Abstract
Host-driven macrophage apoptosis contributes to innate immunity during bacterial infection. Neisseria meningitidis inhibits apoptosis in a variety of cells, but its impact on macrophage apoptosis is unknown. We demonstrate that N. meningitidis prevents macrophage apoptosis via genes encoding nitric oxide detoxification and a porin, PorB.
Neisseria meningitidis is a leading cause of meningitis and sepsis in young adults (6). Macrophages are abundant in the nasopharynx (14) and phagocytose meningococci (15, 16). Although macrophages are constitutively resistant to apoptosis (9), microbial factors induce macrophage apoptosis, facilitating immune evasion and pathogen survival (4, 21). Alternatively, macrophage apoptosis can represent a delayed process favoring the host defense, enhancing bacterial clearance and the resolution of inflammation (1).
We have investigated macrophage apoptosis following infection by N. meningitidis, an organism which is readily phagocytosed and killed by macrophages and not associated with prolonged intracellular survival (7, 16). N. meningitidis inhibits apoptosis in lymphocytes and epithelial cells and has not been demonstrated to induce apoptosis, but nevertheless, its effect on the survival of differentiated macrophages has not been studied (12, 17).
To examine macrophage survival, we infected human monocyte-derived macrophages (MDM) isolated from healthy volunteers (5) or U937 cells (European Collection of Cell Cultures) differentiated to a macrophage phenotype (3) with nonopsonized serogroup B N. meningitidis strain MC58 (B:15:P1.7.16b) at a multiplicity of infection (MOI) of 50, with washing at 4 h to remove noninternalized bacteria. Bacterial internalization, cell viability, or apoptosis was determined by fluorescence microscopy, and results were recorded as means and standard errors of the means (SEM), with significance defined as results having P values of <0.05 following testing with Prism 4.02 software (GraphPad Inc.) (5). Four hours after infection, the mean number of internalized MC58 N. meningitidis organisms per cell was 12.1 ± 3.1 for MDM and 7.2 ± 1.0 for U937 cells. Viable intracellular bacteria remained detectable for up to 20 h after infection (data not shown). Macrophage death was undetectable at representative early or late times postinfection (Fig. 1A). Since opsonic conditions influence late host-driven macrophage apoptosis (1), bacteria were opsonized with immune serum (derived from convalescing patients infected with serogroup B meningococci) for 30 min at 37°C (7, 16). Opsonization did not alter macrophage viability (Fig. 1A). There was no increase in terminal UTP nick end labeling (Intergen)-positive apoptotic cells (5) after N. meningitidis infection (Fig. 1B).
FIG. 1.
N. meningitidis internalization fails to induce macrophage apoptosis. (A) MDM were infected with nonopsonized (Op−) or opsonized (Op+) N. meningitidis strain MC58, and at 4 or 20 h postinfection, the cell density was assessed and normalized to that of mock-infected (MI) cells (n = 6). (B) Percentage of terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end-labeled MDM after mock infection or infection with opsonized MC58 or S. pneumoniae (Spn), used as a control for MDM apoptosis. Data are means + SEM (n = 3). The positive control (DNase I treated) was set to 100%, and the negative control was set to 0% per the manufacturer's instructions. (C) Levels of apoptosis, as assessed by nuclear morphology after 4′,6′-diamidino-2-phenylindole (DAPI) staining, in MDM 4 or 20 h after mock infection or infection with an N. meningitidis strain lacking porB or the parental strain H44/76 (n = 4). The positive control (pneumococcal infection) value was 19.1 ± 0.9%. (D) Levels of apoptosis, as assessed by nuclear morphology after DAPI staining, in U937 cells, differentiated with 10 ng/ml phorbol 12-myristate 13-acetate (Sigma-Aldrich) for 3 days to produce adherent cells, 20 h after mock infection or infection with the porB mutant or H44/76 (n = 4). The positive control (pneumococcal infection) value was 18 ± 0.6%.
The meningococcal porin PorB binds to the voltage-dependent anion channel, stabilizing mitochondria and preventing mitochrondrial outer membrane permeabilization and apoptosis (12). To investigate whether macrophage survival was due to PorB, we infected MDM and U937 cells with the porB-deficient mutant H(III)5 (CE2002), generously provided by J. Tommassen, University of Utrecht (19), or the parental H44/76 (B:15:P1.16) strain. Levels of apoptosis were similar after infection with the porB-deficient mutant or the parental strain in MDM (Fig. 1C) and U937 cells (Fig. 1D).
Nitrosative stress is required for some forms of macrophage apoptosis during bacterial infection (10). N. meningitidis contains genes that detoxify nitric oxide (NO) (2) and decrease the susceptibility to intracellular killing (18). To investigate if NO detoxification systems contribute to macrophage viability, we studied macrophage apoptosis following infection with N. meningitidis MC58 and isogenic strains lacking norB (which encodes an NO reductase that reduces NO to nitrous oxide) or cycP (which encodes cytochrome c′, which contains a heme molecule and is predicted to bind and remove NO, although the exact mechanism is unclear) (2). As we have previously observed (18), infection with wild-type N. meningitidis resulted in low levels of macrophage NO, comparable to those during mock infection (Fig. 2). The absence of the norB or cycP gene resulted in an increased abundance of macrophage NO. These results were confirmed by quantifying NO production, as measured by chemiluminescence involving the reduction of nitrite to NO, determined with a Sievers model 280 NO analyzer (20). Twenty hours after NO loading with 10 μM of the NO donor S-nitroso-N-acetylpenicillamine (SNAP), MDM cultures infected with wild-type N. meningitidis demonstrated negligible NO (98% reduction), while those infected with mutant bacteria demonstrated detectable NO (80% reduction) compared to mock infection (data not shown). The infection of MDM with both norB- and cycP-deficient mutants induced macrophage apoptosis (Fig. 3A and B). Opsonization was not essential for apoptosis induction by these mutants, and apoptosis was only detected at the 20-h time point, and not at 4 h (Fig. 3B). Similarly, low-level apoptosis was seen at 20 h in U937 cells (Fig. 3C and D). The apoptosis was caspase mediated (Fig. 4).
FIG. 2.
Enhanced nitric oxide production in macrophages infected with N. meningitidis mutants. Monocyte-derived macrophages were mock infected (MI) or infected with nonopsonized N. meningitidis parental MC58 cells or a strain lacking norB or cycP, and 20 h after infection, nitric oxide was detected by flow cytometry using 4-amino-5-methylamino-2′,7′-difluorescein diacetate (DAF-FM; Molecular Probes). Cells were unstained (U/S, upper panels) or stained with DAF-FM (lower panels). Representative dot plots from one experiment are shown (representative of three independent experiments). The percentages of positive cells are shown in the upper right corners of the graphs.
FIG. 3.
Macrophage resistance to apoptosis during N. meningitidis infection involves bacterial resistance to nitrosative stress. (A) Representative pictures of MDM demonstrating condensed or fragmented apoptotic nuclei (arrowheads) 20 h after mock infection (MI) or infection with the nonopsonized N. meningitidis parental MC58 strain or a strain lacking norB or cycP, as assessed by DAPI staining and imaged by a Nikon Eclipse TE300 microscope. (B) Levels of apoptosis in MDM, as assessed by nuclear morphology after DAPI staining, 4 or 20 h after mock infection or infection with nonopsonized (Op−) or opsonized (Op+) MC58 or the norB or cycP mutant (n = 12). (C and D) Levels of apoptosis in U937 cells, as assessed by nuclear morphology after DAPI staining, 20 h after mock infection or infection with MC58 or the norB or cycP mutant. **, P < 0.01; ***, P < 0.001 (analysis of variance [ANOVA] with Tukey's posttest).
FIG. 4.
Apoptosis induced by N. meningitidis mutants lacking genes encoding nitric oxide detoxification is caspase dependent. The graph shows levels of apoptosis, as assessed by nuclear morphology after DAPI staining, in monocyte-derived macrophages 20 h after mock infection (MI) or infection with the N. meningitidis parental MC58 strain or a strain lacking norB or cycP following treatment with 50 μM of the pan-caspase inhibitor zVAD [N-benzyloxycarbonyl-Val-Ala-Asp (O-methyl) fluoromethyl ketone; Enzyme Systems Products] or a 50 μM control, zFA (N-benzyloxycarbonyl-Phe-Ala fluoromethyl ketone; Enzyme Systems Products) (n = 5). Data are means + SEM. ***, P < 0.001 by ANOVA with Tukey's posttest.
The failure of the porB-deficient mutant to induce apoptosis in macrophages could be interpreted as evidence that porB plays no role in regulating macrophage apoptosis or that without nitrosative stress in macrophages, inhibition of apoptosis by porB becomes redundant. We examined if the porB-sufficient strain could inhibit a proapoptotic stimulus, using conditions of enhanced nitrosative stress induced by supplying exogenous NO after N. meningitidis infection (13). Macrophages have an increased susceptibility to NO-mediated apoptosis during the phagocytosis of bacteria (10), and this involves the mitochondrial pathway of apoptosis that porB expression blocks (12). Macrophages infected with the porB-deficient strain, but not the parental strain, were susceptible to increased levels of caspase-dependent apoptosis in the presence of exogenous NO after incubation with 50 μM SNAP (Fig. 5). Twenty hours after this dose, mock-infected MDM cultures had approximately 15.3 μM NO, as measured by chemiluminescence as described above. Thus, the host-pathogen interaction during N. meningitidis infection involves a raised threshold for induction of the mitochondrial pathway of apoptosis. PorB contributes to this, but other microbial factors, including the ability to withstand nitrosative stress and potentially other, as yet unidentified microbial factors, influence the resistance to apoptosis.
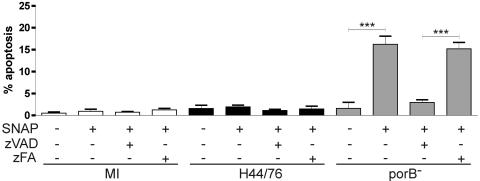
FIG. 5.
Macrophages infected with N. meningitidis lacking porB undergo caspase-dependent apoptosis in the presence of exogenous nitric oxide. The graph shows levels of apoptosis, as assessed by nuclear morphology after DAPI staining, in monocyte-derived macrophages 20 h after mock infection (MI) or infection with the N. meningitidis parental strain H44/76 or a mutant lacking porB, treated with (+) or without (−) the nitric oxide donor SNAP (50 μM; Calbiochem), the pan-caspase inhibitor zVAD (50 μM), or control zFA (50 μM) (n = 5). Data are means + SEM. ***, P < 0.001 by ANOVA with Tukey's posttest.
It remains unclear whether prolongation of macrophage survival after N. meningitidis phagocytosis benefits the host or the pathogen. Pathogen-mediated inhibition of macrophage apoptosis by Mycobacterium tuberculosis prolongs bacterial survival (8). N. meningitidis cells are readily killed following phagocytosis (7, 16), and thus it seems that resistance to apoptosis does not compromise microbiologic clearance, unlike the situation with organisms that are harder to kill (11). Nevertheless, the prolonged survival of a small proportion of meningococci in macrophages could harm the host response if the failure of apoptosis induction leads to high-level expression of proinflammatory cytokines, tissue injury, and sepsis. Invasive meningococcal disease may be associated with the failure of the host to down-regulate an overly vigorous inflammatory response (6). The failure of the macrophage to switch to apoptosis induction and downsize the immune response during infection is likely to contribute to this excessive proinflammatory response.
In summary, we provide evidence that macrophage apoptosis does not follow N. meningitidis internalization. Microbiologic factors, including porB and resistance to nitrosative stress, contribute to the absence of apoptosis. Further investigation is required to determine whether this finding contributes to meningococcal disease outcomes.
Acknowledgments
This work was supported by a Wellcome Trust advanced clinical fellowship to D.H.D. (065054) and Wellcome Trust awards to J.W.B.M. and R.C.R. (056362/98 and 069791/03).
Editor: F. C. Fang
REFERENCES
- 1.Ali, F., M. E. Lee, F. Iannelli, G. Pozzi, T. J. Mitchell, R. C. Read, and D. H. Dockrell. 2003. Streptococcus pneumoniae-associated human macrophage apoptosis after bacterial internalization via complement and Fcgamma receptors correlates with intracellular bacterial load. J. Infect. Dis. 188:1119-1131. [DOI] [PubMed] [Google Scholar]
- 2.Anjum, M. F., T. M. Stevanin, R. C. Read, and J. W. Moir. 2002. Nitric oxide metabolism in Neisseria meningitidis. J. Bacteriol. 184:2987-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callahan, M. K., M. S. Halleck, S. Krahling, A. J. Henderson, P. Williamson, and R. A. Schlegel. 2003. Phosphatidylserine expression and phagocytosis of apoptotic thymocytes during differentiation of monocytic cells. J. Leukoc. Biol. 74:846-856. [DOI] [PubMed] [Google Scholar]
- 4.DeLeo, F. R. 2004. Modulation of phagocyte apoptosis by bacterial pathogens. Apoptosis 9:399-413. [DOI] [PubMed] [Google Scholar]
- 5.Dockrell, D. H., M. Lee, D. H. Lynch, and R. C. Read. 2001. Immune-mediated phagocytosis and killing of Streptococcus pneumoniae are associated with direct and bystander macrophage apoptosis. J. Infect. Dis. 184:713-722. [DOI] [PubMed] [Google Scholar]
- 6.Emonts, M., J. A. Hazelzet, R. de Groot, and P. W. Hermans. 2003. Host genetic determinants of Neisseria meningitidis infections. Lancet Infect. Dis. 3:565-577. [DOI] [PubMed] [Google Scholar]
- 7.Jack, D. L., M. E. Lee, M. W. Turner, N. J. Klein, and R. C. Read. 2005. Mannose-binding lectin enhances phagocytosis and killing of Neisseria meningitidis by human macrophages. J. Leukoc. Biol. 77:328-336. [DOI] [PubMed] [Google Scholar]
- 8.Lammas, D. A., C. Stober, C. J. Harvey, N. Kendrick, S. Panchalingam, and D. S. Kumararatne. 1997. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z(P2X7) receptors. Immunity 7:433-444. [DOI] [PubMed] [Google Scholar]
- 9.Liu, H., H. Perlman, L. J. Pagliari, and R. M. Pope. 2001. Constitutively activated Akt-1 is vital for the survival of human monocyte-differentiated macrophages. Role of Mcl-1, independent of nuclear factor (NF)-kappaB, Bad, or caspase activation. J. Exp. Med. 194:113-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marriott, H. M., F. Ali, R. C. Read, T. J. Mitchell, M. K. Whyte, and D. H. Dockrell. 2004. Nitric oxide levels regulate macrophage commitment to apoptosis or necrosis during pneumococcal infection. FASEB J. 18:1126-1128. [DOI] [PubMed] [Google Scholar]
- 11.Marriott, H. M., C. D. Bingle, R. C. Read, K. E. Braley, G. Kroemer, P. G. Hellewell, R. W. Craig, M. K. Whyte, and D. H. Dockrell. 2005. Dynamic changes in Mcl-1 expression regulate macrophage viability or commitment to apoptosis during bacterial clearance. J. Clin. Investig. 115:359-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massari, P., Y. Ho, and L. M. Wetzler. 2000. Neisseria meningitidis porin PorB interacts with mitochondria and protects cells from apoptosis. Proc. Natl. Acad. Sci. USA 97:9070-9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Messmer, U. K., and B. Brune. 1996. Nitric oxide-induced apoptosis: p53-dependent and p53-independent signalling pathways. Biochem. J. 319:299-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pipkorn, U., G. Karlsson, and L. Enerback. 1988. A brush method to harvest cells from the nasal mucosa for microscopic and biochemical analysis. J. Immunol. Methods 112:37-42. [DOI] [PubMed] [Google Scholar]
- 15.Read, R. C., A. Fox, K. Miller, T. Gray, N. Jones, R. Borrows, D. M. Jones, and R. G. Finch. 1995. Experimental infection of human nasal mucosal explants with Neisseria meningitidis. J. Med. Microbiol. 42:353-361. [DOI] [PubMed] [Google Scholar]
- 16.Read, R. C., S. Zimmerli, C. Broaddus, D. A. Sanan, D. S. Stephens, and J. D. Ernst. 1996. The (α2-8)-linked polysialic acid capsule of group B Neisseria meningitidis modifies multiple steps during interaction with human macrophages. Infect. Immun. 64:3210-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson, K., M. Taraktsoglou, K. S. Rowe, K. G. Wooldridge, and D. A. Ala'Aldeen. 2004. Secreted proteins from Neisseria meningitidis mediate differential human gene expression and immune activation. Cell Microbiol. 6:927-938. [DOI] [PubMed] [Google Scholar]
- 18.Stevanin, T. M., J. W. Moir, and R. C. Read. 2005. Nitric oxide detoxification systems enhance survival of Neisseria meningitidis in human macrophages and in nasopharyngeal mucosa. Infect. Immun. 73:3322-3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tommassen, J., P. Vermeij, M. Struyve, R. Benz, and J. T. Poolman. 1990. Isolation of Neisseria meningitidis mutants deficient in class 1 (porA) and class 3 (porB) outer membrane proteins. Infect. Immun. 58:1355-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang, Y., and N. Hogg. 2004. Formation and stability of S-nitrosothiols in RAW 264.7 cells. Am. J. Physiol. Lung Cell Mol. Physiol. 287:L467-L474. [DOI] [PubMed] [Google Scholar]
- 21.Zychlinsky, A., and P. Sansonetti. 1997. Perspectives series: host/pathogen interactions. Apoptosis in bacterial pathogenesis. J. Clin. Investig. 100:493-495. [DOI] [PMC free article] [PubMed] [Google Scholar]