Abstract
Gamma interferon (IFN-γ)-induced effector mechanisms have potent antichlamydial activities that are critical to host defense. The most prominent and well-studied effectors are indoleamine dioxygenase (IDO) and nitric oxide (NO) synthase. The relative contributions of these mechanisms as inhibitors of chlamydial in vitro growth have been extensively studied using different host cells, induction mechanisms, and chlamydial strains with conflicting results. Here, we have undertaken a comparative analysis of cytokine- and lipopolysaccharide (LPS)-induced IDO and NO using an extensive assortment of human and murine host cells infected with human and murine chlamydial strains. Following cytokine (IFN-γ or tumor necrosis factor alpha) and/or LPS treatment, the majority of human cell lines induced IDO but failed to produce NO. Conversely, the majority of mouse cell lines studied produced NO, not IDO. Induction of IDO in human cell lines inhibited growth of L2 and mouse pneumonitis agent, now referred to as Chlamydia muridarum MoPn equally in all but two lines, and inhibition was completely reversible by the addition of tryptophan. IFN-γ treatment of mouse cell lines resulted in substantially greater reduction of L2 than MoPn growth. However, despite elevated NO production by murine cells, blockage of NO synthesis with the l-arginine analogue N-monomethyl-l-arginine only partially rescued chlamydial growth, suggesting the presence of another IFN-γ-inducible antichlamydial mechanism unique to murine cells. Moreover, NO generated from the chemical nitric oxide donor sodium nitroprusside showed little direct effect on chlamydial infectivity or growth, indicating a natural resistance to NO. Finally, IFN-γ-inducible IDO expression in human HeLa cells was inhibited following exogenous NO treatment, resulting in a permissive environment for chlamydial growth. In summary, cytokine- and LPS-inducible effectors produced by human and mouse cells differ and, importantly, these host-specific effector responses result in chlamydial strain-specific antimicrobial activities.
Chlamydiae are obligate intracellular gram-negative eubacteria that exhibit a highly specialized biphasic developmental cycle (38). Currently, the genus is divided into four species: Chlamydia trachomatis, C. psittaci, C. pneumoniae, and C. pecorum (54). C. trachomatis and C. pneumoniae are important human pathogens, whereas C. psittaci and C. pecorum are primarily pathogens of animals and birds, rarely infecting humans. C. trachomatis has been divided into three biovars: trachoma, lymphogranuloma venereum (LGV), and murine (mouse pneumonitis agent, now referred to as C. muridarum MoPn). MoPn is not a natural human pathogen. LGV biovar strains (L1, L2, and L3) are the most invasive, readily causing systemic infections (16), whereas strains of the trachoma biovar are limited to columnar epithelial cells at mucosal surfaces (54, 56). The trachoma biovar consists of 12 serovars designated A to K, including serovar Ba. Infection with serovar A, B, Ba, or C causes trachoma, the world's leading cause of preventable blindness, affecting 400 million people who live in areas where the disease is endemic. Infection with any of the serovars D through K causes genital tract disease. C. trachomatis is now the most common sexually transmitted bacterial pathogen, with an estimated 90 million cases occurring each year worldwide. Moreover, genital C. trachomatis infection has been identified as a potent cofactor facilitating the transmission of human immunodeficiency virus (9).
Knowledge about immunity to chlamydial infection in humans is limited. The bulk of information on innate and adaptive immunity to infection has come from studies using murine models, the most relevant of these being infection of the female mouse genital tract with the MoPn strain, which produces infection and disease characteristics similar to those found in the human female genital tract. Using this model, studies of gene knockout mice, adoptive transfer of lymphocytes, and in vivo depletion of lymphocytes have clearly demonstrated a major role for the cell-mediated immune response in immunity to infection (37). In particular, adaptive protective immunity is dependent on an interleukin-12 (IL-12) CD4+ TH1 response (10, 37). Gamma interferon (IFN-γ) is the predominate cytokine produced following resolution of infection but, interestingly, its protective role in vivo varies depending on the chlamydial challenge strain; clearance of the mouse-adapted MoPn strain is independent of the cytokine, whereas clearance of C. trachomatis human strains is IFN-γ dependent (37).
The inhibitory effects of IFN-γ on chlamydia replication in vitro have been extensively studied, and the findings have yielded marked inconsistencies in chlamydial strain susceptibility and effector antichlamydial mechanisms. Collectively, these studies have employed multiple cell lines (macrophages, fibroblasts, and epithelial cells) differing in host origin (human and murine), chlamydial strains (human and murine), and culture conditions. Given the pleiotropic signaling capabilities and host and tissue specificities of IFN-γ (44), the genomic differences among chlamydial strains studied (51, 52), and the inherent variation from laboratory to laboratory in experimental design, the inconsistencies in results are perhaps not surprising. Nevertheless, it is important that the reasons for these inconsistencies be identified, since a more accurate understanding of the IFN-γ-mediated antichlamydial activities is important for the design of pathogenesis models for the study of persistence and future vaccine development.
The known molecular basis of the antimicrobial action of IFN-γ includes the induction of indoleamine-2,3-deoxygenase (IDO), inducible nitric oxide synthase (iNOS), p47 GTPases, and alterations in iron metabolism (2, 20, 55, 58). Of these effector responses, the most extensively studied with regards to chlamydiae are IDO and iNOS (2, 53). IDO degrades tryptophan to kynurenine (63). The action of IDO effectively deprives the intracellular chlamydial reticulate bodies of tryptophan, inhibiting their growth and replication. Treatment of chlamydia-infected HeLa cells with IFN-γ results in the development of persistent infections characterized by the formation of aberrant but viable forms of the organism (1). In HeLa cells, the effects of IFN-γ on chlamydial growth can be reversed by the addition of excess tryptophan, implicating IDO and tryptophan starvation as the primary antimicrobial mechanism in human cells. Furthermore, the recently identified immunoregulatory role of IDO-induced tryptophan depletion (35, 36) may influence the in vivo infection outcome.
iNOS catalyzes the production of reactive nitrogen species, including nitric oxide (NO) (5). In cell lines and inbred mouse strains, resistance to microbial growth is often associated with production of NO, and iNOS inhibitors have been shown to worsen the course of disease caused by many pathogens (32). Contradictory results, from significant inhibition to no inhibitory activity, have been reported for the effects of NO on chlamydial growth in cell culture systems (14, 18, 19, 29, 33, 45, 46). Since NO alters several host cell activities, including mitochondrial ATP production (6, 7), it is difficult to differentiate between direct inhibitory action on chlamydiae and indirect effects as a result of inhibition of host cell functions of which chlamydiae are known to depend on for growth and survival. In contrast, all results from in vivo studies, using mice genetically deficient in iNOS, indicate that the production of NO is not essential for resolution of chlamydial genital infection (28, 42, 46). Indeed, iNOS knockout mice resolve both primary and secondary genital chlamydial infections with kinetics similar to those of wild-type mice (28). Studies in iNOS and p47(phox) gene knockout mice do, however, suggest a role for reactive nitrogen species in protection from disseminating and persistent chlamydial infections (47, 48), where macrophages and not epithelial cells are the targets of infection. In addition, NO is known to have immunoregulatory properties (5, 20). While the antimicrobial potential of iNOS is well established in mouse cells and mice, the role in human cells remains controversial (5, 32, 39).
Here we present results of a comprehensive study, employing human and mouse cell lines, designed to provide a better understanding of the mechanisms underlying innate host defense against infection by mouse- (C. muridarum strain MoPn) and human- (C. trachomatis serovar L2) adapted chlamydial strains. The results presented indicate that the cytokine-induced effector mechanisms responsible for antichlamydial activity are distinct in mouse and human cells. Furthermore, mouse- and human-adapted chlamydia have evolved to counter the species-specific antibacterial defense mechanisms of their natural host. The implications of this work in regards to the interpretation of in vitro data and the use of the mouse model for chlamydial vaccine studies are discussed.
MATERIALS AND METHODS
Cytokines, antibodies, and other reagents.
Recombinant human and mouse IFN-γ were purchased from PharMingen, San Diego, CA. Escherichia coli B5:055-derived lipopolysaccharide (LPS) was obtained from Calbiochem, San Diego, CA. Recombinant human IL-1β, recombinant tumor necrosis factor alpha (TNF-α), the iNOS inhibitor N-monomethyl-l-arginine (NMMA), nitric oxide donor sodium nitroprusside (SNP), arginine, tryptophan, modified Griess reagent, and various tissue culture medium supplements, including recombinant keratinocyte growth factor, β-estradiol, recombinant epidermal growth factor, transferrin, bovine insulin, progesterone, and hydrocortisone, were purchased from Sigma Chemical Co., St. Louis, MO. Primary antibodies against human β-actin and iNOS and murine β-actin and iNOS were obtained from Sigma Chemical Co. and Santa Cruz Biotechnology, Santa Cruz, CA, respectively. The human IDO mouse monoclonal antibody was a kind gift from O. Takikawa (Department of Pharmacology, Hokkaido University). Secondary goat anti-mouse and goat anti-rabbit horseradish peroxidase-conjugated antibodies were purchased from Cedarlane, Hornby, Ontario, Canada. The enhanced chemiluminescence detection system was obtained from Amersham Biosciences, Oakville, Ontario, Canada. The lactate dehydrogenase-based cytotoxicity assay kit was purchased from Roche Applied Sciences, Laval, Quebec, Canada. All other chemicals were of reagent grade or higher.
Chlamydiae.
C. trachomatis strains L2/LGV-434 and C. muridarum (MoPn) strain Weiss were propagated in HeLa cell monolayers in T-150 flasks containing high-glucose (4.5 mg/ml) Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum (FBS) (DMEM-10) at 37°C in 5% CO2. Infectious elementary bodies (EBs) were purified by density gradient centrifugation, and the infection-forming units (IFU) of purified EBs were determined by titration on HeLa cell monolayers as previously described (11).
Cell lines and culture conditions.
A summary of the cell lines and culture conditions employed for this study are presented in Table 1. All cells were grown in tissue culture flasks under the conditions recommended by the supplier (American Type Culture Collection, Rockville, MD, or the referenced provider). All cell culture media were purchased from Invitrogen, Burlington, Ontario, Canada. For consistency, concentrations of tryptophan and arginine, the substrates for indoleamine 2,3-dioxygenase and inducible nitric oxide synthase, respectively, were standardized in all media. Final concentrations were 10 mg/liter tryptophan and 200 mg/liter arginine.
TABLE 1.
Human and mouse cell line characteristics and growth conditions
| Cell line | ATCC no. (reference) | Source | Tissue | Morphology | Medium |
|---|---|---|---|---|---|
| HeLa229 | CCL-2.1 | Human | Cervix; adenocarcinoma | Epithelial | DMEM-4.5 mg/ml glucose (high glucose), 10% FBS, 2 mM glutamine |
| HL | Human | Unknown | Epithelial | DMEM high glucose, 10% FBS, 2 mM glutamine | |
| A549 | CCL-185 | Human | Lung; carcinoma | Epithelial | Ham's F-12K, 10% FBS, 2 mM glutamine |
| RT4 | HTB-2 | Human | Urinary bladder | Epithelial | McCoy's 5A, 10% FBS, 2 mM glutamine |
| T24 | HTB-4 | Human | Urinary bladder | Epithelial | McCoy's 5A, 10% FBS, 2 mM glutamine |
| BeWo | CCL-98 | Human | Placenta; choriocarcinoma | Epithelial | Ham's F-12K, 10% FBS, 2 mM glutamine |
| IEC4.1 | H. Caldwell (33) | Murine | Intestinal | Epithelial | 1:1 DMEM high glucose-Ham's F-12, epidermal growth factor (10 ng/ml), 1% hormone mixture (insulin [500 μg/ml], sodium selenite [173 ng/ml], triiodothyronine [3.4 ng/ml], prostaglandin E1 [125 ng/ml], transferrin [500 μg/ml], hydrocortisone [1.8 μg/ml]), 2 mM glutamine, 10% FBS |
| TM3 | CRL-1714 | Murine | Testis; Leydig cell | Epithelial | 1:1 DMEM high glucose-Ham's F-12 high glucose, 2 mM glutamine, 10% FBS |
| MLE15 | J. Whitsett (67) | Murine | Lung | Epithelial | 1:1 DMEM high glucose-Ham's F-12, insulin (5 μg/ml), transferrin (10 μg/ml) sodium selenite (173 ng/ml), hydrocortisone (1.8 μg/ml), beta-estradiol (600 μg/ml), 2 mM glutamine, 10% FBS |
| BM12.4 or BM1.11 | R. Johnson (32) | Murine | Primary upper genital tract | Epithelial | 1:1 DMEM high (4.5 mg/ml) glucose-Ham's F-12 Kaighn's modification, bovine insulin (10 mg/ml), human recombinant keratinocyte growth factor (1.25 μg/ml), 2 mM g lutamine, 10% FBS |
| McCoy | CRL-1696 | Murine | Unknown | Fibroblast | MEM, 10% FBS, 2 mM glutamine |
| L-929 | CCL-1 | Murine | Aveolar | Fibroblast | MEM, 10% FBS, 2 mM glutamine |
| THP-1 | TIB-202 | Human | Monocyte; acute leukemia | Monocyte | RPMI 1640, high glucose, 0.05 mM 2-mercaptoethanol, 2 mM glutamine, 10% FBS |
| RAW 264.7 | TIB-71 | Murine | Abelson murine leukemia | Macrophage | DMEM high glucose, 2 mM glutamine, 10% FBS |
| RAW NO− | CRL-2278 | Murine | Abelson murine leukemia | Macrophage | RPMI 1640 high glucose, 2 mM glutamine, 10% FBS |
| J774 | TIB-67 | Murine | Reticulum cell sarcoma | Macrophage | DMEM high glucose, 2 mM glutamine, 10% FBS |
Effects of exogenous IFN-γ, LPS, and SNP on chlamydial growth in vitro.
Triplicate cultures of the various cell lines were established in six-well plates (∼2 × 106 cells/well) in the recommended medium (Table 1), supplemented with 10% FBS. Cells were grown for 24 h, and then fresh medium was added with no supplements or 20 U/ml murine or human IFN-γ, 20 ng/ml Escherichia coli B5:055 lipopolysaccharide, IFN-γ (20 U/ml) plus LPS (20 ng/ml), or TNF-α (20 ng/ml), IL-1β (20 ng/ml), and IFN-γ (20 U/ml) plus LPS (20 ng/ml), or the indicated concentration of SNP, and incubation was continued for 24 h. This condition is referred to as a 24-h pretreatment in the presence of the indicated supplement(s). For some experiments, cells were not pretreated, and in these cases the indicated supplement(s) was added immediately after chlamydial infection. Cell monolayers were left uninfected or were infected with C. trachomatis L2 or C. muridarum at a multiplicity of infection of 0.6, as determined by titration on HeLa cells. Depending on the cell line being used, there was some variation in the percentage of cells infected, but it typically fell in the range of 40 to 60%. Monolayers were refed with medium alone or medium containing the homologous cytokine and/or LPS or the indicated concentration of SNP, and incubation was continued. For certain experiments, the iNOS inhibitor NMMA was added at a final concentration of 1 mM. After 48 h, cell monolayers and media were harvested, and cells were pelleted by centrifugation, then lysed by sonication, and assayed for chlamydial growth by titration of recoverable IFU on HeLa cell monolayers. A commercial cytotoxicity assay kit (Roche Applied Sciences, Laval, Quebec, Canada), measuring medium lactate dehydrogenase activity, was used to assess the effect of SNP on host cell viability. SNP solution was prepared immediately before use in the experiments.
Determination of intracellular tryptophan levels and medium nitrite concentrations.
As a measure of IDO activity, intracellular tryptophan levels were determined as previously described (64). Briefly, T-75 tissue culture flasks of uninfected or chlamydia-infected cells were treated with the indicated cytokine(s)/LPS or left untreated as a control. At the time indicated, cultures were extensively washed with ice-cold Hank's balanced salt solution, 250 μl 10% trichloroacetic acid was added to each flask, and the cells were scraped from the surface and incubated on ice for 30 min. The trichloroacetic acid-precipitated material was pelleted by centrifugation, and the tryptophan-containing supernatant was neutralized using 78.1:21.9 (vol/vol) freon-tri-N-octylamine (61). Tryptophan was separated and quantified by high-performance liquid chromatography (HPLC) on a 12.5-cm Whatman μBondapak C18 column using 1 mM KH2PO4 (pH 4) in 10% methanol at 1 ml/min (66). Tryptophan was identified and quantified by comparing the absorbance and retention time to that of a known standard. Data were analyzed with Beckman System Gold software. Results are presented as the means of two independent experiments with ranges shown.
As a measure of iNOS activity, medium nitrite levels were quantified in culture supernatants by the Griess reagent method. Briefly, equal volumes of twofold-diluted supernatants and the Griess reagent (1% sulfanilamide, 0.1% naphthylethylene diamine dihydrochloride, 2.5% H3PO4; 50 μl each) were mixed in 96-well plates, and the absorbance associated with color change was measured within 10 min at a 540-nm wavelength in a Microplate Autoreader spectrophotometer (Bio-Rad Laboratories, Hercules, CA). Nitrite concentration was calculated using a sodium nitrite standard curve generated for each assay. Results are presented as the means of two independent experiments with ranges shown.
Statistical analyses.
Differences between groups were analyzed for statistical significance using Student's two-tailed t test.
RT-PCR analysis.
T-75 tissue culture flasks of cells were uninfected or infected with C. trachomatis serovar L2 or C. muridarum at a multiplicity of infection of 0.6 IFU/cell and then cultured in DMEM-10 supplemented with 20 U/ml IFN-γ, 20 ng/ml LPS, or 20 U/ml IFN-γ and 20 ng/ml LPS. After incubation for 24 h at 37°C, the culture medium was removed and the monolayer was rinsed with Hank's balanced salt solution. Total RNA was prepared from the cells using TRIzol reagent according to the manufacturer's instructions (Invitrogen). Next, 1 μg of total RNA was treated with DNase I (Invitrogen), reverse transcribed using random hexamer primers and Thermoscript reverse transcriptase (Invitrogen), and then treated with RNase H (Invitrogen). The nucleotide sequences of the primers used for PCR were as follows: human β-actin, 5′-CATGTACGTTGCTATCCAGGC and 3′-CTCCTTAATGTCACGCACGAT; mouse β-actin, 5′-GAAATCGTGCGTGACATCAAAG and 3′-TGTAGTTTCATGGATGCCACAG; human IDO, 5′-GGCTTTGCTCTGCCAAATCC and 3′-TTCTCAACTCTTTCTCGAAGCTG; mouse IDO, 5′-GCTTTCCTCTACCACATCCAC and 3′-CAGGCGCTGTAACCTGTGT; human iNOS, 5′-GCATCACCAAGTACGAGTG and 3′-CCCGGTACTCATTCTGCA; mouse iNOS, 5′-GTTCTCAGCCCAACAATACAAGA and 3′-GTGGACGGGTCGATGTCAC. For conventional PCR, 1 μl of cDNA was amplified in a 50-μl reaction mixture containing 0.2 μM primers, 0.2 mM deoxynucleoside triphosphates, 1.5 mM MgCl2, 1 × Taq reaction buffer, and 5 U Taq DNA polymerase (Invitrogen). The cycling program was 3 min at 94°C followed by 30 cycles of 30 s at 94°C, 30 s at 60°C, and 60 s at 72°C, with a final extension of 7 min at 72°C. Products were separated on a 2% Tris-borate-EDTA-agarose gel, visualized by ethidium bromide staining, and photographed. For all reverse transcription-PCR (RT-PCR) results reported, no signal was detected in control samples lacking reverse transcriptase.
Western blot analysis.
Uninfected or chlamydia-infected cells were cultured in T-75 culture flasks in the recommended medium (Table 1) containing no supplements or 20 U/ml murine or human IFN-γ, 20 ng/ml Escherichia coli B5:055 lipopolysaccharide, or IFN-γ (20 U/ml) plus LPS (20 ng/ml). After incubation at 37°C for 24 h, the cells were harvested by gentle scraping in 500 μl of 2× Laemmli sample buffer supplemented with 5% (vol/vol) 14.2 M β-mercaptoethanol and denatured at 95°C for 5 min. Proteins were separated on 12% sodium dodecyl sulfate-polyacrylamide gels and blotted onto nitrocellulose membranes (Bio-Rad, Inc.). Membranes were preblocked in phosphate-buffered saline (PBS) plus 5% milk powder overnight at 4°C with shaking. Blots were incubated with the indicated primary antibody in PBS-5% milk powder for 1 h at room temperature with shaking. Membranes were washed three times (once for 15 min and twice for 5 min) in PBS-5% powdered milk. Washed membranes were incubated with secondary anti-mouse or anti-rabbit horseradish peroxidase-conjugated antibodies for 1 h at room temperature in PBS-5% powdered milk and then washed as described above. Proteins were detected by using enhanced chemiluminescence following the manufacturer's instructions. Duplicate blots were probed with monoclonal antibody to β-actin to approximate equal loading of all samples.
RESULTS
Induction of iNOS and IDO in various host cell lines.
In total, 7 human and 10 mouse cell lines, including epithelial, fibroblast, and monocyte/macrophage cells, were tested for induction of IDO and iNOS following treatment with IFN-γ and/or lipopolysaccharide (Table 2). With few exceptions, there was a distinct dichotomy, with human cells inducing IDO and mouse cells inducing iNOS. Treatment with IFN-γ alone or IFN-γ-LPS resulted in IDO activity in all human cell lines tested, with the exception of BeWo cells. BeWo cells originate from a naturally occurring human choriocarcinoma, and it has previously been shown that, despite expressing a functional IFN-γ receptor, they do not induce IDO expression in response to IFN-γ treatment (21). In most instances there was no enhancement of IDO activity when cells were treated with IFN-γ-LPS compared to IFN-γ alone. The one exception was THP-1 cells, which showed little IDO activity when treated with IFN-γ alone but substantial activity when treated with IFN-γ-LPS. Treatment with LPS alone did not induce IDO in any cell line, irrespective of tissue or species origin. The only mouse cell line that showed IDO activity, albeit weak, in response to IFN-γ or IFN-γ-LPS was MLE15, a mouse lung epithelial line.
TABLE 2.
Summary of IDO and iNOS activities in human and mouse cells
| Cell lineb | Production with additive(s)c
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| None
|
IFN-γ
|
LPS
|
IFN-γ-LPS
|
IFN-γ-TNF-α-IL-1β-LPS
|
||||||
| IDO | iNOS | IDO | iNOS | IDO | iNOS | IDO | iNOS | IDO | iNOS | |
| HeLa (HE) | —a | — | 94 | — | — | — | 96 | — | 92 | — |
| HL (HE) | — | — | 95 | — | — | — | 92 | — | NDd | ND |
| A549 (HE) | — | — | 86 | — | — | — | 88 | — | 91 | — |
| RT-4 (HE) | — | — | 93 | — | — | — | 96 | — | 36 | 27 |
| T24 (HE) | — | — | 91 | — | — | — | 90 | — | 87 | — |
| BeWo (HE) | — | — | — | — | — | — | — | — | ND | |
| IEC4.1 (ME) | — | — | — | — | — | — | — | — | ND | ND |
| TM3 (ME) | — | — | — | — | — | — | — | — | ND | ND |
| MLE-15 (ME) | — | — | 39 | — | — | — | 42 | — | ND | ND |
| BM12.4 (ME) | — | — | — | — | — | — | — | — | ND | ND |
| BM1.11 (ME) | — | — | — | — | — | — | — | — | ND | ND |
| McCoy (MF) | — | — | — | 44 | — | 3 | — | 51 | ND | ND |
| L929 (MF) | — | — | — | 19 | — | 4 | — | 24 | ND | ND |
| THP-1 (HM) | — | — | 11 | — | — | — | 90 | — | ND | ND |
| RAW 264.7 (MM) | — | — | — | 32 | — | 70 | — | 118 | ND | ND |
| RAW 264 NO− (MM) | — | — | — | — | — | 6 | — | 59 | ND | ND |
| J774 (MM) | — | — | — | 6 | — | 32 | — | 82 | ND | ND |
—, no evidence of IDO or iNOS production as judged by RT-PCR, Western blotting, and/or bioassay. When IDO was detected results are given as percent reduction in tryptophan pool compared to untreated control, and when iNOS was detected results are given in μM nitrite produced. Results presented are the averages of two determinations. For tryptophan the averaged 100% control values in pmole/107 cells are as follows: HeLa, 7,653; HL, 7,450; A549, 8,054; RT-4, 6,371; T24, 6,543; BeWo, 7,606; MLE15, 5,276.
HE, human epithelial line; ME, murine epithelial line; MF, murine fibroblast; HM, human macrophage; MM, murine macrophage.
Cell culture additives: IFN-γ, 20 U/ml; LPS, 20 ng/ml; TNF-α, 20 ng/ml; IL-1β, 20 ng/ml.
ND, not determined.
No human cell line tested induced iNOS in response to IFN-γ and/or LPS. In keeping with previous findings (27), the human bladder epithelial line RT-4 showed induction of iNOS. iNOS induction was maximal when RT-4 cells were exposed to a cytokine cocktail (IFN-γ-TNF-α-IL-1β) and LPS. Interestingly, even the closely related T-24 uroepithelial line did not induce iNOS following treatment with all four reagents. In contrast to human cells, mouse fibroblasts (McCoy and L929) and macrophage/monocyte lineages (RAW 264.7, RAW NO 264.7, and J774) induced iNOS in response to IFN-γ alone or IFN-γ-LPS. An exception is the RAW 264.7 NO− line, a clone derived from RAW 264.7 which, unlike the parental line, does not induce iNOS upon treatment with LPS or IFN-γ alone but requires IFN-γ-LPS for full activation (50). Nitric oxide was not produced by fibroblasts in response to LPS alone. Finally, iNOS was not induced in any epithelial line under the culture conditions tested.
Growth of C. trachomatis L2 and C. muridarum in various host cell lines.
The ability of the various host cell lines employed to support chlamydial growth was assessed by titrating the number of infectious EB produced at 48 h postinfection (Tables 3 and 4). All non-macrophage/monocyte cell lines tested supported good growth of both C. trachomatis L2 and C. muridarum MoPn, showing between 1 and 2 log10 increases in IFU recovered over input IFU. Furthermore, there was no specific cell line that supported markedly better growth of L2 compared to MoPn or vice versa. Both chlamydial strains grew well in THP-1 cells, a human monocyte line, with yields close to that seen in nonmacrophage lines. In general, MoPn grew better than L2 in mouse macrophage/monocyte cells. The lowest yield obtained was from L2-infected RAW264.7 cells, where an increase of approximately twofold over input IFU was recovered.
TABLE 3.
Summary of the effects of IDO and iNOS activities on L2 growtha
| Cell lineb | Production or growth with additive(s)c
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| None
|
IFN-γ
|
LPS
|
IFN-γ-LPS
|
IFN-γ-TNF-α-IL-1β-LPS
|
|||||||||||
| IDO | iNOS | IFU | IDO | iNOS | IFU | IDO | iNOS | IFU | IDO | iNOS | IFU | IDO | iNOS | IFU | |
| HeLa (HE) | —d | — | 8.30 ± 0.76 | 91 | — | 5.14 ± 0.33 | — | — | 8.28 ± 0.74 | 87 | — | 5.16 ± 0.31 | 88 | — | 5.22 ± 0.46 |
| HL (HE) | — | — | 7.53 ± 0.60 | 89 | — | 5.35 ± 0.35 | — | — | 7.80 ± 0.63 | 93 | — | 5.10 ± 0.40 | NDe | ND | ND |
| A549 (HE) | — | — | 7.71 ± 0.65 | 94 | — | 5.39 ± 0.40 | — | — | 7.69 ± 0.71 | 88 | — | 5.33 ± 0.29 | 87 | — | 5.41 ± 0.32 |
| RT-4 (HE) | — | — | 7.53 ± 0.71 | 92 | — | 5.59 ± 0.36 | — | — | 7.50 ± 0.60 | 89 | — | 5.21 ± 0.33 | 49 | 7 | 5.19 ± 0.29 |
| T24 (HE) | — | — | 7.96 ± 0.72 | 92 | — | 5.56 ± 0.60 | — | — | 7.80 ± 0.71 | 94 | — | 5.60 ± 0.36 | 91 | — | 5.67 ± 0.45 |
| BeWo (HE) | — | — | 7.49 ± 0.63 | — | — | 7.46 ± 0.54 | — | — | 7.51 ± 0.59 | — | — | 7.47 ± 0.55 | ND | ND | ND |
| IEC4.1 (ME) | — | — | 6.96 ± 0.59 | — | — | 5.16 ± 0.43 | — | — | 6.79 ± 0.55 | — | — | 5.32 ± 0.38 | ND | ND | ND |
| TM3 (ME) | — | — | 6.83 ± 0.55 | — | — | 5.16 ± 0.34 | — | — | 6.82 ± 0.50 | — | — | 5.26 ± 0.42 | ND | ND | ND |
| MLE-15 (ME) | — | — | 8.19 ± 0.73 | 39 | — | 7.90 ± 0.71 | — | — | 8.20 ± 0.62 | 41 | — | 7.80 ± 0.59 | ND | ND | ND |
| BM12.4 (ME) | — | 4 | 8.37 ± 0.68 | — | 17 | 6.72 ± 0.71 | — | 3 | 8.24 ± 0.71 | — | 22 | 6.45 ± 0.61 | ND | ND | ND |
| BM1.11 (ME) | — | 12 | 7.17 ± 0.64 | — | 25 | 5.37 ± 0.46 | — | 14 | 7.24 ± 0.65 | — | 29 | 5.46 ± 0.33 | ND | ND | ND |
| McCoy (MF) | — | 3 | 8.20 ± 0.81 | — | 21 | 6.18 ± 0.50 | — | 6 | 8.19 ± 0.70 | — | 25 | 6.15 ± 0.42 | ND | ND | ND |
| L929 (MF) | — | 2 | 8.11 ± 0.77 | — | 11 | 6.31 ± 0.53 | — | 2 | 7.99 ± 0.63 | — | 13 | 6.27 ± 0.57 | ND | ND | ND |
| THP-1 (HM) | — | — | 7.96 ± 0.62 | 94 | — | 5.66 ± 0.39 | — | — | 7.89 ± 0.52 | 93 | — | 5.36 ± 0.51 | ND | ND | ND |
| RAW 264 (MM) | — | 12 | 6.19 ± 0.73 | — | 88 | 4.53 ± 0.47 | — | 82 | 4.34 ± 0.33 | — | 90 | 4.25 ± 0.34 | ND | ND | ND |
| RAW NO− (MM) | — | 1 | 6.29 ± 0.71 | — | 39 | 4.76 ± 0.52 | — | 11 | 6.19 ± 0.47 | — | 47 | 4.62 ± 0.29 | ND | ND | ND |
| J774 (MM) | — | 8 | 6.23 ± 0.66 | — | 50 | 5.17 ± 0.49 | — | 31 | 6.15 ± 0.59 | — | 42 | 5.20 ± 0.38 | ND | ND | ND |
Growth was assessed by determining the number of infectious EBs recovered 48 h Postinfection. Growth data are presented as IFU (log10) and represent the means ± standard errors SE of triplicate determinations. Tryptophan and nitrite data presented are the averages of two determinations. For tryptophan the averaged 100% control values in pmol/107 cells are as follows: HeLa, 7,247; HL, 7,432; A549, 7,789; RT-4, 6,459; T24, 6,483; BeWo, 7,256; MLE15, 5,019.
HE, human epithelial line; ME, murine epithelial; MF, murine fibroblast; HM, human monocyte; MM, murine macrophage.
Cell culture additives: IFN-γ, 20 U/ml; LPS, 20 ng/ml; TNF-α, 20 ng/ml; IL-1β, 20 ng/ml.
—, no evidence of IDO or iNOS production as judged by RT-PCR, western blotting, and/or bioassay.
ND, not determined.
TABLE 4.
Summary of the effects of IDO and iNOS activities on MoPn growtha
| Cell lineb | Production or growth with additive(s)c
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| None
|
IFN-γ
|
LPS
|
IFN-γ-LPS
|
|||||||||
| IDO | iNOS | IFU | IDO | iNOS | IFU | IDO | iNOS | IFU | IDO | iNOS | IFU | |
| HeLa (HE) | —d | — | 8.26 ± 0.57 | 90 | — | 5.26 ± 0.41 | — | — | 8.22 ± 0.69 | 92 | — | 5.18 ± 0.43 |
| HL (HE) | — | — | 7.93 ± 0.63 | 93 | — | 5.79 ± 0.33 | — | — | 8.11 ± 0.81 | 94 | — | 5.69 ± 0.54 |
| A549 (HE) | — | — | 8.12 ± 0.78 | 91 | — | 5.57 ± 0.37 | — | — | 7.84 ± 0.75 | 91 | — | 5.91 ± 0.45 |
| RT-4 (HE) | — | — | 7.79 ± 0.82 | 88 | — | 5.80 ± 0.42 | — | — | 7.72 ± 0.82 | 93 | — | 5.51 ± 0.44 |
| T24 (HE) | — | — | 7.88 ± 0.49 | 93 | — | 6.10 ± 0.59 | — | — | 7.99 ± 0.67 | 89 | — | 6.15 ± 0.61 |
| BeWo (HE) | — | — | 8.12 ± 0.66 | — | — | 8.11 ± 0.68 | — | — | 8.1 ± 0.69 | — | — | 8.11 ± 0.74 |
| IEC4.1 (HE) | — | — | 7.11 ± 0.53 | — | — | 6.53 ± 0.71 | — | — | 7.11 ± 0.72 | — | — | 6.60 ± 0.61 |
| TM3 (ME) | — | — | 6.93 ± 0.38 | — | — | 6.53 ± 0.62 | — | — | 6.79 ± 0.71 | — | — | 6.33 ± 0.54 |
| MLE-15 (ME) | — | — | 8.39 ± 0.87 | 44 | — | 8.40 ± 0.77 | — | — | 8.41 ± 0.73 | 42 | — | 8.41 ± 0.41 |
| BM12.4 (ME) | — | 2 | 8.40 ± 0.54 | — | 7 | 8.39 ± 0.81 | — | 2 | 8.41 ± 0.68 | — | 10 | 8.38 ± 0.67 |
| BM1.11 (ME) | — | 5 | 8.12 ± 0.61 | — | 10 | 8.11 ± 0.83 | — | 6 | 8.12 ± 0.77 | — | 12 | 7.85 ± 0.72 |
| McCoy (MF) | — | 2 | 7.70 ± 0.71 | — | 15 | 6.74 ± 0.53 | — | 2 | 7.73 ± 0.56 | — | 20 | 6.70 ± 0.59 |
| L929 (MF) | — | 3 | 8.10 ± 0.76 | — | 6 | 7.21 ± 0.66 | — | — | 7.89 ± 0.62 | — | 7.2 | 7.17 ± 0.68 |
| THP-1 (HM) | — | — | 7.53 ± 0.48 | 93 | — | 5.59 ± 0.59 | — | — | 7.32 ± 0.69 | 94 | — | 5.31 ± 0.39 |
| RAW 264 (MM) | — | 16 | 6.44 ± 0.61 | — | 41 | 4.88 ± 0.51 | — | 32 | 5.27 ± 0.42 | — | 56 | 5.11 ± 0.46 |
| RAW NO− (MM) | — | — | 7.20 ± 0.77 | — | 21 | 6.46 ± 0.53 | — | 6 | 7.12 ± 0.58 | — | 59 | 6.29 ± 0.51 |
| J774 (MM) | — | 7 | 6.67 ± 0.69 | — | 52 | 5.36 ± 0.37 | — | 29 | 6.67 ± 0.71 | — | 54 | 5.58 ± 0.44 |
Growth was assessed by determining the number of infectious EBs recovered 48 h postinfection. Growth data are presented as IFU (log10) and represent the means ± standard errors of triplicate determinations. Tryptophan and nitrite data presented are the averages of two determinations. For tryptophan the averaged 100% control values in pmol/107 cells are as follows: HeLa, 7,734; HL, 7,653; A549, 7,813; RT-4, 6,216; T24, 6,167; BeWo, 7,237; MLE15, 5,106.
HE, human epithelial line; ME, murine epithelial; MF, murine fibroblast; HM, human monocyte; MM, murine macrophage.
Cell culture additives: IFN-γ, 20 U/ml; LPS, 20 ng/ml.
—, no evidence of IDO or iNOS production.
Effect of chlamydial infection on IDO and iNOS induction.
To determine whether chlamydial infection had an effect on IDO and iNOS induction in the various cell lines cultured under the different conditions, cells were infected with C. trachomatis serovar L2 or C. muridarum MoPn. With all human- and mouse-derived cell lines, infection alone with either L2 (Table 3) or MoPn (Table 4) had little or no effect on IDO expression. Furthermore, with the exception of THP-1s, where infection of IFN-γ-treated cells increased IDO expression, infection of cultures did not substantially alter the IDO expression patterns seen with IFN-γ and/or LPS treatment in the absence of infection. With human RT-4 cells, the only human cell line producing NO, treated with IFN-γ-TNF-α-IL-1β and LPS, infection with L2 (Table 3) or MoPn (data not shown) consistently reduced NO production. Similarly, infection of mouse fibroblasts (McCoy and L929) treated with IFN-γ or IFN-γ-LPS with either L2 or MoPn resulted in a reduction in NO production. Upon infection, mouse fibroblasts and both primary upper genital tract epithelial lines (BM12.4 and BM1.11) (30) produced a small amount of NO even in the absence of cytokine-LPS stimulation. RAW 264.7 and J774 macrophage lines showed substantial induction of iNOS following infection alone (no cytokines or LPS present), regardless of the chlamydial strain used. In comparison to uninfected cells, chlamydia-infected RAW 264.7 NO− cells produced NO following treatment with IFN-γ alone, and infection of IFN-γ-treated RAW 264.7 and J774 cells showed increased NO production compared to their uninfected counterparts. These results suggest that chlamydia infection provides an additional signal(s) which is required for, or can enhance, iNOS induction in professional phagocytes. Presumably, this is similar to the role played by LPS in uninfected cells.
Effect of IDO and iNOS induction on chlamydial growth.
With the exception of BeWo cells, which do not induce IDO in response to cytokine treatment (21), treatment of all human cell lines with IFN-γ or IFN-γ-LPS led to IDO induction and a reduction in intracellular tryptophan pools (Table 2). Regardless of the cell line used, the reduced tryptophan pools had a dramatic effect on the growth of L2 and MoPn, with a reduction of 2 to 3 log10 in recoverable IFU (P < 0.0001) (Tables 3 and 4). With RT-4 cells, treatment with IFN-γ-TNF-α-IL-1β-LPS induced IDO and iNOS, which resulted in a greater than 2 log10 reduction in recoverable IFU (P < 0.0001) compared to control untreated cultures. This reduction was essentially equivalent to that seen with IFN-γ alone, a condition under which no iNOS induction occurred (see later).
With mouse cells, in contrast to what was seen with human cells and IDO induction, it was not possible to consistently correlate inhibition of L2 or MoPn growth with the induction of iNOS (Tables 3 and 4). For example, MoPn-infected primary genital epithelial lines (BM12.4, BM1.11) treated with IFN-γ upregulated iNOS, but organism growth was essentially unaffected. Similarly, when the same cell lines were treated with LPS and infected with L2 there was induction of iNOS but little or no inhibition of IFU recovery, compared to control untreated cultures. In general, compared to MoPn, L2 growth was more sensitive to the effects of IFN-γ or IFN-γ-LPS in mouse nonprofessional phagocytes, irrespective of being fibroblast or epithelial in origin. In contrast, with macrophage cell lines growth inhibition resulting from IFN-γ or IFN-γ-LPS treatment was similar for L2 and MoPn.
Molecular mechanisms of chlamydial inhibition in human cells.
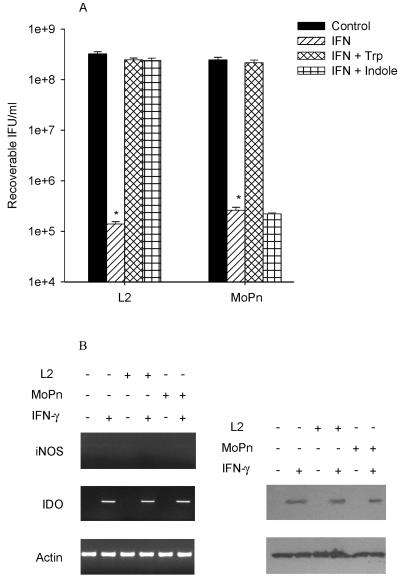
A more detailed characterization of the host response was undertaken in two epithelial lines, HeLa and RT-4, and one macrophage/monocyte line, THP-1, to provide insight into the molecular mechanisms responsible for the antichlamydial activity in human cells. HeLa cells treated with IFN-γ or IFN-γ-LPS had decreased tryptophan pools, no production of NO, and a greater than 3 log10 inhibition of growth (P < 0.0001) (Fig. 1A). Results from RT-PCR experiments with HeLa cells treated with IFN-γ showed induction of IDO but not iNOS. In agreement with these findings, Western blot analysis indicated there was clear upregulation of IDO protein following IFN-γ treatment, and these effects were not changed by infection with MoPn or L2 (Fig. 1B). For L2 and MoPn, growth inhibition caused by IFN-γ or IFN-γ-LPS was reversed by the addition of excess tryptophan. In contrast, indole reversed the IFN-γ-induced growth inhibition of L2 but not MoPn. This is in agreement with previous findings (12, 22) and is consistent with the fact that unlike MoPn, L2 encodes a functional tryptophan synthase (22). Similar results were obtained with other human epithelial cells tested, including HL, A549, and T24 (data not shown). These results suggest that the primary mechanism responsible for the IFN-γ-induced antichlamydial activity in epithelial cells is tryptophan degradation.
FIG. 1.
Effect of IFN-γ on the growth of C. trachomatis L2 and C. muridarum MoPn (A) and on the expression of iNOS and IDO (B) in HeLa cells. HeLa cells were untreated or pretreated with IFN-γ (20 U/ml) for 24 h, infected with chlamydiae, and then incubated in the complete culture medium described in Table 1. After 48 h of incubation, the cultures were harvested and chlamydial growth was assessed by determination of IFU following passing onto fresh HeLa monolayers. Growth data are presented as IFU (log10) and represent the means ± standard errors of triplicate determinations. *, P < 0.0001 compared to the untreated control culture. For RT-PCR and Western blot analyses, HeLa cells were untreated or pretreated with IFN-γ for 24 h and then left uninfected or infected with chlamydiae. Following incubation for a further 24 h, cells were harvested, RNA was isolated, cDNA was synthesized, and PCR was carried out using primers specific for human iNOS, IDO, and actin. For Western blot analyses, cells were harvested after 24 h and the cell pellets were resuspended in Laemmli sample buffer and proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted to nitrocellulose. See Materials and Methods for details.
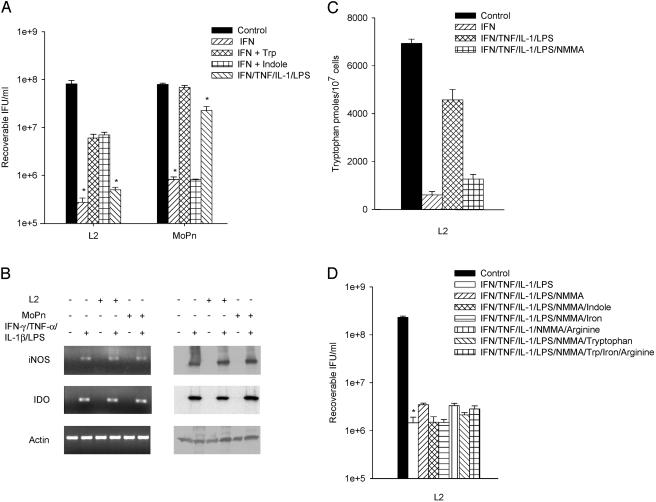
As a result of their ability to produce NO, RT-4 cells provide a unique opportunity to study the interplay between IDO and iNOS and the resulting effects on chlamydia growth. RT-4 cells treated with IFN-γ or IFN-γ-LPS showed decreased tryptophan pools but no nitric oxide production (Table 2). For MoPn, the IFN-γ-induced growth inhibition was significantly reversed with tryptophan but not indole. Surprisingly with L2, growth inhibition was only partially reversed by the addition of tryptophan or indole (Fig. 2A). Infected RT-4 cells treated with the cytokine cocktail (IFN-γ-IL-1β-TNF-α) and LPS resulted in induction of both IDO and iNOS gene expression and protein production (Fig. 2B). Under these conditions, NO was generated and there was a substantial reversal of the decrease seen in the tryptophan pools (Fig. 2C). This result is in keeping with the observation that IDO activity is inhibited by NO (60). Interestingly, the reversal in the decrease in tryptophan pools coincides with a substantial increase in IFU recovery for MoPn but not L2. It was not possible to completely reverse the growth inhibition, which resulted from the cytokine cocktail-LPS treatment, by the addition of indole, iron, or arginine (Fig. 2D). Furthermore, as expected, addition of the iNOS inhibitor NMMA blocked NO production, which gave rise to an increase in tryptophan pools (Fig. 2C). Interestingly, even though the tryptophan pools recovered when NO production was inhibited, there was only a partial recovery of L2 growth and this was not further enhanced by the addition of excess tryptophan or indole (Fig. 2D). In total, results from these experiments indicate that there is an antichlamydial activity induced in RT-4 cells that is not dependent on NO production or tryptophan degradation.
FIG. 2.
Effect of IFN-γ and IFN-γ-TNF-α-IL-1β-LPS on the growth of C. trachomatis L2 and C. muridarum MoPn (A and D) and on the expression of iNOS and IDO (B and C) in RT-4 cells. RT-4 cells were untreated or pretreated with IFN-γ (20 U/ml) for 24 h. Cells exposed to IFN-γ (20 U/ml)-TNF-α (20 ng/ml)-IL-1β (20 ng/ml)-LPS (20 ng/ml) were not pretreated. Following chlamydia infection, cells were incubated for a further 48 h in the complete medium described in Table 1 plus any indicated supplements. Final concentrations of tryptophan, indole, and NMMA were 500 μg/ml, 100 μM, and 1 mM, respectively. Cultures were harvested and processed for the various assays as described in the legend to Fig. 1. Growth data are presented as IFU (log10) and represent the means ± standard errors of triplicate determinations. Intracellular tryptophan pools were measured by HPLC. Data are presented as pmol tryptophan/107 cells and represent the means of two independent experiments with ranges shown. *, P < 0.0001 compared to the untreated control culture.
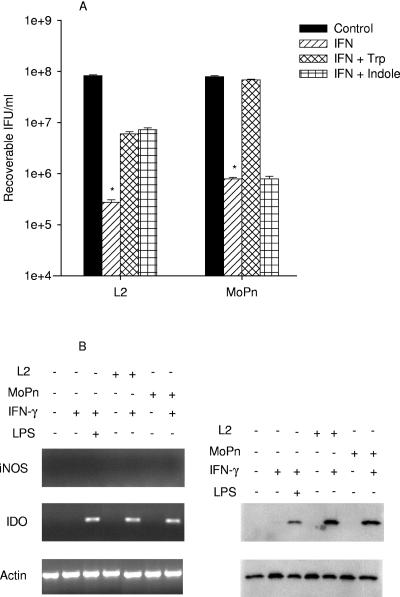
THP-1 cells do not produce NO under any of the treatment conditions tested. In addition, they do not induce much IDO activity in response to IFN-γ alone (Table 2) but do if treated with IFN-γ-LPS (Table 2 and Fig. 3). As shown in Fig. 3B, when L2- or MoPn-infected THP-1 cells are treated with IFN-γ, there is upregulation of IDO transcript and protein and a decrease in tryptophan pools (Table 3 and 4). This indicates that chlamydial infection can substitute for LPS but not IFN-γ at least in terms of induction of IDO activity. For MoPn, similar to what was found with RT-4 cells, the antichlamydial activity could be partially reversed by the addition of excess tryptophan but not indole. Furthermore, also like what was seen with RT-4 cells, for L2 the IFN-γ-induced antichlamydial activity was only partially reversed by the addition of excess tryptophan or indole (Fig. 3A).
FIG. 3.
Effect of IFN-γ or IFN-γ-LPS on the growth of C. trachomatis L2 and C. muridarum MoPn (A) and on the expression of iNOS and IDO (B) in THP-1 monocyte cells. THP-1 cells were untreated or pretreated with IFN-γ (20 U/ml) or IFN-γ (20 U/ml)-LPS (20 ng/ml) for 24 h, infected with chlamydiae, and then incubated in the complete culture medium described in Table 1. Cultures were harvested and processed for the various assays as described in the legend to Fig. 1. Growth data are presented as IFU (log10) and represent the means ± standard errors of triplicate determinations. *, P < 0.0001 compared to the untreated control culture.
Molecular mechanisms of chlamydial inhibition in mouse cells.
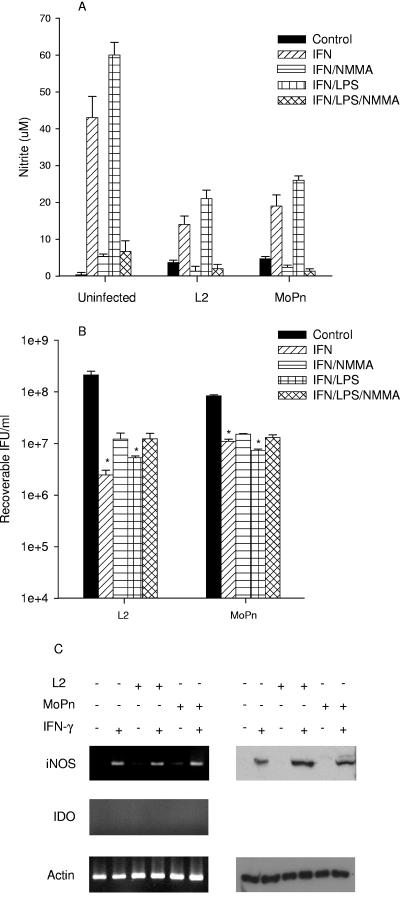
Similar experiments were done in McCoy (fibroblast), BM12.4 (epithelial), and RAW264.7 (monocyte/macrophage) cell lines as representative examples of mouse cells. Culturing McCoy cells in the presence of IFN-γ or IFN-γ-LPS resulted in a 2 log10 decrease in L2 growth and less than 1 log10 reduction in MoPn growth (P < 0.0001) (Fig. 4). There was no induction of IDO or reduction in tryptophan pools under any of the conditions tested. Growth inhibition in either case was not reversed by the addition of excess tryptophan or indole (data not shown). In response to IFN-γ or IFN-γ-LPS, McCoy cells induced iNOS gene and protein expression and NO was produced (Fig. 4A and C). Inhibition of NO production by the addition of NMMA resulted in a partial reversal of the growth reduction seen for L2 and MoPn (Fig. 4B). Identical results were found with L929, another mouse fibroblast line commonly used for chlamydial studies (data not shown).
FIG. 4.
Effect of IFN-γ or IFN-γ-LPS on the expression of iNOS and IDO (A and C) and on the growth of C. trachomatis L2 and C. muridarum MoPn (B) in McCoy cells. McCoy cells were uninfected or infected with chlamydiae and then incubated in the complete culture medium described in Table 1 plus IFN-γ (20 U/ml) or IFN-γ (20 U/ml)-LPS (20 ng/ml) with or without NMMA (1 mM). Cultures were harvested and processed for the various assays 24 h later as described in the legend to Fig. 1. Growth data are presented as IFU (log10) and represent the means ± standard errors of triplicate determinations. *, P < 0.0001 compared to the untreated control culture. Medium nitrite levels were determined after 48 h of incubation using the Griess reagent. Data are presented as μM nitrite and represent the means of two independent experiments with ranges shown.
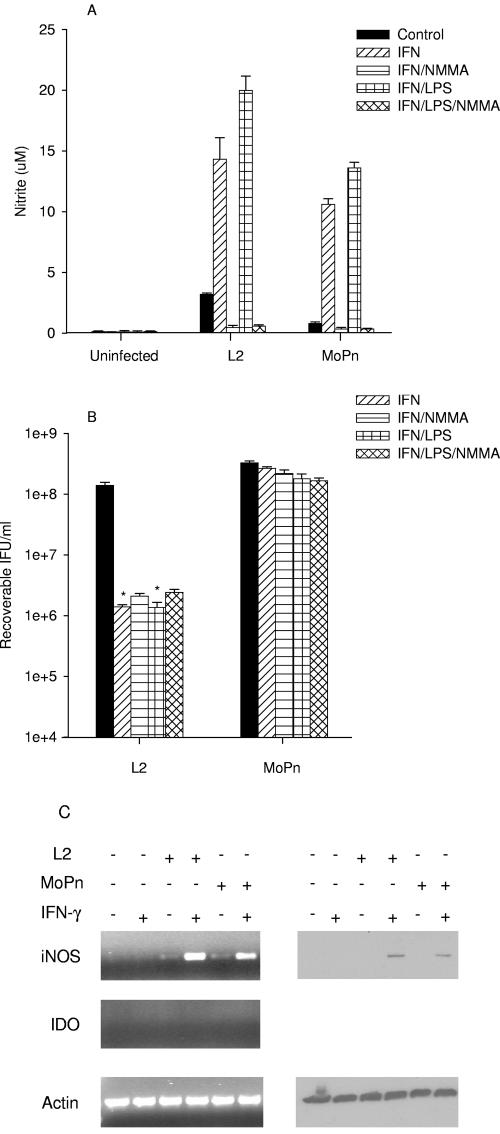
In response to IFN-γ and/or LPS, BM12.4 uninfected or infected cells did not induce IDO gene expression or degrade tryptophan (Table 2). iNOS gene expression was not induced when BM12.4 cells were treated with IFN-γ or IFN-γ-LPS in the absence of infection (Fig. 5). In contrast, iNOS gene and protein expression was upregulated and NO was produced following infection with L2 or MoPn, and the induction was enhanced further by treatment with IFN-γ or IFN-γ-LPS (Fig. 5A and C). In contrast to the results observed with McCoy cells, there was essentially no inhibition of MoPn growth under any of the culture conditions tested (Fig. 5B). There was a reduction of 2 log10 (P < 0.001) in the growth of L2 when BM12.4 cells were treated with IFN-γ or IFN-γ-LPS. Inhibition of NO production, by the addition of NMMA, did not reverse the inhibition of L2 growth (Fig. 5B). These results suggest that there is an IFN-γ-inducible L2-specific antichlamydial activity induced in BM12.4 cells separate from tryptophan degradation or NO production.
FIG. 5.
Effect of IFN-γ or IFN-γ-LPS on the expression of iNOS and IDO (A and C) and on the growth of C. trachomatis L2 and C. muridarum MoPn (B) in BM12.4 cells. BM12.4 cells were uninfected or infected with chlamydiae and then incubated in the complete culture medium described in Table 1 plus IFN-γ (20 U/ml) or IFN-γ (20 U/ml)-LPS (20 ng/ml) with or without NMMA (1 mM). Cultures were harvested and processed for the various assays 24 h later as described in the legend to Fig. 1. Growth data are presented as IFU (log10) and represent the means ± standard errors of triplicate determinations. *, P < 0.0001 compared to the untreated control culture. Medium nitrite levels were determined after 48 h of incubation using the Griess reagent. Data are presented as μM nitrite and represent the means of two independent experiments with ranges shown.
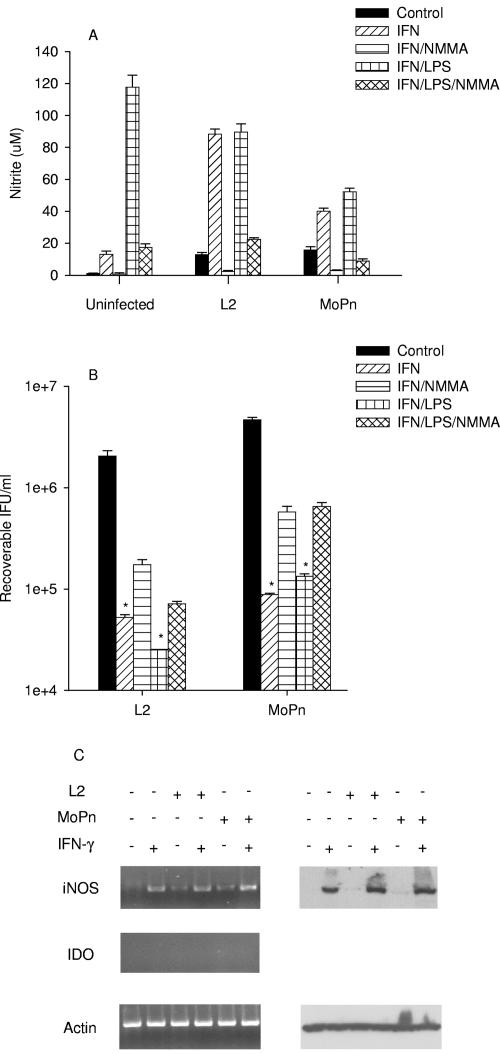
In contrast to results obtained with the human monocyte/macrophage line THP-1, which requires two external stimuli for activation, RAW 264.7 mouse macrophages responded to a single stimulus (IFN-γ, LPS, or chlamydial infection) (Table 2). IDO was not induced under any of the conditions tested. There was a small induction of iNOS gene and protein expression, with concomitant NO production, by infection alone, and this was substantially enhanced by IFN-γ (see Fig. 8, below). There was about a 2 log10 reduction of growth of L2 and MoPn in IFN-γ- or IFN-γ-LPS-treated RAW 264.7 cells when compared to untreated controls (P < 0.0001) (Fig. 6). Addition of NMMA blocked NO production and, in contrast to results observed with BM12.4 cells, there was an increase (0.5 to 1.0 log10) in L2 and MoPn IFU recovered. These results suggest that NO production is an important component of the antichlamydial effector mechanism in mouse macrophages.
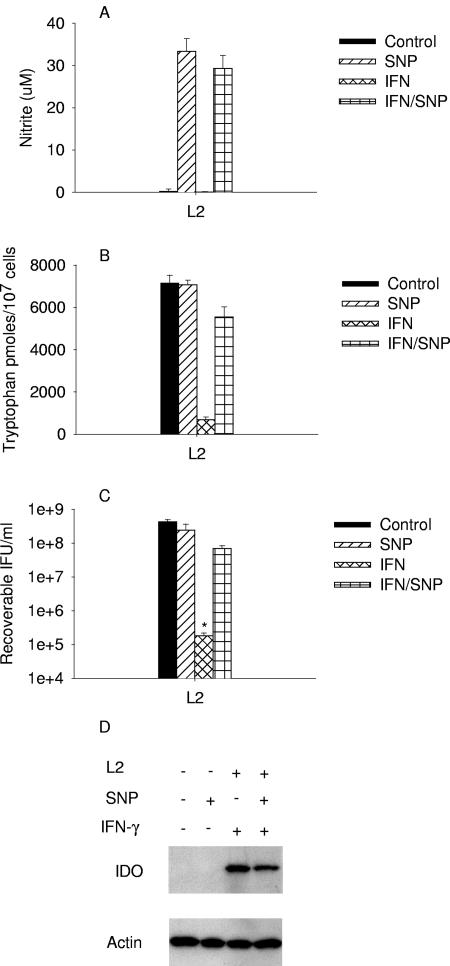
FIG. 8.
Effect of SNP-produced nitric oxide on IDO activity and C. trachomatis L2 growth in HeLa cells. HeLa cells were untreated or pretreated with IFN-γ for 24 h, then infected with C. trachomatis L2, and then cultured in complete medium (Table 1) supplemented with IFN-γ (20 U/ml) and/or sodium nitroprusside (0.5 mM). Following incubation for 48 h, medium nitrite levels were determined using Griess reagent, intracellular tryptophan pools were measured by HPLC, chlamydiae growth was assessed by titrating recoverable IFU, and IDO protein was detected by Western blotting. Growth data are presented as IFU (log10) and represent the means ± standard errors of triplicate determinations. *, P < 0.0001 compared to the untreated control culture. See the legend to Fig. 1 and Materials and Methods for details. Medium nitrite levels were determined after 48 h of incubation using the Griess reagent. Data are presented as μM nitrite (A) or pmol tryptophan/107 cells (B and C) and represent the means of two independent experiments with ranges shown. Intracellular tryptophan pools were measured by HPLC.
FIG. 6.
Effect of IFN-γ or IFN-γ-LPS on the expression of iNOS and IDO (A and C) and on the growth of C. trachomatis L2 and C. muridarum MoPn (B) in RAW 264.7 macrophages. RAW 264.7 macrophages were uninfected or infected with chlamydiae and then incubated in the complete culture medium described in Table 1 plus IFN-γ (20 U/ml) or IFN-γ (20 U/ml)-LPS (20 ng/ml) with or without NMMA (1 mM). Cultures were harvested and processed for the various assays 24 h later as described in the legend to Fig. 1. Growth data are presented as IFU (log10) and represent the means ± standard errors of triplicate determinations. *, P < 0.0001 compared to the untreated control culture. Medium nitrite levels were determined after 48 h of incubation using the Griess reagent. Data are presented as μM nitrite and represent the means of two independent experiments with ranges shown.
Effect of exogenous NO donor on chlamydial growth.
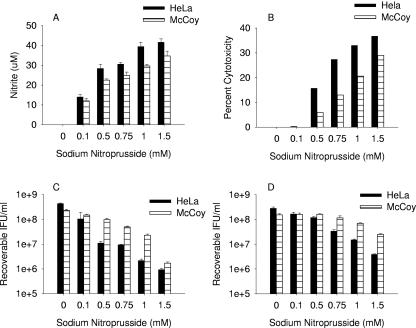
In addition to NO, IFN-γ treatment of cells results in the induction of several antibacterial activities (4, 53), making it difficult to assign inhibitory activity to any one particular effector mechanism. The chemical NO donor sodium nitroprusside was used to assess the effects of NO on chlamydial growth. Since NO is toxic to cells in culture (6-8), we first assessed the effects of NO on host cell viability. Addition of SNP to the culture medium resulted in the production of NO (Fig. 7A), and there was a correlation between the amounts of NO generated, host cell toxicity (Fig. 7B), and inhibition of L2 (Fig. 7C) and MoPn (Fig. 7D) growth. There was increased inhibition of chlamydial growth at higher SNP concentrations, but there was also a substantial decrease in host cell viability, making it difficult to differentiate host- and chlamydia-specific effects. Incubation of C. trachomatis L2 EBs at 4°C in the presence of various concentrations of SNP (NO, 30 to 200 μM) had no subsequent effect on organism viability or growth (data not shown).
FIG. 7.
Effect of sodium nitroprusside-produced nitric oxide on HeLa and McCoy cells (A and B) and on the growth of C. trachomatis L2 (C) and C. muridarum (D) in HeLa and McCoy cells. HeLa and McCoy cells were infected with chlamydiae and then cultured in the appropriate complete medium (Table 1) plus the indicated concentration of SNP. After culturing for 48 h, medium nitrite levels were determined using the Griess reagent, host cell cytotoxicity was measured by assaying medium lactate dehydrogenase activity, and chlamydia growth was assessed by titrating recoverable IFU. Growth data are presented as IFU (log10) and represent the means ± standard errors of triplicate determinations. See the legend to Fig. 1 and Materials and Methods for details. Medium nitrite levels were determined after 48 h of incubation using the Griess reagent. Data are presented as μM nitrite and represent the means of two independent experiments with ranges shown.
Interpretation of the results with RT-4 cells, which produce both IDO and iNOS, is complicated, since it is known that NO inhibits IDO activity (59). HeLa cells and SNP were used to directly assess the effects of NO on the antichlamydial activity of IDO-induced tryptophan degradation (Fig. 8). Incubation of HeLa cells in the presence of 0.5 mM SNP resulted in the production of NO, no induction of IDO, no tryptophan degradation, and essentially no inhibition of chlamydial growth. Even though there was no change in IDO protein levels, the exogenous NO donor did inhibit IFN-γ-induced IDO activity as determined by the recovery of intracellular tryptophan pools and reversal of the inhibition of chlamydial growth (Fig. 8). Together these results indicate that NO can reverse the antichlamydial activity of IDO in HeLa cells.
DISCUSSION
The mouse model has been particularly useful in elucidating the basis of chlamydial immunity and has firmly established the critical role of CD4+ type 1 T cells (37). Not surprisingly, in vitro and in vivo studies indicate that IFN-γ is a critical cytokine for controlling chlamydial infection, and this has been demonstrated in both mice and humans (10, 37). Numerous studies have shown that there is a dramatic difference in the susceptibility of different chlamydial strains to IFN-γ effector mechanisms in different host species, tissues, and cells in culture (10, 37, 49). Multiple observations suggest that this is due to differences in the antichlamydial effector mechanisms induced by IFN-γ in various hosts and the presence of chlamydial strain-specific virulence genes (51, 52). Our results further support this hypothesis.
IFN-γ-induced tryptophan degradation and NO production have been implicated as primary antichlamydial effector mechanisms (10, 37, 53, 65). There was a clear distinction in the response to IFN-γ stimulation between mouse and human cell lines. With the exception of BeWo cells which, despite expressing functional IFN-γ receptors (24) do not induce IDO in response to IFN-γ (21), all human cells tested expressed functional IDO, as assessed by degradation of intracellular tryptophan, after treatment with IFN-γ or IFN-γ-LPS. LPS or infection alone did not result in IDO induction under any conditions tested. This indicates that under our culture conditions, IFN-γ is the inducer of IDO. iNOS was never induced in human cells by chlamydial infection or following treatment with IFN-γ, LPS, or IFN-γ-LPS.
In all cases, IDO-induced tryptophan degradation resulted in a dramatic inhibition, a 2 to 4 log10 reduction in IFU recovery of chlamydial growth. In the majority of cases the effects of IFN-γ could be completely reversed by tryptophan, indicating that tryptophan degradation is a key antichlamydial effector mechanism induced by IFN-γ in human cell lines. Interestingly, with L2-infected THP-1 and RT-4 cells, the reversal by tryptophan was only partial. For L2, as would be expected given the decrease seen in tryptophan pools, we found that trpRBA expression was upregulated in RT-4 and THP-1 cells treated with IFN-γ (data not shown). Furthermore, despite showing only partial reversal of growth inhibition, the induction of trpRBA expression seen in the presence of IFN-γ was repressed by tryptophan or indole (data not shown). In total, these results indicate that there are at least two antichlamydial effector mechanisms induced by IFN-γ in RT-4 and THP-1 cells. Part of the inhibition results from tryptophan deprivation, with the remainder associated with a yet-to-be-identified effector(s). The additional antichlamydial effector is more effective against L2 than MoPn. It is possible that the unidentified effectors could be different in RT-4s and THP-1s.
In mouse cells the response to cytokine/LPS stimulation was different than that seen in human cells. The only mouse cell line that showed induction of IDO in response to any culture condition tested was the lung epithelial line, MLE15. The level of IDO activity in MLE15 cells was low, as assessed by the relatively small decrease in tryptophan pools, which had minimal effect on chlamydial growth. While it is known that IDO is expressed in a variety of mouse tissues (25, 57) and plays an important role as an immune regulator (34, 36), it does not appear to play a role in any of the mouse cell lines tested in our study. In support of our in vitro data, recent in vivo studies with IDO knockout mice indicated that IDO is not necessary for clearance of human C. trachomatis strains or MoPn from the mouse genital tract (40). The fact that C. muridarum does not encode tryptophan synthase (51) also suggests that MoPn does not encounter tryptophan starvation during the natural infection process. In contrast to what is seen with chlamydiae, IDO expression is important for clearance of Listeria monocytogenes infection of mouse placenta (31) and acute Toxoplasma gondii infection in mice (23). Clearly, the role of IDO in controlling infection in mice depends on a complex interplay between various factors, including infected tissue/cells, cytokines present in the environment, and the infecting pathogen.
With the exception of IEC4.1, TM3, and MLE15, which produce little NO, all mouse cell lines tested produced substantial quantities of NO in response to cytokine or LPS treatment and/or chlamydial infection. In contrast to our results, Igietseme et al. found that chlamydiae-infected TM3 cells produced NO when cocultured with a chlamydia-specific, cytokine-secreting, murine T-lymphocyte clone (29). The difference in results is likely due to the ability of the T-cell clone to secrete a variety of cytokines in addition to IFN-γ, including TNF-α, a cytokine known to regulate iNOS expression (5). In support of this we found that the stimulus required for iNOS induction varies from cell line to cell line, some requiring multiple stimuli and others responding to a single stimulus. With the nonprofessional phagocyte mouse cell lines that produce NO in response to cytokine-LPS treatment, a common finding was that chlamydial infection resulted in decreased NO production, compared to the uninfected controls treated in the same manner. This may result from competition from chlamydiae for iNOS substrate (arginine) or cofactors (NADPH and/or tetrahydrobiopterin); alternatively, since iNOS is associated with the actin cytoskeleton (62), it may be a reflection of the cytoskeletal rearrangement caused by chlamydial infection (13).
Interpretation of the data with respect to any direct effect of cytokine-LPS-induced NO on chlamydial growth is complicated by the multitude of potential effector mechanisms induced in the different host cell lines as well as the variety of effects NO has on host cell functions (6-8). These complications likely account for the contradictory results reported in the literature in regards to the antichlamydial activities of NO in vitro (14, 19, 27, 29, 33, 53). In addition, in professional phagocytes, which produce reactive oxygen intermediates, NO can react with O2− to form peroxynitrite (ONOO−), which possesses potent antimicrobial activity (3, 5). In keeping with these observations, our results indicate that the effects of NO on chlamydial growth in vitro were variable and dependent on the cell line used. In agreement with previous reports (14, 33, 46), we found that blocking of IFN-γ-induced NO production with NMMA in McCoy, L929 fibroblasts, and RAW264.7 macrophage cells only partially reversed the inhibition of chlamydial growth, a result suggesting that a second IFN-γ-induced antichlamydial effector may be involved. Reactive oxygen intermediates are a potential alternate effector in RAW264.7 cells.
Of particular interest is the response of the primary upper genital tract epithelial lines (BM12.4 and BM1.11), which are the natural host cell for chlamydiae in the mouse genital tract infection model. These cells produce low levels of NO in response to infection alone, with the level being greatly enhanced by IFN-γ. Despite producing NO, growth of MoPn is unaffected by IFN-γ in these cells. In addition, while L2 replication was reduced by 2 log10 by IFN-γ in these cells, the inhibition was not reversed when NO production was blocked with the iNOS inhibitor NMMA. These results indicate that there is an L2-specific antichlamydial effector mechanism induced in the primary upper genital tract epithelial cells that is independent of NO production. A similar finding was reported by Perry et al., who showed that human C. trachomatis strains were much more sensitive to the inhibitory effects of IFN-γ in mouse cells than were the murine-adapted MoPn cells (43). Furthermore, it has recently been shown that the IFN-γ-inducible p47GTPase, ligp1, is the effector responsible for inhibiting the growth of human chlamydial strains in primary mouse epithelial cells (40). It has been postulated that the natural resistance of MoPn to this effector mechanism may be associated with the presence of three copies of a large cytotoxin gene in the MoPn genome (40, 51).
We used SNP, a chemical NO generator, to directly assess the effects of NO on chlamydial growth. The data from these experiments indicate that direct exposure of chlamydial EBs to noncytotoxic levels of NO has little or no effect on their infectivity or subsequent growth. At higher concentrations in cell culture, the antichlamydial effects of NO correlate with host cell toxicity. In total, the results of all our in vitro experiments suggest that, in nonprofessional phagocytes, NO has little direct effect on chlamydial growth. These findings are consistent with numerous in vivo studies employing iNOS knockout mice, where it has been demonstrated that NO has no effect on clearance of primary or secondary genital tract infection (28, 40, 42, 46). Interestingly, dissemination of chlamydia from the site of inoculation to spleen and lungs has been observed in iNOS knockout mice (28), and it was found that viable MoPn persists for longer periods of time in iNOS−/− mice than in their wild-type counterparts (47). These findings are consistent with the hypothesis that macrophages may be involved in dissemination and persistence of in vivo chlamydial infection (42, 47) and our in vitro data indicating that there is only partial reversal of the IFN-γ-induced growth inhibition in RAW264.7 cells when NO production is blocked with NMMA. Furthermore, the finding that, compared to iNOS knockout animals, even more extensive dissemination of chlamydiae and greatly pronounced clinical disease occurs in IFN-γ knockout mice (15, 41) is in keeping with an only partial reversal of in vitro growth inhibition by iNOS inhibitors. In total, these data suggest that there is an additional IFN-γ-inducible effector mechanism, in addition to iNOS, which is important for controlling chlamydial growth in macrophages.
Of all cell lines examined only the human uroepithelial line, RT-4, induced significant amounts of both iNOS and IDO. Induction of IDO required exposure to IFN-γ alone where, in agreement with others (17, 26, 27), treatment with a cytokine cocktail was required for simultaneous induction of IDO and iNOS. The induction of both effector mechanisms by the same cells under defined culture conditions allowed us to assess the consequences of the interaction between NO and IDO. Culturing RT-4 cells in IFN-γ alone lead to IDO induction, tryptophan degradation, and inhibition of chlamydial growth. Unlike other human nonmacrophage cell lines examined, the inhibition of L2 growth by IFN-γ in RT-4 cells was only partially reversed by tryptophan or indole. In contrast, IFN-γ-induced inhibition of MoPn growth in RT-4 cells was reversed by tryptophan but not indole. Treatment with the cytokine-LPS cocktail resulted in induction of IDO and iNOS. Under these conditions, NO was produced and, even though IDO protein was still present, there was an increase in the tryptophan pools. If NO production was blocked with NMMA, tryptophan pools decreased. These findings are consistent with the ability of NO to inhibit IDO activity (26, 59). The NO-induced increase in tryptophan pools did not result in a significant recovery of L2 growth. In addition, even when NO production was blocked with NMMA, addition of tryptophan or indole only resulted in partial rescue of L2 growth. Furthermore, addition of iron or arginine also resulted in only partial recovery. Igietseme et al. (27) reported similar findings using RT-4 cells infected with C. trachomatis serovar E. In their study addition of iron, tryptophan, and arginine to infected RT-4 cells treated with IFN-γ-TNF-α-IL-1β and LPS only resulted in a 60% reversal of inhibition of inclusion formation. While the complexity of the experimental system sets a limit on the interpretation of the data, in total the results again indicate that there is an additional effector mechanism responsible for the antichlamydial activity seen in RT-4 cells.
As a more direct approach to address the question of the importance of an interaction between NO and IDO, we used the chemical NO donor SNP with IFN-γ-treated HeLa cells, a system where the antichlamydial effects of IFN-γ are completely reversible with tryptophan. The results of these studies clearly indicate that exogenously produced NO inhibits IDO activity, there is a concomitant increase in tryptophan pools, and there is a reversal of the IFN-γ-induced inhibition of chlamydial growth.
In summary, the results presented here provide a better understanding of the IFN-γ-induced antichlamydial effector mechanisms in human and mouse cells. The effectors induced are dependent not only on the host species (human or mouse) but also on the tissue source (epithelial, fibroblast, or macrophage/monocyte). In human cells the major effector mechanism is IDO, catalyzed tryptophan degradation. In mouse cells iNOS is commonly induced by IFN-γ, with the exception of macrophages, where it likely works in concert with reactive oxygen intermediates (i.e., formation of ONOO−); NO possess little antichlamydial activity on its own. It seems likely that the primary antichlamydial effector mechanism in mouse cells is the recently described IFN-γ-inducible p47GTPase, ligp1 (40). Importantly, chlamydia susceptibility to the antimicrobial effectors is strain dependent. As an immune evasion strategy, human-adapted genital C. trachomatis serovars encode a functional tryptophan synthase, while the mouse-adapted MoPn encodes large cytotoxins. These are strain-specific virulence factors which are encoded within the plasticity zone, a highly variable region of the chlamydial chromosome initially hypothesized to be important in determining host range and tissue tropism (51). In total, our results suggest that chlamydiae and their natural hosts have coevolved. These findings also provide a reasonable explanation for much of the controversial data in the literature with regard to the relative contribution of IFN-γ-induced effector mechanisms in controlling chlamydial growth. Many of these studies were conducted using different host cells and various chlamydial strains. It is now clear that studies addressing the role of the various antichlamydial effector mechanisms are best addressed in matched host/chlamydial sets. Finally, with regard to use of the murine model for assessing the potential of experimental chlamydial vaccines, given the obvious host adaptation chlamydia has undergone, it seems most prudent to use MoPn as the challenge strain when a genetically wild-type mouse is used as host.
Acknowledgments
We acknowledge R. Johnson and J. Whitsett for the BM12.4 and BM1.11 and the MLE15 cells, respectively. We thank G. Byrne for helpful discussions.
This research was supported by grants from the Canadian Institutes of Health Research (GR-13301) to G.M. and the National Institutes of Health (R21AI054875-02).
Editor: D. L. Burns
REFERENCES
- 1.Beatty, W. L., G. I. Byrne, and R. P. Morrison. 1993. Morphologic and antigenic characterization of interferon gamma-mediated persistent Chlamydia trachomatis infection in vitro. Proc. Natl. Acad. Sci. USA 90:3998-4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beatty, W. L., R. P. Morrison, and G. I. Byrne. 1994. Persistent chlamydiae: from cell culture to a paradigm for chlamydial pathogenesis. Microbiol. Rev. 58:686-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bogdan, C. 2001. Nitric oxide and the immune response. Nat. Immunol. 2:907-916. [DOI] [PubMed] [Google Scholar]
- 4.Bogdan, C. 1997. Of microbes, macrophages and nitric oxide. Behring Inst. Mitt. 99:58-72. [PubMed] [Google Scholar]
- 5.Bogdan, C., M. Rollinghoff, and A. Diefenbach. 2000. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr. Opin. Immunol. 12:64-76. [DOI] [PubMed] [Google Scholar]
- 6.Brown, G. C. 2001. Regulation of mitochondrial respiration by nitric oxide inhibition of cytochrome c oxidase. Biochim. Biophys. Acta 1504:46-57. [DOI] [PubMed] [Google Scholar]
- 7.Brown, G. C., and V. Borutaite. 2004. Inhibition of mitochondrial respiratory complex I by nitric oxide, peroxynitrite and S-nitrosothiols. Biochim. Biophys. Acta 1658:44-49. [DOI] [PubMed] [Google Scholar]
- 8.Brown, G. C., and V. Borutaite. 2001. Nitric oxide, mitochondria, and cell death. IUBMB Life 52:189-195. [DOI] [PubMed] [Google Scholar]
- 9.Brunham, R. C., J. Kimani, J. Bwayo, G. Maitha, I. Maclean, C. Yang, C. Shen, S. Roman, N. J. Nagelkerke, M. Cheang, and F. A. Plummer. 1996. The epidemiology of Chlamydia trachomatis within a sexually transmitted diseases core group. J. Infect. Dis. 173:950-956. [DOI] [PubMed] [Google Scholar]
- 10.Brunham, R. C., and J. Rey-Ladino. 2005. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat. Rev. Immunol. 5:149-161. [DOI] [PubMed] [Google Scholar]
- 11.Caldwell, H. D., J. Kromhout, and J. Schachter. 1981. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect. Immun. 31:1161-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caldwell, H. D., H. Wood, D. Crane, R. Bailey, R. B. Jones, D. Mabey, I. Maclean, Z. Mohammed, R. Peeling, C. Roshick, J. Schachter, A. W. Solomon, W. E. Stamm, R. J. Suchland, L. Taylor, S. K. West, T. C. Quinn, R. J. Belland, and G. McClarty. 2003. Polymorphisms in Chlamydia trachomatis tryptophan synthase genes differentiate between genital and ocular isolates. J. Clin. Investig. 111:1757-1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carabeo, R. A., S. S. Grieshaber, E. Fischer, and T. Hackstadt. 2002. Chlamydia trachomatis induces remodeling of the actin cytoskeleton during attachment and entry into HeLa cells. Infect. Immun. 70:3793-3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, B., R. Stout, and W. F. Campbell. 1996. Nitric oxide production: a mechanism of Chlamydia trachomatis inhibition in interferon-gamma-treated RAW264.7 cells. FEMS Immunol. Med. Microbiol. 14:109-120. [DOI] [PubMed] [Google Scholar]
- 15.Cotter, T. W., K. H. Ramsey, G. S. Miranpuri, C. E. Poulsen, and G. I. Byrne. 1997. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect. Immun. 65:2145-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cunningham, D. S. 1995. Immune response characteristics in women with chlamydial genital tract infection. Gynecol. Obstet. Investig. 39:54-59. [DOI] [PubMed] [Google Scholar]
- 17.Daubener, W., V. Posdziech, U. Hadding, and C. R. MacKenzie. 1999. Inducible anti-parasitic effector mechanisms in human uroepithelial cells: tryptophan degradation vs. NO production. Med. Microbiol. Immunol. (Berlin) 187:143-147. [DOI] [PubMed] [Google Scholar]
- 18.Dessus-Babus, S., T. L. Darville, F. P. Cuozzo, K. Ferguson, and P. B. Wyrick. 2002. Differences in innate immune responses (in vitro) to HeLa cells infected with nondisseminating serovar E and disseminating serovar L2 of Chlamydia trachomatis. Infect. Immun. 70:3234-3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devitt, A., P. A. Lund, A. G. Morris, and J. H. Pearce. 1996. Induction of alpha/beta interferon and dependent nitric oxide synthesis during Chlamydia trachomatis infection of McCoy cells in the absence of exogenous cytokine. Infect. Immun. 64:3951-3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisenstein, T. K. 2001. Implications of Salmonella-induced nitric oxide (NO) for host defense and vaccines: NO, an antimicrobial, antitumor, immunosuppressive and immunoregulatory molecule. Microbes Infect. 3:1223-1231. [DOI] [PubMed] [Google Scholar]
- 21.Entrican, G., S. Wattegedera, M. Chui, L. Oemar, M. Rocchi, and C. McInnes. 2002. Gamma interferon fails to induce expression of indoleamine 2,3-dioxygenase and does not control the growth of Chlamydophila abortus in BeWo trophoblast cells. Infect. Immun. 70:2690-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fehlner-Gardiner, C., C. Roshick, J. H. Carlson, S. Hughes, R. J. Belland, H. D. Caldwell, and G. McClarty. 2002. Molecular basis defining human Chlamydia trachomatis tissue tropism. A possible role for tryptophan synthase. J. Biol. Chem. 277:26893-26903. [DOI] [PubMed] [Google Scholar]
- 23.Fujigaki, S., M. Takemura, H. Hamakawa, M. Seishima, and K. Saito. 2003. The mechanism of interferon-gamma-induced anti-Toxoplasma gondii by indoleamine 2,3-dioxygenase and/or inducible nitric oxide synthase vary among tissues. Adv. Exp. Med. Biol. 527:97-103. [DOI] [PubMed] [Google Scholar]
- 24.Fulop, V., M. A. Steller, R. S. Berkowitz, and D. J. Anderson. 1992. Interferon-gamma receptors on human gestational choriocarcinoma cell lines: quantitative and functional studies. Am. J. Obstet. Gynecol. 167:524-530. [DOI] [PubMed] [Google Scholar]
- 25.Hansen, A. M., C. Driussi, V. Turner, O. Takikawa, and N. H. Hunt. 2000. Tissue distribution of indoleamine 2,3-dioxygenase in normal and malaria-infected tissue. Redox Rep. 5:112-115. [DOI] [PubMed] [Google Scholar]
- 26.Hucke, C., C. R. MacKenzie, K. D. Adjogble, O. Takikawa, and W. Daubener. 2004. Nitric oxide-mediated regulation of gamma interferon-induced bacteriostasis: inhibition and degradation of human indoleamine 2,3-dioxygenase. Infect. Immun. 72:2723-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Igietseme, J. U., G. A. Ananaba, D. H. Candal, D. Lyn, and C. M. Black. 1998. Immune control of chlamydial growth in the human epithelial cell line RT4 involves multiple mechanisms that include nitric oxide induction, tryptophan catabolism and iron deprivation. Microbiol. Immunol. 42:617-625. [DOI] [PubMed] [Google Scholar]
- 28.Igietseme, J. U., L. L. Perry, G. A. Ananaba, I. M. Uriri, O. O. Ojior, S. N. Kumar, and H. D. Caldwell. 1998. Chlamydial infection in inducible nitric oxide synthase knockout mice. Infect. Immun. 66:1282-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Igietseme, J. U., I. M. Uriri, M. Chow, E. Abe, and R. G. Rank. 1997. Inhibition of intracellular multiplication of human strains of Chlamydia trachomatis by nitric oxide. Biochem. Biophys. Res. Commun. 232:595-601. [DOI] [PubMed] [Google Scholar]
- 30.Johnson, R. M. 2004. Murine oviduct epithelial cell cytokine responses to Chlamydia muridarum infection include interleukin-12-p70 secretion. Infect. Immun. 72:3951-3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mackler, A. M., E. M. Barber, O. Takikawa, and J. W. Pollard. 2003. Indoleamine 2,3-dioxygenase is regulated by IFN-gamma in the mouse placenta during Listeria monocytogenes infection. J. Immunol. 170:823-830. [DOI] [PubMed] [Google Scholar]
- 32.MacMicking, J., Q. W. Xie, and C. Nathan. 1997. Nitric oxide and macrophage function. Annu. Rev. Immunol. 15:323-350. [DOI] [PubMed] [Google Scholar]
- 33.Mayer, J., M. L. Woods, Z. Vavrin, and J. B. Hibbs, Jr. 1993. Gamma interferon-induced nitric oxide production reduces Chlamydia trachomatis infectivity in McCoy cells. Infect. Immun. 61:491-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mellor, A. L., B. Baban, P. Chandler, B. Marshall, K. Jhaver, A. Hansen, P. A. Koni, M. Iwashima, and D. H. Munn. 2003. Cutting edge: induced indoleamine 2,3 dioxygenase expression in dendritic cell subsets suppresses T cell clonal expansion. J. Immunol. 171:1652-1655. [DOI] [PubMed] [Google Scholar]
- 35.Mellor, A. L., D. Munn, P. Chandler, D. Keskin, T. Johnson, B. Marshall, K. Jhaver, and B. Baban. 2003. Tryptophan catabolism and T cell responses. Adv. Exp. Med. Biol. 527:27-35. [DOI] [PubMed] [Google Scholar]
- 36.Mellor, A. L., and D. H. Munn. 2004. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat. Rev. Immunol. 4:762-774. [DOI] [PubMed] [Google Scholar]
- 37.Morrison, R. P., and H. D. Caldwell. 2002. Immunity to murine chlamydial genital infection. Infect. Immun. 70:2741-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moulder, J. W. 1991. Interaction of chlamydiae and host cells in vitro. Microbiol. Rev. 55:143-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nathan, C., and M. U. Shiloh. 2000. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. USA 97:8841-8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nelson, D. E., D. P. Virok, H. Wood, C. Roshick, R. M. Johnson, W. M. Whitmire, D. D. Crane, O. Steele-Mortimer, L. Kari, G. McClarty, and H. D. Caldwell. 2005. Chlamydial IFN-γ immune evasion is linked to host infection tropism. Proc. Natl. Acad. Sci. USA 102:10658-10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perry, L. L., K. Feilzer, and H. D. Caldwell. 1997. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J. Immunol. 158:3344-3352. [PubMed] [Google Scholar]
- 42.Perry, L. L., K. Feilzer, and H. D. Caldwell. 1998. Neither interleukin-6 nor inducible nitric oxide synthase is required for clearance of Chlamydia trachomatis from the murine genital tract epithelium. Infect. Immun. 66:1265-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perry, L. L., H. Su, K. Feilzer, R. Messer, S. Hughes, W. Whitmire, and H. D. Caldwell. 1999. Differential sensitivity of distinct Chlamydia trachomatis isolates to IFN-gamma-mediated inhibition. J. Immunol. 162:3541-3548. [PubMed] [Google Scholar]
- 44.Platanias, L. C. 2005. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 5:375-386. [DOI] [PubMed] [Google Scholar]
- 45.Qiu, H., J. Yang, H. Bai, Y. Fan, S. Wang, X. Han, L. Chen, and X. Yang. 2004. Less inhibition of interferon-gamma to organism growth in host cells may contribute to the high susceptibility of C3H mice to Chlamydia trachomatis lung infection. Immunology 111:453-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramsey, K. H., G. S. Miranpuri, C. E. Poulsen, N. B. Marthakis, L. M. Braune, and G. I. Byrne. 1998. Inducible nitric oxide synthase does not affect resolution of murine chlamydial genital tract infections or eradication of chlamydiae in primary murine cell culture. Infect. Immun. 66:835-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramsey, K. H., G. S. Miranpuri, I. M. Sigar, S. Ouellette, and G. I. Byrne. 2001. Chlamydia trachomatis persistence in the female mouse genital tract: inducible nitric oxide synthase and infection outcome. Infect. Immun. 69:5131-5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramsey, K. H., I. M. Sigar, S. V. Rana, J. Gupta, S. M. Holland, and G. I. Byrne. 2001. Role for inducible nitric oxide synthase in protection from chronic Chlamydia trachomatis urogenital disease in mice and its regulation by oxygen free radicals. Infect. Immun. 69:7374-7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rank, R. 1999. Models of immunity, p. 239-296. In R. S. Stephens (ed.), Chlamydia: intracellular biology, pathogenesis, and immunity. ASM Press, Washington, D.C.
- 50.Raschke, W. C., S. Baird, P. Ralph, and I. Nakoinz. 1978. Functional macrophage cell lines transformed by Abelson leukemia virus. Cell 15:261-267. [DOI] [PubMed] [Google Scholar]
- 51.Read, T. D., R. C. Brunham, C. Shen, S. R. Gill, J. F. Heidelberg, O. White, E. K. Hickey, J. Peterson, T. Utterback, K. Berry, S. Bass, K. Linher, J. Weidman, H. Khouri, B. Craven, C. Bowman, R. Dodson, M. Gwinn, W. Nelson, R. DeBoy, J. Kolonay, G. McClarty, S. L. Salzberg, J. Eisen, and C. M. Fraser. 2000. Genome sequences of Chlamydia trachomatis MoPn and Chlamydia pneumoniae AR39. Nucleic Acids Res. 28:1397-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Read, T. D., G. S. Myers, R. C. Brunham, W. C. Nelson, I. T. Paulsen, J. Heidelberg, E. Holtzapple, H. Khouri, N. B. Federova, H. A. Carty, L. A. Umayam, D. H. Haft, J. Peterson, M. J. Beanan, O. White, S. L. Salzberg, R. C. Hsia, G. McClarty, R. G. Rank, P. M. Bavoil, and C. M. Fraser. 2003. Genome sequence of Chlamydophila caviae (Chlamydia psittaci GPIC): examining the role of niche-specific genes in the evolution of the Chlamydiaceae. Nucleic Acids Res. 31:2134-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rottenberg, M. E., A. Gigliotti-Rothfuchs, and H. Wigzell. 2002. The role of IFN-gamma in the outcome of chlamydial infection. Curr. Opin. Immunol. 14:444-451. [DOI] [PubMed] [Google Scholar]
- 54.Schachter, J. 1999. Infection and disease epidemiology, p. 129-170. In R. S. Stephens (ed.), Chlamydia: intracellular biology, pathogenesis, and immunity. ASM Press, Washington, D.C.
- 55.Shtrichman, R., and C. E. Samuel. 2001. The role of gamma interferon in antimicrobial immunity. Curr. Opin. Microbiol. 4:251-259. [DOI] [PubMed] [Google Scholar]
- 56.Stamm, W. 1999. Chlamydia infections of the adult. McGraw Hill, New York, N.Y.
- 57.Takikawa, O., Y. Tagawa, Y. Iwakura, R. Yoshida, and R. J. Truscott. 1999. Interferon-gamma-dependent/independent expression of indoleamine 2,3-dioxygenase. Studies with interferon-gamma-knockout mice. Adv. Exp. Med. Biol. 467:553-557. [DOI] [PubMed] [Google Scholar]
- 58.Taylor, G. A., C. G. Feng, and A. Sher. 2004. p47 GTPases: regulators of immunity to intracellular pathogens. Nat. Rev. Immunol. 4:100-109. [DOI] [PubMed] [Google Scholar]
- 59.Thomas, S. R., D. Mohr, and R. Stocker. 1994. Nitric oxide inhibits indoleamine 2,3-dioxygenase activity in interferon-gamma primed mononuclear phagocytes. J. Biol. Chem. 269:14457-14464. [PubMed] [Google Scholar]
- 60.Thomas, S. R., and R. Stocker. 1999. Redox reactions related to indoleamine 2,3-dioxygenase and tryptophan metabolism along the kynurenine pathway. Redox Rep. 4:199-220. [DOI] [PubMed] [Google Scholar]
- 61.Tipples, G., and G. McClarty. 1991. Isolation and initial characterization of a series of Chlamydia trachomatis isolates selected for hydroxyurea resistance by a stepwise procedure. J. Bacteriol. 173:4932-4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Webb, J. L., M. W. Harvey, D. W. Holden, and T. J. Evans. 2001. Macrophage nitric oxide synthase associates with cortical actin but is not recruited to phagosomes. Infect. Immun. 69:6391-6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Widner, B., M. Ledochowski, and D. Fuchs. 2000. Interferon-gamma-induced tryptophan degradation: neuropsychiatric and immunological consequences. Curr. Drug Metab. 1:193-204. [DOI] [PubMed] [Google Scholar]
- 64.Wood, H., C. Fehlner-Gardner, J. Berry, E. Fischer, B. Graham, T. Hackstadt, C. Roshick, and G. McClarty. 2003. Regulation of tryptophan synthase gene expression in Chlamydia trachomatis. Mol. Microbiol. 49:1347-1359. [DOI] [PubMed] [Google Scholar]
- 65.Yang, X. 2003. Role of cytokines in Chlamydia trachomatis protective immunity and immunopathology. Curr. Pharm. Des. 9:67-73. [DOI] [PubMed] [Google Scholar]
- 66.Yong, S., and S. Lau. 1979. Rapid separation of tryptophan, kynurenines, and indoles using reversed-phase high-performance liquid chromatography. J. Chromatogr. 175:343-346. [DOI] [PubMed] [Google Scholar]