Abstract
Leishmania major parasites lacking the GDP-mannose transporter, termed Δlpg2 parasites, fail to induce disease in mice but persist long-term. We previously found that Δlpg2 organisms protect BALB/c mice from virulent L. major challenge. In contrast, we report here that Δlpg2 parasites induce protective immunity in C57BL/6 mice only when administered with CpG-containing oligodeoxynucleotides, indicating that parasite persistence alone is not sufficient to maintain protective immunity to L. major.
Following infection with Leishmania major, mice that have achieved a clinical cure harbor persistent parasites and maintain a life-long immunity to reinfection (1, 3, 11). Persistent live parasites are required for the maintenance and/or renewal of anti-Leishmania effector memory cells that produce gamma interferon (IFN-γ), mediate a delayed-type hypersensitivity (DTH) response, and induce rapid protection against virulent challenge (13). Although they are required for durable immunity, persistent virulent parasites may cause disease recrudescence in immunocompromised individuals. Therefore, the generation of live, attenuated parasites that persist without causing disease would be a reasonable approach to generating a vaccine for leishmaniasis. BALB/c mice are extremely susceptible to L. major in part due to the development of a Th2 response (8). To determine if Δlpg2 parasites can protect mice that do not develop a dominant Th2 response, we tested the protective potential of Δlpg2 mutants in C57BL/6 mice. C57BL/6 mice develop a Th1 response following L. major infection and heal over 2 to 3 months. Following resolution of a primary infection, these mice exhibit substantial protective immunity to reinfection (7). In this report we show that although Δlpg2 parasites are maintained long-term in C57BL/6 mice, they provide minimal protection to virulent challenge, although when they were delivered with CpG oligodeoxynucleotides (ODNs), significant protection was observed.
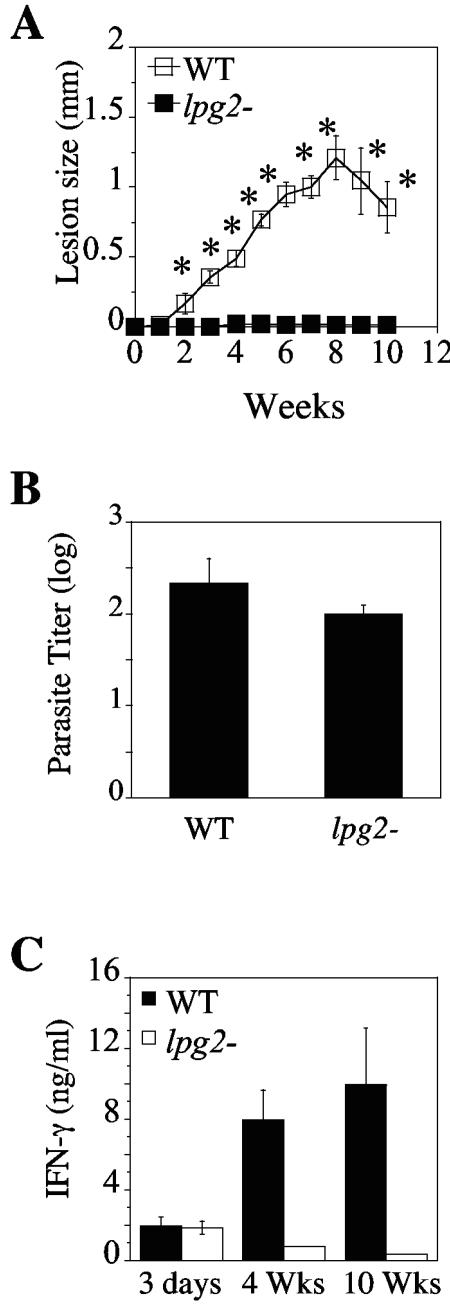
C57BL/6 mice (6 to 8 weeks old; Jackson Laboratories, Bar Harbor, ME) were infected subcutaneously with 5 × 106 stationary-phase L. major substrain LV39 clone 5 (MRHO/SU/59/P; wild type [WT]) or the Δlpg2 mutant (9). The course of infection and parasite titers in the infected feet were then assessed. As expected, C57BL/6 mice infected with WT parasites developed small lesions that started to resolve spontaneously around week 8. In contrast, and consistent with our previous reports (9, 10), no lesions were observed in Δlpg2 mutant-infected C57BL/6 mice (Fig. 1A). The lack of disease in Δlpg2 mutant-infected mice was not due to their destruction, as comparable numbers of parasites could be reisolated from the footpads of WT or Δlpg2 mutant-infected animals by the limiting dilution assay at 10 weeks postinfection (Fig. 1B).
FIG. 1.
C57BL/6 mice infected with Δlpg2 mutants exhibit no pathology but are unable to maintain a strong Th1 response. (A) Mice were vaccinated subcutaneously in their footpads with 5 × 106 stationary-phase promastigotes of L. major LV39 (WT) or Δlpg2 parasites. Lesion progression was monitored weekly. Results are expressed as mean footpad thickness increases ± standard errors of the means. (B) Mice were sacrificed 10 weeks (Wks) after infection to assess parasite burden. (C) Leishmania-specific recall responses by LN cell suspensions from C57BL/6 mice infected for 3 days, 4 weeks, or 10 weeks with 5 × 106 WT or Δlpg2 L. major parasites. Data shown represent the mean increases ± standard deviations for three mice per group.
We then compared the immune responses in C57BL/6 mice following infection with WT or Δlpg2 mutant parasites. At various time points after infection (3 days, 4 weeks, and 10 weeks), single-cell suspensions from draining lymph nodes (LNs) were cultured in the presence of leishmanial freeze-thawed antigen (the equivalent of 107 parasites/ml), and the supernatants were collected and assayed for IFN-γ production. At 3 days postinfection, comparable levels of IFN-γ were detected after in vitro restimulation of cells from mice infected with WT and Δlpg2 parasites (Fig. 1C), indicating that the ability of Δlpg2 mutants to induce an early Th1 response in C57BL/6 mice was equivalent to that of WT parasites. However, this response rapidly waned; the levels of IFN-γ produced by cells from mice infected with Δlpg2 parasites significantly decreased at 4 weeks and were almost undetectable at 10 weeks postinfection. In contrast, IFN-γ production by cells from WT-infected mice increased over time (Fig. 1C). This lack of IFN-γ production by cells from Δlpg2 mutant-infected mice was not due to a shift to a Th2 cytokine response, as we also were unable to detect any measurable production of IL-4 or IL-10 by cells from Δlpg2 mutant-infected mice (data not shown). Taken together, these data indicate that the ability of Δlpg2 mutants to continuously stimulate an effector T-cell response is dramatically impaired.
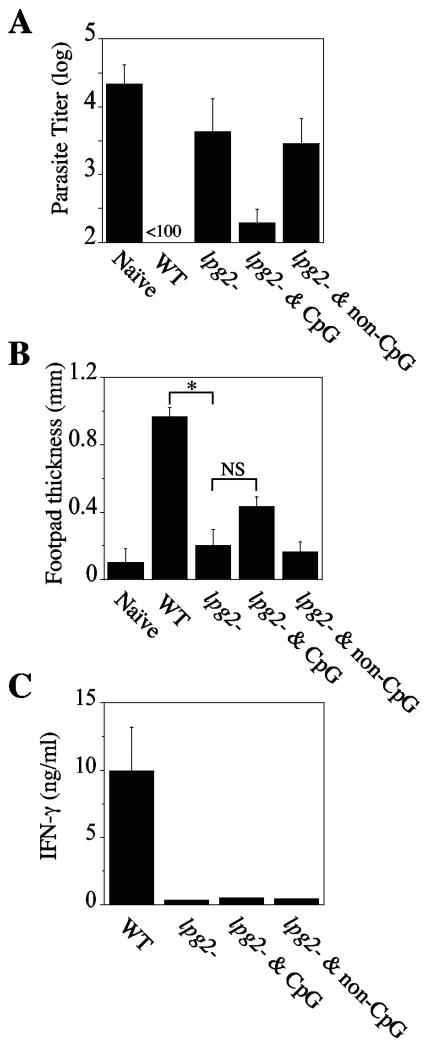
To determine whether Δlpg2 parasites can confer protection in C57BL/6 mice, after 10 weeks of infection with Δlpg2 or WT parasites, these animals, or naïve controls, were challenged in their contralateral footpads with WT L. major parasites. Parasite loads were then compared between naïve and infected animals 5 weeks after challenge. As shown in Fig. 2A, WT-infected mice had significantly lower parasite loads than naïve (unvaccinated) animals, which harbored >104 parasites in their lesions. Surprisingly—and unlike BALB/c mice—C57BL/6 mice vaccinated with Δlpg2 parasites had lower parasite loads, but not significantly so, than naïve controls. Furthermore, C57BL/6 mice previously infected with Δlpg2 parasites for 10 weeks mounted a very weak DTH response, comparable to that exhibited by naïve animals, while healed WT mice exhibited a robust DTH response typical of a classical anti-Leishmania immunity (Fig. 2B).
FIG. 2.
Vaccination with live Δlpg2 mutant parasites and CpG ODNs confers significant resistance to L. major. (A) Naïve unvaccinated mice and mice vaccinated for 10 weeks with 5 × 106 stationary-phase Δlpg2 promastigotes alone or in combination with either 50 μg of CpG ODNs or 50 μg of non-CpG ODNs were challenged with 2 × 106 WT L. major parasites in their contralateral uninfected footpads, and the parasite load was estimated by limiting dilution analysis 5 weeks after challenge. Data shown represent the mean increases ± standard deviations for three mice per group. (B) The increase in footpad thickness was measured after 72 h to assess the DTH response in naïve and challenged mice. Asterisks and NS indicate statistically significant or not significant differences, respectively. (C) Prior to challenge, draining LNs from three mice per group were isolated, single-cell suspensions were restimulated in the presence of leishmanial freeze-thawed antigen for 72 h, and the production of IFN-γ was measured by enzyme-linked immunosorbent assay. Data are representative of three experiments with similar results.
The inability of Δlpg2 parasites to confer significant protection to C57BL/6 mice could be related to the minimal immune response seen prior to challenge (Fig. 2C), although in Δlpg2 mutant-infected BALB/c mice—which were protected from challenge with WT parasites—a similarly weak immune response was observed (10). Nevertheless, we tested whether enhancing the immune response with adjuvants would promote increased protective immunity. CpG ODNs, potent inducers of a Th1 response, have been widely used as effective adjuvants for diseases requiring Th1 immune responses, such as leishmaniasis (5, 6, 12). We therefore asked whether coinjection of Δlpg2 parasites with CpG ODNs would enhance the Δlpg2 mutant-induced protection. C57BL/6 mice were immunized with Δlpg2 parasites in combination with a single dose (50 μg/mouse) of immunostimulatory CpG ODNs (sequence 1826 [5′-TCC ATG ACG TTC CTG ACG TT-3′]) or non-CpG ODNs (sequence 1982 [5′-TCC AGG ACT TCT CTC AGG TT-3′] used as a control) (Coley Pharmaceutical Group, Inc., Wellesley, MA). When challenged with WT parasites, Δlpg2 mutant- plus CpG ODN-vaccinated mice showed a hundredfold reduction in parasite burdens compared to those in naïve unvaccinated animals (Fig. 2A). Interestingly, the protective immunity induced by inclusion of CpG ODNs with the Δlpg2 parasites was not associated with a strong effector T-cell response at the time of challenge. Thus, Δlpg2 mutant- plus CpG ODN-vaccinated-mice did not mount a significantly stronger DTH response than mice receiving Δlpg2 parasites alone (Fig. 2B). Moreover, lymph node cells taken from Δlpg2 mutant- plus CpG ODN-vaccinated-mice prior to challenge did not produce significantly more IFN-γ than Δlpg2 mutant-infected mice (Fig. 2C). Taken together, these data indicate that mice vaccinated with Δlpg2 parasites in combination with a single dose of CpG ODNs are protected against WT parasites in the absence of a strong immune response.
A striking feature of infection with Δlpg2 parasites in BALB/c mice was the rapid waning of the effector immune response, in spite of the long-term maintenance of protection (10). We saw a similar pattern in C57BL/6 mice infected with Δlpg2 parasites, even when CpG ODNs were included in the vaccine. Thus, these results argue that the minimal immune response seen in Δlpg2 mutant-infected C57BL/6 mice at 10 weeks of infection is not likely to be the reason that these animals are not strongly protected. Studies are under way to define the mechanism involved in the protective immunity associated with Δlpg2 parasites. However, whatever the mechanism, it is clear from the data reported in this study and our previous work using Δlpg2 parasites in BALB/c mice that the presence of DTH and large numbers of IFN-γ-producing T cells prior to challenge are not required for protective immunity. Additional studies with more-sensitive assays will be required to determine if any effector T cells are present prior to challenge.
A substantial body of literature indicates that parasite persistence is associated with optimal immunity to L. major (3, 11, 13), which suggests that the best vaccine for leishmaniasis may require live, attenuated parasites. Späth et al. found that Δlpg2 parasites persist in BALB/c mice without disease (9), and we reported that Δlpg2 mutant-infected BALB/c mice are resistant to challenge with virulent L. major (10). Here we show that while C57BL/6 mice infected with Δlpg2 parasites alone are not protected against challenge with virulent L. major, mice vaccinated with CpG ODNs as an adjuvant with Δlpg2 parasites, exhibited significant protection. Furthermore, mice receiving this live, attenuated vaccine were resistant to rechallenge for at least 10 weeks after the immunization. These results are similar to those obtained when mice were immunized with virulent parasites in conjunction with CpG ODNs, but they have the advantage of the use of an attenuated organism (4). Taken together with our previous study of BALB/c mice (10), these results indicate that attenuated parasites are able to induce long-term immunity to leishmaniasis. However, the ability of Δlpg2 parasites to provide protection in BALB/c mice (10), but not in C57BL/6 mice, was a surprise, although we cannot rule out the possibility that different doses of parasites, or additional injections of the parasites, might give better protection. Nevertheless, while previous studies have demonstrated that the presence of WT L. major parasites in C57BL/6 mice is associated with immunity to reinfection (1, 2, 11), our data unexpectedly indicate that the presence of persistent parasites alone is not sufficient to maintain protective immunity. Thus, future studies using Δlpg2 parasites may help to identify the essential characteristics associated with persistent infection that promote the maintenance of protective immunity.
Acknowledgments
This work was supported by Public Health Service grant AI-059396 from the National Institute of Allergy and Infectious Disease.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Aebischer, T., S. F. Moody, and E. Handman. 1993. Persistence of virulent Leishmania major in murine cutaneous leishmaniasis: a possible hazard for the host. Infect. Immun. 61:220-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belkaid, Y., K. F. Hoffmann, S. Mendez, S. Kamhawi, M. C. Udey, T. A. Wynn, and D. L. Sacks. 2001. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J. Exp. Med. 194:1497-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belkaid, Y., C. A. Piccirillo, S. Mendez, E. M. Shevach, and D. L. Sacks. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420:502-507. [DOI] [PubMed] [Google Scholar]
- 4.Mendez, S., K. Tabbara, Y. Belkaid, S. Bertholet, D. Verthelyi, D. Klinman, R. A. Seder, and D. L. Sacks. 2003. Coinjection with CpG-containing immunostimulatory oligodeoxynucleotides reduces the pathogenicity of a live vaccine against cutaneous leishmaniasis but maintains its potency and durability. Infect. Immun. 71:5121-5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rhee, E. G., S. Mendez, J. A. Shah, C. Y. Wu, J. R. Kirman, T. N. Turon, D. F. Davey, H. Davis, D. M. Klinman, R. N. Coler, D. L. Sacks, and R. A. Seder. 2002. Vaccination with heat-killed leishmania antigen or recombinant leishmanial protein and CpG oligodeoxynucleotides induces long-term memory CD4+ and CD8+ T cell responses and protection against Leishmania major infection. J. Exp. Med. 195:1565-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roman, M., E. Martin-Orozco, J. S. Goodman, M. D. Nguyen, Y. Sato, A. Ronaghy, R. S. Kornbluth, D. D. Richman, D. A. Carson, and E. Raz. 1997. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat. Med. 3:849-854. [DOI] [PubMed] [Google Scholar]
- 7.Sacks, D., and N. Noben-Trauth. 2002. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2:845-858. [DOI] [PubMed] [Google Scholar]
- 8.Scott, P., P. Natovitz, R. L. Coffman, E. Pearce, and A. Sher. 1988. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J. Exp. Med. 168:1675-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Späth, G. F., L. F. Lye, H. Segawa, D. L. Sacks, S. J. Turco, and S. M. Beverley. 2003. Persistence without pathology in phosphoglycan-deficient Leishmania major. Science 301:1241-1243. [DOI] [PubMed] [Google Scholar]
- 10.Uzonna, J. E., G. F. Spath, S. M. Beverley, and P. Scott. 2004. Vaccination with phosphoglycan-deficient Leishmania major protects highly susceptible mice from virulent challenge without inducing a strong Th1 response. J. Immunol. 172:3793-3797. [DOI] [PubMed] [Google Scholar]
- 11.Uzonna, J. E., G. Wei, D. Yurkowski, and P. Bretscher. 2001. Immune elimination of Leishmania major in mice: implications for immune memory, vaccination, and reactivation disease. J. Immunol. 167:6967-6974. [DOI] [PubMed] [Google Scholar]
- 12.Walker, P. S., T. Scharton-Kersten, A. M. Krieg, L. Love-Homan, E. D. Rowton, M. C. Udey, and J. C. Vogel. 1999. Immunostimulatory oligodeoxynucleotides promote protective immunity and provide systemic therapy for leishmaniasis via IL-12- and IFN-gamma-dependent mechanisms. Proc. Natl. Acad. Sci. USA 96:6970-6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaph, C., J. Uzonna, S. M. Beverley, and P. Scott. 2004. Central memory T cells mediate long-term immunity to Leishmania major in the absence of persistent parasites. Nat. Med. 10:1104-1110. [DOI] [PubMed] [Google Scholar]