Abstract
Analysis of human buccal epithelial cells frequently reveals an intracellular polymicrobial consortium of bacteria. Although several oral bacteria have been demonstrated to invade cultured epithelial cells, several others appear unable to internalize. We hypothesized that normally noninvasive bacteria may gain entry into epithelial cells via adhesion to invasive bacteria. Fusobacterium nucleatum is capable of binding to and invading oral epithelial cells. By contrast, Streptococcus cristatus binds weakly to host cells and is not internalized. F. nucleatum and S. cristatus coaggregate strongly via an arginine-sensitive interaction. Coincubation of KB or TERT-2 epithelial cells with equal numbers of F. nucleatum and S. cristatus bacteria led to significantly increased numbers of adherent and internalized streptococci. F. nucleatum also promoted invasion of KB cells by other oral streptococci and Actinomyces naeslundii. Dissection of fusobacterial or streptococcal adhesive interactions by using sugars, amino acids, or antibodies demonstrated that this phenomenon is due to direct attachment of S. cristatus to adherent and invading F. nucleatum. Inhibition of F. nucleatum host cell attachment and invasion with galactose, or fusobacterial-streptococcal coaggregation by the arginine homologue l-canavanine, abrogated the increased S. cristatus adhesion to, and invasion of, host cells. In addition, polyclonal antibodies to F. nucleatum, which inhibited fusobacterial attachment to both KB cells and S. cristatus, significantly decreased invasion by both species. Similar decreases were obtained when epithelial cells were pretreated with cytochalasin D, staurosporine, or cycloheximide. These studies indicate that F. nucleatum may facilitate the colonization of epithelial cells by bacteria unable to adhere or invade directly.
The oral epithelium is heavily colonized by a diverse array of bacteria, apparently without detriment to the host. Such colonization is likely the result of complex interbacterial and host-bacterial interactions. The presence of certain microbes may predispose to colonization by others by production of favorable growth conditions, promotion of adhesion, or suppression of host immune factors (2).
Periodontitis occurs when plaque accumulates on the surface of the tooth, deepening the gingival crevice (50). Deep pockets favor the growth of proteolytic gram-negative anaerobes. These bacteria promote destruction of the tissues supporting the tooth (50) and may invade adjacent pocket epithelial cells (62). In addition, bacteria associated with disease can also be detected on other soft tissues (29). These bacteria are hypothesized to act as a reservoir for the reinfection of sites following dental treatment (44, 65), in keeping with the persistent nature of periodontal disease (10, 65). Beyond the oral cavity, oral bacteria have been implicated in several systemic conditions, including atherosclerosis (7, 33), stroke (18, 66), and low weight and preterm birth of infants (31, 36).
The buccal mucosa is colonized by a wide range of different species, including those associated with periodontal disease (29). Such bacteria have been found to exist in polymicrobial communities within buccal epithelial cells (44, 45). The polymicrobial nature of these communities, together with their intracellular location, may explain how strict anaerobes are able to survive outside the gingival crevice. In addition, invasion of host cells by bacteria protects against host immune system components and salivary flow. Since not all bacteria appear to be capable of invading host cells independently (9, 14, 23, 63), it is possible that interbacterial interactions are necessary to create such intracellular diversity.
Fusobacterium nucleatum, a gram-negative fusiform anaerobe, is able to adhere to and invade human epithelial cells (14, 64, 67). Additionally, F. nucleatum adheres to erythrocytes (6, 12, 48), polymorphonuclear leukocytes (20, 30, 37), and lymphocytes (57, 58, 64). It also binds fibronectin (1) and plasminogen (5). Likewise, F. nucleatum is able to adhere to a wide range of other oral bacteria (coaggregation), including both early (Actinomyces spp., Streptococcus spp.) (16, 48, 52) and late (Porphyromonas gingivalis, Treponema denticola) (21, 22, 48) colonizers. The interaction of F. nucleatum with host cells or other bacteria is often inhibited by lactose (or galactose) (14, 22, 30, 48) or l-arginine (6, 20, 27, 51, 52, 58).
The role of F. nucleatum in periodontitis is unclear. F. nucleatum is the most numerous gram-negative bacterium in the oral cavity during health, but the mass of F. nucleatum increases significantly during active periodontal disease (59). In addition, several studies indicate that fusobacteria, with other bacteria, penetrate the epithelium during periodontitis (3). It is possible that F. nucleatum plays an indirect role in the progression of periodontitis by forming a bridge between the primary colonizers of the tooth surface (streptococci and actinomycetes) and later colonizers such as P. gingivalis, Tannerella forsythia, and T. denticola, which are strongly associated with periodontal disease (21, 59). One early colonizer that F. nucleatum adheres to is Streptococcus cristatus, a member of the mitis group of streptococci that has been isolated from both dental plaque and the soft tissues of the oral cavity (15, 56). This bacterium coaggregates strongly with F. nucleatum in an l-arginine-sensitive manner. The interaction is believed to rely on a high-molecular-weight, serine-rich protein on the surface of S. cristatus (16). Although this interaction has been well characterized, the fusobacterial adhesin has not been conclusively identified. Coaggregation between F. nucleatum and S. cristatus can lead to the formation of “corncobs,” whereby large numbers of streptococci can attach to a single F. nucleatum cell (25). These interactions may be beneficial for the fusobacteria since S. cristatus promotes the survival of F. nucleatum in saliva (43).
Recent work has suggested that coaggregation may promote attachment to epithelial cells by nonoral species (24). We hypothesized that the invasive nature of F. nucleatum, combined with its ability to coaggregate with a range of oral bacteria, might enable this bacterium to facilitate the internalization of normally noninvasive bacteria in epithelial cells.
MATERIALS AND METHODS
Bacterial strains and culture.
S. cristatus American Type Culture Collection (ATCC) 49999 (CC5A), S. cristatus ATCC 51110, Streptococcus gordonii DL-1, S. sanguinis SK36, Actinomyces naeslundii ATCC 12104, and F. nucleatum subsp. polymorphum ATCC 10953 were cultured at 37°C in an anaerobic environment (N2-H2-CO2 at 8:1:1). Streptococci were cultured in Todd-Hewitt broth (Sigma, St. Louis, MO) and A. naeslundii and F. nucleatum in TSY, which is tryptic soy broth (Becton Dickinson, Sparks, MD) supplemented with 0.1% yeast extract (Becton Dickenson), hemin (5 μg/ml; Sigma), and menadione (1 μg/ml; Sigma). Bacteria were recovered by centrifugation (7,000 × g, 15 min) and washed in phosphate-buffered saline (PBS) before adjustment to 108 CFU/ml in buffer by alteration of the optical density (OD) to a reference standard. If two different bacteria were to be used, these were mixed by vortexing for 10 s prior to use in adhesion or invasion assays. Bacteria were enumerated by anaerobic culture on TSY supplemented with 1.5% agar (Sigma) and 5% sterile defibrinated sheep blood.
Selection of a spontaneous coaggregation-deficient F. nucleatum mutant.
F. nucleatum cells were subjected to selection pressures in order to isolate spontaneous mutants defective in adhesive properties. It has been hypothesized that the outer membrane protein FomA is important in coaggregation (17). In addition, this protein is believed to mediate immunoglobulin binding (13). We thus hypothesized that isolates defective in salivary immunoglobulin A (S-IgA) binding might be defective in coaggregation. Approximately 108 F. nucleatum cells were mixed with purified S-IgA (0.05 mg/ml; Sigma) by vortexing for 30 s at room temperature. Agglutinated cells were allowed to settle and the supernatant recovered and cultured as described above. This procedure was repeated 10 times before supernatants were plated on TSY agar as described above. Individual colonies were assessed for S-IgA binding by dot blotting and agglutination in the presence of S-IgA. Isolate 21 was selected for deficiency in IgA binding. That isolate was also defective in coaggregation with S. cristatus CC5A as determined by the assays described below.
Coaggregation assay.
The effects of various inhibitors or polyclonal antisera on coaggregation between F. nucleatum and S. cristatus were assessed by various techniques, including microscopy, a visual scoring assay, and a quantitative assay based on that of Lafontaine et al. (24). Briefly, F. nucleatum cells (1 ml, OD620 = 1.0) were washed and fixed in methanol (15 min at room temperature [RT]), washed again, and biotinylated (1 mg/ml Sulfo-NHS-LC-biotin; Pierce, Rockford, IL) for 30 min at RT with rotation. Cells were washed and mixed with 10 μl streptavidin-coated magnetic beads (Dynal Biotech ASA, Oslo, Norway) for 30 min at RT with rotation. Fusobacterium-bead complexes were reduced to 200 μl by centrifugation and resuspension and mixed with 0.8 ml of S. cristatus (OD620 = 1.0) by vortexing for 5 s. A magnetic stand was used to remove magnetic bead-fusobacterium-streptococcus aggregates. Analysis of the amount of remaining S. cristatus in the supernatant revealed the degree of attachment to F. nucleatum isolates.
Generation of antibodies.
F. nucleatum or S. cristatus bacteria were cultured as described above, washed three times with PBS, and fixed with 99% methanol at 4°C overnight. Methanol was removed by washing in PBS. Fixed cells were sent to Pacific Immunology Corp. (Ramona, Calif.) for the generation of polyclonal antisera in rabbits. Immune sera were screened against preimmune blood samples to confirm the generation of specific antibodies. In addition, antiserum raised against F. nucleatum was screened to ensure no cross-reactivity with S. cristatus and vice versa.
Epithelial cell culture.
The KB cell line was kindly provided by Mark Herzberg (University of Minnesota) and maintained in Dulbecco minimal essential medium (GIBCO, Invitrogen, Grand Island, NY) supplemented with 10% fetal bovine serum (GIBCO) at 37°C in a humidified atmosphere of 5% CO2 as described previously (35). KB cells were originally described as an oral epithelial cancer line and have been extensively used as a tissue culture model in studying the interactions between oral bacteria, including F. nucleatum, and epithelial cells (14, 32, 46, 47, 54, 60). Although KB cells deposited with ATCC are now considered to have been contaminated with HeLa cells, they are not cytogenetically identical to ATCC HeLa cells (26). It is also important to note that the HeLa cell line is of epithelial origin, and oral bacterial gene expression on invasion of HeLa cells seems similar to what occurs in the mouth (40). In addition, F. nucleatum appears to invade KB cells in a manner similar to that of primary human gingival epithelial cells (14). Moreover, the line of KB cells employed in this study was not obtained from ATCC and it is phenotypically distinct from HeLa cells and KB cells ordered from ATCC (Mark Herzberg, personal communication). We therefore continue to use the original name in this report, pending definitive resolution of the identity of this cell line.
Oral keratinocyte TERT-2 cells (8) were supplied by the Brigham and Women's Hospital Cell Culture Core (Boston, MA) and were cultured in keratinocyte serum-free medium (K-SFM; Invitrogen) supplemented with CaCl2 (0.3 mM), bovine pituitary extract (30 μg/ml), and epidermal growth factor (0.1 ng/ml).
Epithelial cells were cultured in T75 flasks at 37°C with 5% CO2 until approximately 70% confluent. Cells were detached with trypsin and EDTA (Invitrogen), recovered by centrifugation, and used to seed 24-well plates at 105 cells per well. Plates were incubated for 24 h as described above prior to use in assays. Metabolic inhibitors (cytochalasin D at 1 μg/ml, staurosporine at 1 μM, and cycloheximide at 100 μg/ml) were preincubated with monolayers for 1 h (37°C, 5% CO2) prior to addition of bacteria. Concentrations of inhibitors were maintained throughout the course of the assay. Monolayers were also grown on gelatin-coated coverslips for use in immunofluorescent labeling and analysis by confocal microscopy or scanning electron microscopy (SEM).
Adhesion assay.
Adhesion of F. nucleatum and S. cristatus to epithelial cells was determined by plate counts of associated bacteria and by laser scanning confocal microscopy (see below). KB or TERT-2 cells were prepared as described above, washed with Dulbecco's PBS (DPBS), and incubated with bacteria in Earle's balanced salt solution, with the bacteria at a multiplicity of infection of 10, in the presence of the indicated concentrations of various inhibitors for 4 h at 37°C in 5% CO2. Nonadherent bacteria were removed by three rounds of washing with DPBS before lysis of the monolayer with 1 ml double-distilled H2O. Lysates were diluted in 10-fold steps with minimal essential medium containing 10 mM l-arginine (to disrupt coaggregates) and portions plated on TSY agar (as described above) and incubated under anaerobic conditions for 48 to 72 h to enumerate surviving bacteria. Although this is described as adhesion, viable counts included adherent and internalized bacteria.
Invasion assay.
Invasion was assessed by the antibiotic protection assay as described previously (35) and by laser scanning confocal microscopy. Briefly, KB or TERT-2 cells were washed with DPBS and incubated with bacteria for 4 h at 37°C in 5% CO2. Nonadherent bacteria were removed by washing with PBS. Monolayers were then incubated with 1 ml of minimal essential medium containing 200 μg/ml gentamicin (Sigma), 5 μg/ml penicillin G (Sigma), and 300 μg/ml metronidazole (Sigma) for 1.5 h. Antibiotics were then removed by washing with DPBS and epithelial cells lysed with 1 ml sterile, double-distilled H2O. Surviving bacteria were enumerated as described for adhesion assays. To ensure the effectiveness of the antimicrobials in coincubation experiments, coaggregates of 107 F. nucleatum bacteria and the other bacteria used were exposed to the antibiotics described under identical conditions, broken up with arginine, and plated for viable colonies. Fewer than 10 CFU/ml were recovered.
Inhibition of bacterial adhesion, invasion, or coaggregation.
F. nucleatum or S. cristatus cells were preincubated with the indicated concentrations of galactose (inhibitor of F. nucleatum adhesion/invasion), l-canavanine (analogue of l-arginine and inhibitor of coaggregation), anti-S. cristatus serum, or anti-F. nucleatum serum for 15 min at RT prior to use in assays. None of these reagents exhibited toxicity to either bacteria or KB cells at the concentrations used.
Confocal microscopy.
Monolayers grown on gelatin-coated coverslips were incubated with bacteria under various conditions as described above for adhesion assay. Coverslips were washed in DPBS and fixed in −20°C methanol at 4°C for 20 min. Adherent streptococci were detected with anti-S. cristatus serum (diluted 1:400), followed by fluorescein isothiocyanate (FITC)-conjugated anti-rabbit serum (1:400; Sigma). F. nucleatum was stained with anti-F. nucleatum serum (1:200) and tetramethyl rhodamine isothiocyanate (TRITC)-conjugated anti-rabbit antibodies (1:400; Sigma). Thus, in coincubation adhesion assays S. cristatus cells appeared yellow and F. nucleatum red.
Dual labeling of infected monolayers was employed to discriminate intracellular from extracellular bacteria (4, 53). Monolayers were grown on gelatin-coated coverslips and incubated with bacteria as described above. After 4 h, coverslips were washed in ice-cold PBS and incubated with a 1:200 dilution of antibody to F. nucleatum or S. cristatus for 20 min at 4°C. Coverslips were washed and incubated with 1:200 anti-rabbit FITC-conjugated antibody and incubated as described above to stain extracellular bacteria. Coverslips were then washed and permeabilized with −20°C methanol for 30 min at 4°C. After washing, internalized bacteria were stained with primary antibody and anti-rabbit TRITC-conjugated secondary antibody as described above. Thus, extracellular bacteria appeared yellow and intracellular bacteria red when images taken with red and green filters were merged. F. nucleatum could be distinguished from S. cristatus on the basis of cellular morphology.
SEM.
Coverslips of epithelial cells incubated with bacteria were washed and fixed with 2% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4 with 0.15% alcian blue 8GX) for 1 h (11). Samples were washed in 0.1 M cacodylate buffer for (5 min), followed by secondary fixation (1% OsO4 in 0.1 M cacodylate buffer) for 1 h. Samples were washed with 0.1 M cacodylate buffer (5 min), followed by dehydration with ethanol and critical point dried. Dehydrated samples were coated for 12 min with platinum using an Ion Tech argon ion beam coater and imaged on a Hitachi S-4700 field emission SEM operated at 5,000 eV.
Statistics.
Statistical analyses were performed with Student's t test by using the Bonferroni correction for multiple comparisons. Values that were statistically significantly different from control values are indicated by asterisks in the figures. Error bars indicate the mean average ± standard deviation of three independent experiments performed in triplicate.
RESULTS
Coaggregation.
Examination of the coaggregation of F. nucleatum and S. cristatus confirmed previous reports that l-arginine but not d-galactose significantly inhibited this interaction (19). However, preliminary experiments indicated that, over 4 h incubation at 37°C, l-arginine but not l-canavanine (an l-arginine analogue) lost its ability to inhibit coaggregation between F. nucleatum and S. cristatus, most likely due to hydrolysis by the streptococci (15). l-Canavanine inhibited coaggregation by approximately 80% at 1 mM. In addition, antibodies to F. nucleatum but not S. cristatus inhibited coaggregation (by approximately 50% at a 1:1,000 dilution). A spontaneous mutant of F. nucleatum defective in immunoglobulin binding (isolate 21) was 95% reduced in streptococcal adhesion relative to the wild type (data not shown).
F. nucleatum enhances S. cristatus adhesion to and invasion of human epithelial cells.
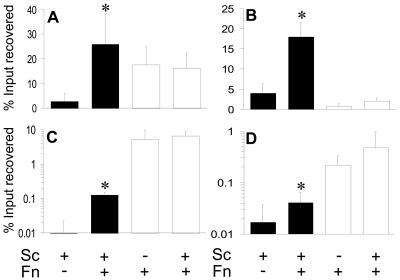
Viable counts of F. nucleatum or S. cristatus incubated with KB or TERT-2 cells were used to determine bacterial adhesion or invasion. F. nucleatum adhered strongly to KB cells (17.6% input, Fig. 1A) but S. cristatus did not (2.9%, Fig. 1A). However, coincubation of F. nucleatum and S. cristatus with KB cells boosted streptococcal attachment almost 10-fold (26%, Fig. 1A) but did not significantly affect F. nucleatum adhesion (16.%, Fig. 1A). F. nucleatum adhesion to TERT-2 cells was much lower (0.8% of input recovered, Fig. 1B) but was enhanced in the presence of S. cristatus (2%, Fig. 1B). Despite the lower level of adhesion, F. nucleatum enhanced S. cristatus binding in a manner similar to that for KB cells (from 4% to 18%, Fig. 1B).
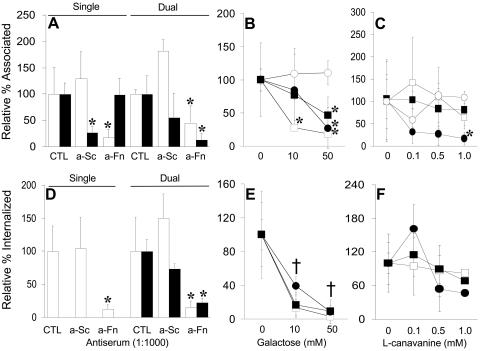
FIG. 1.
Percentages of CFU of S. cristatus (Sc; filled bars) or F. nucleatum (Fn; open bars) recovered from epithelial cells after 4 h of incubation (relative to the input) independently or in dual-incubation assays. Graphs indicate either total associated bacteria (adherent plus internalized; A and B) or those surviving incubation with antibiotics (internalized; C and D). Assays were performed with KB (A and C) or TERT-2 (B and D) epithelial cells. Error bars indicate the mean average ± the standard deviation of four independent experiments performed in triplicate. Values that differ from controls by a statistically significant amount are indicated (*).
Invasion of epithelial cells was determined by the antibiotic protection assay. F. nucleatum invaded KB cells with high efficiency (up to 5% of input recovered, Fig. 1C). By contrast, S. cristatus invasion was at the lower limit of detection (<0.01% of input, Fig. 1C). However, in the presence of F. nucleatum, the number of recovered S. cristatus bacteria dramatically increased to 0.1% of the input (Fig. 1C) while the number of internalized F. nucleatum bacteria was not significantly changed (Fig. 1C). F. nucleatum invaded TERT-2 cells less efficiently than KB cells (0.22% of input recovered) but similarly enhanced S. cristatus internalization from 0.02% to 0.041% of the input. F. nucleatum invasion was doubled in the presence of S. cristatus (0.48%).
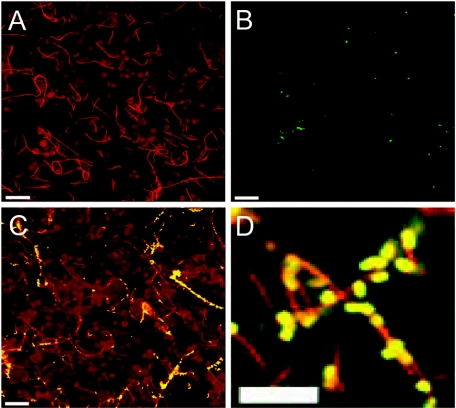
The viable-count data were confirmed by confocal microscopy (Fig. 2 and 3). F. nucleatum bacteria adhered in large numbers to KB cells (Fig. 2A), but S. cristatus bacteria did not (Fig. 2B). However, coincubation of F. nucleatum and S. cristatus promoted streptococcal adhesion significantly (Fig. 2C). Microscopy at high magnification indicated that most of the streptococci were closely associated with F. nucleatum (Fig. 2D). Interestingly, some F. nucleatum cells appeared to bind many S. cristatus cells while others appeared to bind few or no streptococci (Fig. 2C).
FIG. 2.
Confirmation of adhesion of F. nucleatum or S. cristatus to KB cells by confocal microscopy. Fixed monolayers with adherent bacteria were stained with polyclonal antisera to F. nucleatum or S. cristatus and FITC- or TRITC-conjugated antibodies. F. nucleatum only, red in panel A; S. cristatus only, green or yellow in panel B; F. nucleatum and S. cristatus together, panels C and D. Scale bars represent 20 μm (A, B, and C) or 10 μm (D).
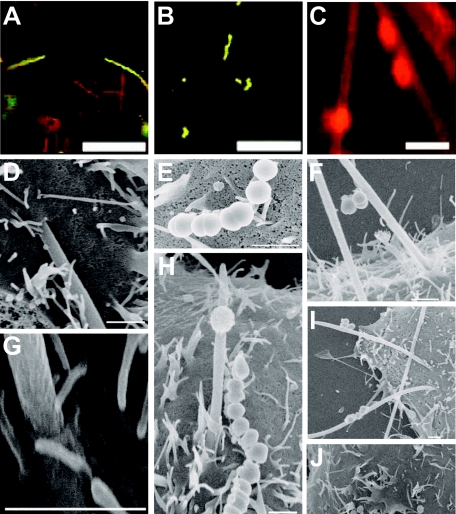
FIG. 3.
Assessment of internalization of F. nucleatum or S. cristatus in KB epithelial cells by confocal microscopy and SEM. Dual-antibody staining demonstrated that F. nucleatum could adhere to (yellow) and invade (red) KB cells (A). S. cristatus attached to but did not invade (B) unless in the presence of F. nucleatum, when internalization of both species occurred (C). SEM of KB cells incubated with F. nucleatum (D and G) confirmed internalization of this species and lack of internalization of S. cristatus (E). Examination of KB monolayers incubated with both F. nucleatum and S. cristatus revealed the direct attachment of streptococci to F. nucleatum bacteria that were in the process of internalization (H, F, and I). SEM of KB cells incubated in buffer only (J) demonstrated that cellular projections were not due to the presence of bacteria. Scale bars represent 20 μm (A and B), 2 μm (C), or 1 μm (D, E, F, G, H, and I).
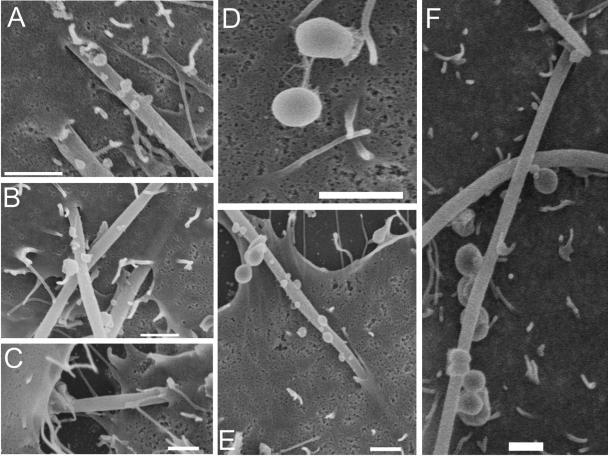
Invasion data were confirmed by dual-antibody staining and SEM (Fig. 3). Dual-antibody staining demonstrated adherent and internalized F. nucleatum (Fig. 3A). Internalized S. cristatus bacteria were not observed (Fig. 3B), except in the presence of F. nucleatum (Fig. 3C). SEM demonstrated that F. nucleatum invaded KB epithelial cells in a polar manner (Fig. 3D and G), rather than along the length of the bacterium. This is in keeping with previous observations (14). The host cell membrane exhibited projections. These did not appear to be caused by the bacteria, since epithelial cells incubated in buffer alone had similar characteristics (Fig. 3J). S. cristatus bacteria attached in low numbers, and adhesion appeared to be mediated via polar tufts (Fig. 3E). Although some large streptococcal-fusobacterial coaggregates were observed (data not shown), the majority consisted of only a few bacteria (Fig. 3F, H, and I). F. nucleatum bacteria in the process of invasion were observed with adherent S. cristatus bacteria (Fig. 3F, H, and I), suggesting that the streptococci internalize via adhesion to invading F. nucleatum. SEM of TERT-2 cells incubated with F. nucleatum and/or S. cristatus revealed results similar to those for KB cells. F. nucleatum were frequently observed invading epithelial cells (Fig. 4A and B). However, many fusobacteria exhibited membrane blebbing (Fig. 4A, B, E, and F). Occasionally, F. nucleatum cells were observed apparently migrating between epithelial cells (Fig. 4C). S. cristatus adhered to but was not observed to invade the TERT-2 cells (Fig. 4D). Dual-incubation experiments revealed S. cristatus attached to F. nucleatum cells in the process of invasion (Fig. 4E and F).
FIG. 4.
Invasion of TERT-2 cells by F. nucleatum or S. cristatus. F. nucleatum readily invaded TERT-2 cells (A and B), although blebbing of the fusobacterial membrane was observed for the majority of the bacteria (A, B, E, and F). There was evidence of intercellular migration by F. nucleatum (C). S. cristatus was rarely observed to adhere directly to epithelial cells; attachment occurred via polar tufts of fibrils (D). In coincubation experiments, invading F. nucleatum bacteria were observed with attached S. cristatus (E and F). Bars represent 1 μm.
Enhanced S. cristatus attachment and invasion are mediated by adhesion to adherent or invading F. nucleatum.
Although microscopy strongly suggested that enhanced S. cristatus adhesion and invasion were due to adhesion to F. nucleatum, various approaches were employed to dissect each of the interactions.
Adhesion of S. cristatus to KB cells was inhibited by anti-S. cristatus serum (diluted 1:1,000) by approximately 70% (Fig. 5A) but was unaffected by anti-F. nucleatum serum, which acted as a negative control (Fig. 5A). By contrast, F. nucleatum attachment was inhibited by anti-F. nucleatum serum (>80%) but not by anti-S. cristatus serum (Fig. 5A). In coincubation assays, antiserum to F. nucleatum inhibited the adhesion of both species while antibodies to S. cristatus had no significant effect on the adhesion of either bacterium (Fig. 5A).
FIG. 5.
Adhesion to (A) and invasion of (D) KB cells by S. cristatus, F. nucleatum, or both species together were examined in the presence of anti-S. cristatus serum (a-Sc) or anti-F. nucleatum serum (a-Fn). Adhesion of or invasion by S. cristatus (filled bars) and F. nucleatum (open bars) is shown relative to the level obtained in the absence of either antiserum. The effects of increasing concentrations of galactose or l-canavanine on adhesion (B and C, respectively) or invasion (E and F, respectively) were assessed for S. cristatus only (open circles), F. nucleatum only (open squares), or S. cristatus and F. nucleatum together (filled circles and squares, respectively). Values that differ from controls (CTL) by a statistically significant amount are indicated (*). If all the values for a given concentration are statistically significantly different from the control value, this is indicated by the symbol †. Since S. cristatus internalization alone remained at the limit of detection in the presence of galactose or l-canavanine, those values are not shown.
F. nucleatum internalization was inhibited by anti-F. nucleatum (90%) but not anti-S. cristatus serum (Fig. 5D). Neither antiserum affected S. cristatus internalization, which remained at the limit of detection (data not shown). Inhibition of streptococcal adhesion with anti-S. cristatus serum had no significant effect on the internalization of S. cristatus in coincubation experiments, but F. nucleatum invasion was promoted (Fig. 4D). By contrast, anti-F. nucleatum serum significantly decreased the internalization of both F. nucleatum and S. cristatus (Fig. 5D).
Since antibodies to F. nucleatum inhibited both fusobacterial adhesion and coaggregation, we investigated the role of each mechanism by inhibition with either galactose (adhesion and invasion) or l-canavanine (coaggregation). Galactose had no effect on S. cristatus adhesion (Fig. 5B) but caused a dose-dependent decrease in F. nucleatum attachment at 10 mM and 50 mM (Fig. 5B). Increasing concentrations of galactose decreased the attachment of both species in coincubation assays (this became statistically significant at 50 mM) (Fig. 5B). l-Canavanine had no significant effect on the adhesion of either F. nucleatum or S. cristatus independently (Fig. 5C). However, dual-incubation assays in the presence of 1 mM l-canavanine showed a significant decrease in the number of bound streptococci relative to controls (Fig. 5C).
The same conditions were utilized in invasion assays. Neither galactose or l-canavanine affected S. cristatus internalization (data not shown). Increasing concentrations of d-galactose inhibited invasion of KB cells by F. nucleatum in a dose-dependent manner (Fig. 5E) and significantly decreased invasion by both species in dual-incubation experiments (Fig. 5E). l-Canavanine caused a slight inhibition of fusobacterial invasion (Fig. 5F). Internalization of both F. nucleatum and S. cristatus was promoted by 0.1 mM l-canavanine in coinvasion experiments, but S. cristatus internalization was reduced at 1.0 mM (although not statistically significantly) (Fig. 5F). This may indicate that smaller coaggregates are more invasive than larger ones.
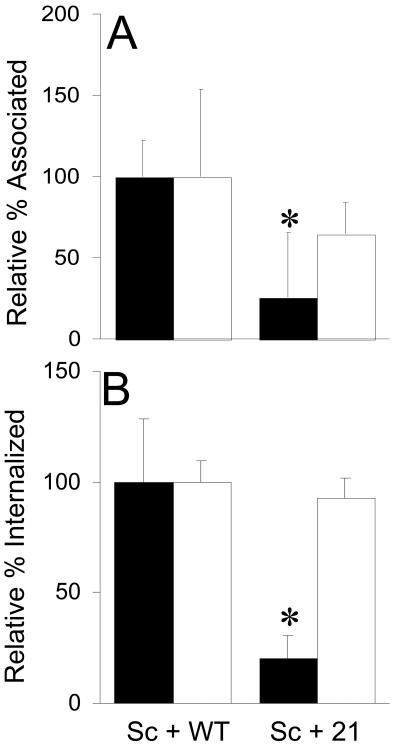
In order to further assess the role of coaggregation in the promotion of S. cristatus adhesion and invasion, we employed coaggregation-defective F. nucleatum isolate 21 (Fig. 6). Although displaying similar levels of adhesion and internalization, this isolate failed to support streptococcal adhesion (Fig. 6A) or invasion (Fig. 6B) to levels associated with wild-type F. nucleatum (Fig. 6).
FIG. 6.
Effect of a coaggregation-defective F. nucleatum isolate on S. cristatus adhesion or internalization. Adhesion to (A) or invasion of (B) KB cells by S. cristatus (filled bars) in the presence of wild-type F. nucleatum (WT, open bars) or isolate 21, a coaggregation-defective F. nucleatum isolate (21, open bars), was measured as described in Materials and Methods. Values are relative to those obtained with S. cristatus incubated in the presence of wild-type F. nucleatum.
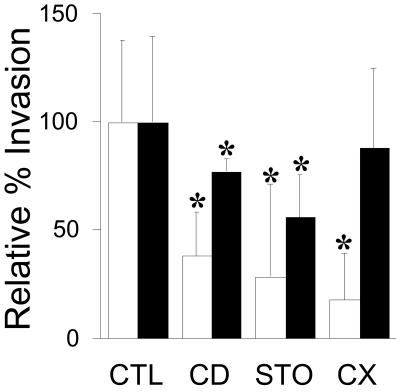
Finally, various metabolic inhibitors previously reported to reduce F. nucleatum invasion were assessed for the ability to inhibit Fusobacterium-enhanced S. cristatus invasion (Fig. 7). Cytochalasin D, staurosporine, and cycloheximide (which target actin, protein kinases, and host cell protein synthesis, respectively) each significantly inhibited invasion by both F. nucleatum and S. cristatus in coincubation experiments, with the exception of cycloheximide (Fig. 7). This inhibitor significantly reduced invasion by F. nucleatum but not that by S. cristatus.
FIG. 7.
Assessment of the effect of cytochalasin D (CD), staurosporine (STO), or cycloheximide (CX), relative to buffer only (control [CTL]), to inhibit invasion of KB cells by S. cristatus (filled bars) and F. nucleatum (open bars) in dual-incubation experiments. Relative percent inhibition is shown, and all values differed statistically significantly from those of untreated cells, except for S. cristatus in the presence of cycloheximide.
F. nucleatum promotes invasion of epithelial cells by other oral bacteria.
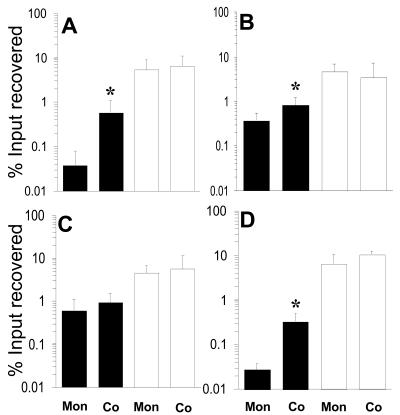
Having demonstrated that F. nucleatum facilitates the invasion of epithelial cells by S. cristatus, we sought to determine if this phenomenon is common to other bacteria. These assays focused on early colonizers that do not readily invade. F. nucleatum significantly enhanced invasion by S. cristatus ATCC 51110 (Fig. 8A), S. sanguinis SK36 (Fig. 8B), and A. naeslundii ATCC 12104 (Fig. 8D). F. nucleatum also promoted invasion by S. gordonii DL-1, but this was not statistically significant (Fig. 8C).
FIG. 8.
Effect of F. nucleatum on the internalization of various oral bacteria by KB cells. Percentages of recovered F. nucleatum (open bars) and other bacteria (closed bars) relative to the input were measured as described in Materials and Methods. Data from monoincubation (Mon) or coincubation (Co) assays are shown. Panels: A, S. cristatus ATCC 51110; B, S. sanguis SK36; C, S. gordonii DL-1; D, A. naeslundii ATCC 12104. Values that differ between invasion alone and in the presence of F. nucleatum by a statistically significant amount are indicated (*).
DISCUSSION
Whether in health or in disease, the oral cavity is host to a wide range of different bacteria. Coupled with the diverse environment of the mouth, this supports many complex interactions. In this report, we demonstrate that an invasive bacterium is able to support attachment to and invasion of human epithelial cells by an otherwise noninvasive species. Specifically, F. nucleatum dramatically increases adhesion to and invasion of KB or TERT-2 epithelial cells by S. cristatus. This process requires attachment of S. cristatus to F. nucleatum cells that subsequently adhere to and invade epithelial cells. In addition to S. cristatus, this mechanism appears to extend to other noninvasive streptococci and A. naeslundii. These data are in support of our hypothesis that invasive bacteria can transport noninvasive species into host cells via interbacterial binding.
Although the mechanism of invasion by F. nucleatum is not as well characterized as for P. gingivalis or Actinobacillus actinomycetemcomitans, it is an active process dependent on the host cytoskeleton and protein synthesis by both the host and the bacterium (14). It appears that the presence of S. cristatus had no significant effect on these processes since F. nucleatum invasion was not significantly affected. The scanning electron micrographs presented here suggest an invasion mechanism that may be similar to that of Streptococcus pyogenes invasion of HEp-2 epithelial cells and endothelial human umbilical vein endothelial cells, in which the bacteria interact with host cell caveolae (42). A similar mechanism has recently been demonstrated for P. gingivalis invasion of KB cells (54).
A difference in the ability of F. nucleatum to attach to and invade TERT-2 cells compared with KB cells (as determined by CFU counts and the antibiotic protection assay) was not confirmed by SEM. F. nucleatum appeared to bind to and invade TERT-2 cells in numbers and a manner comparable to its binding and invasion of KB cells. The blebbing of F. nucleatum cells that were in contact with TERT-2 cells may indicate that this cell line produces elements toxic to fusobacteria (but not streptococci). Since dead F. nucleatum bacteria do not invade epithelial cells (data not shown), it is hypothesized that any such antimicrobial defense may take hours to have an effect.
There have been many in vitro studies on the scope of interactions between oral bacteria and speculation on their role in vivo (21, 28, 38, 39). Bacterial interactions may modulate colonization by other species. S. cristatus down-regulates fimA gene expression in P. gingivalis (68), which may result in decreased adhesion to host surfaces and biofilm formation. Beyond the oral cavity, Moraxella catarrhalis has been shown to promote attachment of S. pyogenes to epithelial cells but inhibit streptococcal invasion (24). In addition, bacteria may compete directly for host cell receptors (55, 61). Interbacterial interactions may promote the colonization of more than one species by providing a suitable environment or substrate for attachment (21, 22, 24, 25, 28, 34, 39, 41, 49). Such interactions are believed to play an important role in the development of dental plaque (21, 38, 39, 41). In addition, coaggregation facilitates the invasion of dentinal tubules by species not able to invade directly (28). This report illustrates another aspect of interbacterial binding. The ability of a bacterium to invade host cells has long been considered a virulence factor. It is possible that in the presence of certain other species the ability to coaggregate with invasive organisms is an effective back door to internalization in host cells.
It is likely that the colonization of oral surfaces is a dynamic process, with bacteria from one site colonizing other sites via salivary flow. The dissociation of polymicrobial complexes from the plaque biofilm may lead to attachment to, and invasion of, the oral mucosa by fusobacteria and associated organisms. Since F. nucleatum has been demonstrated to coaggregate with representatives of every oral genus tested (21), it is possible that a wide range of otherwise noninvasive bacteria may be internalized in the oral epithelium via this mechanism. In addition to dental plaque, many of the soft tissues of the oral cavity support polymicrobial consortia of bacteria, some of which are intracellular (44, 45). These intracellular polymicrobial communities are similar in composition to dental plaque, and it has been hypothesized that they may act as reservoirs of bacteria for the recolonization of remote sites within the oral cavity (44, 45). In order to act as a reservoir, internalized bacteria must survive within the host cell. Currently, the fate of intracellular F. nucleatum is unknown beyond the duration of the assays detailed. It is also unclear whether coinvasion of F. nucleatum with S. cristatus will affect the survival of either species. Further experiments are necessary to determine the fate of internalized bacteria, the range of organisms that can be internalized via F. nucleatum or other invasive bacteria, and the effect, if any, of adding additional species to this model.
In summary, this report demonstrates the ability of an invasive bacterium to transport a noninvasive species into epithelial cells via a combination of coaggregation and invasion mechanisms.
Acknowledgments
Mark Herzberg (University of Minnesota) is acknowledged for providing KB cells. Ruoqiong Chen is acknowledged for assistance with confocal microscopy. Kelly Johnston is acknowledged for assistance with coaggregation assays. Angela Nobbs, Karen Ross, and Kaia Sappington are acknowledged for helpful conversations.
This study was supported by USPHS grant DE 14214 from the National Institute of Dental and Craniofacial Research, Bethesda, MD. T.J.G. was supported by a summer research fellowship from MinnCResT (5T32 DE 007288).
Editor: F. C. Fang
REFERENCES
- 1.Babu, J. P., J. W. Dean, and M. J. Pabst. 1995. Attachment of Fusobacterium nucleatum to fibronectin immobilized on gingival epithelial cells or glass coverslips. J. Periodontol. 66:285-290. [DOI] [PubMed] [Google Scholar]
- 2.Brogden, K. A., J. M. Guthmiller, and C. E. Taylor. 2005. Human polymicrobial infections. Lancet 365:253-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carranza, F. A, Jr., R. Saglie, M. G. Newman, and P. L. Valentin. 1983. Scanning and transmission electron microscopic study of tissue-invading microorganisms in localized juvenile periodontitis. J. Periodontol. 54:598-617. [DOI] [PubMed] [Google Scholar]
- 4.Clark, M. A., B. H. Hirst, and M. A. Jepson. 1998. Inoculum composition and Salmonella pathogenicity island 1 regulate M-cell invasion and epithelial destruction by Salmonella typhimurium. Infect. Immun. 66:724-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darenfed, H., D. Grenier, and D. Mayrand. 1999. Acquisition of plasmin activity by Fusobacterium nucleatum subsp. nucleatum and potential contribution to tissue destruction during periodontitis. Infect. Immun. 67:6439-6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dehazya, P., and R. S. Coles, Jr. 1980. Agglutination of human erythrocytes by Fusobacterium nucleatum: factors influencing hemagglutination and some characteristics of the agglutinin. J. Bacteriol. 143:205-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desvarieux, M., R. T. Demmer, T. Rundek, B. Boden-Albala, D. R. Jacobs, Jr., R. L. Sacco, and P. N. Papapanou. 2005. Periodontal microbiota and carotid intima-media thickness: the Oral Infections and Vascular Disease Epidemiology Study (INVEST). Circulation 111:576-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickson, M. A., W. C. Hahn, Y. Ino, V. Ronfard, J. Y. Wu, R. A. Weinberg, D. N. Louis, F. P. Li, and J. G. Rheinwald. 2000. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol. Cell. Biol. 20:1436-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorn, B. R., K. L. Leung, and A. Progulske-Fox. 1998. Invasion of human oral epithelial cells by Prevotella intermedia. Infect. Immun. 66:6054-6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebersole, J. L., D. Cappelli, and S. C. Holt. 2001. Periodontal diseases: to protect or not to protect is the question? Acta Odontol. Scand. 59:161-166. [DOI] [PubMed] [Google Scholar]
- 11.Erlandsen, S. L., C. J. Kristich, G. M. Dunny, and C. L. Wells. 2004. High-resolution visualization of the microbial glycocalyx with low-voltage scanning electron microscopy: dependence on cationic dyes. J. Histochem. Cytochem. 52:1427-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falkler, W. A, Jr., and C. E. Hawley. 1977. Hemagglutinating activity of Fusobacterium nucleatum. Infect. Immun. 15:230-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo, M., Y. W. Han, A. Sharma, and E. De Nardin. 2000. Identification and characterization of human immunoglobulin G Fc receptors of Fusobacterium nucleatum. Oral Microbiol. Immunol. 15:119-123. [DOI] [PubMed] [Google Scholar]
- 14.Han, Y. W., W. Shi, G. T. Huang, S. Kinder Haake, N. H. Park, H. Kuramitsu, and R. J. Genco. 2000. Interactions between periodontal bacteria and human oral epithelial cells: Fusobacterium nucleatum adheres to and invades epithelial cells. Infect. Immun. 68:3140-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Handley, P., A. Coykendall, D. Beighton, J. M. Hardie, and R. A. Whiley. 1991. Streptococcus crista sp. nov., a viridans streptococcus with tufted fibrils, isolated from the human oral cavity and throat. Int. J. Syst. Bacteriol. 41:543-547. [DOI] [PubMed] [Google Scholar]
- 16.Handley, P. S., F. F. Correia, K. Russell, B. Rosan, and J. M. DiRienzo. 2005. Association of a novel high molecular weight, serine-rich protein (SrpA) with fibril-mediated adhesion of the oral biofilm bacterium Streptococcus cristatus. Oral Microbiol. Immunol. 20:131-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen, H. B., J. Skeidsvoll, A. Fjellbirkeland, B. Hogh, P. Puntervoll, H. Kleivdal, and J. Tommassen. 1996. Cloning of the fomA gene, encoding the major outer membrane porin of Fusobacterium nucleatum ATCC 10953. Microb. Pathog. 21:331-342. [DOI] [PubMed] [Google Scholar]
- 18.Joshipura, K. 2002. The relationship between oral conditions and ischemic stroke and peripheral vascular disease. J. Am. Dent. Assoc. 133:23S-30S. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman, J., and J. M. DiRienzo. 1989. Isolation of a corncob (coaggregation) receptor polypeptide from Fusobacterium nucleatum. Infect. Immun. 57:331-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinder-Haake, S., and R. A. Lindemann. 1997. Fusobacterium nucleatum T18 aggregates human mononuclear cells and inhibits their PHA-stimulated proliferation. J. Periodontol. 68:39-44. [DOI] [PubMed] [Google Scholar]
- 21.Kolenbrander, P. E. 2000. Oral microbial communities: biofilms, interactions, and genetic systems. Annu. Rev. Microbiol. 54:413-437. [DOI] [PubMed] [Google Scholar]
- 22.Kolenbrander, P. E., and R. N. Andersen. 1989. Inhibition of coaggregation between Fusobacterium nucleatum and Porphyromonas (Bacteroides) gingivalis by lactose and related sugars. Infect. Immun. 57:3204-3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kremer, B. H., A. J. Herscheid, W. Papaioannou, M. Quirynen, and T. J. van Steenbergen. 1999. Adherence of Peptostreptococcus micros morphotypes to epithelial cells in vitro. Oral Microbiol. Immunol. 14:49-55. [DOI] [PubMed] [Google Scholar]
- 24.Lafontaine, E. R., D. Wall, S. L. Vanlerberg, H. Donabedian, and D. D. Sledjeski. 2004. Moraxella catarrhalis coaggregates with Streptococcus pyogenes and modulates interactions of S. pyogenes with human epithelial cells. Infect. Immun. 72:6689-6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lancy, P., Jr., J. M. Dirienzo, B. Appelbaum, B. Rosan, and S. C. Holt. 1983. Corncob formation between Fusobacterium nucleatum and Streptococcus sanguis. Infect. Immun. 40:303-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavappa, K. 1978. Survey of ATCC stocks of human cell lines for HeLa contamination. In Vitro 14:469-475. [DOI] [PubMed] [Google Scholar]
- 27.Levesque, C., J. Lamothe, and M. Frenette. 2003. Coaggregation of Streptococcus salivarius with periodontopathogens: evidence for involvement of fimbriae in the interaction with Prevotella intermedia. Oral Microbiol. Immunol. 18:333-337. [DOI] [PubMed] [Google Scholar]
- 28.Love, R. M., D. Malcolm, D. McMillan, Y. Park, and H. F. Jenkinson. 2000. Coinvasion of dentinal tubules by Porphyromonas gingivalis and Streptococcus gordonii depends upon binding specificity of streptococcal antigen I/II adhesin. Infect. Immun. 68:1359-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mager, D. L., L. A. Ximenez-Fyvie, A. D. Haffajee, and S. S. Socransky. 2003. Distribution of selected bacterial species on intraoral surfaces. J. Clin. Periodontol. 30:644-654. [DOI] [PubMed] [Google Scholar]
- 30.Mangan, D. F., M. J. Novak, S. A. Vora, J. Mourad, and P. S. Kriger. 1989. Lectinlike interactions of Fusobacterium nucleatum with human neutrophils. Infect. Immun. 57:3601-3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marin, C., J. J. Segura-Egea, A. Martinez-Sahuquillo, and P. Bullon. 2005. Correlation between infant birth weight and mother's periodontal status. J. Clin. Periodontol. 32:299-304. [DOI] [PubMed] [Google Scholar]
- 32.Meyer, D., J. Lippmann, and P. Fives-Taylor. 1996. Invasion of epithelial cells by Actinobacillus actinomycetemcomitans: a dynamic, multistep process. Infect. Immun. 64:2988-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nieminen, M. S., K. Mattila, and V. Valtonen. 1993. Infection and inflammation as risk factors for myocardial infarction. Eur. Heart J. 14:12-16. [PubMed] [Google Scholar]
- 34.Nilius, A. M., S. C. Spencer, and L. G. Simonson. 1993. Stimulation of in vitro growth of Treponema denticola by extracellular growth factors produced by Porphyromonas gingivalis. J. Dent. Res. 72:1027-1031. [DOI] [PubMed] [Google Scholar]
- 35.Nisapakultorn, K., K. F. Ross, and M. C. Herzberg. 2001. Calprotectin expression in vitro by oral epithelial cells confers resistance to infection by Porphyromonas gingivalis. Infect. Immun. 69:4242-4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Offenbacher, S., V. Katz, G. Fertik, J. Collins, D. Boyd, G. Maynor, R. McKaig, and J. J. Beck. 1996. Periodontal infection as a possible risk factor for preterm low birth weight. Periodontology 67:1103-1113. [DOI] [PubMed] [Google Scholar]
- 37.Ozaki, M., Y. Miyake, M. Shirakawa, T. Takemoto, H. Okamoto, and H. Suginaka. 1990. Binding specificity of Fusobacterium nucleatum to human erythrocytes, polymorphonuclear leukocytes, fibroblasts, and HeLa cells. J. Periodontal Res. 25:129-134. [DOI] [PubMed] [Google Scholar]
- 38.Palmer, R. J., Jr., M. C. Kazmerzak, M. C. Hansen, and P. E. Kolenbrander. 2001. Mutualism versus independence: strategies of mixed-species oral biofilms in vitro using saliva as the sole nutrient source. Infect. Immun. 69:5794-5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palmer, R. J., Jr., S. M. Gordon, J. O. Cisar, and P. E. Kolenbrander. 2003. Coaggregation-mediated interactions of streptococci and actinomyces detected in initial human dental plaque. J. Bacteriol. 185:3400-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richardson, J., J. Craighead, S. Cao, and M. Handfield. 2005. Concurrence between the gene expression pattern of Actinobacillus actinomycetemcomitans in localized aggressive periodontitis and in human epithelial cells. J. Med. Microbiol. 54:497-504. [DOI] [PubMed] [Google Scholar]
- 41.Rickard, A. H., P. Gilbert, N. J. High, P. E. Kolenbrander, and P. S. Handley. 2003. Bacterial coaggregation: an integral process in the development of multi-species biofilms. Trends Microbiol. 11:94-100. [DOI] [PubMed] [Google Scholar]
- 42.Rohde, M., E. Müller, G. S. Chhatwal, and S. R. Talay. 2003. Host cell caveolae act as an entry-port for group A streptococci. Cell. Microbiol. 5:323-342. [DOI] [PubMed] [Google Scholar]
- 43.Rudney, J. D., and C. A. Strait. 2000. Effects of Streptococcus crista and human saliva on the viability of Fusobacterium nucleatum ATCC 10953. Arch. Oral Biol. 45:667-674. [DOI] [PubMed] [Google Scholar]
- 44.Rudney, J. D., R. Chen, and G. J. Sedgewick. 2001. Intracellular Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in buccal epithelial cells collected from human subjects. Infect. Immun. 69:2700-2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rudney, J. D., R. Chen, and G. J. Sedgewick. 2005. Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, and Tannerella forsythensis are components of a polymicrobial intracellular flora within human buccal cells. J. Dent. Res. 84:59-63. [DOI] [PubMed] [Google Scholar]
- 46.Sandros, J., C. Karlsson, D. Lappin, P. Madianos, D. Kinane, and P. Papapanou. 2000. Cytokine responses of oral epithelial cells to Porphyromonas gingivalis infection. J. Dent. Res. 79:1808-1814. [DOI] [PubMed] [Google Scholar]
- 47.Scannapieco, F., B. Wang, and H. Shiau. 2001. Oral bacteria and respiratory infection: effects on respiratory pathogen adhesion and epithelial cell proinflammatory cytokine production. Ann. Periodontol. 6:78-86. [DOI] [PubMed] [Google Scholar]
- 48.Shaniztki, B., D. Hurwitz, N. Smorodinsky, N. Ganeshkumar, and E. I. Weiss. 1997. Identification of a Fusobacterium nucleatum PK1594 galactose-binding adhesin which mediates coaggregation with periopathogenic bacteria and hemagglutination. Infect. Immun. 65:5231-5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharma, A., S. Inagaki, W. Sigurdson, and H. K. Kuramitsu. 2005. Synergy between Tannerella forsythia and Fusobacterium nucleatum in biofilm formation. Oral Microbiol. Immunol. 20:39-42. [DOI] [PubMed] [Google Scholar]
- 50.Smalley, J. W. 1994. Pathogenic mechanisms in periodontal disease. Adv. Dent. Res. 8:320-328. [DOI] [PubMed] [Google Scholar]
- 51.Takemoto, T., M. Ozaki, M. Shirakawa, T. Hino, and H. Okamoto. 1993. Purification of arginine-sensitive hemagglutinin from Fusobacterium nucleatum and its role in coaggregation. J. Periodontal. Res. 28:21-26. [DOI] [PubMed] [Google Scholar]
- 52.Takemoto, T., T. Hino, M. Yoshida, K. Nakanishi, M. Shirakawa, and H. Okamoto. 1995. Characteristics of multimodal co-aggregation between Fusobacterium nucleatum and streptococci. J. Periodontal. Res. 30:252-257. [DOI] [PubMed] [Google Scholar]
- 53.Talay, S. R., A. Zock, M. Rohde, G. Molinari, M. Oggioni, G. Pozzi, C. A. Guzman, and G. S. Chhatwal. 2000. Co-operative binding of human fibronectin to Sfbl protein triggers streptococcal invasion into respiratory epithelial cells. Cell. Microbiol. 2:521-535. [DOI] [PubMed] [Google Scholar]
- 54.Tamai, R., Y. Asai, and T. Ogawa. 2005. Requirement for intercellular adhesion molecule 1 and caveolae in invasion of human oral epithelial cells by Porphyromonas gingivalis. Infect. Immun. 73:6290-6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Toleman, M., E. Aho, and M. Virji. 2001. Expression of pathogen-like Opa adhesins in commensal Neisseria: genetic and functional analysis. Cell. Microbiol. 3:33-44. [DOI] [PubMed] [Google Scholar]
- 56.Trüper, H. G., and D. C. Clari. 1997. Taxonomic note: necessary correction of specific epithets formed as substantives (nouns) “in apposition.” Int. J. Syst. Bacteriol. 47:908-909. [Google Scholar]
- 57.Tuttle, R. S., and D. F. Mangan. 1990. Interaction of Fusobacterium nucleatum 191 with human peripheral blood lymphocytes. J. Periodontal Res. 25:364-371. [DOI] [PubMed] [Google Scholar]
- 58.Tuttle, R. S., N. A. Strubel, J. Mourad, and D. F. Mangan. 1992. A non-lectin-like mechanism by which Fusobacterium nucleatum 10953 adheres to and activates human lymphocytes. Oral Microbiol. Immunol. 7:78-83. [DOI] [PubMed] [Google Scholar]
- 59.van Winkelhoff, A. J., B. G. Loos, W. A. van der Reijden, and U. van der Velden. 2002. Porphyromonas gingivalis, Bacteroides forsythus and other putative periodontal pathogens in subjects with and without periodontal destruction. J. Clin. Periodontol. 29:1023-1028. [DOI] [PubMed] [Google Scholar]
- 60.Vernier, A., M. Diab, M. Soell, G. Haan-Archipoff, A. Beretz, D. Wachsmann, and J. Klein. 1996. Cytokine production by human epithelial and endothelial cells following exposure to oral viridans streptococci involves lectin interactions between bacteria and cell surface receptors. Infect Immun. 64:3016-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Virji, M., D. Evans, J. Griffith, D. Hill, L. Serino, A. Hadfield, and S. M. Watt. 2000. Carcinoembryonic antigens are targeted by diverse strains of typable and non-typable Haemophilus influenzae. Mol. Microbiol. 36:784-795. [DOI] [PubMed] [Google Scholar]
- 62.Vitkov. L., W. D. Krautgartner, and M. Hannig. 2005. Bacterial internalization in periodontitis. Oral Microbiol. Immunol. 20:317-321. [DOI] [PubMed] [Google Scholar]
- 63.Watanabe, K., O. Yilmaz, S. F. Nakhjiri, C. M. Belton, and R. J. Lamont. 2001. Association of mitogen-activated protein kinase pathways with gingival epithelial cell responses to Porphyromonas gingivalis infection. Infect. Immun. 69:6731-6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weiss, E. I., B. Shaniztki, M. Dotan, N. Ganeshkumar, P. E. Kolenbrander, and Z. Metzger. 2000. Attachment of Fusobacterium nucleatum PK1594 to mammalian cells and its coaggregation with periodontopathogenic bacteria are mediated by the same galactose-binding adhesin. Oral Microbiol. Immunol. 15:371-377. [DOI] [PubMed] [Google Scholar]
- 65.Wong, M. Y., C. L. Lu, C. M. Liu, and L. T. Hou. 1999. Microbiological response of localized sites with recurrent periodontitis in maintenance patients treated with tetracycline fibers. J. Periodontol. 70:861-868. [DOI] [PubMed] [Google Scholar]
- 66.Wu, T., M. Trevisan, R. J. Genco, J. P. Dorn, K. L. Falkner, and C. T. Sempos. 2000. Periodontal disease and risk of cerebrovascular disease: the first national health and nutrition examination survey and its follow-up study. Arch. Intern. Med. 160:2749-2755. [DOI] [PubMed] [Google Scholar]
- 67.Xie, H., R. J. Gibbons, and D. I. Hay. 1991. Adhesive properties of strains of Fusobacterium nucleatum of the subspecies nucleatum, vincentii and polymorphum. Oral Microbiol. Immunol. 6:257-263. [DOI] [PubMed] [Google Scholar]
- 68.Xie, H., G. S. Cook, J. W. Costerton, G. Bruce, T. M. Rose, and R. J. Lamont. 2000. Intergeneric communication in dental plaque biofilms. J. Bacteriol. 182:7067-7069. [DOI] [PMC free article] [PubMed] [Google Scholar]