The genus Salmonella contains a group of closely related organisms that are pathogenic for humans and other vertebrates. The human disease manifestations caused most frequently by Salmonella serotypes worldwide are typhoid fever and gastroenteritis (reviewed in reference 102). Both illnesses clearly differ with regard to their etiology. Typhoid fever is caused by Salmonella enterica serotype Typhi, a strictly human-adapted pathogen. In contrast, gastroenteritis is caused by zoonotic nontyphoidal Salmonella serotypes, with Salmonella enterica serotypes Typhimurium and Enteritidis being isolated most frequently (67).
A second difference between typhoid fever and gastroenteritis is the host response elicited at the site where both infections originate, the intestinal mucosa. Gastroenteritis is a typical diarrheal disease characterized by a massive neutrophil influx in the terminal ileum and colon and the predominance of neutrophils in stool samples of patients (16, 41, 66). In contrast, typhoid fever is not a typical diarrheal disease and the intestinal pathology is characterized by a predominantly mononuclear infiltrate (i.e., macrophages and dendritic cells) (41, 57, 77, 83, 110). Although a fraction (approximately one-third) of typhoid fever patients develop diarrhea, the fecal leukocyte populations in these patients are dominated by mononuclear cells, while neutrophils are scarce (41).
Serotype Typhi and nontyphoidal Salmonella serotypes further differ in their abilities to survive and persist in human tissue. Gastroenteritis is an infection that usually remains localized to the intestine and mesenteric lymph nodes. The incubation period is short (12 to 72 h) and is followed by a short episode of disease (<10 days), suggesting that the infection is efficiently cleared with the onset of adaptive immune responses. In contrast, typhoid fever is a systemic infection during which serotype Typhi colonizes the liver, spleen, and bone marrow in addition to the intestine (i.e., Peyer's patches where perforation may occur) and the mesenteric lymph nodes. Furthermore, typhoid fever is characterized by a considerably longer incubation period (median of 5 to 9 days) and longer duration of symptoms (fever persists for approximately 3 weeks) than that observed in patients infected with nontyphoidal Salmonella serotypes.
The differences in the host responses and disease manifestations of typhoid fever and gastroenteritis suggest that serotype Typhi and nontyphoidal Salmonella serotypes cause disease by different mechanisms. Recent advances in our understanding of the mechanisms by which nontyphoidal Salmonella serotypes cause gastroenteritis have helped in defining the unique properties that enable serotype Typhi to trigger host responses in humans that lead to the development of typhoid fever rather than gastroenteritis.
INFLAMMATORY DIARRHEA: A STEREOTYPIC HOST RESPONSE TO MICROBIAL INVASION
Based on the presence or absence of leukocytes in stool samples of patients, diarrheal pathogens can be differentiated into those causing inflammatory diarrhea (the presence of neutrophils or lactoferrin in stool samples) and those causing secretory diarrhea (no fecal leukocytes). Bacterial pathogens associated with secretory diarrhea (e.g., Vibrio cholerae, enterotoxigenic Escherichia coli, enteropathogenic E. coli, or enterohemorrhagic E. coli) generally have in common that they are noninvasive and cause little or no inflammation in the intestinal mucosa. In contrast, pathogens causing inflammatory diarrhea (e.g., enteroinvasive E. coli, Shigella spp., Campylobacter spp., or nontyphoidal Salmonella serotypes) readily invade the intestinal epithelium and trigger a massive neutrophil influx in the intestine. Recent work on the mechanism of serotype Typhimurium-induced gastroenteritis has made it increasingly clear that the severe neutrophil infiltration that characterizes inflammatory diarrhea is due largely to bacterial invasion followed by innate immune recognition (reviewed in reference 20).
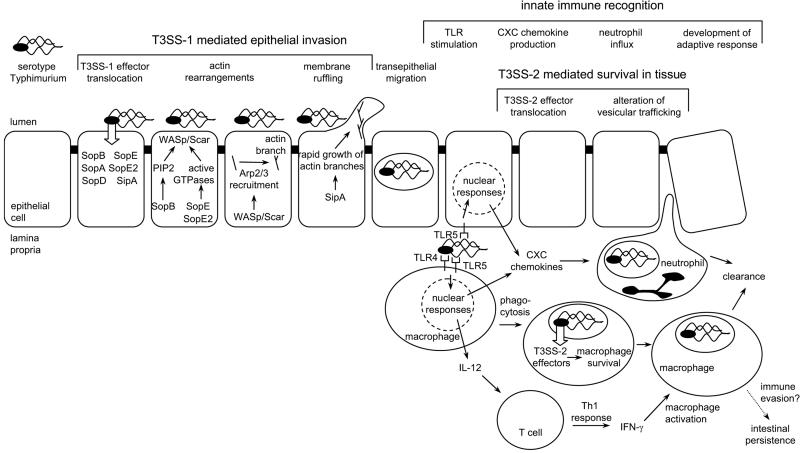
Serotype Typhimurium readily enters enterocytes and M cells during infection of bovine ligated ileal loops, an in vivo model for human gastroenteritis (29, 95, 101). Analysis of this process using human epithelial cell lines shows that Salmonella serotypes trigger entry by injecting proteins, termed effectors, into host cells using type III secretion system 1 (T3SS-1), encoded by Salmonella pathogenicity island 1 (SPI1) (31, 32, 70). Six effectors, including two guanine nucleotide exchange factors for Rho family GTPases (SopE and SopE2) (40, 111), an inositol phosphate phosphatase (SopB, also known as SigD) (84), an actin-binding protein (SipA, also known as SspA) (130), and two effectors with unknown activities (SopA and SopD), act in concert to mediate serotype Typhimurium entry into intestinal epithelial cells (71, 94, 129) and are required for triggering inflammation in the intestinal mucosa (50, 119, 126, 127). Phosphatidylinositol phosphates (generated by SopB) and activated Rho family GTPases (produced by SopE and SopE2) act in concert to activate WASp/Scar proteins (reviewed in reference 45), which have been shown to be required for serotype Typhimurium invasion (115). Activated WASp/Scar proteins recruit the Arp2/3 complex (108, 111), which in turn initiates the formation of new branches on actin filaments (78). SipA accelerates the growth of new branches by lowering the critical concentration required for actin polymerization (130) and by locally inhibiting ADF/cofilin-directed actin disassembly (65). Rapid growth of the barbed ends of the new actin branches pushes the membrane forward (74), leading to the formation of membrane ruffles and bacterial internalization by macropinocytosis (28, 33) (Fig. 1).
FIG. 1.
Pathogenesis of serotype Typhimurium-induced gastroenteritis in humans. Serotype Typhimurium T3SS-1 injects effector proteins (SipA, SopA, SopB, SopD, SopE, and SopE2) into epithelial cells, which mediate bacterial invasion. Phosphatidylinositol phosphates (PIP2) produced by SopB and Rho family GTPases activated by SopE/SopE2 act in concert to activate WASp/Scar proteins, which in turn recruit the Arp2/3 complex to form new branches on actin filaments. SipA accelerates the growth of new branches, resulting in the formation of membrane ruffles and bacterial internalization. Once serotype Typhimurium has crossed the epithelial barrier, its presence in the lamina propria is detected by pathogen recognition receptors (TLR4 and TLR5) and the organism is internalized by phagocytes. TLR signaling in epithelial cells and mononuclear cells (macrophages and dendritic cells) results in the release of neutrophil chemoattractants (CXC chemokines) which trigger the massive neutrophil influx that is the hallmark of inflammatory diarrhea. Serotype Typhimurium is able to survive within macrophages by using its T3SS-2 to translocate into the host cell cytosol effectors that enable the pathogen to evade phagocyte killing mechanisms by altering vesicular trafficking. TLR signaling also triggers the release of IL-12 from mononuclear cells, thereby initiating the development of a protective Th1 response. With the development of adaptive immune responses (e.g., IFN-γ activation of macrophages), host phagocytes are able to clear the infection and symptoms subside. Some bacteria escape clearance and persist for 1 or 2 months in an intestinal niche. Recently identified mechanisms for immune evasion by serotype Typhimurium could be important during this phase of intestinal persistence (4, 10, 53).
Subsequent to invasion, serotype Typhimurium traverses the epithelium to reach the lamina propria, where bacteria always have an intracellular location within phagocytes (neutrophils and mononuclear cells) (29, 95, 101). Intestinal epithelial cells can differentiate commensal bacteria present in the intestinal lumen from serotype Typhimurium organisms present in the lamina propria by expressing a pathogen recognition receptor, termed Toll-like receptor 5 (TLR5), exclusively on their basolateral surfaces (34). The presence of bacteria in the lamina propria is further detected by phagocytes which express a variety of pathogen recognition receptors on their surfaces, including TLR1, TLR2, TLR4, TLR5, and TLR6 (reviewed in reference 49). TLR1, TLR2, TLR4, TLR5, and TLR6 are specialized in recognizing products, termed pathogen-associated molecular patterns (PAMPs), that are unique to bacteria and are not made by the host. PAMPs that are important for the recognition of serotype Typhimurium include flagella (detected by TLR5) (43) and the lipid A moiety of lipopolysaccharide (LPS) (detected by the TLR4/CD14/MD2 complex) (68, 89, 109, 121). Tissue culture models demonstrate that the detection of serotype Typhimurium in the lamina propria through TLRs that are expressed on macrophages and on intestinal epithelial cells initiates the induction of a proinflammatory gene expression profile in these host cells, which includes the expression of neutrophil chemoattractants (CXC chemokines), such as interleukin 8 (IL-8) (81, 97, 124). CXC chemokine production in infected tissue precedes the massive neutrophil influx in the intestinal mucosa that characterizes serotype Typhimurium-induced gastroenteritis (101, 125) (Fig. 1).
The preferred niche for the survival of serotype Typhimurium in the intestinal mucosa appears to be within macrophages (reviewed in reference 100). Survival within macrophages requires the action of a second type III secretion system (T3SS-2) encoded by SPI2 (85) (Fig. 1). Inactivation of T3SS-2 results in a reduced severity of neutrophil infiltration in the intestinal mucosa at later times (>24 h) after oral infection of calves (113) or streptomycin-treated mice (13, 39) with serotype Typhimurium and markedly reduces bacterial recovery from intestinal tissues (114). The dependence of this inflammatory reaction on MyD88, an intracellular adaptor protein that integrates signals from several TLRs, suggests that T3SS-2-mediated bacterial survival in the lamina propria is continuously monitored by pathogen recognition receptors (39).
The mechanisms by which other pathogens cause inflammatory diarrhea appear to be slight variations of the above theme. For instance, Shigella flexneri uses a type III secretion system for the invasion of epithelial cells which mediates entry by mechanisms similar to those of T3SS-1 of serotype Typhimurium (reviewed in reference 99). However, once within an epithelial cell, the S. flexneri T3SS enables this pathogen to escape from the vacuole and enter the cytosol. S. flexneri is detected within the cytosol of host cells by the nucleotide-binding oligomerization domain 1 (Nod1) protein (37), a cytosolic pathogen recognition receptor that induces expression of a proinflammatory cytokine profile upon recognition of a PAMP present in the bacterial peptidoglycan (36). Once S. flexneri translocates through M cells or is released from enterocytes, its interaction with phagocytes in the lamina propria results in an inflammatory response that is similar to that seen during serotype Typhimurium infection (reviewed in reference 99). Since bacterial invasion is detected by a small number of pathogen recognition receptors that trigger the bulk of transcriptional changes through activation of NF-κB, AP-1, and IRF3, it may not be surprising that the host responses to different invasive enteric pathogens (e.g., enteroinvasive E. coli, Shigella spp., Campylobacter spp., or nontyphoidal Salmonella serotypes) are very similar. Even an infection with an invasive parasite (i.e., Entamoeba histolytica) triggers changes in host gene expression in the human colonic mucosa that are very similar to those accompanying infection with S. flexneri (128). The picture emerging from these studies is that microbial invasion of the intestinal mucosa is recognized by phagocytes and epithelial cells through stimulation of pathogen recognition receptors that trigger a stereotypic host response leading to neutrophil influx and inflammatory diarrhea.
TYPHOID FEVER: EVASION OF INNATE IMMUNE RECOGNITION IN THE INTESTINE
The above considerations illustrate that bacterial invasion (mediated by T3SS-1), bacterial survival in intestinal tissue (mediated by T3SS-2), and recognition of bacterial PAMPs (i.e., flagella and LPS) by cells in the intestinal mucosa are the main factors responsible for the massive neutrophil influx triggered during serotype Typhimurium infection in humans (Fig. 1). Since serotype Typhi does not cause a massive neutrophil influx in the intestine, it seems surprising that this pathogen can invade epithelial cells using its T3SS-1 (1, 23, 93), survive in macrophages using its T3SS-2 (55, 76), and express PAMPs (i.e., flagella and LPS) that, when purified, are potent inducers of proinflammatory cytokine secretion in human monocytes (5, 122, 123). Whole-genome sequencing shows that sopA (in strains CT18 and Ty2) and sopE2 (in strain CT18) are pseudogenes in serotype Typhi (17, 86), and it has been speculated that these mutations may explain the low propensity of serotype Typhi to cause diarrhea (63). However, a more recent analysis shows that introduction of the remaining T3SS-1 effectors of serotype Typhi (i.e., SipA, SopB, and SopD) can complement a serotype Typhimurium sipAsopABDE2 mutant for eliciting neutrophil influx and fluid accumulation in bovine ligated ileal loops (93). Thus, serotype Typhi appears to possess all the factors (T3SS-1, T3SS-2, and PAMPs) that make serotype Typhimurium into a pathogen causing inflammatory diarrhea. It is therefore puzzling that invasion of the human intestinal mucosa by serotype Typhi does not trigger a stereotypic host response resulting in neutrophil infiltration.
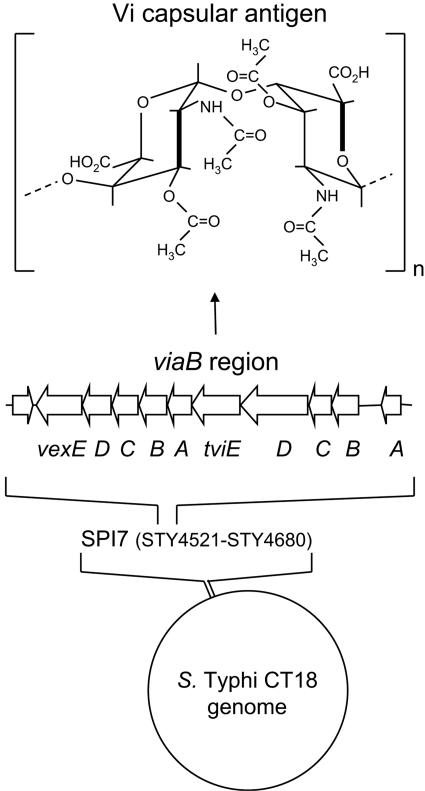
Human colonic epithelial cell lines can be used to model early interactions of serotype Typhi with the intestinal mucosa, while macrophage-like cell lines can be used to model interactions that occur subsequently in the lamina propria. Microarray analysis shows that unlike serotype Typhimurium, serotype Typhi does not trigger a classical proinflammatory gene expression program in epithelial cells (124). Serotype Typhimurium can trigger the migration of neutrophils across a monolayer of polarized colonic epithelial cells, but serotype Typhi is not able to elicit this response (64). Furthermore, stimulation of macrophage-like cells with serotype Typhi results in markedly reduced IL-8 production compared to stimulation with serotype Typhimurium (92). These in vitro observations raise the possibility that unlike serotype Typhimurium, serotype Typhi expresses a virulence factor that allows it to down-regulate a TLR-mediated host response in the intestinal mucosa that leads to neutrophil infiltration. This virulence factor has recently been identified as the Vi capsular antigen (92), a linear polymer of α-1,4 (2-deoxy)-2-N-acetylgalacturonic acid variously O acetylated at the C-3 position (44) (Fig. 2).
FIG. 2.
Vi capsular antigen of serotype Typhi. Whole-genome sequencing of serotype Typhi strain CT18 reveals the presence of a 135-kb DNA region, termed SPI7, that is absent from the serotype Typhimurium genome (bottom). A DNA region within SPI7, termed the viaB locus, encodes proteins for the biosynthesis and the export of the Vi capsular antigen (middle). The Vi antigen is a linear polymer of α-1,4 (2-deoxy)-2-N-acetyl 3-acetyl-galacturonic acid whose predicted structure (44) is shown at the top.
Genes required for the biosynthesis and the export of the Vi capsular antigen are encoded by the viaB locus, which is located on a 134-kb serotype Typhi DNA region, termed SPI7, that is absent from the serotype Typhimurium chromosome (86) (Fig. 2). The Vi capsular polysaccharide is expressed during human infection, as indicated by protection against typhoid fever following vaccination with the Vi antigen (56). A number of studies suggest that serotype Typhi isolates from blood of patients invariably express the Vi antigen (reviewed in reference 96). However, SPI7 is genetically unstable and can be lost upon laboratory passage of serotype Typhi (6, 79). Furthermore, the amount of Vi antigen produced by serotype Typhi can decrease following multiple subcultures on agar (26). An increase in the isolation of Vi agglutination-negative serotype Typhi has recently been reported from India (69, 98). In one of these studies, Vi-negative serotype Typhi isolates were identified by a purely agglutination-based approach (69). The second study identified 74 Vi-negative serotype Typhi isolates by slide agglutination, but only two of these were viaB negative as determined by PCR (98). A more recent analysis from Pakistan shows that 12 of 2,222 human serotype Typhi isolates were Vi negative by slide agglutination. However, 11 of these 12 Vi agglutination-negative isolates were Vi positive when analyzed by immunofluorescence microscopy (118). Thus, identification of Vi-negative serotype Typhi by purely agglutination-based approaches should be viewed with caution. Given the instability upon laboratory passage of Vi antigen biosynthesis, the above epidemiological observations suggest a remarkable conservation of capsule expression among primary human serotype Typhi isolates.
In vivo studies exploring the selective forces responsible for maintaining the ability to produce the Vi antigen among human serotype Typhi isolates are sparse. In their original description, Felix and Pitt introduced the term Vi (virulence) antigen, because noncapsulated serotype Typhi strains were attenuated in a mouse model (27). Loss of the Vi antigen results in an approximately 10,000-fold reduction in the virulence of serotype Typhi after intraperitoneal infection of mucin-treated mice (47). Since the mucin-treated mouse model is not a close mimic of typhoid fever, volunteer studies have been essential for establishing the role of the Vi antigen during pathogenesis. After infection with approximately 107 organisms, disease rates are significantly higher in volunteers infected with capsulated serotype Typhi strains (with a disease rate of 51%; 80% of the volunteers were blood culture positive) than in those infected with passaged derivatives lacking the Vi antigen (with a disease rate of 26%; 40% were blood culture positive). The same study shows that the fraction of volunteers developing disease after infection with approximately 105 organisms of a capsulated serotype Typhi strain (disease rate, 28%) is similar to the fraction of volunteers developing disease when they were infected with a 100-fold-higher dose of a noncapsulated serotype Typhi strain (disease rate, 26%) (48). Although noncapsulated serotype Typhi strains can still cause typhoid fever, these in vivo data suggest that the loss of the Vi antigen results in considerable attenuation.
While serotype Typhi isolates from human blood are typically capsulated, some isolates from stool samples do not express the Vi antigen (14). These data seem to be consistent with the idea that capsule expression is not required in the intestinal lumen. However, the interpretation of these results is complicated by the finding that capsule-specific bacteriophages were isolated from stools in many of these cases, raising the possibility that phages may have selected for Vi-negative bacteria during isolation (14). More-recent in vitro studies show that under conditions of high osmolarity, such as those encountered in the intestinal lumen, capsule expression is switched off (87), while flagella and T3SS-1 genes are expressed (2). These data suggest that the Vi antigen may not be expressed in the intestinal lumen, thereby enhancing the ability of serotype Typhi to invade the intestinal epithelium. With decreased osmolarity, which is encountered in tissue or blood, the two-component regulatory systems RcsBC and OmpR EnvZ activate Vi antigen expression, while the expression of flagella and T3SS-1 genes is reduced (2, 87, 116). Thus, production of the Vi antigen may be induced while bacteria pass through the intestinal epithelium or subsequently in intestinal tissue when bacteria are engulfed by host phagocytes. Consistent with this idea, expression of the viaB genes is induced during serotype Typhi infection of human macrophages in vitro (15). These expression data hint towards a possible role of the Vi antigen during the interaction of serotype Typhi with components of human tissue or blood. One Vi-mediated mechanism for survival in tissue or blood was suggested by the finding that expression of a capsule enables serotype Typhi to resist phagocytosis in vitro (25, 59). It remains unclear how preventing phagocytosis would benefit serotype Typhi because its main virulence strategy is thought to be survival within macrophages. Serotype Typhi likely has a short extracellular phase when it infects new macrophages in tissue, and the Vi antigen may enhance bacterial survival during this extracellular phase because capsulated bacteria exhibit increased serum resistance (42, 59). However, the in vivo relevance of these virulence mechanisms remains speculative.
Recent experiments using tissue culture models and human colonic tissue explants suggest a new function for the Vi antigen: the evasion of innate immune recognition in the intestinal mucosa. The Vi antigen reduces IL-8 production elicited by serotype Typhi in human colonic epithelial cell lines (92, 107). Furthermore, noncapsulated serotype Typhi causes more IL-8 and more tumor necrosis factor alpha (TNF-α) expression in human macrophage-like cells than do capsulated bacteria (46, 92). Capsulated bacteria appear to elicit decreased IL-8 production in vitro because the Vi antigen interferes with the stimulation of TLR5 and TLR4/MD2/CD14 by flagella and LPS, respectively (46, 92). The capsule may prevent inflammation by reducing bacterial adhesion to and invasion of the intestinal epithelium (2, 72). However, this explanation requires that the Vi antigen would be produced in the intestinal lumen, an assumption not currently supported by studies of capsule expression. The notion that the capsule may inhibit bacterial invasion in vivo is also at odds with the highly invasive character of typhoid fever. For example, during challenge of chimpanzees, serotype Typhi readily penetrates the gut epithelium without causing appreciable inflammation (30). This observation seems to be consistent with bacterial invasion followed by inhibition of TLR stimulation, but it does not support the view that bacterial invasion is inhibited in vivo. An alternative mechanism by which the Vi antigen may inhibit inflammation is by physically masking PAMPs and/or by acting as a physical barrier to their release, thereby interfering with TLR stimulation. The finding that the Vi antigen blocks the agglutination of serotype Typhi with anti-LPS serum (25, 27) and inhibits Saccharomyces cerevisiae agglutination mediated by type 1 fimbriae (72) supports the idea that the capsule can physically mask surface structures. Finally, a third possible mechanism by which the capsule may inhibit inflammation is suggested by the finding that purified Vi antigen binds to prohibitin at the surfaces of intestinal epithelial cells and reduces extracellular signal-regulated kinase phosphorylation and IL-8 production in response to phorbol 12-myristate 13-acetate stimulation (91, 107). These data raise the possibility that the Vi antigen may exhibit properties of an anti-inflammatory drug.
Experiments with human colonic tissue explants have recently provided an important link between in vitro data suggesting that the Vi antigen reduces TLR signaling (46, 92, 107) and the clinical observation that neutrophils are scarce in intestinal infiltrates of typhoid fever patients (41, 57, 77, 83, 110). Analysis of the host response elicited during infection of human colonic explants reveals that unlike the capsulated serotype Typhi wild type, which does not elicit IL-8 production, a noncapsulated serotype Typhi mutant elicits IL-8 expression at levels similar to those seen during serotype Typhimurium infection (92). Thus, a capsule-mediated suppression of IL-8 production can be observed in a model that closely resembles host pathogen interaction in vivo. Collectively, these data suggest that expression of the Vi antigen allows serotype Typhi to evade a TLR-mediated host response triggering neutrophil infiltration in the intestinal mucosa.
Given the role of the Vi antigen in preventing neutrophil infiltration, it may seem surprising that volunteers ingesting a serotype Typhi galE viaB vaccine candidate do not develop inflammatory diarrhea (47). A likely explanation for this finding is that a strain carrying a mutation in galE, a gene encoding the enzyme uridine-5′-diphosphate galactose epimerase, required for the biosynthesis of the LPS outer core, is not able to elicit neutrophil infiltration. The finding that a serotype Typhimurium galE mutant is able neither to elicit neutrophil influx in bovine ligated ileal loops nor to cause diarrhea after oral infection of calves provides compelling experimental support for this idea (11, 12).
In addition to serotype Typhi, the viaB locus is also present in S. enterica serotype Paratyphi C (a typhoidal Salmonella serotype) and some isolates of S. enterica serotype Dublin (a nontyphoidal Salmonella serotype). Serotype Dublin does not cause gastroenteritis in humans but is associated with a bacteremic disease, with high fever and few intestinal manifestations (24, 112). These observations show that the viaB locus is consistently absent from Salmonella serotypes that are associated with human gastroenteritis. The importance of the Vi antigen has been called into question because the genomes of S. enterica serotypes Paratyphi A and Paratyphi B do not contain the viaB locus, although both pathogens can cause typhoid fever in humans (63). However, the expectation that all typhoidal Salmonella serotypes share genetic determinants that distinguish them from Salmonella serotypes causing gastroenteritis has proven to be unrealistic. A recent analysis of S. enterica subspecies I serotypes using a serotype Typhimurium microarray supplemented with open reading frames from serotype Typhi did not identify any genes that are conserved among typhoidal Salmonella serotypes but that are absent from nontyphoidal Salmonella serotypes (90). Thus, neither the genes in the viaB region nor any other virulence genes are conserved among typhoidal Salmonella serotypes but are absent from Salmonella serotypes causing gastroenteritis in humans. Phylogenetic analysis by multilocus enzyme electrophoresis shows that the ability to cause typhoid fever evolved independently in four distinct S. enterica lineages, represented by serotypes Typhi, Paratyphi A, Paratyphi B, and Paratyphi C (105). Since typhoidal Salmonella serotypes do not share any unique virulence factors, it seems likely that pathogens in each lineage developed the ability to cause typhoid fever by incorporating different genetic material. This process included acquisition of the viaB region by serotypes Typhi and Paratyphi C (but not by other typhoidal Salmonella serotypes). A possible conclusion from these evolutionary considerations is that serotypes Paratyphi A and Paratyphi B cause typhoid fever by somewhat different mechanisms than serotypes Typhi and Paratyphi C.
FUTURE DIRECTIONS FOR RESEARCH
Recent findings on the role of the Vi antigen in evading innate immunity not only explain why neutrophils are scarce in intestinal infiltrates of typhoid fever patients but also shed a new light on several other clinical observations. For example, a polymorphism in the TLR5 gene that introduces a premature stop codon has no measurable effect on clinical parameters associated with typhoid fever, including fever clearance time, pathogen burden, disease severity, and the age at acquisition of disease (21). The redundancy of the innate immune system may account for this observation. Alternatively, it is possible that no essential role for TLR5 during typhoid fever is detectable because expression of the Vi antigen blocks TLR5 signaling in patients.
Serum levels of pyrogenic cytokines, such as TNF-α and IL-1β, are elevated in typhoid fever patients compared to levels in healthy individuals (8, 54) but are low compared to cytokine levels measured in the sera of patients with sepsis (35, 117, 120). During gram-negative sepsis, patients with high concentrations of bacteria in their blood have a worse prognosis than patients with lower concentrations of bacteria (9, 18, 22). In contrast, quantitative blood cultures from typhoid fever patients show no significant association between the concentration of bacteria in blood and the severity of symptoms (7). The capsule-mediated suppression of TNF-α production in human monocytes (46) may help explain why typhoid fever patients have relatively low serum concentrations of pyrogenic cytokines and do not develop septic shock.
Patients with mutations in genes that encode components of the IL-12/IL-23/gamma interferon (IFN-γ) axes show increased susceptibility to infections with nontyphoidal Salmonella serotypes but not to infections with serotype Typhi (58, 60). The IL-12/IL-23/IFN-γ axis is a major immunoregulatory system that bridges innate and adaptive immunity. Upon TLR activation, macrophages and dendritic cells produce IL-12 and IL-23 (104). These cytokines in turn stimulate T cells and natural killer cells to produce IFN-γ and promote a Th1 response (Fig. 1). IL-12 is elevated in sera from patients with gastroenteritis caused by nontyphoidal Salmonella serotypes (73). Furthermore, the production of the Th1-inducing cytokines IL-12 and IFN-γ is important for the control of serotype Typhimurium infection in mice (3, 62, 82). It is tempting to speculate that patients with a defective IL-12/IL-23/IFN-γ axis are not more susceptible to serotype Typhi because the Vi antigen prevents TLR activation in both genetically normal and in IL-12/IL-23/IFN-γ axis-deficient patients. In other words, unlike nontyphoidal Salmonella serotypes, serotype Typhi may be able to evade adaptive immunity by reducing TLR signaling using its capsule. A proof of principle for this mechanism of immune evasion is provided by the finding that impaired TLR signaling due to the absence of the adaptor MyD88 causes a defective Th1 response against herpes simplex virus 2 in mice (103). It remains unclear whether IL-12 levels are elevated during typhoid fever, because a recent study on this subject did not distinguish between typhoid fever patients and patients with systemic disease caused by nontyphoidal Salmonella serotypes (73).
Recent epidemiological observations made among patients with AIDS in Africa seem to support the concept that serotype Typhi is able to evade adaptive immunity. In healthy individuals, nontyphoidal Salmonella serotypes are unable to overcome defense mechanisms that limit bacterial dissemination from the intestinal mucosa to systemic sites of infection. As a result, bacteremia is a rare complication during nontyphoidal salmonellosis but may occur in patients with impaired immunity (19, 61). The increasing prevalence of patients with AIDS has made nontyphoidal salmonellosis a leading cause of bacteremia in sub-Saharan Africa (38, 51, 52, 75, 80). While the frequency of bacteremia caused by nontyphoidal Salmonella serotypes is considerably higher among human immunodeficiency virus-positive patients than among human immunodeficiency virus-negative patients in Africa, the frequencies of typhoid fever do not differ between these patient groups (88, 106). These epidemiological observations suggest that systemic spread of nontyphoidal Salmonella serotypes is normally prevented by adaptive immune responses that are defective in AIDS patients. In contrast, serotype Typhi is able to overcome adaptive immune mechanisms that prevent systemic spread and can thus cause typhoid fever in both healthy individuals and AIDS patients with similar efficiencies. This hypothesis implies that unlike nontyphoidal Salmonella serotypes, serotype Typhi possesses a unique virulence factor that allows it to evade an aspect of adaptive immunity in humans. The Vi capsular antigen is an attractive candidate for such a virulence determinant.
Acknowledgments
Work in A.J.B.'s laboratory is supported by USDA/NRICGP grant 2002-35204-12247 and Public Health Service grants AI040124, AI044170, and AI065534.
Editor: J. B. Kaper
REFERENCES
- 1.Altmeyer, R. M., J. K. McNern, J. C. Bossio, I. Rosenshine, B. B. Finlay, and J. E. Galán. 1993. Cloning and molecular characterization of a gene involved in Salmonella adherence and invasion of cultured epithelial cells. Mol. Microbiol. 7:89-98. [DOI] [PubMed] [Google Scholar]
- 2.Arricau, N., D. Hermant, H. Waxin, C. Ecobichon, P. S. Duffey, and M. Y. Popoff. 1998. The RcsB-RcsC regulatory system of Salmonella typhi differentially modulates the expression of invasion proteins, flagellin and Vi antigen in response to osmolarity. Mol. Microbiol. 29:835-850. [DOI] [PubMed] [Google Scholar]
- 3.Bao, S., K. W. Beagley, M. P. France, J. Shen, and A. J. Husband. 2000. Interferon-gamma plays a critical role in intestinal immunity against Salmonella typhimurium infection. Immunology 99:464-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergman, M. A., L. A. Cummings, S. L. Barrett, K. D. Smith, J. C. Lara, A. Aderem, and B. T. Cookson. 2005. CD4+ T cells and toll-like receptors recognize Salmonella antigens expressed in bacterial surface organelles. Infect. Immun. 73:1350-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bignold, L. P., S. D. Rogers, T. M. Siaw, and J. Bahnisch. 1991. Inhibition of chemotaxis of neutrophil leukocytes to interleukin-8 by endotoxins of various bacteria. Infect. Immun. 59:4255-4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bueno, S. M., C. A. Santiviago, A. A. Murillo, J. A. Fuentes, A. N. Trombert, P. I. Rodas, P. Youderian, and G. C. Mora. 2004. Precise excision of the large pathogenicity island, SPI7, in Salmonella enterica serovar Typhi. J. Bacteriol. 186:3202-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler, T., W. R. Bell, J. Levin, N. N. Linh, and K. Arnold. 1978. Typhoid fever. Studies of blood coagulation, bacteremia, and endotoxemia. Arch. Intern. Med. 138:407-410. [DOI] [PubMed] [Google Scholar]
- 8.Butler, T., M. Ho, G. Acharya, M. Tiwari, and H. Gallati. 1993. Interleukin-6, gamma interferon, and tumor necrosis factor receptors in typhoid fever related to outcome of antimicrobial therapy. Antimicrob. Agents Chemother. 37:2418-2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butler, T., J. Levin, N. N. Linh, D. M. Chau, M. Adickman, and K. Arnold. 1976. Yersinia pestis infection in Vietnam. II. Quantitative blood cultures and detection of endotoxin in the cerebrospinal fluid of patients with meningitis. J. Infect. Dis. 133:493-499. [DOI] [PubMed] [Google Scholar]
- 10.Cheminay, C., A. Mohlenbrink, and M. Hensel. 2005. Intracellular Salmonella inhibit antigen presentation by dendritic cells. J. Immunol. 174:2892-2899. [DOI] [PubMed] [Google Scholar]
- 11.Clarke, R. C., and C. L. Gyles. 1986. Galactose epimerase mutants of Salmonella typhimurium as live vaccines for calves. Can. J. Vet. Res. 50:165-173. [PMC free article] [PubMed] [Google Scholar]
- 12.Clarke, R. C., and C. L. Gyles. 1987. Virulence of wild and mutant strains of Salmonella typhimurium in ligated intestinal segments of calves, pigs, and rabbits. Am. J. Vet. Res. 48:504-510. (Erratum, 48: 879.) [PubMed] [Google Scholar]
- 13.Coburn, B., Y. Li, D. Owen, B. A. Vallence, and B. B. Finlay. 2005. Salmonella enterica serovar Typhimurium pathogenicity island 2 is necessary for complete virulence in a mouse model of infectious enterocolitis. Infect. Immun. 73:3219-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craigie, J., and K. F. Brandon. 1936. The identification of the V and W forms of B. typhosus and the occurrence of the V form in cases of typhoid fever and carriers. J. Pathol. Bacteriol. 43:249-260. [Google Scholar]
- 15.Daigle, F., J. E. Graham, and R. Curtiss III. 2001. Identification of Salmonella typhi genes expressed within macrophages by selective capture of transcribed sequences (SCOTS). Mol. Microbiol. 41:1211-1222. [DOI] [PubMed] [Google Scholar]
- 16.Day, D. W., B. K. Mandal, and B. C. Morson. 1978. The rectal biopsy appearances in Salmonella colitis. Histopathology 2:117-131. [DOI] [PubMed] [Google Scholar]
- 17.Deng, W., S. R. Liou, G. Plunkett III, G. F. Mayhew, D. J. Rose, V. Burland, V. Kodoyianni, D. C. Schwartz, and F. R. Blattner. 2003. Comparative genomics of Salmonella enterica serovar Typhi strains Ty2 and CT18. J. Bacteriol. 185:2330-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dietzman, D. E., G. W. Fischer, and F. D. Schoenknecht. 1974. Neonatal Escherichia coli septicemia—bacterial counts in blood. J. Pediatr. 85:128-130. [DOI] [PubMed] [Google Scholar]
- 19.Diez Dorado, R., A. Tagarro Garcia, F. Baquero-Artigao, M. J. Garcia-Miguel, M. J. Uria Gonzalez, P. Pena Garcia, and F. del Castillo Martin. 2004. Non-typhi Salmonella bacteremia in children: an 11-year review. An. Pediatr. (Barcelona) 60:344-348. (In Spanish.) [DOI] [PubMed] [Google Scholar]
- 20.Dorsey, C. W., M. Raffatellu, R. A. Kingsley, and A. J. Bäumler. 2005. Mechanisms of Salmonella enterica serotype Typhimurium intestinal colonization, p. 302-312. In J. Natarro, P. S. Cohen, H. L. Mobley, and J. N. Weiser (ed.), Colonization of mucosal surfaces. ASM Press, Washington, D.C.
- 21.Dunstan, S. J., T. R. Hawn, N. T. Hue, C. P. Parry, V. A. Ho, H. Vinh, T. S. Diep, D. House, J. Wain, A. Aderem, T. T. Hien, and J. J. Farrar. 2005. Host susceptibility and clinical outcomes in toll-like receptor 5-deficient patients with typhoid fever in Vietnam. J. Infect. Dis. 191:1068-1071. [DOI] [PubMed] [Google Scholar]
- 22.DuPont, H. L., and W. W. Spink. 1969. Infections due to gram-negative organisms: an analysis of 860 patients with bacteremia at the University of Minnesota Medical Center, 1958-1966. Medicine (Baltimore) 48:307-332. [DOI] [PubMed] [Google Scholar]
- 23.Elsinghorst, E. A., L. S. Baron, and D. J. Kopecko. 1989. Penetration of human intestinal epithelial cells by Salmonella: molecular cloning and expression of Salmonella typhi invasion determinants in Escherichia coli. Proc. Natl. Acad. Sci. USA 86:5173-5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fang, F. C., and J. Fierer. 1991. Human infection with Salmonella dublin. Medicine (Baltimore) 70:198-207. [DOI] [PubMed] [Google Scholar]
- 25.Felix, A., S. S. Bhatnagar, and R. M. Pitt. 1934. Observations on the properties of the Vi antigen of B. typhosus. Br. J. Exp. Pathol. 15:346-354. [Google Scholar]
- 26.Felix, A., K. S. Krikorian, and R. Reitler. 1935. The occurrence of typhoid bacilli containing Vi antigens in cases of typhoid fever and of Vi antibody in their sera. J. Hyg. (London) 35:421-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Felix, A., and R. M. Pitt. 1934. A new antigen of B. typhosus. Lancet 227:186-191. [Google Scholar]
- 28.Frances, C. L., T. A. Ryan, B. D. Jones, S. J. Smith, and S. Falkow. 1993. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature 364:639-642. [DOI] [PubMed] [Google Scholar]
- 29.Frost, A. J., A. P. Bland, and T. S. Wallis. 1997. The early dynamic response of the calf ileal epithelium to Salmonella typhimurium. Vet. Pathol. 34:369-386. [DOI] [PubMed] [Google Scholar]
- 30.Gaines, S., H. Sprinz, J. G. Tully, and W. D. Tigertt. 1968. Studies on infection and immunity in experimental typhoid fever. VII. The distribution of Salmonella typhi in chimpanzee tissue following oral challenge, and the relationship between the numbers of bacilli and morphologic lesions. J. Infect. Dis. 118:293-306. [DOI] [PubMed] [Google Scholar]
- 31.Galán, J. E., and R. Curtiss III. 1989. Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc. Natl. Acad. Sci. USA 86:6383-6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galyov, E. E., M. W. Wood, R. Rosqvist, P. B. Mullan, P. R. Watson, S. Hedges, and T. S. Wallis. 1997. A secreted effector protein of Salmonella dublin is translocated into eukaryotic cells and mediates inflammation and fluid secretion in infected ileal mucosa. Mol. Microbiol. 25:903-912. [DOI] [PubMed] [Google Scholar]
- 33.Garcia-delPortillo, F., and B. B. Finlay. 1994. Salmonella invasion of nonphagocytic cells induces formation of macropinosomes in the host cell. Infect. Immun. 62:4641-4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gewirtz, A. T., T. A. Navas, S. Lyons, P. J. Godowski, and J. L. Madara. 2001. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 167:1882-1885. [DOI] [PubMed] [Google Scholar]
- 35.Girardin, E., G. E. Grau, J. M. Dayer, P. Roux-Lombard, and P. H. Lambert. 1988. Tumor necrosis factor and interleukin-1 in the serum of children with severe infectious purpura. N. Engl. J. Med. 319:397-400. [DOI] [PubMed] [Google Scholar]
- 36.Girardin, S. E., I. G. Boneca, L. A. Carneiro, A. Antignac, M. Jehanno, J. Viala, K. Tedin, M. K. Taha, A. Labigne, U. Zahringer, A. J. Coyle, P. S. DiStefano, J. Bertin, P. J. Sansonetti, and D. J. Philpott. 2003. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science 300:1584-1587. [DOI] [PubMed] [Google Scholar]
- 37.Girardin, S. E., R. Tournebize, M. Mavris, A. L. Page, X. Li, G. R. Stark, J. Bertin, P. S. DiStefano, M. Yaniv, P. J. Sansonetti, and D. J. Philpott. 2001. CARD4/Nod1 mediates NF-kappaB and JNK activation by invasive Shigella flexneri. EMBO Rep. 2:736-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gordon, M. A., A. L. Walsh, M. Chaponda, D. Soko, M. Mbvwinji, M. E. Molyneux, and S. B. Gordon. 2001. Bacteraemia and mortality among adult medical admissions in Malawi—predominance of non-typhi salmonellae and Streptococcus pneumoniae. J. Infect. 42:44-49. [DOI] [PubMed] [Google Scholar]
- 39.Hapfelmeier, S., B. Stecher, M. Barthel, M. Kremer, A. J. Muller, M. Heikenwalder, T. Stallmach, M. Hensel, K. Pfeffer, S. Akira, and W. D. Hardt. 2005. The Salmonella pathogenicity island (SPI)-2 and SPI-1 type III secretion systems allow Salmonella serovar typhimurium to trigger colitis via MyD88-dependent and MyD88-independent mechanisms. J. Immunol. 174:1675-1685. [DOI] [PubMed] [Google Scholar]
- 40.Hardt, W. D., L. M. Chen, K. E. Schuebel, X. R. Bustelo, and J. E. Galan. 1998. S. typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell 93:815-826. [DOI] [PubMed] [Google Scholar]
- 41.Harris, J. C., H. L. Dupont, and R. B. Hornick. 1972. Fecal leukocytes in diarrheal illness. Ann. Intern. Med. 76:697-703. [DOI] [PubMed] [Google Scholar]
- 42.Hashimoto, Y., N. Li, H. Yokoyama, and T. Ezaki. 1993. Complete nucleotide sequence and molecular characterization of ViaB region encoding Vi antigen in Salmonella typhi. J. Bacteriol. 175:4456-4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:1099-1103. [DOI] [PubMed] [Google Scholar]
- 44.Heyns, K., and G. Kiessling. 1967. Strukturaufklarung des Vi-antigens aus Citrobacter freundii (E. coli) 5396/38. Carbohydr. Res. 3:340-353. [Google Scholar]
- 45.Higgs, H. N., and T. D. Pollard. 2001. Regulation of actin filament network formation through ARP2/3 complex: activation by a diverse array of proteins. Annu. Rev. Biochem. 70:649-676. [DOI] [PubMed] [Google Scholar]
- 46.Hirose, K., T. Ezaki, M. Miyake, T. Li, A. Q. Khan, Y. Kawamura, H. Yokoyama, and T. Takami. 1997. Survival of Vi-capsulated and Vi-deleted Salmonella typhi strains in cultured macrophage expressing different levels of CD14 antigen. FEMS Microbiol. Lett. 147:259-265. [DOI] [PubMed] [Google Scholar]
- 47.Hone, D. M., S. R. Attridge, B. Forrest, R. Morona, D. Daniels, J. T. LaBrooy, R. C. Bartholomeusz, D. J. Shearman, and J. Hackett. 1988. A galE via (Vi antigen-negative) mutant of Salmonella typhi Ty2 retains virulence in humans. Infect. Immun. 56:1326-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hornick, R. B., S. E. Greisman, T. E. Woodward, H. L. DuPont, A. T. Dawkins, and M. J. Snyder. 1970. Typhoid fever: pathogenesis and immunologic control (First of two parts). N. Engl. J. Med. 283:686-691. [DOI] [PubMed] [Google Scholar]
- 49.Iwasaki, A., and R. Medzhitov. 2004. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5:987-995. [DOI] [PubMed] [Google Scholar]
- 50.Jones, M. A., M. W. Wood, P. B. Mullan, P. R. Watson, T. S. Wallis, and E. E. Galyov. 1998. Secreted effector proteins of Salmonella dublin act in concert to induce enteritis. Infect. Immun. 66:5799-5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kankwatira, A. M., G. A. Mwafulirwa, and M. A. Gordon. 2004. Non-typhoidal salmonella bacteraemia—an under-recognized feature of AIDS in African adults. Trop. Doct. 34:198-200. [DOI] [PubMed] [Google Scholar]
- 52.Kassa-Kelembho, E., C. D. Mbolidi, Y. B. Service, J. Morvan, and P. Minssart. 2003. Bacteremia in adults admitted to the Department of Medicine of Bangui Community Hospital (Central African Republic). Acta Trop. 89:67-72. [DOI] [PubMed] [Google Scholar]
- 53.Kawasaki, K., R. K. Ernst, and S. I. Miller. 2005. Purification and characterization of deacylated and/or palmitoylated lipid A species unique to Salmonella enterica serovar Typhimurium. J. Endotoxin Res. 11:57-61. [DOI] [PubMed] [Google Scholar]
- 54.Keuter, M., E. Dharmana, M. H. Gasem, J. van der Ven-Jongekrijg, R. Djokomoeljanto, W. M. Dolmans, P. Demacker, R. Sauerwein, H. Gallati, and J. W. van der Meer. 1994. Patterns of proinflammatory cytokines and inhibitors during typhoid fever. J. Infect. Dis. 169:1306-1311. [DOI] [PubMed] [Google Scholar]
- 55.Khan, S. A., R. Stratford, T. Wu, N. McKelvie, T. Bellaby, Z. Hindle, K. A. Sinha, S. Eltze, P. Mastroeni, D. Pickard, G. Dougan, S. N. Chatfield, and F. R. Brennan. 2003. Salmonella typhi and S typhimurium derivatives harbouring deletions in aromatic biosynthesis and Salmonella pathogenicity island-2 (SPI-2) genes as vaccines and vectors. Vaccine 21:538-548. [DOI] [PubMed] [Google Scholar]
- 56.Klugman, K. P., I. T. Gilbertson, H. J. Koornhof, J. B. Robbins, R. Schneerson, D. Schulz, M. Cadoz, and J. Armand. 1987. Protective activity of Vi capsular polysaccharide vaccine against typhoid fever. Lancet ii:1165-1169. [DOI] [PubMed] [Google Scholar]
- 57.Kraus, M. D., B. Amatya, and Y. Kimula. 1999. Histopathology of typhoid enteritis: morphologic and immunophenotypic findings. Mod. Pathol. 12:949-955. [PubMed] [Google Scholar]
- 58.Lammas, D. A., E. De Heer, J. D. Edgar, V. Novelli, A. Ben-Smith, R. Baretto, P. Drysdale, J. Binch, C. MacLennan, D. S. Kumararatne, S. Panchalingam, T. H. Ottenhoff, J. L. Casanova, and J. F. Emile. 2002. Heterogeneity in the granulomatous response to mycobacterial infection in patients with defined genetic mutations in the interleukin 12-dependent interferon-gamma production pathway. Int. J. Exp. Pathol. 83:1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Looney, R. J., and R. T. Steigbigel. 1986. Role of the Vi antigen of Salmonella typhi in resistance to host defense in vitro. J. Lab. Clin. Med. 108:506-516. [PubMed] [Google Scholar]
- 60.MacLennan, C., C. Fieschi, D. A. Lammas, C. Picard, S. E. Dorman, O. Sanal, J. M. MacLennan, S. M. Holland, T. H. Ottenhoff, J. L. Casanova, and D. S. Kumararatne. 2004. Interleukin (IL)-12 and IL-23 are key cytokines for immunity against Salmonella in humans. J. Infect. Dis. 190:1755-1757. [DOI] [PubMed] [Google Scholar]
- 61.Mandal, B. K., and J. Brennand. 1988. Bacteraemia in salmonellosis: a 15 year retrospective study from a regional infectious diseases unit. BMJ 297:1242-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mastroeni, P., J. A. Harrison, J. A. Chabalgoity, and C. E. Hormaeche. 1996. Effect of interleukin 12 neutralization on host resistance and gamma interferon production in mouse typhoid. Infect. Immun. 64:189-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McClelland, M., K. E. Sanderson, S. W. Clifton, P. Latreille, S. Porwollik, A. Sabo, R. Meyer, T. Bieri, P. Ozersky, M. McLellan, C. R. Harkins, C. Wang, C. Nguyen, A. Berghoff, G. Elliott, S. Kohlberg, C. Strong, F. Du, J. Carter, C. Kremizki, D. Layman, S. Leonard, H. Sun, L. Fulton, W. Nash, T. Miner, P. Minx, K. Delehaunty, C. Fronick, V. Magrini, M. Nhan, W. Warren, L. Florea, J. Spieth, and R. K. Wilson. 2004. Comparison of genome degradation in Paratyphi A and Typhi, human-restricted serovars of Salmonella enterica that cause typhoid. Nat. Genet. 36:1268-1274. [DOI] [PubMed] [Google Scholar]
- 64.McCormick, B. A., S. I. Miller, D. Carnes, and J. L. Madara. 1995. Transepithelial signaling to neutrophils by salmonellae: a novel virulence mechanism for gastroenteritis. Infect. Immun. 63:2302-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McGhie, E. J., R. D. Hayward, and V. Koronakis. 2004. Control of actin turnover by a salmonella invasion protein. Mol. Cell 13:497-510. [DOI] [PubMed] [Google Scholar]
- 66.McGovern, V. J., and L. J. Slavutin. 1979. Pathology of salmonella colitis. Am. J. Surg. Pathol. 3:483-490. [DOI] [PubMed] [Google Scholar]
- 67.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Medzhitov, R., P. Preston-Hurlburt, and C. A. Janeway, Jr. 1997. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388:394-397. [DOI] [PubMed] [Google Scholar]
- 69.Mehta, G., and S. C. Arya. 2002. Capsular Vi polysaccharide antigen in Salmonella enterica serovar Typhi isolates. J. Clin. Microbiol. 40:1127-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mills, D. M., V. Bajaj, and C. A. Lee. 1995. A 40kb chromosomal fragment encoding Salmonella typhimurium invasion genes is absent from the corresponding region of the Escherichia coli K-12 chromosome. Mol. Microbiol. 15:749-759. [DOI] [PubMed] [Google Scholar]
- 71.Mirold, S., K. Ehrbar, A. Weissmuller, R. Prager, H. Tschape, H. Russmann, and W. D. Hardt. 2001. Salmonella host cell invasion emerged by acquisition of a mosaic of separate genetic elements, including Salmonella pathogenicity island 1 (SPI1), SPI5, and sopE2. J. Bacteriol. 183:2348-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miyake, M., L. Zhao, T. Ezaki, K. Hirose, A. Q. Khan, Y. Kawamura, R. Shima, M. Kamijo, T. Masuzawa, and Y. Yanagihara. 1998. Vi-deficient and nonfimbriated mutants of Salmonella typhi agglutinate human blood type antigens and are hyperinvasive. FEMS Microbiol. Lett. 161:75-82. [DOI] [PubMed] [Google Scholar]
- 73.Mizuno, Y., H. Takada, A. Nomura, C. H. Jin, H. Hattori, K. Ihara, T. Aoki, K. Eguchi, and T. Hara. 2003. Th1 and Th1-inducing cytokines in Salmonella infection. Clin. Exp. Immunol. 131:111-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mogilner, A., and G. Oster. 1996. Cell motility driven by actin polymerization. Biophys. J. 71:3030-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Molyneux, E. 2004. Bacterial infections in children with HIV/AIDS. Trop. Doct. 34:195-198. [DOI] [PubMed] [Google Scholar]
- 76.Monack, D. M., C. S. Detweiler, and S. Falkow. 2001. Salmonella pathogenicity island 2-dependent macrophage death is mediated in part by the host cysteine protease caspase-1. Cell. Microbiol. 3:825-837. [DOI] [PubMed] [Google Scholar]
- 77.Mukawi, T. J. 1978. Histopathological study of typhoid perforation of the small intestines. Southeast Asian J. Trop. Med. Public Health 9:252-255. [PubMed] [Google Scholar]
- 78.Mullins, R. D., J. A. Heuser, and T. D. Pollard. 1998. The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc. Natl. Acad. Sci. USA 95:6181-6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nair, S., S. Alokam, S. Kothapalli, S. Porwollik, E. Proctor, C. Choy, M. McClelland, S. L. Liu, and K. E. Sanderson. 2004. Salmonella enterica serovar Typhi strains from which SPI7, a 134-kilobase island with genes for Vi exopolysaccharide and other functions, has been deleted. J. Bacteriol. 186:3214-3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nathoo, K. J., S. Chigonde, M. Nhembe, M. H. Ali, and P. R. Mason. 1996. Community-acquired bacteremia in human immunodeficiency virus-infected children in Harare, Zimbabwe. Pediatr. Infect. Dis. J. 15:1092-1097. [DOI] [PubMed] [Google Scholar]
- 81.Nau, G. J., A. Schlesinger, J. F. Richmond, and R. A. Young. 2003. Cumulative Toll-like receptor activation in human macrophages treated with whole bacteria. J. Immunol. 170:5203-5209. [DOI] [PubMed] [Google Scholar]
- 82.Nauciel, C., and F. Espinasse-Maes. 1992. Role of gamma interferon and tumor necrosis factor alpha in resistance to Salmonella typhimurium infection. Infect. Immun. 60:450-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nguyen, Q. C., P. Everest, T. K. Tran, D. House, S. Murch, C. Parry, P. Connerton, V. B. Phan, S. D. To, P. Mastroeni, N. J. White, T. H. Tran, V. H. Vo, G. Dougan, J. J. Farrar, and J. Wain. 2004. A clinical, microbiological, and pathological study of intestinal perforation associated with typhoid fever. Clin. Infect. Dis. 39:61-67. [DOI] [PubMed] [Google Scholar]
- 84.Norris, F. A., M. P. Wilson, T. S. Wallis, E. E. Galyov, and P. W. Majerus. 1998. SopB, a protein required for virulence of Salmonella dublin, is an inositol phosphate phosphatase. Proc. Natl. Acad. Sci. USA 95:14057-14059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ochman, H., F. C. Soncini, F. Solomon, and E. A. Groisman. 1996. Identification of a pathogenicity island for Salmonella survival in host cells. Proc. Natl. Acad. Sci. USA 93:7800-7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Parkhill, J., G. Dougan, K. D. James, N. R. Thomson, D. Pickard, J. Wain, C. Churcher, K. L. Mungall, S. D. Bentley, M. T. Holden, M. Sebaihia, S. Baker, D. Basham, K. Brooks, T. Chillingworth, P. Connerton, A. Cronin, P. Davis, R. M. Davies, L. Dowd, N. White, J. Farrar, T. Feltwell, N. Hamlin, A. Haque, T. T. Hien, S. Holroyd, K. Jagels, A. Krogh, T. S. Larsen, S. Leather, S. Moule, P. O'Gaora, C. Parry, M. Quail, K. Rutherford, M. Simmonds, J. Skelton, K. Stevens, S. Whitehead, and B. G. Barrell. 2001. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature 413:848-852. [DOI] [PubMed] [Google Scholar]
- 87.Pickard, D., J. Li, M. Roberts, D. Maskell, D. Hone, M. Levine, G. Dougan, and S. Chatfield. 1994. Characterization of defined ompR mutants of Salmonella typhi: ompR is involved in the regulation of Vi polysaccharide expression. Infect. Immun. 62:3984-3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pithie, A. D., A. S. Malin, and V. J. Robertson. 1993. Salmonella and shigella bacteraemia in Zimbabwe. Cent. Afr. J. Med. 39:110-112. [PubMed] [Google Scholar]
- 89.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 90.Porwollik, S., E. F. Boyd, C. Choy, P. Cheng, L. Florea, E. Proctor, and M. McClelland. 2004. Characterization of Salmonella enterica subspecies I genovars by use of microarrays. J. Bacteriol. 186:5883-5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Qadri, A. 1997. Identification of specific recognition molecules on murine mononuclear phagocytes and B lymphocytes for Vi capsular polysaccharide: modulation of MHC class II expression on stimulation with the polysaccharide. Immunology 92:146-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Raffatellu, M., D. Chessa, R. P. Wilson, R. Dusold, S. Rubino, and A. J. Bäumler. 2005. The Vi-capsular antigen of Salmonella enterica serotype Typhi reduces Toll-like receptor-dependent IL-8 expression in the intestinal mucosa. Infect. Immun. 73:3367-3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Raffatellu, M., Y.-H. Sun, R. P. Wilson, Q. T. Tran, D. Chessa, H. L. Andrews-Polymenis, S. D. Lawhon, J. F. Figueiredo, R. M. Tsolis, L. G. Adams, and A. J. Bäumler. 2005. Host restriction of Salmonella enterica serotype Typhi is not caused by functional alteration of SipA, SopB, or SopD. Infect. Immun. 73:7817-7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Raffatellu, M., R. P. Wilson, D. Chessa, H. Andrews-Polymenis, Q. T. Tran, S. Lawhon, S. Khare, L. G. Adams, and A. J. Bäumler. 2005. SipA, SopA, SopB, SopD, and SopE2 contribute to Salmonella enterica serotype Typhimurium invasion of epithelial cells. Infect. Immun. 73:146-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Reis, B. P., S. Zhang, R. M. Tsolis, A. J. Bäumler, L. G. Adams, and R. L. Santos. 2003. The attenuated sopB mutant of Salmonella enterica serovar Typhimurium has the same tissue distribution and host chemokine response as the wild type in bovine Peyer's patches. Vet. Microbiol. 97:269-277. [DOI] [PubMed] [Google Scholar]
- 96.Robbins, J. D., and J. B. Robbins. 1984. Reexamination of the protective role of the capsular polysaccharide (Vi antigen) of Salmonella typhi. J. Infect. Dis. 150:436-449. [DOI] [PubMed] [Google Scholar]
- 97.Rosenberger, C. M., M. G. Scott, M. R. Gold, R. E. Hancock, and B. B. Finlay. 2000. Salmonella typhimurium infection and lipopolysaccharide stimulation induce similar changes in macrophage gene expression. J. Immunol. 164:5894-5904. [DOI] [PubMed] [Google Scholar]
- 98.Saha, M. R., T. Ramamurthy, P. Dutta, and U. Mitra. 2000. Emergence of Salmonella typhi Vi antigen-negative strains in an epidemic of multidrug-resistant typhoid fever cases in Calcutta, India. Natl. Med. J. India 13:164. [PubMed] [Google Scholar]
- 99.Sansonetti, P. 2002. Host-pathogen interactions: the seduction of molecular cross talk. Gut 50(Suppl. 3):III2-III8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Santos, R. L., and A. J. Bäumler. 2004. Cell tropism of Salmonella enterica. Int. J. Med. Microbiol. 294:225-233. [DOI] [PubMed] [Google Scholar]
- 101.Santos, R. L., S. Zhang, R. M. Tsolis, A. J. Bäumler, and L. G. Adams. 2002. Morphologic and molecular characterization of Salmonella typhimurium infection in neonatal calves. Vet. Pathol. 39:200-215. [DOI] [PubMed] [Google Scholar]
- 102.Santos, R. L., S. Zhang, R. M. Tsolis, R. A. Kingsley, L. G. Adams, and A. J. Bäumler. 2001. Animal models of Salmonella infections: enteritis vs. typhoid fever. Mircrob. Infect. 3:1335-1344. [DOI] [PubMed] [Google Scholar]
- 103.Sato, A., and A. Iwasaki. 2004. Induction of antiviral immunity requires Toll-like receptor signaling in both stromal and dendritic cell compartments. Proc. Natl. Acad. Sci. USA 101:16274-16279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schuetze, N., S. Schoeneberger, U. Mueller, M. A. Freudenberg, G. Alber, and R. K. Straubinger. 2005. IL-12 family members: differential kinetics of their TLR4-mediated induction by Salmonella Enteritidis and the impact of IL-10 in bone marrow-derived macrophages. Int. Immunol. 17:649-659. [DOI] [PubMed] [Google Scholar]
- 105.Selander, R. K., P. Beltran, N. H. Smith, R. Helmuth, F. A. Rubin, D. J. Kopecko, K. Ferris, B. D. Tall, A. Cravioto, and J. M. Musser. 1990. Evolutionary genetic relationships of clones of Salmonella serovars that cause human typhoid and other enteric fevers. Infect. Immun. 58:2262-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Seydi, M., M. Soumare, A. I. Sow, B. M. Diop, and P. S. Sow. 2005. Current aspects of Salmonella bacteremia cases in the Ibrahima Diop Mar Infectious Diseases clinic, Fann National Hospital Center (Senegal). Med. Mal. Infect. 35:23-27. (In French.) [DOI] [PubMed] [Google Scholar]
- 107.Sharma, A., and A. Qadri. 2004. Vi polysaccharide of Salmonella typhi targets the prohibitin family of molecules in intestinal epithelial cells and suppresses early inflammatory responses. Proc. Natl. Acad. Sci. USA 101:17492-17497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shi, J., G. Scita, and J. E. Casanova. 2005. Wave2 signaling mediates invasion of polarized epithelial cells by Salmonella typhimurium. J. Biol. Chem. 280:29849-29855. [DOI] [PubMed] [Google Scholar]
- 109.Shimazu, R., S. Akashi, H. Ogata, Y. Nagai, K. Fukudome, K. Miyake, and M. Kimoto. 1999. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 189:1777-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sprinz, H., E. J. Gangarosa, M. Williams, R. B. Hornick, and T. E. Woodward. 1966. Histopathology of the upper small intestines in typhoid fever. Biopsy study of experimental disease in man. Am. J. Dig. Dis. 11:615-624. [DOI] [PubMed] [Google Scholar]
- 111.Stender, S., A. Friebel, S. Linder, M. Rohde, S. Mirold, and W. D. Hardt. 2000. Identification of SopE2 from Salmonella typhimurium, a conserved guanine nucleotide exchange factor for Cdc42 of the host cell. Mol. Microbiol. 36:1206-1221. [DOI] [PubMed] [Google Scholar]
- 112.Taylor, D. N., J. M. Bied, J. S. Munro, and R. A. Feldman. 1982. Salmonella dublin infections in the United States, 1979-1980. J. Infect. Dis. 146:322-327. [DOI] [PubMed] [Google Scholar]
- 113.Tsolis, R. M., L. G. Adams, T. A. Ficht, and A. J. Bäumler. 1999. Contribution of Salmonella typhimurium virulence factors to diarrheal disease in calves. Infect. Immun. 67:4879-4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tsolis, R. M., S. M. Townsend, E. A. Miao, S. I. Miller, T. A. Ficht, L. G. Adams, and A. J. Bäumler. 1999. Identification of a putative Salmonella enterica serotype Typhimurium host range factor with homology to IpaH and YopM by signature-tagged mutagenesis. Infect. Immun. 67:6385-6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Unsworth, K. E., M. Way, M. McNiven, L. Machesky, and D. W. Holden. 2004. Analysis of the mechanisms of Salmonella-induced actin assembly during invasion of host cells and intracellular replication. Cell. Microbiol. 6:1041-1055. [DOI] [PubMed] [Google Scholar]
- 116.Virlogeux, I., H. Waxin, C. Ecobichon, J. O. Lee, and M. Y. Popoff. 1996. Characterization of the rcsA and rcsB genes from Salmonella typhi: rcsB through tviA is involved in regulation of Vi antigen synthesis. J. Bacteriol. 178:1691-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Waage, A., P. Brandtzaeg, A. Halstensen, P. Kierulf, and T. Espevik. 1989. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J. Exp. Med. 169:333-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wain, J., D. House, A. Zafar, S. Baker, S. Nair, C. Kidgell, Z. Bhutta, G. Dougan, and R. Hasan. 2005. Vi antigen expression in Salmonella enterica serovar Typhi clinical isolates from Pakistan. J. Clin. Microbiol. 43:1158-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wood, M. W., M. A. Jones, P. R. Watson, S. Hedges, T. S. Wallis, and E. E. Galyov. 1998. Identification of a pathogenicity island required for Salmonella enteropathogenicity. Mol. Microbiol. 29:883-891. [DOI] [PubMed] [Google Scholar]
- 120.Wortel, C. H., M. A. von der Mohlen, S. J. van Deventer, C. L. Sprung, M. Jastremski, M. J. Lubbers, C. R. Smith, I. E. Allen, and J. W. ten Cate. 1992. Effectiveness of a human monoclonal anti-endotoxin antibody (HA-1A) in gram-negative sepsis: relationship to endotoxin and cytokine levels. J. Infect. Dis. 166:1367-1374. [DOI] [PubMed] [Google Scholar]
- 121.Wright, S. D., R. A. Ramos, P. S. Tobias, R. J. Ulevitch, and J. C. Mathison. 1990. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249:1431-1433. [DOI] [PubMed] [Google Scholar]
- 122.Wyant, T. L., M. K. Tanner, and M. B. Sztein. 1999. Potent immunoregulatory effects of Salmonella typhi flagella on antigenic stimulation of human peripheral blood mononuclear cells. Infect. Immun. 67:1338-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wyant, T. L., M. K. Tanner, and M. B. Sztein. 1999. Salmonella typhi flagella are potent inducers of proinflammatory cytokine secretion by human monocytes. Infect. Immun. 67:3619-3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zeng, H., A. Q. Carlson, Y. Guo, Y. Yu, L. S. Collier-Hyams, J. L. Madara, A. T. Gewirtz, and A. S. Neish. 2003. Flagellin is the major proinflammatory determinant of enteropathogenic Salmonella. J. Immunol. 171:3668-3674. [DOI] [PubMed] [Google Scholar]
- 125.Zhang, S., L. G. Adams, J. Nunes, S. Khare, R. M. Tsolis, and A. J. Bäumler. 2003. Secreted effector proteins of Salmonella enterica serotype Typhimurium elicit host-specific chemokine profiles in animal models of typhoid fever and enterocolitis. Infect. Immun. 71:4795-4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang, S., R. L. Santos, R. M. Tsolis, S. Mirold, W.-D. Hardt, L. G. Adams, and A. J. Bäumler. 2002. Phage mediated horizontal transfer of the sopE1 gene increases enteropathogenicity of Salmonella enterica serotype Typhimurium for calves. FEMS Microbiol. Lett. 217:243-247. [DOI] [PubMed] [Google Scholar]
- 127.Zhang, S., R. L. Santos, R. M. Tsolis, S. Stender, W.-D. Hardt, A. J. Bäumler, and L. G. Adams. 2002. SipA, SopA, SopB, SopD, and SopE2 act in concert to induce diarrhea in calves infected with Salmonella enterica serotype Typhimurium. Infect. Immun. 70:3843-3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang, Z., and S. L. Stanley, Jr. 2004. Stereotypic and specific elements of the human colonic response to Entamoeba histolytica and Shigella flexneri. Cell. Microbiol. 6:535-554. [DOI] [PubMed] [Google Scholar]
- 129.Zhou, D., L. M. Chen, L. Hernandez, S. B. Shears, and J. E. Galan. 2001. A Salmonella inositol polyphosphatase acts in conjunction with other bacterial effectors to promote host cell actin cytoskeleton rearrangements and bacterial internalization. Mol. Microbiol. 39:248-259. [DOI] [PubMed] [Google Scholar]
- 130.Zhou, D., M. S. Mooseker, and J. E. Galan. 1999. Role of the S. typhimurium actin-binding protein SipA in bacterial internalization. Science 283:2092-2095. [DOI] [PubMed] [Google Scholar]