Abstract
Intimin facilitates intestinal colonization by enterohemorrhagic Escherichia coli O157:H7; however, the importance of intimin binding to its translocated receptor (Tir) as opposed to cellular coreceptors is unknown. The intimin-Tir interaction is needed for optimal actin assembly under adherent bacteria in vitro, a process which requires the Tir-cytoskeleton coupling protein (TccP/EspFU) in E. coli O157:H7. Here we report that E. coli O157:H7 tir mutants are at least as attenuated as isogenic eae mutants in calves and lambs, implying that the role of intimin in the colonization of reservoir hosts can be explained largely by its binding to Tir. Mutation of tccP uncoupled actin assembly from the intimin-Tir-mediated adherence of E. coli O157:H7 in vitro but did not impair intestinal colonization in calves and lambs, implying that pedestal formation may not be necessary for persistence. However, an E. coli O157:H7 tccP mutant induced typical attaching and effacing lesions in a bovine ligated ileal loop model of infection, suggesting that TccP-independent mechanisms of actin assembly may operate in vivo.
Enterohemorrhagic Escherichia coli (EHEC) is a zoonotic enteric pathogen of worldwide importance and may cause severe diarrheal disease, hemorrhagic colitis, and hemolytic-uremic syndrome in humans (27). Healthy ruminants are the primary reservoir of EHEC, and human infections are frequently associated with direct or indirect contact with ruminant feces (33, 38). EHEC is closely related to enteropathogenic E. coli (EPEC), which is a major cause of infantile diarrhea, and both pathovars have the ability to induce attaching and effacing (A/E) lesions on intestinal epithelia. A/E lesions are characterized by localized effacement of microvilli, intimate adherence of bacteria to the apical plasma membrane, and formation of filamentous (F)-actin-enriched pedestal structures beneath adherent bacteria.
The genes that determine the A/E phenotype are located within the locus of enterocyte effacement (LEE) (35) and encode a filamentous type III secretion system, which delivers bacterial effector proteins into host cells that subvert, inhibit, or activate cellular processes (21). Both EPEC and EHEC insert their own receptors into the target cell plasma membrane. This molecule (Tir; translocated intimin receptor) is inserted into the host cell membrane in a hairpin loop topology with the central loop of the molecule exposed to the host cell surface and accessible for interaction with an LEE-encoded bacterial outer membrane adhesin called intimin (11, 13, 29). The intracellular amino and carboxyl termini of Tir interact with a number of focal adhesion and cytoskeletal proteins and contribute to pedestal formation (reviewed in reference 6). The mechanisms by which actin polymerization is stimulated by EPEC O127:H6 Tir and EHEC O157:H7 Tir are different. Actin assembly by EHEC O157:H7 Tir requires the type III secreted protein TccP/EspFU, which couples Tir with the neural Wiskott-Aldrich syndrome protein (N-WASP), an activator of the cellular Arp2/3 complex that is required for pedestal formation (8, 22). Mutation of tccP prevents actin nucleation under adherent EHEC O157:H7 in vitro but not intimin-Tir-mediated adherence (8, 22); however, the consequences of this event in vivo are unknown. In contrast, actin nucleation by EPEC O127:H7 Tir involves tyrosine phosphorylation of a C-terminal 12-amino-acid motif that recruits the host adaptor protein Nck and in turn N-WASP (5, 7, 23). EHEC O157:H7 Tir lacks the critical tyrosine Y474 residue, and the 12-amino-acid region required for Nck recruitment is not conserved (14). As a consequence, EHEC O157:H7 Tir is not tyrosine phosphorylated and cannot functionally substitute for Tir in EPEC O127:H6 or the A/E mouse pathogen Citrobacter rodentium (12, 28).
E. coli O157:H7 intimin is required for bacterial adherence to cultured cells and actin assembly under adherent bacteria in vitro (17, 24, 51). In vivo studies using different animal models have demonstrated that in comparison to the wild type, intimin-deficient EHEC O157:H7 is defective in intestinal colonization and, in some models, in the induction of enteritis (9, 10, 15, 25, 26, 36, 42, 47, 50). Intimin exists in at least six phylogenetically distinct subtypes which exhibit marked sequence divergence in their carboxyl-terminal cell-binding domains (1, 18, 19, 39, 40, 47). The carboxyl-terminal domain of intimin mediates the interaction with Tir; however, interactions between intimin and cellular coreceptors, including β1-integrins and surface-localized nucleolin, have been described previously (20, 43). β1-Integrins are not essential for the adherence of EPEC and actin nucleation in vitro (32). Intimin subtypes α, β, and γ, which confer a tropism for distinct intestinal sites, bind to nucleolin with comparable affinities but with lower avidity than to Tir (44). The relative importance of the intimin-Tir interaction as opposed to interactions with cellular coreceptors in vivo has so far received little attention.
The aim of this study was to assess the importance of the intimin-Tir interaction in colonization of reservoir hosts and to elucidate the function of TccP/EspFU, and consequently of actin pedestal formation, in intestinal colonization and A/E lesion formation.
In this study, the importance of the intimin-Tir interaction was studied using two different E. coli O157:H7 strains in two reservoir hosts. Animal procedures have been described in detail previously (46) and were performed in accordance with the Animals (Scientific Procedures) Act of 1986 and approved by the local Ethical Review Committee. Fecal shedding data were statistically analyzed for the effect of mutation by means of an F test, with age as a cofactor and data taken as repeated measurements (Proc Mixed, Statistical Analysis System, 1995; SAS Institute, North Carolina). P values of <0.05 were taken to be significant.
Calf studies were performed using single and double nonpolar eae and tir deletion mutants of the stx-negative E. coli O157:H7 strain 85-170 Nalr (Table 1). The 85-170 Nalr Δeae mutant has been described previously (4, 18). The 85-170 Nalr Δtir and 85-170 Nalr Δeae Δtir mutant strains were constructed by Stevens et al. (46) and Vlisidou et al. (49), respectively. Studies of lambs were performed with the stx-negative strain NCTC12900 Nalr, an isogenic NCTC12900 Nalr eae::Camr mutant (31), and with NCTC12900 Nalr tir::Strr. The tir mutant of NCTC12900 Nalr was made by the insertion of a nonpolar streptomycin resistance cassette into tir followed by allelic exchange using a positive-selection suicide vector. Primers TirF (5′-CTGGCGGCGTCTGAGATAACACT-3′) and TirR (5′-CCCCGCACTTGGATTTC-3′) were used to amplify an internal 1,317-bp fragment of tir from NCTC12900 Nalr. The amplicon was purified and ligated into the multiple cloning site of the cloning vector pCR2.1 (Invitrogen, Paisley, United Kingdom), producing pLMF1. A blunt-ended 1.2-kb PvuII fragment from plasmid p723 (2) containing a nonpolar streptomycin resistance cassette was cloned into a unique HpaI site within the tir gene, yielding pLMF2. A SpeI fragment and a NotI fragment of pLMF2 carrying tir::Strr was then subcloned into the SrfI site of pERFORMK (3), and the resulting plasmid (pLMF3) was introduced into NCTC12900 Nalr by conjugation from E. coli K-12 S17-1λpir with selection for streptomycin and nalidixic acid resistance. Colonies from filter matings were tested for loss of the antibiotic conferred by the suicide vector by replica plating onto minimal medium supplemented with the antibiotic conferred by the suicide vector. All eae and tir mutants were verified by PCR analysis using primers flanking the targeted genes or by Southern hybridization. Western blotting using specific antisera confirmed the absence of intimin and Tir in the respective mutants, and no polar effects on the expression of eae or tir were detected by analysis of total or secreted proteins (data not shown). Fluorescent actin staining tests confirmed that each mutant was unable to nucleate actin at sites of attachment to HeLa cells, and introduction of the cloned eae or tir gene restored fluorescent actin staining reactivity to the respective mutants (data not shown).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Description | Reference(s) |
|---|---|---|
| Strains | ||
| 85-170 Nalr | EHEC O157:H7 spontaneous stx1 and stx2 mutant, nalidixic acid resistant | 46 |
| 85-170 Nalr Δeae (ICC170) | 85-170 Nalr lacking eae | 18 |
| 85-170 Nalr Δtir | 85-170 Nalr lacking tir | 46 |
| 85-170 Nalr Δeae Δtir | 85-170 Nalr lacking eae and tir | 49 |
| ICC203 | 85-170 Nalr ΔtccP::Kanr | This study |
| NCTC12900 Nalr | EHEC O157:H7 naturally stx1 and stx2 mutant, Nalr | 31,50 |
| NCTC12900 Nalreae::Camr | NCTC12900 Nalr lacking eae | 31 |
| NCTC12900 Nalrtir::Strr | NCTC12900 Nalr lacking tir | This study |
| Plasmids | ||
| pLMF1 | pCR2.1 derivative containing a 1,317-bp tir amplicon from NCTC12900 | This study |
| pLMF2 | pCR2.1 derivative containing a 1,317-bp tir amplicon from NCTC12900 Nalr, disrupted by the streptomycin resistance cassette from p723 | This study |
| pLMF3 | pERFORMK derivative containing the tir::strr construct from pLMF2 | This study |
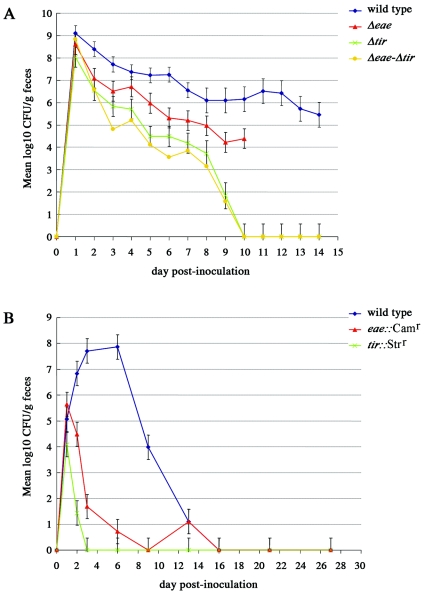
Conventional Friesian bull calves 11 to 16 days old were individually challenged by the oral route with 2.44 × 1010 ± 0.9× 1010 CFU (mean ± standard error of the mean) of strain 85-170 Nalr Δeae (n = 4), 1.17 × 1010 ± 0.55 × 1010 CFU of 85-170 Nalr Δtir (n = 4), 5.00 × 1010 ± 0.93 × 1010 CFU of 85-170 Nalr Δeae Δtir (n = 5), or 2.60 × 1010 ± 0.42 × 1010 CFU of the wild-type strain (n = 7) as described previously (46). No significant effect of age on the course of fecal excretion of 85-170 Nalr was detected (P > 0.05); therefore, statistical analysis was performed taking into account only the effect of mutation. All 85-170 Nalr mutants were found to be defective in colonization compared to that of the wild type (Fig. 1A). The 85-170 Nalr Δtir and Δeae Δtir mutants were shed in significantly lower numbers than the wild type from day 2 postinoculation (P < 0.05). The excretion of the 85-170 Nalr Δtir mutant followed a trend similar to that of the double mutant, indicating that the deletion of eae did not cause further attenuation. Both the 85-170 Nalr Δtir and 85-170 Nalr Δeae Δtir mutants were eliminated by day 10 postinoculation. The 85-170 Nalr Δeae mutant was shed in significantly lower numbers than the wild type from day 5 postinoculation, with the number of bacteria consistently recovered in the feces being at least 1 order of magnitude lower (P < 0.05), except on day 8 postinoculation, when no statistically significant difference was observed (P = 0.1136). The 85-170 Nalr Δeae mutant was recovered in significantly higher numbers than the isogenic Δtir and Δeae Δtir mutants on days 9 and 3 postinoculation, respectively (P < 0.05).
FIG. 1.
Course of fecal excretion of E. coli O157:H7 eae and tir mutants in 14-day-old calves (A) and six-week-old lambs (B). The level of colonization is indicated by the mean log-transformed viable count (CFU/g) of E. coli O157:H7 in fecal samples taken twice daily for 12 days (A) or at intervals for 27 days (B). The data for the 85-170 Nalr Δtir single mutant have been described previously (16) and are shown for the purpose of comparison. The error bars indicate the standard errors of the means.
Similar trends were observed following the inoculation of groups of six 6-week-old lambs with NCTC12900 Nalr, NCTC12900 Nalr eae::Camr, or NCTC12900 Nalr tir::Strr (Fig. 1B). Significant reductions in the fecal shedding of both mutants relative to that of the wild type were detected from day 2 postinoculation (P values of 0.0006 and <0.0001, respectively). Consistent with the results obtained using the calf model, the NCTC12900 Nalr tir::Strr mutant was shed in lower numbers and for a shorter duration than the isogenic eae mutant. Indeed, the NCTC12900 Nalr tir::Strr mutant could not be detected even after enrichment from day 3 postinoculation, whereas the NCTC12900 Nalr eae::Camr mutant was eliminated on day 16 postinoculation.
While differences in the kinetics of fecal excretion of the eae and tir mutants were detected between the calf and lamb models, the data imply that the role of intimin in the colonization of the bovine and ovine intestines can be explained largely by its binding to Tir as opposed to cellular coreceptors. If interactions with cellular coreceptors had been of key importance, then it may have been anticipated that eae mutants would be more attenuated than those lacking tir. The role of Tir in intestinal colonization by rabbit EPEC and EHEC O157:H7 in infant rabbits (34, 42) as well as C. rodentium in mice (12) has been confirmed. In addition, a vaccine containing secreted proteins from E. coli O157:H7 lacking tir was not as efficient in cattle as was an identical formulation from an isogenic wild-type isolate (41). However, this is the first report of the relative attenuation of isogenic eae and tir mutants of E. coli O157:H7 strains in reservoir hosts.
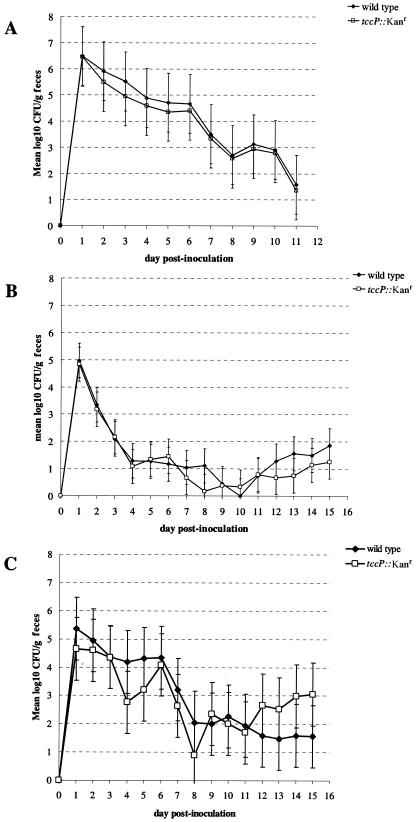
To determine whether actin assembly dependent on the Tir-cytoskeleton coupling protein is necessary for intestinal colonization in calves and sheep, tccP was replaced in strain 85-170 Nalr by a λRed-mediated insertion of a nonpolar kanamycin resistance cassette using primers tccP-flank-f1, tccP-flank-r1, tccP-flank-f2, and tccP-flank-r2, as described previously (22). The ΔtccP::Kanr mutant (strain ICC203) was confirmed to adhere to HeLa cells but to be unable to nucleate F-actin under sites of attachment (Fig. 3B). Initially, mixed-oral-infection experiments were performed. Groups of three 14-day-old Friesian bull calves and six 6-week-old lambs were orally coinoculated with 1.41 × 1010 ± 0.21 × 1010 and 8.00 × 109 ± 0.35 × 109 CFU of the 85-170 Nalr wild type and strain ICC203, respectively, and the course of fecal excretion of the bacteria was followed for 2 weeks. Wild-type and mutant bacteria were enumerated by plating serial dilutions of feces onto sorbitol MacConkey agar supplemented with potassium tellurite and with either nalidixic acid only or both nalidixic acid and kanamycin as described previously (16). The tccP mutant competed equally with the wild-type strain in both animal models, and no statistically significant differences in fecal excretion of the two strains could be detected at any time postinoculation (Fig. 2A and B). Cross-complementation of the tccP defect by wild-type bacteria is considered unlikely, since EHEC mutants deficient in type III secretion or in the production of effector proteins (e.g., Map or NleD) were found to be attenuated by screening pools of 95 signature-tagged transposon mutants in which the vast majority of bacteria were wild-type strains with respect to LEE function (16, 48). However, to address this possibility, two groups of three 6-week-old lambs were separately inoculated with 85-170 Nalr or ICC203. Consistent with the phenotype in coinfection experiments, the ΔtccP::Kanr mutation had no significant effect on the fecal excretion of EHEC O157:H7 following a single infection of lambs (Fig. 2C). These data suggest that it may be possible to uncouple a pedestal formation from intimin-Tir-mediated colonization; therefore, it was necessary to confirm that actin assembly by the E. coli O157:H7 ΔtccP::Kanr mutant had been abolished in vivo.
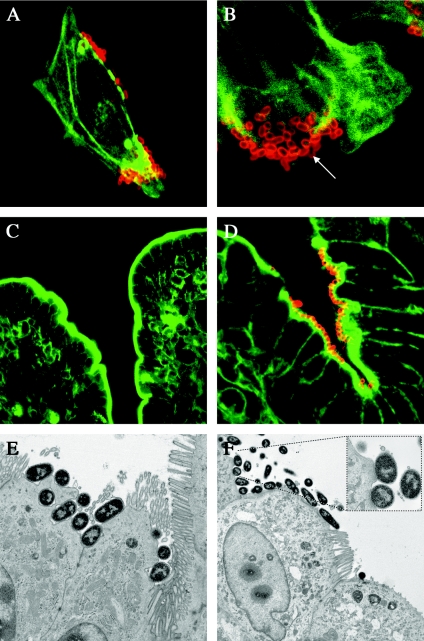
FIG. 3.
TccP is not essential for the induction of A/E lesions in calves. HeLa cells were infected with 85-170 Nalr (A) and ICC203 (ΔtccP::Kanr mutant) (B) for 8 h. The arrow shows an absence of F-actin nucleation under adherent ICC203, consistent with previous observations (8, 22). Fifty-micrometer-thick vibrating-microtome sections of bovine ileum 12 h after inoculation with sterile LB medium (C) and ICC203 (D) in the presence of 5 mM NE are shown. Bacteria were detected with rabbit anti-O157 lipopolysaccharide typing serum and anti-rabbit immunoglobulin-Alexa568 (red) as described previously (45). Filamentous actin was stained with fluorescein isothiocyanate phalloidin (green). Magnification, ×630. Transmission electron micrographs showing A/E lesions induced by the 85-170 Nalr wild type (E) and strain ICC203 (F) in bovine ileal loops in the presence of 5 mM NE are also shown.
FIG. 2.
Colonization of the bovine and ovine intestine by 85-170 Nalr ΔtccP::Kanr (ICC203) in mixed-infection experiments with the wild-type strain in 14-day-old calves (A) and 6-week-old lambs (B). The level of colonization is indicated by the mean log-transformed viable count (CFU/g) of fecal samples taken once daily for 11 days postinoculation. The error bars indicate the standard errors of the means. To assess the possibility of cross-complementation of the tccP defect, 85-170 Nalr and ICC203 were fed separately to groups of three lambs (C). No statistically significant difference was observed between colonization in lambs infected with ICC203 and that in the wild-type control (C).
While EHEC O157:H7 has been reported to form A/E lesions in neonatal colostrum-deprived calves and at the terminal part of the rectum in adult cattle (10, 37), we have observed, by confocal microscopy, only sparse microcolonies of adherent 85-170 Nalr at distal intestinal sites in calves of the age used and have so far failed to detect A/E lesions by transmission electron microscopy (data not shown). However, we recently reported that 85-170 Nalr can be induced to form extensive A/E lesions on the ileal mucosa of 28-day-old conventional calves in the presence of the neuroendocrine hormone norepinephrine (NE) (49). Following these observations, we used the bovine ligated intestinal loop assay to assess the role of TccP in the ability of EHEC O157:H7 to adhere to ileal mucosa and to induce the formation of A/E lesions in the presence of 5 mM NE. Mid-ileal loops were constructed in a 28-day-old conventional Friesian bull calf, and the loops were inoculated with the NE-treated 85-170 Nalr wild type, the isogenic tccP mutant ICC203, or sterile medium as a control. Tissue sections were excised 12 h after loop inoculation and analyzed by confocal laser scanning microscopy and transmission electron microscopy as described previously (45). ICC203 was able to form microcolonies and disrupt the rim of F-actin staining around villi, consistent with microvillus effacement (Fig. 3D). Using transmission electron microscopy, characteristic A/E lesions were observed on the epithelial surfaces of loops infected with the NE-treated 85-170 Nalr wild type (Fig. 3E) and strain ICC203 (Fig. 3F). In both cases, relatively few bacteria were observed on raised electron-dense pedestals; rather, EHEC O157:H7 often adhered to the surfaces of enterocytes in cup-shaped depressions. No endogenous EHEC O157:H7 was detected in control loops filled with sterile medium (Fig. 3C). The finding that at least some tccP-deficient bacteria induced apparently normal pedestal structures implies that actin assembly may occur via TccP-independent mechanisms in vivo. This notion is supported by the finding that a C. rodentium Δtir mutant expressing a Y471F variant of Tir, unable to assemble actin under adherent bacteria in vitro, colonized the intestines of mice efficiently and was observed to form normal A/E lesions on the colonic mucosa (12).
In summary, we report that Tir is an essential colonization factor for E. coli O157:H7 in calves and sheep and mediates bacterial attachment to intestinal mucosa by serving as the primary receptor for intimin. The finding that tir mutants are more attenuated than isogenic eae mutants implies that Tir may facilitate intestinal colonization in other ways. We have also shown that the Tir-cytoskeleton coupling protein (TccP) is not required by E. coli O157:H7 to colonize the intestines of calves and sheep and induce attaching and effacing lesions, suggesting that pedestal formation in vitro and A/E lesion formation in vivo may occur by distinct mechanisms. Bacterial factors mediating actin assembly in a continuous mammalian cell line may be redundant or functionally replaced by bacterial or host factors in vivo, and this study underlines the importance of studying bacterial gene function in target animal hosts wherever possible.
Acknowledgments
We gratefully acknowledge Lisa Faulkner.
We also acknowledge the support of the Department for the Environment, Food and Rural Affairs (grant OZ0707) and the Wellcome Trust.
Editor: J. T. Barbieri
REFERENCES
- 1.Adu-Bobie, J., L. R. Trabulsi, M. M. S. Carneiro-Sampaio, G. Dougan, and G. Frankel. 1998. Identification of immunodominant regions within the C-terminal cell binding domain of intimin α and intimin β from enteropathogenic Escherichia coli. Infect. Immun. 66:5643-5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexeyev, M. F., I. N. Shokolenko, and T. P. Croughan. 1995. Improved antibiotic-resistance gene cassettes and omega elements for Escherichia coli vector construction and in vitro deletion/insertion mutagenesis. Gene 160:63-67. [DOI] [PubMed] [Google Scholar]
- 3.Allen-Vercoe, E., M. Dibb-Fuller, C. J. Thorns, and M. J. Woodward. 1997. SEF17 fimbriae are essential for the convoluted colonial morphology of Salmonella enteritidis. FEMS Microbiol. Lett. 153:33-42. [DOI] [PubMed] [Google Scholar]
- 4.Best, A., R. M. La Ragione, A. R. Sayers, and M. J. Woodward. 2005. Role for flagella but not intimin in the persistent infection of the gastrointestinal tissues of specific-pathogen-free chicks by Shiga toxin-negative Escherichia coli O157:H7. Infect. Immun. 73:1836-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campellone, K. G., A. Giese, D. J. Tipper, and J. M. Leong. 2002. A tyrosine-phosphorylated 12-amino-acid sequence of enteropathogenic Escherichia coli Tir binds the host adaptor protein Nck and is required for Nck localization to actin pedestals. Mol. Microbiol. 43:1227-1241. [DOI] [PubMed] [Google Scholar]
- 6.Campellone, K. G., and J. M. Leong. 2003. Tails of two Tirs: actin pedestal formation by enteropathogenic E. coli and enterohemorrhagic E. coli O157:H7. Curr. Opin. Microbiol. 6:82-90. [DOI] [PubMed] [Google Scholar]
- 7.Campellone, K. G., S. Rankin, T. Pawson, M. W. Kirschner, D. J. Tipper, and J. M. Leong. 2004. Clustering of Nck by a 12-residue Tir phosphopeptide is sufficient to trigger localized actin assembly. J. Cell Biol. 164:407-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campellone, K. G., D. Robbins, and J. M. Leong. 2004. EspFU is a translocated EHEC effector that interacts with Tir and N-WASP and promotes Nck-independent actin assembly. Dev. Cell 7:217-228. [DOI] [PubMed] [Google Scholar]
- 9.Cornick, N. A., S. L. Booher, and H. W. Moon. 2002. Intimin facilitates colonization by Escherichia coli O157:H7 in adult ruminants. Infect. Immun. 70:2704-2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dean-Nystrom, E. A., B. T. Bosworth, H. W. Moon, and A. D. O'Brien. 1998. Escherichia coli O157:H7 requires intimin for enteropathogenicity in calves. Infect. Immun. 66:4560-4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deibel, C., S. Kramer, T. Chakraborty, and F. Ebel. 1998. EspE, a novel secreted protein of attaching and effacing bacteria, is directly translocated into infected host cells, where it appears as a tyrosine-phosphorylated 90 kDa protein. Mol. Microbiol. 28:463-474. [DOI] [PubMed] [Google Scholar]
- 12.Deng, W., B. A. Vallance, Y. Li, J. L. Puente, and B. B. Finlay. 2003. Citrobacter rodentium translocated intimin receptor (Tir) is an essential virulence factor needed for actin condensation, intestinal colonization and colonic hyperplasia in mice. Mol. Microbiol. 48:95-115. [DOI] [PubMed] [Google Scholar]
- 13.DeVinney, R., A. Gauthier, A. Abe, and B. B. Finlay. 1999. Enteropathogenic Escherichia coli: a pathogen that inserts its own receptor into host cells. Cell. Mol. Life Sci. 55:961-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeVinney, R., M. Stein, D. Reinscheid, A. Abe, S. Ruschkowski, and B. B. Finlay. 1999. Enterohemorrhagic Escherichia coli O157:H7 produces Tir, which is translocated to the host cell membrane but is not tyrosine phosphorylated. Infect. Immun. 67:2389-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donnenberg, M. S., J. Yu, and J. B. Kaper. 1993. A second chromosomal gene necessary for intimate attachment of enteropathogenic Escherichia coli to epithelial cells. J. Bacteriol. 175:4670-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dziva, F., P. M. van Diemen, M. P. Stevens, A. J. Smith, and T. S. Wallis. 2004. Identification of Escherichia coli O157:H7 genes influencing colonization of the bovine gastrointestinal tract using signature-tagged mutagenesis. Microbiology 150:3631-3645. [DOI] [PubMed] [Google Scholar]
- 17.Ebel, F., T. Podzadel, M. Rohde, A. U. Kresse, S. Kramer, C. Deibel, C. A. Guzman, and T. Chakraborty. 1998. Initial binding of Shiga toxin-producing Escherichia coli to host cells and subsequent induction of actin rearrangements depend on filamentous EspA-containing surface appendages. Mol. Microbiol. 30:147-161. [DOI] [PubMed] [Google Scholar]
- 18.Fitzhenry, R. J., D. J. Pickard, E. L. Hartland, S. Reece, G. Dougan, A. D. Phillips, and G. Frankel. 2002. Intimin type influences the site of human intestinal mucosal colonisation by enterohaemorrhagic Escherichia coli O157:H7. Gut 50:180-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitzhenry, R. J., M. P. Stevens, C. Jenkins, T. S. Wallis, R. Heuschkel, S. Murch, M. Thomson, G. Frankel, and A. D. Phillips. 2003. Human intestinal tissue tropism of intimin epsilon O103 Escherichia coli. FEMS Microbiol. Lett. 218:311-316. [DOI] [PubMed] [Google Scholar]
- 20.Frankel, G., O. Lider, R. Hershkoviz, A. P. Mould, S. G. Kachalsky, D. C. Candy, L. Cahalon, M. J. Humphries, and G. Dougan. 1996. The cell-binding domain of intimin from enteropathogenic Escherichia coli binds to beta1 integrins. J. Biol. Chem. 271:20359-20364. [DOI] [PubMed] [Google Scholar]
- 21.Garmendia, J., G. Frankel, and V. F. Crepin. 2005. Enteropathogenic and enterohemorrhagic Escherichia coli infections: translocation, translocation, translocation. Infect. Immun. 73:2573-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garmendia, J., A. D. Phillips, M. F. Carlier, Y. Chong, S. Schuller, O. Marches, S. Dahan, E. Oswald, R. K. Shaw, S. Knutton, and G. Frankel. 2004. TccP is an enterohaemorrhagic Escherichia coli O157:H7 type III effector protein that couples Tir to the actin-cytoskeleton. Cell. Microbiol. 6:1167-1183. [DOI] [PubMed] [Google Scholar]
- 23.Gruenheid, S., R. DeVinney, F. Bladt, D. Goosney, S. Gelkop, G. D. Gish, T. Pawson, and B. B. Finlay. 2001. Enteropathogenic E. coli Tir binds Nck to initiate actin pedestal formation in host cells. Nat. Cell Biol. 3:856-859. [DOI] [PubMed] [Google Scholar]
- 24.Jerse, A. E., and J. B. Kaper. 1991. The eae gene of enteropathogenic Escherichia coli encodes a 94-kilodalton membrane protein, the expression of which is influenced by the EAF plasmid. Infect. Immun. 59:4302-4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jordan, D. M., S. L. Booher, and H. W. Moon. 2005. Escherichia coli O157:H7 does not require intimin to persist in pigs. Infect. Immun. 73:1865-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Judge, N. A., H. S. Mason, and A. D. O'Brien. 2004. Plant cell-based intimin vaccine given orally to mice primed with intimin reduces time of Escherichia coli O157:H7 shedding in feces. Infect. Immun. 72:168-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaper, J. B., J. P. Nataro, and H. L. Mobley. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2:123-140. [DOI] [PubMed] [Google Scholar]
- 28.Kenny, B. 1999. Phosphorylation of tyrosine 474 of the enteropathogenic Escherichia coli (EPEC) Tir receptor molecule is essential for actin nucleating activity and is preceded by additional host modifications. Mol. Microbiol. 31:1229-1241. [DOI] [PubMed] [Google Scholar]
- 29.Kenny, B., R. DeVinney, M. Stein, D. J. Reinscheid, E. A. Frey, and B. B. Finlay. 1997. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91:511-520. [DOI] [PubMed] [Google Scholar]
- 30.Knutton, S., I. Rosenshine, M. J. Pallen, I. Nisan, B. C. Neves, C. Bain, C. Wolff, G. Dougan, and G. Frankel. 1998. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 17:2166-2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.La Ragione, R. M., N. M. Ahmed, A. Best, D. Clifford, U. Weyer, W. A. Cooley, L. Johnson, G. R. Pearson, and M. J. Woodward. 2005. Colonization of 8-week-old conventionally reared goats by Escherichia coli O157:H7 after oral inoculation. J. Med. Microbiol. 54:485-492. [DOI] [PubMed] [Google Scholar]
- 32.Liu, H., L. Magoun, and J. M. Leong. 1999. β1-Chain integrins are not essential for intimin-mediated host cell attachment and enteropathogenic Escherichia coli-induced actin condensation. Infect. Immun. 67:2045-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Locking, M. E., S. J. O'Brien, W. J. Reilly, E. M. Wright, D. M. Campbell, J. E. Coia, L. M. Browning, and C. N. Ramsay. 2001. Risk factors for sporadic cases of Escherichia coli O157 infection: the importance of contact with animal excreta. Epidemiol. Infect. 127:215-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marchès, O., J.-P. Nougayrède, S. Boullier, J. Mainil, G. Charlier, I. Raymond, P. Pohl, M. Boury, J. De Rycke, A. Milon, and E. Oswald. 2000. Role of Tir and intimin in the virulence of rabbit enteropathogenic Escherichia coli serotype O103:H2. Infect. Immun. 68:2171-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDaniel, T. K., K. G. Jarvis, M. S. Donnenberg, and J. B. Kaper. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. USA 92:1664-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKee, M. L., A. R. Melton-Celsa, R. A. Moxley, D. H. Francis, and A. D. O'Brien. 1995. Enterohemorrhagic Escherichia coli O157:H7 requires intimin to colonize the gnotobiotic pig intestine and to adhere to HEp-2 cells. Infect. Immun. 63:3739-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naylor, S. W., A. J. Roe, P. Nart, K. J. Spears, D. G. E. Smith, J. C. Low, and D. L. Gally. 2005. Escherichia coli O157:H7 forms attaching and effacing lesions at the terminal rectum of cattle and colonization requires the LEE4 operon. Microbiology 151:2773-2781. [DOI] [PubMed] [Google Scholar]
- 38.O'Brien, S. J., G. K. Adak, and C. Gilham. 2001. Contact with farming environment as a major risk factor for Shiga toxin (Vero cytotoxin)-producing Escherichia coli O157 infection in humans. Emerg. Infect. Dis. 7:1049-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phillips, A. D., and G. Frankel. 2000. Intimin-mediated tissue specificity in enteropathogenic Escherichia coli interaction with human intestinal organ cultures. J. Infect. Dis. 181:1496-1500. [DOI] [PubMed] [Google Scholar]
- 40.Phillips, A. D., J. Giron, S. Hicks, G. Dougan, and G. Frankel. 2000. Intimin from enteropathogenic Escherichia coli mediates remodelling of the eukaryotic cell surface. Microbiology 146:1333-1344. [DOI] [PubMed] [Google Scholar]
- 41.Potter, A. A., S. Klashinsky, Y. Li, E. Frey, H. Townsend, D. Rogan, G. Erickson, S. Hinkley, T. Klopfenstein, R. A. Moxley, D. R. Smith, and B. B. Finlay. 2004. Decreased shedding of Escherichia coli O157:H7 by cattle following vaccination with type III secreted proteins. Vaccine 22:362-369. [DOI] [PubMed] [Google Scholar]
- 42.Ritchie, J. M., C. M. Thorpe, A. B. Rogers, and M. K. Waldor. 2003. Critical roles for stx2, eae, and tir in enterohemorrhagic Escherichia coli-induced diarrhea and intestinal inflammation in infant rabbits. Infect. Immun. 71:7129-7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sinclair, J. F., and A. D. O'Brien. 2002. Cell surface-localized nucleolin is a eukaryotic receptor for the adhesin intimin-gamma of enterohemorrhagic Escherichia coli O157:H7. J. Biol. Chem. 277:2876-2885. [DOI] [PubMed] [Google Scholar]
- 44.Sinclair, J. F., and A. D. O'Brien. 2004. Intimin types alpha, beta, and gamma bind to nucleolin with equivalent affinity but lower avidity than to the translocated intimin receptor. J. Biol. Chem. 279:33751-33758. [DOI] [PubMed] [Google Scholar]
- 45.Stevens, M. P., O. Marchès, J. Campbell, V. Huter, G. Frankel, A. D. Phillips, E. Oswald, and T. S. Wallis. 2002. Intimin, Tir, and Shiga toxin 1 do not influence enteropathogenic responses to Shiga toxin-producing Escherichia coli in bovine ligated intestinal loops. Infect. Immun. 70:945-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stevens, M. P., A. J. Roe, I. Vlisidou, P. M. van Diemen, R. M. La Ragione, A. Best, M. J. Woodward, D. L. Gally, and T. S. Wallis. 2004. Mutation of toxB and a truncated version of the efa-1 gene in Escherichia coli O157:H7 influences the expression and secretion of locus of enterocyte effacement-encoded proteins but not intestinal colonization in calves or sheep. Infect. Immun. 72:5402-5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tzipori, S., F. Gunzer, M. S. Donnenberg, L. de Montigny, J. B. Kaper, and A. Donohue-Rolfe. 1995. The role of the eaeA gene in diarrhea and neurological complications in a gnotobiotic piglet model of enterohemorrhagic Escherichia coli infection. Infect. Immun. 63:3621-3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Diemen, P. M., F. Dziva, M. P. Stevens, and T. S. Wallis. 2005. Identification of enterohemorrhagic Escherichia coli O26:H− genes required for intestinal colonization in calves. Infect. Immun. 73:1735-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vlisidou, I., M. Lyte, P. M. van Diemen, P. Hawes, P. Monaghan, T. S. Wallis, and M. P. Stevens. 2004. The neuroendocrine stress hormone norepinephrine augments Escherichia coli O157:H7-induced enteritis and adherence in a bovine ligated ileal loop model of infection. Infect. Immun. 72:5446-5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woodward, M. J., A. Best, K. A. Sprigings, G. R. Pearson, A. M. Skuse, A. Wales, C. M. Hayes, J. M. Roe, J. C. Low, and R. M. La Ragione. 2003. Non-toxigenic Escherichia coli O157:H7 strain NCTC12900 causes attaching-effacing lesions and eae-dependent persistence in weaned sheep. Int. J. Med. Microbiol. 293:299-308. [DOI] [PubMed] [Google Scholar]
- 51.Yu, J., and J. B. Kaper. 1992. Cloning and characterization of the eae gene of enterohaemorrhagic Escherichia coli O157:H7. Mol. Microbiol. 6:411-417. [DOI] [PubMed] [Google Scholar]