Abstract
The receptors involved in the sampling of particulate microbial antigens by the gut are largely unknown. Here we demonstrate for the first time in an in vitro M-cell model and in situ in isolated murine intestinal segments that the receptors TLR-4, PAF-R, and α5β1 integrin are all involved in mediating bacterial uptake associated with transcytosis. The pattern of expression of TLR-4 and α5β1 integrin differed between M cells and enterocytes. There was increased apical expression of TLR-4 in M-cell cultures, and it was present on the apical surface of murine M cells but not enterocytes in situ. In contrast, PAF-R was expressed equally by both cell types in vitro and was abundantly expressed throughout the intestinal epithelium. Inhibition of TLR-4 and PAF-R, but not TLR-2, reduced gram-negative bacterial uptake by both cell types, whereas inhibition of the apically expressed α5β1 integrin significantly reduced the ability of M cells to translocate bacteria. Hence, the involvement of each receptor was dependent not only on differences in the level of receptor expression but the cellular localization. Using bacteria that had mutations that affected the bacterial lipooligosaccharide structure indicated that the oligosaccharide moiety was important in bacterial uptake. Taken together, the data suggest that pathogen-associated molecular pattern interactions with pattern recognition receptors are key factors in M-cell recognition of intestinal antigens for mucosal immune priming.
The sampling of particulate microbial antigen by epithelial cells of the gastrointestinal tract resulting in stimulation of the mucosal immune system has long been thought of as a specific receptor-driven event. These receptors are considered to be specific to the microfold (M) cells that are found within the follicle-associated epithelia (FAE) that overlie the mucosal immune inductive tissue of the Peyer's patches of the intestine (6, 16, 17). Researchers have demonstrated that interactions between pathogenic bacteria and the gut epithelia rely on several pathways, such as receptor-mediated uptake as demonstrated by Yersinia enterocolytica invasin-β1 integrin interactions (8); type III secretion systems, such as the translocated intimin receptor (Tir)-dependent disruption of the intestinal epithelia by enteropathogenic Escherichia coli (13); and the paracellular invasion strategy used by Campylobacter enteritis (10). However, the species-specific variability of potential M-cell receptors (12) and a failure to find receptors that are specific to human M cells that are not present elsewhere on the gut lining has thwarted research efforts to target mucosal antigen uptake for the induction of mucosal immune responses.
Prokaryotes contain molecular motifs that are unique to microorganisms. These motifs, known as common microbial pathogen-associated molecular patterns (PAMPs), are recognized by the innate immune system through pattern recognition receptors (PRRs), either through direct receptor-bacterial ligand or endogenous adaptor-bacterial molecule interactions (7). For example, Toll-like receptor 4 (TLR4), in association with CD14 and MD-2, recognizes lipopolysaccharide (LPS) expressed by gram-negative bacteria (2); TLR-2 recognizes lipotechoic acids expressed by gram-positive bacteria (23); platelet-activating factor receptor (PAF-R), the receptor for PAF, which is a phospholipid that has messenger functions, also binds to the phosphorylcholine (ChoP) moiety of the surface lipooligosaccharide (LOS) molecules on bacteria (22); and, α5β1 integrin binds, through endogenous fibronectin, to fibronectin-binding proteins expressed by many bacteria (19). To date, with the exception of α5β1 integrin, the expression of PRRs by follicular associated epithelial cells has not been described, and neither has their involvement in gut microbial particulate antigen sampling been demonstrated. We approached this challenge from two directions and have now identified specific receptors for particulate microbial antigen uptake and functional differences that allow M cells rather than enterocytes to recognize and deliver antigen (transcytosis) to the underlying gut-associated lymphoid tissues (GALT). An in vitro coculture model of M cells was developed (14) and subsequently modified and validated (24). Approximately 30% of the cells within the coculture monolayers display M-cell characteristics such as enhanced energy-dependent microsphere transcytosis, loss of apical alkaline phosphatase expression, and redistribution of α5β1 integrin (24). This model has allowed us to describe the relative distribution and expression levels of four specific PRRs on M cells, to demonstrate their involvement in antigen sampling, and to substantiate these results in situ in a mouse intestinal segment model.
MATERIALS AND METHODS
Cell culture.
The growth of the Caco-2 and the culture of M cells was performed as described previously (24). Briefly, Caco-2 cells were cultured in 75-cm2 tissue culture flasks in Caco-2 medium (Dulbecco modified Eagle medium with GlutaMax I, 4,500 g of glucose/liter, pyridoxal [Invitrogen, Mount Waverley, Victoria, Australia] supplemented with 10% [vol/vol] fetal calf serum [CSL, Parkville, Victoria, Australia], 1% [vol/vol] nonessential amino acids [Invitrogen], and 1% [vol/vol] antibiotic and antimycotic [Invitrogen]) in a humidified atmosphere at 37°C in 5% CO2, until they had reached confluence. After trypsinization of the Caco-2 cells, the cells were seeded onto the uppermost surfaces of inverted 6.5-mm, 3.0-μm-pore-size, polycarbonate transwells (Corning/Costar, Lindfield, NSW, Australia) and incubated overnight at 37°C to allow the cells to attach. The transwells were reinserted into their 24-well dishes and cultured in Caco-2 medium for 21 to 28 days to allow the cells to fully differentiate. Prior to coculture, the transepithelial electrical resistances (TEER) of the cells were checked by use of an EVOM (World Precision Instruments, Coherent Scientific Pty., Ltd, Hilton, South Australia), and cultures with readings greater than 150 Ω · cm2 were considered suitable for coculture. M-cell cocultures were produced by introducing 0.1 ml of 106 cells/ml freshly isolated murine Peyer's patch cells in Caco-2 medium. The cultures were then incubated at 37°C in 5% CO2 for 2 days prior to experimentation. TEER levels were continually monitored and increased to greater than 300 Ω · cm2 throughout the use of the cultured cells.
Identification and quantification of PRRs In vitro.
Culture media from the 2-day-old M cell cocultures and Caco-2 monocultures were discarded, and the cells were washed three times with Dulbecco phosphate-buffered saline (D-PBS; Invitrogen). The cells were then fixed in 10% neutral buffered formalin for 10 min and washed again. Nonspecific binding sites were blocked by incubation of the samples in 2% (wt/vol) bovine serum albumin (BSA; Sigma, Sydney, NSW, Australia) in D-PBS for 1 h at 37°C. The samples were probed with 1 μg each of rabbit anti-TLR-2 (Zymed, Bio-Scientific, Pty., Ltd., Gymea, NSW, Australia), anti-TLR-4 (Zymed), or anti-PAF-R (Cayman Chemical, Sapphire Bioscience, Pty., Ltd., Crows Nest, NSW, Australia)/ml for 1 h at 37°C and then washed three times in D-PBS. Primary antibodies were probed with donkey anti-rabbit Alexa 488 conjugate (1:200; Molecular Probes, Mount Waverley, Victoria, Australia) for 1 h at 37°C, before washing and counterstaining with 10 μg of DAPI (4′,6′-diamidino-2-phenylindole; Sigma)/ml for 15 min at room temperature. The transwell membranes were then excised with a scalpel blade and mounted onto poly-l-lysine-coated microscope slides. Glass coverslips were mounted with ProLong AntiFade Kit (Molecular Probes) according to the manufacturer's instructions.
The slides were imaged by fluorescence microscopy and a MicroMax cooled charge-coupled device camera (Princeton Instruments, Trenton, NJ). The total area of staining of TLR-2, TLR-4, and PAF-R for each slide was analyzed by MetaMorph software (Universal Imaging Corp., Ltd., Marlow, Buckinghamshire, United Kingdom). Three areas of five transwells for each receptor and culture system were analyzed.
Identification of PRRs in murine intestine.
Eight-week-old male specific-pathogen-free BALB/c mice were killed by cervical dislocation, and their intestines were excised. (The use of animals in this study was approved by the University of Canberra Animal Ethics Committee.) Rings of tissue ca. 5 mm in length from a section of the jejunum proximal to the stomach containing Peyer's patches and villus epithelium were removed and fixed overnight in 10% (vol/vol) neutral buffered formalin. Tissue samples were then processed for paraffin embedding with a Hypercenter XL tissue processor, and 5-μm sections were cut from the embedded material with a microtome (model HM325; Microm GmbH, Waldorf, Germany), and mounted onto poly-l-lysine-coated microscope slides. The samples were stored at 4°C until further processing. The samples were then dewaxed and rehydrated according to standard procedures before immunofluorescence staining.
To unmask antigens that were cross-linked by the fixation procedure and incapable of binding to primary antibodies, the sections were subjected to antigen retrieval. A 10× stock of pH 7.8 citrate buffer (containing 3.2 g of sodium citrate, 5.0 g of sodium EDTA, and 2.5 g of Tris base/liter) was diluted 1/10, and 500 μl of Tween 20 was added. This solution was dispensed into 30-ml microscope slide staining chambers and immersed into a water bath at 95°C to equilibrate. Once the citrate buffer solution had reached 95°C, the slides were inserted into the chambers and immersed into the water bath for 20 min. The slides were then removed and rinsed three times in deionized water.
Nonspecific binding sites in the tissue sections were blocked in blocking buffer (2% [wt/vol] BSA in D-PBS) for 1 h at 37°C, and then primary antibodies directed against TLR-2, TLR-4, and PAF-R were added at a concentration of 1 μg/ml in blocking buffer, and the samples were incubated for 1 h at 37°C. After three washes in D-PBS, secondary conjugate antibodies (1-in-200 dilution) and 1 μg of TRITC (tetramethyl rhodamine isothiocyanate) Ulex europeaus I lectin (UEA-1; to identify murine M cells; Sigma)/ml, each in blocking buffer, were added for 1 h at 37°C. Control sections were stained with only conjugate to control for nonspecific staining by the secondary antibodies. After the samples were washed, the sections were counterstained with 1 μg of DAPI/ml for 15 min and washed in deionized water, and then coverslips were mounted with ProLong mounting medium. The medium was allowed to set overnight before the slides were sealed with clear nail varnish. The slides were photographed as described above for the in vitro samples.
Bacteria.
All bacteria used in the present study have been previously described. The two nontypeable H. influenzae (NTHi) wild-type clinical isolates used were NTHi 289 (5) and NTHi 2019 (3), and the mutant NTHi strains were NTHi 2019 (licD::kan) and NTHi 2019 (pgmB::erm) (22). NTHi was grown overnight at 37°C in 5% CO2 on brain heart infusion agar supplemented with 50 ml of defibrinated horse blood per liter of agar. The agar for the mutant strains was supplemented with kanamycin (15 μg/ml) or erythromycin (5 μg/ml).
Receptor antagonism of in vitro bacterial translocation.
The harvested bacteria were labeled with 10 μM chloromethylfluorescein diacetate (CMFDA; Molecular Probes) in D-PBS overnight at 37°C and then washed in D-PBS. The bacteria were killed by suspension in 1% (vol/vol) formaldehyde for 2 h at 37°C and then washed three times. The killed, labeled bacteria were stored at 4°C, protected from light, until required (within 1 week).
The lower chambers (apical surface) of the M-cell cocultures and Caco-2 monocultures were incubated in the presence of the specific receptor antagonists in Caco-2 medium for 1 h at 37°C in 5% CO2. The concentration of the agonists were anti-TLR-2, anti-TLR-4, and anti-α5β1 integrin at 5 μg/ml and PAF-16 antagonist at 10 μM.
The inhibitor solutions were replaced with 600 μl of 5 × 108 CFU/ml of CMFDA-labeled killed NTHi in respective inhibitor solution, and the cultures were incubated in a shaking incubator (Bioline; Edwards Instrument Company) at 37°C for 2 h. The numbers of bacteria translocated from the lower chamber (apical surface) to the upper chamber (basolateral surface) was analyzed by counting labeled NTHi in the upper chamber media by using a Coulter EPICS-XL/MCL flow cytometer.
Receptor antagonism of ex vivo bacterial translocation.
Male specific-pathogen-free BALB/c mice were killed by cervical dislocation, and their intestines were excised above the cecum. Lengths of jejunum (4 cm), just below the stomach and containing at least one Peyer's patch, were removed. One end of each isolated intestinal segment was closed by tying it with suture thread. The intestinal segments were filled by injection with labeled NTHi (3 × 1010 CFU/ml) in HEPES buffered Kreb's Ringer bicarbonate solution (20 mM HEPES, 107 mM NaCl, 5 mM KCl, 3 mM CaCl2, 1 mM MgSO4, 7 mM NaHCO3, 0.1% [wt/vol] BSA, and 10 mM glucose) with antagonist (0.5 μg of anti-TLR-2, anti-TLR-4, or anti-α5β1 integrin/ml or 10 μM PAF-16 antagonist). Control segments were filled with only bacteria and buffer. The open end was tied with a suture, and the segments were incubated in the aerated HEPES buffered Kreb's Ringer bicarbonate solution for 2 h at 37°C. One segment was used for each combination of antagonist and bacteria. The experiments using these segments were conducted within 2 h of collection of the tissue since this was the time interval determined suitable for minimal segment deterioration (21).
After incubation, the intestinal segments were removed, and the Peyer's patches were cut out. The Peyer's patch segments were fixed, processed, paraffin embedded, and mounted on poly-l-lysine-coated slides. The samples were washed in deionized water prior to immunofluorescence staining. The tissue sections were permeabilized in 0.05% Tween 20 for 15 min at room temperature to allow access to intracellular antigens. The slides were then blocked in 2% (vol/vol) BSA in D-PBS to block nonspecific binding by antibodies. The CMFDA dye, conjugated to bacterial proteins, was probed with 1 μg of anti-fluorescein antibody/ml in 2% BSA in D-PBS, followed by the addition of 1 μg of Alexa 488 conjugate/ml. M cells were detected with 1 μg of TRITC UEA-1/ml. The slides were counterstained with 1 μg of DAPI/ml, and coverslips were mounted with the ProLong Antifade Kit and allowed to set overnight before sealing them with nail varnish.
The slides were observed with a Nikon TE300 inverted epifluorescence microscope using a ×100 PlanFluor objective. The degree of uptake of bacteria by Peyer's patches and villus segments in untreated and treated intestines was measured by scoring each section between 0 and 5 as described in Table 1.
TABLE 1.
Scoring system for analysis of uptake of bacteria into murine Peyer's patches
| Score | No. of bacteria counted |
|---|---|
| 5 | >50 |
| 4 | 16-50 |
| 3 | 6-15 |
| 2 | 3-5 |
| 1 | 1 or 2 |
| 0 | 0 |
Statistical analysis.
Statistical significance between results was analyzed by using SPSS software. Quantitative differences for receptors in vitro were assessed by using a Student's t test, receptor antagonism of bacterial translocation in vivo was analyzed by using a Mann-Whitney test, and receptor antagonism of bacterial translocation ex vivo was examined by a one-sided multivariate General Linear Model with Dunnet's post hoc analysis. P values of <0.05 were considered statistically significant.
RESULTS
Expression intensity and location of receptors differs on M cells compared to enterocytes.
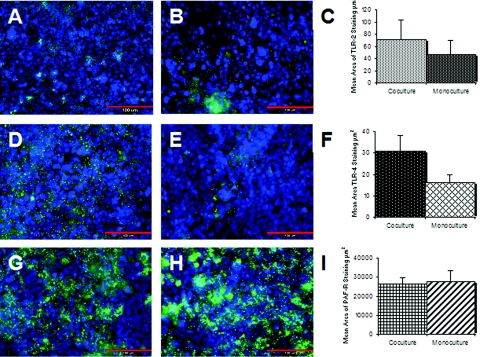
The expression and function of PAF-R and the PRRs, TLR-2 and TLR-4, were examined in M-cell cocultures and Caco-2 monocultures by immunofluorescence microscopy and quantitative image analysis (Fig. 1). TLR-4 expression was significantly upregulated in the in vitro M-cell cocultures compared to the Caco-2 cell monocultures. In contrast, TLR-2 and PAF-R were equally expressed in both culture systems. These in vitro findings were substantiated in situ by immunofluorescence photomicrography of paraffin-embedded sections of murine villi and FAE (Fig. 2). In vivo and in vitro observations showed that TLR-4 expression was restricted to the apical surfaces of M cells. In contrast, PAF-R was abundantly expressed throughout the murine intestine and TLR-2 was poorly expressed in situ, and it was not possible to obtain quality photomicrographs that accurately represented results across the entire histological section. Visual examination of the sections indicated that there was no apparent difference in the expression of TLR-2 by M cells and enterocytes.
FIG. 1.
Expression of PRRs by in vitro M-cell cocultures and Caco-2 monocultures as measured by immunofluorescence microscopy and image analysis with MetaMorph software. (A to C) Expression of TLR-2. TLR-2 was probed with anti-TLR-2 antibody and antibody conjugated Alexa 488 (green). (A) Expression of TLR-2 in M-cell cocultures; (B) expression of TLR-2 in Caco-2 monocultures; (C) image analysis of expression of TLR-2. Image analysis demonstrates that there was no difference in expression between the culture systems (P = 0.217). (D to F) Expression of TLR-4. TLR-4 was probed with anti-TLR-4 antibody and antibody conjugated Alexa 488 (green). (D) Expression of TLR-4 by M-cell cocultures; (E) expression of TLR-4 by Caco-2 monocultures; (F) image analysis of expression of TLR-4. Image analysis demonstrates that TLR-4 was significantly upregulated in the M-cell cocultures (P = 0.043). (G to I) Expression of PAF-R. PAF-R was detected with anti-PAF-R antibody and antibody-conjugated Alexa 488 conjugate (green). (G) Expression of PAF-R by M-cell cocultures; (H) expression of PAF-R by Caco-2 monocultures; (I) image analysis of PAF-R expression. Image analysis demonstrated that PAF-R was expressed equally by both cell culture systems (P=0.432). In each photomicrograph, nuclei are counterstained blue with DAPI. Size bar, 100 μm. The image analysis data are expressed as the mean± the standard error of the mean (SEM).
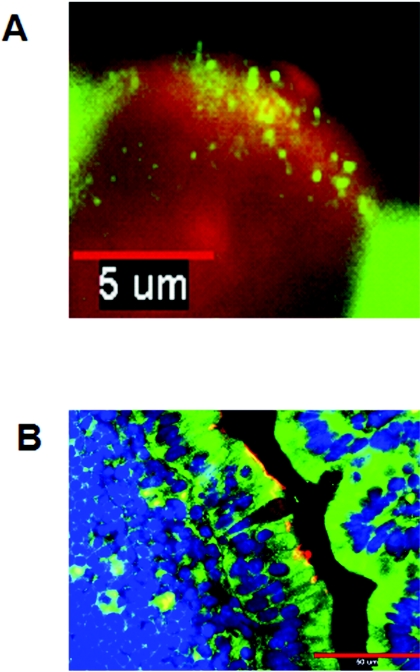
FIG. 2.
Expression of PRRs by murine M cells and enterocytes in an intestinal segment as shown by immunofluorescence microscopy. M cells were identified by the M-cell-specific Ulex europeaus (I) TRITC conjugate (red). TLR-4 and PAF-R were probed with anti-TLR-4 (A) and anti-PAF-R (B) and stained with antibody-conjugated Alexa 488 (green for both TLR-4 and PAF-R). (A) TLR-4 (green) was expressed on the apical surfaces of murine M cells (red) and intracellularly in adjacent follicle-associated enterocytes, as seen in this representative longitudinal section. Size bar, 5 μm. (B) PAF-R (green) was expressed ubiquitously throughout the murine intestine, including M cells (red). The section is counterstained blue with DAPI. Size bar,50 μm.
M cells preferentially transport whole killed cell bacteria.
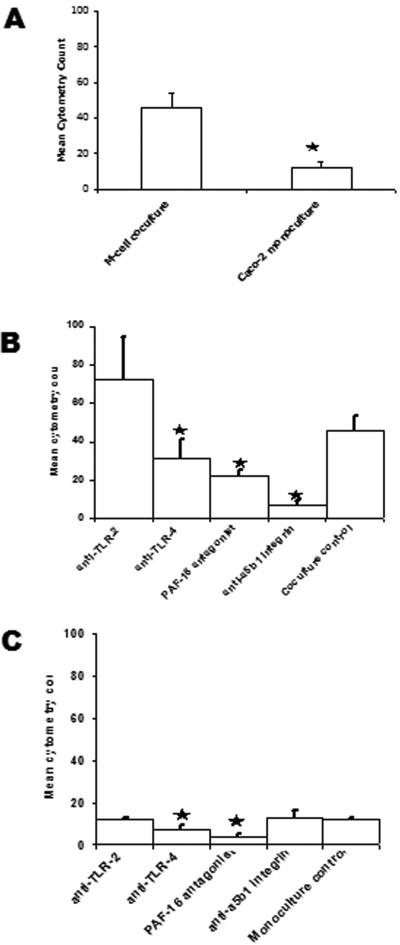
A formalin-killed NTHi vaccine administered orally to both rodents and humans has demonstrated protection against subsequent respiratory challenge or infection with this microbe (4, 5) To investigate whether or not this vaccine initiated this immunity through PRRs on M cells, we have used killed NTHi in these studies. Formaldehyde-killed CMFDA-labeled NTHi was added to the lower chambers of M-cell cocultures and Caco-2 monocultures, and flow cytometry analysis was used to measure the number of bacteria moving from the lower to the upper chambers, representing transcytosis from the apical to the basolateral surfaces of the cells. In this model, there was a three- to fourfold increase in the amount of NTHi moving from the lower to the upper chambers, indicating that NTHi was preferentially transported by the M-cell cocultures (Fig. 3A).
FIG. 3.
NTHi translocation by M-cell cocultures and Caco-2 monocultures. Translocation of fluorescently labeled NTHi 289 from the lower chamber (apical surface) to the upper chamber (basal surface) was measured by flow cytometry analysis of the culture media. (A) M-cell cocultures translocated more NTHi 289 than Caco-2 monocultures (P = 0.009). (B) In the M-cell cocultures, specific blocking of TLR-4 (P = 0.046), PAF-R (P = 0.004), and α5β1 integrin (P < 0.001) inhibited transcytosis of killed NTHi. (C) In the Caco-2 monocultures, blocking of TLR-4 (P = 0.017) and PAF-R (P < 0.001) inhibited translocation of NTHi 289 by Caco-2 cells. The data are expressed as means ± the SEM, and the “*” indicates a significant difference from the control with the P values indicated above.
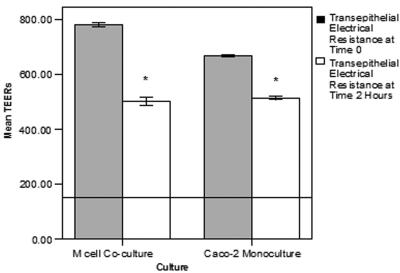
Although the addition of killed NTHi did cause some reduction in the TEER measurements, an indication of the tight-junctional integrity of both M-cell cocultures and Caco-2 monocultures, the decreases were not sufficient to compromise the epithelial barrier function that occurs at readings lower than 150 Ω · cm2 (Fig. 4).
FIG. 4.
Effects of killed NTHi translocation on transepithelial electrical resistance in M-cell cocultures and Caco-2 monocultures. The TEERs of the M-cell cocultures and Caco-2 monocultures were determined by an EVOM meter on addition of killed NTHi to the apical chambers and after 2 h of incubation. Introduction of the NTHi to the cell cultures did result in a significant decrease in the TEER in both culture systems (P < 0.001) with the decrease being slightly greater in the M cell cocultures. The absolute levels of TEERs did not drop sufficiently to compromise the epithelial barrier function that occurs when the TEER drops to 150 Ω · cm2 (indicated by the line across the graph). The data are expressed as means ± the SEM, and the “*” indicates a significant difference from the control with the P values indicated above.
Role of the LOS structure in bacterial uptake by M cells.
To determine that the transcytosis was associated with a PAMP moiety, NTHi with mutations in genes responsible for the synthesis of specific components within the LOS structure were compared to the wild-type parent NTHi strain. NTHi 2019 licD::kan, a HI1540-null mutant that lacks ChoP (22), was preferentially transcytosed by the M-cell cocultures similar to transcytosis observed for the wild-type NTHi 2019 strain (Table 2). In contrast, NTHi 2019 pgmB::erm, a HI0750-null mutant lacking oligosaccharide, displayed no differences in transport by either the M-cell cocultures or the Caco-2 monocultures. This result suggests that the oligosaccharide component on the NTHi was important in the preferential transcytosis by the M-cell cocultures. It should be noted that the NTHi 2019 pgmB::erm also lacks the ChoP moiety, indicating that although the system may have sufficient redundancy to accommodate the loss of a ChoP-PAF-R interaction, the additional loss of the LOS molecules significantly affected the ability of the cells to transcytose the bacteria.
TABLE 2.
Apical to basolateral chamber transport of labeled NTHi with LOS mutations
| Bacterium | Median flow cytometry count (95% CI)a
|
Pd | |
|---|---|---|---|
| M-cell coculturesb | Caco-2 monoculturesc | ||
| NTHi 2019 | 170 (95-360) | 51 (23-96) | <0.001 |
| NTHi 2019 (pgmB::erm) | 131 (85-202) | 90 (61-162) | 0.254 |
| NTHi 2019 (licD::kan) | 187 (161-262) | 62 (42-91) | <0.001 |
95% CI, %95 confidence interval.
Mean of five replicates.
Mean of six replicates.
Mann-Whitney test.
Blocking of receptors specifically inhibits whole killed cell bacterial transport.
Blocking of specific receptors with either receptor specific antibody or a mimetic antagonist demonstrated that blocking of PAF-R, and the PRR TLR-4 similarly reduced the capacity of both M-cell cocultures and Caco-2 monocultures to translocate NTHi (Fig. 3B and C). In contrast, blocking α5β1 integrin did not affect translocation of NTHi by the Caco-2 monocultures but did significantly inhibit the translocation by M-cell cocultures. Inhibition of TLR-2 did not affect the translocation of NTHi in either culture system, an expected outcome for a gram-negative microorganism.
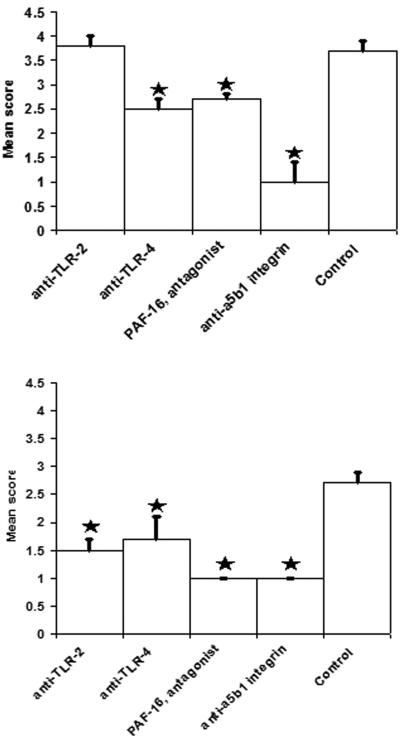
Studies using isolated murine intestinal loops ex vivo concurred with the in vitro data for TLR-4 and PAF-R. Unlike the Caco-2 cell monocultures, blocking of α5β1 integrin did reduce translocation of NTHi across the intestinal epithelia; however, a greater reduction was observed in the Peyer's patches, confirming the importance of α5β1 integrin in M-cell sampling and transport to the Peyer's patches (Fig. 5).
FIG. 5.
Blocking PRRs with specific receptor antagonists inhibits translocation of NTHi in situ in mouse intestinal segments. (A and B) Specific blocking of TLR-4 (P = 0.004), PAF-R (P = 0.013) and α5β1 integrin (P < 0.001) inhibited uptake of NTHi 289 into Peyer's patches (A) and TLR-2 (P = 0.003), TLR-4 (P < 0.001), PAF-R (P < 0.001), and α5β1 integrin (P < 0.001) (B) inhibited uptake of NTHi 289 into the intestinal villus. The data are expressed as means ± the SEM, and the “*” indicates a significant difference from control with the P values indicated above.
DISCUSSION
To study the bacterial-M-cell interactions in vitro, we used the M-cell coculture model originally described by Kerneis et al. (14) and subsequently validated by Tyrer et al. (24). This model consisted of a bicameral coculture of human enterocyte-like Caco-2 cells and freshly isolated murine Peyer's patch cells, separated by a semipermeable membrane (24). Approximately 30% of the monolayer cells develop an M-like cell phenotype in the cocultures, as indicated by measurement of the apical expression of α5β1 integrin. These in vitro M cells were shown to display many other characteristics exhibited by M cells in vivo, such as reduced apical expression of digestive enzymes and enhanced energy-dependent transcytosis of particulates. Hence, it is an excellent in vitro model to investigate biochemical and functional differences between M cells and adjacent enterocytes.
Although this is the first study to identify the distribution of TLR-2, TLR-4, and PAF-R in an M-cell model in vitro and by M cells and FAE overlying the Peyer's patches in situ, earlier studies have examined the expression of these receptors in the intestinal villi (1, 15). These earlier studies demonstrated low-level expression of TLR-2 and TLR-4 in the villi of the jejunum, and these patterns of receptor expression were considered to reflect hyporesponsiveness of the mucosal immune system in response to the high levels of immunostimulatory bacterial products, such as LPS, within the lumen of the gut (1). In contrast, the Peyer's patches are immunological inductive sites, and one may expect that the expression of receptors involved in the generation of immune responses could be upregulated. We have demonstrated that the expression of the LPS receptor, TLR-4, is upregulated in vitro in M-cell cocultures and on the apical surfaces of murine M cells and, hence, may play an important role in the induction of mucosal immune responses to gram-negative bacteria.
Expression of the lipotechoic acid receptor TLR-2 was low in both cocultures and monocultures and barely detectable in murine villi and Peyer's patches. Although this pattern of expression suggests a bias in the gut for providing mechanisms for inducing immune responses against gram-negative bacteria, receptors, such as PAF-R, can bind both gram-negative and gram-positive bacteria (11, 20, 22). Low levels of TLR-2 expression by intestinal epithelial cells, including on Caco-2 cells, has also previously been reported and these cells were found to be broadly unresponsive to a range of gram-positive ligands (15).
Oral immunization as a vaccination route is not a new concept and has significant potential application; however, this approach for widespread use in vaccine delivery has been limited by the challenges in targeting such vaccines to the GALT and more specifically to M-cell antigen sampling receptors. It is well established that oral immunization of humans and animals with whole killed cell NTHi (formalin killed) induces protection against acute exacerbations of chronic obstructive pulmonary disease in humans (4) and enhances bacterial clearance in animals challenged with live NTHi in the middle ear and lung (5). The intestinal antigen sampling mechanisms responsible for the induction of these immune processes have not yet been elucidated. The present study has shown that formalin killed NTHi was transported in both in vitro and in situ models, indicating that, at least for NTHi, live bacteria were not essential for translocation. Therefore, we hypothesized that the intestinal sampling of NTHi, and hence induction of protective mucosal immune responses, was initially mediated by PRRs on the surface of M cells.
To determine that a PAMP-PRR interaction was mediating transcytosis through M cells, two mutant strains of NTHi that have defects in the synthesis of LOS components were examined. The H. influenzae LOS lacks an O antigen and is composed of nonrepeating oligosaccharides consisting of glucose, galactose, N-acetylglucosamine, ChoP, or N-acetyl-neuraminic acid in different combinations (18). Mutation in the pgmB gene abolishes phosphoglucomutase activity, resulting in the production of truncated LOS molecules lacking oligosaccharide chains external to the core region (22). The absence of the oligosaccharide side chains did affect transcytosis; however, this mutation also affects the presence of other moieties, such as ChoP. The mutation in licD prevents production of ChoP. This mutant was relevant in that PAF (1-alkyl-2-acetyl-sn-glycero-3-phosphocholine) is an acetyl-ChoP molecule and antigen mimicry from the ChoP+LOS of the wild-type NTHi with PAF-R was possible. Although there was no significant difference in PAF-R expression between the culture systems or along the murine intestine, inhibition of PAF-R significantly reduced transcytosis in both M-cell cocultures and the Caco-2 monocultures. However, the lack of ChoP did not affect the transcytosis of the NTHi, suggesting that it may be the oligosaccharide component that was important in any receptor-ligand interaction. Two structures on the LOS that may play an important role are the terminal sugars, sialic acid and galactose, either as the digalactose or lactosamine moiety. These are important moieties for the sialic acid Siglec receptors and asialoglycoprotein receptors on macrophages and dendritic cells (9, 25) and so may be important PAMPs on the LOS that could be associated with receptors that are as yet uncharacterized on M cells.
The inhibition of these particular receptors associated with PAMP-PRR interactions demonstrated their importance in the uptake of NTHi from the intestinal lumen. In particular, the significance of α5β1 integrin was demonstrated by the translocation of antigen through the M cells and the capacity to affect this through inhibition of this integrin when it is apically expressed. This preferential transcytosis through M cells would enable the bacteria to be processed by the GALT for the induction of antigen-specific immunity.
One obvious hypothesis to test was that the level of PRR expression, particularly the TLRs, may differ between enterocytes and M cells. However, our data would suggest that surface expression alone was not the sole indicator of receptor functionality for the uptake of NTHi by M cells. Our previous study demonstrated that α5β1 integrin relocates from basolateral and lateral surfaces to the apical surface (24) with differentiation of Caco-2 cells into M cells, which is a classical differential characteristic between M cells and enterocytes (22). Although the patterns of expression of TLR-4 (Fig. 1F) and α5β1 integrin (24) differed on M cells and enterocytes in vitro, it was only the increase in apical expression of α5β1 integrin and not TLR-4 (Fig. 3) that was shown to enhance the translocation of NTHi across M cells. This suggests that upregulation of receptor expression alone does not necessarily reflect enhanced functionality and that it is both the expression and location of this receptor on the apical surface that is critical for M cells to transcytose NTHi. Such differential functionality may reflect downstream intracellular signaling initiated by the different receptors on the M cells.
In conclusion, our data suggest that there are multiple PAMP-PRR interactions, through the expression of different receptors, that ensure adequate redundancy in M-cell antigen sampling so that immune priming can occur in response to specific pathogens. In support of our hypothesis, the protective responses after oral immunization with killed NTHi appear to be initially mediated by PRR recognition of PAMPs, with the PRR α5β1 integrin being the most significant receptor associated with translocation of NTHi through M cells. This finding contributes significantly to research focused on targeting vaccines or therapeutic agents through the M cells to the intestinal lymphoid tissues. So, in developing prophylactics and therapeutics, where M-cell recognition is important for mucosal immune priming or immune modulation, the ability to target and optimize PAMP-PRR interaction is important.
Acknowledgments
We thank Robert Clancy and Paul Pavli for critical evaluation of a draft manuscript.
This study was funded by an ARC SPIRT grant, and P.T. was supported by Provalis, Plc. (United Kingdom).
Editor: J. D. Clements
REFERENCES
- 1.Abreu, M. T., P. Vora, E. Faure, L. S. Thomas, E. T. Arnold, and M. Arditi. 2001. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J. Immunol. 167:1609-1616. [DOI] [PubMed] [Google Scholar]
- 2.Akashi, S., S. Saitoh, Y. Wakabayashi, T. Kikuchi, N. Takamura, Y. Nagai, Y. Kusumoto, K. Fukase, S. Kusumoto, Y. Adachi, A. Kosugi, and K. Miyake. 2003. Lipopolysaccharide interaction with cell surface Toll-like receptor 4-MD-2: higher affinity than that with MD-2 or CD14. J. Exp. Med. 198:1035-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campagnari, A. A., M. R. Gupta, K. C. Dudas, T. F. Murphy, and M. A. Apicella. 1987. Antigenic diversity of lipooligosaccharides of nontypeable Haemophilus influenzae. Infect. Immun. 55:882-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foxwell, A. R., A. W. Cripps, and K. B. Dear. 2003. Haemophilus influenzae oral vaccination against acute bronchitis. Cochrane Database Syst. Rev:CD001958. [DOI] [PubMed]
- 5.Foxwell, A. R., J. M. Kyd, and A. W. Cripps. 1998. Kinetics of inflammatory cytokines in the clearance of nontypeable Haemophilus influenzae from the lung. Immunol. Cell Biol. 76:556-559. [DOI] [PubMed] [Google Scholar]
- 6.Gebert, A., H.-J. Rothkotter, and R. Pabst. 1996. M cells in Peyer's patches of the intestine. Int. Rev. Cytol. 167:91-159. [DOI] [PubMed] [Google Scholar]
- 7.Girardin, S. E., P. J. Sansonetti, and D. J. Philpott. 2002. Intracellular versus extracellular recognition of pathogens—common concepts in mammals and flies. Trends Microbiol. 10:193-199. [DOI] [PubMed] [Google Scholar]
- 8.Grassl, G. A., E. Bohn, Y. Muller, O. T. Buhler, and I. B. Autenrieth. 2003. Interaction of Yersinia enterocolitica with epithelial cells: invasin beyond invasion. Int. J. Med. Microbiol. 293:41-54. [DOI] [PubMed] [Google Scholar]
- 9.Hartnell, A., J. Steel, H. Turley, M. Jones, D. G. Jackson, and P. R. Crocker. 2001. Characterization of human sialoadhesin, a sialic acid binding receptor expressed by resident and inflammatory macrophage populations. Blood 97:288-296. [DOI] [PubMed] [Google Scholar]
- 10.Harvey, P., T. Battle, and S. Leach. 1999. Different invasion phenotypes of Campylobacter isolates in Caco-2 cell monolayers. J. Med. Microbiol. 48:461-469. [DOI] [PubMed] [Google Scholar]
- 11.Ishizuka, S., M. Yamaya, T. Suzuki, K. Nakayama, M. Kamanaka, S. Ida, K. Sedizawa, and H. Sasaki. 2001. Acid exposure stimulates the adherence of Streptococcus pneumoniae to cultured human airway epithelial cells: effects on platelet-activating factor receptor expression. Am. J. Respir. Cell Mol. Biol. 24:459-468. [DOI] [PubMed] [Google Scholar]
- 12.Jepson, M. A., C. M. Mason, N. L. Simmons, and B. H. Hirst. 1995. Enterocytes in the follicle-associated epithelia of rabbit small intestine display distinctive lectin-binding properties. Histochem. Cell Biol. 103:131-134. [DOI] [PubMed] [Google Scholar]
- 13.Kenny, B. 2002. Mechanism of action of EPEC type III effector molecules. Int. J. Med. Microbiol. 291:469-477. [DOI] [PubMed] [Google Scholar]
- 14.Kerneis, S., A. Bogdanova, J.-P. Kraehenbuhl, and E. Pringault. 1997. Conversion by Peyer's patch lymphocytes of human enterocytes into M cells that transport bacteria. Science 277:949-952. [DOI] [PubMed] [Google Scholar]
- 15.Melmed, G., L. S. Thomas, N. Lee, S. Y. Tesfay, K. Lukasek, K. S. Michelsen, Y. Zhou, B. Hu, M. Arditi, and M. T. Abreu. 2003. Human intestinal epithelial cells are broadly unresponsive to Toll-like receptor 2-dependent bacterial ligands: implications for host-microbial interactions in the gut. J. Immunol. 170:1406-1415. [DOI] [PubMed] [Google Scholar]
- 16.Neutra, M. R., N. J. Mantis, and J.-P. Kraenhebuhl. 2001. Collaboration of epithelial cells with organized mucosal lymphoid tissue. Nat. Immunol. 2:1004-1009. [DOI] [PubMed] [Google Scholar]
- 17.Pabst, R. 1987. The anatomical basis for the immune function of the gut. Anat. Embryol. 176:135-144. [DOI] [PubMed] [Google Scholar]
- 18.Risberg, A., H. Masoud, A. Martin, J. C. Richards, E. R. Moxon, and E. K. Schweda. 1999. Structural analysis of the lipooligosaccharide oligosaccharide epitopes expressed by a capsule-deficient strain of Haemophilus influenzae Rd. Eur. J. Biochem. 261:171-180. [DOI] [PubMed] [Google Scholar]
- 19.Secott, T. E., T. L. Lin, and C. C. Wu. 2004. Mycobacterium avium subsp. paratuberculosis fibronectin attachment protein facilitates M-cell targeting and invasion through a fibronectin bridge with host integrins. Infect. Immun. 72:3724-3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soares, A. C., V. S. Pinho, D. G. Souza, T. Shimizu, S. Ishii, J. R. Nicoli, and M. M. Teixeira. 2002. Role of the platelet-activating factor (PAF) receptor during pulmonary infection with gram-negative bacteria. Br. J. Pharmacol. 137:621-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soderholm, J. D., J. Hedman, P. Artursson, L. Franzen, J. Larsson, N. Pantzar, J. Permert, and G. Olaison. 1998. Integrity and metabolism of human ileal mucosa in vitro in the Ussing chamber. Acta Physiol. Scand. 162:47-56. [DOI] [PubMed] [Google Scholar]
- 22.Swords, W. E., B. A. Buscher, K. ver Steeg Ii, A. Preston, W. A. Nichols, J. N. Weiser, B. W. Gibson, and M. A. Apicella. 2000. Nontypeable Haemophilus influenzae adhere to and invade human bronchial epithelial cells via an interacting of lipooligosaccharide with the PAF receptor. Mol. Microbiol. 37:13-27. [DOI] [PubMed] [Google Scholar]
- 23.Travassos, L. H., S. E. Girardin, D. J. Philpott, D. Blanot, M. A. Nahori, C. Werts, and I. G. Boneca. 2004. Toll-like receptor 2-dependent bacterial sensing does not occur via peptidoglycan recognition. EMBO Rep. 5:1000-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tyrer, P., A. R. Foxwell, J. M. Kyd, M. Harvey, P. Sizer, and A. W. Cripps. 2002. Validation and quantitation of an in vitro M cell model. Biochem. Biophys. Res. Commun. 299:377-383. [DOI] [PubMed] [Google Scholar]
- 25.Valladeau, J., V. Duvert-Frances, J. J. Pin, M. J. Kleijmeer, S. Ait-Yahia, O. Ravel, C. Vincent, F. J. Vega, A. Helms, D. Gorman, S. Zurawski, M., G. Zurawski, J. Ford, and S. Saeland. 2001. Immature human dendritic cells express asialoglycoprotein receptor isoforms for efficient receptor-mediated endocytosis. J. Immunol. 167:5767-5774. [DOI] [PubMed] [Google Scholar]