Abstract
Ehrlichia canis major immunoreactive proteins of 36 and 19 kDa elicit the earliest detectable antibody responses during the acute phase of canine monocytic ehrlichiosis. Genes encoding the major immunoreactive 36-kDa protein of E. canis and the corresponding ortholog of E. chaffeensis (47 kDa) were identified and the proteins characterized. The molecular masses of the strongly immunoreactive recombinant proteins were larger than predicted (26.7 and 32.9 kDa, respectively) but were consistent with those of the corresponding native proteins (36 and 47 kDa). Similar to other reported ehrlichial immunoreactive glycoproteins, carbohydrate was detected on the recombinant expressed proteins, indicating that they were glycoproteins. Both glycoproteins (gp36 and gp47) have carboxy-terminal serine/threonine-rich tandem repeat regions containing repeats that vary in number (4 to 16 repeats) and amino acid sequence among different isolates of each species. E. canis gp36 was recognized by early acute-phase antibodies (day 14), and species-specific antibody epitopes were mapped to C-terminal nonhomologous repeat units of gp36 and gp47. Periodate treatment of recombinant gp36 reduced the antibody reactivity, and nonglycosylated synthetic peptide repeat units from E. canis gp36 and E. chaffeensis gp47 were substantially less immunoreactive than corresponding recombinant peptides, demonstrating that glycans are important epitope determinants that are structurally conserved on the recombinant proteins expressed in Escherichia coli. E. canis gp36 and E. chaffeensis gp47 were differentially expressed only on the surface of dense-cored ehrlichiae and detected in the Ehrlichia-free supernatants, indicating that these proteins are released extracellularly during infection.
Ehrlichiae are tick-transmitted, obligately intracellular gram-negative bacteria that reside primarily within cytoplasmic vacuoles (early endosomes) of professional phagocytes, including macrophages and neutrophils (28). Ehrlichia canis causes canine monocytic ehrlichiosis, a serious and sometimes fatal globally distributed disease (36). Ehrlichia chaffeensis causes human monocytotropic ehrlichiosis (HME), an emerging life-threatening disease in humans (28), in the United States and also causes mild to severe disease in canines (5). The importance of E. canis as a veterinary pathogen in conjunction with the recent identification of E. chaffeensis as the cause of an emerging tick-borne zoonosis has highlighted the need for improved diagnostics and vaccines for both veterinary and human ehrlichioses and thus the need for the identification of immunoreactive proteins.
A small subset of proteins expressed by E. canis and E. chaffeensis react strongly with antibodies and are considered to be the major immunoreactive proteins (6, 7, 15). Several of these proteins have been molecularly characterized, including the 200- and 140/120-kDa proteins and the 28/30-kDa multigene family of proteins (19, 24, 25, 27, 31, 38-40), all of which are glycoproteins (16, 20, 34). Until recently, bacteria were thought to be incapable of protein glycosylation, but numerous glycoproteins have recently been identified in both intracellular and extracellular pathogenic bacteria, including Ehrlichia (4, 16, 33, 37). Glycoproteins in pathogenic bacteria that have been functionally characterized include adhesins, toxins, and proteins involved in structural stability or mobility (37). Some bacterial glycoproteins are highly immunogenic, highlighting a potential role in the development of protective immunity (4).
Several glycoproteins, including surface-exposed proteins, have been identified in Ehrlichia species. E. chaffeensis gp120 and E. canis gp140 are major immunoreactive surface protein orthologs that have repeat units with high serine and threonine content and are involved in ehrlichial attachment to the host cell (30). The gp200 orthologs are the largest major immunoreactive proteins of Ehrlichia spp. and are found primarily in the ehrlichial cytoplasm (16). The proteins in the p28/p30 multigene family are major constituents of the outer membrane and are thought to play a role in surface antigenic diversity and perhaps immune evasion (27, 31, 40). Glycosylation and phosphorylation of the p28/p30 proteins have been reported in E. chaffeensis (34). Little is known regarding the role of these glycoproteins as immunoprotective antigens, although partial protection in mice has been observed after immunization with recombinant p28/p30 (27).
The differential expression of ehrlichial antigens in tick and mammalian cells cultured in vitro has been reported previously (34), and ehrlichial antigens expressed in the tick or soon after inoculation in the host are likely to elicit the earliest host immune response. The kinetics of the antibody response that develops to the major immunoreactive proteins of E. canis has been investigated in experimentally infected dogs (15). Two proteins of approximately 19 and 37 kDa were found to elicit the earliest acute-phase antibody response, while the antibody response to p28/p30 major outer membrane proteins as well as others developed 2 weeks later. A total of eight major immunoreactive proteins were recognized by antibodies in convalescent-phase sera 6 weeks after inoculation (15).
We describe in this report the identification of the third pair of molecularly and antigenically divergent major immunoreactive glycoprotein orthologs of Ehrlichia canis (36 kDa) and E. chaffeensis (47 kDa). These glycoproteins have tandem repeat units that contain major B-cell epitopes with carbohydrate determinants, which contribute substantially to the immunoreactivity of these proteins. Differential expression of these glycoproteins was observed only on the dense-cored morphological form of the bacterium, and the gp36 and gp47 proteins are surface exposed and secreted extracellularly.
MATERIALS AND METHODS
Culture and purification of ehrlichiae.
E. canis (Jake, Oklahoma, and Demon isolates) and E. chaffeensis (Arkansas and Sapulpa isolates) were propagated as previously described (17). Ehrlichiae were purified by size exclusion chromatography over Sephacryl S-1000 (Amersham Biosciences, Piscataway, N.J.) as previously described (32). The fractions containing bacteria were frozen and utilized as antigen and DNA sources.
Construction and screening of the E. canis genomic library.
An E. canis Jake strain genomic library was constructed using an HpaII restriction endonuclease and screened as previously described (17).
DNA sequencing.
Library inserts, plasmids, and PCR products were sequenced with an ABI Prism 377XL DNA sequencer (Perkin-Elmer Applied Biosystems, Foster City, Calif.) at the University of Texas Medical Branch Protein Chemistry Core Laboratory.
Glycoprotein gene analysis.
Nucleic acid and amino acid alignments were performed with MegAlign (Lasergene v5.08; DNAStar, Madison, Wis.). The gp36 and gp47 protein sequences were evaluated for potential mucin-type O-linked glycosylation on serines and threonines with the computational algorithm NetOGlyc v3.1 (http://www.cbs.dtu.dk/services/NetOGlyc/) (12). The Tandem Repeats Finder database (http://tandem.bu.edu/trf/trf.html) (3) was used to analyze the tandem repeats of the genes encoding gp36 of E. canis (Jake, Oklahoma, and Demon); gp47 of E. chaffeensis (Arkansas and Sapulpa); “mucinlike” proteins of Ehrlichia ruminantium strains Highway (AF308673), Welgevonden (CR767821), and Gardel (CR925677); gp140 of E. canis Jake strain (AF112369); and gp120 of E. chaffeensis strains Arkansas (ECU49426) and Sapulpa (ECU74670). The analysis was done to determine period sizes, numbers of repeats, and percentages of homology among the repeats. The gp36 and gp47 sequences were tested for the presence of signal sequences with the computational algorithm SignalP trained on gram-negative bacteria (www.cbs.dtu.dk/services/SignalP-2.0/) (22). Percentages of identity between orthologs were calculated by aligning the sequences with the Clustal W algorithm in MegAlign (Lasergene v5.08; DNAStar) and calculating the sequence pair distances.
PCR amplification of the Ehrlichia glycoprotein genes.
Oligonucleotide primers for the amplification of the E. canis gp36 and E. chaffeensis gp47 genes were designed using the PrimerSelect program (Lasergene v5.08; DNAStar, Madison Wis.). Oligonucleotides corresponding to nucleotides 28 to 47 (5′-ATG CTT CAT TTA ACA ACA GA; forward) and 794 to 816 (5′-AGA ATC TAA ATC TAA AAG TCC AG; reverse) within the open reading frame (ORF) were used to amplify the E. canis gp36 gene, and oligonucleotide primers corresponding to nucleotides 4 to 22 (5′-CTT CAT TTA ACA ACA GAA A; forward) and 902 to 924 (5′-TTG AGC AGC CAT ATC TTC TTC AT; reverse) within the ORF were used to amplify the E. chaffeensis gp47 gene. The region of E. canis gp36 corresponding to the amino terminus of its product (bases 28 to 345) was amplified with oligonucleotide primers corresponding to nucleotides 28 to 47 (5′-ATG CTT CAT TTA ACA ACA GA; forward) and nucleotides 321 to 345 (5′-TTG ATA AGC ATG CAC AGA AAT AAA G; reverse), and the region corresponding to the carboxy-terminal region of its product (bases 370 to 816) was amplified with primers specific for nucleotides 370 to 392 (5′-GGA AAT CCA TCA CGT CCT GCT AT; forward) and 794 to 816 (5′-AGA ATC TAA ATC TAA AAG TCC AG; reverse). The region of E. chaffeensis gp47 corresponding to the amino-terminal region of its product (bases 4 to 459) was produced by amplifying respective DNA with primers corresponding to nucleotides 4 to 22 (5′-CTT CAT TTA ACA ACA GAA A forward) and nucleotides 436 to 459 (5′-AAC TGG AAC CAC TAT ACT GTC ACT; reverse), and the region corresponding to the carboxy-terminal region of its product (bases 439 to 924) was amplified with primers specific for nucleotides 439 to 463 (5′-GAC AGT ATA GTG GTT CCA GTT CTT G; forward) and 902 to 924 (5′-TTG AGC AGC CAT ATC TTC TTC AT; reverse). E. canis or E. chaffeensis DNA used as a template was amplified using PCR master mix (F. Hoffmann-La Roche Ltd., Basel, Switzerland) with a thermal cycling profile of 95°C for 4 min and 30 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 1 min followed by a 72°C extension for 7 min and a 4°C hold.
Cloning and expression of recombinant Ehrlichia glycoproteins.
The amplified PCR products were cloned directly into the pBAD-Thio TOPO expression vector (Invitrogen, Carlsbad, Calif.). TOP10 E. coli (Invitrogen) was transformed with the plasmid containing the E. canis gp36 or E. chaffeensis gp47 genes, and positive transformants were screened by PCR to confirm insertion and orientation and were sequenced to determine the reading frames of the genes. Recombinant protein expression was induced with 0.2% arabinose for 3 h at 37°C. Bacteria were pelleted (5,000 × g for 20 min) and resuspended in phosphate-buffered saline, and recombinant proteins were purified under native conditions as previously described (10).
Cloning and expression of recombinant Ehrlichia glycopeptides.
Complementary oligonucleotides encoding the E. canis gp36 single repeat unit (TEDSVSAPA; 9-mer), a region overlapping two tandem repeat units (SVSAPATEDSVS; 12-mer), two tandem repeat units (TEDSVSAPATEDSVSAPA; 18-mer), and the E. chaffeensis gp47 single repeat unit (ASVSEGDAVVNAVSQETPA; 19-mer) were synthesized (Sigma-Genosys, The Woodlands, TX). The oligonucleotides for each coding strand contained additional 5′ CACC nucleotides for directional TOPO vector cloning. Oligonucleotides for the E. canis gp36 single repeat (9-mer; forward, 5′-CACC ACT GAA GAT TCT GTT TCT GCT CCA GCT; reverse complement, 5′-AGC TGG AGC AGA AAC AGA ATC TTC AGT), the region overlapping two repeat units (12-mer; forward, 5′-CACC TCT GTT TCT GCT CCA GCT ACT GAA GAT TCT GTT TCT; reverse complement, 5′-AGA AAC AGA ATC TTC AGT AGC TGG AGC AGA AAC AGA), two repeat units (18-mer; forward, 5′-CACC ACT GAA GAT TCT GTT TCT GCT CCA GCT ACT GAA GAT TCT GTT TCT GCT CCA GCT; reverse complement, 5′-AGC TGG AGC AGA AAC AGA ATC TTC AGT AGC TGG AGC AGA AAC AGA ATC TTC AGT), and the E. chaffeensis single repeat unit (19-mer; forward, 5′-CACC GCT AGT GTA TCT GAA GGA GAT GCA GTA GTA AAT GCT GTA AGC CAA GAA ACT CCT GCA; reverse complement, 5′-TGC AGG AGT TTC TTG GCT TAC AGC ATT TAC TAC TGC ATC TCC TTC AGA TAC ACT AGC) were resuspended in water (200 μM), combined, diluted to 100 μM in oligonucleotide-annealing buffer (10 mM Tris-HCl [pH 7.5], 100 mM NaCl, 1 mM EDTA), and then heated to 95°C for 15 min and allowed to slowly cool to room temperature. This mixture was subsequently used for standard cloning into the pBAD directional TOPO expression vector (Invitrogen) to express E. canis gp36 (9-mer, 12-mer, and 18-mer peptides) and E. chaffeensis gp47 (19-mer single repeat peptide) as thioredoxin fusion proteins.
Synthetic peptides.
Peptides corresponding to the repeat regions of gp36 (9-mer, TEDSVSAPA; 12-mer, SVSAPATEDSVS; 18-mer, TEDSVSAPATEDSVSAPA) and gp47 (19-mer, ASVSEGDAVVNAVSQETPA) were synthesized (Bio-Synthesis, Inc., Lewisville, TX) and resuspended in water.
Gel electrophoresis and Western immunoblotting.
Purified E. canis or E. chaffeensis whole-cell lysates or recombinant proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose, and Western blotting was performed as previously described (15), except that primary dog antibodies were diluted (1:500). Anti-E. canis and -E. chaffeensis dog sera were obtained from experimentally infected dogs (E. canis #2995 and E. chaffeensis #2495 and #2251). Antibody kinetics to E. canis gp36 were determined using 15 sera from dogs experimentally infected with E. canis as previously described (15). Sera from HME patients were a kind gift from Focus Technologies (Cypress, Calif.) and were used at a 1:100 dilution for Western immunoblots.
Carbohydrate detection.
Glycan detection on the recombinant proteins was performed using a carbohydrate detection kit (Bio-Rad, Hercules, Calif.) as previously described (20).
Antibody production.
Five BALB/c mice (Jackson Laboratories, Bar Harbor, Maine) were immunized with recombinant E. canis Oklahoma strain gp36 or E. chaffeensis Arkansas strain gp47 proteins. Recombinant protein (100 μg) in 0.1 ml was mixed with an equal volume of Freund's complete adjuvant (Sigma, St. Louis, Mo.) for the first intraperitoneal injection and with Freund's incomplete adjuvant for the subsequent injections. The mice were given injections twice at 2-week intervals.
ELISA.
Recombinant proteins and synthetic peptides (1.25 μg/well; 100 μl) in phosphate-buffered saline were adsorbed to enzyme-linked immunosorbent assay (ELISA) plates (Nunc-Immuno plates with MaxiSorp surface; NUNC, Roskilde, Denmark) overnight at 4°C with gentle agitation and subsequently washed three times with 200 μl Tris-buffered saline-Tween 20 (TBST), blocked with 3% bovine serum albumin (BSA) in TBST for 1 h at room temperature with agitation, and washed again. Convalescent anti-E. canis dog serum (1:5,000) or anti-E. chaffeensis dog serum (1:500) diluted in 3% BSA TBST was added to each well (100 μl) and incubated at room temperature for 1 h with gentle agitation. The plates were washed four times, an alkaline phosphatase-labeled goat anti-dog immunoglobulin G (IgG) (heavy plus light chains [H+L]) secondary antibody (1:3,000) (Kirkegaard & Perry Laboratories) in 3% BSA TBST was added, and the plates were incubated for 1 h. The plates were washed four times, and substrate (100 μl) (BluePhos; Kirkegaard & Perry Laboratories) was added to each well. The plates were incubated for 30 min in the dark with agitation, color development was read on a microplate reader (Versamax; Molecular Devices, Sunnyvale, Calif.) at A650, and data were analyzed (SoftmaxPro v4.0; Molecular Devices). Optical density readings of the bar graph represent the mean of two wells with the optical density of the buffer-only blank wells subtracted. Periodate treatment of the recombinant gp36 was carried out for 20 min in 100 mM sodium acetate/5 mM EDTA buffer with 100 mM sodium metaperiodate. Sham-treated control protein was incubated in the same buffers in the absence of periodate. The ELISA procedure was performed as described above, except 1× milk diluent/blocking solution (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) was used.
Immunogold electron microscopy.
Immunogold electron microscopy was performed as previously described (10) with mouse anti-recombinant E. canis gp36 (1:10,000), anti-recombinant E. chaffeensis gp47 (1:1,000), or normal mouse (1:1,000; control) serum.
Analysis of secreted immunoreactive proteins.
E. canis- or E. chaffeensis-infected DH82 cells were monitored until 90 to 100% of the monolayer cells were infected. Three days prior to supernatant harvest, the culture medium (Dulbecco's minimal essential medium supplemented with 10% bovine calf serum) was completely removed and replaced with serum-free Dulbecco's minimal essential medium. Culture supernatants were collected without disturbing the cell monolayer and centrifuged (5,000 × g for 20 min) to pellet cells and bacteria. Supernatants were subsequently concentrated 40-fold (Amicon Ultra centrifugal filter devices with a 10-kDa molecular mass cutoff; Millipore, Billerica, Mass.). Cell culture supernatants (2 μl) were diluted 1:2 in LDS sample buffer, separated by gel electrophoresis, and transferred to nitrocellulose; immunoreactive proteins were detected by Western immunoblotting using anti-E. canis polyclonal antibody (1:500) as described previously (15).
Indirect fluorescent antibody (IFA) analysis and confocal microscopy.
Antigen slides were prepared from DH82 cells infected with E. canis (Jake isolate) or E. chaffeensis (Arkansas isolate) as described previously (17). Monospecific rabbit serum against the recombinant E. canis disulfide bond formation protein (Dsb) (18) diluted 1:100 was added to each well (15 μl) and allowed to incubate for 30 min. Slides were washed, and either mouse anti-gp36 or mouse anti-gp47 serum (1:100 dilutions) or negative mouse serum (1:100) was added and incubated for 30 min. Alexa Fluor 488 goat anti-rabbit IgG (H+L) secondary antibody (Molecular Probes, Eugene, Oreg.) diluted 1:100 was added and incubated for 30 min, followed by washing and subsequent addition of and incubation with rhodamine-labeled goat anti-mouse IgG (H+L) secondary antibody (Kirkegaard & Perry Laboratories). Slides were viewed in the Optical Imaging Laboratory at UTMB using a Zeiss LSM-510 META confocal microscope.
Nucleotide sequence accession numbers.
The E. canis gp36 gene sequences from the Jake, Oklahoma, and Demon isolates and E. chaffeensis gp47 gene sequences from the Arkansas and Sapulpa isolates were deposited into GenBank and assigned the following accession numbers: DQ085427 (Jake), DQ085428 (Oklahoma), DQ085429 (Demon), DQ085430 (Arkansas), and DQ085431 (Sapulpa).
RESULTS
Molecular identification of the E. canis 36-kDa major immunoreactive protein.
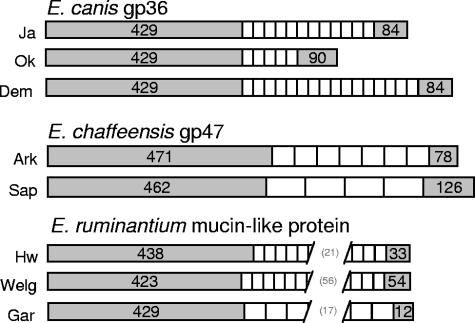
Expression library screening identified a positive clone with a 1.5-kb insert containing an 840-bp ORF encoding a predicted protein with 279 amino acids and a molecular mass of 29.3 kDa (26.7 without the predicted 23-amino-acid signal peptide) with homology to highly glycosylated eukaryotic mucin proteins. Considering the previous correlation of glycoproteins with major immunoreactive proteins, this candidate was of particular interest. The gene contained 12 tandem repeats at the 3′ end of the ORF (Fig. 1) encoding nine amino acids (Table 1). The NetOGlyc O-linked glycosylation prediction server identified serines and threonines within the tandem repeat (TEDSVSAPA) that were the sites of glycosylation (Table 1). A BLAST search of the E. chaffeensis genome sequence database (http://tigrblast.tigr.org/ufmg/index.cgi?database= e_chaffeensis%7Cseq) with the E. canis gp36 sequence identified a 951-bp homologous gene encoding a 316-amino-acid protein with seven 19-mer tandem repeats (ASVSEGDAVVNAVSQETPA) and a predicted mass of 32.9 kDa. The amino acid sequence upstream of the tandem repeat region was 48.3% identical to that of E. canis, but tandem repeat regions exhibited very limited homology (16.5% of amino acids). We subsequently determined that these glycoproteins were orthologous with the mucin-like protein previously described in E. ruminantium (2, 8) based on amino acid homology and genomic synteny. Comparison of the E. ruminantium Welgevonden strain mucin-like protein to E. canis gp36 and E. chaffeensis gp47 found that the prerepeat region is 34 to 35% conserved (amino acid) and of very low identity (14.5 to 18%) in the repeat region. The mucin-like protein of the E. ruminantium Gardel strain is conserved with the Welgevonden strain in the N-terminal nonrepeat region (80.6% amino acids) but has a divergent tandem repeat (Table 1) with low homology (17.5% amino acids) to the repeat of Welgevonden.
FIG. 1.
Genetic organization of the known mucin-like orthologs of E. canis, E. chaffeensis, and E. ruminantium. White bars represent the tandem repeat regions, and the gray bars represent length (base pairs) of regions upstream or downstream of the tandem repeats. E. canis strains illustrated include Jake (Ja), Oklahoma (Ok), and Demon (Dem); E. chaffeensis strains include Arkansas (Ark) and Sapulpa (Sap); and E. ruminantium strains include Highway (Hw), Welgevonden (Welg), and Gardel (Gar). Numbers in parentheses in the E. ruminantium repeat regions are the total number of repeat units present in each strain.
TABLE 1.
Summary of Ehrlichia tandem repeats present in glycoprotein orthologs
| Source | Strain | Repeat
|
Consensus tandem repeat sequence (amino acid) | ||
|---|---|---|---|---|---|
| Length (bp) | No. | Homology (%) | |||
| E. canis gp36 | Jake | 27 | 12.2 | 100 | TEDSVSAPA |
| Oklahoma | 27 | 5.2 | 100 | TEDSVSAPA | |
| Demon | 27 | 16.2 | 100 | TEDSVSAPA | |
| E. chaffeensis gp47 | Arkansas | 57 | 7.0 | 99 | ASVSEGDAVVNAVSQETPA |
| Sapulpa | 99 | 4.5 | 99 | EGNASEPVVSQEAAPVSESGDAANPVSSSENAS | |
| E. ruminantium mucin-like protein | Highway Welgevonden Gardel | 27 27 66 | 21.7 56.0 16.9 | 99 95 99 | VTSSPEGSV VTSSPEGSV SSEVTESNQGSSASVVGDAGVQ |
The genes encoding gp36 of E. canis Oklahoma and Demon strains as well as gp47 of the E. chaffeensis Sapulpa strain were sequenced to identify strain variations. Different E. canis strains had identical tandem repeat sequences but differed in the number of the repeats (Table 1). Interestingly, the Sapulpa strain of E. chaffeensis encoded a set of tandem repeats (4.5 repeats of 33 amino acids) entirely different from that found in the E. chaffeensis Arkansas strain (7 repeats of 19 amino acids). The E. chaffeensis gp47 amino acid sequences upstream of the repeat region were 97.5% homologous between strains but had a low homology (38.3%) in the tandem repeats. The region just upstream of the Arkansas tandem repeats encodes the amino acids Glu-Gly-Asn, which are the first three amino acids of the Sapulpa strain repeat, suggesting a more recent tandem repeat diversification. The nucleic acid sequence within the tandem repeats of each gp47 gene was highly conserved (at least 99%) within each strain (Table 1). Genome sequencing of several ehrlichial species and strains has allowed for comparisons of the gene loci. Interestingly, a hypothetical protein gene paralog containing no repeats was found immediately upstream of the genes encoding the mucin-like proteins of E. ruminantium (8). This paralog is also found in the E. ruminantium Gardel strain, but a paralog without repeats is not found in E. chaffeensis or E. canis.
Although the tandem repeat sequences varied greatly among species and strains, a conservation of amino acid usage was noted among the repeats. A total of 10 amino acids was used in the all of the repeats, with a particularly high occurrence of serine, threonine, alanine, proline, valine, and glutamic acid. Analysis of the glycoprotein amino acid sequence upstream of the repeats comparing it to that including the repeats until the termination codon demonstrated a substantial increase in usage of these amino acids (Table 2). Furthermore, glycosylation sites predicted by NetOGlyc were found only within the tandem repeats of the proteins. The threonine residues within the E. chaffeensis repeat were predicted sites for glycan attachment, and serine residues exhibited a high potential but were not identified as glycan attachment sites. Similarly, the E. ruminantium Gardel strain mucin-like protein contained a threonine and several serine residues that were slightly below the predicted threshold as sites of glycan attachment. Analysis of G+C content of the glycoprotein genes demonstrated a significant difference between the repeat regions (∼44%) and the nonrepeat regions (∼32%) among all genes.
TABLE 2.
Amino acid analysis of ehrlichial glycoproteinsa
| Source | Strain | Ser
|
Thr
|
Ala
|
Pro
|
Val
|
Glu
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-rpt | Rpt | Non-rpt | Rpt | Non-rpt | Rpt | Non-rpt | Rpt | Non-rpt | Rpt | Non-rpt | Rpt | ||
| E. canis gp36 | Jake | 6.4 | 22.0 | 4.1 | 11.9 | 5.3 | 22.0 | 4.1 | 11.0 | 5.3 | 11.0 | 3.5 | 11.0 |
| Oklahoma | 6.4 | 21.7 | 4.1 | 13.0 | 5.3 | 21.7 | 4.1 | 10.9 | 5.3 | 10.9 | 3.5 | 10.9 | |
| Demon | 6.4 | 22.1 | 4.1 | 11.7 | 5.3 | 22.1 | 4.1 | 11.0 | 5.3 | 11.0 | 3.5 | 11.0 | |
| E. chaffeensis gp47 | Arkansas | 9.2 | 15.8 | 3.3 | 4.5 | 6.0 | 21.8 | 3.3 | 5.3 | 8.7 | 21.1 | 5.4 | 10.5 |
| Sapulpa | 9.9 | 21.8 | 3.3 | 1.4 | 6.0 | 17.0 | 3.3 | 9.5 | 8.8 | 12.2 | 5.0 | 15.7 | |
| E. ruminantium mucin-like protein | Highway Welgevonden Gardel | 8.9 8.2 8.8 | 32.3 27.2 27.4 | 6.4 6.3 5.0 | 11.3 11.1 4.6 | 2.6 1.9 2.5 | 1.0 12.3 8.3 | 1.9 1.9 1.9 | 11.3 11.1 0 | 8.3 7.6 7.6 | 21.5 15.3 18.8 | 6.4 7.6 7.6 | 11.3 11.1 9.1 |
Non-rpt, all amino acids outside the tandem repeats; Rpt, tandem repeats.
Immunoreactivity and glycosylation of gp36 and gp47.
The recombinant E. canis gp36 reacted strongly with serum antibodies from a dog experimentally infected with E. canis (Fig. 2A). The fusion protein exhibited a molecular mass of approximately 60 kDa, which was significantly larger than that predicted by amino acid sequence (26.7 kDa), even with the addition of the 13-kDa expression partner. Expression of gp36 in the pBAD-Thio vector resulted in a shift in molecular weight larger than that seen in a separate vector without the thioredoxin fusion, but the recombinant protein cleaved free of thioredoxin exhibited a molecular mass of 36 kDa (data not shown). Carbohydrate was detected on the recombinant gp36 (Fig. 2B). The recombinant E. chaffeensis gp47 also exhibited strong immunoreactivity (Fig. 2C) and migrated at 60 kDa (larger than the predicted mass of 32.9 kDa plus 13 kDa from the fusion partner), and carbohydrate was detected (Fig. 2D). The recombinant thioredoxin control did not react with anti-E. canis or anti-E. chaffeensis dog serum (see Fig. 5).
FIG. 2.

E. canis gp36 and E. chaffeensis gp47 immunoreactivity and carbohydrate detection. Western immunoblot of recombinant Jake strain gp36 reacted with anti-E. canis dog serum (#2995) (A); carbohydrate detection (B). Western immunoblot of recombinant Arkansas strain gp47 reacted with anti-E. chaffeensis dog serum (#2495) (C); carbohydrate detection (D).
FIG. 5.

Western immunoblots of thioredoxin control (A to C, lanes 1) and the E. canis gp36 single repeat fusion protein (9 amino acids) (A and B, lanes 2) reacted with anti-thioredoxin (panel A) and anti-E. canis dog serum (#2995) (panel B). (C) Western immunoblot of the E. chaffeensis Arkansas strain gp47 single repeat fusion protein (19 amino acids) (lane 2) reacted with anti-E. chaffeensis dog serum (#2495).
Identification of native gp36 and gp47.
A native E. canis protein of a molecular mass of 36 kDa, which corresponded to the ∼37-kDa protein previously described (15), reacted by Western immunoblotting with monospecific mouse antiserum produced against the recombinant protein (Fig. 3A). A less prominent protein was also visualized at 34 kDa. Mouse anti-recombinant gp47 identified a 47-kDa protein in E. chaffeensis whole-cell lysates (Fig. 3B). Negative mouse serum was not reactive with E. canis or E. chaffeensis antigen (data not shown). E. chaffeensis whole-cell lysates were reacted with 10 HME patient sera that had detectable E. chaffeensis antibodies by IFA analysis. Seven of 10 sera recognized an immunoreactive 47-kDa protein identical in mass to the protein recognized by anti-recombinant gp47 serum by Western immunoblotting (Fig. 3B).
FIG. 3.
(A) Western immunoblot of E. canis Jake strain lysate with anti-recombinant gp36 (lane 1) and anti-E. canis dog serum (lane 2). (B) Reactivity of E. chaffeensis Arkansas lysate with HME patient sera (lanes 1 to 10), mouse anti-recombinant E. chaffeensis gp47 serum, and anti-E. chaffeensis dog serum (#2251).
Early antibody response to gp36.
Kinetic studies of the host response to E. canis demonstrated that an ∼37-kDa antigen was recognized earliest by antibodies in acute-phase sera from dogs experimentally infected with E. canis (15). Western immunoblotting confirmed that the recombinant gp36 was not recognized by preinoculation sera (day 0) (Fig. 4), but that antibodies were produced against gp36 in the early acute phase (day 14) (Fig. 4), reproducing kinetics similar to those reported with the corresponding native 37-kDa major immunoreactive antigen of E. canis (15). The antibody response against gp36 remained very strong through convalescence (day 56) (Fig. 4).
FIG. 4.
Kinetic antibody responses to E. canis Oklahoma strain gp36 (days [d] 0, 14, and 56) from 15 dogs (lanes 1 to 15) experimentally infected with E. canis.
Immunoreactivity of the gp36 and gp47 tandem repeats.
Western immunoblotting demonstrated that the carboxy-terminal tandem repeat region was highly immunoreactive, but the homologous amino-terminal regions preceding the tandem repeat regions of gp36 and gp47 were not immunoreactive (data not shown). A single repeat expressed as a recombinant fusion protein was recognized by anti-E. canis dog serum (Fig. 5A). The fusion protein containing the 9-mer demonstrated an electrophoretic shift larger than that corresponding to the predicted mass (∼1 kDa), suggesting that the peptide was posttranslationally modified and corroborating the NetOGlyc prediction that identified the repeat units as sites of glycan attachment (Fig. 5B). A fusion protein containing a single 19-mer repeat of E. chaffeensis gp47 was also recognized by anti-E. chaffeensis dog serum and migrated to a position indicating that it was ∼5 kDa larger (∼20 kDa) than predicted (15 kDa with fusion protein) (Fig. 5C). gp36 and gp47 were antigenically distinct, as neither reacted with heterologous antisera (Fig. 6A and B). Corroborating the importance of this epitope, monospecific antiserum produced against recombinant E. canis Oklahoma strain gp36 was capable of recognizing recombinant proteins from the Oklahoma, Jake, and Demon strains, but monospecific antiserum produced against E. chaffeensis Arkansas strain gp47 did not react with Sapulpa strain whole-cell lysate (data not shown).
FIG. 6.
E. canis gp36 and E. chaffeensis gp47 species-specific epitopes. Western immunoblots of native E. canis Jake strain gp36 (lanes 1), gp36 single repeat recombinant protein (lanes 2), native E. chaffeensis Arkansas strain gp47 (lanes 3), and gp47 single repeat recombinant protein reacted with anti-recombinant gp36 (A) and anti-recombinant gp47 (B) sera.
Carbohydrate is an important gp36 epitope determinant.
As each tandem repeat unit of E. canis gp36 was a predicted site of glycosylation and found to contain a major B-cell epitope, we hypothesized that attached glycans were important epitope determinants. To test this, recombinant E. canis gp36 was treated with periodate to test antibody recognition following structural modification of the glycan. The sham-treated gp36 reacted strongly with anti-E. canis dog serum by ELISA, while the periodate-treated recombinant gp36 was substantially reduced (Fig. 7A). To further confirm this observation, ELISA was used to test the recognition of recombinant fusion proteins with a single repeat (9-mer; TEDSVSAPA), a 12-mer (SVSAPATEDSVS), and two tandem repeat units (18-mer; TEDSVSAPATEDSVSAPA) from gp36 in comparison with nonglycosylated synthetic peptides with the identical sequences. Whereas all of the recombinant proteins were recognized with immune dog serum, the synthetic single repeat (9-mer) was not recognized at all, and the overlapping peptide (12-mer) exhibited minimal reactivity, demonstrating the importance of posttranslational modification for antibody binding to these peptides (Fig. 7B). The tandem repeat (18-mer) synthetic peptide was recognized by dog serum, although not as well as the recombinant, demonstrating the presence of a linear amino acid-based epitope present in a tandem repeat unit-containing peptide (18-mer) (Fig. 7B). Recognition of the recombinant E. chaffeensis repeat fusion protein exhibited an absorbance by ELISA higher than that of synthetic peptide (Fig. 7C).
FIG. 7.
Contribution of glycans to the antibody reactivity of E. canis Jake strain gp36 and E. chaffeensis gp47 as determined by ELISA. (A) Antibody reactivities of untreated and periodate-treated recombinant E. canis gp36 with anti-E. canis dog serum (#2995). (B) Immunoreactivities of the recombinant E. canis gp36 repeat fusion peptides containing the 9-mer, 12-mer, and 18-mer compared to those of aglycosylated synthetic peptides. (C) Immunoreactivities of the recombinant E. chaffeensis gp47 repeat fusion peptide (19-mer) and aglycosylated synthetic peptide with anti-E. chaffeensis dog serum (#2495). OD, optical density.
Cellular localization and secretion of gp36 and gp47.
Ehrlichiae exist in two distinct morphological forms known as reticulate and dense-cored cells (29). The localization of E. canis gp36 (Fig. 8A) and E. chaffeensis gp47 (Fig. 8B) by immunogold electron microscopy found that these proteins were differentially expressed only on the surface of the dense-cored form of the bacteria. gp36 and gp47 were also associated with the morula membranes containing the dense-cored morphological forms of the bacteria (Fig. 8A and B). The differential expressions of E. canis gp36 and E. chaffeensis gp47 were further confirmed by IFA analysis and confocal microscopy with infected cell slides by use of antisera against the conserved Dsb protein in addition to gp36 or gp47. The IFA demonstrated that E. canis gp36 (Fig. 9A) and E. chaffeensis gp47 (Fig. 9B) were expressed on a subset of ehrlichiae compared to Dsb (constitutively expressed).
FIG. 8.
Immunogold-labeled electron micrographs of E. canis gp36 and E. chaffeensis gp47 localization. (A) E. canis morulae containing the reticulate (R) and dense-cored (DC) morphological forms. (B) E. chaffeensis morulae containing the reticulate and dense-cored morphological forms.
FIG. 9.
Confocal immunofluorescent photomicrographs of E. canis gp36 and E. chaffeensis gp47 expression. Cells infected with E. canis (A) or E. chaffeensis (B) were dually stained with anti-ehrlichial Dsb (18) (green; constitutive; top and bottom) and with anti-gp36 (red; top) or anti-gp47 (red; bottom) sera; merged photomicrographs demonstrate singly (green) and dually (yellow) labeled ehrlichiae.
E. canis gp36 and E. chaffeensis gp47 were also found to be the predominant immunoreactive proteins secreted into the cell culture supernatant (Fig. 10A and B, lanes 1). E. canis gp36 and E. chaffeensis gp47 were conclusively identified in the supernatant fractions with anti-recombinant gp36 and gp47 sera (Fig. 10A and B, lanes 2). The gp36 and gp47 proteins were not observed in uninfected DH82 supernatants (data not shown).
FIG. 10.

Secreted E. canis and E. chaffeensis immunoreactive proteins. (A) Western immunoblots of concentrated supernatants from E. canis-infected DH82 cells with anti-E. canis dog serum (lane 1) and mouse anti-recombinant E. canis gp36 serum (lane 2). (B) Western immunoblots of supernatants from E. chaffeensis-infected DH82 cells with anti-E. chaffeensis dog serum (lane 1) and mouse anti-recombinant E. chaffeensis gp47 serum (lane 2).
DISCUSSION
Our previous study of the kinetic antibody responses to E. canis revealed two major immunoreactive antigens (36- and 19-kDa proteins) as dominant targets of the early host antibody response (15). Antigens recognized early in the host immune response provide evidence of those that may be especially important in the initial stages of infection of the mammalian host and thus are high-priority targets for molecular identification and for vaccine development. In this study, we have conclusively identified and molecularly characterized the E. canis 36-kDa major immunoreactive protein and found that, consistent with several other major ehrlichial immunoreactive proteins, it is glycosylated. In addition, a divergent and antigenically distinct 47-kDa ortholog in E. chaffeensis, also a major immunoreactive protein consistently recognized by antibodies from HME patients, was identified. Other major immunoreactive proteins that have been molecularly characterized in E. canis include three glycoproteins (gp200, gp140, and p28/p30) (16, 20, 34), and the identification of the gp36 in E. canis further supports glycosylation as an important ehrlichial posttranslational modification on several surface-exposed proteins.
E. canis gp36 and E. chaffeensis gp47 have considerable nucleic acid and amino acid divergence in regions containing the serine/threonine-rich tandem repeats. The recent genome sequence analysis of E. ruminantium (8) has identified a high frequency of genes containing tandem repeat units. None of the known glycoprotein repeat regions share significant conserved sequences among themselves, but we discovered that all have high serine and threonine content in addition to alanine, proline, valine, and glutamic acid residues, which have been reported to serve as recognition motifs for O-glycan attachment (23, 35). The E. canis gp140 and the E. chaffeensis gp120 orthologs have longer, genetically divergent tandem repeats (20) that also exhibit the amino acid bias. The repeat units contained the only sites of O-glycan attachment, as predicted by NetOGlyc, and not all of the serines and threonines were identified as potential glycan attachment sites. However, as NetOGlyc was developed with data from eukaryotic glycoproteins, it is quite possible that the threshold for ehrlichial glycosylation is lower and that these are sites of glycosylation.
As with other known ehrlichial glycoprotein orthologs, gp36 and gp47 are antigenically divergent. The consistent immunodominance and divergence of the tandem repeats of E. chaffeensis gp47 suggest that the immune response creates strong selective pressures to alter the sequence of the repeats. However, all E. canis strains tested contained identical tandem repeats but had variable repeat numbers (5 to 16), even though gp36 is strongly recognized by antibody. E. chaffeensis exhibited more divergence (amino acid sequence and repeat number) in the tandem repeat regions from the two isolates we examined (Arkansas and Sapulpa). Three of 10 HME patient sera tested did not react with the gp47 from the E. chaffeensis Arkansas strain, and the divergence in the repeat region and the lack of cross-reactive antibodies could explain the inconsistency of gp47 recognition by sera from different patients. A search for orthologous tandem repeat DNA sequences throughout the genome does not detect pseudogenes or other sources for the divergent repeats. The high level of sequence conservation within the tandem repeats of each strain suggests that duplication occurred after diversification of the repeat-encoding DNA. The discovery of a new pair of surface-expressed orthologs with repeat units is a point of interest in an obligate intracellular organism that has undergone reductive genome evolution (1) and thus leads to the hypothesis that there may be a selective advantage to increasing and retaining the glycosylated repeat units of these proteins.
Although gp36 and gp47 have considerable homology in the amino-terminal regions upstream of the repeat regions, the immunoreactive regions were localized to the carboxy-terminal regions of the proteins, which contain the tandem repeats. We determined that single repeats from E. canis gp36 (9-mer) and E. chaffeensis gp47 (19-mer) expressed as recombinant peptides were sufficient for antibody recognition by immune sera, demonstrating that they contain repeated epitopes. Similarly, the repeat regions of E. canis gp120 and E. chaffeensis gp140 contain major antibody epitopes, although the contribution of glycans to the reactivity of these proteins is not known (20). Interestingly, periodate treatment of E. canis gp36 greatly reduced antibody recognition by convalescent dog serum, providing the first evidence of nonpeptide epitope determinants for complete antibody recognition of ehrlichial glycoproteins. This was corroborated by using synthetic peptides of the E. canis gp36 repeat (9-mer, 12-mer, and 18-mer) and the E. chaffeensis repeat (19-mer) in comparison with recombinant fusion proteins expressing the same peptides. The synthetic peptides, especially the shorter peptides, were recognized far less efficiently by immune dog serum than the recombinant forms, demonstrating the importance of carbohydrate modification in optimal antibody recognition. Of note is the lower immunoreactivity of synthetic peptides by ELISA, which occurs despite the fact that the synthetic peptides containing the epitope are likely to be present in high numbers compared to those of the synthetic peptides, due to the additional protein mass of the thioredoxin fusion partner (13 kDa). The immunoreactivity of the repeat unit peptides (E. canis 18-mer; E. chaffeensis 19-mer) is consistent with previous observations reported using a three-repeat (27-mer) peptide from the E. ruminantium mucin-like protein ortholog (2). However, the antibody recognition of the E. ruminantium 27-mer synthetic repeat did not exhibit a high absorbance by ELISA, even with low serum dilutions (1:25 to 1:200) (2), suggesting that the immunoreactivity of this repeat unit may also be stronger as a recombinant protein.
In the absence of organelles for protein trafficking, it was long believed that prokaryotes did not contain the cellular machinery needed to modify proteins with carbohydrates. Even E. coli, used for many years to express aglycosylated eukaryotic proteins, has been found to modify its own proteins with carbohydrate moieties (13). Several human bacterial pathogens have now been discovered to express glycoproteins, and the few prokaryotic glycoproteins functionally characterized contribute to adhesion, structural stability, and mobility; they are also targets of the immune system (4, 33, 37). These characteristics demonstrate the potential roles of bacterial glycoproteins in pathogenesis and immunity (4). Significantly, the existence of carbohydrate-dependent antibody epitopes of recombinant gp36 and gp47 expressed in E. coli demonstrates that glycans and attachment sites are conserved between native ehrlichial proteins and recombinant glycoproteins expressed in E. coli. This suggests that the mechanisms for glycosylation are conserved between Ehrlichia and E. coli. However, glycosyltransferases homologous to those present in E. coli have not been identified in the E. canis genome (14), suggesting that these enzymes contain unique sequences and are among the hypothetical proteins of unknown function. Based on the dependence of posttranslational modification for glycoprotein epitope reactivity, the recombinant ehrlichial glycoproteins appear to be very similar to the native proteins in structure and composition and thus are appropriate surrogates for native proteins in studies to determine functions and roles as immunoprotective antigens.
Several ehrlichial proteins, including gp120 and the ferric ion-binding protein, have been identified outside the bacterial cell. Furthermore, E. chaffeensis gp120 has been demonstrated to be differentially expressed on the surface of dense-cored E. chaffeensis and extracellularly on fibrils in the morula matrix. Immunogold electron microscopy demonstrated that E. canis gp36 and E. chaffeensis gp47 are also differentially expressed on the surface of dense-cored ehrlichiae. The dense-cored and reticulate cell morphological forms of ehrlichiae are thought to be homologous to the infectious elementary bodies of Chlamydia trachomatis and the metabolically active reticulate body, respectively. This observation suggests that these orthologous glycoproteins may play an important role in ehrlichial infection. The cell surface expression of these glycoproteins leads to the hypothesis that they could function as adhesins. Carbohydrate-lectin interactions are common means of bacterial adhesion, and E. chaffeensis has been demonstrated to use L- and E-selectins to mediate cellular binding (41). Furthermore, repeat-containing proteins from Anaplasma marginale, including the mucin-like protein ortholog from Ehrlichia ruminantium, adhere to tick cells (9).
gp36 and gp47 are minor constituents in whole-cell lysates, but we found substantial amounts of both in supernatants of infected cells. Interestingly, these were the abundant immunoreactive proteins found in the supernatants in which other known surface proteins, such as p28/p30, were not detected, indicating that gp36 was indeed secreted and was not associated with intact outer membranes in the supernatants. The secretion of gp36 in the tick salivary gland or in the mammalian host may provide a partial explanation for the early host immune response to this glycoprotein. A signal sequence suggesting the involvement of a sec-dependent secretion mechanism, such as type II or type IV (21), was identified on gp36. Genes encoding type IV secretion machinery have been identified in E. canis, E. chaffeensis, and E. ruminantium (8, 11, 26) as the role of type IV secretion of bacterial virulence factors has become better recognized (18).
Kinetics of the antibody response to E. canis gp36 suggest that this antigen may be useful for recombinant immunodiagnostics. Current commercially available diagnostic assays for canine monocytic ehrlichiosis are based on p28/p30 proteins, and gp36 may provide substantially increased sensitivity for this application. Furthermore, antibodies directed at the E. canis gp36 antigen were not cross-reactive with E. chaffeensis, which suggests that it could also be useful in developing species-specific immunodiagnostic assays. Similar serologic species specificity has been reported for antigenically divergent E. canis and E. chaffeensis orthologs gp120/gp140 as well as the gp200s (16, 39).
Acknowledgments
We thank David H. Walker and Xue-jie Yu for critical reviews and suggestions. We acknowledge Xiaofeng Zhang and Ana Maria Cardenas for thoughtful discussions and technical support.
This work was supported by the Clayton Foundation for Research, the John Sealy Memorial Foundation, and the Sealy Center for Vaccine Development.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Andersson, S. G., and C. G. Kurland. 1998. Reductive evolution of resident genomes. Trends Microbiol. 6:263-268. [DOI] [PubMed] [Google Scholar]
- 2.Barbet, A. F., W. M. Whitmire, S. M. Kamper, B. H. Simbi, R. R. Ganta, A. L. Moreland, D. M. Mwangi, T. C. McGuire, and S. M. Mahan. 2001. A subset of Cowdria ruminantium genes important for immune recognition and protection. Gene 275:287-298. [DOI] [PubMed] [Google Scholar]
- 3.Benson, G. 1999. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res. 27:573-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benz, I., and M. A. Schmidt. 2002. Never say never again: protein glycosylation in pathogenic bacteria. Mol. Microbiol. 45:267-276. [DOI] [PubMed] [Google Scholar]
- 5.Breitschwerdt, E. B., B. C. Hegarty, and S. I. Hancock. 1998. Sequential evaluation of dogs naturally infected with Ehrlichia canis, Ehrlichia chaffeensis, Ehrlichia equi, Ehrlichia ewingii, or Bartonella vinsonii. J. Clin. Microbiol. 36:2645-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, S. M., L. C. Cullman, and D. H. Walker. 1997. Western immunoblotting analysis of the antibody responses of patients with human monocytotropic ehrlichiosis to different strains of Ehrlichia chaffeensis and Ehrlichia canis. Clin. Diagn. Lab. Immunol. 4:731-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, S. M., J. S. Dumler, H. M. Feng, and D. H. Walker. 1994. Identification of the antigenic constituents of Ehrlichia chaffeensis. Am. J. Trop. Med. Hyg. 50:52-58. [PubMed] [Google Scholar]
- 8.Collins, N. E., J. Liebenberg, E. P. de Villiers, K. A. Brayton, E. Louw, A. Pretorius, F. E. Faber, H. van Heerden, A. Josemans, M. van Kleef, H. C. Steyn, M. F. van Strijp, E. Zweygarth, F. Jongejan, J. C. Maillard, D. Berthier, M. Botha, F. Joubert, C. H. Corton, N. R. Thomson, M. T. Allsopp, and B. A. Allsopp. 2005. The genome of the heartwater agent Ehrlichia ruminantium contains multiple tandem repeats of actively variable copy number. Proc. Natl. Acad. Sci. USA 102:838-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de la Fuente, J., J. C. Garcia-Garcia, A. F. Barbet, E. F. Blouin, and K. M. Kocan. 2004. Adhesion of outer membrane proteins containing tandem repeats of Anaplasma and Ehrlichia species (Rickettsiales: Anaplasmataceae) to tick cells. Vet. Microbiol. 98:313-322. [DOI] [PubMed] [Google Scholar]
- 10.Doyle, C. K., X. Zhang, V. L. Popov, and J. W. McBride. 2005. An immunoreactive 38-kilodalton protein of Ehrlichia canis shares structural homology and iron-binding capacity with the ferric ion-binding protein family. Infect. Immun. 73:62-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felek, S., H. Huang, and Y. Rikihisa. 2003. Sequence and expression analysis of virB9 of the type IV secretion system of Ehrlichia canis strains in ticks, dogs, and cultured cells. Infect. Immun. 71:6063-6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Julenius, K., A. Molgaard, R. Gupta, and S. Brunak. 2005. Prediction, conservation analysis, and structural characterization of mammalian mucin-type O-glycosylation sites. Glycobiology 15:153-164. [DOI] [PubMed] [Google Scholar]
- 13.Lindenthal, C., and E. A. Elsinghorst. 1999. Identification of a glycoprotein produced by enterotoxigenic Escherichia coli. Infect. Immun. 67:4084-4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mavromatis, K., C. K. Doyle, A. Lykidis, N. Ivanova, P. Chain, F. Larimer, J. Copeland, J. C. Detter, M. Land, P. M. Richardson, X. J. Yu, D. H. Walker, J. W. McBride, and N. C. Kyrpides. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 15.McBride, J. W., R. E. Corstvet, S. D. Gaunt, C. Boudreaux, T. Guedry, and D. H. Walker. 2003. Kinetics of antibody response to Ehrlichia canis immunoreactive proteins. Infect. Immun. 71:2516-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McBride, J. W., J. E. Comer, and D. H. Walker. 2003. Novel immunoreactive glycoprotein orthologs of Ehrlichia spp. Ann. N. Y. Acad. Sci. 990:678-684. [DOI] [PubMed] [Google Scholar]
- 17.McBride, J. W., R. E. Corstvet, E. B. Breitschwerdt, and D. H. Walker. 2001. Immunodiagnosis of Ehrlichia canis infection with recombinant proteins. J. Clin. Microbiol. 39:315-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McBride, J. W., L. M. Ndip, V. L. Popov, and D. H. Walker. 2002. Identification and functional analysis of an immunoreactive DsbA-like thio-disulfide oxidoreductase of Ehrlichia spp. Infect. Immun. 70:2700-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McBride, J. W., X. Yu, and D. H. Walker. 2000. A conserved, transcriptionally active p28 multigene locus of Ehrlichia canis. Gene 254:245-252. [DOI] [PubMed] [Google Scholar]
- 20.McBride, J. W., X. J. Yu, and D. H. Walker. 2000. Glycosylation of homologous immunodominant proteins of Ehrlichia chaffeensis and E. canis. Infect. Immun. 68:13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagai, H., and C. R. Roy. 2003. Show me the substrates: modulation of host cell function by type IV secretion systems. Cell. Microbiol. 5:373-383. [DOI] [PubMed] [Google Scholar]
- 22.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 23.O'Connell, B., L. A. Tabak, and N. Ramasubbu. 1991. The influence of flanking sequences on O-glycosylation. Biochem. Biophys. Res. Commun. 180:1024-1030. [DOI] [PubMed] [Google Scholar]
- 24.Ohashi, N., Y. Rikihisa, and A. Unver. 2001. Analysis of transcriptionally active gene clusters of major outer membrane protein multigene family in Ehrlichia canis and E. chaffeensis. Infect. Immun. 69:2083-2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohashi, N., A. Unver, N. Zhi, and Y. Rikihisa. 1998. Cloning and characterization of multigenes encoding the immunodominant 30-kilodalton major outer membrane proteins of Ehrlichia canis and application of the recombinant protein for serodiagnosis. J. Clin. Microbiol. 36:2671-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohashi, N., N. Zhi, Q. Lin, and Y. Rikihisa. 2002. Characterization and transcriptional analysis of gene clusters for a type IV secretion machinery in human granulocytic and monocytic ehrlichiosis agents. Infect. Immun. 70:2128-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohashi, N., N. Zhi, Y. Zhang, and Y. Rikihisa. 1998. Immunodominant major outer membrane proteins of Ehrlichia chaffeensis are encoded by a polymorphic multigene family. Infect. Immun. 66:132-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paddock, C. D., and J. E. Childs. 2003. Ehrlichia chaffeensis: a prototypical emerging pathogen. Clin. Microbiol. Rev. 16:37-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Popov, V. L., S. M. Chen, H. M. Feng, and D. H. Walker. 1995. Ultrastructural variation of cultured Ehrlichia chaffeensis. J. Med. Microbiol. 43:411-421. [DOI] [PubMed] [Google Scholar]
- 30.Popov, V. L., X. J. Yu, and D. H. Walker. 2000. The 120-kDa outer membrane protein of Ehrlichia chaffeensis: preferential expression on dense-core cells and gene expression in Escherichia coli associated with attachment and entry. Microb. Pathog. 28:71-80. [DOI] [PubMed] [Google Scholar]
- 31.Reddy, G. R., C. R. Sulsona, A. F. Barbet, S. M. Mahan, M. J. Burridge, and A. R. Alleman. 1998. Molecular characterization of a 28 kDa surface antigen gene family of the tribe Ehrlichieae. Biochem. Biophys. Res. Commun. 247:636-643. [DOI] [PubMed] [Google Scholar]
- 32.Rikihisa, Y., S. A. Ewing, J. C. Fox, A. G. Siregar, F. H. Pasaribu, and M. B. Malole. 1992. Analyses of Ehrlichia canis and a canine granulocytic Ehrlichia infection. J. Clin. Microbiol. 30:143-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt, M. A., L. W. Riley, and I. Benz. 2003. Sweet new world: glycoproteins in bacterial pathogens. Trends Microbiol. 11:554-561. [DOI] [PubMed] [Google Scholar]
- 34.Singu, V., H. Liu, C. Cheng, and R. R. Ganta. 2005. Ehrlichia chaffeensis expresses macrophage- and tick cell-specific 28-kilodalton outer membrane proteins. Infect. Immun. 73:79-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thanka Christlet, T. H., and K. Veluraja. 2001. Database analysis of O-glycosylation sites in proteins. Biophys. J. 80:952-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Troy, G. C., and S. D. Forrester. 1990. Canine ehrlichiosis, p. 404-418. In C. E. Green (ed.), Infectious diseases of the dog and cat. The W. B. Saunders Co., Philadelphia, Pa.
- 37.Upreti, R. K., M. Kumar, and V. Shankar. 2003. Bacterial glycoproteins: functions, biosynthesis and applications. Proteomics 3:363-379. [DOI] [PubMed] [Google Scholar]
- 38.Yu, X. J., P. Crocquet-Valdes, and D. H. Walker. 1997. Cloning and sequencing of the gene for a 120-kDa immunodominant protein of Ehrlichia chaffeensis. Gene 184:149-154. [DOI] [PubMed] [Google Scholar]
- 39.Yu, X. J., J. W. McBride, C. M. Diaz, and D. H. Walker. 2000. Molecular cloning and characterization of the 120-kilodalton protein gene of Ehrlichia canis and application of the recombinant 120-kilodalton protein for serodiagnosis of canine ehrlichiosis. J. Clin. Microbiol. 38:369-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu, X. J., J. W. McBride, X. F. Zhang, and D. H. Walker. 2000. Characterization of the complete transcriptionally active Ehrlichia chaffeensis 28 kDa outer membrane protein multigene family. Gene 248:59-68. [DOI] [PubMed] [Google Scholar]
- 41.Zhang, J. Z., J. W. McBride, and X. J. Yu. 2003. L-selectin and E-selectin expressed on monocytes mediating Ehrlichia chaffeensis attachment onto host cells. FEMS Microbiol. Lett. 227:303-309. [DOI] [PubMed] [Google Scholar]