The sphingolipids are a class of lipids that serve as integral components of eukaryotic cell membranes (21, 49). Although previously considered to be simply structural molecules, sphingolipids have more recently been shown to act as signaling molecules in many cellular functions and to play crucial roles in the regulation of pathobiological processes, such as cancer, cardiovascular and neurodegenerative disorders, and inflammation or infectious diseases. In mammalian cells, ceramide, sphingosine, sphingosine-1-phosphate, and glucosylceramide are the most studied sphingolipids, and they regulate important processes, including the stress response, cell proliferation, apoptosis, angiogenesis, genetic diseases, and resistance to chemotherapy (7, 51, 80, 103, 105, 120). In other eukaryotes, such as fungi, sphingolipids have been implicated in the heat stress response (59, 110), endocytosis (97, 151), signal transduction (104), apoptosis (11), and fungal pathogenesis (11, 83). Interestingly, a mathematical model interconnecting the sphingolipid intracellular network has recently been proposed in the nonpathogenic fungus Saccharomyces cerevisiae (2); however, whether this interconnection is also present in pathogenic fungi or in mammalian cells awaits further investigations.
In the area of microbial pathogenesis, sphingolipids play a role in the regulation of the delicate balance between the microbe and the host. Microorganisms that do not produce sphingolipids, including most bacteria and viruses, are able to utilize host sphingolipids to promote their virulence. Thus, in the context of bacterium- and virus-host interaction, the host is typically the source of sphingolipids, whereas in the context of the protozoan- and fungus-host interaction, both host and pathogen sphingolipids are involved. The determination of which sphingolipid(s) (host, microbe, or both) modulate the host-parasite interaction is particularly significant not only because it may provide important insights into the development of new therapeutic strategies but also because the outcome of this sphingolipid interaction may either lead to commensalism or to host damage/disease (9), thus expanding the function of a specific sphingolipid outside of the organism from which it originated. For instance, a mammalian sphingolipid acquired by a microbe may be used to exert a novel or different function through conversion by microbial enzyme into new sphingolipids or by hiding microbial cells from the host immune response and allowing colonization or dormancy without damage to the host. On the other hand, a microbial sphingolipid acquired by the host may interfere with host intracellular signaling and thereby alter the removal and destruction of the microbial cell, or it may elicit an autoimmune response through molecular mimicry. The goal of this review is to introduce and discuss microbial sphingolipids and their corresponding metabolizing enzymes as regulators of pathogenesis and to propose new hypotheses and perspectives toward a better understanding of the host-microbe interaction mediated by sphingolipids.
SPHINGOLIPIDS AND THEIR METABOLISM
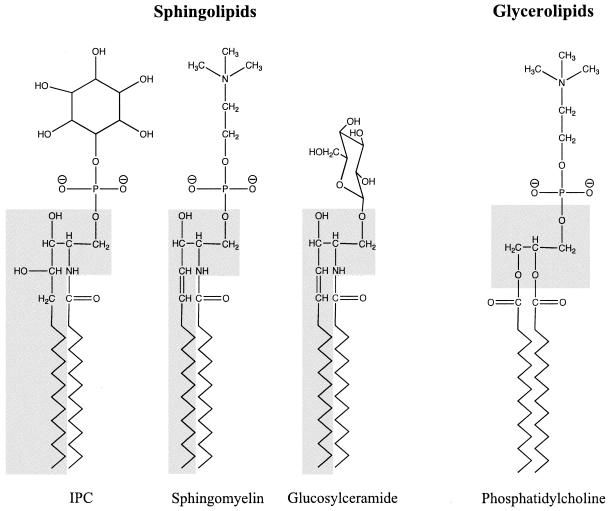
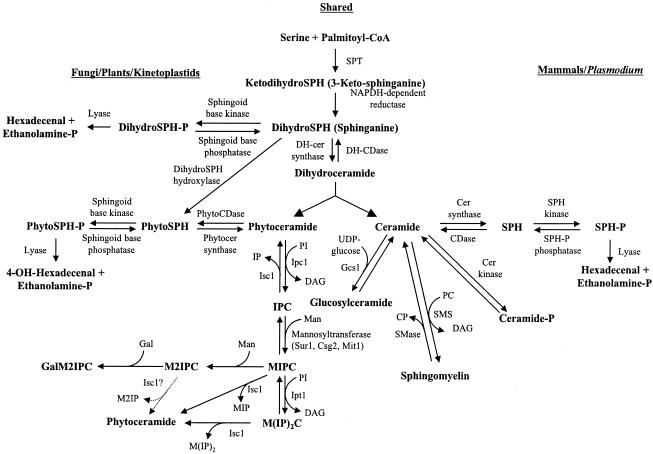
The basic structure of a sphingolipid consists of a long-chain sphingoid base backbone (distinguishing it from glycerolipids which have a glycerol backbone) linked to a fatty acid via an amide bond with the 2-amino group and to a polar head group at the C-1 position via an ester bond (Fig. 1). The synthesis of sphingolipids is highly conserved among eukaryotes and begins with the condensation of serine and palmitoyl coenzyme A catalyzed by serine palmitoyltransferase (SPT) (Fig. 2). Recent work suggests that phosphatidylserine may serve as the serine donor in this reaction (94). After the synthesis of dihydroceramide, the pathway can be generally divided into mammalian- and fungal/plant-specific branches. In mammalian systems, ceramide is formed and is incorporated into complex sphingolipids such as sphingomyelin and glycosphingolipids. Fungal and plant cells produce phytoceramide, which has an additional hydroxyl group at the C-4 position and is used to form complex sphingolipids, including inositolphosphoryl ceramide (IPC) and its mannosylated derivatives. Many fungi also contain ceramide to produce glucosylceramide (139), a glycosphingolipid that is absent in the nonpathogenic fungal model S. cerevisiae. Sphingolipid biosynthesis appears to be conserved among protozoa, but whereas Plasmodium falciparum produces mammalian-like sphingolipids such as sphingomyelin (3, 25, 44), the kinetoplastid protozoa (including Leishmania species) produce fungal/plant-like IPCs (17, 62). For comprehensive reviews discussing the similarities and differences of sphingolipid metabolism among eukaryotes, we suggest the following reviews (20, 22, 50, 104).
FIG. 1.
Structure of sphingolipids. Sphingolipids, such as IPC, sphingomyelin, and GlcCer contain a long-chain sphingoid base backbone (highlighted in gray) linked to a fatty acid via an amide bond with the 2-amino group and to a polar head group at the C-1 position via an ester bond. In contrast, glycerolipids, such as phosphatidylcholine, have a glycerol backbone.
FIG. 2.
Sphingolipid biosynthetic pathways. A schema of sphingolipids and their metabolizing enzymes in fungal/plant/kinetoplastid protozoan and mammalian/Plasmodium systems. SPT, serine palmitoyltransferase; DH-CDase, dihydroceramidase; SPH, sphingosine; Cer, ceramide; CDase, ceramidase; PhytoCDase, phytoceramidase; Gcs1, glucosylceramide synthase; Ipc1, inositol phosphorylceramide synthase 1; Isc1, inositol phosphosphingolipids-phospholipase C1; Sur1 (also called Csg1), Suppressor of rvs161 and rvs167 mutations 1; Csg2, calcium sensitive growth 2; Mit1, mannose:inositol phospho-ceramide transferase; Ipt1, inositolphosphotransferase 1; DAG, diacylglycerol; IPC, inositol phosphorylceramide; MIPC, mannose-inositol-P-ceramide; M(IP)2C, mannose-(inositol-P)2-ceramide; M2IPC, dimannose-inositol-P-ceramide; GalM2IPC, galactose-dimannose-inositol-P-ceramide; MIP, mannose-inositol-P; M2IP, dimannose-inositol-P; IP, inositol-phosphate; PI, phosphatidylinositol; CP, choline phosphate; PC, phosphatidylcholine; SMase, sphingomyelinase; SMS, sphingomyelin synthase. (Adapted from reference 51 with permission of the publisher.)
THE SPHINGOLIPID-INFECTION CONNECTION: MODEL SYSTEMS FOR SPHINGOLIPID-MEDIATED PATHOGENESIS
Bacteria.
Most prokaryotic cells do not contain sphingolipids. However, some bacteria, including the genera Sphingobacterium (99, 147, 148), Sphingomonas (142, 146), and Bacteroides (63, 122) and Bdellovibrio stolpii (140) are able to synthesize sphingolipids. Sphingomonas spp. are infrequent human pathogens, although they can cause osteomyelitis and septic shock (10, 57). Interestingly, Sphingomonas capsulata is a gram-negative bacterium that does not produce lipopolysaccharide. However, it does produce glycosphingolipids, which are localized in the cell wall and serve as lipid antigens for innate recognition by natural killer T (NKT) cells (93) and stimulate monokine production by mononuclear cells (68). Other Sphingomonas species, such as S. paucimobilis, are known to possess a homodimer serine palmitoyl transferase (SPT) enzyme, but the role of this enzyme in the pathogenesis of these bacteria has yet to be explored.
Among bacteria that do not synthesize sphingolipids, some pathogenic bacteria have developed mechanisms by which they can utilize host sphingolipids to promote their pathogenicity. For example, many intracellular bacterial pathogens can use ceramide-enriched lipid rafts as a port of entry into macrophages (reviewed in reference 90). Other microbes are able to scavenge host sphingolipids and to incorporate them into bacterial membranes, as in the case of Chlamydia trachomatis (39, 40). Toxin-producing bacteria may also target host sphingolipids. For example, β-toxin from Staphylococcus aureus or α-toxin produced by Clostridium perfringens can hydrolyze host sphingomyelin (23, 67, 98). Also, cholera toxin and botulinum neurotoxin can bind to glycosphingolipids such as the ganglioside GM1 (15, 96, 135, 149). However, recent work suggests that gangliosides produced by the host cell may actually help protect against C. perfringens α-toxin (28). We discuss below how prokaryotes use host sphingolipids during multiple steps of the infection process. We propose that bacteria utilize different host sphingolipids for their survival in the host, either extracellularly or intracellularly.
(i) Chlamydia trachomatis.
C. trachomatis is a gram-negative, obligate intracellular bacterium that is the etiologic agent of the most common sexually transmitted bacterial disease in the United States. It can also cause trachoma, an infection of the eye, which may lead to blindness and adult inclusion conjunctivitis. The microbe enters the host cell in the form of infectious elementary bodies (EBs) and resides in a special organelle termed the inclusion body. After uptake by host cells, the EBs differentiate into metabolically active reticulate bodies. The infectious cycle is completed after proliferation and release of bacteria, in the form of EBs, back into the extracellular environment.
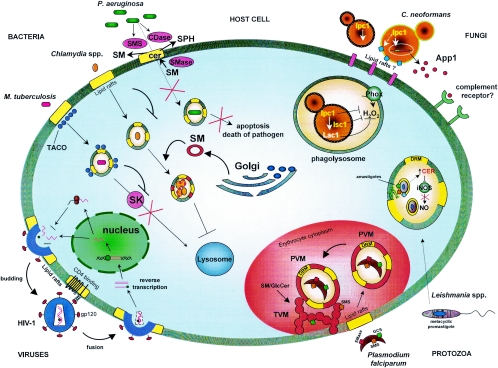
C. trachomatis lacks the proper enzymes for sphingolipid metabolism (121) but, interestingly, the bacterium membranes do contain up to 4% sphingolipids (100, 145), suggesting that C. trachomatis may be able to scavenge lipids from the host. Indeed, the chlamydial inclusion body fuses with vesicles containing sphingomyelin that originate from the Golgi of macrophages (26, 39). The sphingomyelin from the Golgi may disguise the bacterial inclusion body and prevent fusion with lysosomes. Host sphingomyelin taken up by the chlamydial inclusion body is eventually incorporated into the bacterial membranes (39, 40), and this process may be important for the intracellular growth and replication of C. trachomatis (137). The molecular mechanism by which sphingomyelin incorporation favors intracellular survival of the pathogen has not been explored, although it is tempting to speculate that the microbe containing sphingomyelin would be more resistant to damage by oxidative stress (Fig. 3). This mechanism could lead to colonization (survival without proliferation) or microbial proliferation with consequent host cell disruption.
FIG. 3.
Model representing the involvement of sphingolipids in microbial pathogenesis.
(ii) Mycobacterium tuberculosis.
M. tuberculosis is a facultative intracellular bacterium and the etiologic agent of tuberculosis, a serious global human infectious disease. Survival of this pathogen within host macrophages is attributed to its ability to prevent fusion of the phagosome with the lysosomal compartment (70). Almost concomitantly, two groups were able to demonstrate a role for host lipid rafts in promoting the intracellular survival of M. tuberculosis (31, 118). More recently, it has been proposed that host sphingolipids play a critical role in controlling M. tuberculosis proliferation in vivo. Specifically, Malik et al. showed that M. tuberculosis is able to repress the rise in Ca2+ levels that typically occurs in macrophages upon ingestion of opsonized organisms (85). This increase in Ca2+ is required for activation and maturation of the macrophage (reviewed in reference 70). Interestingly, host sphingosine kinase (SK) activity and, thus, sphingosine-1-phosphate (S1P) levels, are decreased after mycobacterial infection (86). S1P had previously been shown to regulate cellular levels of Ca2+ (119). Inhibition of SK upon mycobacterial infection suggests that a bacterium-derived component is able to decrease S1P in order to prevent the rise in Ca2+ that would normally lead to maturation and/or acidification of the macrophage phagolysosome and subsequent killing of the internalized bacteria (Fig. 3) (86). Most recently, it has been suggested that M. tuberculosis can inhibit the translocation of SK to the phagosome membrane (128). Identification and characterization of the Mycobacterium component inhibiting SK would be helpful in determining the role of this inhibitory mechanism in favoring colonization and dormancy over bacterial proliferation and host damage and increase our understanding of the pathogenesis of M. tuberculosis.
(iii) Pseudomonas aeruginosa.
Chronic infection by the gram-negative bacterium P. aeruginosa is often associated with cystic fibrosis (CF), a disease caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) protein. The adherence of P. aeruginosa to CF respiratory epithelial cells is increased compared to normal cells (115), and it has been suggested that P. aeruginosa binds to asialylated gangliosides such as asialoGM1, a sphingolipid that is increased on the surface of CF cells (116). Characteristically, P. aeruginosa produces the exoprotein neuraminidase, which cleaves sialylic acid from cell surface sialylated gangliosides, to enhance adherence of bacteria to the respiratory epithelium (8, 115). Thus, one could hypothesize that altering the expression of asialoGM1 might reduce the adherence of the microbe to epithelial cells in CF patients and inhibit infection. However, there is some evidence which suggests that binding of P. aeruginosa to asialoGM1 can actually increase the uptake of bacteria into host cells (13). Given that P. aeruginosa is an extracellular opportunistic pathogen and would want to evade uptake by host cells, it is not yet clear if this interaction with asialoGM1 is beneficial or detrimental to the pathogen.
It has also been shown that interaction of P. aeruginosa with host epithelial cells activates host acid sphingomyelinase, leading to generation of plasma membrane ceramide-enriched platforms (37). The formation of these platforms enables internalization of P. aeruginosa which triggers apoptosis of the host cell and will ultimately lead to the killing of the pathogen (37). Localization of CFTR protein to lipid rafts is required for uptake of P. aeruginosa by host cells (66). In CF patients, the mutated form of CFTR protein does not localize to rafts, altering the microbe internalization and increasing the extracellular proliferation and the severity of the disease (66).
Intriguingly, P. aeruginosa also produces and secretes sphingolipid-metabolizing enzymes. These include hemolytic phospholipase C (PlcH), which can synthesize sphingomyelin from ceramide, and alkaline ceramidase, which can break down ceramide (82, 106). Disruption of the gene encoding PlcH, and resultant loss of sphingomyelin synthase activity decreases the virulence of P. aeruginosa in a mouse model (108), suggesting that P. aeruginosa may use PlcH, as well as alkaline ceramidase, to metabolize host ceramide, thereby inhibiting formation of anti-Pseudomonas ceramide-enriched rafts (Fig. 3).
It is interesting that both PlcH and alkaline ceramidase have been shown to have dual activities. PlcH has sphingomyelinase activity (107), whereas alkaline ceramidase has ceramide synthase activity at neutral pH (65). One could hypothesize that whether these enzymes express one activity versus the other depends on substrate availability and/or reaction conditions (e.g., pH). However, the significance of the ability of these enzymes to catalyze either reaction under different conditions is still unclear in the context of pathogenesis.
Viruses.
Sphingolipids play an important role in many aspects of viral replication, including the initial infection of mammalian cells, depression of the host immune response, and assembly and budding of newly synthesized viral components (90). Interestingly, recent work by Wilson et al. indicate that viruses of the genus Coccolithovirus may contain genes involved in ceramide biosynthesis (143). Although the possibility of sphingolipid generation by viruses needs further exploration, the role of host sphingolipids in viral infection has been best-studied with human immunodeficiency virus (HIV).
HIV type 1 (HIV-1) is the causative agent of AIDS. Lipid rafts of CD4+ T cells appear to play an integral role in the replication cycle of HIV-1 (reviewed in references 90 and 29). The first step of HIV-1 infection is the binding of viral gp120 to the host cell surface receptor CD4, a raft-associated protein (Fig. 3) (16, 84, 111). This interaction appears to trigger lipid raft reorganization to cluster additional cofactors required for subsequent viral fusion and entry into the host cells (89). These cofactors include other chemokine receptors, as well as glycosphingolipids, including galactocerebroside, globotriaosylceramide (Gb3), and ganglioside GM3 (45, 52, 58). Viral budding also occurs through lipid rafts, since host lipids and proteins associated with host membrane rafts appear in the viral envelope of newly synthesized HIV-1 virions (101).
Because sphingolipids and lipid rafts are critical to the infection by HIV-1 (fusion, entry, and budding), targeting these molecules may give rise to novel antiretroviral therapies. Several studies have investigated cholesterol-depletion as a means of disrupting lipid rafts to prevent HIV-1 entry (78, 79, 102). Very recently, it has been proposed that an increase of ceramide levels in CD4+ lymphocytes and in monocyte-derived macrophages using pharmacological agents, such as N-(4-hydroxyphenyl) retinamide and fenretinide, or by treatment with sphingomyelinase or by addition of long-chain ceramide may block HIV infection, perhaps by disrupting normal lipid raft organization and function which would inhibit HIV fusion (27). Since HIV infection predisposes a patient to further infection by opportunistic pathogens (in essence facilitating the pathogenicity of these microbes), treatment with compounds that alter lipid-raft-mediated HIV infection would not only protect the patients from immunodepression but also prevent establishment of opportunistic infections by microorganisms that cause disease through a lipid-raft mediated mechanism.
Protozoa.
Protozoa do synthesize sphingolipids, but whereas some protozoa have biosynthetic pathways which produce sphingomyelin, similar to mammalian cells, others produce IPCs, similar to fungal and plant cells.
(i) Plasmodium falciparum.
P. falciparum is the etiologic agent of malaria. Infective forms or sporozoites reside in the salivary glands of the Anopheles mosquito and are introduced into the mammalian host when the mosquito takes a blood meal. The sporozoites migrate to the liver where they proliferate and then enter the bloodstream, where they infect erythrocytes. Erythrocytes present an interesting host environment, since they are terminally differentiated cells that lack organelles, are nonendocytic, and are unable to synthesize proteins or lipids de novo. To enter erythrocytes, Plasmodia first attach to the host cell membrane (Fig. 3). Invagination of the erythrocyte membrane leads to uptake of Plasmodia within a parasitophorous vacuolar membrane (PVM), where they proliferate to form a schizont that consists of many individual merozoites (1). Rupture of the infected erythrocyte releases the merozoites, which can proceed to infect other erythrocytes. Some merozoites develop into male and female gametocytes that can eventually be ingested by a mosquito. The gametocytes fuse within the gut of the mosquito to produce sporozoites, which then migrate to the salivary glands to repeat the infectious cycle.
Lipid rafts of host erythrocytes have been shown to be critical for the formation of the PVM (42). The erythrocytes' rafts are associated with particular GPI-anchored, transmembrane, and cytosolic proteins. Several of these host raft-associated proteins, including the transmembrane protein Duffy, are also found in detergent-resistant membranes (DRM) of the PVM, whereas other major erythrocyte membrane proteins that do not associate with rafts are not found in the PVM (72), suggesting that lipid rafts are a key platform for the entry of Plasmodium into the erythrocytes. Synthesis of sphingomyelin regulates the accumulation of the DRM-associated proteins in the PVM (72), although the molecular mechanism of this phenomenon has not yet been elucidated.
Various mammalian-like sphingolipid biosynthetic activities have been demonstrated in Plasmodium. Both sphingomyelin synthase (SMS) (3, 24, 25, 44) and glucosylceramide synthase (GCS) (14, 32) activities have been observed, suggesting that the Plasmodium has a conserved mammalian-like sphingolipid biosynthetic pathway. SMS enzyme is localized in the parasite Golgi but can be actively transported out of the parasite into the tubulovesicular membrane (TVM), a structure which buds from the PVM during parasite development (Fig. 3). Within the infected erythrocyte, which lacks endocytic activity (43), the TVM provides a key means of communication between the intracellular parasite and extracellular domain for uptake of nutrients and host proteins (72, 74). It has been demonstrated that SMS activity is essential for TVM development (reviewed in reference 41) and that inhibition of this activity leads to death of the parasite (73), suggesting sphingomyelin synthesis is crucial to the survival and virulence of Plasmodium. The role of GCS in the intraerythrocytic development of the parasite is still unclear because, although inhibition of GCS activity decreases formation of glucosylceramide (GlcCer), this apparently does not affect the development of the parasite inside the erythrocyte (14, 32). Perhaps GCS (or GlcCer) may be important only at certain stages of the intraerythrocyte development of the parasite.
In addition, a neutral sphingomyelinase capable of generating ceramide from sphingomyelin has been identified in P. falciparum (47, 48). Hanada and coworkers propose the intriguing hypothesis that plasmodial SMase activity degrades host SM to produce ceramide, which is then used by plasmodial SMS and GCS to produce SM and GlcCer in the TVM (Fig. 3). As a consequence, targeting SMase, SMS, and/or GCS could inhibit growth of the parasite (14, 73). Conversely, excessive accumulation of ceramide may also be detrimental for the pathogen since treatment with exogenous ceramide inhibits parasitic growth through a nonapoptotic mechanism (71, 109). Indeed, the antimalarial drugs artemisinin and mefloquine may exert their antiparasitic effects through the activation of SMase (109). The consequent increase of ceramide would decrease the level of glutathione, thereby preventing degradation of ferroprotoporfirin IX, which is toxic to plasmodia. The exact molecular mechanism by which these antimalarial drugs activate neutral sphingomyelinase activity is still under investigation.
(ii) Kinetoplastid parasites.
The kinetoplastid protozoa include Leishmania spp., which cause leishmaniases, and Trypanosoma spp., which cause African sleeping sickness (Trypanosoma brucei) and Chagas disease (T. cruzi). Although sphingolipids have been identified in all of these organisms, we will focus our attention here on Leishmania spp. because sphingolipid metabolism in these protozoa has been studied in greater detail than in Trypanosoma spp.
Leishmania major is carried by the phlebotomine sandflies (genus Phlebotomus or Lutzomyia). In the sandfly, Leishmania exists as a flagellated promastigote, which differentiates from a replicating procyclic form to a nonreplicating metacyclic form. The infectious metacyclics are transferred through the sandfly proboscis into the mammalian host where they enter macrophages. Inside the macrophage, they differentiate into nonflagellated amastigotes and replicate, ultimately killing the host cell. In the culturing of parasites, the log phase represents the procyclic promastigotes, the stationary phase induces differentiation into infective metacyclic promastigotes, and amastigotes form within the phagolysosome of the host macrophage and cause pathology in the host organism.
Several studies indicate that Leishmania produce IPCs (17, 62) and, as such, are more similar to fungal cells. Among the Leishmania surface membrane glycolipids, which include lipophosphoglycan and glycosylinositolphospholipids, IPCs have been found to be the most abundant class (18, 112). However, thus far, no IPC synthase has been identified in this parasite. Sphingolipid synthesis has been studied in L. major by knocking out SPT2/LCB2, the catalytic subunit of serine palmitoyl transferase, to generate sphingolipid-null mutants (19, 153). Interestingly, the loss of de novo sphingolipid synthesis did not affect the viability of the promastigote parasite. However, these mutants were defective in their ability to differentiate into the metacyclic (or infective) form. Accordingly, these parasites had poor infectivity of isolated host macrophages and delayed infectivity in murine models in vivo, indicating that sphingolipids are important for the initial invasion of the host by Leishmania. Although the Spt2/Lcb2 knockout mutant had a delayed onset of infection, once lesions appeared, their progression was comparable to that of wild-type strains (19, 153). These studies suggest that de novo sphingolipid synthesis is not necessary for proliferation of amastigotes inside the phagolysosome. This hypothesis is supported by the observation that Spt2/Lcb2 is downregulated to undetectable levels in wild-type amastigotes compared to promastigote forms of the parasite.
More interesting, however, is the fact that wild-type and Spt2/Lcb2 knockout amastigotes contain Leishmania-specific IPCs, indicating that sphingolipids play an important role in this intracellular proliferation (152). How these amastigotes generate complex sphingolipids when Spt2/Lcb2 activity is undetectable is currently unknown. Recent evidence suggests that Leishmania is capable of salvaging host sphingolipids (152) and can remodel them to generate parasite-specific sphingolipids. Thus, Leishmania may possess an Ipc1-like enzyme that uses host ceramide and host or parasitic phosphatidylinositol to make IPCs (Fig. 3). What causes Leishmania to downregulate Spt2/Lcb2 and how they acquire and remodel host sphingolipids are also unknown.
On the other hand, Leishmania may also alter host sphingolipid metabolism within the host cell itself. For instance, L. donovani induces the generation of ceramide by murine macrophages, thereby increasing the intracellular survival of the parasite (33, 34). The increase of ceramide inhibits AP-1 and NF-κB transcription factors via downregulation of classical protein kinase C (PKC) activity and mitogen-activated protein phosphorylation. These events serve to inhibit nitric oxide synthesis (Fig. 3), thereby preventing macrophage activation and the killing of the parasite (33, 34). It appears that the effect of host ceramide on Leishmania is opposite to the effect of ceramide on Plasmodia. This interesting difference might be due to the branch of sphingolipid (fungus-like versus mammalian-like) present in the two parasites and/or to the particular environment in which they reside (erythrocytes versus macrophages). It is unknown what stimulates the generation of ceramide, but it is tempting to speculate that inhibition of mammalian PKC is due to the ceramide formed by the action of plasmodial sphingomyelinase.
As sphingolipid-synthesizing organisms, Leishmania may also use its own sphingolipids as part of its signaling machinery for the expression of virulence factors. It has been observed that the Spt2/Lcb2 knockout mutant accumulates abnormal intracellular lipid vesicles, which may implicate sphingolipids in the regulation of intracellular trafficking (19, 153). Also, a study by Denny et al. suggests that the expression of GP63 (also called leishmanolysin), an important virulence factor of Leishmania (61), is dysregulated in the Spt2/Lcb2 knockout mutants (19), although this result was not reproduced in a study by Zhang et al. (152).
In addition to playing an important role in survival and proliferation in the host, sphingolipids from amastigotes of Leishmania amazonensis induce a selective loss of CD4+ cells and inhibit lymphoproliferation through a PKC-dependent mechanism, without affecting the expression of immune surface markers (CD3, CD4, CD8, CD14, CD19, and CD45) (35). Intriguingly, inhibition of host PKC activity is also caused by Leishmania promastigotes, but in this case the effect is due to lipophosphoglycans instead of sphingolipids. This is an interesting finding because it has been proposed that amastigote sphingolipids may act as immunomodulators during the progression of the infection, whereas the lipophosphoglycan in promastigotes acts during the early stage of macrophage infection (35).
Fungi.
Fungi produce sphingolipids of the IPC lineage, and IPC synthase homologs (encoded by the AUR1 gene [also called IPC1] in S. cerevisiae) have been identified in many fungal organisms (54). However, the roles of sphingolipids in fungal infectious diseases are not well established because the biological functions of fungal sphingolipids have been studied almost exclusively in nonpathogenic fungi such as S. cerevisiae. In addition, since S. cerevisiae does not produce cerebrosides (for example, GlcCer), their roles and functions in fungi are largely unexplored. It is interesting that pathogenic fungi do produce GlcCer, and very recently it has been shown that Cryptococcus neoformans GlcCer synthase (GCS1) is required for pathogenicity of this fungus (113).
During the infection process, it is not yet known whether fungal cells can incorporate host sphingolipids into fungal membranes or modify host sphingolipids to alter cell membrane structure or signaling pathways. There is some evidence that host sphingolipids such as lactosylceramide can mediate the adherence of fungal cells to epithelial and phagocytic cells (60). On the other hand, fungi are themselves sphingolipid-producing organisms and could use sphingolipids as important signaling molecules, particularly in mediating the expression of virulence factors.
(i) Candida albicans.
Although the presence of sphingolipids and sphingolipid-metabolizing enzymatic activities have been demonstrated in C. albicans, their role in the physiology, biology, and pathobiology of this important human pathogen has yet to be elucidated. Glucosylceramides were first shown to be produced by C. albicans by Matsubara et al. (91). Recently, the gene encoding for GlcCer synthase has been isolated in C. albicans, but its role in the pathogenicity of this fungus has yet to be determined (75). Wells et al. showed that the hyphal form of C. albicans produces large amounts of complex sphingolipids, such as IPC, MIPC, and M(IP)2C (141). However, whether sphingolipid production is higher in hyphal compared to yeast forms of Candida is not known. Sphingolipid metabolism could be important considering that the yeast-hyphal switch is a “pro-virulence” transformation. Known inhibitors of Ipc1 activity inhibit growth of C. albicans similarly to the inhibition exerted in other fungi (87, 88, 154). A “new” complex sphingolipid, consisting of dimannose inositol phosphoceramide (M2IPC), possibly derived from MIPC, has been characterized in C. albicans (133). This sphingolipid was actually previously described in other fungi, such as Histoplasma capsulatum (6). The interesting feature of M2IPC is that its lipophilic property allows the diffusion of this complex sphingolipid to the cell wall. In contrast, other complex sphingolipids, such as IPC, MIPC, and M(IP)2C, are thought to be anchored to the plasma membrane because they are much less polar. Whether this specific localization in the cell wall can exert a signaling function to host cells, such as macrophages or epithelial cells, has yet to be elucidated. Very recently, a C. albicans strain lacking this lipid has been produced, but this strain also lacks MIPC and M(IP)2C, indicating that the deleted gene (MIT1) encodes for MIPC synthase activity, a precursor of M2IPC and M(IP)2C (95). It was interesting that although yeast cell growth at 37°C and yeast-hyphal transitions were not affected, the C. albicans Δmit2 mutant showed a significant decrease in virulence. The latter observation should prompt additional investigations to understand sphingolipid mannosylation at the plasma membrane and cell wall and their role in the regulation of Candida pathogenicity.
(ii) Cryptococcus neoformans.
C. neoformans is an opportunistic fungal pathogen which enters a human host through the respiratory tract. Upon immunosuppression, yeast cells can migrate through the bloodstream to the brain and cause a life-threatening meningoencephalitis. Cryptococcosis is found primarily among AIDS patients, cancer patients, and others undergoing transplantation procedures or on immunosuppressive therapy. Current anticryptococcal treatments are unable to completely eradicate the infection, forcing patients to be maintained on life-long antifungal therapy with the consequent selection of resistant strains and the development of relapses.
C. neoformans has several virulence factors that enable it to cause disease, including a large polysaccharide capsule, ability to grow at 37°C (temperature of mammalian hosts) and low pH (environment within phagolysosome), and melanin, a black pigment produced by the oxidation of diphenolic substrates (such as catecholamines) through the action of laccase, an enzyme localized in the fungal cell wall. Melanin protects the fungus from the host immune response.
In previous studies, Luberto et al. established that the sphingolipid enzyme Ipc1 plays a crucial role in the regulation of the pathogenesis of C. neoformans (83). Ipc1 is encoded by the IPC1 gene, also called AUR1. This enzyme catalyzes the transfer of a phosphoryl-inositol group from phosphatidylinositol to phytoceramide (53, 54, 69) to produce the complex sphingolipid IPC, as well as a molecule of diacylglycerol (Fig. 2). In C. neoformans, diacylglycerol regulates activation of Pkc1 through the C1 domain, and this activation is required for the proper localization and function of the enzyme laccase (Lac1) at the cell wall (Fig. 3) (55, 56). Among the virulence traits that Ipc1 has been shown to regulate are growth at low pH (83), melanin production (55, 56), and secretion of antiphagocytic protein 1 (App1) (Fig. 3) (81).
Other sphingolipid-metabolizing enzymes have been identified and characterized in C. neoformans. One of these enzymes is inositol phosphosphingolipid phospholipase C (Isc1), which metabolizes complex sphingolipids into phytoceramide and inositol phosphate (Fig. 3) and exerts a key role in virulence of C. neoformans (J. Shea and M. Del Poeta, manuscript in preparation) and (117). Isc1 is the fungal counterpart to sphingomyelinase in mammalian cells but possesses unique biochemical characteristics different from the mammalian and the S. cerevisiae enzymes (J. L. Henry and M. Del Poeta, manuscript in preparation). Another sphingolipid-metabolizing enzyme characterized in C. neoformans is GlcCer synthase 1 (Gcs1) (113).
C. neoformans is a facultative intracellular pathogen that can grow within the phagolysosome of macrophages. In previous studies, it has been shown that an increase in fungal complex sphingolipids, due to the upregulation of Ipc1, significantly increases the size of the phagolysosome (83). Whether this alteration of the phagolysosome has implications in the overall membrane structure of the host organelle is currently under investigation. It is also interesting to hypothesize that fungal sphingolipids might be incorporated into the membrane of the phagolysosome, altering its membrane potential, signaling function, and/or fluidity. Since host sphingolipid-metabolizing enzymes cannot metabolize or remodel fungal sphingolipids, their incorporation into membranes of the phagolysosome could lead to its early disruption, the lysing of the host cell, and/or extracellular dissemination of the fungus.
(iii) Aspergillus spp. and other filamentous fungi.
Aspergillus spp. are filamentous fungi which commonly cause disease of the respiratory system. The Aspergillus IPC synthase homolog, named AurA, has been identified and isolated (54, 69), and complex sphingolipids are present in Aspergillus spp. (38). In A. nidulans, a nonpathogenic model of Aspergillus, SPT and IPC synthase activities are required for cell cycle progression and polarized cell growth (12), but their role in the pathogenicity of A. fumigatus has yet to be addressed. Recent work suggests that sphingoid bases induce apoptosis in A. nidulans through a caspase-dependent mechanism (11). Aspergillus spp. also produce GlcCer and galactosylceramide (130, 138), and disruption of their synthesis by using pharmacological inhibitors causes defects in both germination and hyphal growth (76), suggesting that they might play an important role in pathogenicity. Some Aspergillus spp. show sphingomyelinase activity (92), and this is interesting because, thus far, sphingomyelinase activity has been demonstrated in the nonpathogenic fungus S. cerevisiae but not in C. neoformans and C. albicans (117).
Although Aspergillus spp. possess known complex sphingolipids, other filamentous fungi do not. For instance, zygomycetes, such as Mucor spp. (the agents responsible for mucormycosis), do not possess Ipc1-derived sphingolipids, such as IPC, MIPC, and M(IP)2C. These fungi are resistant to aureobasidin A, an IPC synthase inhibitor, suggesting that they do not possess the Ipc1 enzyme. Instead, they contain neutral glycosphingolipids not previously described in other fungal organisms. The role of these neutral glycosphingolipids in the physiopathology of these fungi has yet to be elucidated (4).
In Histoplasma capsulatum, two interesting studies identified complex sphingolipids present in the mycelial and yeast formsof this dimorphic fungus (5, 6). In particular, the authors ofthose studies identified a dimmanosylinositolphosphoceramide (M2IPC-compound V) and galactosyldimmanosylinositolphosphoceramide (GalM2IPC), with the latter existing in two forms depending on the addition of a galactofuranose residue at the 6-position of mannose (compound VI) or the addition of galactopyranose at the 4-position of mannose (compound VIII). Interestingly, whereas compound VIII is present in both yeast and hyphal forms, compounds V and VI are only present in the yeast form of H. capsulatum, which is the in vivo form of the fungus that survives within macrophages. Antibodies that react with compounds V, VI, and VIII have been found in sera of patients with histoplasmosis; however, whether these human antibodies would have a protective effect against dissemination of the disease is not known. A class of acidic glycosphingolipids (glycoinositol phosphorylceramide) has been described in the nonpathogenic basidiomycete Agaricus blazei and in pathogenic euascomycetes such as Aspergillus fumigatus, Histoplasma capsulatum, and Sporothrix schenckii (77). This class of sphingolipids shows a very high diversity in their glycosylinositol and ceramide structure especially between the yeast and hyphal form of S. schenckii (77). In addition, GlcCer has been described in both the yeast and hyphal form of H. capsulatum (131), as well as in Paracoccidioides brasiliensis (124), S. schenckii (129), and other filamentous fungi pathogenic to humans or plants (reviewed in reference 139). The characterization of these glycosphingolipids could contribute to a better understanding of fungal pathogenesis.
WHEN SPHINGOLIPIDS COLLIDE: HOW THE BALANCE OF HOST AND MICROBIAL SPHINGOLIPIDS DETERMINES PATHOGENICITY
Although sphingolipids and their metabolism may aid microbial pathogenesis, it also appears that sphingolipids may be key players in the host's defenses against infections. In the case of extracellular pathogens, such as P. aeruginosa, sphingolipid-enriched rafts facilitate the phagocytosis and eventual lysis of bacteria (37). In the case of intracellular pathogens, the host may initiate sphingolipid-mediated pathways which enable containment or dormancy of the microbe or enhance the clearance of the microorganism. For example, generation of sphingosine-1-phosphate by sphingosine kinase is crucial to the initiation of macrophage activation in M. tuberculosis infection (30, 85, 86). The particular microbial component responsible for this type of host response is yet to be identified. Studies with M. tuberculosis and C. neoformans, a eukaryotic intracellular pathogen, as genetic models may help discern whether microbial sphingolipids may, in fact, be the trigger of these host defense pathways.
Alternatively, the host cell may directly target the pathogen's sphingolipid metabolism through the production of molecules such as antimicrobial peptides or antibodies. It has been shown that fungal cells deficient in sphingolipid biosynthesis are more resistant to plant antimicrobial peptides such as DmAMP1 (125, 126). Also, the plant defensin RsAFP2 and the insect defensin-like peptide heliomicin interact with fungal glucosylceramides (127). Fungal GlcCer evokes the production of human antibodies during cryptococcosis (132) or histoplasmosis (5). In addition, it has been demonstrated that treatment of in vitro cultures of C. neoformans with human anti-GlcCer antibody inhibits fungal growth (114), suggesting that such a host immune response could counteract in vivo fungal proliferation.
Mounting an immune response against microbial sphingolipids, however, could have detrimental effects on the host cell through the phenomenon of molecular mimicry and the development of autoimmunity. Bacterial glycosphingolipids have been shown to be stimulators of NKT cells (64, 93, 144), which play a key role in the regulation of the host's immune response against viral, bacterial, fungal, and parasitic infections (134). Interestingly, a subpopulation of NKT cells, invariant NKT cells, reacts against microbial glycosphingolipids and controls autoimmunity (136). Thus, it is possible that some pathophysiological features associated with infections could be explained by an autoimmune response against host glycosphingolipids due to molecular mimicry by microbial sphingolipids. This hypothesis has been postulated to explain the demyelinating encephalopathy in African trypanosomiasis (36). In bacteria, molecular mimicry exists between lipopolysaccharides and gangliosides (mainly glycosphingolipids), and it has been suggested to be responsible for the neuropathy after Mycoplasma infection (123) or neuromuscular paralysis (or Guillain-Barre syndrome) due to infections by Campylobacter jejuni or other microbes (150). It is also worth noting that P. aeruginosa ceramidase is significantly similar to the human alkaline ceramidase and that M. tuberculosis also has a gene encoding a protein that is significantly homologous to the P. aeruginosa ceramidase. These similarities between prokaryotes and eukaryotes might suggest that, in addition to autoimmunity against sphingolipid molecules, molecular mimicry may also exist in regard to enzymes of sphingolipid metabolism and/or synthesis. Potentially, this pathophysiological mechanism could also explain clinical manifestations present in other infections (e.g., cryptococcosis, histoplasmosis, and paracoccidioidomycosis) in which production of antibodies against glycosphingolipids has been demonstrated. This area of investigation is particularly attractive.
The interaction between microbial and host sphingolipids could explain many aspects of the immune system. As signaling molecules, sphingolipids can trigger actions and responses that would either prevent host damage from the microbe (or from the host itself) without the establishment of disease or favor the initiation of damage signals that could promote a pathological process. There have been recent successes in demonstrating that inhibition of microbe-utilized sphingolipids limits the pathogenicity of these organisms. Thus, a better understanding of sphingolipid-mediated microbial pathogenesis and how sphingolipid interactions can be modified to benefit the host may forge inroads into new therapeutic strategies and provide alternatives to traditional chemotherapeutic approaches to infectious disease.
ADDENDUM
During the revision of this minireview, a review by K. Hanada was published (46). We recommend reading this review, which focuses on the biochemistry of sphingolipids and recent advances in sphingolipid biology related to infectious diseases.
Acknowledgments
We are grateful to Edward Balish, Yusuf Hannun, and Jennifer Schnellmann for critical reading and editing of the manuscript. We also thank all members of the Del Poeta and Luberto laboratories for helpful discussions.
M.D.P. is supported by the Burroughs Wellcome Fund, the National Institutes of Health grant AI56168, and the Centers of Biomedical Research Excellence (COBRE) Program of the National Center for Research Resources grant RR17677 (Project 2). C.L. is supported by the COBRE Program of the National Center for Research Resources Grant RR17677 (Project 6) and by the National Science Foundation/EPSCoR Grant #EPS-0132573. L.J.H. is supported in part by Medical Scientist Training Grant GM08716 from the NIH. M.D.P. is a Burroughs Wellcome New Investigator in Pathogenesis of Infectious Diseases.
Editor: J. B. Kaper
REFERENCES
- 1.Aikawa, M., L. H. Miller, J. Johnson, and J. Rabbege. 1978. Erythrocyte entry by malarial parasites: a moving junction between erythrocyte and parasite. J. Cell Biol. 77:72-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez-Vasquez, F., K. J. Sims, L. A. Cowart, Y. Okamoto, E. O. Voit, and Y. A. Hannun. 2005. Simulation and validation of modeled sphingolipid metabolism in Saccharomyces cerevisiae. Nature 433:425-430. [DOI] [PubMed] [Google Scholar]
- 3.Ansorge, I., D. Jeckel, F. Wieland, and K. Lingelbach. 1995. Plasmodium falciparum-infected erythrocytes utilize a synthetic truncated ceramide precursor for synthesis and secretion of truncated sphingomyelin. Biochem. J. 308(Pt. 1):335-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aoki, K., R. Uchiyama, S. Yamauchi, T. Katayama, S. Itonori, M. Sugita, N. Hada, J. Yamada-Hada, T. Takeda, H. Kumagai, and K. Yamamoto. 2004. Newly discovered neutral glycosphingolipids in aureobasidin A-resistant zygomycetes: identification of a novel family of Gala-series glycolipids with core Gal alpha 1-6Gal beta 1-6Gal beta sequences. J. Biol. Chem. 279:32028-32034. [DOI] [PubMed] [Google Scholar]
- 5.Barr, K., R. A. Laine, and R. L. Lester. 1984. Carbohydrate structures of three novel phosphoinositol-containing sphingolipids from the yeast Histoplasma capsulatum. Biochemistry 23:5589-5596. [DOI] [PubMed] [Google Scholar]
- 6.Barr, K., and R. L. Lester. 1984. Occurrence of novel antigenic phosphoinositol-containing sphingolipids in the pathogenic yeast Histoplasma capsulatum. Biochemistry 23:5581-5588. [DOI] [PubMed] [Google Scholar]
- 7.Bell, R., D. Burns, T. Okazaki, and Y. Hannun. 1992. Network of signal transduction pathways involving lipids: protein kinase C-dependent and -independent pathways. Adv. Exp. Med. Biol. 318:275-284. [DOI] [PubMed] [Google Scholar]
- 8.Cacalano, G., M. Kays, L. Saiman, and A. Prince. 1992. Production of the Pseudomonas aeruginosa neuraminidase is increased under hyperosmolar conditions and is regulated by genes involved in alginate expression. J. Clin. Investig. 89:1866-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casadevall, A., and L. A. Pirofski. 2003. The damage-response framework of microbial pathogenesis. Nat. Rev. Microbiol. 1:17-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charity, R. M., and A. F. Foukas. 2005. Osteomyelitis and secondary septic arthritis caused by Sphingomonas paucimobilis. Infection 33:93-95. [DOI] [PubMed] [Google Scholar]
- 11.Cheng, J., T. S. Park, L. C. Chio, A. S. Fischl, and X. S. Ye. 2003. Induction of apoptosis by sphingoid long-chain bases in Aspergillus nidulans. Mol. Cell. Biol. 23:163-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng, J., T. S. Park, A. S. Fischl, and X. S. Ye. 2001. Cell cycle progression and cell polarity require sphingolipid biosynthesis in Aspergillus nidulans. Mol. Cell. Biol. 21:6198-6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Comolli, J. C., L. L. Waite, K. E. Mostov, and J. N. Engel. 1999. Pili binding to asialo-GM1 on epithelial cells can mediate cytotoxicity or bacterial internalization by Pseudomonas aeruginosa. Infect. Immun. 67:3207-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Couto, A. S., C. Caffaro, M. L. Uhrig, E. Kimura, V. J. Peres, E. F. Merino, A. M. Katzin, M. Nishioka, H. Nonami, and R. Erra-Balsells. 2004. Glycosphingolipids in Plasmodium falciparum. Presence of an active glucosylceramide synthase. Eur. J. Biochem. 271:2204-2214. [DOI] [PubMed] [Google Scholar]
- 15.Cuatrecasas, P. 1973. Vibrio cholerae choleragenoid: mechanism of inhibition of cholera toxin action. Biochemistry 12:3577-3581. [DOI] [PubMed] [Google Scholar]
- 16.Dalgleish, A. G., P. C. Beverley, P. R. Clapham, D. H. Crawford, M. F. Greaves, and R. A. Weiss. 1984. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature 312:763-767. [DOI] [PubMed] [Google Scholar]
- 17.De-Majumdar, T. 1992. Leishmania donovani: purification and partial characterization of a glycophosphosphingolipid antigen expressed on promastigote surface. Exp. Parasitol. 74:251-260. [DOI] [PubMed] [Google Scholar]
- 18.Denny, P. W., M. C. Field, and D. F. Smith. 2001. GPI-anchored proteins and glycoconjugates segregate into lipid rafts in Kinetoplastida. FEBS Lett. 491:148-153. [DOI] [PubMed] [Google Scholar]
- 19.Denny, P. W., D. Goulding, M. A. Ferguson, and D. F. Smith. 2004. Sphingolipid-free Leishmania are defective in membrane trafficking, differentiation, and infectivity. Mol. Microbiol. 52:313-327. [DOI] [PubMed] [Google Scholar]
- 20.Denny, P. W., and D. F. Smith. 2004. Rafts and sphingolipid biosynthesis in the kinetoplastid parasitic protozoa. Mol. Microbiol. 53:725-733. [DOI] [PubMed] [Google Scholar]
- 21.Dickson, R. C., and R. L. Lester. 1999. Metabolism and selected functions of sphingolipids in the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta 1438:305-321. [DOI] [PubMed] [Google Scholar]
- 22.Dickson, R. C., and R. L. Lester. 2002. Sphingolipid functions in Saccharomyces cerevisiae. Biochim. Biophys. Acta 1583:13-25. [DOI] [PubMed] [Google Scholar]
- 23.Doery, H. M., B. J. Magnusson, I. M. Cheyne, and J. Gulasekharam. 1963. A phospholipase in staphylococcal toxin which hydrolyzes sphingomyelin. Nature 198:1091-1092. [DOI] [PubMed] [Google Scholar]
- 24.Elmendorf, H. G., and K. Haldar. 1993. Identification and localization of ERD2 in the malaria parasite Plasmodium falciparum: separation from sites of sphingomyelin synthesis and implications for organization of the Golgi. EMBO J. 12:4763-4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elmendorf, H. G., and K. Haldar. 1994. Plasmodium falciparum exports the Golgi marker sphingomyelin synthase into a tubovesicular network in the cytoplasm of mature erythrocytes. J. Cell Biol. 124:449-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fields, K. A., and T. Hackstadt. 2002. The chlamydial inclusion: escape from the endocytic pathway. Annu. Rev. Cell Dev. Biol. 18:221-245. [DOI] [PubMed] [Google Scholar]
- 27.Finnegan, C. M., S. S. Rawat, A. Puri, J. M. Wang, F. W. Ruscetti, and R. Blumenthal. 2004. Ceramide, a target for antiretroviral therapy. Proc. Natl. Acad. Sci. USA 101:15452-15457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flores-Diaz, M., A. Alape-Giron, G. Clark, B. Catimel, Y. Hirabayashi, E. Nice, J. M. Gutierrez, R. Titball, and M. Thelestam. 2005. A cellular deficiency of gangliosides causes hypersensitivity to Clostridium perfringens phospholipase C. J. Biol. Chem. 280:26680-26689. [DOI] [PubMed] [Google Scholar]
- 29.Fust, G., Z. Beck, D. Banhegyi, J. Kocsis, A. Biro, and Z. Prohaszka. 2005. Antibodies against heat shock proteins and cholesterol in HIV infection. Mol. Immunol. 42:79-85. [DOI] [PubMed] [Google Scholar]
- 30.Garg, S. K., E. Volpe, G. Palmieri, M. Mattei, D. Galati, A. Martino, M. S. Piccioni, E. Valente, E. Bonanno, P. De Vito, P. M. Baldini, L. G. Spagnoli, V. Colizzi, and M. Fraziano. 2004. Sphingosine 1-phosphate induces antimicrobial activity both in vitro and in vivo. J. Infect. Dis. 189:2129-2138. [DOI] [PubMed] [Google Scholar]
- 31.Gatfield, J., and J. Pieters. 2000. Essential role for cholesterol in entry of mycobacteria into macrophages. Science 288:1647-1650. [DOI] [PubMed] [Google Scholar]
- 32.Gerold, P., and R. T. Schwarz. 2001. Biosynthesis of glycosphingolipids de novo by the human malaria parasite Plasmodium falciparum. Mol. Biochem. Parasitol. 112:29-37. [DOI] [PubMed] [Google Scholar]
- 33.Ghosh, S., S. Bhattacharyya, S. Das, S. Raha, N. Maulik, D. K. Das, S. Roy, and S. Majumdar. 2001. Generation of ceramide in murine macrophages infected with Leishmania donovani alters macrophage signaling events and aids intracellular parasitic survival. Mol. Cell Biochem. 223:47-60. [DOI] [PubMed] [Google Scholar]
- 34.Ghosh, S., S. Bhattacharyya, M. Sirkar, G. S. Sa, T. Das, D. Majumdar, S. Roy, and S. Majumdar. 2002. Leishmania donovani suppresses activated protein 1 and NF-κB activation in host macrophages via ceramide generation: involvement of extracellular signal-regulated kinase. Infect. Immun. 70:6828-6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giorgio, S., M. R. Santos, A. H. Straus, H. K. Takahashi, and C. L. Barbieri. 2003. Effect of glycosphingolipids purified from Leishmania amazonensis amastigotes on human peripheral lymphocytes. Clin. Diagn. Lab. Immunol. 10:469-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Girard, M., S. Bisser, P. Buscher, B. Bouteille, J. L. Preud'homme, and M. O. Jauberteau. 2000. Cross-reactivity of anti-galactocerebroside autoantibodies with a Trypanosoma brucei proteolipidic epitope. Clin. Exp. Immunol. 119:516-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grassme, H., V. Jendrossek, A. Riehle, G. von Kurthy, J. Berger, H. Schwarz, M. Weller, R. Kolesnick, and E. Gulbins. 2003. Host defense against Pseudomonas aeruginosa requires ceramide-rich membrane rafts. Nat. Med. 9:322-330. [DOI] [PubMed] [Google Scholar]
- 38.Hackett, J. A., and P. J. Brennan. 1977. The isolation and biosynthesis of the ceramide-phosphoinositol of Aspergillus niger. FEBS Lett. 74:259-263. [DOI] [PubMed] [Google Scholar]
- 39.Hackstadt, T., D. D. Rockey, R. A. Heinzen, and M. A. Scidmore. 1996. Chlamydia trachomatis interrupts an exocytic pathway to acquire endogenously synthesized sphingomyelin in transit from the Golgi apparatus to the plasma membrane. EMBO J. 15:964-977. [PMC free article] [PubMed] [Google Scholar]
- 40.Hackstadt, T., M. A. Scidmore, and D. D. Rockey. 1995. Lipid metabolism in Chlamydia trachomatis-infected cells: directed trafficking of Golgi- derived sphingolipids to the chlamydial inclusion. Proc. Natl. Acad. Sci. USA 92:4877-4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haldar, K. 1996. Sphingolipid synthesis and membrane formation by Plasmodium. Trends Cell Biol. 6:398-405. [DOI] [PubMed] [Google Scholar]
- 42.Haldar, K., N. Mohandas, B. U. Samuel, T. Harrison, N. L. Hiller, T. Akompong, and P. Cheresh. 2002. Protein and lipid trafficking induced in erythrocytes infected by malaria parasites. Cell Microbiol. 4:383-395. [DOI] [PubMed] [Google Scholar]
- 43.Haldar, K., and L. Uyetake. 1992. The movement of fluorescent endocytic tracers in Plasmodium falciparum infected erythrocytes. Mol. Biochem. Parasitol. 50:161-177. [DOI] [PubMed] [Google Scholar]
- 44.Haldar, K., L. Uyetake, N. Ghori, H. G. Elmendorf, and W. L. Li. 1991. The accumulation and metabolism of a fluorescent ceramide derivative in Plasmodium falciparum-infected erythrocytes. Mol. Biochem. Parasitol. 49:143-156. [DOI] [PubMed] [Google Scholar]
- 45.Hammache, D., N. Yahi, M. Maresca, G. Pieroni, and J. Fantini. 1999. Human erythrocyte glycosphingolipids as alternative cofactors for human immunodeficiency virus type 1 (HIV-1) entry: evidence for CD4-induced interactions between HIV-1 gp120 and reconstituted membrane microdomains of glycosphingolipids (Gb3 and GM3). J. Virol. 73:5244-5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanada, K. 2005. Sphingolipids in infectious diseases. Jpn. J. Infect. Dis. 58:131-148. [PubMed] [Google Scholar]
- 47.Hanada, K., T. Mitamura, M. Fukasawa, P. A. Magistrado, T. Horii, and M. Nishijima. 2000. Neutral sphingomyelinase activity dependent on Mg2+ and anionic phospholipids in the intraerythrocytic malaria parasite Plasmodium falciparum. Biochem. J. 346:671-677. [PMC free article] [PubMed] [Google Scholar]
- 48.Hanada, K., N. M. Palacpac, P. A. Magistrado, K. Kurokawa, G. Rai, D. Sakata, T. Hara, T. Horii, M. Nishijima, and T. Mitamura. 2002. Plasmodium falciparum phospholipase C hydrolyzing sphingomyelin and lysocholinephospholipids is a possible target for malaria chemotherapy. J. Exp. Med. 195:23-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hannun, Y. A., and C. Luberto. 2000. Ceramide in the eukaryotic stress response. Trends Cell. Biol. 10:73-80. [DOI] [PubMed] [Google Scholar]
- 50.Hannun, Y. A., and C. Luberto. 2004. Lipid metabolism: ceramide transfer protein adds a new dimension. Curr. Biol. 14:R163-165. [PubMed] [Google Scholar]
- 51.Hannun, Y. A., C. Luberto, and K. M. Argraves. 2001. Enzymes of sphingolipid metabolism: from modular to integrative signaling. Biochemistry 40:4893-4903. [DOI] [PubMed] [Google Scholar]
- 52.Harouse, J. M., S. Bhat, S. L. Spitalnik, M. Laughlin, K. Stefano, D. H. Silberberg, and F. Gonzalez-Scarano. 1991. Inhibition of entry of HIV-1 in neural cell lines by antibodies against galactosyl ceramide. Science 253:320-323. [DOI] [PubMed] [Google Scholar]
- 53.Heidler, S. A., and J. A. Radding. 1995. The AUR1 gene in Saccharomyces cerevisiae encodes dominant resistance to the antifungal agent aureobasidin A (LY295337). Antimicrob. Agents Chemother. 39:2765-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heidler, S. A., and J. A. Radding. 2000. Inositol phosphoryl transferases from human pathogenic fungi. Biochim. Biophys. Acta 1500:147-152. [DOI] [PubMed] [Google Scholar]
- 55.Heung, L. J., A. E. Kaiser, C. Luberto, and M. Del Poeta. 2005. The role and mechanism of diacylglycerol-protein kinase C1 signaling in melanogenesis by Cryptococcus neoformans. J. Biol. Chem. 280:28547-28555. [DOI] [PubMed] [Google Scholar]
- 56.Heung, L. J., C. Luberto, A. Plowden, Y. A. Hannun, and M. Del Poeta. 2004. The sphingolipid pathway regulates protein kinase C 1 (Pkc1) through the formation of diacylglycerol (DAG) in Cryptococcus neoformans. J. Biol. Chem. 279:21144-21153. [DOI] [PubMed] [Google Scholar]
- 57.Hsueh, P. R., L. J. Teng, P. C. Yang, Y. C. Chen, H. J. Pan, S. W. Ho, and K. T. Luh. 1998. Nosocomial infections caused by Sphingomonas paucimobilis: clinical features and microbiological characteristics. Clin. Infect. Dis. 26:676-681. [DOI] [PubMed] [Google Scholar]
- 58.Hug, P., H. M. Lin, T. Korte, X. Xiao, D. S. Dimitrov, J. M. Wang, A. Puri, and R. Blumenthal. 2000. Glycosphingolipids promote entry of a broad range of human immunodeficiency virus type 1 isolates into cell lines expressing CD4, CXCR4, and/or CCR5. J. Virol. 74:6377-6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jenkins, G. M., A. Richards, T. Wahl, C. Mao, L. Obeid, and Y. Hannun. 1997. Involvement of yeast sphingolipids in the heat stress response of Saccharomyces cerevisiae. J. Biol. Chem. 272:32566-32572. [DOI] [PubMed] [Google Scholar]
- 60.Jimenez-Lucho, V., V. Ginsburg, and H. C. Krivan. 1990. Cryptococcus neoformans, Candida albicans, and other fungi bind specifically to the glycosphingolipid lactosylceramide (Galβ1-4Glcβ1-1Cer), a possible adhesion receptor for yeasts. Infect. Immun. 58:2085-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Joshi, P. B., B. L. Kelly, S. Kamhawi, D. L. Sacks, and W. R. McMaster. 2002. Targeted gene deletion in Leishmania major identifies leishmanolysin (GP63) as a virulence factor. Mol. Biochem. Parasitol. 120:33-40. [DOI] [PubMed] [Google Scholar]
- 62.Kaneshiro, E. S., K. Jayasimhulu, and R. L. Lester. 1986. Characterization of inositol lipids from Leishmania donovani promastigotes: identification of an inositol sphingophospholipid. J. Lipid Res. 27:1294-1303. [PubMed] [Google Scholar]
- 63.Kato, M., Y. Muto, K. Tanaka-Bandoh, K. Watanabe, and K. Ueno. 1995. Sphingolipid composition in Bacteroides species. Anaerobe 1:135-139. [DOI] [PubMed] [Google Scholar]
- 64.Kinjo, Y., D. Wu, G. Kim, G. W. Xing, M. A. Poles, D. D. Ho, M. Tsuji, K. Kawahara, C. H. Wong, and M. Kronenberg. 2005. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature 434:520-525. [DOI] [PubMed] [Google Scholar]
- 65.Kita, K., N. Okino, and M. Ito. 2000. Reverse hydrolysis reaction of a recombinant alkaline ceramidase of Pseudomonas aeruginosa. Biochim. Biophys. Acta 1485:111-120. [DOI] [PubMed] [Google Scholar]
- 66.Kowalski, M. P., and G. B. Pier. 2004. Localization of cystic fibrosis transmembrane conductance regulator to lipid rafts of epithelial cells is required for Pseudomonas aeruginosa-induced cellular activation. J. Immunol. 172:418-425. [DOI] [PubMed] [Google Scholar]
- 67.Krug, E. L., and C. Kent. 1984. Phospholipase C from Clostridium perfringens: preparation and characterization of homogeneous enzyme. Arch. Biochem. Biophys. 231:400-410. [DOI] [PubMed] [Google Scholar]
- 68.Krziwon, C., U. Zahringer, K. Kawahara, B. Weidemann, S. Kusumoto, E. T. Rietschel, H. D. Flad, and A. J. Ulmer. 1995. Glycosphingolipids from Sphingomonas paucimobilis induce monokine production in human mononuclear cells. Infect. Immun. 63:2899-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kuroda, M., T. Hashida-Okado, R. Yasumoto, K. Gomi, I. Kato, and K. Takesako. 1999. An aureobasidin A resistance gene isolated from Aspergillus is a homolog of yeast AUR1, a gene responsible for inositol phosphorylceramide (IPC) synthase activity. Mol. Gen. Genet. 261:290-296. [DOI] [PubMed] [Google Scholar]
- 70.Kusner, D. J. 2005. Mechanisms of mycobacterial persistence in tuberculosis. Clin. Immunol. 114:239-247. [DOI] [PubMed] [Google Scholar]
- 71.Labaied, M., A. Dagan, M. Dellinger, M. Geze, S. Egee, S. L. Thomas, C. Wang, S. Gatt, and P. Grellier. 2004. Anti-plasmodium activity of ceramide analogs. Malaria J. 3:49-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lauer, S., J. VanWye, T. Harrison, H. McManus, B. U. Samuel, N. L. Hiller, N. Mohandas, and K. Haldar. 2000. Vacuolar uptake of host components, and a role for cholesterol and sphingomyelin in malarial infection. EMBO J. 19:3556-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lauer, S. A., N. Ghori, and K. Haldar. 1995. Sphingolipid synthesis as a target for chemotherapy against malaria parasites. Proc. Natl. Acad. Sci. USA 92:9181-9185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lauer, S. A., P. K. Rathod, N. Ghori, and K. Haldar. 1997. A membrane network for nutrient import in red cells infected with the malaria parasite. Science 276:1122-1125. [DOI] [PubMed] [Google Scholar]
- 75.Leipelt, M., D. Warnecke, U. Zahringer, C. Ott, F. Muller, B. Hube, and E. Heinz. 2001. Glucosylceramide synthases, a gene family responsible for the biosynthesis of glucosphingolipids in animals, plants, and fungi. J. Biol. Chem. 276:33621-33629. [DOI] [PubMed] [Google Scholar]
- 76.Levery, S. B., M. Momany, R. Lindsey, M. S. Toledo, J. A. Shayman, M. Fuller, K. Brooks, R. L. Doong, A. H. Straus, and H. K. Takahashi. 2002. Disruption of the glucosylceramide biosynthetic pathway in Aspergillus nidulans and Aspergillus fumigatus by inhibitors of UDP-Glc:ceramide glucosyltransferase strongly affects spore germination, cell cycle, and hyphal growth. FEBS Lett. 525:59-64. [DOI] [PubMed] [Google Scholar]
- 77.Levery, S. B., M. S. Toledo, A. H. Straus, and H. K. Takahashi. 2001. Comparative analysis of glycosylinositol phosphorylceramides from fungi by electrospray tandem mass spectrometry with low-energy collision-induced dissociation of Li+ adduct ions. Rapid Commun. Mass Spectrom. 15:2240-2258. [DOI] [PubMed] [Google Scholar]
- 78.Liao, Z., L. M. Cimakasky, R. Hampton, D. H. Nguyen, and J. E. Hildreth. 2001. Lipid rafts and HIV pathogenesis: host membrane cholesterol is required for infection by HIV type 1. AIDS Res. Hum. Retrovir. 17:1009-1019. [DOI] [PubMed] [Google Scholar]
- 79.Liao, Z., D. R. Graham, and J. E. Hildreth. 2003. Lipid rafts and HIV pathogenesis: virion-associated cholesterol is required for fusion and infection of susceptible cells. AIDS Res. Hum. Retrovir. 19:675-687. [DOI] [PubMed] [Google Scholar]
- 80.Luberto, C., and Y. A. Hannun. 1999. Sphingolipid metabolism in the regulation of bioactive molecules. Lipids 34:S5-S11. [DOI] [PubMed] [Google Scholar]
- 81.Luberto, C., B. Martinez-Marino, D. Taraskiewicz, B. Bolanos, P. Chitano, D. L. Toffaletti, G. M. Cox, J. R. Perfect, Y. A. Hannun, E. Balish, and M. Del Poeta. 2003. Identification of App1 as a regulator of phagocytosis and virulence of Cryptococcus neoformans. J. Clin. Investig. 112:1080-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Luberto, C., M. J. Stonehouse, E. A. Collins, N. Marchesini, S. El-Bawab, A. I. Vasil, M. L. Vasil, and Y. A. Hannun. 2003. Purification, characterization, and identification of a sphingomyelin synthase from Pseudomonas aeruginosa: PlcH is a multifunctional enzyme. J. Biol. Chem. 278:32733-32743. [DOI] [PubMed] [Google Scholar]
- 83.Luberto, C., D. L. Toffaletti, E. A. Wills, S. C. Tucker, A. Casadevall, J. R. Perfect, Y. A. Hannun, and M. Del Poeta. 2001. Roles for inositol-phosphoryl ceramide synthase 1 (IPC1) in pathogenesis of Cryptococcus neoformans. Genes Dev. 15:201-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maddon, P. J., A. G. Dalgleish, J. S. McDougal, P. R. Clapham, R. A. Weiss, and R. Axel. 1986. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell 47:333-348. [DOI] [PubMed] [Google Scholar]
- 85.Malik, Z. A., G. M. Denning, and D. J. Kusner. 2000. Inhibition of Ca2+ signaling by Mycobacterium tuberculosis is associated with reduced phagosome-lysosome fusion and increased survival within human macrophages. J. Exp. Med. 191:287-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Malik, Z. A., C. R. Thompson, S. Hashimi, B. Porter, S. S. Iyer, and D. J. Kusner. 2003. Cutting edge: Mycobacterium tuberculosis blocks Ca2+ signaling and phagosome maturation in human macrophages via specific inhibition of sphingosine kinase. J. Immunol. 170:2811-2815. [DOI] [PubMed] [Google Scholar]
- 87.Mandala, S. M., R. A. Thornton, J. Milligan, M. Rosenbach, M. Garcia-Calvo, H. G. Bull, G. Harris, G. K. Abruzzo, A. M. Flattery, C. J. Gill, K. Bartizal, S. Dreikorn, and M. B. Kurtz. 1998. Rustmicin, a potent antifungal agent, inhibits sphingolipid synthesis at inositol phosphoceramide synthase. J. Biol. Chem. 273:14942-14949. [DOI] [PubMed] [Google Scholar]
- 88.Mandala, S. M., R. A. Thornton, M. Rosenbach, J. Milligan, M. Garcia-Calvo, H. G. Bull, and M. B. Kurtz. 1997. Khafrefungin, a novel inhibitor of sphingolipid synthesis. J. Biol. Chem. 272:32709-32714. [DOI] [PubMed] [Google Scholar]
- 89.Manes, S., G. del Real, R. A. Lacalle, P. Lucas, C. Gomez-Mouton, S. Sanchez-Palomino, R. Delgado, J. Alcami, E. Mira, and A. C. Martinez. 2000. Membrane raft microdomains mediate lateral assemblies required for HIV-1 infection. EMBO Rep. 1:190-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Manes, S., G. del Real, and A. C. Martinez. 2003. Pathogens: raft hijackers. Nat. Rev. Immunol. 3:557-568. [DOI] [PubMed] [Google Scholar]
- 91.Matsubara, T., A. Hayashi, Y. Banno, T. Morita, and Y. Nozawa. 1987. Cerebroside of the dimorphic human pathogen, Candida albicans. Chem. Phys Lipids 43:1-12. [DOI] [PubMed] [Google Scholar]
- 92.Matsuoka, S., H. Kimura, A. Kiuchi, H. Ohkawa, and K. Yagi. 1987. Purification and properties of a phospholipase C that has high activity toward sphingomyelin from Aspergillus saitoi. Biotechnol. Appl. Biochem. 9:401-409. [PubMed] [Google Scholar]
- 93.Mattner, J., K. L. Debord, N. Ismail, R. D. Goff, C. Cantu III, D. Zhou, P. Saint-Mezard, V. Wang, Y. Gao, N. Yin, K. Hoebe, O. Schneewind, D. Walker, B. Beutler, L. Teyton, P. B. Savage, and A. Bendelac. 2005. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434:525-529. [DOI] [PubMed] [Google Scholar]
- 94.Meyer, S. G., and H. de Groot. 2003. [14C]serine from phosphatidylserine labels ceramide and sphingomyelin in L929 cells: evidence for a new metabolic relationship between glycerophospholipids and sphingolipids. Arch. Biochem. Biophys. 410:107-111. [DOI] [PubMed] [Google Scholar]
- 95.Mille, C., G. Janbon, F. Delplace, S. Ibata-Ombetta, C. Gaillardin, G. Strecker, T. Jouault, P. A. Trinel, and D. Poulain. 2004. Inactivation of CaMIT1 inhibits Candida albicans phospholipomannan beta-mannosylation, reduces virulence, and alters cell wall protein beta-mannosylation. J. Biol. Chem. 279:47952-47960. [DOI] [PubMed] [Google Scholar]
- 96.Moss, J., P. H. Fishman, V. C. Manganiello, M. Vaughan, and R. O. Brady. 1976. Functional incorporation of ganglioside into intact cells: induction of choleragen responsiveness. Proc. Natl. Acad. Sci. USA 73:1034-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Munn, A. L., and H. Riezman. 1994. Endocytosis is required for the growth of vacuolar H+-ATPase-defective yeast: identification of six new END genes. J. Cell Biol. 127:373-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nagahama, M., K. Michiue, and J. Sakurai. 1996. Membrane-damaging action of Clostridium perfringens alpha-toxin on phospholipid liposomes. Biochim. Biophys. Acta 1280:120-126. [DOI] [PubMed] [Google Scholar]
- 99.Naka, T., N. Fujiwara, I. Yano, S. Maeda, M. Doe, M. Minamino, N. Ikeda, Y. Kato, K. Watabe, Y. Kumazawa, I. Tomiyasu, and K. Kobayashi. 2003. Structural analysis of sphingophospholipids derived from Sphingobacterium spiritivorum, the type species of genus Sphingobacterium. Biochim. Biophys. Acta 1635:83-92. [DOI] [PubMed] [Google Scholar]
- 100.Newhall, W. J. 1988. Macromolecular and antigenic composition of chlamydiae, p. 48-70. In A. L. Barron (ed.), Microbiology of Chlamydia. CRC, Boca Raton, Fla.
- 101.Nguyen, D. H., and J. E. Hildreth. 2000. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J. Virol. 74:3264-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nguyen, D. H., and D. D. Taub. 2004. Targeting lipids to prevent HIV infection. Mol. Interv. 4:318-320. [DOI] [PubMed] [Google Scholar]
- 103.Obeid, L. M., C. M. Linardic, L. A. Karolak, and Y. A. Hannun. 1993. Programmed cell death induced by ceramide. Science 259:1769-1771. [DOI] [PubMed] [Google Scholar]
- 104.Obeid, L. M., Y. Okamoto, and C. Mao. 2002. Yeast sphingolipids: metabolism and biology. Biochim. Biophys. Acta 1585:163-171. [DOI] [PubMed] [Google Scholar]
- 105.Ogretmen, B., and Y. A. Hannun. 2001. Updates on functions of ceramide in chemotherapy-induced cell death and in multidrug resistance. Drug Resist. Update 4:368-377. [DOI] [PubMed] [Google Scholar]
- 106.Okino, N., M. Tani, S. Imayama, and M. Ito. 1998. Purification and characterization of a novel ceramidase from Pseudomonas aeruginosa. J. Biol. Chem. 273:14368-14373. [DOI] [PubMed] [Google Scholar]
- 107.Ostroff, R. M., A. I. Vasil, and M. L. Vasil. 1990. Molecular comparison of a nonhemolytic and a hemolytic phospholipase C from Pseudomonas aeruginosa. J. Bacteriol. 172:5915-5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ostroff, R. M., B. Wretlind, and M. L. Vasil. 1989. Mutations in the hemolytic-phospholipase C operon result in decreased virulence of Pseudomonas aeruginosa PAO1 grown under phosphate-limiting conditions. Infect. Immun. 57:1369-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pankova-Kholmyansky, I., A. Dagan, D. Gold, Z. Zaslavsky, E. Skutelsky, S. Gatt, and E. Flescher. 2003. Ceramide mediates growth inhibition of the Plasmodium falciparum parasite. Cell Mol. Life Sci. 60:577-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Patton, J. L., B. Srinivasan, R. C. Dickson, and R. L. Lester. 1992. Phenotypes of sphingolipid-dependent strains of Saccharomyces cerevisiae. J. Bacteriol. 174:7180-7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Popik, W., T. M. Alce, and W. C. Au. 2002. Human immunodeficiency virus type 1 uses lipid raft-colocalized CD4 and chemokine receptors for productive entry into CD4+ T cells. J. Virol. 76:4709-4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ralton, J. E., K. A. Mullin, and M. J. McConville. 2002. Intracellular trafficking of glycosylphosphatidylinositol (GPI)-anchored proteins and free GPIs in Leishmania mexicana. Biochem. J. 363:365-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rittershaus, P. C., J. Allegood, T. B. Kechichian, A. H. J. Merrill, C. Luberto, and M. Del Poeta. 2005. Glucosylceramide is required for virulence of Cryptococcus neoformans. Submitted.
- 114.Rodrigues, M. L., L. R. Travassos, K. R. Miranda, A. J. Franzen, S. Rozental, W. de Souza, C. S. Alviano, and E. Barreto-Bergter. 2000. Human antibodies against a purified glucosylceramide from Cryptococcus neoformans inhibit cell budding and fungal growth. Infect. Immun. 68:7049-7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Saiman, L., G. Cacalano, D. Gruenert, and A. Prince. 1992. Comparison of adherence of Pseudomonas aeruginosa to respiratory epithelial cells from cystic fibrosis patients and healthy subjects. Infect. Immun. 60:2808-2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Saiman, L., and A. Prince. 1993. Pseudomonas aeruginosa pili bind to asialoGM1 which is increased on the surface of cystic fibrosis epithelial cells. J. Clin. Investig. 92:1875-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shea, J., J. Garcia, R. Iatta, A. Bielawska, J. Bielawski, C. Luberto, and M. Del Poeta. 2005. Abstr. 105th Gen. Meet. Am. Soc. Microbiol., F-026, p. 67.
- 118.Shin, J. S., Z. Gao, and S. N. Abraham. 2000. Involvement of cellular caveolae in bacterial entry into mast cells. Science 289:785-788. [DOI] [PubMed] [Google Scholar]
- 119.Spiegel, S., and S. Milstien. 2002. Sphingosine 1-phosphate, a key cell signaling molecule. J. Biol. Chem. 277:25851-25854. [DOI] [PubMed] [Google Scholar]
- 120.Spiegel, S., and S. Milstien. 2003. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat. Rev. Mol. Cell. Biol. 4:397-407. [DOI] [PubMed] [Google Scholar]
- 121.Stephens, R. S., S. Kalman, C. Lammel, J. Fan, R. Marathe, L. Aravind, W. Mitchell, L. Olinger, R. L. Tatusov, Q. Zhao, E. V. Koonin, and R. W. Davis. 1998. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science 282:754-759. [DOI] [PubMed] [Google Scholar]
- 122.Stoffel, W., K. Dittmar, and R. Wilmes. 1975. Sphingolipid metabolism in Bacteroideaceae. Hoppe-Seyler's Z. Physiol. Chem. 356:715-725. [DOI] [PubMed] [Google Scholar]
- 123.Susuki, K., M. Odaka, M. Mori, K. Hirata, and N. Yuki. 2004. Acute motor axonal neuropathy after Mycoplasma infection: evidence of molecular mimicry. Neurology 62:949-956. [DOI] [PubMed] [Google Scholar]
- 124.Takahashi, H. K., S. B. Levery, M. S. Toledo, E. Suzuki, M. E. Salyan, S. Hakomori, and A. H. Straus. 1996. Isolation and possible composition of glucosylceramides from Paracoccidioides brasiliensis. Braz. J. Med. Biol. Res. 29:1441-1444. [PubMed] [Google Scholar]
- 125.Thevissen, K., B. P. A. Cammue, K. Lemaire, J. Winderickx, R. C. Dickson, R. L. Lester, K. K. A. Ferket, F. Van Even, A. H. A. Parret, and W. F. Broekaert. 2000. A gene encoding a sphingolipid biosynthesis enzyme determines the sensitivity of Saccharomyces cerevisiae to an antifungal plant defensin from dahlia (Dahlia merckii). Proc. Natl. Acad. Sci. USA 97:9531-9536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Thevissen, K., J. Idkowiak-Baldys, Y. Im, J. Takemoto, I. E. J. A. Francois, K. K. A. Ferket, A. M. Aerts, E. M. K. Meert, J. Winderickx, J. Roosen, and B. P. A. Cammue. 2005. SKN1, a novel plant defensin-sensitivity gene in Saccharomyces cerevisiae, is implicated in sphingolipid biosynthesis. FEBS Lett. 579:1973-1977. [DOI] [PubMed] [Google Scholar]
- 127.Thevissen, K., D. C. Warnecke, I. E. Francois, M. Leipelt, E. Heinz, C. Ott, U. Zahringer, B. P. Thomma, K. K. Ferket, and B. P. Cammue. 2004. Defensins from insects and plants interact with fungal glucosylceramides. J. Biol. Chem. 279:3900-3905. [DOI] [PubMed] [Google Scholar]
- 128.Thompson, C. R., S. S. Iyer, N. Melrose, R. VanOosten, K. Johnson, S. M. Pitson, L. M. Obeid, and D. J. Kusner. 2005. Sphingosine kinase 1 (SK1) is recruited to nascent phagosomes in human macrophages: inhibition of SK1 translocation by Mycobacterium tuberculosis. J. Immunol. 174:3551-3561. [DOI] [PubMed] [Google Scholar]
- 129.Toledo, M. S., S. B. Levery, J. Glushka, A. H. Straus, and H. K. Takahashi. 2001. Structure elucidation of sphingolipids from the mycopathogen Sporothrix schenckii: identification of novel glycosylinositol phosphorylceramides with core manα1→6Ins linkage. Biochem. Biophys. Res. Commun. 280:19-24. [DOI] [PubMed] [Google Scholar]
- 130.Toledo, M. S., S. B. Levery, A. H. Straus, E. Suzuki, M. Momany, J. Glushka, J. M. Moulton, and H. K. Takahashi. 1999. Characterization of sphingolipids from mycopathogens: factors correlating with expression of 2-hydroxy fatty acyl (E)-Delta 3-unsaturation in cerebrosides of Paracoccidioides brasiliensis and Aspergillus fumigatus. Biochemistry 38:7294-7306. [DOI] [PubMed] [Google Scholar]
- 131.Toledo, M. S., S. B. Levery, E. Suzuki, A. H. Straus, and H. K. Takahashi. 2001. Characterization of cerebrosides from the thermally dimorphic mycopathogen Histoplasma capsulatum: expression of 2-hydroxy fatty N-acyl (E)-Delta(3)-unsaturation correlates with the yeast-mycelium phase transition. Glycobiology 11:113-124. [DOI] [PubMed] [Google Scholar]
- 132.Toledo, M. S., E. Suzuki, S. B. Levery, A. H. Straus, and H. K. Takahashi. 2001. Characterization of monoclonal antibody MEST-2 specific to glucosylceramide of fungi and plants. Glycobiology 11:105-112. [DOI] [PubMed] [Google Scholar]
- 133.Trinel, P. A., E. Maes, J. P. Zanetta, F. Delplace, B. Coddeville, T. Jouault, G. Strecker, and D. Poulain. 2002. Candida albicans phospholipomannan, a new member of the fungal mannose inositol phosphoceramide family. J. Biol. Chem. 277:37260-37271. [DOI] [PubMed] [Google Scholar]
- 134.Van der Vliet, H. J., J. W. Molling, B. M. von Blomberg, N. Nishi, W. Kolgen, A. J. van den Eertwegh, H. M. Pinedo, G. Giaccone, and R. J. Scheper. 2004. The immunoregulatory role of CD1d-restricted natural killer T cells in disease. Clin. Immunol. 112:8-23. [DOI] [PubMed] [Google Scholar]
- 135.Van Heyningen, W. E., C. C. Carpenter, N. F. Pierce, and W. B. Greenough III. 1971. Deactivation of cholera toxin by ganglioside. J. Infect. Dis. 124:415-418. [DOI] [PubMed] [Google Scholar]
- 136.Van Kaer, L. 2005. α-Galactosylceramide therapy for autoimmune diseases: prospects and obstacles. Nat. Rev. Immunol. 5:31-42. [DOI] [PubMed] [Google Scholar]
- 137.Van Ooij, C., L. Kalman, I. van, M. Nishijima, K. Hanada, K. Mostov, and J. N. Engel. 2000. Host cell-derived sphingolipids are required for the intracellular growth of Chlamydia trachomatis. Cell Microbiol. 2:627-637. [DOI] [PubMed] [Google Scholar]
- 138.Villas Boas, M. H., H. Egge, G. Pohlentz, R. Hartmann, and E. B. Bergter. 1994. Structural determination of N-2′-hydroxyoctadecenoyl-1-O-β-d-glucopyranosyl-9-methyl-4, 8-sphingadienine from species of Aspergillus. Chem. Phys. Lipids 70:11-19. [DOI] [PubMed] [Google Scholar]
- 139.Warnecke, D., and E. Heinz. 2003. Recently, discovered functions of glucosylceramides in plants and fungi. Cell Mol. Life Sci. 60:919-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Watanabe, Y., M. Nakajima, T. Hoshino, K. Jayasimhulu, E. E. Brooks, and E. S. Kaneshiro. 2001. A novel sphingophosphonolipid head group 1-hydroxy-2-aminoethyl phosphonate in Bdellovibrio stolpii. Lipids 36:513-519. [DOI] [PubMed] [Google Scholar]
- 141.Wells, G. B., R. C. Dickson, and R. L. Lester. 1996. Isolation and composition of inositolphosphorylceramide-type sphingolipids of hyphal forms of Candida albicans. J. Bacteriol. 178:6223-6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.White, D. C., S. D. Sutton, and D. B. Ringelberg. 1996. The genus Sphingomonas: physiology and ecology. Curr. Opin. Biotechnol. 7:301-306. [DOI] [PubMed] [Google Scholar]
- 143.Wilson, W. H., D. C. Schroeder, M. J. Allen, M. T. G. Holden, J. Parkhill, B. G. Barrell, C. Churcher, N. Hamlin, K. Mungall, H. Norbertczak, M. A. Quail, C. Price, E. Rabbinowitsch, D. Walker, M. Craigon, D. Roy, and P. Ghazal. 2005. Complete genome sequence and lytic phase transcription profile of a coccolithovirus. Science 309:1090-1092. [DOI] [PubMed] [Google Scholar]
- 144.Wu, D., G. W. Xing, M. A. Poles, A. Horowitz, Y. Kinjo, B. Sullivan, V. Bodmer-Narkevitch, O. Plettenburg, M. Kronenberg, M. Tsuji, D. D. Ho, and C. H. Wong. 2005. Bacterial glycolipids and analogs as antigens for CD1d-restricted NKT cells. Proc. Natl. Acad. Sci. USA 102:1351-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wylie, J., G. Hatch, and G. McClarty. 1997. Host cell phospholipids are trafficked to and then modified by Chlamydia trachomatis. J. Bacteriol. 179:7233-7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yabuuchi, E., I. Yano, H. Oyaizu, Y. Hashimoto, T. Ezaki, and H. Yamamoto. 1990. Proposals of Sphingomonas paucimobilis gen. nov. and comb. nov., Sphingomonas parapaucimobilis sp. nov., Sphingomonas yanoikuyae sp. nov., Sphingomonas adhaesiva sp. nov., Sphingomonas capsulata comb. nov., and two genospecies of the genus Sphingomonas. Microbiol. Immunol. 34:99-119. [DOI] [PubMed] [Google Scholar]
- 147.Yano, I., S. Imaizumi, I. Tomiyasu, and E. Yabuuchi. 1983. Separation and analysis of free ceramides containing 2-hydroxy fatty acids in Sphingobacterium species. FEMS Microbiol. Lett. 20:449-453. [Google Scholar]
- 148.Yano, I., I. Tomiyasu, and E. Yabuuchi. 1982. Long chain base composition of strains of three species of Sphingobacterium gen. nov. FEMS Microbiol. Lett. 15:303-307. [Google Scholar]
- 149.Yowler, B. C., and C. L. Schengrund. 2004. Glycosphingolipids: sweets for botulinum neurotoxin. Glycoconj. J. 21:287-293. [DOI] [PubMed] [Google Scholar]
- 150.Yuki, N., K. Susuki, M. Koga, Y. Nishimoto, M. Odaka, K. Hirata, K. Taguchi, T. Miyatake, K. Furukawa, T. Kobata, and M. Yamada. 2004. Carbohydrate mimicry between human ganglioside GM1 and Campylobacter jejuni lipooligosaccharide causes Guillain-Barre syndrome. Proc. Natl. Acad. Sci. USA 101:11404-11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zanolari, B., S. Friant, K. Funato, C. Sutterlin, B. J. Stevenson, and H. Riezman. 2000. Sphingoid base synthesis requirement for endocytosis in Saccharomyces cerevisiae. EMBO J. 19:2824-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang, K., F. F. Hsu, D. A. Scott, R. Docampo, J. Turk, and S. M. Beverley. 2005. Leishmania salvage and remodeling of host sphingolipids in amastigote survival and acidocalcisome biogenesis. Mol. Microbiol. 55:1566-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang, K., M. Showalter, J. Revollo, F. F. Hsu, J. Turk, and S. M. Beverley. 2003. Sphingolipids are essential for differentiation but not growth in Leishmania. EMBO J. 22:6016-6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhong, W., M. W. Jeffries, and N. H. Georgopapadakou. 2000. Inhibition of inositol phosphorylceramide synthase by aureobasidin A in Candida and Aspergillus species. Antimicrob. Agents Chemother. 44:651-653. [DOI] [PMC free article] [PubMed] [Google Scholar]