Abstract
The development of new approaches to combat anthrax requires that the pathogenesis and host response to Bacillus anthracis spores be better understood. We investigated the roles that macrophages and neutrophils play in the progression of infection by B. anthracis in a mouse model. Mice were treated with a macrophage depletion agent (liposome-encapsulated clodronate) or with a neutrophil depletion agent (cyclophosphamide or the rat anti-mouse granulocyte monoclonal antibody RB6-8C5), and the animals were then infected intraperitoneally or by aerosol challenge with fully virulent, ungerminated B. anthracis strain Ames spores. The macrophage-depleted mice were significantly more susceptible to the ensuing infection than the saline-pretreated mice, whereas the differences observed between the neutropenic mice and the saline-pretreated controls were generally not significant. We also found that augmenting peritoneal neutrophil populations before spore challenge did not increase resistance of the mice to infection. In addition, the bacterial load in macrophage-depleted mice was significantly greater and appeared significantly sooner than that observed with the saline-pretreated mice. However, the bacterial load in the neutropenic mice was comparable to that of the saline-pretreated mice. These data suggest that, in our model, neutrophils play a relatively minor role in the early host response to spores, whereas macrophages play a more dominant role in early host defenses against infection by B. anthracis spores.
Bacillus anthracis is the etiological agent of anthrax and a proven agent of bioterrorism (12, 16, 27, 41). B. anthracis sporulates in the presence of environmental stresses, such as inadequate nutrient supply or desiccation (31). The spore, the infectious form of the organism, can remain dormant and viable for decades until favorable conditions are encountered. Upon infection, the spore germinates (42) and begins to outgrow into toxin-producing (2, 4), replicating bacilli, which can ultimately kill the infected host (26). Little information exists concerning the earliest stages of spore germination in vivo and the nature of the host response to the spores. It has been proposed that macrophages phagocytose spores, promote spore germination, and play a role that has been likened to a “Trojan horse” during an infection with B. anthracis spores (11, 21-24, 30). However, macrophages have also been shown to be sporicidal in vitro (3, 47, 59, 60, 64) and have a protective role for the host infected with B. anthracis spores (7).
Neutrophils are widely considered the first responders to an infection and can be observed at sites of infection significantly sooner than migrating macrophages (38). The roles of neutrophils during an infection with B. anthracis also remain unclear. The few accounts available suggest that they may play a more minor role, secondary to that of macrophages, in host defenses against anthrax (20, 50, 64). Nevertheless, the relative role of these phagocytes in the host response and their possible interactions during the response to B. anthracis in vivo remain equivocal.
Mice that are treated with clodronate-containing liposomes experience a significant depletion of their macrophages (54, 55). We showed previously that macrophage-depleted mice exhibit significantly shorter mean times to death and lower survival rates when challenged with B. anthracis spores than do mice retaining their native macrophage populations (7). This was the case when mice were challenged with spores either parenterally or via an aerosolized inoculum (7). The mechanisms for this increased susceptibility of the macrophage-depleted mice were unknown. In the current study, we further characterized the in vivo germination rates and pathogenesis of B. anthracis spores in macrophage-depleted mice. We also began to evaluate the roles of neutrophils during in vivo spore germination by comparing the responses of neutropenic and normal mice to infection with B. anthracis.
MATERIALS AND METHODS
Reagents and animals.
Dichloromethylene diphosphonate (clodronate) was a gift from Roche (Mannheim, Germany). Cyclophosphamide (Cytoxan) was purchased from Mead Johnson (Bristol-Myers Squibb Co., Princeton, N.J.). The hybridoma cell line expressing the rat anti-mouse granulocyte monoclonal antibody (MAb) RB6-8C5 was a gift of Robert Coffman (DNAX Research Institute of Molecular and Cellular Biology, Inc., Palo Alto, Calif.). Hybridomas were grown using Integra CL1000 flasks. Antibodies were collected from supernatants, immunoglobulin G (IgG) purified on a Sepharose A column via high-performance liquid chromatography, and stored at −20°C until use. The antibody preparations were tested for the presence of endotoxin, and it was determined that there was approximately 4 endotoxin units/mg of antibody (Cambrex, Walkersville, Md.). Pathogen-free BALB/c mice (Hc+) (62) were obtained from the National Cancer Institute, Fort Detrick (Frederick, Md.). Pathogen-free SJL/J (Hc+) (62) mice were obtained from Jackson Laboratories (Bar Harbor, Maine). All mice were female and between 6 and 9 weeks old at the time of use.
In vivo phagocyte depletion.
Macrophages were depleted in vivo by use of clodronate-loaded liposomes. The clodronate-loaded liposomes were prepared as previously described (56). Mice were injected intraperitoneally (i.p.) with 400 μl clodronate liposomes and intravenously with 200 μl. Intranasal (i.n.) instillation of the clodronate liposomes was also performed as described previously (7, 35). Mice were anesthetized with 100 μl of a solution of ketamine, acepromazine, and xylazine injected intramuscularly before 100 μl of clodronate liposomes was deposited onto the nares to be inhaled. All clodronate liposome treatments were performed 48 h before challenge with B. anthracis Ames strain spores, as previously reported (7, 65).
Mice were rendered neutropenic either by i.p. injection of 200 mg of cyclophosphamide/kg of body weight (10, 58) or by i.p. injection of 0.4 mg of MAb RB6-8C5 (6, 9, 19). Injections to induce neutropenia were performed 4 days before spore challenge when using cyclophosphamide and 2 days before spore challenge when using RB6-8C5. To determine the extent of neutropenia, blood was collected from the retro-orbital sinuses of anesthetized, randomly selected, uninfected mice and was analyzed with an Abbott Cell-Dyn 3700 system, which is a multiparameter, automated hematology analyzer designed for in vitro diagnostic use. On average, neutropenic mice contained approximately 95% fewer circulating neutrophils than saline-pretreated mice regardless of depletion agent used. When RB6-8C5 was used as the depleting agent, the differential blood counts obtained from treated mice revealed no change in circulating monocyte populations (including macrophages), an approximately 40% decrease in circulating eosinophil populations, and an approximately 50% decrease in circulating basophil and lymphocyte populations. In some experiments, control mice were pretreated with saline, and in others, rat IgG (reagent grade, from serum) (Sigma, St. Louis, Mo.) was used. We and others (6, 9) noted that there is no apparent difference between these two control treatments.
Spore preparations.
Spores of the fully virulent B. anthracis Ames strain were prepared as previously described (36, 59, 64), except the sporulating cultures were incubated between 48 and 50 h before harvest. In addition, as described previously (59, 64), the spores were purified by centrifugation of the spore suspensions through a density gradient medium (58 ml Hypaque 76 [Nycomed] into 42 ml water), and the pellets were collected and washed three additional times with water for injection (Medical Marketing, Inc., Lutherville, Md.).
Spore challenge and infection models.
The purified spores were heat activated (65°C for 30 min) before each challenge experiment (36). The spore challenges were administered i.p. as 200 μl of heat-activated spores suspended in water for injection. In addition, spore challenges were also administered by i.n. instillation (37) and by aerosol exposure (7, 8, 17). Administered spore doses for each experiment are indicated in Results. Mice infected with B. anthracis spores were monitored several times each day, and morbidity and mortality rates were recorded for 14 days.
In vivo time course experiments.
For time course experiments, mice were challenged as described above. Mice were then euthanized at specified time points after spore challenge. With the i.p. model of infection, mice were euthanized and subjected to peritoneal lavage procedures (64) in which they were injected i.p. with 7 ml ice-cold phosphate-buffered saline (PBS) and 3 ml of air. The fluid was then drawn up and immediately placed on ice. Half of the collected lavage fluid was plated onto trypticase soy agar (TSA) plates to obtain total counts, and the other half was heated at 65°C for 30 min to kill vegetative cells (36), immediately placed on ice, and then plated onto TSA plates. The counts obtained from the heated lavage samples represent the number of dormant/heat-resistant spores present in the lavage fluid.
In time course studies with the inhalation model of infection, mice were euthanized and subjected to bronchioalveolar lavage (BAL) procedures (23). Briefly, mice were euthanized and their tracheas were cannulated. The lungs were then lavaged with 1 ml of ice-cold PBS. The collected BAL fluid was treated as described above for the peritoneal lavage fluid. In addition, the lungs were harvested so as to exclude the majority of the trachea but to retain the mediastinal area. The lungs were rinsed with ice-cold PBS, suspended in 10 ml of ice-cold PBS, and then homogenized in a chilled glass dounce tissue grinder (Corning). An aliquot of the resulting lung homogenate was plated onto TSA plates to obtain total counts, while an aliquot was heated, chilled immediately on ice, and then plated to obtain ungerminated (heat-resistant) spore counts within the homogenate. In some experiments, the spleens were also removed from mice that had been exposed to aerosolized spores. The spleens were rinsed in Hanks' balanced salt solution and homogenized in the same manner as described above for lungs. Lavage fluid samples and homogenate samples were also mounted onto slides with a Cytospin centrifuge (Shandon, Inc., Pittsburgh, Pa.). The slides were stained with malachite green (to stain ungerminated spores) and counterstained with Diff-Quik (Harleco, Philadelphia, Pa.) (64).
In experiments done to compare and evaluate the effects of homogenization procedures on the spores, mice were challenged i.n. with 1.5 × 107 spores. One hour later, the mice were euthanized and the lungs were harvested as described above. The lungs from one group of infected mice were homogenized in 1 ml of ice-cold PBS containing 2.5-mm zirconia/silica beads (Biospec Products, Bartlesville, Okla.) for 90 s by use of a Fast Prep instrument (bead beater) from Q-Biogene (Carlsbad, Calif.). The lungs from another group of infected mice were homogenized in 10 ml of ice-cold PBS with a glass dounce tissue grinder (our standard homogenization procedure as described above), and lungs from a third group of infected mice were homogenized in 1 ml of ice-cold PBS with a glass dounce tissue grinder. All samples were kept ice-cold before plating and/or heating. The resulting homogenates were plated (heated and unheated) to obtain total counts and ungerminated spore counts as described above.
Phagocyte augmentation.
To augment their peritoneal macrophage populations, mice were injected i.p. with 1 ml of 2% starch 4 days before i.p. spore challenge (64). Mice were also injected i.p. with 1 ml of 2% starch 4 h before i.p. spore challenge to augment their peritoneal neutrophil populations (64). Other mice were injected i.p. with neutrophils collected from starch-treated mice. Four hours after the starch inoculation, cellular exudates were harvested from the mice by peritoneal lavage. The cells were washed twice in saline and resuspended in saline, and viable cells were counted by trypan blue dye exclusion before injection into naïve recipient mice.
In vitro spore germination assay.
To determine the tendency of spores to germinate in mouse peritoneal fluid, we performed a fluorescence-based in vitro spore germination assay. As previously described, this assay measures the uptake of the fluorescent Syto 9 dye (Molecular Probes, Eugene, Oreg.) by germinating spores (61). This was performed as a kinetic assay that measured the fluorescence every minute for 1 h (61) and also in a modified assay that measured fluorescence every 15 min for 4 h. A defined germination medium, AAC (l-alanine, adenosine, and Casamino Acids), was used as a positive control for the assay, while buffer alone was included as a negative control (61). Peritoneal lavage fluids were collected from saline-treated mice or clodronate liposome-treated mice and filtered through a 0.8-mm syringe filter to remove any cells present. In one experiment, samples were concentrated 10-fold with a Centricon device (YM-3) (Millipore, Bedford, Mass.).
Statistical analyses.
Survival rates were compared between each treatment group and control group by Fisher's exact tests with permutation adjustment for multiple comparisons. Kaplan-Meier/product-limit estimation was used to construct survival curves and to compute mean survival times. Survival curves were compared between each treatment group and control group by log rank tests with Hochberg adjustment for multiple comparisons. Mean times to death were compared between each treatment group and control group by t tests with permutation adjustment for multiple comparisons. The above analyses were conducted using SAS version 8.2 (SAS Institute, Inc., SAS OnlineDoc, version 8, Cary, N.C., 2000). The in vitro germination kinetics of spores in different peritoneal fluids were analyzed by a four-parameter logistic regression model available on the SigmaPlot PC software program (61). When comparing total counts recovered from lavage fluids, statistical significance (P < 0.05) was determined by the two-tailed Student t test with the GraphPad Prism software (GraphPad, San Diego, Calif.).
RESULTS
Effects of depleting specific host phagocyte populations on the bacterial burden observed after i.p. challenge with B. anthracis spores.
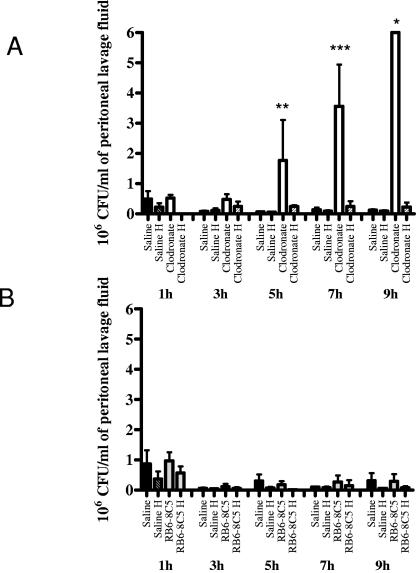
Mice were treated intravenously and i.p. with clodronate liposomes to induce macrophage depletion. Other mice were rendered neutropenic by i.p. administration of the rat anti-mouse granulocyte MAb RB6-8C5. Two days later, the mice were challenged i.p. with ungerminated B. anthracis Ames strain spores. Mice were euthanized at sequential time points postchallenge, and peritoneal lavages were performed. After removal of an aliquot for microscopic evaluation, half of the peritoneal lavage fluid was immediately plated for viable counts, and the remainder of the lavage fluid samples was heated and plated to obtain counts of heat-resistant, ungerminated spores. Significantly greater numbers of B. anthracis were isolated from the peritoneal lavage fluids of macrophage-depleted mice than from the lavage fluids from mice with native macrophage populations. In time course studies, this difference was appreciable at approximately 4 to 5 h postchallenge (Fig. 1A). Whereas the macrophage-depleted mice exhibited a significant increase in bacteria present in lavage fluid with time, the saline-pretreated mice exhibited consistently low levels of bacteria in the lavage fluid for at least 24 h postchallenge (Fig. 1A and data not shown). In contrast, the neutropenic mice did not have a significantly greater bacterial burden during the first 9 h after i.p. infection with B. anthracis spores than control mice pretreated with either saline or normal rat IgG (Fig. 1B). These data suggest that in the peritoneal model of infection, macrophages are necessary for the host to limit spore outgrowth and bacillary replication during the earliest stages of the infection.
FIG. 1.
Effect of macrophage or neutrophil depletion on bacterial load associated with an i.p. infection with B. anthracis spores. Time course study of bacteria collected from peritoneal lavage fluid of (A) mice retaining normal macrophage populations (saline) or mice that underwent macrophage depletion (clodronate) or (B) mice retaining normal neutrophil populations (rat IgG) or mice that underwent neutrophil depletion (RB6-8C5). Total bacterial counts are depicted, and counts obtained from heated fractions (H) of lavage fluid are also shown. Counts obtained from heated fractions correspond to numbers of dormant/heat-resistant spores. Panel A is a combination of two experiments where the i.p. challenge dose was approximately 3 × 103 spores. Panel B is representative data from an experiment where the i.p. challenge dose was approximately 3.2 × 103 spores. *, note that the clodronate bar at the 8-h time point should be larger, as the bacterial colonies exceeded the maximum countable number of bacteria for this experiment (>2.5 × 103/ml), but is depicted this way for illustrative purposes. The total number of bacteria recovered from the macrophage-depleted mice was significantly greater than the total number of bacteria recovered from saline-treated mice (**, P = 0.0065; ***, P < 0.0001). All values represent the averages from at least two mice ± standard deviations.
These observations were not dependent upon the amount of spores in the challenge dose (note the different scales used for Fig. 1 and Fig. 2). In initial experiments (Fig. 1), BALB/c mice were challenged i.p. with approximately 3 × 103 spores (representing approximately six 50% lethal doses [48]), and in later experiments (Fig. 2) the mice were challenged i.p. with approximately 2 × 107 spores (representing approximately 4 × 104 50% lethal doses [reference 48 and data not shown]). The difference in bacterial load attributed to clodronate liposome treatment was observed approximately 5 h postchallenge for both the low-dose (Fig. 1A) and the high-dose (Fig. 2A) challenges. While saline-pretreated mice had minimal outgrowth of B. anthracis spores, the macrophage-depleted mice sustained rapid outgrowth of B. anthracis spores to vegetative bacilli (Fig. 3). Again, as observed with the low-dose challenge (Fig. 1B), the neutropenic mice did not have a significantly increased bacterial burden compared to the saline control mice after i.p. challenge with the significantly higher bolus of spores (Fig. 2B and data not shown).
FIG. 2.
Effect of macrophage or neutrophil depletion on bacterial load associated with an i.p. infection with a high dose of B. anthracis spores. Time course study of bacteria collected from peritoneal lavage fluid of (A) mice retaining normal macrophage populations (saline) or mice that underwent macrophage depletion (clodronate) or (B) mice retaining normal neutrophil populations (saline) or mice that underwent neutrophil depletion (RB6-8C5). Total bacterial counts are depicted, and counts obtained from heated fractions (H) of lavage fluid are also shown. Counts obtained from heated fractions correspond to numbers of dormant/heat-resistant spores. Panel A presents data from an experiment where the i.p. challenge dose was approximately 1.8 × 107 spores. Panel B presents data from an experiment where the i.p. challenge dose was approximately 2.5 × 107 spores. Similar results were obtained in at least two additional experiments. *, note that the clodronate bar at the 9-h time point should be larger, as the bacterial colonies exceeded the maximum countable number of bacteria for this experiment (>5 × 106/ml), but is depicted this way for illustrative purposes. The total number of bacteria recovered from the macrophage-depleted mice was significantly greater than the total number of bacteria recovered from saline-treated mice (**, P = 0.0015; ***, P < 0.0001). All values represent the averages from at least two mice ± standard deviations.
FIG. 3.
Time course of infection, as detected by microscopic examination of peritoneal lavage fluids. Cytospin preparations were prepared from peritoneal lavage fluid samples collected from saline-pretreated mice retaining native macrophage populations (S) and mice that had been depleted of macrophages by administration of clodronate liposomes (C). Samples were examined at 1 h, 3 h, 5 h, 7 h, and 9 h after i.p. challenge with approximately 1.8 × 107 B. anthracis Ames strain spores. The 1-h samples were concentrated approximately fourfold before being mounted on the slide to visualize spores (arrows). In both the S 1-h and the C 1-h samples, ungerminated and germinated spores were observed, as determined by malachite green/Diff-Quik staining. The magnification was ×60, except for the 1-h samples, which were observed at ×100 to visualize spores.
It has been well documented that susceptibility to infection with B. anthracis varies greatly between mouse strains (62, 63). To confirm that these observations were not entirely dependent upon the strain of mouse challenged, we repeated these macrophage depletion experiments with SJL/J mice (Hc+). Preliminary work suggested that the SJL/J mice are slightly more susceptible to infection with the B. anthracis Ames strain than the BALB/c mice (data not shown), even though both strains are proficient for the complement component C5 (Hc+). However, when the time course experiments were repeated, nearly identical results were obtained (data not shown). The saline-treated SJL/J mice contained the infection, while the macrophage-depleted SJL/J mice had significant bacterial growth, as determined by an increase in bacteria obtained from peritoneal lavage fluid and microscopic evaluation (data not shown). This increase in B. anthracis was observed approximately 5 h postchallenge for both the BALB/c and the SJL/J mice.
In vitro germination of spores in the presence of mouse peritoneal fluid.
It was necessary to determine if the increased bacterial load associated with macrophage depletion (clodronate liposome treatment) was due to the deficiency of macrophages. An alternative explanation could be that the peritoneal cavity of macrophage-depleted mice was more favorable for rapid spore germination due to possible intracellular germinants being released upon apoptosis of macrophages. Experiments were designed to determine if peritoneal lavage fluid from macrophage-depleted mice could stimulate spore germination at rates comparable to peritoneal lavage fluid from saline-treated mice. By use of a spectrofluorometric assay, spore germination in the presence of the peritoneal fluid samples was monitored every minute for 1 h in one experiment (data not shown) and every 15 min for 4 h in another (Fig. 4). In both assays, we found no appreciable differences in rate or extent of spore germination in the presence of the peritoneal fluid samples collected from saline-treated mice or macrophage-depleted mice (Fig. 4). Neither peritoneal lavage fluid sample stimulated significant germination. This was also the case for peritoneal lavage fluid that had been concentrated approximately 10-fold to mimic undiluted intraperitoneal fluid as much as possible (data not shown). Only spores suspended in a defined germination medium (AAC) germinated significantly (Fig. 4).
FIG. 4.
In vitro fluorescent germination assay (60) of the germination potential of intraperitoneal fluids obtained from saline-pretreated control mice (▴) and macrophage-depleted clodronate-treated mice (▪). A synthetic germination medium, AAC (60), was used as a positive control (○), and buffer alone was used as a negative control (•). There were no appreciable differences between the extents of germination associated with the negative control, buffer alone, compared to either peritoneal fluid sample. RFU, relative fluorescence units.
Alveolar macrophage-depleted mice retain significantly more spores within the bronchioalveolar spaces of the lungs than do normal mice after challenge with aerosolized B. anthracis spores.
Alveolar macrophages, a unique subset of macrophages found in the connective tissues of the septum and in the air spaces of the lung alveoli, are of particular importance when investigating inhalation anthrax. We showed previously that mice that undergo alveolar macrophage depletion have a significantly lower survival rate and significantly shorter mean time to death than saline-pretreated mice (7). In the current study, alveolar macrophage-depleted mice and saline-pretreated mice were challenged with B. anthracis Ames spores by aerosol exposure and euthanized at predetermined time points postchallenge, and BAL fluids were collected. Quantitative viable counts of both heated and unheated samples of the BAL fluids were determined. In three separate experiments, the saline-pretreated mice had significantly fewer spores in the BAL fluids collected postchallenge than did alveolar macrophage-depleted mice (Fig. 5). There was no significant germination associated with the spores recovered from BAL fluids from either macrophage-depleted or saline-treated mice; nearly all of the bacteria counted were heat resistant, illustrating that predominantly ungerminated spores remained within the bronchioalveolar spaces of the lungs (Fig. 5).
FIG. 5.
Clearance of spores from lungs depleted of alveolar macrophages. BALs were performed on mice at various times after challenge with aerosolized spores. The inhaled dose was approximately 3 × 105 B. anthracis Ames spores. BAL fluid was plated directly onto TSA plates, and half of the BAL fluid was heated (H) before being plated onto TSA plates. Bacterial counts obtained from heated fractions correspond to numbers of dormant/heat-resistant spores. All values represent the averages from two mice ± standard deviations (except the saline 72-h bar, which represents one mouse). Similar results were obtained in two additional experiments. *, 5/15 clodronate liposome-treated mice were found dead at this time. **, 2/15 clodronate liposome-treated mice and 1/15 saline-treated mice were found dead at this time. The number of spores recovered from clodronate-treated mice at 24 h postchallenge was statistically greater than the number of spores collected from saline-treated mice (***, P = 0.037; ****, P = 0.013).
Alveolar macrophage depletion is associated with an increased bacterial burden in lung tissue in mice after challenge with aerosolized B. anthracis spores.
To further characterize spore germination in lung tissues, lung homogenates were examined. As shown in Fig. 6 and 7, germination and outgrowth were observed with the alveolar macrophage-depleted mice as early as 48 h postexposure to aerosolized spores. However, the saline-pretreated mice harbored a generally constant number of bacteria even after 96 h postchallenge (Fig. 7). The lung tissue collected did not include most of the trachea but did contain the mediastinal region. Thus, the precise site of spore germination within the alveolar macrophage-depleted mice remains in question.
FIG. 6.
Effect of alveolar macrophage depletion on the number of bacteria recovered from the lungs postchallenge with aerosolized spores. The inhaled dose was approximately 6 × 105 B. anthracis Ames spores. Mice were euthanized at selected time points postchallenge, and the lungs were lavaged and removed as to include both lobes and mediastinal area but to exclude the majority of the trachea. Lung homogenate fluids (homogenates generated with our standard tissue grinder procedure) were divided, with half being plated directly onto TSA plates and half being heated (H) before being plated onto TSA plates. Bacterial counts obtained from heated fractions correspond to numbers of dormant/heat-resistant spores. All values represent the averages from at least two mice (except the clodronate liposome 72-h bar, which represents the sole clodronate liposome-treated survivor of the experiment) ± standard deviations. Similar results were obtained in two additional experiments. *, note that the clodronate (clodronate liposomes) bars at the 48-h and 72-h time points should be larger, as the bacterial colonies exceeded the maximum countable number of bacteria for this experiment (>1 × 105/ml), but are depicted this way for illustrative purposes. **, 1/19 clodronate liposome-treated mice was found dead at this time. ***, 8/19 clodronate liposome-treated mice and 4/19 saline-treated mice were found dead at this time.
FIG. 7.
Microscopic examination of lung homogenates. Both mice were euthanized 48 h postchallenge with an aerosolized dose of approximately 8.2 × 104 B. anthracis Ames spores. Lung homogenate from a saline-pretreated mouse retaining native alveolar macrophage populations (S) and lung homogenate from a clodronate liposome-treated mouse (macrophage depleted) (C) are shown. Bacilli can be observed in the macrophage-depleted mouse but are not observed in the saline-pretreated mouse.
We also evaluated homogenization protocols to ensure that our use of a glass dounce tissue grinder was adequate compared to more recently published methods of tissue homogenization (13, 37). In these experiments, BALB/c mice were challenged i.n. with approximately 1.5 × 107 spores. One hour later, the mice were euthanized and the lungs were removed as described above. Both the glass dounce tissue grinder and bead beating with silica/zirconia beads homogenized mouse lungs to similar extents, as determined visually and microscopically. However, we observed a striking difference in percentage of heat-sensitive bacterial cells recovered from the homogenates between the two methods of homogenization. The use of glass dounce tissue grinders in our standard procedure consistently resulted in the recovery of mostly heat-resistant (dormant) spores. However, approximately 95% ± 2% (n = 3) of the bacterial counts obtained with the bead beater method were heat sensitive (germinated spores or bacilli). In addition, the volume of buffer in which the lungs were homogenized affected the heat sensitivity of the spores. When the lungs were homogenized in 1 ml of ice-cold PBS with a glass dounce tissue grinder, approximately 52% ± 11% (n = 2) of the bacteria recovered were heat sensitive. In contrast, when the lungs were homogenized in 10 ml of ice-cold PBS, only 23% ± 14% (n = 2) of the recovered bacteria were heat sensitive. These data indicate that the spores recovered from the lungs subjected to mechanical homogenization with a bead beater are significantly altered and that the majority of the spores are heat sensitive. Thus, the procedure used to process tissue samples is critical when evaluating and interpreting the fate of spores in vivo.
Alveolar macrophage depletion also influenced bacterial loads observed in the spleens of mice post-aerosol challenge. Harvill et al. recently reported that the numbers of viable bacteria recovered from spleens of mice challenged with aerosolized spores can vary dramatically (28). In our experiments, there were no detectable bacteria isolated from the spleens of mice with intact native alveolar macrophage populations (limit of detection, 100 CFU per spleen), but clodronate liposome-treated mice had spleens that were positive for B. anthracis at either 24 h (one of three mice examined) or 48 h (two of three mice examined) postchallenge (data not shown).
Effect of induced neutropenia on survival rates of mice after challenge with B. anthracis spores.
We established that macrophage depletion via clodronate liposomes renders mice significantly more susceptible to infection with B. anthracis (7). It has been shown that macrophage depletion may also result in a slight decrease in neutrophil populations (32, 33, 39). Thus, to further characterize our macrophage depletion model we must also address the roles of neutrophils in the host immune response to the infection. In this study, we examined the effects of neutrophil depletion on the times to death and survival rates of mice after challenge with B. anthracis spores. When neutropenic (cyclophosphamide-treated or RB6-8C5-treated) and saline-pretreated control mice were challenged i.p. with approximately 8 × 102 B. anthracis Ames spores, there were no statistically significant differences in times to death or survival rates between the three groups (Fig. 8). Similar results were obtained from three independent experiments, and none of the experiments yielded statistically significant differences between treatment groups.
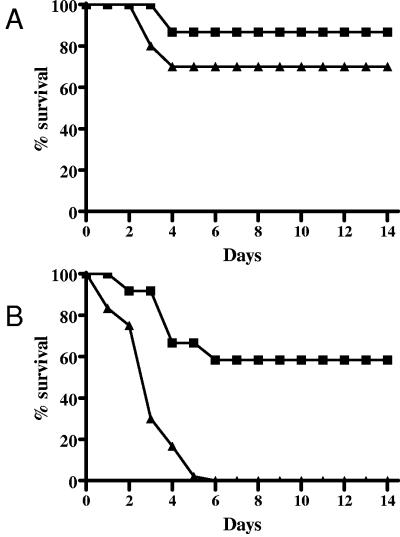
FIG. 8.
Effect of induced neutropenia on survival rates of mice challenged i.p. with B. anthracis Ames spores. Mice were pretreated with saline i.p. (▪), RB6-8C5 i.p. (▴), or cyclophosphamide i.p. (○). The mice received an i.p. injection of approximately 1 × 103 B. anthracis Ames spores. The mice were observed for 14 days postchallenge. Survival rates and times to death were not significantly different. Similar results were obtained in two additional experiments.
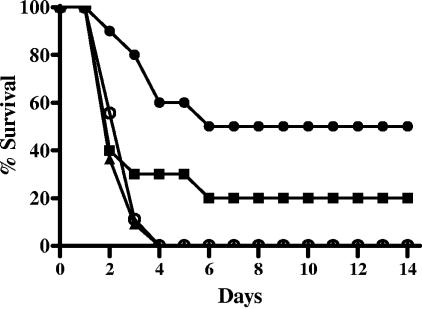
When the mice were challenged with a low dose of aerosolized spores (approximately 9 × 103 inhaled spores), there were no statistically significant differences in the survival rates or mean times to death of the control saline-treated mice and neutropenic (RB6-8C5-treated) mice (Fig. 9A). However, when the aerosolized spore challenge was increased approximately 10 times, to 8 × 104 inhaled spores, there were statistically significant differences observed in the survival rates (P = 0.005) between the control saline-treated mice (58%) and neutropenic mice (0%) but there were no significant differences in mean times to death (P = 0.33) (Fig. 9B).
FIG. 9.
Effect of induced neutropenia on survival rates of mice challenged with aerosolized B. anthracis Ames spores. Mice were pretreated with saline i.p. (▪) or RB6-8C5 i.p. (▴). The mice received an inhaled dose of (A) approximately 9 × 103 or (B) approximately 8 × 104 B. anthracis Ames spores. The mice were observed for 14 days postchallenge.
Effect of augmenting neutrophil populations on survival of mice challenged i.p. with B. anthracis spores.
In our earlier report, we demonstrated that by augmenting native peritoneal macrophage populations with RAW 264.7 cells we significantly increased survival rates of mice subsequently infected i.p. with B. anthracis spores (7), and this effect of the additional macrophages was dose related (7). In this report, we investigated the possibility of a corresponding effect of neutrophil supplementation on B. anthracis infection by using two approaches: (i) directly stimulating neutrophil influx in mice by i.p. injection of soluble starch before spore challenge and (ii) injecting mice i.p. just before spore challenge with neutrophils collected exogenously from uninfected, starch-elicited mice.
BALB/c mice were treated with 2% starch, and cell exudates were harvested 4 h later, at which time the responding inflammatory cells were predominantly neutrophils (64). The cells were washed in saline, and neutrophils were counted; their viability as determined by trypan blue exclusion was >95%. Approximately 2 × 106 neutrophils were delivered to each naïve mouse by i.p. injection. The mice were then challenged i.p. with B. anthracis Ames strain spores. Augmenting peritoneal neutrophil populations in this manner did not offer the recipient mice any protection from the infection. There was no increase in survival rate or time to death (data not shown).
Other mice were treated with starch and then challenged directly with B. anthracis spores 4 h later. In addition, one group of mice was injected i.p. with starch 4 days prior to infection, so that the inflammatory response would be dominated by macrophages at the time of spore challenge (64). The mice were then observed for 14 days, and mortality rates were recorded. We found no significant differences between mice with an augmented neutrophil population, neutrophil-depleted mice, and saline-pretreated mice (Fig. 10). However, the mice that received the i.p. injection of starch 4 days before the spore challenge were partially protected (Fig. 10). Moreover, when the experiment was repeated with a higher bolus of spores (approximately 1.7 × 103 spores) there was again no difference between mice with augmented neutrophil populations, neutropenic mice, and saline-pretreated mice (data not shown). However, the mice receiving a starch injection 4 days before spore challenge had a significantly longer mean time to death than mice pretreated with saline (P = 0.0361). These data suggest that a robust macrophage response to B. anthracis spores is clearly advantageous to the host, whereas the neutrophil response is of secondary importance during infection.
FIG. 10.
Effect of augmenting peritoneal neutrophil or macrophage populations on survival rates of mice challenged i.p. with B. anthracis spores. Mice were pretreated with saline i.p. (▪), RB6-8C5 i.p. (▴), 1 ml of 2% starch i.p. 4 h before spore challenge (○), or 1 ml of 2% starch i.p. 4 days before spore challenge (•). The mice were challenged i.p. with approximately 9 × 102 spores and observed for 14 days postchallenge.
DISCUSSION
The putative roles of macrophages during infection with B. anthracis have been addressed by numerous groups (3, 11, 21-24, 30, 47, 59, 60, 64). We previously showed that mice specifically depleted of macrophages are significantly more susceptible to infection with B. anthracis spores (7). This increased susceptibility was observed regardless of inoculation route (i.p., subcutaneous, or inhalation) (7). In this study, we examined putative mechanisms leading to the increased susceptibility. Saline-pretreated mice challenged i.p. with B. anthracis strain Ames spores were able to limit, and, depending on the dose, resolve the B. anthracis infection. However, when the mice were depleted of macrophages by treatment with clodronate-containing liposomes, a significant degree of spore germination and bacillary replication was observed at very early time points postchallenge. This was evident when we examined the bacterial burden found in the peritoneal cavities of infected mice (Fig. 1 to 3).
Interestingly, these observations were similar to those obtained when examining mice that had been exposed to an aerosolized challenge of B. anthracis Ames spores. Mice that retained their native alveolar macrophages showed no signs of significant spore germination or bacillary replication in bacteria recovered from BAL fluid or in lung homogenates (Fig. 5 to 7). These data support the observation that after inhalation of spores the bacteria retained in the BAL fluid are predominantly heat-resistant ungerminated spores (24, 47, 50). However, when mice depleted of their alveolar macrophages were examined, although there was no detectable germination in the BAL fluid (Fig. 5), large numbers of bacilli were observed in the lung homogenates (Fig. 6 and 7).
During these studies, we also evaluated several methods for tissue homogenization and the potential effects of these procedural differences on spore germination. It has been well established that there are numerous factors that can initiate spore germination and subsequent loss of heat resistance. These include physical injury to the spore (15), increased pressure (18, 42, 68), and exposure to defined germinants (42, 43). We showed that the technique used to homogenize tissues can significantly influence the proportion of heat-sensitive spores recovered and ultimately the interpretation of spore germination rates in vivo. We observed that processing lungs of infected mice with a bead beater apparatus was associated with the recovery of mostly heat-sensitive spores, whereas homogenizing lungs with a tissue grinder in a large volume of cold PBS resulted in recovery of mostly heat-resistant spores. Using a tissue grinder in a reduced volume of buffer induced intermediate levels of germination, possibly due to the increased concentration of potential germinants released from the lung tissues or an increase in heat production. Thus, we hypothesize that a combination of factors associated with tissue homogenization by using a bead beater apparatus, including spore abrasion, heat production, and an increased concentration of germinants in small sample volumes, may induce spore germination after the lung has been removed from the euthanized animal. Accordingly, it appears to be critical to optimize and standardize tissue collection and processing procedures to accurately evaluate in vivo spore germination.
It has been demonstrated that administrating liposome-encapsulated clodronate specifically depletes macrophages and monocyte precursors (29) while not directly affecting dendritic cells (55, 56) or neutrophils (49, 56). There is a clear correlation between the macrophage response to the spores and the rapidity of progression of the B. anthracis infection in our mouse models. However, it has been shown that macrophage-depleted mice can have a somewhat reduced neutrophil response, as resident macrophages can secrete chemoattractants, such as macrophage inflammatory protein 2 (MIP-2), resulting in the recruitment of neutrophils to the site of infection (32, 33, 39). Because macrophages and neutrophils can act in concert, it is necessary to examine the effects of induced neutropenia on the earliest stages of in vivo spore germination.
Mice were rendered neutropenic by administration of either the pharmaceutical agent cyclophosphamide (Cytoxan) or the rat anti-mouse granulocyte MAb RB6-8C5. In initial experiments, we determined that the MAb RB6-8C5 was less detrimental to the health of the mice, more specific for neutrophil depletion, and just as efficient at inducing neutropenia compared to cyclophosphamide (data not shown). Thus, subsequent experiments were performed using the MAb as the neutrophil depletion agent. There were only small (statistically insignificant) differences in the survival rates of normal and neutropenic mice challenged i.p. with spores (Fig. 8) or by aerosol with a low dose of spores (Fig. 9A). In addition, time course studies illustrated that, in the intraperitoneal model of infection, there were no significant differences in the bacterial concentrations present in peritoneal lavage fluids obtained from normal mice and neutropenic mice (Fig. 1B and 2B).
The results obtained from the low-dose aerosol challenge are noteworthy because we have shown that challenging macrophage-depleted mice with a similarly low dose of aerosolized spores resulted in >90% mortality, which was significantly greater than the mortality rate observed for mice with a normal complement of alveolar macrophages (7). Thus, these results suggest that alveolar macrophages play a more direct role in host defense against inhaled B. anthracis spores than do neutrophils. However, when the aerosolized challenge dose was increased, there were significant differences in survival rates between neutropenic and saline control mice (Fig. 9B).
These relatively minor differences (Fig. 8 and 9) may not be directly due to the absence of neutrophils. It has been shown that neutrophilic responses in mice to B. anthracis strain Sterne spores delivered by the i.p. route peak at approximately 6 h after infection (64). Thus, if neutrophils had a significant direct effect on the outcome of a B. anthracis infection (i.e., by direct killing or translocation of spores by neutrophils), a very early effect on either survival rate or bacterial burden in challenged mice should be observed. However, no significant effects of neutropenia on the bacterial load within 9 h (Fig. 1 and 2) or on survival within 48 h of aerosol exposure (Fig. 9A and B) were observed. In addition, mice were not protected from a peritoneal infection when the peritoneal neutrophil population was augmented (Fig. 10).
These results do not entirely rule out an important role for neutrophils in the host response to a B. anthracis infection. However, they suggest the possibility that the increase in susceptibility (depending upon dose of spores administered) of neutropenic mice may be the result of more-general alterations of the immune system in response to administration of the MAb RB6-8C5. These may include moderately lower numbers of CD8+ T cells (44, 45, 54), different cytokine and chemokine profiles (5, 25), and ultimately reduced levels of recruited and/or activated macrophages. By analyzing blood samples from control mice, we observed that RB6-8C5 was efficient at depleting circulating neutrophil populations by at least 95%; however, lymphocyte numbers also declined (data not shown). This reduction in lymphocyte numbers has been shown to be attributed predominantly to a drop in CD8+ T lymphocytes (44). It has been reported that neutrophil depletion induced by administering RB6-8C5 can result in up to 50% reduction of CD8+ T-cell populations (51, 54). This partial depletion of CD8+ T cells may alter cytokine levels (e.g., gamma interferon), subsequently altering macrophage activation (52) and possibly killing of the B. anthracis spores by macrophages. Others have reported that neutrophil depletion can result in a less robust Th1-cell-mediated immune response. Tateda et al. showed that early recruitment of neutrophils is critical to Th1 polarization in a mouse model of Legionella pneumophila (53), again suggesting crucial roles for neutrophils in immune modulation.
Very little is known about the role of neutrophils during infection with B. anthracis of either experimental animal models or humans. Several groups have addressed the effects of anthrax toxins on neutrophils (1, 14, 34, 40, 46, 57, 66, 67). However, the interaction between B. anthracis spores and neutrophils has not been studied in great detail. Ross suggested that the role of neutrophils in a guinea pig aerosol model may be minimal (50). In these experiments, a slight increase in neutrophils in the walls of the alveoli was noted, but their significance was equivocal (50). Results of our previous studies suggested that the roles of neutrophils during an anthrax infection would most likely be secondary to those of macrophages (64). In these studies, peritoneal exudates from A/J mice (sensitive to Sterne spore challenge [63]) and CBA/J mice (more resistant to Sterne spore challenge [63]) were examined (64). While there was an initial lag in neutrophil recruitment in the A/J mice, the lag was temporary, and equal numbers of neutrophils were observed with both A/J mice and CBA/J mice in response to i.p. injection with viable Sterne spores. In addition, it was shown that neutrophils obtained from either A/J mice or CBA/J mice could phagocytose and kill spores similarly (64). However, it was reported that the more sensitive A/J strain also had a reduced total accumulated number of macrophages at the initial site of infection that was not corrected with time as was the lag in neutrophil accumulation (64). Thus, the results correlated the increased susceptibility of the A/J mice with reduced numbers of monocyte precursor cells and inadequate numbers of functional macrophages rather than a delayed neutrophil response (64).
The importance of neutrophils in human anthrax infection is also largely unexplored. In 2001, Grinberg et al. reexamined the human specimens obtained from autopsies of victims of the inhalational anthrax epidemic in Sverdlovsk, Russia, in 1979 (20). Upon quantitative microscopic analysis of the samples, they determined that patients with a short survival time had an inflammatory response dominated by the presence of neutrophils, whereas an inflammatory response that included larger numbers of mononuclear cells (including macrophages) correlated with increased patient survival time (20). Taken together with experimental animal models (48, 62), these results suggest the importance of a robust macrophage response to a challenge with B. anthracis spores.
In conclusion, these data suggested that in our BALB/c mouse models of the early pathogenesis of anthrax, macrophages were critical to host survival of either an intraperitoneal or inhalation infection with B. anthracis spores. In addition, in our models, neutrophils apparently played a secondary, but necessary, role in a fully functional innate immune response to spores.
Acknowledgments
The research described herein was sponsored by the Medical Biological Defense Research Program, U.S. Army Medical Research and Materiel Command, project no. 02-4-5C-023.
We thank A. Bassett, T. Dimezzo, J. Bashaw, and L. Hildebrand for their invaluable technical assistance and S. Norris for her expert statistical analysis.
Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the U.S. Army.
Research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adheres to the principles stated in the Guide for the Care and Use of Laboratory Animals, National Research Council, 1996. The facility where this research was conducted is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Editor: J. D. Clements
REFERENCES
- 1.Abalakin, V. A., E. G. Sirina, and T. D. Cherkasova. 1990. The effect of lethal anthrax toxin on the functional activity of peritoneal mononuclear phagocytes and polymorphonuclear neutrophils in mice. Zh. Mikrobiol. Epidemiol. Immunobiol. 2:62-67. (In Russian.) [PubMed] [Google Scholar]
- 2.Bhatnagar, R., and S. Batra. 2001. Anthrax toxin. Crit. Rev. Microbiol. 27:167-200. [DOI] [PubMed] [Google Scholar]
- 3.Bozue, J. A., N. Parthasarathy, L. R. Phillips, C. K. Cote, P. F. Fellows, I. Mendelson, A. Shafferman, and A. M. Friedlander. 2005. Construction of a rhamnose mutation in Bacillus anthracis affects adherence to macrophages but not virulence in guinea pigs. Microb. Pathog. 38:1-12. [DOI] [PubMed] [Google Scholar]
- 4.Brossier, F., and M. Mock. 2001. Toxins of Bacillus anthracis. Toxicon 39:1747-1755. [DOI] [PubMed] [Google Scholar]
- 5.Cassatella, M. A. 1995. The production of cytokines by polymorphonuclear neutrophils. Immunol. Today 16:21-26. [DOI] [PubMed] [Google Scholar]
- 6.Conlan, J. W., and R. J. North. 1994. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J. Exp. Med. 179:259-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cote, C. K., K. M. Rea, S. L. Norris, N. Van Rooijen, and S. L. Welkos. 2004. The use of a model of in vivo macrophage depletion to study the role of macrophages during infection with Bacillus anthracis spores. Microb. Pathog. 37:169-175. [DOI] [PubMed] [Google Scholar]
- 8.Cown, W. B., T. W. Kethley, and E. L. Fincher. 1957. The critical-orifice liquid impinger as a sampler for bacterial aerosols. Appl. Microbiol. 5:119-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Czuprynski, C. J., J. F. Brown, N. Maroushek, R. D. Wagner, and H. Steinberg. 1994. Administration of anti-granulocyte mAb RB6-8C5 impairs the resistance of mice to Listeria monocytogenes infection. J. Immunol. 152:1836-1846. [PubMed] [Google Scholar]
- 10.Denning, D. W., L. Hall, M. Jackson, and S. Hollis. 1995. Efficacy of D0870 compared with those of itraconazole and amphotericin B in two murine models of invasive aspergillosis. Antimicrob. Agents Chemother. 39:1809-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dixon, T. C., A. A. Fadl, T. M. Koehler, J. A. Swanson, and P. C. Hanna. 2000. Early Bacillus anthracis-macrophage interactions: intracellular survival and escape. Cell. Microbiol. 2:453-463. [DOI] [PubMed] [Google Scholar]
- 12.Dixon, T. C., M. Meselson, J. Guillemin, and P. C. Hanna. Anthrax. N. Engl. J. Med. 341:815-826. [DOI] [PubMed]
- 13.Drysdale, M., S. Heninger, J. Hutt, Y. Chen, C. R. Lyons, and T. M. Koehler. 2005. Capsule synthesis by Bacillus anthracis is required for dissemination in murine inhalation anthrax. EMBO J. 24:221-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.During, R. L., W. Li, B. Hao, J. M. Koenig, D. S. Stephens, C. P. Quinn, and F. S. Southwick. 2005. Anthrax lethal toxin paralyzes neutrophil actin-based motility. J. Infect. Dis. 192:837-845. [DOI] [PubMed] [Google Scholar]
- 15.Fitz-James, P. C. 1953. The structure of spores as revealed by mechanical disruption. J. Bacteriol. 66:312-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedlander, A. M. 2000. Anthrax: clinical features, pathogenesis, and potential biological warfare threat. Curr. Clin. Top. Infect. Dis. 20:335-349. [PubMed] [Google Scholar]
- 17.Friedlander, A. M., S. L. Welkos, M. L. M. Pitt, J. W. Ezzell, P. L. Worsham, K. J. Rose, B. E. Ivins, J. R. Lowe, G. B. Howe, P. Miksell, et. al. 1993. Post-exposure prophylaxis against experimental inhalation anthrax. J. Infect. Dis. 167:129-142. [DOI] [PubMed] [Google Scholar]
- 18.Furukawa, S., M. Shimoda, and I. Hayakawa. 2003. Mechanism of the inactivation of bacterial spores by reciprocal pressurization treatment. J. Appl. Microbiol. 94:836-841. [DOI] [PubMed] [Google Scholar]
- 19.Gregory, S. H., A. J. Sagnimeni, and E. J. Wing. 1996. Bacteria in the bloodstream are trapped in the liver and killed by immigrating neutrophils. J. Immunol. 157:2514-2520. [PubMed] [Google Scholar]
- 20.Grinberg, L. M., F. A. Abramova, O. V. Yampolskaya, D. H. Walker, and J. H. Smith. 2001. Quantitative pathology of inhalational anthrax I: quantitative microscopic findings. Mod. Pathol. 14:482-495. [DOI] [PubMed] [Google Scholar]
- 21.Guidi-Rontani, C., M. Levy, H. Ohayon, and M. Mock. 2001. Fate of germinated Bacillus anthracis spores in primary murine macrophages. Mol. Microbiol. 42:931-938. [DOI] [PubMed] [Google Scholar]
- 22.Guidi-Rontani, C. 2002. The alveolar macrophage: the Trojan horse of Bacillus anthracis. Trends Microbiol. 10:405-409. [DOI] [PubMed] [Google Scholar]
- 23.Guidi-Rontani, C., Y. Pereira, S. Ruffie, J.-C. Sirard, M. Weber-Levy, and M. Mock. 1999. Identification and characterization of a germination operon on the virulence plasmid pXO1 of Bacillus anthracis. Mol. Microbiol. 33:407-414. [DOI] [PubMed] [Google Scholar]
- 24.Guidi-Rontani, C., M. Weber-Levy, E. Labruyere, and M. Mock. 1999. Germination of Bacillus anthracis spores within alveolar macrophages. Mol. Microbiol. 31:9-17. [DOI] [PubMed] [Google Scholar]
- 25.Hachicha, M., P. Rathanaswami, P. H. Naccache, and S. R. McColl. 1998. Regulation of chemokine gene expression in human peripheral blood neutrophils phagocytosing microbial pathogens. J. Immunol. 160:449-454. [PubMed] [Google Scholar]
- 26.Hanna, P. 1998. Anthrax pathogenesis and host response. Curr. Top. Microbiol. Immunol. 225:13-35. [DOI] [PubMed] [Google Scholar]
- 27.Hanna, P. C., and J. A. W. Ireland. 1999. Understanding Bacillus anthracis pathogenesis. Trends Microbiol. 7:180-182. [DOI] [PubMed] [Google Scholar]
- 28.Harvill, E. T., G. Lee, V. K. Grippe, and T. J. Merkel. 2005. Complement depletion renders C57BL/6 mice sensitive to the Bacillus anthracis Sterne strain. Infect. Immun. 73:4420-4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huitinga, I., J. G. Damoiseaux, N. van Rooijen, E. A. Dopp, and C. D. Dijkstra. 1992. Liposome mediated affection of monocytes. Immunobiology 185:11-19. [DOI] [PubMed] [Google Scholar]
- 30.Ireland, J., and P. C. Hanna. 2002. Macrophage-enhanced germination of Bacillus anthracis endospores requires gerS. Infect. Immun. 70:5870-5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jedrzejas, M. J., and W. J. Huang. 2003. Bacillus species proteins involved in spore formation and degradation: from identification in the genome, to sequence analysis, and determination of function and structure. Crit. Rev. Biochem. Mol. Biol. 38:173-198. [DOI] [PubMed] [Google Scholar]
- 32.Knudsen, E., H. B. Benestad, T. Seierstad, and P. O. Iversen. 2004. Macrophages in spleen and liver direct the migration pattern of rat neutrophils during inflammation. Eur. J. Haematol. 73:109-122. [DOI] [PubMed] [Google Scholar]
- 33.Knudsen, E., P. O. Iversen, N. Van Rooijen, and H. B. Benestad. 2002. Macrophage-dependent regulation of neutrophil mobilization and chemotaxis during development of sterile peritonitis in the rat. Eur. J. Haematol. 69:284-296. [DOI] [PubMed] [Google Scholar]
- 34.Kumar, P., N. Ahuja, and R. Bhatnagar. 2002. Anthrax edema toxin requires influx of calcium for inducing cyclic AMP toxicity in target cells. Infect. Immun. 70:4997-5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leemans, J. C., N. P. Juffermans, S. Florquin, N. van Rooijen, M. J. Vervoordeldonk, A. Verbon, S. J. van Deventer, and T. van der Poll. 2001. Depletion of alveolar macrophages exerts protective effects in pulmonary tuberculosis in mice. J. Immunol. 166:4604-4611. [DOI] [PubMed] [Google Scholar]
- 36.Leighton, T. J., and R. H. Doi. 1971. The stability of messenger ribonucleic acid during sporulation in Bacillus subtilis. J. Biol. Chem. 246:3189-3195. [PubMed] [Google Scholar]
- 37.Lyons, C. R., J. Lovchik, J. Hutt, M. F. Lipscomb, E. Wang, S. Heninger, L. Berliba, and K. Garrison. 2004. Murine model of pulmonary anthrax: kinetics of dissemination, histopathology, and mouse strain susceptibility. Infect. Immun. 72:4801-4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayer-Scholl, A., P. Averhoff, and A. Zychlinsky. 2004. How do neutrophils and pathogens interact? Curr. Opin. Microbiol. 7:62-66. [DOI] [PubMed] [Google Scholar]
- 39.Misawa, R., C. Kawagishi, N. Watanabe, and Y. Kobayashi. 2001. Infiltration of neutrophils following injection of apoptotic cells into the peritoneal cavity. Apoptosis 6:411-417. [DOI] [PubMed] [Google Scholar]
- 40.Moayeri, M., N. W. Martinez, J. Wiggins, H. A. Young, and S. H. Leppla. 2004. Mouse susceptibility to anthrax lethal toxin is influenced by genetic factors in addition to those controlling macrophage sensitivity. Infect. Immun. 72:4439-4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mock, M., and A. Fouet. 2001. Anthrax. Annu. Rev. Microbiol. 55:647-671. [DOI] [PubMed] [Google Scholar]
- 42.Moir, A., B. M. Corfe, and J. Behravan. 2002. Spore germination. Cell. Mol. Life Sci. 59:403-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moir, A., and D. Smith. 1990. The genetics of bacterial spore germination. Annu. Rev. Microbiol. 44:5331-5553. [DOI] [PubMed] [Google Scholar]
- 44.Montes de Oca, R., A. J. Buendía, L. Del Río, J. Sánchez, J. Salinas, and J. A. Navarro. 2000. Polymorphonuclear neutrophils are necessary for the recruitment of CD8+ T cells in the liver in a pregnant mouse model of Chlamydophila abortus (Chlamydia psittaci serotype 1) infection. Infect. Immun. 68:1746-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Montes de Oca, R., A. J. Buendia, J. Sanchez, L. Del Rio, J. Seva, J. A. Navarro, and J. Salinas. 2000. Limited role of polymorphonuclear neutrophils in a pregnant mouse model of secondary infection by Chlamydophila abortus (Chlamydia psittaci serotype 1). Microb. Pathog. 29:319-327. [DOI] [PubMed] [Google Scholar]
- 46.O'Brien, J., A. Friedlander, T. Dreier, J. Ezzell, and S. Leppla. 1985. Effects of anthrax toxin components on human neutrophils. Infect. Immun. 47:306-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pickering, A. K., M. Osorio, G. M. Lee, V. K. Grippe, M. Bray, and T. J. Merkel. 2004. Cytokine response to infection with Bacillus anthracis spores. Infect. Immun. 72:6382-6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Popov, S. G., T. G. Popova, E. Grene, F. Klotz, J. Cardwell, C. Bradburne, Y. Jama, M. Maland, J. Wells, A. Nalca, T. Voss, C. Bailey, and K. Alibek. 2004. Systemic cytokine response in murine anthrax. Cell. Microbiol. 6:225-233. [DOI] [PubMed] [Google Scholar]
- 49.Qian, Q., M. A. Jutila, N. Van Rooijen, and J. E. Cutler. 1994. Elimination of mouse splenic macrophages correlates with increased susceptibility to experimental disseminated candidiasis. J. Immunol. 152:5000-5008. [PubMed] [Google Scholar]
- 50.Ross, J. M. 1957. The pathogenesis of anthrax following the administration of spores by the respiratory route. J. Pathol. Bacteriol. 73:485-494. [Google Scholar]
- 51.Rousseau, D., S. Demartino, B. Ferrua, J. F. Michiels, F. Anjuere, K. Fragaki, Y. Le Fichoux, and J. Kubar. 2001. In vivo involvement of polymorphonuclear neutrophils in Leishmania infantum infection. BMC Microbiol. 1:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith, L. E., M. Rodrigues, and D. G. Russell. 1991. The interaction between CD8+ cytotoxic T cells and Leishmania-infected macrophages. J. Exp. Med. 174:499-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tateda, K., T. A. Moore, J. C. Deng, M. W. Newstead, X. Zeng, A. Matsukawa, M. S. Swanson, K. Yamaguchi, and T. J. Standiford. 2001. Early recruitment of neutrophils determines subsequent T1/T2 host responses in a murine model of Legionella pneumophila pneumonia. J. Immunol. 166:3355-3361. [DOI] [PubMed] [Google Scholar]
- 54.Tumpey, T. M., S. H. Chen, J. E. Oakes, and R. N. Lausch. 1996. Neutrophil-mediated suppression of virus replication after herpes simplex virus type 1 infection of the murine cornea. J. Virol. 70:898-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Rooijen, N., N. Kors, and G. Kraal. 1989. Macrophage subset repopulation in the spleen: differential kinetics after liposome-mediated elimination. J. Leukoc. Biol. 45:97-104. [DOI] [PubMed] [Google Scholar]
- 56.Van Rooijen, N., and A. Sanders. 1994. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J. Immunol. Methods 174:83-93. [DOI] [PubMed] [Google Scholar]
- 57.Wade, B. H., G. G. Wright, E. L. Hewlett, S. H. Leppla, and G. L. Mandell. 1985. Anthrax toxin components stimulate chemotaxis of human polymorphonuclear neutrophils. Proc. Soc. Exp. Biol. Med. 179:159-162. [DOI] [PubMed] [Google Scholar]
- 58.Warn, P. A., J. Morrissey, C. B. Moore, and D. W. Denning. 2000. In vivo activity of amphotericin B lipid complex in immunocompromised mice against fluconazole-resistant or fluconazole-susceptible Candida tropicalis. Antimicrob. Agents Chemother. 44:2664-2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Welkos, S., A. Friedlander, S. Weeks, S. Little, and I. Mendelson. 2002. In-vitro characterisation of the phagocytosis and fate of anthrax spores in macrophages and the effects of anti-PA antibody. J. Med. Microbiol. 51:821-831. [DOI] [PubMed] [Google Scholar]
- 60.Welkos, S., S. Little, A. Friedlander, D. Fritz, and P. Fellows. 2001. The role of antibodies to Bacillus anthracis and anthrax toxin components in inhibiting the early stages of infection by anthrax spores. Microbiology 147:1677-1685. [DOI] [PubMed] [Google Scholar]
- 61.Welkos, S. L., C. K. Cote, K. M. Rea, and P. H. Gibbs. 2004. A microtiter fluorometric assay to detect the germination of Bacillus anthracis spores and the germination inhibitory effects of antibodies. J. Microb. Methods 56:253-265. [DOI] [PubMed] [Google Scholar]
- 62.Welkos, S. L., and A. M. Friedlander. 1988. Pathogenesis and genetic control of resistance to the Sterne strain of Bacillus anthracis. Microb. Pathog. 4:53-69. [DOI] [PubMed] [Google Scholar]
- 63.Welkos, S. L., T. J. Keener, and P. H. Gibbs. 1986. Differences in susceptibility of inbred mice to Bacillus anthracis. Infect. Immun. 51:795-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Welkos, S. L., R. W. Trotter, D. M. Becker, and G. O. Nelson. 1989. Resistance to the Sterne strain of B. anthracis: phagocytic cell responses of resistant and susceptible mice. Microb. Pathog. 7:15-36. [DOI] [PubMed] [Google Scholar]
- 65.Wijburg, O. L., C. P. Simmons, N. van Rooijen, and R. A. Strugnell. 2000. Dual role for macrophages in vivo in pathogenesis and control of murine Salmonella enterica var. Typhimurium infections. Eur. J. Immunol. 30:944-953. [DOI] [PubMed] [Google Scholar]
- 66.Wright, G. G., and G. L. Mandell. 1986. Anthrax toxin blocks priming of neutrophils by lipopolysaccharide and by muramyl dipeptide. J. Exp. Med. 164:1700-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu, A. G., D. Alibek, Y. L. Li, C. Bradburne, C. L. Bailey, and K. Alibek. 2003. Anthrax toxin induces hemolysis: an indirect effect through polymorphonuclear cells. J. Infect. Dis. 188:1138-1141. [DOI] [PubMed] [Google Scholar]
- 68.Wuytack, E. Y., J. Soons, F. Poschet, and C. W. Michiels. 2000. Comparative study of pressure- and nutrient-induced germination of Bacillus subtilis spores. Appl. Environ. Microbiol. 66:257-261. [DOI] [PMC free article] [PubMed] [Google Scholar]