Abstract
Mycoplasma hyopneumoniae is the causative agent of porcine enzootic pneumonia, a chronic and economically significant respiratory disease that affects swine production worldwide. M. hyopneumoniae adheres to and adversely affects the function of ciliated epithelial cells of the respiratory tract, and the cilium adhesin (Mhp183, P97) is intricately but not exclusively involved in this process. Although binding of pathogenic bacteria to glycosaminoglycans is a recognized step in pathogenesis, knowledge of glycosaminoglycan-binding proteins in M. hyopneumoniae is lacking. However, heparin and other sulfated polysaccharides are known to block the binding of M. hyopneumoniae to purified swine respiratory cilia. In this study, four regions within the cilium adhesin were examined for the ability to bind heparin. Cilium adhesin fragments comprising 653 amino acids of the N terminus and 301 amino acids of the C terminus (containing two repeat regions, R1 and R2) were cloned and expressed. These fragments bound heparin in a dose-dependent and saturable manner with physiologically significant binding affinities of 0.27 ± 0.02 μM and 1.89 ± 0.33 μM, respectively. Heparin binding of both fragments was strongly inhibited by the sulfated polysaccharides fucoidan and mucin but not by chondroitin sulfate B. When the C-terminal repeat regions R1 and R2 were cloned separately and expressed, heparin-binding activity was lost, suggesting that both regions are required for heparin binding. The ability of the cilium adhesin to bind heparin indicates that this molecule plays a multifunctional role in the adherence of M. hyopneumoniae to host respiratory surfaces and therefore has important implications with respect to the pathogenesis of this organism.
Mycoplasma hyopneumoniae is the etiological agent of porcine enzootic pneumonia, a chronic respiratory disease that causes significant economic losses to the swine industry (30). Infections are established via colonization of porcine respiratory epithelia, a process initiated by the adherence of bacterial cells to host cilia. Successful colonization results in ciliostasis and shedding of cilia from the epithelial surface, thereby disrupting the mucociliary escalator and leaving the host susceptible to secondary infections (3, 9).
Studies have shown that the cilium adhesin P97 is essential for the adherence of M. hyopneumoniae and the onset of disease (15, 37). The cilium adhesin gene (mhp183) encodes a 126-kDa preprotein. The full-length protein is barely present in in vitro-grown cells due to posttranslational processing. A major cleavage event at position 195 generates the functional adhesin (P97) and the N-terminal fragment (P22). P97 is further cleaved to yield a host of other peptides (10). While the functions of these peptides (apart from P97) are unknown, proteomic and immunogold studies demonstrate that they remain associated with the cell surface or the surrounding matrix (10).
Like several other characterized mycoplasma adhesins, P97 contains repetitive elements within the C-terminal portion of its amino acid sequence (4, 8, 15). P97 contains two such repeat regions, designated R1 and R2, with R1 (sequence AAKPV/E) being identified as the cilium-binding epitope (16). Size variation in the cilium adhesin protein of different M. hyopneumoniae strains (95 to 97 kDa) has been accounted for by differences in the number of copies of R1, which varies from 9 in the nonadherent J strain to 15 in the pathogenic 232 strain (35). A minimum of eight copies of the R1 repeat is necessary for cilium binding, but this is not sufficient to confer the ability to adhere in vivo (16, 23). These data suggest that there are additional proteins and mechanisms involved in adherence to host epithelia.
The importance of binding to host extracellular matrix (ECM) components in initiating pathogenic processes has been well documented. By binding to ECM proteins such as fibronectin, vitronectin, laminin, and collagen, pathogenic microbes are able to mediate interactions with host cells (27) and, in some cases, enhance their invasive potential (12, 18, 28). Furthermore, it has been demonstrated that many pathogens are able to recruit exogenous glycosaminoglycans (e.g., heparin, heparan sulfate, and chondroitin sulfate B) to their surfaces to facilitate interactions between the pathogen and host ECM components, which are themselves glycosaminoglycan-binding proteins (13, 21, 34). Binding to glycosaminoglycans is also believed to enable pathogenic bacteria to evade the host immune system (7, 12) and modulate the inflammatory response (13) by allowing bridging interactions with host chemokines and cytokines. Thus, glycosaminoglycan-binding proteins are considered to be of central importance to the infection process.
Previous experiments have revealed that adherence of purified recombinant P97 to cilia is inhibited by sulfated glycosaminoglycans (15). These findings suggest a possible interaction between the P97 protein and sulfated glycosaminoglycans, which may act as receptor analogs for the M. hyopneumoniae adhesin (15, 36, 38); however; these studies did not directly demonstrate such an interaction. Furthermore, the domains within the cilium adhesin responsible for this interaction were not identified. In this study, we identify regions within the cilium adhesin that interact with the glycosaminoglycan heparin. The ability of M. hyopneumoniae cell surface molecules to bind heparin may play a significant role in the pathogenesis of this organism.
MATERIALS AND METHODS
Bacterial strains and plasmids.
M. hyopneumoniae strain J (NCTC 10110) was cultivated to mid-log phase (medium pH range, 6.8 to 7.2) in modified Friis broth at 37°C on a rotary shaker as described previously (33). The Escherichia coli plasmid expression and maintenance strains were M15(pREP4) and JM109, respectively, and they were grown in Luria-Bertani medium (31) at 37°C. Broth cultures were incubated on a rotary shaker at 200 to 250 rpm to achieve aeration. For selection of transformants, Luria-Bertani medium was supplemented with 100 μg/ml ampicillin and 50 μg/ml kanamycin as required.
DNA extraction.
Chromosomal DNA was extracted from the J strain of M. hyopneumoniae by a phenol-chloroform method, followed by dialysis against TE buffer as described previously (11). Plasmid DNA was extracted from E. coli with either the QIAGEN mini-prep or midi-prep kit according to the manufacturer's instructions (QIAGEN, Alameda, Calif.).
Plasmid constructs.
Adhesin molecules from two different strains of M. hyopneumoniae were used to generate polyhistidine fusion proteins of different regions of the coding sequence. Because UGA codons encode tryptophan in mycoplasmas, they cause translation problems in E. coli, resulting in premature stops during the expression of cloned sequences. To circumvent this problem, three clones were generated by amplifying chromosomal DNA from strain J with PCR primers designed to avoid in-frame TGA codons. An additional construct was generated with a clone containing a large fragment of the P97 sequence from strain P5722 (19) in which the TGA codons had been mutagenized to TGGs. This approach was deemed valid because the sequence difference between these two P97 homologs is less than 4%, with most of the difference accounted for by variation in the number of R1 and R2 repeats.
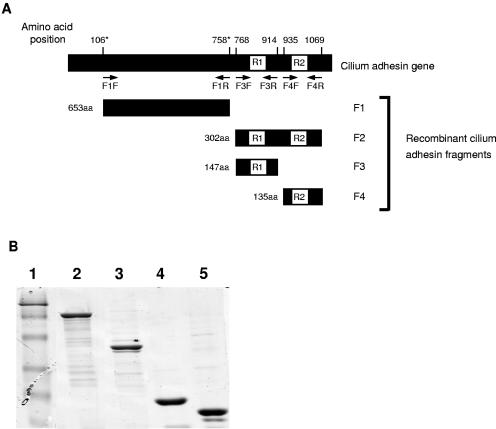
The first construct contained the region coding for the N terminus of the cilium adhesin (amino acids 106 to 758 of the mutagenized gene sequence) and was referred to as F1P97. The PCR template for this construct was pISM405, in which the TGA codons had been converted to TGGs (10). The second construct, referred to as F2P97 (amino acids 768 to 1069 of the J strain sequence), contained the cilium adhesin repeat domains coding for both R1, the cilium-binding epitope, and R2. This gene fragment was further subcloned to yield two additional constructs referred to as F3P97 (amino acids 768 to 914 of the J strain sequence) and F4P97 (amino acids 935 to 1069 of the J strain sequence), which contained the R1 and R2 regions, respectively (Fig. 1).
FIG. 1.
(A) Diagrammatic representation of the cilium adhesin gene. Arrows indicate the locations of primers used to amplify DNA fragments coding for F1P97 to F4P97. Amino acid (aa) positions corresponding to the N and C termini of F1P97 to F4P97 are indicated. Numbers marked with asterisks indicate positions corresponding to the cilium adhesin of M. hyopneumoniae strain P5722 (GenBank accession no. AAB03497); all other numbers correspond to the cilium adhesin of M. hyopneumoniae strain J (GenBank accession no. AAC35402). The F1P97 to F4P97 adhesin fragment sizes are indicated. (B) SDS-polyacrylamide gel showing the purified, recombinant F1P97 to F4P97 cilium adhesin fragments. Lane 1, prestained protein molecular weight marker (MBI Fermentas, Hanover, Maryland; band sizes correspond to 118, 86, 47, 34, 26, and 19 kDa); lane 2, F1P97; lane 3, F2P97; lane 4, F3P97; lane 5, F4P97. All cilium adhesin fragments run at higher molecular weights than predicted due to abnormal migration patterns.
To clone specific sequences, gene fragments were amplified via PCR with Taq polymerase (QIAGEN). The primers used for amplification of the gene fragments encoding F1P97, F3P97, and F4P97 were F1F (5′-GGGGATCCCAAGATCCTGAATATACC) and F1R (5′-GGCTGCAGTTAGGCTGCTTTAAGGAAAAATGC) (underlined sequences represent BamHI and PstI restriction sites, respectively), F3F (5′-GGGTCGACAAATTAGACGATAATCTTCAG) and F3R (5′-GGCTGCAGTTACTCGCTTTGATGAACTAGTTC) (underlined sequences represent SalI and PstI restriction sites, respectively), and F4F (5′-GGGGATCCCAGGAAGTCAAGGTAACTAGT) and F4R (5′-GGCTGCAGCCCGGGTTAGGATCACCGGATTTTGAATC) (underlined sequences represent BamHI and PstI restriction sites, respectively). Primers F3F and F4R were used to amplify the gene fragment corresponding to F2P97. Cilium adhesin fragments F1P97 to F4P97 were digested with the appropriate restriction enzymes and ligated into linearized pQE-9 with 1 U of T4 DNA ligase (Roche, Basel, Switzerland) at 10°C overnight. Ligated plasmids were electroporated into E. coli M15(pREP4) cells with a Bio-Rad Gene Pulser (Bio-Rad Laboratories, Hercules, Calif.) at 2.5 kV, 25 μF, and 200 Ω according to the manufacturer's instructions. All plasmid constructs were confirmed via DNA sequencing.
Protein expression and purification.
All cilium adhesin fragments were expressed as hexahistidyl fusion proteins and purified by nickel affinity chromatography. Briefly, E. coli M15(pREP4) cells were grown to mid-log phase and induced with a final concentration of isopropyl-β-d-thiogalactopyranoside of 1 mM. Cells were harvested by centrifugation (4,000 × g for 20 min) and then lysed in 8 M urea buffer (8 M urea, 0.01 M Tris, 0.1 M NaH2PO4, pH 8.0) with gentle rocking for 1 h. Cell debris was pelleted by centrifugation at 10,000 × g for 30 min. To the supernatant, 0.25 volume of a 50% Ni-nitrilotriacetic acid slurry (QIAGEN) was added and the solution was allowed to mix gently for 1 h. The solution was loaded onto a glass column and washed twice with 8 M urea buffer (pH 6.3) to remove unbound protein. Bound protein was eluted with low-pH urea buffers (pH 5.9 and pH 4.5). Proteins were analyzed for purity by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and then dialyzed for 48 h with multiple buffer changes against phosphate-buffered saline (PBS; 10 mM sodium phosphate, 150 mM sodium chloride, pH 7.4)-0.1% SDS or PBS-5% glycerol to remove the urea. The concentration of the purified proteins was estimated by the Bradford assay (Bio-Rad Laboratories).
Production of polyclonal antisera.
Antisera to the F1P97 to F4P97 adhesin fragments were generated via primary and secondary intramuscular injections of antigen into New Zealand White rabbits at 2-week intervals. Antigens (approximately 0.5 mg) were prepared for injection by mixing equal volumes of purified protein (approximately 500 μl) and Freund's incomplete adjuvant (Sigma Aldrich, St. Louis, Missouri). Preimmune serum was collected prior to the primary injection and served as control antiserum. A trial bleeding was performed 10 to 14 days after the secondary injection of antigen, and immune responses to the antigen at this stage were assessed via immunoblotting. Positive sera were collected via cardiac bleeding, during which the rabbits were anesthetized and euthanized as previously described (32).
Heparin-binding assays.
For ligand dot blot assays, purified proteins were diluted to 5 μg/ml in PBS. To denature samples, proteins were diluted in cracking buffer (60 mM Tris, pH 6.8, 1% SDS, 1% β-mercaptoethanol, 10% glycerol) and boiled for 5 min. A 100-μl volume of each sample was spotted onto a Hybond-C super nitrocellulose membrane (Amersham Pharmacia Biotech, Uppsala, Sweden) that had been prewetted in Tris-buffered saline (TBS; 10 mM Tris, 150 mM sodium chloride, pH 7.4) with a Bio-Dot microfiltration apparatus (Bio-Rad Laboratories). The membrane was blocked with 300 μl per well of blocking solution (1% bovine serum albumin [BSA] in TBS) and then washed twice under vacuum with 300 μl per well of TTBS wash solution (0.05% Tween 20 in TBS). The membrane was removed from the manifold and placed in a dish containing 100 μg/ml biotinylated heparin (Sigma) diluted in 1% BSA-TTBS. The membrane was incubated for 1 h with gentle rocking and then washed 3 × 10 min in TTBS. Streptavidin peroxidase (Roche) was diluted 1:3,000 in 1% BSA-TTBS and added to the membrane. After 1 h of incubation, the blot was washed 3 × 10 min with TTBS. The membrane was equilibrated with 100 mM Tris (pH 7.6) and developed with 3,3′-diaminobenzidine (Sigma) solution. To ensure that equivalent amounts of protein had been blotted in each position, a duplicate blot was stained with amido black protein stain (0.1% amido black, 7% [vol/vol] acetic acid) immediately after spotting of the proteins onto nitrocellulose.
For microtiter plate binding assays, 96-well plates (Linbro/Titertek; ICN Biomedicals Inc., Aurora, Ohio) were coated overnight in a humidified chamber with 0.5 μg per well purified protein diluted in 100 μl carbonate coating buffer (18 mM NaHCO3, 27 mM Na2CO3, pH 9.5). Following overnight incubation, the plates were washed five times with PBS-0.2% Tween 20 (200 μl wash solution per well) with an automatic 96PW plate washer (SLT Labinstruments, Crailsheim, Germany). The wells were blocked with 100 μl of blocking solution (2% skim milk in PBS) for 1 h in a humidified chamber. The plates were washed five times, and biotinylated heparin which had been prediluted in 1% skim milk-PBS was added in increasing concentrations to the wells. Final concentrations of biotinylated heparin were 0, 1, 5, 10, 20, 50, and 100 μg/ml. Aliquots of 100 μl of each concentration were added to microtiter plate wells; the plates were then incubated for 1 h. After washing the plates as described above, 100 μl of streptavidin-peroxidase (Roche) diluted 1:3,000 in 1% skim milk-PBS was added to each well and the mixture was incubated for 1 h. The plates were washed and developed with 1 mM 2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) substrate (Sigma) in citrate buffer (100 mM citric acid, 200 mM Na2HPO4, pH 4.2) which had been activated with hydrogen peroxide. Plates were developed with shaking on a Titramax 1000 microtiter plate shaker (Heidolph, Schwabach, Germany), and the optical density at 414 nm was measured with a Multiskan Ascent plate reader (Thermo Labsystems, Franklin, Mass.) at 7-, 15-, 25-, and 45-min intervals.
Microtiter plate assays for determining the specificity of heparin binding were performed as described above but with serial twofold dilutions of biotinylated heparin, beginning at a saturating concentration (100 μg/ml for F1P97 and 50 μg/ml for F2P97), each of which was premixed with a 50-fold excess of unlabeled heparin prior to addition to the plates.
Competitive-binding assays were also performed as above but with the addition of a 1-, 5-, 10-, 20-, 30-, 40-, or 50-fold excess of a range of sulfated polysaccharides. Inhibitors were premixed with biotinylated heparin immediately prior to addition to the wells and included unlabeled heparin, fucoidan, mucin, and chondroitin sulfate B (Sigma). In all microtiter plate assays, controls were performed with uncoated wells, wells where no heparin was added, or wells where no streptavidin-peroxidase was added. Controls which employed protein-specific antiserum and horseradish peroxidase-labeled anti-rabbit immunoglobulin G (Chemicon, Temecula, Calif.) were also conducted to ensure that proteins were adhering to the microtiter plate wells. All experiments were performed in triplicate. Results were graphed by GraphPad Prism version 4.02 for Windows (GraphPad Software, San Diego, Calif.; www.graphpad.com) by nonlinear regression.
RESULTS
Bioinformatic identification of putative heparin-binding motifs.
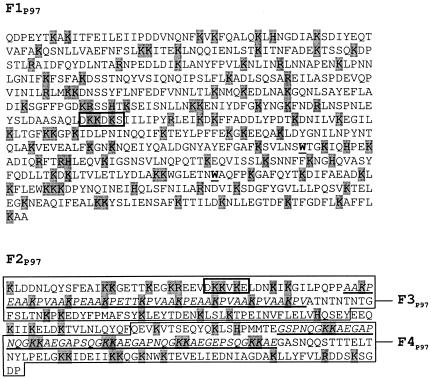
The cilium adhesin was examined for potential heparin-binding sites within its amino acid sequence by using established criteria (5, 6). In many instances, glycosaminoglycan-binding proteins are reported to contain consensus sequences of two types: XBBXBX and XBBBXXBX, where B represents a basic residue and X is any other amino acid. The F1P97 and F2P97-F3P97 domains of the cilium adhesin each contain one motif that matches the former consensus sequence (DKKDKS and DKKVKE, respectively) (Fig. 2). Several heparin-binding proteins have been identified that do not contain the consensus sequences but possess sequences that are relatively rich in basic residues (20, 29). Both F1P97 and F2P97 also contain relatively large numbers of basic amino acids across the lengths of their sequences (Fig. 2).
FIG. 2.
Amino acid sequences of F1P97 to F4P97. The sequences shown correspond to the M. hyopneumoniae strains and numbering described in Fig. 1A. F3P97 and F4P97 are indicated by large boxes. Small boxes indicate motifs matching the heparin-binding consensus sequences. Basic residues are shaded. Tryptophan residues that represent mutagenized TGG codons are underlined. Repeat regions 1 and 2 (R1 and R2) are italicized and underscored.
Heparin-binding assays.
To determine whether the cilium adhesin fragments bound to heparin and to assess the importance of their protein conformation in this process, ligand blot assays were performed. Standard ligand blot assays involving the transfer of proteins from denaturing polyacrylamide gels to polyvinylidene difluoride gave only weak or unreliable results (data not shown). In a dot blot assay, strong and reproducible reactions with biotinylated heparin were observed for undenatured F1P97 and F2P97, while a weak reaction was observed for undenatured F4P97 (Fig. 3). Interestingly, the same protein fragments did not show a strong reaction with heparin when they were denatured prior to blotting, indicating that protein conformation may play a role in the interaction with heparin.
FIG. 3.
Dot blot assay of denatured and undenatured cilium adhesin fragments reacted with biotinylated heparin (A) and stained with amido black (B). Position 1, undenatured F1P97; position 2, denatured F1P97; position 3, undenatured F2P97; position 4, denatured F2P97; position 5, undenatured F3P97; position 6, denatured F3P97; position 7, undenatured F4P97; position 8, denatured F4P97.
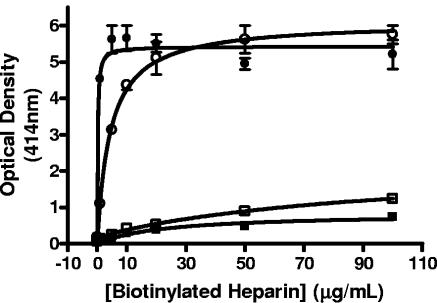
Quantification of heparin binding by the cilium adhesin domains and determination of binding affinities was performed by a microtiter plate binding assay. Recombinant cilium adhesin fragments F1P97 to F4P97 were shown to reliably bind to microtiter plates by interacting with fragment-specific antibodies (data not shown). Experiments were then performed with increasing amounts of biotinylated heparin up to a saturating concentration. Figure 4 indicates the total binding (specific and nonspecific) of the cilium adhesin fragments to heparin. Both F1P97 and F2P97 were found to bind heparin in a dose-dependent and saturable manner. In contrast, F3P97 and F4P97 showed low-level, nonsaturable binding, indicating a nonspecific interaction. Interestingly, despite being subfragments of F2P97, F3P97 and F4P97 continued to show low-level binding when pooled and assayed for heparin-binding activity (data not shown). This result supports the ligand dot blot data, which suggest that the conformation of F2P97 is essential for heparin binding.
FIG. 4.
Binding of biotinylated heparin to immobilized cilium adhesin domains F1P97 (•), F2P97 (○), F3P97 (▪), and F4P97 (□). Increasing concentrations of biotinylated heparin were added until saturation was reached. Absorbances were read after 45 min of incubation in the presence of substrate. Error bars indicate the standard deviation from the mean for triplicate readings. Replicate experiments resulted in similar binding curves.
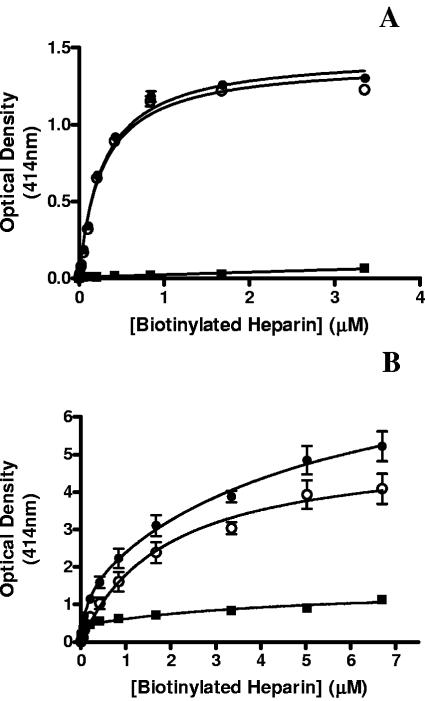
To determine whether the binding of F1P97 and F2P97 to heparin was the result of a specific interaction, binding assays were undertaken in which biotinylated heparin was added in the presence or absence of a 50-fold concentration of unlabeled heparin. Binding of heparin to both F1P97 and F2P97 was shown to be highly specific with minimal nonspecific binding observed (Fig. 5A and B). Affinity constants were determined by using the specific binding data (total binding minus nonspecific binding) and were found to be 0.27 ± 0.02 μM and 1.89 ± 0.33 μM for F1P97 and F2P97, respectively. These values are comparable to those derived from replicate experiments.
FIG. 5.
Heparin-binding curves for F1P97 (A) and F2P97 (B) showing total binding (•), nonspecific binding (▪), and specific binding (○). Total binding was determined by the standard microtiter plate assay protocol, while nonspecific binding was determined in the presence of a 50-fold excess of unlabeled heparin. The specific binding curves were derived by subtracting the nonspecific binding data from the total binding data. Error bars indicate the standard deviation from the mean for triplicate readings.
Inhibition of heparin binding by sulfated polysaccharides.
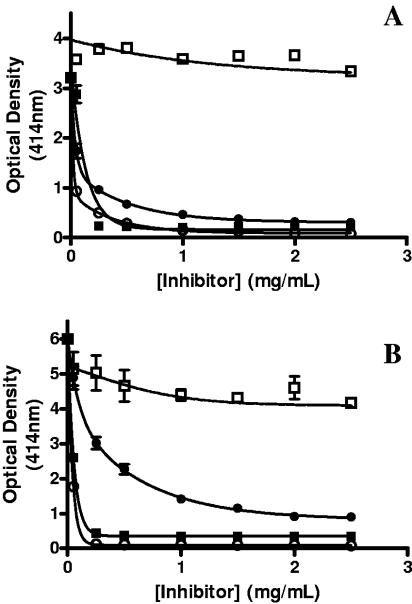
The nature of the interaction between the cilium adhesin fragments and heparin was further characterized in competitive-binding experiments with the sulfated polysaccharides heparin, fucoidan, mucin, and chondroitin sulfate B. For both F1P97 and F2P97, biotinylated heparin binding was significantly inhibited by heparin. Furthermore, for both F1P97 and F2P97, fucoidan and mucin were shown to be strong competitors while chondroitin sulfate B did not inhibit the interaction of heparin with either adhesin domain (Fig. 6A and B). Notably, both fucoidan and mucin gave stronger inhibition than unlabeled heparin. These results suggest that both F1P97 and F2P97 are able to bind fucoidan and mucin in addition to heparin.
FIG. 6.
Competitive-binding assays showing inhibition of heparin binding to F1P97 (A) and F2P97 (B) by various sulfated polysaccharides, i.e., unlabeled heparin (•), fucoidan (▪), mucin (○), and chondroitin sulfate B (□). Microtiter plate binding assays were performed by the standard protocol but with an excess of each inhibitor. Error bars indicate the standard deviation from the mean for triplicate readings.
DISCUSSION
In order to establish infection, bacterial pathogens must first adhere to and colonize host epithelia. For many bacteria, the initial site of contact with host tissues is at the level of the ECM; therefore, the ability to bind components of the ECM is widespread among pathogenic microbes (12, 13, 22, 34). Because of their fastidious nature and obligate parasitic mode of life, mycoplasmas require particularly efficient mechanisms for adherence. For M. hyopneumoniae, knowledge about the mechanisms of adhesion to host tissues is currently confined to the cilium adhesin and its interaction with the cilia of the porcine respiratory tract (15, 17, 23, 37). No ECM-binding proteins have been identified in this organism; however, previous studies indicated that binding of the cilium adhesin to cilia could be inhibited by heparin, providing indirect evidence that the adhesin is able to bind this molecule (15, 38).
In this study, two nonoverlapping regions of the M. hyopneumoniae cilium adhesin, F1P97 and F2P97, were shown to bind heparin in a dose-dependent, saturable manner. These results indicate that there are at least two distinct heparin-binding domains within the adhesin protein. Both of these regions contain clusters of basic amino acids that match the consensus for glycosaminoglycan-binding motifs (6). One of these protein regions, F2P97, contains both of the cilium adhesin repeat regions, R1 and R2. The heparin-binding consensus motif of F2P97 lies within the F3P97 region (Fig. 1A), which suggests that F3P97 should also bind heparin. As F3P97 was not shown to bind heparin in either the ligand blot or the microtiter plate assay, it must be concluded that the heparin-binding motif in F2P97 alone was not sufficient to confer binding activity, indicating that additional sequences are required. F4P97 also failed to show any significant interaction with heparin in microtiter plate assays, indicating that while R1 and R2 are unable to bind heparin independently, the intact F2P97 fragment (containing both of these regions) displays heparin-binding activity. The fact that both of the cilium adhesin repeat regions are required for heparin binding suggests that this interaction is conformation dependent rather than due to the presence of a specific linear amino acid motif. Furthermore, the idea that protein conformation plays a role in heparin binding is supported by the ligand blot assay data, which demonstrated that undenatured F1P97 and F2P97 were able to bind heparin while the denatured forms were unable to do so.
Alternatively, since 20 amino acids (914 to 935) were omitted during the construction of F3P97 and F4P97, it is possible that this sequence may be responsible for the heparin-binding activity observed for F2P97. However, an analysis of this sequence failed to identify a heparin-binding motif or a basic amino acid density that might facilitate binding.
Homologues of the cilium adhesin Mhp183 among different M. hyopneumoniae strains are highly conserved, with the majority of sequence changes resulting from variations in the number of repeats within the R1 and R2 regions. Because these repeat regions are contained within the heparin-binding F2P97 fragment, the cilium adhesins from different strains of M. hyopneumoniae might be expected to bind heparin with various affinities. The M. hyopneumoniae J strain used in this study has a cilium adhesin with only nine copies of the R1 repeat, while pathogenic strains have been shown to contain up to 15 copies. An increase in the number of copies of R1 may therefore confer an even greater affinity for heparin since this repeat is rich in basic residues. Further experiments are required to confirm this.
Studies on the proteolytic cleavage of Mhp183 have revealed a complex processing pattern. One major processing event cleaves an N-terminal 22-kDa fragment (P22), generating the “mature” P97 adhesin (10, 37). A second major event (at position 862 for the J strain) removes a C-terminal 28-kDa fragment (P28) that separates the cilium-binding epitope, R1, from R2. This event generates a central adhesin fragment that contains R1 and is approximately 66 kDa in size (P66) (10). Since both R1 and R2 are necessary for the F2P97 fragment of the adhesin to bind heparin, the cleavage event that generates P28 is likely to have an impact on the ability of some cilium adhesin cleavage products to bind glycosaminoglycans. However, this cleavage event may not have a detrimental effect on the ability of P66 to bind heparin since this is likely to be conferred by the second heparin-binding site found in F1P97. The presence of two distally located heparin-binding sites may allow M. hyopneumoniae to alter its surface architecture without a corresponding loss of heparin-binding activity.
For some proteins, the ability to bind heparin has been attributed to the presence of relatively high numbers of evenly spaced basic amino acid residues rather than the presence of specific linear motifs (20, 29). The positively charged basic residues are believed to interact with the exposed, negatively charged sulfate groups of heparin and other glycosaminoglycans (5, 6, 20). This may also be true of cilium adhesin regions F1P97 and F2P97, which are relatively rich in lysine residues. Further evidence for this type of interaction can be derived from competitive-binding assays. Binding of both F1P97 and F2P97 to heparin was disrupted by the addition of fucoidan, a highly sulfated fucose polymer derived from marine algae, but not by chondroitin sulfate B. While not present in mammalian systems, fucoidan is useful in binding studies due to the high degree of sulfation of its carbohydrate chains and for this reason is frequently found to be a potent inhibitor of protein-heparin interactions (14, 24). The strong inhibition of heparin binding by fucoidan highlights the significance of sulfate groups in this interaction.
Mucin, another highly sulfated compound, was also found to be a powerful inhibitor in our competitive-binding assays. Mucins are abundant in the mucosal secretions lining various epithelial surfaces (25). Thus, the ability of the cilium adhesin fragments to bind mucin may in itself be highly significant, since the mucosal layer is often considered the first defense against pathogens that colonize epithelial surfaces (1). Interactions with the mucous layer of the respiratory tract would likely precede binding of M. hyopneumoniae to cilia; therefore, binding to mucins may represent an important first step in the pathogenesis of this organism. Further studies are required to characterize this interaction.
The binding affinities of both F1P97 (0.27 ± 0.02 μM) and F2P97 (1.89 ± 0.33 μM) for heparin are within the range reported for other heparin-binding proteins (0.3 nM to 4 μM) such as antithrombin (2), interleukin-2 and -12 (14, 24), clusterin (26), and the mycobacterial heparin-binding hemagglutinin (29), suggesting that these interactions are of physiological significance. The ability of the M. hyopneumoniae cilium adhesin to bind to components of the ECM, as well as to cilia, indicates that this molecule plays a multifunctional role in adherence to the porcine respiratory tract. While more experiments are required to establish the precise role of the adhesin's interaction with glycosaminoglycans in vivo, studies of microbial pathogenesis continue to highlight the importance of binding to heparin and other sulfated polysaccharides in the establishment, maintenance, and dissemination of infection (13, 21, 27, 34). Many pathogens, including Neisseria, Yersinia, and Staphylococcus species, exploit the widespread ability of host proteins to bind heparin. By recruiting heparin to their surfaces, these organisms acquire the ability to interact with a multitude of host molecules and circumvent the need for individual ligand receptors (13). For organisms such as M. hyopneumoniae, which have small genomes and therefore a limited repertoire of proteins, the ability to bind exogenous heparin may significantly increase the potential for host-parasite interactions at the site of colonization. Host molecules known to interact with heparin include ECM components, such as fibronectin and vitronectin, and cytokines and inflammatory mediators (13). Thus, the ability of M. hyopneumoniae to bind heparin may have important implications with respect to modulation of the host immune system and inflammatory response in addition to adherence of the pathogen to host tissues.
Acknowledgments
This work was partly funded by an ARC-Linkage grant (LP0455306) and by the McGarvie Smith Trust.
We are grateful for the skillful assistance of Katrina Stewart in cloning fragments F2P97, F3P97, and F4P97.
Editor: J. T. Barbieri
REFERENCES
- 1.Alvarez, R. A., M. W. Blaylock, and J. B. Baseman. 2003. Surface localized glyceraldehyde-3-phosphate dehydrogenase of Mycoplasma genitalium binds mucin. Mol. Microbiol. 48:1417-1425. [DOI] [PubMed] [Google Scholar]
- 2.Barrow, R. T., E. T. Parker, S. Krishnaswamy, and P. Lollar. 1994. Inhibition by heparin of the human blood coagulation intrinsic pathway factor X activator. J. Biol. Chem. 269:26796-26800. [PubMed] [Google Scholar]
- 3.Blanchard, B., M. M. Vena, A. Cavalier, J. Le Lannic, J. Gouranton, and M. Kobisch. 1992. Electron microscopic observation of the respiratory tract of SPF piglets inoculated with Mycoplasma hyopneumoniae. Vet. Microbiol. 30:329-341. [DOI] [PubMed] [Google Scholar]
- 4.Boguslavsky, S., D. Menaker, I. Lysnyansky, T. Liu, S. Levisohn, R. Rosengarten, M. Garcia, and D. Yogev. 2000. Molecular characterization of the Mycoplasma gallisepticum pvpA gene which encodes a putative variable cytadhesin protein. Infect. Immun. 68:3956-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardin, A. D., D. A. Demeter, H. J. Weintraub, and R. L. Jackson. 1991. Molecular design and modeling of protein-heparin interactions. Methods Enzymol. 203:556-583. [DOI] [PubMed] [Google Scholar]
- 6.Cardin, A. D., and H. J. Weintraub. 1989. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis 9:21-32. [DOI] [PubMed] [Google Scholar]
- 7.Chen, T., J. Swanson, J. Wilson, and R. J. Belland. 1995. Heparin protects Opa+ Neisseria gonorrhoeae from the bactericidal action of normal human serum. Infect. Immun. 63:1790-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dallo, S. F., A. Chavoya, and J. B. Baseman. 1990. Characterization of the gene for a 30-kilodalton adhesion-related protein of Mycoplasma pneumoniae. Infect. Immun. 58:4163-4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeBey, M. C., and R. F. Ross. 1994. Ciliostasis and loss of cilia induced by Mycoplasma hyopneumoniae in porcine tracheal organ cultures. Infect. Immun. 62:5312-5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Djordjevic, S. P., S. J. Cordwell, M. A. Djordjevic, J. Wilton, and F. C. Minion. 2004. Proteolytic processing of the Mycoplasma hyopneumoniae cilium adhesin. Infect. Immun. 72:2791-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Djordjevic, S. P., G. J. Eamens, H. Ha, M. J. Walker, and J. C. Chin. 1998. Demonstration that Australian Pasteurella multocida isolates from sporadic outbreaks of porcine pneumonia are non-toxigenic (toxA−) and display heterogeneous DNA restriction endonuclease profiles compared with toxigenic isolates from herds with progressive atrophic rhinitis. J. Med. Microbiol. 47:679-688. [DOI] [PubMed] [Google Scholar]
- 12.Dubreuil, J. D., G. D. Giudice, and R. Rappuoli. 2002. Helicobacter pylori interactions with host serum and extracellular matrix proteins: potential role in the infectious process. Microbiol. Mol. Biol. Rev. 66:617-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duensing, T. D., J. S. Wing, and J. P. van Putten. 1999. Sulfated polysaccharide-directed recruitment of mammalian host proteins: a novel strategy in microbial pathogenesis. Infect. Immun. 67:4463-4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasan, M., S. Najjam, M. Y. Gordon, R. V. Gibbs, and C. C. Rider. 1999. IL-12 is a heparin-binding cytokine. J. Immunol. 162:1064-1070. [PubMed] [Google Scholar]
- 15.Hsu, T., S. Artiushin, and F. C. Minion. 1997. Cloning and functional analysis of the P97 swine cilium adhesin gene of Mycoplasma hyopneumoniae. J. Bacteriol. 179:1317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu, T., and F. C. Minion. 1998. Identification of the cilium binding epitope of the Mycoplasma hyopneumoniae P97 adhesin. Infect. Immun. 66:4762-4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu, T., and F. C. Minion. 1998. Molecular analysis of the P97 cilium adhesin operon of Mycoplasma hyopneumoniae. Gene 214:13-23. [DOI] [PubMed] [Google Scholar]
- 18.Joh, D., E. R. Wann, B. Kreikemeyer, P. Speziale, and M. Hook. 1999. Role of fibronectin-binding MSCRAMMs in bacterial adherence and entry into mammalian cells. Matrix Biol. 18:211-223. [DOI] [PubMed] [Google Scholar]
- 19.King, K. W., D. H. Faulds, E. L. Rosey, and R. J. Yancey, Jr. 1997. Characterization of the gene encoding Mhp1 from Mycoplasma hyopneumoniae and examination of Mhp1's vaccine potential. Vaccine 15:25-35. [DOI] [PubMed] [Google Scholar]
- 20.Margalit, H., N. Fischer, and S. A. Ben-Sasson. 1993. Comparative analysis of structurally defined heparin binding sequences reveals a distinct spatial distribution of basic residues. J. Biol. Chem. 268:19228-19231. [PubMed] [Google Scholar]
- 21.Menozzi, F. D., K. Pethe, P. Bifani, F. Soncin, M. J. Brennan, and C. Locht. 2002. Enhanced bacterial virulence through exploitation of host glycosaminoglycans. Mol. Microbiol. 43:1379-1386. [DOI] [PubMed] [Google Scholar]
- 22.Menozzi, F. D., J. H. Rouse, M. Alavi, M. Laude-Sharp, J. Muller, R. Bischoff, M. J. Brennan, and C. Locht. 1996. Identification of a heparin-binding hemagglutinin present in mycobacteria. J. Exp. Med. 184:993-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minion, F. C., C. Adams, and T. Hsu. 2000. R1 region of P97 mediates adherence of Mycoplasma hyopneumoniae to swine cilia. Infect. Immun. 68:3056-3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Najjam, S., R. V. Gibbs, M. Y. Gordon, and C. C. Rider. 1997. Characterization of human recombinant interleukin 2 binding to heparin and heparan sulfate using an ELISA approach. Cytokine 9:1013-1022. [DOI] [PubMed] [Google Scholar]
- 25.Nieuw Amerongen, A. V., J. G. Bolscher, E. Bloemena, and E. C. Veerman. 1998. Sulfomucins in the human body. Biol. Chem. 379:1-18. [DOI] [PubMed] [Google Scholar]
- 26.Pankhurst, G. J., C. A. Bennett, and S. B. Easterbrook-Smith. 1998. Characterization of the heparin-binding properties of human clusterin. Biochemistry 37:4823-4830. [DOI] [PubMed] [Google Scholar]
- 27.Patti, J. M., and M. Hook. 1994. Microbial adhesins recognizing extracellular matrix macromolecules. Curr. Opin. Cell Biol. 6:752-758. [DOI] [PubMed] [Google Scholar]
- 28.Pethe, K., S. Alonso, F. Biet, G. Delogu, M. J. Brennan, C. Locht, and F. D. Menozzi. 2001. The heparin-binding haemagglutinin of Mycobacterium tuberculosis is required for extrapulmonary dissemination. Nature 412:190-194. [DOI] [PubMed] [Google Scholar]
- 29.Pethe, K., M. Aumercier, E. Fort, C. Gatot, C. Locht, and F. D. Menozzi. 2000. Characterization of the heparin-binding site of the mycobacterial heparin-binding hemagglutinin adhesin. J. Biol. Chem. 275:14273-14280. [DOI] [PubMed] [Google Scholar]
- 30.Ross, R. F. 1992. Mycoplasmal disease, p. 537-551. In D. J. Taylor (ed.), Diseases of swine. Iowa State University Press, Ames.
- 31.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed., vol. 1. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 32.Scarman, A. L., J. C. Chin, G. J. Eamens, S. F. Delaney, and S. P. Djordjevic. 1997. Identification of novel species-specific antigens of Mycoplasma hyopneumoniae by preparative SDS-PAGE ELISA profiling. Microbiology 143:663-673. [DOI] [PubMed] [Google Scholar]
- 33.Sheldrake, R. F., and L. F. Romalis. 1992. Evaluation of an enzyme-linked immunosorbent assay for the detection of Mycoplasma hyopneumoniae antibody in porcine serum. Aust. Vet. J. 69:255-258. [DOI] [PubMed] [Google Scholar]
- 34.Wadstrom, T., and A. Ljungh. 1999. Glycosaminoglycan-binding microbial proteins in tissue adhesion and invasion: key events in microbial pathogenicity. J. Med. Microbiol. 48:223-233. [DOI] [PubMed] [Google Scholar]
- 35.Wilton, J. L., A. L. Scarman, M. J. Walker, and S. P. Djordjevic. 1998. Reiterated repeat region variability in the ciliary adhesin gene of Mycoplasma hyopneumoniae. Microbiology 144:1931-1943. [DOI] [PubMed] [Google Scholar]
- 36.Zhang, Q., T. F. Young, and R. F. Ross. 1994. Glycolipid receptors for attachment of Mycoplasma hyopneumoniae to porcine respiratory ciliated cells. Infect. Immun. 62:4367-4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang, Q., T. F. Young, and R. F. Ross. 1995. Identification and characterization of a Mycoplasma hyopneumoniae adhesin. Infect. Immun. 63:1013-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang, Q., T. F. Young, and R. F. Ross. 1994. Microtiter plate adherence assay and receptor analogs for Mycoplasma hyopneumoniae. Infect. Immun. 62:1616-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]