Ag85 components (14, 21). Thus, strong IL-2 and IFN-γ production was observed in response to peptide 25 (spanning aa 241 to 260) and peptide 27 (aa 261 to 280) of Ag85A and to peptide 27 (aa 240 to 260) and peptide 30 (aa 262 to 279) of Ag85B. IL-2 responses against peptides from Ag85C were absent in spleen cell cultures from M. avium subsp. paratuberculosis-infected mice, whereas some IFN-γ was detected in response to peptide 3 (aa 21 to 40) and peptides 26 to 27 (aa 251 to 280). However, these IFN-γ levels were about 10-fold lower that the IFN-γ responses induced by the Ag85A or Ag85B peptides.
FIG. 2.
Characterization of cross-reactive T-cell epitopes of Ag85A, Ag85B and Ag85C in M. avium subsp. paratuberculosis-infected B6 bg/bg mice, using synthetic peptides spanning the mature sequences of Ag85A, Ag85B, and Ag85C from M. bovis BCG. IL-2 (left) and IFN-γ (right) production was analyzed in 24-h and 72-h culture supernatants, respectively, of spleen cells from a pool of five animals infected intravenously with 3 × 105 CFU of M. avium subsp. paratuberculosis and stimulated with overlapping synthetic peptides (10 μg/ml).
Proliferative responses against culture filtrate from M. avium subsp. paratuberculosis ATCC 19698 and Ag85 following oral infection with M. avium subsp. paratuberculosis ATCC 19698 in cattle.
In view of the strong reactivity of experimentally infected mice against culture filtrate from M. avium subsp. paratuberculosis and its Ag85 components, we decided to analyze whether a similar reactivity could also be induced by an experimental infection of a target species for Johne's disease, i.e., Bos taurus. As M. avium subsp. paratuberculosis infection is naturally acquired in young calves through ingestion of contaminated colostrum and milk, we infected five 2- to 3-week-old calves by the oral route with 10 mg (108 CFU) of M. avium subsp. paratuberculosis ATCC 19698 cells per day for 10 consecutive days and monitored the animals for 125 weeks.
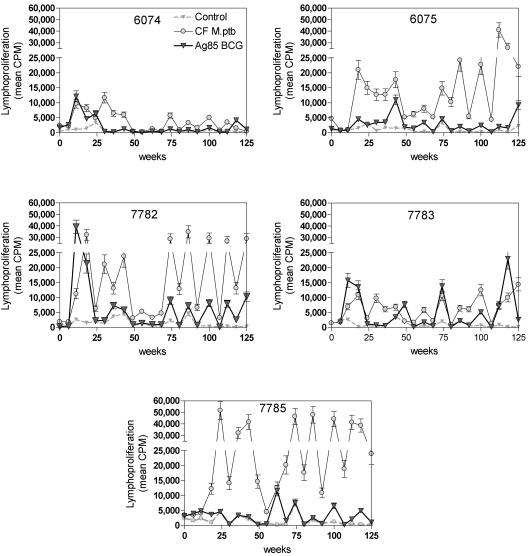
As shown in Fig. 3, proliferative responses against culture filtrate from M. avium subsp. paratuberculosis could be detected as early as 10 weeks p.i. in all five animals. Three animals (6074, 7782, and 7783) also demonstrated a proliferative response to Ag85, which, at this time point, was comparable in magnitude to the M. avium subsp. paratuberculosis CF-specific response. Between weeks 50 and 70 postinfection, proliferative responses to M. avium subsp. paratuberculosis CF declined but subsequently reappeared in four animals out of five. Animal 6074 reacted only weakly to CF in this second period; animal 7785 showed the strongest responses. Consistent Ag85-specific proliferative responses were detected in animals 7782, 7783, and 7785 from week 70 onwards. As reported by Koo et al. (26), cattle lymphoproliferative responses fluctuated considerably over time irrespective of background responses in unstimulated cells.
FIG. 3.
Bovine proliferative responses (mean cpm ± SD) of unstimulated PBMCs (Control) or of PBMCs stimulated with culture filtrate from M. avium subsp. paratuberculosis ATCC 19698 and Ag85 from M. bovis BCG following oral infection with 109 CFU of M. avium subsp. paratuberculosis ATCC 19698 in five animals, as analyzed over a follow-up period of 125 weeks postinfection.
Ex vivo IFN-γ production in response to Ag85 following oral infection with M. avium subsp. paratuberculosis ATCC 19698 in cattle.
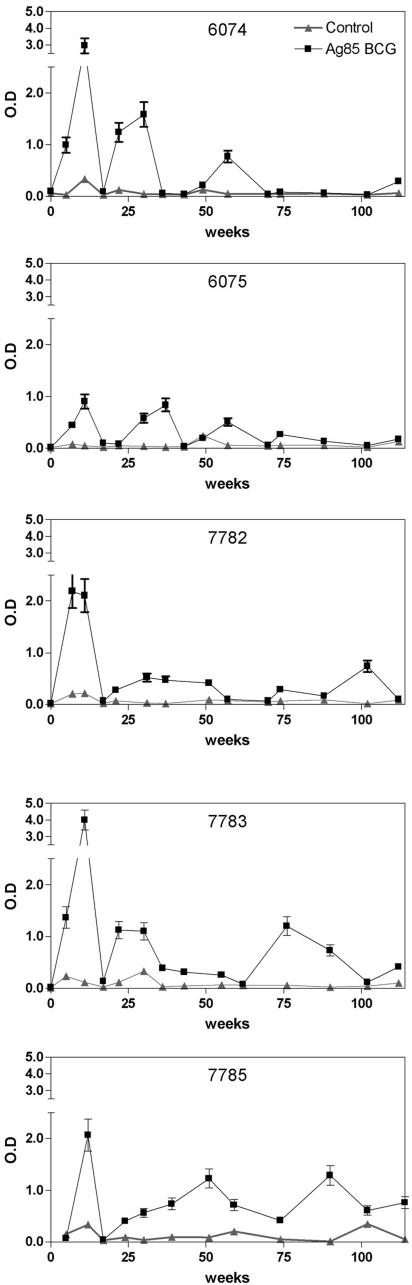
All five animals demonstrated an early peak of Ag85-specific IFN-γ at about 10 weeks of infection (Fig. 4). After a sharp decline around week 20, IFN-γ responses reappeared and could be detected throughout the whole follow-up period (albeit with some variation) in animals 7783 and 7785. Weak Ag85-specific IFN-γ responses were detected in animal 7782, whereas in animals 6074 and 6075 ex vivo IFN-γ levels were undetectable from week 70 onwards.
FIG. 4.
Ex vivo IFN-γ production in unstimulated cultures (Control) or in cultures stimulated with Ag85 of whole blood following oral infection with 109 CFU of M. avium subsp. paratuberculosis ATCC 19698 in five animals, as analyzed over a follow-up period of 125 weeks postinfection. Data are represented as optical density values (mean ± SD).
Proliferative and cultured IFN-γ responses at 114 weeks following oral infection with M. avium subsp. paratuberculosis ATCC 19698.
A more detailed representation of the proliferative responses against CF from M. avium subsp. paratuberculosis and M. bovis BCG and from PPD-A, PPD-B, and Ag85 (from BCG) as tested on week 114 p.i. is shown in Table 2. Animal 6074 did not react to any of the antigens, whereas the four other animals showed a positive proliferative response following stimulation with M. avium subsp. paratuberculosis CF and PPD-A. Responses to PPD-B were two- to sevenfold lower than to PPD-A in animals 6075, 7782, and 7785 and of comparable magnitude in animal 7783. Responses to CF from BCG were lower than to CF from M. avium subsp. paratuberculosis in animals 6075, 7782, 7783, and 7785. Purified Ag85 induced positive proliferative responses in all animals except animal 6074. As for the responses to M. avium subsp. paratuberculosis CF and PPD-A, the highest Ag85-specific proliferation was observed in animal 7785. IFN-γ production was also assessed at week 114 p.i. in 6-day culture supernatants (Table 3). In animal 6074, IFN-γ responses to M. avium subsp. paratuberculosis CF and PPD-A hardly exceeded background levels (400 pg/ml). In contrast, the other animals demonstrated IFN-γ levels ranging between 2,000 and 13,000 pg/ml in response to M. avium subsp. paratuberculosis CF and PPD-A. IFN-γ responses to PPD-B were low in animals 6074 and 6075 but detectable in the three other animals. Finally, a significant IFN-γ response to Ag85 was detected in two animals, i.e., 7783 and 7785.
TABLE 2.
Bovine lymphoproliferative responses at week 114 after oral infection with 109 CFU of M. avium subsp. paratuberculosis ATCC 19698
| Antigen | Proliferative response (cpm) in animala
|
||||
|---|---|---|---|---|---|
| 6074 | 6075 | 7782 | 7783 | 7785 | |
| Medium | 143 ± 20 | 335 ± 25 | 157 ± 23 | 133 ± 38 | 174 ± 45 |
| CF M. avium subsp. paratuberculosis | 276 ± 34 | 19,670 ± 3,374 | 15,790 ± 468 | 7,536 ± 390 | 27,835 ± 2,190 |
| CF BCG | 148 ± 48 | 1,350 ± 86 | 970 ± 46 | 2,098 ± 180 | 4,232 ± 233 |
| PPD-A | 184 ± 6 | 23,141 ± 2,203 | 10,347 ± 804 | 7,706 ± 126 | 34,255 ± 2,210 |
| PPD-B | 112 ± 7 | 1,662 ± 106 | 1,277 ± 143 | 11,123 ± 893 | 12,673 ± 143 |
| Ag85 (BCG) | 205 ± 11 | 1,131 ± 79 | 667 ± 24 | 1,194 ± 71 | 1,608 ± 74 |
Mean cpm ± SD of proliferative responses of whole blood diluted 1:10 and cultured in triplicate for 7 days in the absence (medium) or presence of the various antigens. [3H]thymidine was added to the cultures on the evening of day 6 and cells were harvested after overnight culture.
TABLE 3.
Bovine IFN-γ responses at week 114 after oral infection with 109 CFU of M. avium subsp. paratuberculosis ATCC 19698
| Antigen | IFN-γ production (pg/ml) in animala
|
||||
|---|---|---|---|---|---|
| 6074 | 6075 | 7782 | 7783 | 7785 | |
| Medium | 387 ± 78 | 521 ± 104 | 395 ± 79 | 394 ± 79 | 239 ± 48 |
| CF M. avium subsp. paratuberculosis | 852 ± 170 | 2,386 ± 477 | 3,902 ± 780 | 1,855 ± 371 | 13,240 ± 2,648 |
| PPD-A | 574 ± 115 | 5,648 ± 1,130 | 3,976 ± 795 | 2,823 ± 565 | 13,294 ± 2,659 |
| PPD-B | 440 ± 88 | 690 ± 138 | 1,133 ± 227 | 1,831 ± 366 | 4,547 ± 910 |
| Ag85 (BCG) | 394 ± 79 | 648 ± 130 | 440 ± 88 | 721 ± 144 | 1,787 ± 357 |
IFN-γ production in 6-day culture supernatants of whole blood diluted to 106 white blood cells/ml and cultured in the absence (medium) or presence of the various antigens. Supernatants from three individual wells were pooled for the assay. Values are the means ± SD.
Permissive proliferative responses against Ag85B145-162 peptide.
Analysis of peripheral blood mononuclear cell (PBMC) responses of an 11-month-old bull, infected by the intravenous route with 108 CFU of M. avium subsp. paratuberculosis, using synthetic peptides spanning the entire sequence of mature Ag85A, Ag85B, and Ag85C from BCG, indicated a strong and sustained proliferative response against a peptide from BCG Ag85B spanning amino acids 145 to 162 (Ag85B145-162) (data not shown). We therefore examined the responses against this Ag85B145-162 peptide in the five orally infected animals at different time points after infection. Proliferative values with a stimulation index higher than 2 were considered as positive. As shown in Table 4, at week 13 p.i., only one animal (6074) reacted to the peptide. Subsequently, this animal did not react to the peptide any longer, in line with its overall nonresponsiveness to M. avium subsp. paratuberculosis CF and Ag85 observed throughout the entire follow-up period. All other animals showed significant responses to the Ag85B145-162 peptide on at least two of the four subsequent time points tested. Again, animal 7785 ranked as the strongest reactor, with the highest stimulation index and cpm values. Proliferative responses were also observed against the corresponding peptide region of Ag85A and of Ag85C (data not shown).
TABLE 4.
Permissive proliferative responses against Ag85B145-162 peptide of bovine whole blood PBMCs after oral infection with 109 CFU of M. avium subsp. paratuberculosis ATCC 19698
| No. of weeks p.i. | Proliferative response (cpm) in animala
|
||||
|---|---|---|---|---|---|
| 6074 | 6075 | 7782 | 7783 | 7785 | |
| 13 | 1,888 ± 97 (4.57) | 293 ± 33 (1.14) | 271 ± 30 (0.89) | 279 ± 60 (0.82) | 309 ± 39 (0.97) |
| 28 | 323 ± 18 (0.94) | 814 ± 234 (1.62) | 2,777 ± 509 (4.32) | 3,760 ± 45 (6.96) | 3,713 ± 453 (15.41) |
| 59 | 414 ± 98 (1.38) | 2,390 ± 354 (1.76) | 12,909 ± 1,391 (5.12) | 1,263 ± 133 (1.02) | 6,420 ± 291 (4.09) |
| 82 | 859 ± 65 (1.19) | 3,233 ± 252 (6.97) | 1,167 ± 227 (7.39) | 495 ± 101 (1.65) | 9,694 ± 1,013 (3.61) |
| 114 | 251 ± 39 (1.25) | 1,285 ± 75 (3.84) | 1,238 ± 86 (7.88) | 2,293 ± 111 (17.24) | 6,241 ± 442 (35.86) |
Proliferative responses (mean ± SD of quadruplicate cultures) as detected after 7 days of culture of PBMCs (whole blood diluted 1:10) stimulated with Ag85B145-162 peptide (10 mg/ml). Stimulation indices compared to mean cpm values of unstimulated cells are given in parentheses.
Early proliferative T-cell responses against recombinant Ag85A and Ag85B from M. avium subsp. paratuberculosis in naturally infected calves.
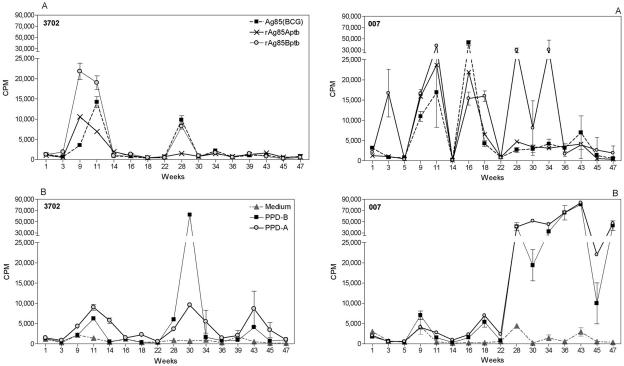
In order to find out whether similar, strong proliferative responses against Ag85 could also be observed in natural M. avium subsp. paratuberculosis infection, we examined two calves born from cows suffering from clinical paratuberculosis. Animal 3702 was 10 weeks of age at onset of the study, and animal 007 was 8 weeks old; both calves were monitored for a period of almost 1 year. As shown in Fig. 5, positive T-cell responses against recombinant Ag85A and Ag85B from M. avium subsp. paratuberculosis could be detected very early in these animals. Ag85-specific responses in animal 3072 waned at week 14 of follow-up and remained subsequently very low, except for one time point at week 28 (when the animal also reacted strongly to PPD-B). Proliferative responses to PPD-A were higher than to PPD-B but lower in magnitude than to the recombinant Ag85 (rAg85) proteins. Animal 007 showed a different lymphoproliferative profile. Positive responses to rAg85 (particularly the rAg85B component) fluctuated but could be detected throughout the entire year of follow-up. Around week 28, responses to PPD-A became positive. These responses were more or less comparable to those against PPD-B and much stronger than in animal 3702.
FIG. 5.
(A) Proliferative response to recombinant Ag85A and recombinant Ag85B proteins from M. avium subsp. paratuberculosis and to native Ag85 from BCG in two calves. (B) Proliferative responses to PPD-B and PPD-A in two calves born from cows with confirmed Johne's disease. Responses of unstimulated cells are shown (broken lines and black triangles). Data represent mean cpm ± SD of quadruplicate cultures of whole-blood PBMCs diluted 1:10 and stimulated for 7 days with the respective antigens.
DISCUSSION
The current vaccines against M. avium subsp. paratuberculosis are based on killed or live attenuated, whole-bacteria preparations. Although these vaccines provide partial protection, by reducing fecal shedding and reducing the number of clinically affected animals in a herd, they do not protect against infection. Animals vaccinated with these whole-bacteria vaccines develop positive tuberculin skin reactions and are of lesser economic value, because trading of PPD-positive animals is not permitted (EU Council Directive 64/432/EEC) (25). Moreover, such animals may develop observable granulomas at the injection site, which can be a problem at culling. An efficient subunit vaccine that would not interfere with bovine tuberculosis diagnosis would be very valuable in the management of paratuberculosis but requires the characterization of immunodominant and protective antigens. A number of immunogenic proteins of M. avium subsp. paratuberculosis have been described (17), but they have mostly been analyzed for diagnostic purposes (4, 38), and little is known about their vaccine potential. Recently, Nagata et al. reported on the expression cloning of three recombinant, IFN-γ-inducing antigens from M. avium subsp. paratuberculosis. Two of these proteins were members of the PPE protein family (35).
Immunization with mycobacterial culture filtrate proteins can protect mice and guinea pigs to some extent against subsequent challenge with M. tuberculosis and M. bovis (1, 39). Here we have analyzed the immunogenicity and the antigenic composition of CF derived from M. avium subsp. paratuberculosis ATCC 19698. First we adapted M. avium subsp. paratuberculosis isolate to grow as a surface pellicle on synthetic Sauton medium supplemented with mycobactin J. This protein-free medium enabled us to separate the CF proteins in 30 fractions with decreasing molecular weight by whole-gel electroelution following SDS-PAGE.
Culture filtrate from M. avium subsp. paratuberculosis was strongly recognized in experimentally infected B6 bg/bg mice and cattle, as indicated by spleen cell IFN-γ secretion and elevated lymphoproliferative responses of PBMCs, respectively. Animals reacted strongly to electroeluted CF fractions containing the Ag85 homologues from M. avium subsp. paratuberculosis (data not shown) and against recombinant E. coli-derived Ag85A and Ag85B from M. avium subsp. paratuberculosis. Cross-reactive responses were observed against Ag85 purified from M. bovis BCG CF and against synthetic peptides from the Ag85A and Ag85B components of this complex. Responses against synthetic peptides from Ag85C were much lower. As the Ag85C homologues from M. avium subsp. paratuberculosis and M. tuberculosis share the highest percentage of identical amino acids, this suggests that low responsiveness to the Ag85C component is probably caused by low expression levels, rather than by low immunogenicity. Low response levels to the Ag85C component have also been described in tuberculosis (14, 32). B6 bg/bg mice experimentally infected with M. avium subsp. paratuberculosis reacted against the same immunodominant Ag85A and Ag85B epitopes as C57BL/6 mice vaccinated with M. bovis BCG or infected with M. tuberculosis, i.e., regions spanning aa 241 to 260 and 261 to 280 (14, 21).
We also infected five 2- to 3-week-old calves with M. avium subsp. paratuberculosis ATCC 19698 by the oral route and monitored the animals for 2 years (125 weeks). CF-specific proliferative responses could be detected at 10 weeks postinfection in all five animals. These responses were sustained for about 50 weeks and then declined and reappeared from about week 70 onwards to remain stable until the end of the 125-week follow-up period in four out of five animals. Proliferative responses against Ag85 were also detected very early in infection with magnitudes as high or even higher than responses against whole CF in three out of five animals. Ex vivo IFN-γ responses to Ag85 showed an early peak in all five animals around week 10 p.i. After a sharp decline around week 20, ex vivo Ag85-specific IFN-γ responses reappeared in three out of five animals. On the whole, the evolution of day 7 proliferative and ex vivo IFN-γ responses to Ag85 correlated well. This early recognition of Ag85 in M. avium subsp. paratuberculosis infection is reminiscent of a household contact study of leprosy patients, in whom early T-cell reactivity against Ag85 complex (from M. bovis BCG) could be detected before reactivity against whole M. leprae bacilli or lepromin (29). CF proteins such as Ag85 are produced by actively replicating, live mycobacteria. In the phagosome of antigen-presenting cells, these proteins can be secreted and processed very early upon infection, before actual destruction of the bacteria releases antigenic components from the cytoplasm (18). This may explain the very early recognition of Ag85 components by the immune system and also the confirmed vaccine potential of this protein family.
In 2003, Bannantine and his colleagues reported on T-cell responses to PPD-A and whole M. avium subsp. paratuberculosis in three newborn calves, infected experimentally with ±1.6 × 107 CFU of M. avium subsp. paratuberculosis given into the tonsillar crypts (50). Positive immune responses were detected beginning around week 26 after infection. Also, in a more recent report, Koo et al. used flow cytometry to analyze proliferative responses against PPD and soluble M. avium subsp. paratuberculosis extract in orally infected calves (26). Persistent proliferative responses were again detected much later in this study (24 weeks after infection). It is probable that the higher infectious dose we used (109 CFU) is the reason for the more rapid appearance of reactive T cells observed in this study.
Ag85 epitope mapping in a young bull showed that one peptide spanning aa 145 to 162 of Ag85B from M. bovis BCG induced strong proliferative T responses after intravenous infection. The sequence of this Ag85B peptide is identical in M. bovis BCG and in M. avium subsp. paratuberculosis (13). Responses to this Ag85B peptide were also detected in the four orally infected calves that reacted against whole Ag85 protein. This peptide was not recognized prior to infection, nor was it recognized in animal 6074, which was nonreactive to Ag85. It has previously been shown that peptides from Ag85A and Ag85B are recognized in a permissive way by PBMCs from healthy PPD-positive human volunteers, with a majority of subjects reacting to a limited number of the same peptides, irrespective of their HLA haplotype (28, 40, 48). Interestingly, the peptide region spanning aa 141 to 160 from Ag85A was strongly recognized by 90% of these subjects (28), and we have identified in this same region a CD4+ and a CD8+ H-2d-restricted epitope (shared with the Ag85B component) and a CD4+ H-2b-restricted epitope in, respectively, BALB/c and C57BL/6 mice (12, 14). With respect to bovine tuberculosis, Lightbody et al. have reported on the permissive recognition of a T-cell epitope of M. bovis Ag85B in 7/13 (54%) tuberculous cattle of mixed breed, but the peptide identified in that study spanned aa 61 to 70 (YYQSGLSIVM) of the mature Ag85B protein (31).
Finally, strong proliferative responses against recombinant Ag85A and Ag85B from M. avium subsp. paratuberculosis could also be detected around 3 to 4 months of age in two young calves born from cows suffering from culture-confirmed Johne's disease. Although natural infection has not been formally proven so far in these two young animals, the positive proliferative responses to PPD-A would suggest that M. avium subsp. paratuberculosis infection was indeed transmitted by the diseased mothers to their offspring. Also, the results generated with the laboratory ATCC 19698 strain of M. avium subsp. paratuberculosis seem to be relevant to natural paratuberculosis infection, but a more detailed analysis and follow-up of the immune responses in naturally infected animals and bacterial culture will be required to confirm their infection status.
In conclusion, we have shown that experimental M. avium subsp. paratuberculosis infection of cattle and mice elicits a rapid and strong T-cell response against CF proteins in general and against mycolyl transferase Ag85A and Ag85B proteins in particular. We have also provided (preliminary) evidence that the Ag85 homologues are recognized very early in natural M. avium subsp. paratuberculosis infection. More work is needed to elucidate the protective potential of the antigen 85 family for the development of a new, subunit-based vaccine. It is interesting here that in a context of vaccination, the Ag85B component of M. avium subsp. paratuberculosis can elicit strong immune responses in mice when administered either as recombinant protein in Ribi adjuvant (34) or as a DNA vaccine (41). Also, mice and cattle vaccinated with an experimental vaccine consisting of whole, irradiated M. avium subsp. paratuberculosis ATCC 19698 in adjuvant showed significant proliferative and IFN-γ responses to recombinant Ag85A and Ag85B from M. avium subsp. paratuberculosis (Rosseels et al., unpublished data).
Acknowledgments
This work was partially supported by grants from the FWO-Vlaanderen (G.0266.00) and from The Brussels Capital region. V. Rosseels holds a grant from DG6 (Former Federal Ministry of Agriculture, now Federal Public Service for Health, Food Chain Security and Environment). V. Roupie is a FRIA bursary.
We thank J. Nyabenda for the generous gifts of bovine and avian PPD. We also are grateful to R. Wattiez and I. Georis, Department of Proteomic and Protein Biochemistry, University of Mons-Hainaut, for sequence analysis. The excellent technical assistance of F. Jurion, K. Palfliet, P.-Y. Adnet, and R. Laali is gratefully acknowledged.
Editor: J. D. Clements
REFERENCES
- 1.Andersen, P. 1994. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect. Immun. 62:2536-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appelberg, R., A. G. Castro, S. Gomes, J. Pedrosa, and M. T. Silva. 1995. Susceptibility of beige mice to Mycobacterium avium: role of neutrophils. Infect. Immun. 63:3381-3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldwin, S. L., C. D'Souza, A. D. Roberts, B. P. Kelly, A. A. Frank, M. A. Liu, J. B. Ulmer, K. Huygen, D. M. Mc Murray, and I. M. Orme. 1998. Evaluation of new vaccines in the mouse and guinea pig model of tuberculosis. Infect. Immun. 66:2951-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bannantine, J. P., E. Baechler, Q. Zhang, L. Li, and V. Kapur. 2002. Genome scale comparison of Mycobacterium avium subsp. paratuberculosis with Mycobacterium avium subsp. avium reveals potential diagnostic sequences. J. Clin. Microbiol. 40:1303-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belisle, J. T., V. D. Vissa, T. Sievert, K. Takayama, P. J. Brennan, and G. S. Besra. 1997. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science 276:1420-1422. [DOI] [PubMed] [Google Scholar]
- 6.Boelaert, F., K. Walravens, P. Biront, J. P. Vermeersch, D. Berkvens, and J. Godfroid. 2000. Prevalence of paratuberculosis (Johne's disease) in the Belgian cattle population. Vet. Microbiol. 77:269-281. [DOI] [PubMed] [Google Scholar]
- 7.Chandler, R. L. 1961. Infection of laboratory animals with Mycobacterium johnei. IV. Comparative susceptibility to infection of C. 57, C.B.A. and Swiss white mice. J. Comp. Pathol. 71:233-242. [DOI] [PubMed] [Google Scholar]
- 8.Cocito, C., P. Gilot, M. Coene, M. De Kesel, P. Poupart, and P. Vannuffel. 1994. Paratuberculosis. Clin. Microbiol. Rev. 7:328-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Content, J., A. de La Cuvellerie, L. De Wit, V. Vincent-Levy-Frebault, J. Ooms, and J. De Bruyn. 1991. The genes coding for the antigen 85 complexes of Mycobacterium tuberculosis and Mycobacterium bovis BCG are members of a gene family: cloning, sequence determination, and genomic organization of the gene coding for antigen 85-C of M. tuberculosis. Infect. Immun. 59:3205-3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coussens, P. M. 2001. Mycobacterium paratuberculosis and the bovine immune system. Animal Health Res. Rev. 2:141-161. [PubMed] [Google Scholar]
- 11.De Bruyn, J., K. Huygen, R. Bosmans, M. Fauville, R. Lippens, J. P. Van Vooren, P. Falmagne, M. Weckx, H. G. Wiker, M. Harboe, and M. Turneer. 1987. Purification, partial characterization and identification of a 32 kDa protein antigen of Mycobacterium bovis BCG. Microb. Pathog. 2:351-366. [DOI] [PubMed] [Google Scholar]
- 12.Denis, O., A. Tanghe, K. Palfliet, F. Jurion, T. P. Van den Berg, A. Vanonckelen, J. Ooms, E. Saman, J. B. Ulmer, J. Content, and K. Huygen. 1998. Vaccination with plasmid DNA encoding mycobacterial antigen 85A stimulates a CD4+ and CD8+ T-cell epitopic repertoire broader than that stimulated by Mycobacterium tuberculosis H37Rv infection. Infect. Immun. 66:1527-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dheenadhayalan, V., K.-S. Shin, C.-F. Chang, C.-D. Chang, S.-J. Wang, P. McDonough, S. Stehman, S. Shin, A. Torres, and Y.-F. Chang. 2002. Cloning and characterization of the genes coding for antigen 85A, 85B and 85C of Mycobacterium avium subsp. paratuberculosis. DNA Seq. 13:287-294. [DOI] [PubMed] [Google Scholar]
- 14.D'Souza, S., V. Rosseels, M. Romano, A. Tanghe, O. Denis, F. Jurion, N. Castiglioni, A. Vanonckelen, K. Palfliet, and K. Huygen. 2003. Mapping of murine Th1 helper T-cell epitopes of mycolyl transferases Ag85A, Ag85B and Ag85C from M. tuberculosis. Infect. Immun. 71:483-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feola, R. P., M. T. Collins, and C. J. Czuprynski. 1999. Hormonal modulation of phagocytosis and intracellular growth of Mycobacterium avium subsp. paratuberculosis in bovine peripheral blood monocytes. Microb. Pathog. 26:1-11. [DOI] [PubMed] [Google Scholar]
- 16.Florido, M., R. Appelberg, I. M. Orme, and A. M. Cooper. 1997. Evidence for a reduced chemokine response in the lungs of beige mice infected with Mycobacterium avium. Immunology 90:600-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris, N. B., and R. G. Barletta. 2001. Mycobacterium avium subsp. paratuberculosis in veterinary medicine. Clin. Microbiol. Rev. 14:489-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harth, G., B. Y. Lee, J. Wang, D. L. Clemens, and M. A. Horwitz. 1996. Novel insights into the genetics, biochemistry, and immunocytochemistry of the 30-kilodalton major extracellular protein of Mycobacterium tuberculosis. Infect. Immun. 64:3038-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huygen, K., D. Abramowicz, P. Vandenbussche, F. Jacobs, J. De Bruyn, A. Kentos, A. Drowart, J.-P. Van Vooren, and M. Goldman. 1992. Spleen cell cytokine secretion in Mycobacterium bovis BCG-infected mice. Infect. Immun. 60:2880-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huygen, K., J. Content, O. Denis, D. L. Montgomery, A. M. Yawman, R. R. Deck, C. M. DeWitt, I. M. Orme, S. Baldwin, C. D'Souza, A. Drowart, E. Lozes, P. Vandenbussche, J.-P. Van Vooren, M. A. Liu, and J. B. Ulmer. 1996. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat. Med. 2:893-898. [DOI] [PubMed] [Google Scholar]
- 21.Huygen, K., E. Lozes, B. Gilles, A. Drowart, K. Palfliet, F. Jurion, I. Roland, M. Art, M. Dufaux, J. Nyabenda, J. De Bruyn, J.-P. Van Vooren, and R. Deleys. 1994. Mapping of Th1 helper T-cell epitopes on major secreted mycobacterial antigen 85A in mice infected with live Mycobacterium bovis BCG. Infect. Immun. 62:363-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamath, A. T., C. G. Feng, M. MacDonald, H. Briscoe, and W. J. Britton. 1999. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis. Infect. Immun. 67:1702-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khalifeh, M. S., and J. R. Stabel. 2004. Upregulation of transforming growth factor-beta and interleukin-10 in cows with clinical Johne's disease. Vet. Immunol. Immunopathol. 9:39-46. [DOI] [PubMed] [Google Scholar]
- 24.Koets, A., V. Rutten, A. Hoek, F. van Mil, K. Müller, D. Bakker, E. Gruys, and W. van Eden. 2002. Progressive bovine paratuberculosis is associated with local loss of CD4+ T cells, increased frequency of γδ T cells, and related changes in T-cell function. Infect. Immun. 70:3856-3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Köhler, H., H. Gyra, K. Zimmer, K. G. Dräger, B. Burkert, B. Lemser, D. Hausleithner, K. Cuszler, W. Klawonn, and R. G. Hesz. 2001. Immune reactions in cattle after immunization with a Mycobacterium paratuberculosis vaccine and implications for the diagnosis of M. paratuberculosis and M. bovis infections. J. Vet. Med. B. 48:185-195. [DOI] [PubMed] [Google Scholar]
- 26.Koo, H. C., Y. H. Park, M. J. Hamilton, G. M. Barrington, C. J. Davies, J. B. Kim, J. L. Dahl, W. R. Waters, and W. C. Davis. 2004. Analysis of the immune response to Mycobacterium avium subsp. paratuberculosis in experimentally infected calves. Infect. Immun. 72:6870-6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsen, A. B., R. S. Merkal, and T. H. Vardaman. 1956. Survival time of Mycobacterium paratuberculosis. Am. J. Vet. Res. 17:549-551. [PubMed] [Google Scholar]
- 28.Launois, P., R. DeLeys, M. N'Diaye Niang, A. Drowart, M. Andrien, P. Dierckx, J.-L. Cartel, J.-L. Sarthou, J.-P. Van Vooren, and K. Huygen. 1994. T-cell-epitope mapping of the major secreted mycobacterial antigen Ag85A in tuberculosis and leprosy. Infect. Immun. 62:3679-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Launois, P., M. N. Niang, J. De Bruyn, J. L. Sarthou, F. Rivier, A. Drowart, J.-P. Van Vooren, J. Millan, and K. Huygen. 1993. The major secreted antigen complex (Ag 85) from Mycobacterium bovis bacille Calmette-Guérin is associated with protective T cells in leprosy: a follow-up study of 45 household contacts. J. Infect. Dis. 167:1160-1167. [DOI] [PubMed] [Google Scholar]
- 30.Leid, J. G., D. Hunter, and C. A. Speer. 2002. Early diagnosis of Johne's disease in the American bison by monoclonal antibodies directed against antigen 85. Ann. N. Y. Acad. Sci. 969:66-72. [DOI] [PubMed] [Google Scholar]
- 31.Lightbody, K. A., R. M. Girvin, D. A. Pollock, D. P. Mackie, S. D. Neill, and J. M. Pollock. 1998. Recognition of a common mycobacterial T cell epitope in MPB59 of Mycobacterium bovis. Immunology 93:314-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim, J.-H., J.-K. Park, E.-K. Jo, C.-H. Song, D. Min, Y.-J. Song, and H.-J. Kim. 1999. Purification and Immunoreactivity of three components from 30/32 kDa antigen 85 complex in Mycobacterium tuberculosis. Infect. Immun. 67:6187-6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McShane, H., A. A. Pathan, C. Sander, S. M. Keating, S. C. Gilbert, K. Huygen, H. A. Fletcher, and A. V. S. Hill. 2004. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat. Med. 10:1240-1244. [DOI] [PubMed] [Google Scholar]
- 34.Mullerad, J., I. Michal, Y. Fishman, A. H. Hovav, R. G. Barletta, and H. Bercovier. 2002. The immunogenicity of Mycobacterium paratuberculosis 85B antigen. Med. Microbiol. Immunol. 190:179-187. [DOI] [PubMed] [Google Scholar]
- 35.Nagata, R., Y. Muneta, K. Yoshihara, Y. Yokomizo, and Y. Mori. 2005. Expression cloning of gamma interferon-inducing antigens of Mycobacterium avium subsp. paratuberculosis. Infect. Immun. 73:3778-3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohara, N., K. Matsuo, R. Yamaguchi, A. Yamazaki, H. Tasaka, and T. Yamada. 1993. Cloning and sequencing of the gene for α antigen from Mycobacterium avium and mapping of the B-cell epitopes. Infect. Immun. 61:1173-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohara, N., N. Ohara-Wada, H. Kitaura, T. Nishiyama, S. Matsumoto, and T. Yamada. 1997. Analysis of the genes encoding the antigen 85 complex and MPT51 from Mycobacterium avium. Infect. Immun. 65:3680-3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olsen, I., H. G. Wiker, E. Johnson, H. Langeggen, and L. J. Reitan. 2001. Elevated antibody responses in patients with Crohn's disease against a 14 kDa secreted protein purified from Mycobacterium avium subsp. paratuberculosis. Scand. J. Immunol. 53:198-203. [DOI] [PubMed] [Google Scholar]
- 39.Pal, P. G., and M. A. Horwitz. 1992. Immunization with extracellular proteins of Mycobacterium tuberculosis induces cell-mediated immune responses and substantial protective immunity in a guinea pig model of pulmonary tuberculosis. Infect. Immun. 60:4781-4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roche, P. W., P. W. Peake, H. Billman-Jacobe, T. Doran, and W. J. Britton. 1994. T-cell determinants and antibody binding sites on the major mycobacterial secretory protein MPB59 of Mycobacterium bovis. Infect. Immun. 62:5319-5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosseels, V., V. Scanlan, A. Vanonckelen, F. Jurion, K. Palfliet, S. Marché, J. Godfroid, K. Walravens, and K. Huygen. 2003. Development of a plasmid DNA based M. paratuberculosis vaccine encoding immunodominant T cell antigens identified in mycobacterial culture filtrate, p. 108-113. In R. A. Juste (ed.), Proceedings of the Seventh International Colloquium on Paratuberculosis. International Association for Paratuberculosis, Madison, Wis.
- 42.Seitz, S. E., L. E. Heider, W. D. Heuston, S. Bech-Nielsen, D. M. Rings, and L. Spangler. 1989. Bovine fetal infection with Mycobacterium paratuberculosis. J. Am. Vet. Med. Assoc. 194:1423-1426. [PubMed] [Google Scholar]
- 43.Shin, S. J., H. S. Yoo, S. P. McDonough, and Y.-F. Chang. 2004. Comparative antibody response of five recombinant antigens in related to bacterial shedding levels and development of serological diagnosis based on 35 kDa antigen for Mycobacterium avium subsp. paratuberculosis. J. Vet. Sci. 5:111-117. [PubMed] [Google Scholar]
- 44.Stabel, J. R., J. P. Goff, D. L. Whipple, M. R. Ackermann, and T. A. Reinhardt. 1996. Low calcium diet and 1,25-dihydroxyvitamin D(3) infusion modulate immune responses during Mycobacterium paratuberculosis infection in beige mice. Vet. Immunol. Immunopathol. 50:127-143. [DOI] [PubMed] [Google Scholar]
- 45.Sung, N., K. Takayama, and M. T. Collins. 2004. Possible association of GroES and antigen 85 proteins with heat resistance of Mycobacterium paratuberculosis. Appl. Environ. Microbiol. 70:1688-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sweeney, R. W. 1996. Transmission of paratuberculosis. Vet. Clin. N. Am. Food Anim. Pract. 12:305-312. [DOI] [PubMed] [Google Scholar]
- 47.Tanghe, A., J. Content, J.-P. Van Vooren, F. Portaels, and K. Huygen. 2001. Protective efficacy of a DNA vaccine encoding Ag85A from Mycobacterium bovis BCG against Buruli Ulcer. Infect. Immun. 69:5403-5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valle, M. T., A. M. Megiovanni, A. Merlo, G. Li Pira, L. Bottone, G. Angelini, L. Bracci, L. Lozzi, K. Huygen, and F. Manca. 2001. Epitope focus, clonal composition and Th1 phenotype of the human CD4 response to the secretory mycobacterial antigen Ag85. Clin. Exp. Immunol. 123:226-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walravens, K., V. Wellemans, V. Weynants, F. Boelaert, V. deBergeyck, J.-J. Letesson, K. Huygen, and J. Godfroid. 2002. Analysis of the antigen-specific IFN-γ producing T-cell subsets in cattle experimentally infected with Mycobacterium bovis. Vet. Immunol. Immunopathol. 84:29-41. [DOI] [PubMed] [Google Scholar]
- 50.Waters, W. R., J. M. Muillner, M. V. Palmer, J. R. Stabel, D. E. Jones, K. A. Koistinen, E. M. Steadham, M. J. Hamilton, W. C. Davis, and J. P. Bannantine. 2003. Early induction of humoral and cellular immune responses during experimental Mycobacterium avium subsp. paratuberculosis infection of calves. Infect. Immun. 71:5130-5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wiker, H. G., and M. Harboe. 1992. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol. Rev. 56:648-661. [DOI] [PMC free article] [PubMed] [Google Scholar]