Abstract
Gamma interferon (IFN-γ) plays a critical role in the protective immune responses against mycobacteria. We previously cloned a cDNA coding for guinea pig IFN-γ (gpIFN-γ) and reported that BCG vaccination induced a significant increase in the IFN-γ mRNA expression in guinea pig cells in response to living mycobacteria and that the virulent H37Rv strain of Mycobacterium tuberculosis stimulated less IFN-γ mRNA than did the attenuated H37Ra strain. In this study, we successfully expressed and characterized recombinant gpIFN-γ with a histidine tag at the N terminus (His-tagged rgpIFN-γ) in Escherichia coli. rgpIFN-γ was identified as an 18-kDa band in the insoluble fraction; therefore, the protein was purified under denaturing conditions and renatured. N-terminal amino acid sequencing of the recombinant protein yielded the sequence corresponding to the N terminus of His-tagged gpIFN-γ. The recombinant protein upregulated major histocompatibility complex class II expression in peritoneal macrophages. The antiviral activity of rgpIFN-γ was demonstrated with a guinea pig fibroblast cell line (104C1) infected with encephalomyocarditis virus. Interestingly, peritoneal macrophages treated with rgpIFN-γ did not produce any nitric oxide but did produce hydrogen peroxide and suppressed the intracellular growth of mycobacteria. Furthermore, rgpIFN-γ induced morphological alterations in cultured macrophages. Thus, biologically active rgpIFN-γ has been successfully produced and characterized in our laboratory. The study of rgpIFN-γ will further increase our understanding of the cellular and molecular responses induced by BCG vaccination in the guinea pig model of pulmonary tuberculosis.
Tuberculosis remains a global health problem, in part because of its occurrence in populations where human immunodeficiency virus/AIDS is also highly prevalent and due to the emergence of multidrug-resistant strains (22). The only vaccine available for tuberculosis is Mycobacterium bovis BCG; however, its efficacy has varied widely in clinical trials (4). Effector mechanisms in antimycobacterial immunity involve macrophages and lymphocytes that act in concert with the help of costimulatory molecules and molecular mediators such as cytokines and chemokines (1). Macrophages act as the primary phagocytic cells for early clearance of mycobacteria. It is known that the immune response against Mycobacterium tuberculosis is mediated primarily by CD4+ and CD8+ T cells but also involves other cell types, including NK cells (14, 33, 39). Both gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) have been identified as important cytokines acting at the effector level of resistance to mycobacteria. TNF-α plays an important role in the formation and maintenance of granulomas and, along with IFN-γ, activates macrophages to produce effector molecules such as reactive oxygen and nitrogen intermediates (15, 17, 27). It is also known that interleukin-12 (IL-12) and IL-23 (18, 46) contribute to the host response to mycobacteria by enhancing the production of IFN-γ and thus the development of Th1 immunity.
The importance of IFN-γ in antimycobacterial immunity has been well documented. For example, IFN-γ is known to activate macrophages by upregulating major histocompatibility complex (MHC) class II molecules, as well as to induce effector molecules such as reactive nitrogen intermediates in murine and human cells (45, 47). Several studies indicate that activation of monocytes and macrophages by IFN-γ results in effective growth restriction and clearance of mycobacteria (11-13). Treatment of mice with neutralizing antibodies to TNF-α and IFN-γ prevented cellular infiltration and reduced the expression of proinflammatory and regulatory cytokines such as TNF-α, IFN-γ, macrophage inflammatory protein 1α, IL-1β, and IL-10 (15). Similarly, disruption of the mouse IFN-γ gene or the IFN-γ receptor gene resulted in an exacerbation of disease after M. tuberculosis or M. bovis infection, reduced expression of MHC class II antigens on macrophages, and lethality in mice given sublethal doses (5, 6, 23, 24, 25). Furthermore, humans with a mutation in the IFN-γ receptor or IFN-γ receptor signal-transducing chain developed disseminated mycobacterial infections (9, 41).
The role of IFN-γ in the guinea pig model of low-dose pulmonary infection has not been studied due to the lack of recombinant protein and antibody. We have found that BCG vaccination induced a significant increase in IFN-γ mRNA expression in spleen or lymph node cells stimulated with increasing doses of purified protein derivative or M. tuberculosis H37Ra or H37Rv, compared to cells from naive animals, by using a guinea pig IFN-γ (gpIFN-γ) cDNA clone (21). The purpose of this study was to express and purify recombinant gpIFN-γ (rgpIFN-γ) protein and test its bioactivity based on its ability to activate macrophages. The results from these studies indicate that treatment with rgpIFN-γ upregulates MHC class II expression, induces H2O2 production, restricts the growth of virulent M. tuberculosis in peritoneal cells, and reduces the cytopathic effect (CPE) in a guinea pig cell line infected with encephalomyocarditis virus (EMCV).
MATERIALS AND METHODS
Cloning and expression of rgpIFN-γ.
The plasmid chosen for expression of rgpIFN-γ was a pQE-30 derivative (QIAGEN, Valencia, CA), and the protocol used was essentially the same as that used earlier in our laboratory for rgpTNF-α and rgpIL-8 expression (29, 32). The plasmid was originally engineered by Magnus Hook (TAMU-HSC IBT, Houston, TX) to include a thrombin cleavage site, THR, between the six-His tag and the multiple cloning sites. gpIFN-γ cDNA provided by one of us (T.Y.) was cloned into the pQE-30 multiple cloning site. Escherichia coli strain M15 transformed with pQE-30/gpIFN-γ was grown at 37°C for approximately 1.5 h (optical density at 600 nm [OD600] = 0.7) in Luria-Bertani medium containing 100 μg/ml ampicillin and 25 μg/ml kanamycin (Sigma). Induction of protein was done with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG; Sigma), and 5 h later, the bacterial cells were harvested by centrifugation at 4,000 × g for 20 min. The cell pellet was frozen at −80°C. An 18-kDa band in the insoluble fraction was identified as a putative six-His-rgpIFN-γ by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis. Cleared lysates (CL) were prepared by lysing the pellet under denaturing conditions by the QIAGEN protocol in the presence of 10 mM benzamidine to limit protease activity. The supernatant collected after centrifugation was mixed with a 50% Ni-nitrilotriacetic acid slurry (QIAGEN) and purified on polypropylene columns (QIAGEN). The immobilized rgpIFN-γ was washed, and the protein was eluted in elution buffer (100 mM NaH2PO4, 10 mM Tris-Cl, pH 4.5) in the presence of 8 M urea. Renaturation of the denatured protein was carried out by dropwise addition of 10 ml of eluted protein to 100 ml of dilution buffer (50 mM Tris, 50 mM NaCl, pH 8.0) containing 14 μl of β-mercaptoethanol in the absence of urea. The folded protein solution was left for 2 to 4 h at room temperature (RT) and transferred to 4°C. Protein was concentrated in Amicon Ultra-15 centrifugal filter devices (Millipore Corp., Bedford, MA) by spinning 15 ml of sample for 50 min at 2,635 × g at 4°C. The protein content was determined by the Bradford assay (Bio-Rad, Richmond, CA). The refolded protein was sequenced by the Edman protein sequencing method on a Hewlett Packard G1000A automated protein sequencer (L. Dangott, Protein Chemistry Laboratory, Texas A&M University). Aliquots of rgpIFN-γ were prepared and stored at −80°C for testing the bioactivity. The level of endotoxin in the folded recombinant protein was tested by the Limulus amebocyte lysate assay (Associates of Cape Cod, Inc., Falmouth, MA) and was found to be 0.075 endotoxin units/μg (17 pg/μg) IFN-γ and was much lower than the acceptable concentration (100 pg/μg) in commercially available recombinant cytokines.
Induction of polyclonal antibody.
Three New Zealand rabbits (Charles River Breeding Laboratories, Inc., Wilmington, MA) were immunized subcutaneously with 200 μg of denatured gpIFN-γ in 500 μl of phosphate-buffered saline (PBS) mixed with 500 μl of TiterMaxGold adjuvant (CytRx Corp., Atlanta, GA). The adjuvant-antigen mixture was injected into five sites (0.2 ml/site). Beginning 4 weeks after the first immunization, the rabbits were boosted three times intramuscularly at 3-week intervals with the same dose of denatured protein-adjuvant. The rabbits were bled both before and at regular intervals after immunization to determine the antibody titer by Western blot analysis and were exsanguinated 5 weeks following the last booster. Sera obtained after centrifugation of blood at 1,520 × g for 20 min were stored in small aliquots at −80°C. The specificity of the serum was tested by Western blot analysis.
Western blot analysis.
Approximately 200 ng of the denatured protein eluted from the Ni column or the folded protein was separated by SDS-PAGE with 10 to 20% Tris-Tricine precast gels under nonreducing conditions and stained by Coomassie blue or SilverXpress (NOVEX-Invitrogen, Carlsbad, CA). The protein was transferred onto polyvinylidene difluoride membranes (Invitrogen) with Tris-glycine transfer buffer (Invitrogen) for 1 h at 100 V. The WesternBreeze chromogenic immunodetection kit (Invitrogen) was used to visualize the immunoreactive protein. Briefly, the blotted polyvinylidene difluoride membranes were blocked with a solution containing Hammersten casein for 30 min at RT and incubated in a primary antibody solution containing 1:1,000 and 1:5,000 dilutions of rabbit anti-rgpIFN-γ antiserum for 1 h and in a secondary antibody solution (alkaline phosphatase-conjugated, affinity-purified anti-rabbit immunoglobulin G [IgG]; Invitrogen) for 30 min. In order to develop the blots, the antibody-bound membranes were incubated at RT with gentle agitation in a solution containing 5-bromo-4-chloro-3-indolyl-1-phosphate (BCIP) and Nitro Blue Tetrazolium. The filters were air dried and photographed.
Viral cytopathicity assay.
The biological activity of the folded IFN-γ was determined based on its ability to reduce the CPE of EMCV in guinea pig fibroblast cell line 104C1 as described previously by us (51). The expanded cells (3 × 104/100 μl/well) were plated in flat-bottom 96-well plates in RPMI medium (Irvine Scientific, Santa Ana, CA) without phenol red containing 4% fetal bovine serum (FBS; Atlanta Biologicals, Norcross, GA), 100 U/ml penicillin (Irvine Scientific), and 100 μg/ml streptomycin (Irvine Scientific) and incubated at 37°C for 24 h. Protein samples were added to the cells after making serial dilutions in complete RPMI medium, and the plates were incubated overnight at 37°C. A human lymphoblastoid IFN-α (hIFN-α, 200 U/ml; ICN Pharmaceuticals, Inc., Costa Mesa, CA) standard was used in each assay. The cells were infected with the virus (multiplicity of infection [MOI], 0.03) for 30 h; the dye, a mixture of 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium monosodium salt and 1-methoxy-5-methylphenazinium methyl sulfate, was added; and the plates were incubated for 1.5 h at 37°C. The color reaction was stopped by adding 1 N H2SO4, and the intensity of the color was measured at OD450 to OD650 in a Dynatech MR5000 automated plate reader (Dynatech Laboratories, Chantilly, VA). The net change in OD was calculated by subtracting the OD of the test well from that of the infected control wells. The titers that showed 50% CPE inhibition were calculated for both hIFN-α and the test samples, and the units of activity in the sample were expressed relative to the hIFN-α standard.
Animals and BCG vaccination.
Randomly bred Hartley strain guinea pigs weighing 200 to 300 g were obtained from Charles River Breeding Laboratories, Inc. (Wilmington, MA). The animals were housed individually in polycarbonate cages in a temperature- and humidity-controlled environment; ambient lighting was automatically controlled to provide 12-h light and 12-h dark cycles. Animals were given commercial chow (Ralston Purina, St. Louis, MO) and tap water ad libitum. All procedures were reviewed and approved by the Texas A&M University Laboratory Animal Care Committee. Guinea pigs were vaccinated intradermally with 0.1 ml (103 viable units) of M. bovis BCG (Danish 1331 strain; Statens Seruminstitut, Copenhagen, Denmark) in the left and right inguinal regions. The lyophilized vaccine was reconstituted with Sauton's medium (Statens Seruminstitut) just before use.
Harvesting of peritoneal cells.
Guinea pigs were euthanized by intramuscular injection of an overdose of sodium pentobarbital (Sleepaway; Fort Dodge Laboratories, Inc.). The peritoneal cavity was opened aseptically, and resident cells were harvested by flushing the peritoneal cavity three times with 20 ml of cold RPMI medium (Irvine Scientific, Santa Ana, CA) containing 20 U of heparin. The recovered cells were kept on ice. Cells were depleted of erythrocytes by incubation in ACK lysing buffer (0.14 M NH4Cl, 1.0 mM KHCO3, 0.1 mM Na2EDTA [pH 7.2 to 7.4]). The cells were washed in RPMI medium, and the viable cells were counted by the trypan blue exclusion method. The cells were suspended at 5 × 106/ml in RPMI medium supplemented with 2 μM glutamine (Irvine Scientific), 0.01 mM 2-mercaptoethanol (Sigma, St. Louis, MO), 100 U of penicillin/ml (Irvine Scientific), 100 μg of streptomycin/ml (Irvine Scientific), and 10% heat-inactivated FBS (Atlanta Biologicals). Cells (2 × 106) were cultured in a 1-ml volume in polypropylene round-bottom tubes (Becton Dickinson, Franklin Lakes, NJ) in the presence or absence of rgpIFN-γ (5 to 2,000 ng/ml) for 24 to 72 h to determine MHC class II expression. For other assays, cells were allowed to adhere for 2 to 3 h in 24- or 96-well microtiter plates (Becton Dickinson Labware, Franklin Lakes, NJ). Nonadherent cells were removed; the monolayers were composed predominantly of macrophages (>95%), as visualized by nonspecific esterase staining (50). These macrophage-enriched cells were then stimulated with various concentrations of rgpIFN-γ.
MHC class II staining and flow cytometry.
The peritoneal cells were stained with monoclonal antibody against guinea pig MHC class II (Biosource International, Camarillo, CA). For each antibody or control, 5 × 105 cells were placed in small polypropylene tubes and pelleted by centrifugation at 200 × g for 10 min at 4°C. The supernatant was removed, and the pellet was resuspended in 50 μl of PBS. FcR binding was effectively blocked by incubating the cell suspension in 10 μl of (1 mg/ml) normal goat IgG (Sigma, St. Louis, MO) for 10 min before the addition of 50 μl of mouse anti-guinea pig MHC class II (1:10) antibody. Both primary and secondary antibody dilutions were made in PBS containing 0.1% goat IgG. The cells were then incubated for 1 h in ice on a shaker. At the end of the incubation, cells were washed three times with Hanks balanced salt solution (HBSS) containing 10% FBS at 200 × g for 10 min. The pellet was resuspended in a 1:10 dilution of the secondary antibody (fluorescein isothiocyanate-conjugated AffiniPure goat anti-mouse IgG [heavy and light chains]; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) and incubated for 1 h in ice on a shaker. The cells were then washed two times and finally suspended in 300 μl HBSS containing 1% paraformaldehyde. Expression of MHC class II on macrophages was determined by flow cytometry, with a FACScalibur and CellQuest (Becton Dickinson Immunocytometry Systems, San Jose, CA) software for data acquisition. Data analysis was performed in FlowJo (Treestar Inc., Ashland, OR). A macrophage region was defined based on forward and side light scattering characteristics, and univariate probability binning (43) was used to define the MHC class-II positive macrophages based on the intensity of fluorescein staining. In the MultiSample Comparison platform, the number of bins was set to ensure an average of at least 10 events per channel in all samples being compared, and a gate for the MHC class II-positive macrophages was defined. The mean fluorescence intensity (MFI) of the MHC class II macrophages was defined as the geometric MFI, expressed in arbitrary linear units, of the positive population defined by probability binning.
Determination of nitrite production.
Peritoneal cells were allowed to adhere to 96-well microtiter plates for 2 to 3 h, and the nonadherent cells were removed. Macrophages were incubated in the presence of rgpIFN-γ (5 to 500 ng/ml) for 24 h and subsequently infected with M. tuberculosis H37Rv (MOI, 0.1) for 24 to 72 h. The supernatants from these cultures were collected at regular time intervals and tested for the presence of nitrite with the Griess reagent (8). Briefly, 50 μl of supernatant was added in triplicate to a 96-well plate and mixed with an equal volume of Griess reagent (1% sulfanilamide, 0.1% naphthylethylenediamine dihydrochloride, 2.5% H3PO4). The absorbance at 540 nm was measured in a microplate reader with NaNO3 as a standard for determining the nitrite concentration.
Effect on H2O2 production.
The H2O2 production assay was based on the horseradish peroxidase-dependent oxidation of phenol red by H2O2 as reported earlier (40). Peritoneal cells from both naive and BCG-vaccinated guinea pigs were plated in 96-well microtiter plates, and the nonadherent cells were removed after 3 h. The cells were stimulated with rgpIFN-γ (5 to 500 ng/ml) for 2 to 3 h and then infected with M. tuberculosis H37Rv (MOI, 0.1) for 24 to 48 h. At appropriate time intervals, media were removed from the wells and 100 μl of the assay solution (28 mM phenol red [Sigma], 100 U/ml horseradish peroxidase type II [Sigma]) in HBSS was added to the test wells. The assay solution containing stimulants (phorbol myristate acetate [PMA; Sigma] at 100 ng/ml) or inhibitors (100 to 200 μM catalase; Sigma) was also added to the appropriate wells. The plates were incubated for 1 h at 37°C in the presence of 5% CO2. The control wells contained unstimulated and uninfected cells. After the incubation, the reaction was stopped by addition of 10 μl of 1 N NaOH to each well. The standards were prepared by making twofold serial dilutions of 100 μM H2O2 (Sigma) in the assay solution and adding 10 μl of 1 N NaOH. The plates were then allowed to equilibrate for 3 min, and the absorbance was measured in a plate reader at 630 nm. The amount of H2O2 released by the rgpIFN-γ-treated cultures was calculated as nanomoles per milliliter from the standard curve.
Effect of rgpIFN-γ on intracellular growth of mycobacteria.
The intracellular growth of mycobacteria was determined by the incorporation of [3H]uracil by metabolically active M. tuberculosis as reported by us previously (3). Peritoneal cells from both naive and BCG-vaccinated guinea pigs were plated (3 × 105 cells/well) in 96-well microtiter plates in complete RPMI medium containing 10% FBS, and the nonadherent cells were removed after 3 h of incubation at 37°C. The cells were treated with various concentrations (50 to 1,000 ng/ml) of rgpIFN-γ for 24 h, at the end of which medium was removed and the cells washed once in antibiotic free RPMI medium. Macrophages were then infected with M. tuberculosis H37Rv (MOI, 1:1) for 3 h, and the extracellular bacteria were removed by washing two times with antibiotic-free medium. In some experiments, rgpIFN-γ was added both before and after phagocytosis, and in other experiments, the recombinant protein was added only after phagocytosis. In all cultures, protein was present throughout the 7-day culture period. Infected macrophages were then incubated in medium containing gentamicin (50 μg/ml) to inhibit the extracellular mycobacteria for 7 days at 37°C. The macrophages were pulsed with 1 μCi of [3H]uracil for 24 h before harvesting. The mycobacteria were killed by incubating the plate at 80°C for 30 min and harvested with a FilterMate harvester, and the [3H]uracil uptake was measured in a scintillation counter (Beckman LS-1801).
Effect of rgpIFN-γ treatment on morphological changes.
The changes in the morphology of resident peritoneal macrophages after rgpIFN-γ (5 to 500 ng/ml) treatment were visualized under an inverted microscope. Macrophages were also cultured in collagen- or fibronectin-coated chamber slides (BD Biosciences, Bedford, MA) in the presence of rgpIFN-γ and stained by the Diff-Quik staining method (American Scientific Products, McGaw Park, IL).
Statistics.
The differences between naive and BCG-vaccinated groups were assessed by analysis of variance (ANOVA), and the differences between the treated and untreated groups were examined by Student t test; values of P < 0.05 were considered statistically significant. There were 3 to 10 guinea pigs per group, and each experiment was performed at least three times.
RESULTS
Purification of rgpIFN-γ.
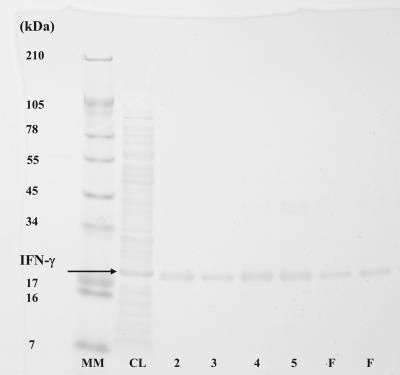
gpIFN-γ cDNA (GenBank accession number E25787) was subcloned into the expression vector pQE30 THR and transformed into E. coli M15, and protein expression was induced with IPTG for 5 h. An 18-kDa band in the insoluble fraction was recognized as the putative His-tagged gpIFN-γ by SDS-PAGE analysis and was purified by Ni affinity chromatography. Figure 1 shows the Coomassie blue-stained SDS-PAGE gel with a distinct band present at 18 kDa in the crude CL (lane 2), which corresponds to the molecular mass of gpIFN-γ; lanes 3 to 6 contain elution fractions (2 to 5) following affinity chromatography; and lanes 7 and 8 contain the folded protein (F). N-terminal sequencing of the recombinant protein preparation yielded the sequence MRGSHHHHHHGALVPRGXYYXQSRF. This corresponds to the predicted N-terminal sequence of His-tagged gpIFN-γ based on the sequence analysis of the cDNA published earlier (21), thus confirming the identity of the purified protein and suggesting the lack of any other protein contaminants in the sample.
FIG. 1.
Coomassie blue-stained SDS-PAGE analysis of crude and purified cell lysate, elution fractions, and folded rgpIFN-γ. The arrow indicates an 18-kDa band in the insoluble fraction which was recognized as a putative six-His-rgpIFN-γ. Lane 1 (MM), molecular mass marker; lane 2, CL; lanes 3 to 6, elution fractions 2 to 5; lanes 7 and 8 (F), folded-protein preparations from two separate purifications.
Sequence alignment of gpIFN-γ (GenBank accession numbers E25787 and Q8CGS0 and reference 21) with human, rat, and mouse IFN-γ (GenBank accession numbers P01579, P01581, and P01580, respectively) revealed 29% overall identity (Fig. 2A), with the human and gpIFN-γ sequences being more closely related to each other (57.14% identity) than the rat and mouse sequences (Fig. 2B).
FIG. 2.
Protein sequence comparison of IFN-γ from different species. (A) ClustalW (version 1.82) alignment of the amino acid sequences of the rat, mouse, guinea pig, and human IFN-γ precursor proteins (accession numbers P01581, P01580, Q8CGS0, and P01579, respectively). Asterisks indicate positions displaying identical amino acid residues in all sequences in the alignment, colons indicate positions showing conserved substitutions, and periods indicate positions displaying semiconserved substitutions. (B) Phylogram showing the relative evolutionary distances among the primary structures of the IFN-γ proteins from the species indicated above. The branch lengths are proportional to the amount of inferred evolutionary change.
Induction of polyclonal antibody.
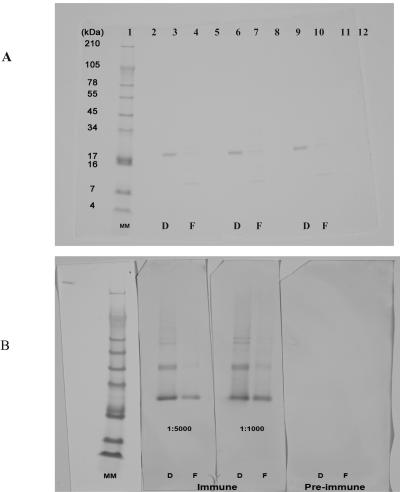
The specificity of the rabbit antiserum was analyzed by Western blotting with the WesternBreeze chromogenic immunodetection kit. Figure 3 illustrates an 18-kDa band of gpIFN-γ in a Coomassie blue-stained SDS-PAGE gel (panel A) and the identification of the protein by the rabbit serum in the Western blotting (panel B). Polyclonal rabbit anti-gpIFN-γ at dilutions of 1:1,000 and 1:5,000 detected both denatured (D, immunizing protein) and folded (F) rgpIFN-γ (200 ng/lane), with the reactivity stronger in the denatured form. No reactivity was seen with preimmune serum (Fig. 3B).
FIG. 3.
Identification of rgpIFN-γ by polyclonal antiserum (1:1,000 and 1:5,000 dilutions) from immunized rabbits. rgpIFN-γ (200 ng/well) was loaded onto duplicate gels and run under nonreducing conditions. One was visualized by Coomassie blue staining (A), and the other was used as a template to compare the results from the Western blot assay (B). MM, molecular mass marker; D, denatured protein used for immunizing rabbits; F, folded protein.
Viral neutralization activity.
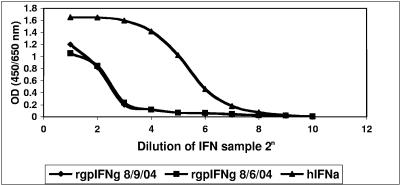
It has been well documented that IFN-γ induces antiviral activity in both human and mouse cells (26, 49). The biological activity of the folded rgpIFN-γ protein was determined based on its ability to reduce a viral (EMCV) CPE in guinea pig fibroblast cell line 104C1 by a method we have published previously (51). Figure 4 shows the results from the CPE inhibition assay of rgpIFN-γ on fibroblasts infected with the virus. The OD readings directly proportional to cell viability indicated that hIFN-α and the two batches of rgpIFN-γ protected guinea pig fibroblasts against a CPE. The level of inhibition was dose dependent and lower in the rgpIFN-γ samples than in the human IFN-α standard. The activity was diluted out at lower concentrations in all of the stimulants. The dilution titer that induced 50% CPE inhibition was 26.3 for 100 U of the human IFN-α standard, 22.8 for rgpIFN-γ batch 1 (8/6/04), and 22.89 for batch 2 (8/9/04). The titer for rgpIFN-γ, after conversion to units of activity based on the human IFN-α standard, was 17 U for batch 1 and 18 U for batch 2. Thus, rgpIFN-γ expressed in E. coli induced antiviral activity in a guinea pig cell line.
FIG. 4.
CPE inhibition assay of rgpIFN in 104C1 cells with EMCV. A 100-μl volume of the guinea pig fibroblast cell line suspension (3 × 104 cells/well) was seeded into 96-well plates and incubated overnight. On the following day, 50 μl of two batches of rgpIFN-γ protein samples (40 to 60 μg/ml) were serially diluted and added to each well and incubated for an additional 20 h. Cells were then infected with EMCV and incubated for 30 h. 2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium monosodium salt and 1-methoxy-5-methylphenazinium methyl sulfate were used for color development, and the reaction was stopped with 1 N H2SO4, and the OD450 and OD650 in each well were measured with a microplate reader for the test and reference wavelengths. The amount of active protein was assessed by preventing a virus-induced CPE in guinea pig cells with human IFN-α as the standard.
Effect on MHC class II expression.
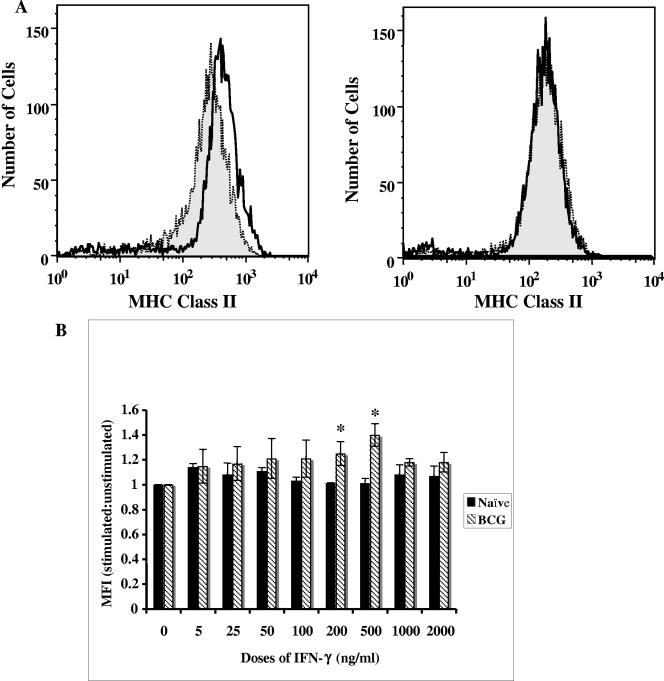
IFN-γ is known to upregulate MHC class II expression in human and mouse macrophages (16, 38, 45). The upregulation of MHC class II expression on guinea pig peritoneal cells was examined following incubation with doses of 5 to 2,000 ng/ml rgpIFN-γ for 24 to 72 h. The cells were stained for surface expression of MHC class II molecules with a mouse anti-guinea pig MHC class II antibody, and the MFI was determined by flow cytometry. Figure 5 shows a representative histogram of MFI in macrophages from naive and BCG-vaccinated animals treated with 500 ng/ml rgpIFN-γ (panel A) and the ratio of MFI of stimulated over unstimulated cultures treated with 5 to 2,000 ng/ml protein (panel B). Treatment of cells from the naive animals resulted in a minimal increase in the expression levels (<15%). However, rgpIFN-γ at all doses induced an increase in MHC class II expression in the BCG-vaccinated group (15 to 40%). The level of expression was significantly enhanced in the cells treated with 200 (P < 0.05) and 500 (P < 0.02) ng/ml of rgpIFN-γ protein. Incubation of the cells with rgpIFN-γ for more than 24 h did not increase the level of MHC class II expression (data not shown). Staining of the cells by the trypan blue exclusion method revealed no cytotoxicity at the higher doses of protein (data not shown).
FIG. 5.
Effect of rgpIFN-γ on MHC class II expression on peritoneal macrophages. Peritoneal cells from naive and BCG-vaccinated guinea pigs were treated with rgpIFN-γ (5 to 2,000 ng/ml) for 18 to 24 h, and the cells were stained with monoclonal antibody against guinea pig MHC class II. The MFIs of MHC class II-positive cells were determined by flow cytometry. The results are expressed in a histogram (A) as MFIs from a representative experiment in which macrophages were treated with 500 ng/ml rgpIFN-γ for 24 h. The ratios of MFIs of stimulated over unstimulated cultures are shown in panel B, and the results are the mean and standard error of the mean from three experiments. There were 5 to 10 animals per group. *, P < 0.05 compared with the untreated control group (ANOVA).
Effect on nitrite production.
Peritoneal macrophages from naive and BCG-vaccinated guinea pigs were cultured with or without rgpIFN-γ (50 to 1,000 ng/ml) for 24 h. M. tuberculosis H37Rv (MOI, 0.1) was added, and cultures were incubated for an additional 24 to 72 h. The supernatants from these cultures were tested for the presence of nitrite by the Griess reaction at regular time intervals. Infected macrophages did not produce any nitrite when incubated with rgpIFN-γ at any of the concentrations tested during the 72-h incubation (data not shown). Similarly, macrophages treated with IFN-γ alone also showed no nitrite production.
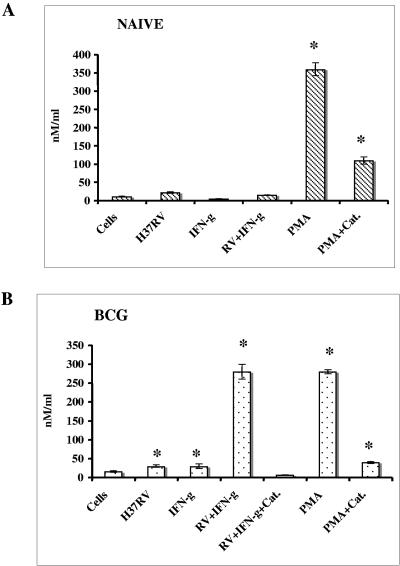
Effect on H2O2 production.
In order to determine whether rgpIFN-γ-treated macrophages generate H2O2, peritoneal macrophages from naive and BCG-vaccinated guinea pigs were incubated with 50 to 500 ng/ml of rgpIFN-γ for 3 h and then infected with virulent M. tuberculosis for 24 to 48 h. At the end of the incubation time, media were removed from the wells and the assay solution containing phenol red and horseradish peroxidase type II was added with or without PMA or catalase. The increased absorbance at an alkaline pH was measured at 630 nm. Figure 6 shows the number of nanomoles of H2O2 produced by macrophages in response to IFN-γ (200 ng/ml) for 3 h, M. tuberculosis (MOI, 0.1) for 24 h, or PMA (100 ng/ml) for 1 h. rgpIFN-γ at doses of 200 to 500 ng/ml induced a significant increase in H2O2 production (P < 0.05) in these macrophages, and the maximum effect was seen in cells treated with 200 ng/ml. The results indicated that macrophages from naive animals were incapable of producing H2O2 after incubation with the recombinant protein and mycobacteria (Fig. 6A). However, stimulation with PMA induced a significant (P < 0.05) increase in the production of H2O2 in these cells and the effect was inhibited (P < 0.05) after the addition of catalase. In contrast, macrophages from BCG-vaccinated guinea pigs treated with 200 ng/ml rgpIFN-γ or M. tuberculosis (Fig. 6B) alone produced significant amounts of H2O2 (P < 0.05). The level of H2O2 production was enhanced synergistically after treatment with the combination of recombinant protein and M. tuberculosis. Incubation with PMA also induced large amounts of H2O2 in these macrophages, and the level was similar to that obtained with the combination treatment. Addition of 100 μM catalase to the cultures neutralized the effect (Fig. 6B). There was no further increase in H2O2 production in these macrophages after 48 h of stimulation with rgpIFN-γ or M. tuberculosis (data not shown).
FIG. 6.
Effect of rgpIFN-γ on H2O2 production. Peritoneal macrophages from naive (A) and BCG-vaccinated (B) guinea pigs plated in 96-well microtiter plates were stimulated with rgpIFN-γ (200 ng/ml) for 3 h and then infected with M. tuberculosis H37Rv (MOI, 0.1) for 24 h. Medium from the wells was removed, and 100 μl of the assay solution containing phenol red, horseradish peroxidase type II in HBSS was added to the test wells. Some wells contained stimulants (PMA, 100 ng/ml) or inhibitors (100 μM catalase [Cat.]). The reaction was stopped after 1 h by the addition of 10 μl of 1 N NaOH, and the absorbance was measured in a plate reader at 630 nm. The results are the mean ± the standard error of the mean from three different experiments involving at least three animals per group and are expressed as nanomoles per milliliter of H2O2 calculated from a standard curve. *, P < 0.05 compared with the untreated control (ANOVA).
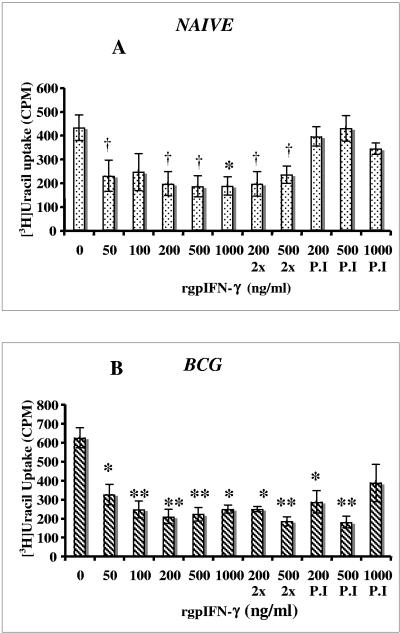
Effect on intracellular growth of M. tuberculosis.
IFN-γ is known to activate mouse macrophages to kill M. tuberculosis (11-13). In order to determine whether rgpIFN-γ would restrict the intracellular growth of M. tuberculosis, macrophages from naive and BCG-vaccinated guinea pigs were cultured with different doses of rgpIFN-γ for 24 h. The medium was removed, fresh medium without antibiotics was added, and the cultures were infected with M. tuberculosis for 3 h. Some macrophage cultures were treated twice with the recombinant cytokine, both before and after infection. rgpIFN-γ was present throughout the 7-day culture period in these two groups. Figure 7 shows the [3H]uracil uptake by M. tuberculosis in macrophages from naive (A) and BCG-vaccinated (B) guinea pigs. rgpIFN-γ treatment reduced the intracellular growth of bacteria in the macrophages from both naive and BCG-vaccinated animals. In the naive group, a significant reduction (P < 0.0.05 to 0.007) in growth was observed at 50, 200, 500, or 1,000 ng/ml of the recombinant protein given 24 h prior to infection with M. tuberculosis compared to the untreated macrophages (Fig. 7A). Additional rgpIFN-γ added after infection (2x) had no effect on bacterial growth. Moreover, no reduction in bacterial growth was seen (Fig. 7A) when rgpIFN-γ was added to the macrophage cultures after infection (P.I). The decrease in bacterial growth varied from 0 to 57% in the naive macrophages after IFN-γ treatment. In BCG-vaccinated animals (Fig. 7B), the intracellular survival of M. tuberculosis was significantly reduced (P < 0.003 to 0.0002) after rgpIFN-γ treatment of macrophages with 50 to 1,000 ng/ml protein added 24 h prior to M. tuberculosis infection. The reduction in growth was 38 to 71% compared with the untreated cultures. The response was only slightly enhanced when macrophages were treated with two doses of 200 and 500 ng/ml protein both before and after infection (2x). Unlike the naive animals, macrophages from vaccinated animals were able to restrict the growth of M. tuberculosis significantly (P < 0.003 and 0.0003) when rgpIFN-γ (200 and 500 ng/ml) was added after infection (P.I) (Fig. 7B). Thus, a single treatment with rgpIFN-γ restricted the survival of intracellular bacteria in macrophages from both naive and BCG-vaccinated guinea pigs. In the BCG-vaccinated animals, rgpIFN-γ was able to reduce bacterial growth in macrophages even when added after infection. The viability of macrophages cultured in the presence of M. tuberculosis and rgpIFN-γ was unaffected at 7 days, as confirmed by trypan blue staining of macrophages (data not shown).
FIG. 7.
Effect of rgpIFN-γ on intracellular growth of M. tuberculosis by [3H]uracil uptake. Macrophages from naive (A) and BCG-vaccinated (B) guinea pigs were treated with various doses (50 to 1,000 ng/ml) of rgpIFN-γ for 24 h. The RPMI medium was removed, fresh medium without antibiotics was added, and the cultures were infected with M. tuberculosis (MOI, 1:1) for 3 h. The extracellular bacteria were removed, and some cultures were further treated with 200 or 500 ng/ml protein or treated with 200, 500, or 1,000 ng/ml only after infection. The [3H]uracil uptake by viable M. tuberculosis was measured on day 7 and is expressed as counts per minute (CPM). The results represent the mean ± the standard error of the mean from five experiments. The differences between naive and BCG-vaccinated groups were examined by ANOVA, and differences between means were compared by Student's t test (†, P < 0.05; *, P < 0.005; **, P < 0.0005 [in comparison with the respective untreated cultures]). P.I, postinfection.
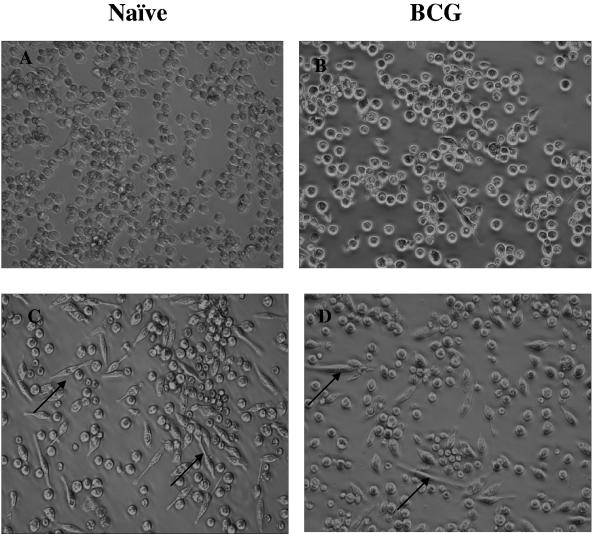
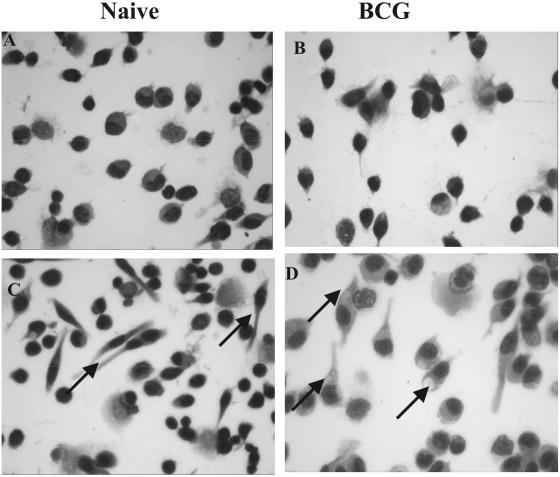
Effect on morphology of macrophages.
We examined the ability of rgpIFN-γ to induce morphological alterations in cultured peritoneal cells. Figure 8 shows resident peritoneal cells from naive (panels A and C) and BCG-vaccinated (panels B and D) guinea pigs cultured with or without rgpIFN-γ (50 ng/ml) for 16 h. rgpIFN-γ induced the formation of multiple spindle-shaped macrophages by 16 h in culture, from both naive (Fig. 8C) and BCG-vaccinated (Fig. 8D) guinea pigs, although the effect was more apparent in the naive animals. Figure 9 shows resident peritoneal macrophages from naive (panels A and C) and BCG-vaccinated (panels B and D) guinea pigs stained by the Diff-Quik method. Macrophages from BCG-vaccinated guinea pigs cultured in chamber slides in the presence of various doses of rgpIFN-γ (50 to 500 ng/ml) for 16 h, followed by Diff-Quik staining, were more vacuolar in appearance (panel D), especially at higher doses (500 ng/ml), suggesting that they are more activated than the cells from the naive group (panel C). Interestingly, as little as 5 ng of rgpIFN-γ protein was capable of inducing this effect in macrophages and the effect was consistently observed with all doses tested (data not shown).
FIG. 8.
Effect of rgpIFN-γ on the morphology of resident peritoneal cells. Total peritoneal cells (106/well) from naive and BCG-vaccinated guinea pigs were cultured in the presence of rgpIFN-γ (50 ng/ml) for 18 h. Photomicrographs show cells from naive guinea pigs left untreated (A) or treated (C) with rgpIFN-γ and cells from BCG-vaccinated guinea pigs left untreated (B) or treated (D). Note the spindle-shaped macrophages in the IFN-γ-treated groups (arrows; magnification, ×200). Results are representative of multiple experiments involving several animals.
FIG. 9.
Effect of rgpIFN-γ on the morphology of resident peritoneal macrophages. Cells from naive (A and C) and BCG-vaccinated (B and D) guinea pigs were cultured as described in Materials and Methods in the presence of IFN-γ (50 to 500 ng/ml) in chamber slides and then stained by the Diff-Quik method. Shown are treated (500 ng/ml) cells from naive (C) and BCG-vaccinated (D) guinea pigs showing vacuoles (arrows) in the macrophages. Magnification, ×600. There were three guinea pigs per group, and the experiments were repeated several times.
DISCUSSION
IFN-γ is a pleiotropic molecule involved in multiple aspects of the immune response, including the enhancement of antigen presentation through MHC class I and II molecules, activation of phagocytic cells to produce effector molecules such as reactive oxygen and nitrogen intermediates, and the induction of bactericidal activity (15, 16, 27, 38) in macrophages. This is the first report of the production, purification, and characterization of rIFN-γ from the guinea pig. IFN-γ was produced in E. coli after subcloning the mature coding region of gpIFN-γ into the pQE30-THR expression vector. rgpIFN-γ was identified as an 18-kDa band in the insoluble fraction and was confirmed by N-terminal sequence analysis. Although the protein was produced in the insoluble fraction, purification steps in the absence of denaturant resulted in the production of folded bioactive protein. The deduced sequence alignment of rgpIFN-γ protein revealed an overall identity with the mouse, rat, and human sequences of 29%; however, its identity with human IFN-γ was 57% (Fig. 2). Western blot analysis confirmed that rabbit polyclonal antisera raised against the denatured protein could detect both denatured and folded bioactive rgpIFN-γ (Fig. 3).
The distinctive antiviral activity of IFN-γ is well documented (26, 49). In the present study, the bioactivity of the recombinant protein was tested for the ability to protect the guinea pig fibroblast cell line from EMCV infection. The results clearly indicate that rgpIFN-γ was effective in preventing a CPE in this cell line. The units of IFN activity in two different recombinant protein batches were quite comparable in our viral cytopathicity assay (Fig. 4). An earlier study in our laboratory (51) demonstrated that culture supernatants from spleen cells from BCG-vaccinated guinea pigs stimulated with either purified protein derivative or MY-1 (an immunostimulatory oligoDNA extract from M. bovis BCG) had antiviral activity in this bioassay. The IFN titers, as defined by 50% CPE inhibition, and the units of activity in terms of the human IFN-α standard in these two studies are quite comparable.
IFN-γ is known to play a central role in resistance against mycobacteria by activating macrophages to produce effector molecules such as reactive nitrogen or oxygen intermediates (11, 13, 37). Although nitric oxide (NO) has been clearly identified as an effector molecule in the killing of mycobacteria in the mouse (11, 12, 17), its role in the induction of intracellular killing by human macrophages in vitro is still debatable (7, 48). Interestingly, in the present study, rgpIFN-γ was incapable of inducing the production of NO from peritoneal macrophages, even after infection with virulent M. tuberculosis. Either the dose of the protein or the duration of treatment used in the present study may not be optimum for the production of NO in vitro by guinea pig macrophages, although under similar experimental conditions, mouse macrophages are known to produce nitrite in response to IFN-γ alone (11, 12, 19). It is also possible that guinea pig macrophages require stimulation with other cytokines in addition to IFN-γ to produce NO. A previous study in our laboratory demonstrated that guinea pig alveolar macrophages were not able to produce reactive nitrogen metabolites, even after stimulation with lipopolysaccharide (LPS) in combination with recombinant murine or human IFN, infection with Listeria monocytogenes, or supernatants obtained from concanavalin A-stimulated lymphocytes (52). This finding is quite consistent with earlier studies with guinea pigs in which peritoneal macrophages were not able to produce detectable NO after virulent and avirulent Legionella pneumophila infection (30). Interestingly, inducible NO synthase activity has been detected in the guinea pig ileum, after hapten administration in the cochlea of guinea pigs, or in the inflammatory cells of guinea pigs with chronic allergic airway inflammation (34, 35, 42). This suggests that mechanisms other than toxic nitrogen intermediates may be responsible for mycobacterial control in the vaccinated guinea pig. On the contrary, rgpIFN-γ and virulent M. tuberculosis, alone or in combination, induced the production of H2O2 in macrophages obtained from BCG-vaccinated guinea pigs (Fig. 6). IFN-γ has been shown to activate murine peritoneal macrophages and human alveolar macrophages to produce H2O2 (37, 44). We have reported that guinea pig neutrophils produced superoxide after incubation with rgpIL-8, and this was stimulated further by exposure of neutrophils to LPS or attenuated M. tuberculosis H37Ra (32).
Production of H2O2 by macrophages from BCG-vaccinated guinea pigs after rgpIFN-γ treatment was associated with a reduction in the intracellular survival of M. tuberculosis (Fig. 7). Interestingly, macrophages from naive animals were incapable of producing H2O2 after 3 h of rgpIFN-γ treatment; however, macrophages from both naive and vaccinated animals were able to restrict mycobacterial growth after rgpIFN-γ treatment. The recombinant protein at very low doses was capable of inducing the antimycobacterial effect in macrophages; however, rgpIFN-γ had to be added before M. tuberculosis infection in order for macrophages from naive animals to restrict bacterial growth. In contrast, macrophages from BCG-vaccinated animals responded by controlling intracellular M. tuberculosis even when rgpIFN-γ was added after infection. IFN-γ is a potent activator of mouse macrophages (2, 11-14), and this activation is followed by an upregulation of MHC class II expression and production of effector molecules such as reactive oxygen and nitrogen intermediates, followed by effective intracellular killing of mycobacteria (8, 12, 15, 27). On the contrary, it is not clear yet whether human macrophages can be activated by IFN-γ to be bactericidal (7, 36, 37). The fact that macrophages from vaccinated guinea pigs respond differently to rgpIFN-γ in vitro indicates that they are already in some state of activation or heightened receptivity to activating signals after vaccination, and a short exposure to rgpIFN-γ induces production of H2O2 in these macrophages compared to the unvaccinated group. Perhaps macrophages from naive animals need a prolonged exposure to recombinant protein before they can be activated to produce effector molecules. Recently, reports from our laboratory have shown that rgpTNF-α stimulated IL-12 mRNA expression in alveolar macrophages and addition of anti-TNF-α polyclonal antibody increased the intracellular survival of both attenuated and virulent strains of M. tuberculosis (3).
It is well established that IFN-γ upregulates expression of cell surface molecules such as MHC class II in mice and humans (7, 37). Our results demonstrate that rgpIFN-γ treatment increased MHC class II expression in peritoneal macrophages obtained from BCG-vaccinated guinea pigs (Fig. 5). Even low doses of rgpIFN-γ (5 to 50 ng/ml) were capable of inducing this effect, which diminished at higher doses (>1,000 ng/ml) of the protein. Interestingly, rgpIFN-γ caused only minimal increases in MHC class II expression in macrophages obtained from naive guinea pigs. These results confirm that rgpIFN-γ purified from E. coli in our laboratory is capable of activating guinea pig macrophages by upregulating MHC class II expression. rgpIFN-γ at low doses induced dramatic changes in the morphology of macrophages. The macrophages assumed a spindle shape in less than 18 h of treatment, while untreated cells were uniformly round (Fig. 8). Although this phenomenon was more apparent in the macrophages from naive animals, cells from BCG-vaccinated guinea pigs also responded in a similar manner. The rgpIFN-γ-treated macrophages from BCG-vaccinated guinea pigs also appeared more vacuolar, indicating that they might have been more activated than the treated cells from the naive group (Fig. 9). This is consistent with our earlier findings that macrophages from BCG-vaccinated animals had enhanced IL-1β, RANTES, IL-8, and TNF-α mRNA levels compared to cells from nonvaccinated animals following stimulation with PMA and LPS or infection with virulent or attenuated M. tuberculosis (20, 28, 31). Interestingly, increased MHC class II expression, production of H2O2, and an increased ability to control intracellular survival of M. tuberculosis even when rgpIFN-γ was added after infection were observed only in macrophages from BCG-vaccinated animals, which further confirms that these macrophages are more activated or more susceptible to activation than cells from naive guinea pigs. These results are consistent with those from a previous report that demonstrated that BCG infection of mice induced activated macrophages in the peritoneal cavity and these macrophages killed tumor targets more effectively than cells from uninfected mice (10).
These results clearly indicate that the rgpIFN-γ produced in our laboratory is capable of inducing antiviral activity, upregulation of MHC class II expression, production of H2O2, inhibition of intracellular growth of virulent M. tuberculosis, and morphological changes in macrophages. The biologic activity of rgpIFN-γ is not due to LPS contamination, as the endotoxin level in our protein samples was much lower than that present in commercial preparations. The availability of rgpIFN-γ will contribute to the elucidation of the mechanisms of BCG-induced resistance in the guinea pig model of pulmonary tuberculosis. We are currently developing monoclonal antibodies against rgpIFN-γ so that quantitative assays of protein production may be applied in this model. Such studies will be valuable in identifying and validating correlates of protection in vaccinated guinea pigs and contribute to the screening of new vaccine candidates against tuberculosis.
Acknowledgments
This work was supported by USPHS NIH grant RO1 AI 15495 to D.N.M.
We are grateful to Jane Miller for help and expertise in fluorescence-activated cell sorter analysis, Larry Dangott for sequence analysis of protein, and Adam Johnson for assistance with photography. We are indebted to Roger Smith III for analyzing the MHC class II data and German Rosas, Seshu Janakiram, and Rajesh Miranda for valuable advice and constructive help in this study.
Editor: J. L. Flynn
REFERENCES
- 1.Barnes, P. F., S. J. Fong, P. J. Brennan, P. E. Twomey, A. Mazumder, and R. L. Modlin. 1990. Local production of tumor necrosis factor and IFN-γ in tuberculous pleuritis. J. Immunol. 145:149-154. [PubMed] [Google Scholar]
- 2.Chan, J., Y. Xing, R. S. Magliozzo, and B. R. Bloom. 1992. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp. Med. 175:1111-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho, H., T. M. Lasco, S. Sedberry, T. Yoshimura, and D. N. McMurray. 2005. Recombinant guinea pig tumor necrosis factor alpha stimulates the expression of interleukin-12 and the inhibition of Mycobacterium tuberculosis growth in macrophages. Infect. Immun. 73:1367-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colditz, G. A., T. F. Brewer, C. S. Berkey, M. E. Wilson, E. Burdick, H. V. Fineberg, and F. Mosteller. 1994. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 271:698-702. [PubMed] [Google Scholar]
- 5.Cooper, A. M. D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalton, D. K., S. Pitts-Meek, S. Keshav, I. S. Figari, A. Bradley, and T. A. Stewart. 1993. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science 259:1739-1742. [DOI] [PubMed] [Google Scholar]
- 7.Denis, M. 1994. Monocytes/macrophages: NO or no NO? J. Leukoc. Biol. 55:682-684. [DOI] [PubMed] [Google Scholar]
- 8.Ding, A. H., C. F. Nathan, and D. J. Stuehr. 1988. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages: comparison of activating cytokines and evidence for independent production. J. Immunol. 141:2407-2412. [PubMed] [Google Scholar]
- 9.Dorman, S. E., and S. M. Holland. 1998. Mutation in the signal-transducing chain of the interferon-gamma receptor and susceptibility to mycobacterial infection. J. Clin. Investig. 101:2364-2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drapier, J. C., and J. B. Hibbs, Jr. 1988. Differentiation of murine macrophages to express nonspecific cytotoxicity for tumor cells results in l-arginine-dependent inhibition of mitochondrial iron-sulfur enzymes in the macrophage effector cells. J. Immunol. 140:2829-2838. [PubMed] [Google Scholar]
- 11.Flesch, I. E., and S. H. Kaufmann. 1990. Activation of tuberculostatic macrophage functions by gamma interferon, interleukin-4, and tumor necrosis factor. Infect. Immun. 58:2675-2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flesch, I. E. A., and S. H. E. Kaufmann. 1991. Mechanisms involved in mycobacterial growth inhibition by gamma interferon-activated bone marrow macrophages: role of reactive nitrogen intermediates. Infect. Immun. 59:3213-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flesch, I. E., J. H. Hess, I. P. Oswald, and S. H. Kaufmann. 1994. Growth inhibition of Mycobacterium bovis by IFN-γ stimulated macrophages: regulation by endogenous tumor necrosis factor-alpha and by IL-10. Int. Immunol. 6:693-700. [DOI] [PubMed] [Google Scholar]
- 14.Flynn, J. L., M. M. Goldstein, K. J. Triebold, D. K. Dalton, B. Koller, and B. R. Bloom. 1992. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. USA 89:12013-12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fulton, S. A., S. M. Reba, R. K. Pai, M. Pennini, M. Torres, C. L. Harding, and W. H. Boom. 2004. Inhibition of major histocompatibility complex II expression and antigen processing in murine alveolar macrophages by Mycobacterium bovis BCG and the 19-kilodalton mycobacterial lipoprotein. Infect. Immun. 72:2101-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gazzinelli, R. T., I. P. Oswald, S. L. James, and A. Sher. 1992. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-γ activated macrophages. J. Immunol. 148:1792-1796. [PubMed] [Google Scholar]
- 18.Holscher, C., R. A. Atkinson, B. Arendse, N. Brown, E. Myburgh, G. Alber, and F. Brombacher. 2001. A protective and agonistic function of IL-12p40 in mycobacterial infection. J. Immunol. 167:6957-6966. [DOI] [PubMed] [Google Scholar]
- 19.Jeevan, A., C. D. Bucana, Z. Dong, V. Dizon, S. L. Thomas, T. E. Lloyd, and M. L. Kripke. 1995. Ultraviolet radiation reduces phagocytosis and intracellular killing of mycobacteria and inhibits nitric oxide production by macrophages in mice. J. Leukoc. Biol. 57:883-890. [DOI] [PubMed] [Google Scholar]
- 20.Jeevan, A., T. Yoshimura, G. Foster, and D. N. McMurray. 2002. Effect of BCG infection on interleukin-1β and RANTES mRNA expression in guinea pig cells exposed to attenuated and virulent mycobacteria. Infect. Immun. 70:1245-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeevan, A., T. Yoshimura, K. E. Lee, and D. N. McMurray. 2003. Differential expression of gamma interferon mRNA induced by attenuated and virulent Mycobacterium tuberculosis in guinea pig cells after Mycobacterium bovis BCG vaccination. Infect. Immun. 71:354-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaufmann, S. H. E. 1987. Towards new leprosy and tuberculosis vaccines. Microbiol. Sci. 4:324-328. [PubMed] [Google Scholar]
- 23.Kemijo, R., J. Le, D. Shapiro, E. A. Havell, S. Huang, M. Aguet, M. Bosland, and J. Vilcek. 1993. Mice that lack the interferon-gamma receptor have profoundly altered responses to infection with Bacillus Calmette-Guerin and subsequent challenge with lipopolysaccharide. J. Exp. Med. 178:1435-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kemijo, R., D. Shapiro, J. Le, S. Huang, M. Aguet, and J. Wilcek. 1993. Generation of nitric oxide and induction of major histocompatibility complex class II antigen in macrophages from mice lacking the interferon gamma receptor. Proc. Natl. Acad. Sci. USA 90:6626-6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kemijo, R., J. Gerecitano, D. Shapiro, S. J. Green, M. Aguet, J. Le, and J. Vilcek. 1995. Generation of nitric oxide and clearance of interferon-gamma after BCG infection are impaired in mice that lack the interferon-gamma receptor. J. Inflamm. 46:23-31. [PubMed] [Google Scholar]
- 26.Kerr, I. M., and G. R. Stark. 1992. The antiviral effects of the interferons and their inhibition. J. Interferon Res. 12:237-240. [DOI] [PubMed] [Google Scholar]
- 27.Kindler, V., A. P. Sappino, G. E. Grau, P. F. Piguet, and P. Vassalli. 1989. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell 56:731-740. [DOI] [PubMed] [Google Scholar]
- 28.Lasco, T. M., T. Yamamoto, T. Yoshimura, S. S. Allen, L. Cassone, and D. N. McMurray. 2003. Effect of Mycobacterium bovis BCG vaccination on mycobacterium-specific cellular proliferation and tumor necrosis factor alpha production from distinct guinea pig leukocyte populations. Infect. Immun. 71:7035-7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lasco, T. M., L. Cassone, H. Kamohara, T. Yoshimura, and D. N. McMurray. 2005. Evaluating the role of tumor necrosis factor-alpha in experimental pulmonary tuberculosis in the guinea pig. Tuberculosis 85:245-258. [DOI] [PubMed] [Google Scholar]
- 30.Levasseur, P. R., D. Lecointe, G. Bertrand, and M. Fay. 1996. Differential nitric oxide (NO) production by macrophages from mice and guinea pigs infected with virulent and avirulent Legionella pneumophila serogroup 1. Clin. Exp. Immunol. 104:48-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyons, M. J., T. Yoshimura, and D. N. McMurray. 2002. Mycobacterium bovis BCG vaccination augments interleukin-8 mRNA expression and protein production in guinea pig alveolar macrophages infected with Mycobacterium tuberculosis. Infect. Immun. 70:5471-5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyons, M. J., T. Yoshimura, and D. N. McMurray. 2004. Interleukin (IL)-8 (CXCL8) induces cytokine expression and superoxide formation by guinea pig neutrophils infected with Mycobacterium tuberculosis. Tuberculosis 84:283-292. [DOI] [PubMed] [Google Scholar]
- 33.Mackaness, G. B. 1969. The immunology of antituberculous immunity. Am. Rev. Respir. Dis. 97:337-344. [DOI] [PubMed] [Google Scholar]
- 34.Michel, O., A. Hess, J. Su, W. Bloch, E. Stennert, and K. Addicks. 2000. Expression of inducible nitric oxide synthase (iNOS/NOS II) in the hydropic cochlea of guinea pigs. Hearing Res. 143:23-28. [DOI] [PubMed] [Google Scholar]
- 35.Miller, M. J., J. H. Thompson, X. J. Zhang, H. Sadowska-Krowicka, J. L. Kakkis, U. K. Munshi, M. Sandoval, J. L. Rossi, S. Eloby-Childress, J. S. Beckman, et al. 1995. Role of inducible nitric oxide synthase expression and peroxynitrite formation in guinea pig ileitis. Gastroenterology 109:1475-1483. [DOI] [PubMed] [Google Scholar]
- 36.Muijsers, R. B. R., N. H. T. ten Hacken, I. V. Ark, G. Folkerts, F. P. Nijkamp, and D. S. Postma. 2001. l-Arginine is not the limiting factor for nitric oxide synthesis by human alveolar macrophages in vitro. Eur. Respir. J. 18:667-671. [DOI] [PubMed] [Google Scholar]
- 37.Nathan, C. F., H. W. Murray, M. E. Wiebe, and B. Y. Rubin. 1983. Identification of interferon gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J. Exp. Med. 158:670-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noss, E. H., R. K. Pai, T. J. Sellati, J. D. Radolf, J. Belisle, D. T. Golenbock, W. H. Boom, and C. V. Harding. 2001. Toll-like receptor 2-dependent inhibition of macrophage class II MHC expression and antigen processing by 19-kDa lipoprotein of Mycobacterium tuberculosis. J. Immunol. 167:910-918. [DOI] [PubMed] [Google Scholar]
- 39.Orme, I. M., E. S. Miller, A. D. Roberts, S. K. Furney, J. P. Griffin, K. M. Dobos, D. Chi, B. Rivoire, and P. J. Brennan. 1992. T lymphocytes mediating protection and cellular cytolysis during the course of Mycobacterium tuberculosis infection. Evidence for different kinetics and recognition of a wide spectrum of protein antigens. J. Immunol. 148:189-196. [PubMed] [Google Scholar]
- 40.Pick, E. 1986. Microassays for superoxide and hydrogen peroxide production and nitroblue tetrazolium reduction using an enzyme immunoassay microplate reader. Methods Enzymol. 132:407-421. [DOI] [PubMed] [Google Scholar]
- 41.Pierre-Audigier, C., E. Jouanguy, S. Lamhamedi, F. Altare, J. Rauzier, V. Vincent, D. Canioni, J. F. Emile, A. Fischer, S. Blanche, J. L. Gaillard, and J. L. Casanova. 1997. Fatal disseminated Mycobacterium smegmatis infection in a child with inherited interferon gamma receptor deficiency. Clin. Infect. Dis. 24:982-984. [DOI] [PubMed] [Google Scholar]
- 42.Prado, C. M., E. A. Leick-Maldonado, V. Arata, D. I. Kasahara, M. A. Martins, and I. F. L. C. Tiberio. 2004. Neurokinins and inflammatory cell iNOS expression in guinea pigs with chronic allergic airway inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 288:L741-L748. [DOI] [PubMed] [Google Scholar]
- 43.Roederer, M., A Treister, W. Moore, and L. A. Herzenberg. 2001. Probability binning comparison: a metric for quantitating univariate distribution differences. Cytometry 45:37-46. [DOI] [PubMed] [Google Scholar]
- 44.Sharp, A., K. Sharp, and D. K. Banerjee. 1986. Effect of interferon on hydrogen peroxide production by cultured mouse peritoneal macrophages. Infect. Immun. 54:597-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steeg, P. A., R. N. Moore, H. M. Johnson, M. Muramatsu, S. Kobayashi, and T. Sudo. 1982. Regulation of murine macrophage Ia antigen expression by a lymphokine with immune interferon activity. J. Exp. Med. 156:1780-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verreck, F. A., T. de Boer, D. M. Langernberg, M. A. Hoeve, M. Kramer, E. Vaisberg, R. Kastelein, A. Kolk, R. de Waal-Malefyt, and T. H. Ottenhoff. 2004. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc. Natl. Acad. Sci. USA 101:4560-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wadee, A. A., R. H. Kuschke, and T. G. Dooms. 1995. The inhibitory effects of Mycobacterium tuberculosis on MHC class II expression by monocytes activated with riminophenazines and phagocyte stimulants. Clin. Exp. Immunol. 100:434-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weinberg, J. B. 1998. Nitric oxide production and nitric oxide synthase type 2 expression by human mononuclear phagocytes: a review. Mol. Med. 4:557-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wheelock. E. F. 1965. Interferon-like virus-inhibitor induced in human leukocytes by phytohemagglutinin. Science 149:310-311. [PubMed] [Google Scholar]
- 50.Yam, L. T., C. Y. Li, and W. H. Crosby. 1971. Cytochemical identification of monocytes and granulocytes. Am. J. Clin. Pathol. 55:283-290. [DOI] [PubMed] [Google Scholar]
- 51.Yamamoto, T., A. Jeevan, K. Ohishi, Y. Nojima, K. Umemori, S. Yamamoto, and D. N. McMurray. 2002. A new system for guinea pig interferon biological activity. J. Interferon Cytokine Res. 22:793-797. [DOI] [PubMed] [Google Scholar]
- 52.Zhang, X., and D. N. McMurray. 1998. Suppression of lymphoproliferation by alveolar macrophages in the guinea pig. Tuber. Lung Dis. 79:119-126. [DOI] [PubMed] [Google Scholar]