Abstract
Protection against intracellular pathogens such as Mycobacterium tuberculosis requires the development of Th1-like T-cell responses. This in turn is dependent on the pattern of cytokine produced from dendritic cells (DCs) after infection. Three heterodimeric cytokines, interleukin-12 (IL-12), IL-23, and IL-27, as well as IL-18, contribute to the differentiation and expansion of naive CD4+ T cells. In this study we compared the effects of plasmids expressing both chains of IL-12, IL-23, or IL-27 as adjuvants for DNA immunization against M. tuberculosis infection. The genes encoding p19 and p40 chains of IL-23 or EBI3 and p28 chains of IL-27 were cloned on either side of a self-cleaving peptide from the FMDV2A protein. The secretion of functional cytokines from transfected cells was detected with bioassays. Supernatant from p2AIL-23-transfected cells induced the release of IL-17 from activated lymphocytes, confirming the presence of bioactive IL-23. Further, supernatant from p2AIL-27-transfected cells stimulated a significant increase in the proliferation of peptide-stimulated transgenic CD4+ T cells. In initial experiments, M. tuberculosis infection of DCs was more potent at inducing IL-12 and IL-23 secretion than infection with the vaccine strain Mycobacterium bovis bacille Calmette-Guérin (BCG), and no significant upregulation of IL-27 was observed. Coimmunization of C57BL/6 mice with DNA expressing M. tuberculosis antigen 85B (Ag85B; DNA85B) and plasmids expressing IL-23 or IL-12 stimulated stronger Ag85B-specific T-cell proliferative and IFN-γ responses than DNA85B alone, whereas the addition of p2AIL-27 had no effect. Interestingly, DNA85B codelivered with p2AIL-12, but not p2AIL-23, reduced the immunoglobulin G antibody response. Both p2AIL-23 and p2AIL-12, but not p2AIL-27, enhanced the protective efficacy of DNA85B against aerosol M. tuberculosis challenge. Therefore, both p2AIL-23 and p2AIL-12 are valuable as cytokine adjuvants for increasing the protective antituberculosis immunity induced by DNA vaccines.
Tuberculosis (TB) is a global health emergency, with an estimated nine million new cases of active disease and approximately 2 million deaths per year (11a). The development of more effective vaccines than the current vaccine Mycobacterium bovis bacillus Calmette-Guérin (BCG) may improve the control of this pandemic. New approaches to the design of TB vaccines include the preparation of recombinant BCG oversecreting mycobacterial antigens (32), attenuated strains of M. tuberculosis (54), and subunit vaccines based on DNA or protein antigens (33, 55). DNA vaccines encoding M. tuberculosis proteins, such as antigen 85A (Ag85A) or Ag85B (DNA85), induce partial protection against experimental TB (34, 36). However, the degree of protection gained from DNA vaccination alone is less than that afforded by BCG vaccination. Strategies to improve antimycobacterial immunity from subunit vaccines include the development of fusion proteins containing multiple protective antigens (46) and the use of immunostimulatory molecules as adjuvants (50).
The development of acquired cellular immunity is critical for the control of M. tuberculosis infection. The key cytokine required for cell-mediated immunity is gamma interferon (IFN-γ), which functions by stimulating infected macrophages to induce phagolysosomal fusion and killing of intracellular bacteria (10, 20). The heterodimeric cytokines interleukin-12 (IL-12) and IL-18 are critical for the induction of Th1-like CD4+ cells and are produced primarily by dendritic cells (DCs) (44, 59, 67). Humans and mice lacking the p40 chain of IL-12 or its receptors are highly susceptibility to M. tuberculosis infection (6, 11). Plasmids expressing either IL-12 or IL-18 have been used as adjuvants in several infectious models (42, 45, 50). Coadministration of plasmids expressing IL-12 or IL-18 increased the IFN-γ T-cell response in DNA vaccination to Ag85B, but only plasmids expressing IL-12 increased protective efficacy (62).
Recently, two further cytokines, IL-23 and IL-27, have been found to contribute to the development of Th1-like CD4+ T-cell responses. The heterodimeric cytokine IL-23 is secreted by activated macrophages and DCs and induces clonal expansion of memory CD4+ T cells (49). IL-23 is composed of a p40 subunit, shared with IL-12, and a unique p19 subunit, signaling through the receptor IL-12Rβ, and a unique IL-23R chain (49). In addition to its direct action on T cells, IL-23 also induces the secretion of IL-12 and IFN-γ by DCs in vitro (4). This suggests that IL-23 has indirect involvement in the activation of antigen-presenting cells (APCs). Studies with gene-deficient mice reveal that a number of roles that were previously accredited to IL-12 may be dependent on IL-23 (12). In M. tuberculosis infection, the absence of the p40 subunit common to IL-12 and IL-23 results in more marked susceptibility to M. tuberculosis infection than IL-12p35 deficiency, suggesting an important role for IL-23 in mycobacterial infections (11). The functions of IL-27, which is comprised of the gene product of the Epstein-Barr virus induced gene 3 (EBI3) and a p28 subunit, are less well defined (17, 53). Monocyte-derived DCs and macrophages secrete IL-27, which stimulates the clonal expansion of naive T cells with or without the presence of IL-12 (53). Initially, IL-27 was recognized as an early acting proinflammatory cytokine (8, 69); however, recent evidence suggests IL-27 has additional immunoregulatory functions (2, 29, 31, 64).
In this report we have examined the effects of three immunostimulatory cytokines delivered as plasmid vectors on the immunogenicity and protective efficacy of antituberculosis DNA vaccines. Codelivery of plasmids expressing IL-12 or IL-23, but not IL-27, with DNA85B increased the expansion of antigen-specific IFN-γ-secreting T cells and enhanced the protective efficacy of DNA vaccine against aerosol M. tuberculosis infection.
MATERIALS AND METHODS
Bacterial growth conditions.
M. tuberculosis H37Rv (ATCC 27294) was grown in Proskauer and Beck liquid medium for 14 days and M. bovis BCG (Pasteur strain) was grown in Middlebrook 7H9 broth supplemented with ADC (Difco Laboratories, Detroit, MI) for 14 days at 37°C. The bacteria were enumerated on oleic acid-albumin-dextrose-catalase (OADC)-enriched Middlebrook 7H11 agar and stored in 30% glycerol-phosphate-buffered saline at −70°C. For plasmid preparations, Escherichia coli MC1061 was grown in Luria-Bertani broth or on Luria-Bertani agar and for large-scale preparations Circlegrow broth (Bio 101, Vista, CA) was used. Ampicillin (100 μg/ml) was supplemented as necessary. The infection experiments with DCs used M. tuberculosis Mt103, a wild-type strain isolated from a patient with tuberculosis (35).
Preparation of vaccines.
Construction of DNA vaccines encoding M. tuberculosis Ag85B (DNA85B) and murine IL-12 (p2AIL-12) has been described previously (36, 50). The gene for the p19 chain of IL-23 was amplified from cDNA obtained by reverse transcription (RT) of mRNA from lipopolysaccharide-stimulated DCs. The genes for EBI3 and p28 chains of IL-27 were obtained from a lipopolysaccharide-stimulated mouse macrophage cell line, RAW264. The genes were inserted into the pCI2A vector, which contains the sequence for the peptide of 2A FMDV protein and the cytomegalovirus promoter (7). For the construction of p2AIL-23, the IL-23p19 gene was placed upstream of the 2A motif with the p40 gene cloned downstream. Similarly, IL-27EBI3 gene was placed upstream of the 2A motif, followed by IL-27p28, which was cloned downstream of the motif. The DNA used in immunizations was prepared by equilibrium centrifugation in a continuous CsCl-ethidium bromide gradient. The Ag85 protein was obtained through the TB Research Material and Vaccine Testing Contract at Colorado State University (NIAID AI-75320).
Transfection of human embryonic kidney (HEK) 293 cells.
Six-well tissue culture plates were seeded with 4 × 105 HEK293 cells overnight. A mixture of plasmid DNA and FuGene (Boehringer Mannheim, Mannheim, Germany) was prepared according to the manufacturer's instructions and added to cells. After 48 h, the supernatants and cells were collected. Secreted IL-12 and IL-23 were determined by enzyme-linked immunosorbent assay (ELISA) for IL-12p70, IL-12p40, and IL-23p19 in HEK293 cell transfected supernatants.
Immunization.
Six- to eight-week-old C57BL/6 female mice were obtained from Animal Resources Centre (Perth, WA, Australia) and maintained in specific-pathogen-free conditions. Mice were immunized with 100 μg of DNA85B mixed with 100 μg of either p2AIL-12, p2AIL-23, p2AIL-27, or control vector by intramuscular (i.m.) injection into each tibialis anterior muscle. Control mice were immunized either i.m. with 200 μg of parental control vector pcDNA3 or subcutaneously with BCG (5 × 105 CFU) at least 100 days prior to challenge.
M. tuberculosis challenge.
Six weeks after the last boost with DNA vaccine, mice were challenged with aerosol M. tuberculosis H37Rv using a Middlebrook airborne infection apparatus (Glas-Col, Terre Haute, IN) with an infective dose of approximately 100 viable bacilli per lung. Four weeks after M. tuberculosis challenge, homogenized lungs were plated in 10-fold dilutions on supplemented Middlebrook 7H11 Bacto agar.
DC preparation and infection.
Bone marrow-derived dendritic cells were generated as previously described (14) with modification. Briefly, cells were incubated with a mixture of hybridoma supernatants from M5.114 (anti-major histocompatibility complex class II), RA3-6B2 (anti-B220), 53-6.7 (anti-CD8), GK1.5 (anti-CD4), and RB6-8C5 (anti-Ly-6G). Cells bound to antibodies were eliminated by using goat anti-rat immunoglobulin G (IgG) microbeads (MACS, Miltenyi Biotech). Bone marrow-derived dendritic cell cultures were infected with BCG or M. tuberculosis Mt103 at a multiplicity of infection of 0.5 for 24 h. Cells were collected for RNA extraction.
Antibody measurement.
To detect serum Ag85-specific antibody levels, plates were first coated with Ag85 (2 μg/ml). Fivefold dilutions of sera were incubated for 1 h prior to the addition of goat anti-mouse IgG-alkaline phosphate-conjugated antibodies (Sigma), and detection was with n-nitrophenyl phosphate (1 mg/ml) (Sigma). The mean absorbance plus three standard deviations of normal mouse sera, diluted at 1:100, was adopted as the cutoff absorbance for determining antibody titers.
Lymphocyte proliferation.
Two weeks after the last immunization, single cell suspensions of splenocytes were prepared in complete RPMI medium containing 10% fetal calf serum, 50 μM β-mercaptoethanol, and 2 mM glutamine. Pooled samples were enriched for T cells by using a nylon wool column and cultured with gamma-irradiated syngeneic splenocytes (2 × 105 cells/well) in 96-well plates. Ag85 (3 μg/ml), concanavalin A (ConA; 3 μg/ml), or medium alone was incubated for 72 h and subsequently pulsed with 1 μCi of [3H]thymidine for 6 h. The results were calculated by subtracting the mean counts per minute in control wells from test samples.
Cytokine assays.
Antigen-specific IFN-γ-secreting cells were measured by ELIspot as previously described (50). For cytokine ELISAs, plates were coated with anti-IL-12p70, IL-12p40, or IL-23p19 antibodies (R&D Biosciences), supernatant samples and standards were added, and anti-IL-12p40biotin (R&D Biosciences) was added, followed by streptavidin-horseradish peroxidase. IL-17 was determined by ELISA, using reagents from R&D Biosciences. Substrate solution was allowed to develop and absorbance measured at a dual wavelength of 405/492 nm. The bioactivity of plasmid-expressed IL-12 has been confirmed (50). The bioactivity of IL-23 was determined by the induction of IL-17 secretion from ConA-activated lymph node cells. To determine the expression of IL-27, RNA was purified from p2AIL-27-transfected HEK293 cells and tested by quantitative real-time PCR (qRT-PCR) for IL-27p28 gene expression. Bioactivity of IL-27 was confirmed by the ability of supernatants to induce the proliferation of naive CD4+ T cells.
IL-23 and IL-27 bioassay.
Lymph nodes were isolated from wild-type mice and stimulated with ConA (1 μg/ml) and IL-2 (50 U/ml) in 48-well plates (3 × 105 cells/well). Supernatant from HEK293 cells transfected with either empty vector, p2AIL-12, or p2AIL-23 was added, and the cells were cultured for 5 days. Release of IL-17 from the ConA-activated lymphocytes was measured by ELISA. For the IL-27 bioassay, CD4+ T cells were purified from lymph nodes of OT-II transgenic C57BL/6 mice (kindly provided by B. Heath, WEHI, Melbourne, Australia) by using magnetic bead sorting (MACS; Miltenyi Biotec) of stained CD4+ T cells. Purified CD4+ T cells were cultured in 96-well plates (2 × 105 cells/well) with syngeneic wild-type irradiated APCs (2 × 105 cells/well). Cells were activated with various doses of the H-2b-restricted ovalbumin (OVA) peptide323-339 (ISQAVHAAHAEINEAGR) and stimulated for 72 h with titrated doses of supernatants from p2AIL-27 or empty vector-transfected HEK293 cells. To assay proliferation of T cells, the incorporation of [3H]thymidine was determined in counts per minute.
RNA extraction.
After infection, 2 × 106 DCs were resuspended in 500 μl of RNAzol-Bee, and RNA extraction was performed as previously described (9). For RT, 5 μg of DNase-treated RNA was mixed with oligo(dT) primers (0.1 μg/μl; Invitrogen) and 10 mM deoxynucleoside triphosphates at 65°C for 5 min and left to cool at 4°C for 1 min. The reaction was performed at 50°C for 50 min. All cDNA samples were diluted 1:25 in diethyl pyrocarbonate-treated water (Gibco, Auckland, New Zealand) to be used in qRT-PCR.
qRT-PCR.
All PCRs were performed with Platinum qPCR Supermix-UCG (Invitrogen), containing a 0.3× concentration of SYBR Green I stock (Molecular Probes, Eugene, OR). Each 20-μl PCR mixture contained 8 μl of dilute cDNA sample, 10 μl of Platinum qPCR Supermix-UDG with SYBR Green I, 200 nM concentrations of each primer, and 1 μl of 50× 6-carboxy-rhodamine reference dye (Invitrogen). The primers are listed in Table 1; oligonucleotides were designed by using Primer Express 1.5 software (PE Applied Biosystems, Foster City, CA). All reactions were performed with a model 7700 sequence detector (PE Applied Biosystems) using the following thermal conditions: stage 1, 50°C for 2 min and 95°C for 10 min; stage 2, 95°C for 25 s and 60°C for 1 min. Stage 2 was repeated for 40 cycles. The data were analyzed by using Sequence detector 1.7 software (PE Applied Biosystems) to calculate the threshold cycle number (CT) and were used to quantify target gene expression of each sample using the comparative CT (2−ΔΔCT) method, which has been previously described (40). The results represent the expression of IL-12p40, IL-23p19, and IL-27p28 mRNA in infected DCs relative to uninfected samples.
TABLE 1.
List of primers used
| Primer | Sequence (5′ to 3′)
|
|
|---|---|---|
| Forward | Reverse | |
| IL-12p40 | CAGAAGCTAACCATCTCCTGG | CCGGAGTAATTTGGTGCTTCA |
| IL-23p19 | GAACAAGATGCTGGATTGCAGAG | TGTGCGTTCCAGGCTAGCA |
| IL-27p28 | TCGATTGCCAGGAGTGAACC | CGAAGTGGTAGCGAGGAAG |
Statistical analysis.
Statistical analysis of the results from immunological assays and log-transformed bacterial counts were conducted by using analysis of variance. Differences with a P value of <0.05 were considered significant.
RESULTS
Induction of IL-12, IL-23, and IL-27 during mycobacterial infection of DCs.
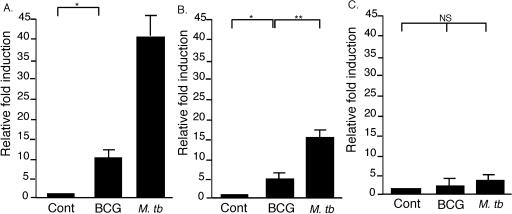
Bone marrow-derived DCs were infected with M. tuberculosis or BCG and evaluated for cytokine expression by qRT-PCR. M. tuberculosis infection induced a higher relative induction of cytokine in DCs compared to BCG infection (Fig. 1). Infection with M. tuberculosis resulted in a fourfold-higher induction of IL-12p40 mRNA compared to the mRNA level after BCG infection (Fig. 1A). M. tuberculosis infection also induced a 10-fold increase in the mRNA level for IL-23p19, with a small but significant induction of IL-23p19 after BCG infection (Fig. 1B). There was no significant increase in IL-27p28 mRNA at 24 h after infection with M. tuberculosis or BCG (Fig. 1C).
FIG. 1.
Expression of IL-12p40, IL-23p19, and IL-27p28 mRNA in DCs infected with mycobacteria. At 24 h after infection with M. tuberculosis Mt103 or BCG, RNA was extracted and reverse transcribed to determine IL-12p40 (A), IL-23p19 (B), and IL-27p28 (C) expression by RT-PCR. The data are the means ± the standard error of the mean (SEM) for the relative induction (n = fold) of cytokine mRNA in triplicate samples compared to uninfected DCs (Cont) and are representative of one of two experiments.
Expression of IL-12, IL-23, and IL-27 by transfected cells.
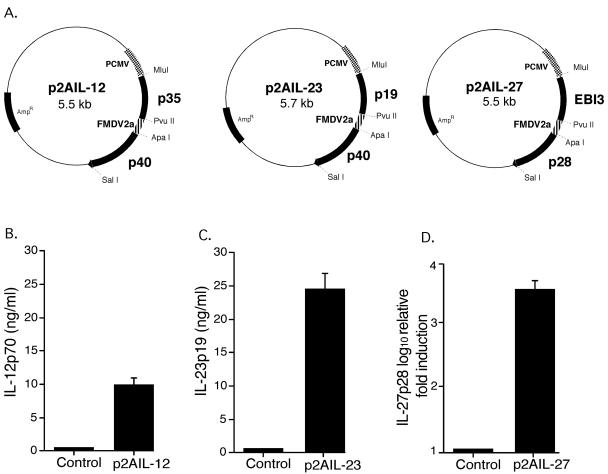
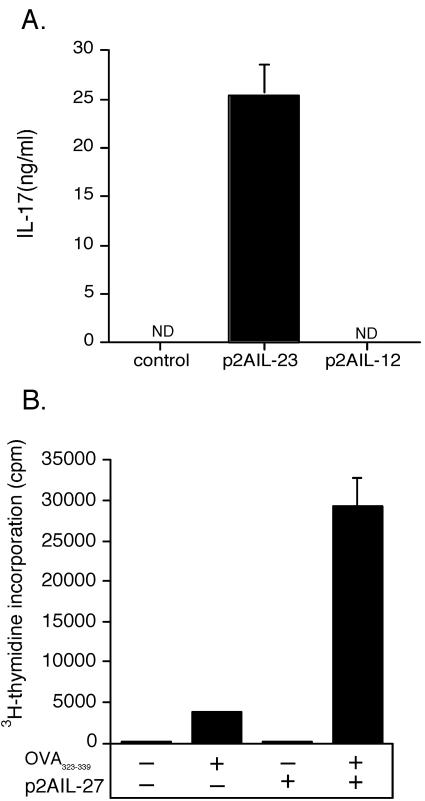
Plasmids expressing the genes for both chains of IL-12, IL-23, and IL-27 linked by the gene for the FMDV 2A polypeptide were constructed (Fig. 2A). IL-12, IL-23, and IL-27 each require the conjugate expression of both subunits by the same cell to form the heterodimeric cytokine. To confirm correct folding and expression of cytokines, HEK293 cells were transfected with p2AIL-12, p2AIL-23, or p2AIL-27. Supernatants from IL-12 and IL-23 HEK293 transfections were collected and tested by using ELISA for IL-12p70, IL-12p40, and IL-23p19. Supernatant from p2AIL-12-transfected cells contained IL-12p70 (Fig. 2B) and IL-12p40 (28 ng/ml [data not shown]). The bioactivity of secreted IL-12 has previously been confirmed (50). Supernatant from p2AIL-23-transfected cells contains IL-23p19 (Fig. 2C) and p40 (data not shown). The induction of mRNA for IL-12p40 and IL-23p19 in transfected HEK293 cells was also confirmed by using qRT-PCR (data not shown). The expression of mRNA for IL-27p28 was confirmed by qRT-PCR in cells transfected with p2AIL-27 (Fig. 2D). To confirm the bioactivity of IL-23, supernatants from p2AIL-23- transfected cells were tested for their capacity to induce IL-17 secretion from activated lymphocytes. Supernatants from control or p2AIL-12-transfected HEK293 cells did not stimulate IL-17 secretion; however, heterodimeric IL-23 expression from p2AIL-23 induced a significant level of IL-17 (Fig. 3A). To determine the activity of IL-27 secretion from p2AIL-27, we investigated its ability to induce the proliferation of naive CD4+ T cells. OVA-specific (OT-II) CD4+ T cells were purified and cultured with titrated doses of supernatant from p2AIL-27 transfected HEK293 cells. In the absence of IL-27, peptide stimulated modest proliferation of OT-II T cells. The addition of IL-27 containing supernatant resulted in a 10-fold enhancement of antigen-specific T-cell proliferation (Fig. 3B).
FIG. 2.
Expression of cytokines from p2AIL-12, p2AIL-23, and p2AIL-27 vectors. (A) Plasmids p2AIL-12, p2AIL-23, and p2AIL-27 contain the coding regions for both chains of each cytokine flanking the self-cleaving 2A polypeptide of FMDV. HEK293 cells were transiently transfected by the three plasmids, and both the supernatants and the cell lysates were collected. (B and C) Secretion of IL-12p70 (B) and IL-23p19 (C) protein into the supernatant was confirmed by ELISA. (D) Expression of mRNA for IL-27p28 was measured by qRT-PCR from cell lysate. The results are the means ± the SEM for three replicate assays and are representative of one of two experiments.
FIG. 3.
Bioactivity of functional IL-23 and IL-27 expressed by the plasmids. Supernatants from p2AIL-23- and p2AIL-27-transfected HEK293 cells were analyzed for functional IL-23 and IL-27, respectively. The bioactivity of IL-23 released from p2AIL-23-transfected cells was tested by the capacity to induce IL-17 secretion from activated lymphocytes. (A) The IL-17 in supernatants was measured by ELISA. Secretion of functional IL-27 from p2AIL-27-transfected cells was tested by the capacity to increase the proliferation of antigen-specific T cell. Purified ovalbumin (OVA)-specific (OT-II) CD4+ T cells were stimulated with OVA323-339 peptide (1 μg/ml), and the cells were cultured with supernatant from p2AIL-27-transfected HEK293 cells (1:100 final dilution) for 3 days (B). The results are the means ± the SEM for triplicate samples and are representative of one of two independent experiments. The differences between the groups were analyzed by analysis of variance. ND, not detected.
Coimmunization with p2AIL-12 and p2AIL-23 enhanced antigen-specific T-cell responses to DNA vaccine antigens.
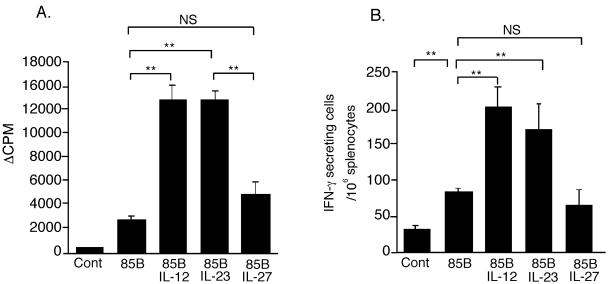
C57BL/6 mice were immunized three times at two weekly intervals with DNA85B alone or in combination with p2AIL-12, p2AIL-23, or p2AIL-27. Splenocytes from mice immunized with DNA85B had a threefold increase in Ag85-specific proliferative response compared to control vector (Fig. 4A). Coimmunization with p2AIL-12 and p2AIL-23 further increased this response by three- to fourfold. There was no difference in T-cell proliferation between mice coimmunized with p2AIL-12 and p2AIL-23. The response from both p2AIL-12- and p2AIL-23-immunized mice was significantly greater than that for p2AIL-27. A similar pattern was observed in Ag85-specific IFN-γ-secreting T-cell responses (Fig. 4B). The number of IFN-γ-secreting lymphocytes was significantly higher in mice coimmunized with DNA85B and p2AIL-12 or p2AIL-23 compared to DNA85B alone. Plasmid IL-27 did not enhance the IFN-γ T-cell response.
FIG. 4.
Coimmunization with p2AIL-12 and p2AIL-23, but not p2AIL-27, increases the antigen-specific proliferative and IFN-γ T-cell responses to DNA85B vaccine. Mice were immunized i.m. three times at 2-week intervals with control vector (200 μg), DNA85B (85B; 100 μg), control vector (100 μg), or DNA85B (100 μg) with each of the cytokine-encoding plasmids (100 μg). At 2 weeks after the final immunization, T cells purified from the spleens were stimulated with syngeneic irradiated splenocytes and Ag85. (A) Proliferation was measured 3 days later by thymidine incorporation. (B) Splenocyte cultures from the five groups were also stimulated with Ag85, and the number of IFN-γ-secreting cells was determined by ELISpot assay after 16 h of incubation. The data are means and SEM for five mice and are representative of one of three experiments. The differences between the groups were analyzed by analysis of variance (P < 0.001 [✽✽]; NS, not significant).
Codelivery of p2AIL-23 and p2AIL-27 has no significant effect on antigen-specific humoral responses.
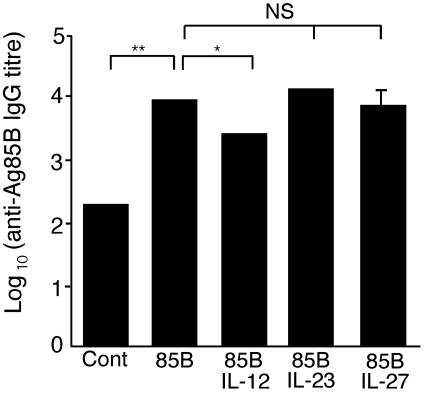
To determine whether coimmunization with p2AIL-23 and p2AIL-27 had an effect on the humoral response, anti-Ag85 IgG antibody titer was measured 2 weeks after the final immunization. DNA85B stimulated a strong IgG response to Ag85, whereas coimmunization with p2AIL-12 resulted in a significant decrease in IgG antibody titer (Fig. 5). In contrast, coimmunization with p2AIL-23 and p2AIL-27 resulted in no change in antibody titer compared to mice immunized with DNA85B alone.
FIG. 5.
Coimmunization with p2AIL-12, but not p2AIL-23 and p2AIL-27, decreases the antigen-specific antibody response. Mice were immunized i.m. three times at 2-week intervals as described in the legend of Fig. 4. Two weeks after the final immunization, sera were collected. The geometric means of the log10 titers of anti-Ag85 IgG antibodies were measured as described in Materials and Methods. The data represent the means ± the SEM for five mice and are representative of two separate experiments. The differences between the groups were analyzed by analysis of variance (P < 0.05 [✽], P < 0.001 [✽✽]; NS, not significant).
Delivery of IL-23, but not IL-27, increased the protective effect of DNA vaccines against M. tuberculosis infection.
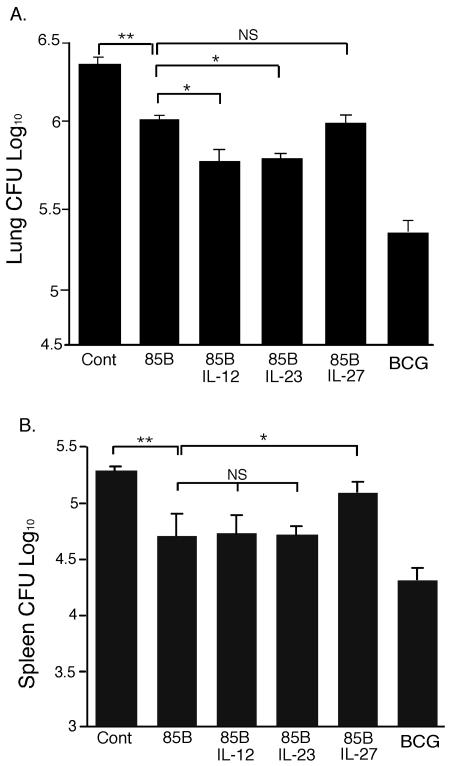
Mice were immunized three times with combinations of DNA85B and p2AIL-12, p2AIL-23, or p2AIL-27 or DNA85B alone and challenged 6 weeks later with aerosol H37Rv M. tuberculosis. DNA85B resulted in a significant reduction in the bacterial load in the lungs compared to control DNA, but the level of protection was not as great as that induced by BCG (Fig. 6A). Coimmunizing with p2AIL-12 or p2AIL-23, but not p2AIL-27, further improved protective efficacy compared to DNA85B. Immunization with DNA85B and BCG decreased the levels of dissemination to the spleen; however, coimmunization with p2AIL-12 or p2AIL-23 did not improve the control of dissemination over that elicited by DNA85B alone. Mice coimmunized with p2AIL-27 and DNA85B showed an increased bacterial burden, suggesting that plasmid-expressed IL-27 had inhibited the protective effect of DNA85B in the spleen (Fig. 6B).
FIG. 6.
Coimmunization with p2AIL-12 and p2AIL-23, but not p2AIL-27, increases the protective effect of DNA85B against M. tuberculosis infection in the lungs. Mice were immunized i.m. three times at 2-week intervals as described in the legend of Fig. 4. At 6 weeks after the final immunization, mice were challenged by aerosol with M. tuberculosis H37Rv. Four weeks later, the bacterial loads in the lungs (A) and spleen (B) were determined. The data are the means ± the SEM for five mice and are representative of two separate experiments. The significances of differences between the groups were analyzed by analysis of variance (P < 0.05 [✽], P < 0.001 [✽✽]; NS, not significant).
DISCUSSION
The response of DCs to mycobacteria is complex, involving phenotypic maturation and the secretion of multiple proinflammatory cytokines (15, 25, 30, 37). Infection of bone marrow-derived DCs with M. tuberculosis resulted in a greater upregulation of genes encoding IL-12 and IL-23 than did infection with BCG at the same level of infection (Fig. 1). Interestingly, there was no detectable increase in the expression of IL-27p28 mRNA in DCs infected with M. tuberculosis or BCG. This implicates both IL-12 and IL-23 in the initial host response to mycobacteria. Heterodimeric cytokines, such as IL-12 and IL-23, require the conjugate expression of both subunit chains from activated DCs. Homodimers of the p40 subunit may have an antagonistic effect on the IL-12R (24). Therefore, we used the self-cleaving polypeptide of the 2A protein from the FMDV (7) to express both chains of IL-12, IL-23, and IL-27 cytokines in transfected cells. In vitro bioassays confirmed the secretion of functional IL-23 and IL-27 (Fig. 3), whereas we have previously shown that the IL-12p70 product (Fig. 2B) is bioactive (50). Codelivery of plasmid IL-23, but not plasmid IL-27, with the antituberculosis DNA vaccine, DNA85B, mimicked the effect of coimmunization with p2AIL-12, leading to the enhanced induction of antigen-specific T cells and increased vaccine efficacy against aerosol M. tuberculosis.
Each of the cytokines, IL-12 IL-23, and IL-27 has been implicated in the development of Th1-like immunity (49, 53, 63). IL-12 has a central role in the differentiation of naive CD4+ T cells into an IFN-γ-secreting T-cell lineage, whereas IL-23 has been considered to act primarily on activated memory CD4+ T cells (49). The receptors for IL-23 (49) and IL-18 are both present on activated T cells, and the IL-18R is upregulated by IL-12 (38). In turn, these cytokines act on the activated CD4+ T cells to increase their clonal expansion and, in the case of IL-23, to bias their cytokine expression from IFN-γ to IL-17 (1). We now demonstrate that coimmunization with p2AIL-12 or p2AIL-23 has equivalent effects in increasing the frequency of Ag85B-specific IFN-γ-secreting T cells with a consequent increase in protective immunity. The one difference between p2AIL-12 and p2AIL-23 was that coimmunization with p2AIL-23 was not associated with the reduction in specific IgG response, which reproducibly accompanies the use of p2AIL-12 as an adjuvant with DNA vaccines (41, 50). In addition to the effects on CD4+ T cells, both plasmid-expressed IL-12 (50) and plasmid-expressed IL-23 stimulate increased CD8+ T-cell responses (43). The addition of plasmids expressing IL-23 to a DNA vaccine expressing hepatitis C envelope protein 2 resulted in long-lasting Th1-like and cytotoxic-T-lymphocyte (CTL) responses, which were stronger than when plasmids expressing IL-12 were used (28). The combined effects of p2AIL-12 and p2AIL-23 as long-term adjuvants on the protective efficacy of DNA vaccines against M. tuberculosis will be assessed in future studies.
In addition to the effect on T cells, both IL-12 and IL-23 may influence the function of APCs. Kinetic studies of gene expression after Salmonella enterica serovar Enteritidis infection of macrophages demonstrated an early, but transient, expression of IL-23p19 mRNA and a delayed, but continuous, expression of IL-12p40 and IL-12p35 mRNA (57). The receptors for IL-23 and IL-12 are both present on DCs (26) and macrophages (51), and the secretion of IL-12 and IL-23 may further stimulate DCs in an autocrine fashion (3, 21). In addition, the activation of DCs through CD40 signaling is of critical importance for the optimal induction of the IL-12 family cytokines and the subsequent development of adaptive immune responses (58). The in vitro stimulation of human and murine APCs with anti-CD40 increased the secretion of IL-12 and IL-23 (16, 65).
The importance of IL-23 in the control of intracellular infections is demonstrated by comparative studies with IL-12p35-deficient mice, which lack IL-12 alone, and IL-12p40-deficient mice, which lack both IL-12 and IL-23. IL-12p40-deficient mice have a more severe course of infection than IL-12p35-deficient mice after infection with Cryptococcus neoformans (13), Salmonella enterica serovar Enteritidis (39), Francisella tularensis (19), and M. tuberculosis (11). Nevertheless, IL-23 is not essential for the development of Th1-like T cells as specific T-cell proliferation and cytokine responses can be induced in IL-23p19-deficient mice, although the downstream production of IL-17 from memory CD4+ T cells was dependent on IL-23 (12). Excess IL-23 may contribute to inflammatory damage as the ubiquitous overexpression of IL-23p19 resulted in multiorgan failure and early death in transgenic mice (66).
Initial in vitro studies with purified IL-27 suggested it also had a role in the clonal expansion of naive CD4+ T cells into an IFN-γ-secreting Th1-like phenotype (53). BCG, Leishmania major or Listeria monocytogenes infection of IL-27R-deficient mice showed a transient reduction of T-cell responses to the pathogen and a diffuse granuloma formation; however, these were restored to normal patterns after 6 weeks (8, 69). These studies suggested that exogenous IL-27 may increase the T-cell response to vaccine antigens. However, plasmids expressing IL-27 when codelivered with DNA85B failed to increase the frequency of Th1-like T cells and to influence the course of M. tuberculosis infection. This finding is consistent with the more recent reports that IL-27 signaling is not required to control M. tuberculosis infection. Paradoxically, IL-27R-deficient mice showed increased clearance of M. tuberculosis during the first 4 to 8 weeks of infection, although there was a late increase in mortality in the IL-27R-deficient mice (31, 52). Moreover, infection with intracellular pathogens such as Trypanosoma cruzi or Toxoplasma gondii also resulted in an enhanced clearance of the organism but a late increase in the inflammatory response leading to death. Therefore, IL-27 signaling may be involved in an in vivo feedback loop controlling inflammatory responses to intracellular pathogens, as well as regulating Th1-like T-cell development (29, 64).
Effective antituberculosis subunit vaccines may contribute to the control of tuberculosis either as primary vaccines to replace BCG or to boost the effects of BCG given as a neonatal vaccine. More than 20 protein or DNA vaccines incorporating individual or fusion antigens from M. tuberculosis have been developed, but few, if any, have reproducibly demonstrated equivalent protection to BCG after aerosol or systemic M. tuberculosis infection (5). Both the Ag 85A and Ag 85B forms of mycolyl transferase are examples of partially protective subunit vaccines against tuberculosis, but neither is the equivalent to BCG. The present study demonstrates that the plasmid expressing IL-23, as well as the plasmid expressing IL-12, increases the immunogenicity and protective efficacy of DNA vaccine expressing Ag 85B in the lungs of challenged mice. Immunization with the plasmid expressing IL-12 is a more effective strategy than immunization with the plasmid expressing protein IL-12 in increasing the protective effect of DNA and protein vaccines against L. major (27). In addition, the plasmid expressing IL-12 increases the protective efficacy of DNA vaccines in a number of other infectious models (22, 23, 47, 48, 56, 60, 61, 68), but this is the first report of plasmid IL-23 enhancing the protective immunity of a subunit vaccine. Therefore, the addition of IL-23 and/or IL-12 may be an effective strategy for increasing the efficacy of DNA vaccines against M. tuberculosis.
Acknowledgments
This study was supported by the National Health and Medical Research Council of Australia. The support of the NSW Health Department through its research and development infrastructure grants program is gratefully acknowledged. T.M.W. is a recipient of the University of Sydney Faculty of Medicine Postgraduate Scholarship, and A.A.R. is supported by the Australian Postgraduate Award.
We thank U. Palendira and A. Kamath for helpful discussions and provision of the p2AIL-12 plasmid.
Editor: D. L. Burns
REFERENCES
- 1.Aggarwal, S., N. Ghilardi, M. H. Xie, F. J. de Sauvage, and A. L. Gurney. 2003. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 278:1910-1914. [DOI] [PubMed] [Google Scholar]
- 2.Artis, D., A. Villarino, M. Silverman, W. He, E. M. Thornton, S. Mu, S. Summer, T. M. Covey, E. Huang, H. Yoshida, G. Koretzky, M. Goldschmidt, G. D. Wu, F. de Sauvage, H. R. Miller, C. J. Saris, P. Scott, and C. A. Hunter. 2004. The IL-27 receptor (WSX-1) is an inhibitor of innate and adaptive elements of type 2 immunity. J. Immunol. 173:5626-5634. [DOI] [PubMed] [Google Scholar]
- 3.Bastos, K. R., C. R. Marinho, R. Barboza, M. Russo, J. M. Alvarez, and M. R. D'Imperio Lima. 2004. What kind of message does IL-12/IL-23 bring to macrophages and dendritic cells? Microbes Infect. 6:630-636. [DOI] [PubMed] [Google Scholar]
- 4.Belladonna, M. L., J. C. Renauld, R. Bianchi, C. Vacca, F. Fallarino, C. Orabona, M. C. Fioretti, U. Grohmann, and P. Puccetti. 2002. IL-23 and IL-12 have overlapping, but distinct, effects on murine dendritic cells. J. Immunol. 168:5448-5454. [DOI] [PubMed] [Google Scholar]
- 5.Britton, W. J., and U. Palendira. 2003. Improving vaccines against tuberculosis. Immunol. Cell Biol. 81:34-45. [DOI] [PubMed] [Google Scholar]
- 6.Casanova, J. L., and L. Abel. 2002. Genetic dissection of immunity to mycobacteria: the human model. Annu. Rev. Immunol. 20:581-620. [DOI] [PubMed] [Google Scholar]
- 7.Chaplin, P. J., E. B. Camon, B. Villarreal-Ramos, M. Flint, M. D. Ryan, and R. A. Collins. 1999. Production of interleukin-12 as a self-processing 2A polypeptide. J. Interferon Cytokine Res. 19:235-241. [DOI] [PubMed] [Google Scholar]
- 8.Chen, Q., N. Ghilardi, H. Wang, T. Baker, M. H. Xie, A. Gurney, I. S. Grewal, and F. J. de Sauvage. 2000. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature 407:916-920. [DOI] [PubMed] [Google Scholar]
- 9.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 10.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper, A. M., A. Kipnis, J. Turner, J. Magram, J. Ferrante, and I. M. Orme. 2002. Mice lacking bioactive IL-12 can generate protective, antigen-specific cellular responses to mycobacterial infection only if the IL-12 p40 subunit is present. J. Immunol. 168:1322-1327. [DOI] [PubMed] [Google Scholar]
- 11a.Corbett, E. L., C. J. Watt, N. Walker, D. Maher, B. G. Williams, M. C. Raviglione, and C. Dye. 2003. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch. Intern. Med. 163:1009-1021. [DOI] [PubMed] [Google Scholar]
- 12.Cua, D. J., J. Sherlock, Y. Chen, C. A. Murphy, B. Joyce, B. Seymour, L. Lucian, W. To, S. Kwan, T. Churakova, S. Zurawski, M. Wiekowski, S. A. Lira, D. Gorman, R. A. Kastelein, and J. D. Sedgwick. 2003. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421:744-748. [DOI] [PubMed] [Google Scholar]
- 13.Decken, K., G. Kohler, K. Palmer-Lehmann, A. Wunderlin, F. Mattner, J. Magram, M. K. Gately, and G. Alber. 1998. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect. Immun. 66:4994-5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demangel, C., A. G. Bean, E. Martin, C. G. Feng, A. T. Kamath, and W. J. Britton. 1999. Protection against aerosol Mycobacterium tuberculosis infection using Mycobacterium bovis bacillus Calmette-Guerin-infected dendritic cells. Eur. J. Immunol. 29:1972-1979. [DOI] [PubMed] [Google Scholar]
- 15.Demangel, C., and W. J. Britton. 2000. Interaction of dendritic cells with mycobacteria: where the action starts. Immunol. Cell Biol. 78:318-324. [DOI] [PubMed] [Google Scholar]
- 16.Demangel, C., U. Palendira, C. G. Feng, A. W. Heath, A. G. Bean, and W. J. Britton. 2001. Stimulation of dendritic cells via CD40 enhances immune responses to Mycobacterium tuberculosis infection. Infect. Immun. 69:2456-2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devergne, O., M. Hummel, H. Koeppen, M. M. Le Beau, E. C. Nathanson, E. Kieff, and M. Birkenbach. 1996. A novel interleukin-12 p40-related protein induced by latent Epstein-Barr virus infection in B lymphocytes. J. Virol. 70:1143-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reference deleted.
- 19.Elkins, K. L., A. Cooper, S. M. Colombini, S. C. Cowley, and T. L. Kieffer. 2002. In vivo clearance of an intracellular bacterium, Francisella tularensis LVS, is dependent on the p40 subunit of interleukin-12 (IL-12) but not on IL-12 p70. Infect. Immun. 70:1936-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frucht, D. M., T. Fukao, C. Bogdan, H. Schindler, J. J. O'Shea, and S. Koyasu. 2001. IFN-gamma production by antigen-presenting cells: mechanisms emerge. Trends Immunol. 22:556-560. [DOI] [PubMed] [Google Scholar]
- 22.Gan, Y., Y. E. Shi, L. Y. Bu, X. H. Zhu, C. X. Ning, and H. G. Zhu. 2004. Immune responses against Schistosoma japonicum after vaccinating mice with a multivalent DNA vaccine encoding integrated membrane protein Sj23 and cytokine interleukin-12. Chin. Med. J. 117:1842-1846. [PubMed] [Google Scholar]
- 23.Garcia-Navarro, R., B. Blanco-Urgoiti, P. Berraondo, R. Sanchez de la Rosa, A. Vales, S. Hervas-Stubbs, J. J. Lasarte, F. Borras, J. Ruiz, and J. Prieto. 2001. Protection against woodchuck hepatitis virus (WHV) infection by gene gun coimmunization with WHV core and interleukin-12. J. Virol. 75:9068-9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gately, M. K., D. M. Carvajal, S. E. Connaughton, S. Gillessen, R. R. Warrier, K. D. Kolinsky, V. L. Wilkinson, C. M. Dwyer, G. F. Higgins, Jr., F. J. Podlaski, D. A. Faherty, P. C. Familletti, A. S. Stern, and D. H. Presky. 1996. Interleukin-12 antagonist activity of mouse interleukin-12 p40 homodimer in vitro and in vivo. Ann. N. Y. Acad. Sci. 795:1-12. [DOI] [PubMed] [Google Scholar]
- 25.Giacomini, E., E. Iona, L. Ferroni, M. Miettinen, L. Fattorini, G. Orefici, I. Julkunen, and E. M. Coccia. 2001. Infection of human macrophages and dendritic cells with Mycobacterium tuberculosis induces a differential cytokine gene expression that modulates T-cell response. J. Immunol. 166:7033-7041. [DOI] [PubMed] [Google Scholar]
- 26.Grohmann, U., M. L. Belladonna, R. Bianchi, C. Orabona, E. Ayroldi, M. C. Fioretti, and P. Puccetti. 1998. IL-12 acts directly on DC to promote nuclear localization of NF-κB and primes DC for IL-12 production. Immunity 9:315-323. [DOI] [PubMed] [Google Scholar]
- 27.Gurunathan, S., C. Prussin, D. L. Sacks, and R. A. Seder. 1998. Vaccine requirements for sustained cellular immunity to an intracellular parasitic infection. Nat. Med. 4:1409-1415. [DOI] [PubMed] [Google Scholar]
- 28.Ha, S. J., D. J. Kim, K. H. Baek, Y. D. Yun, and Y. C. Sung. 2004. IL-23 induces stronger sustained CTL and Th1 immune responses than IL-12 in hepatitis C virus envelope protein 2 DNA immunization. J. Immunol. 172:525-531. [DOI] [PubMed] [Google Scholar]
- 29.Hamano, S., K. Himeno, Y. Miyazaki, K. Ishii, A. Yamanaka, A. Takeda, M. Zhang, H. Hisaeda, T. W. Mak, A. Yoshimura, and H. Yoshida. 2003. WSX-1 is required for resistance to Trypanosoma cruzi infection by regulation of proinflammatory cytokine production. Immunity 19:657-667. [DOI] [PubMed] [Google Scholar]
- 30.Hickman, S. P., J. Chan, and P. Salgame. 2002. Mycobacterium tuberculosis induces differential cytokine production from dendritic cells and macrophages with divergent effects on naive T-cell polarization. J. Immunol. 168:4636-4642. [DOI] [PubMed] [Google Scholar]
- 31.Holscher, C., A. Holscher, D. Ruckerl, T. Yoshimoto, H. Yoshida, T. Mak, C. Saris, and S. Ehlers. 2005. The IL-27 receptor chain WSX-1 differentially regulates antibacterial immunity and survival during experimental tuberculosis. J. Immunol. 174:3534-3544. [DOI] [PubMed] [Google Scholar]
- 32.Horwitz, M. A., G. Harth, B. J. Dillon, and S. Maslesa-Galic. 2000. Recombinant bacillus Calmette-Guerin (BCG) vaccines expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc. Natl. Acad. Sci. USA 97:13853-13858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huygen, K. 2003. On the use of DNA vaccines for the prophylaxis of mycobacterial diseases. Infect. Immun. 71:1613-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huygen, K., J. Content, O. Denis, D. L. Montgomery, A. M. Yawman, R. R. Deck, C. M. DeWitt, I. M. Orme, S. Baldwin, C. D'Souza, A. Drowart, E. Lozes, P. Vandenbussche, J. P. Van Vooren, M. A. Liu, and J. B. Ulmer. 1996. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat. Med. 2:893-898. [DOI] [PubMed] [Google Scholar]
- 35.Jackson, M., S. W. Phalen, M. Lagranderie, D. Ensergueix, P. Chavarot, G. Marchal, D. N. McMurray, B. Gicquel, and C. Guilhot. 1999. Persistence and protective efficacy of a Mycobacterium tuberculosis auxotroph vaccine. Infect. Immun. 67:2867-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamath, A. T., C. G. Feng, M. Macdonald, H. Briscoe, and W. J. Britton. 1999. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis. Infect. Immun. 67:1702-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim, K. D., H. G. Lee, J. K. Kim, S. N. Park, I. S. Choe, Y. K. Choe, S. J. Kim, E. Lee, and J. S. Lim. 1999. Enhanced antigen-presenting activity and tumour necrosis factor-alpha-independent activation of dendritic cells following treatment with Mycobacterium bovis bacillus Calmette-Guerin. Immunology 97:626-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lawless, V. A., S. Zhang, O. N. Ozes, H. A. Bruns, I. Oldham, T. Hoey, M. J. Grusby, and M. H. Kaplan. 2000. Stat4 regulates multiple components of IFN-gamma-inducing signaling pathways. J. Immunol. 165:6803-6808. [DOI] [PubMed] [Google Scholar]
- 39.Lehmann, J., S. Bellmann, C. Werner, R. Schroder, N. Schutze, and G. Alber. 2001. IL-12p40-dependent agonistic effects on the development of protective innate and adaptive immunity against Salmonella enteritidis. J. Immunol. 167:5304-5315. [DOI] [PubMed] [Google Scholar]
- 40.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 41.Martin, E., A. T. Kamath, H. Briscoe, and W. J. Britton. 2003. The combination of plasmid interleukin-12 with a single DNA vaccine is more effective than Mycobacterium bovis (bacille Calmette-Guerin) in protecting against systemic Mycobacterium avium infection. Immunology 109:308-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsui, M., O. Moriya, and T. Akatsuka. 2003. Enhanced induction of hepatitis C virus-specific cytotoxic T lymphocytes and protective efficacy in mice by DNA vaccination followed by adenovirus boosting in combination with the interleukin-12 expression plasmid. Vaccine 21:1629-1639. [DOI] [PubMed] [Google Scholar]
- 43.Matsui, M., O. Moriya, M. L. Belladonna, S. Kamiya, F. A. Lemonnier, T. Yoshimoto, and T. Akatsuka. 2004. Adjuvant activities of novel cytokines, interleukin-23 (IL-23) and IL-27, for induction of hepatitis C virus-specific cytotoxic T lymphocytes in HLA-A*0201 transgenic mice. J. Virol. 78:9093-9104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Micallef, M. J., T. Ohtsuki, K. Kohno, F. Tanabe, S. Ushio, M. Namba, T. Tanimoto, K. Torigoe, M. Fujii, M. Ikeda, S. Fukuda, and M. Kurimoto. 1996. Interferon-gamma-inducing factor enhances T helper 1 cytokine production by stimulated human T cells: synergism with interleukin-12 for interferon-gamma production. Eur. J. Immunol. 26:1647-1651. [DOI] [PubMed] [Google Scholar]
- 45.Okada, E., S. Sasaki, N. Ishii, I. Aoki, T. Yasuda, K. Nishioka, J. Fukushima, J. Miyazaki, B. Wahren, and K. Okuda. 1997. Intranasal immunization of a DNA vaccine with IL-12- and granulocyte-macrophage colony-stimulating factor (GM-CSF)-expressing plasmids in liposomes induces strong mucosal and cell-mediated immune responses against HIV-1 antigens. J. Immunol. 159:3638-3647. [PubMed] [Google Scholar]
- 46.Olsen, A. W., A. Williams, L. M. Okkels, G. Hatch, and P. Andersen. 2004. Protective effect of a tuberculosis subunit vaccine based on a fusion of antigen 85B and ESAT-6 in the aerosol guinea pig model. Infect. Immun. 72:6148-6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Neill, E., I. Martinez, F. Villinger, M. Rivera, S. Gascot, C. Colon, T. Arana, M. Sidhu, R. Stout, D. C. Montefiori, M. Martinez, A. A. Ansari, Z. R. Israel, and E. Kraiselburd. 2002. Protection by SIV VLP DNA prime/protein boost following mucosal SIV challenge is markedly enhanced by IL-12/GM-CSF co-administration. J. Med. Primatol. 31:217-227. [DOI] [PubMed] [Google Scholar]
- 48.Operschall, E., J. Pavlovic, M. Nawrath, and K. Molling. 2000. Mechanism of protection against influenza A virus by DNA vaccine encoding the hemagglutinin gene. Intervirology 43:322-330. [DOI] [PubMed] [Google Scholar]
- 49.Oppmann, B., R. Lesley, B. Blom, J. C. Timans, Y. Xu, B. Hunte, F. Vega, N. Yu, J. Wang, K. Singh, F. Zonin, E. Vaisberg, T. Churakova, M. Liu, D. Gorman, J. Wagner, S. Zurawski, Y. Liu, J. S. Abrams, K. W. Moore, D. Rennick, R. de Waal-Malefyt, C. Hannum, J. F. Bazan, and R. A. Kastelein. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13:715-725. [DOI] [PubMed] [Google Scholar]
- 50.Palendira, U., A. T. Kamath, C. G. Feng, E. Martin, P. J. Chaplin, J. A. Triccas, and W. J. Britton. 2002. Coexpression of interleukin-12 chains by a self-splicing vector increases the protective cellular immune response of DNA and Mycobacterium bovis BCG vaccines against Mycobacterium tuberculosis. Infect. Immun. 70:1949-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parham, C., M. Chirica, J. Timans, E. Vaisberg, M. Travis, J. Cheung, S. Pflanz, R. Zhang, K. P. Singh, F. Vega, W. To, J. Wagner, A. M. O'Farrell, T. McClanahan, S. Zurawski, C. Hannum, D. Gorman, D. M. Rennick, R. A. Kastelein, R. de Waal Malefyt, and K. W. Moore. 2002. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rβ1 and a novel cytokine receptor subunit, IL-23R. J. Immunol. 168:5699-5708. [DOI] [PubMed] [Google Scholar]
- 52.Pearl, J. E., S. A. Khader, A. Solache, L. Gilmartin, N. Ghilardi, F. deSauvage, and A. M. Cooper. 2004. IL-27 signaling compromises control of bacterial growth in mycobacteria-infected mice. J. Immunol. 173:7490-7496. [DOI] [PubMed] [Google Scholar]
- 53.Pflanz, S., J. C. Timans, J. Cheung, R. Rosales, H. Kanzler, J. Gilbert, L. Hibbert, T. Churakova, M. Travis, E. Vaisberg, W. M. Blumenschein, J. D. Mattson, J. L. Wagner, W. To, S. Zurawski, T. K. McClanahan, D. M. Gorman, J. F. Bazan, R. de Waal Malefyt, D. Rennick, and R. A. Kastelein. 2002. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity 16:779-790. [DOI] [PubMed] [Google Scholar]
- 54.Pinto, R., B. M. Saunders, L. R. Camacho, W. J. Britton, B. Gicquel, and J. A. Triccas. 2004. Mycobacterium tuberculosis defective in phthiocerol dimycocerosate translocation provides greater protective immunity against tuberculosis than the existing bacille Calmette-Guerin vaccine. J. Infect. Dis. 189:105-112. [DOI] [PubMed] [Google Scholar]
- 55.Reed, S. G., M. R. Alderson, W. Dalemans, Y. Lobet, and Y. A. Skeiky. 2003. Prospects for a better vaccine against tuberculosis. Tuberculosis 83:213-219. [DOI] [PubMed] [Google Scholar]
- 56.Sakai, T., H. Hisaeda, Y. Nakano, M. Zhang, M. Takashima, K. Ishii, Y. Maekawa, S. Matsumoto, Y. Nitta, J. Miyazaki, S. Yamamoto, and K. Himeno. 2003. Gene gun-based coimmunization of merozoite surface protein-1 cDNA with IL-12 expression plasmid confers protection against lethal Plasmodium yoelii in A/J mice. Vaccine 21:1432-1444. [DOI] [PubMed] [Google Scholar]
- 57.Schuetze, N., S. Schoeneberger, U. Mueller, M. A. Freudenberg, G. Alber, and R. K. Straubinger. 2005. IL-12 family members: differential kinetics of their TLR4-mediated induction by Salmonella enteritidis and the impact of IL-10 in bone marrow-derived macrophages. Int. Immunol. 17:649-659. [DOI] [PubMed] [Google Scholar]
- 58.Schulz, O., A. D. Edwards, M. Schito, J. Aliberti, S. Manickasingham, A. Sher, and C. Reis e Sousa. 2000. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity 13:453-462. [DOI] [PubMed] [Google Scholar]
- 59.Seder, R. A., R. Gazzinelli, A. Sher, and W. E. Paul. 1993. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proc. Natl. Acad. Sci. USA 90:10188-10192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sin, J. I., J. J. Kim, R. L. Arnold, K. E. Shroff, D. McCallus, C. Pachuk, S. P. McElhiney, M. W. Wolf, S. J. Pompa-de Bruin, T. J. Higgins, R. B. Ciccarelli, and D. B. Weiner. 1999. IL-12 gene as a DNA vaccine adjuvant in a herpes mouse model: IL-12 enhances Th1-type CD4+ T cell-mediated protective immunity against herpes simplex virus-2 challenge. J. Immunol. 162:2912-2921. [PubMed] [Google Scholar]
- 61.Suh, Y. S., S. J. Ha, C. H. Lee, J. I. Sin, and Y. C. Sung. 2001. Enhancement of VP1-specific immune responses and protection against EMCV-K challenge by co-delivery of IL-12 DNA with VP1 DNA vaccine. Vaccine 19:1891-1898. [DOI] [PubMed] [Google Scholar]
- 62.Triccas, J. A., L. Sun, U. Palendira, and W. J. Britton. 2002. Comparative affects of plasmid-encoded interleukin 12 and interleukin 18 on the protective efficacy of DNA vaccination against Mycobacterium tuberculosis. Immunol. Cell Biol. 80:346-350. [DOI] [PubMed] [Google Scholar]
- 63.Trinchieri, G. 1993. Interleukin-12 and its role in the generation of TH1 cells. Immunol. Today 14:335-338. [DOI] [PubMed] [Google Scholar]
- 64.Villarino, A., L. Hibbert, L. Lieberman, E. Wilson, T. Mak, H. Yoshida, R. A. Kastelein, C. Saris, and C. A. Hunter. 2003. The IL-27R (WSX-1) is required to suppress T-cell hyperactivity during infection. Immunity 19:645-655. [DOI] [PubMed] [Google Scholar]
- 65.Wesa, A., and A. Galy. 2002. Increased production of pro-inflammatory cytokines and enhanced T cell responses after activation of human dendritic cells with IL-1 and CD40 ligand. BMC Immunol. 3:14. [Online.] http://www.biomedcentral.com/1471-2172/3/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wiekowski, M. T., M. W. Leach, E. W. Evans, L. Sullivan, S. C. Chen, G. Vassileva, J. F. Bazan, D. M. Gorman, R. A. Kastelein, S. Narula, and S. A. Lira. 2001. Ubiquitous transgenic expression of the IL-23 subunit p19 induces multiorgan inflammation, runting, infertility, and premature death. J. Immunol. 166:7563-7570. [DOI] [PubMed] [Google Scholar]
- 67.Wolf, S. F., P. A. Temple, M. Kobayashi, D. Young, M. Dicig, L. Lowe, R. Dzialo, L. Fitz, C. Ferenz, and R. M. Hewick. 1991. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J. Immunol. 146:3074-3081. [PubMed] [Google Scholar]
- 68.Wu, S. F., C. L. Liao, Y. L. Lin, C. T. Yeh, L. K. Chen, Y. F. Huang, H. Y. Chou, J. L. Huang, M. F. Shaio, and H. K. Sytwu. 2003. Evaluation of protective efficacy and immune mechanisms of using a nonstructural protein NS1 in DNA vaccine against dengue 2 virus in mice. Vaccine 21:3919-3929. [DOI] [PubMed] [Google Scholar]
- 69.Yoshida, H., S. Hamano, G. Senaldi, T. Covey, R. Faggioni, S. Mu, M. Xia, A. C. Wakeham, H. Nishina, J. Potter, C. J. Saris, and T. W. Mak. 2001. WSX-1 is required for the initiation of Th1 responses and resistance to Leishmania major infection. Immunity 15:569-578. [DOI] [PubMed] [Google Scholar]