Abstract
Pathogenic Brucella species replicate within mammalian cells, and their type IV secretion system is essential for intracellular survival and replication. The options for biochemical studies on the Brucella secretion system are limited due to the rigidity of the cells and biosafety concerns, which preclude large-scale cell culture and fractionation. To overcome these problems, we heterologously expressed the Brucella suis virB operon in the closely related α2-proteobacterium Agrobacterium tumefaciens and showed that the VirB proteins assembled into a complex. Eight of the twelve VirB proteins were detected in the membranes of the heterologous host with specific antisera. Cross-linking indicated protein-protein interactions similar to those in other type IV secretion systems, and the results of immunofluorescence analysis supported the formation of VirB protein complexes in the cell envelope. Production of a subset of the B. suis VirB proteins (VirB3-VirB12) in A. tumefaciens strongly increased its ability to receive IncQ plasmid pLS1 in conjugation experiments, and production of VirB1 further enhanced the conjugation efficiency. Plasmid recipient competence correlated with periplasmic leakage and the detergent sensitivity of A. tumefaciens, suggesting a weakening of the cell envelope. Heterologous expression thus permits biochemical characterization of B. suis type IV secretion system assembly.
Brucella species are pathogens of mammals, which cause severe infections and abortions in animals and long-lasting febrile diseases in humans (65). They impact agriculture by causing zoonotic diseases of cattle (Brucella abortus), sheep (B. melitensis), and swine (B. suis), which cause substantial economic losses, and they pose a threat for those handling the animals (8, 28). The eradication of Brucella from livestock has succeeded in some parts of the world, but expensive control and surveillance systems are necessary due to the possibility of reinfection of livestock from wildlife. In addition to its threat to commercial agriculture, Brucella is considered as a potential category B bioterror agent (32). Brucella infections are very long-lasting, and current treatment regimens require 6 to 8 weeks of therapy with two antibiotics (61). Several live attenuated vaccines are effective for animals, but safe vaccines for humans are currently not available (28). The threat posed by Brucella infections gives research on the molecular basis of virulence and persistence in the mammalian body a high priority.
Brucella species survive and multiply inside mammalian cells, including cells of the immune system such as macrophages (12, 51). They inhibit apoptosis of infected cells and apparently evade the immune response of their hosts, causing long-lasting infections (48). After entering macrophages via lipid rafts, the Brucella-containing vacuole (BCV) does not fuse with the lysosomes, thus avoiding rapid cell destruction (13). Instead, the BCV follows a novel intracellular trafficking pathway, which interacts with the endoplasmic reticulum (ER), leading to the creation of a specialized vacuole in which the bacteria multiply (37). Brucella species are trophic for cells of the reproductive tissues in their natural animal hosts. Analysis of the genomes of three Brucella species has shown that they are devoid of “classical” virulence factors such as adhesins or toxins (21, 29, 50). One exception is the VirB type IV secretion system (T4SS) that has been identified in several transposon mutagenesis screens as a key virulence factor (20, 31, 49).
T4SSs are a family of multiprotein complexes, which serve to secrete macromolecules across the bacterial envelope. The Brucella virB operon encodes 12 proteins, of which VirB1 to VirB11 show significant similarity to those from other T4SSs. The similarity of the Brucella VirB proteins to components of other T4SSs, including that of the well-studied model organism, the plant pathogen Agrobacterium tumefaciens (11, 14, 15, 49), suggests that Brucella uses it as conduit for the translocation of virulence factors into mammalian cells (12, 48). It is currently unknown at which stage of the infection process the Brucella T4SS secretes virulence factors, how it assembles in the membranes, whether it forms a pilus-like structure, and whether and which host structures it contacts during this process. Analysis of gene regulation shed some light on the time frame of T4SS action. The B. suis virB operon was induced after uptake into mammalian cells, which is well in accord with a requirement for intracellular growth (9). In contrast, the B. abortus virB genes appear to be expressed constitutively (22, 52). In both B. melitensis and B. suis, virB expression is negatively regulated by quorum sensing and dependent on a quorum-sensing regulator (19, 59).
In contrast to work on bacterial uptake, trafficking within infected cells, and gene regulation, relatively little research has been done on the structure and function of the Brucella T4SS. Transposon insertions were likely polar so that conclusions on the effects of single genes could not be made (17, 20, 49). In-frame deletions of B. abortus virB1 and virB2 were shown to inhibit intracellular survival and multiplication in macrophages; however, only deletion of virB2, which encodes a protein similar to the main pilus component in other T4SS (23, 43), attenuated bacterial persistence in a mouse infection model (22). The VirB12 protein, which does not have homologs in other T4SSs, encodes a protein with similarity to outer membrane adhesin in Pseudomonas species (1). Whereas this suggested a role in host cell attachment, it was recently shown that virB12 is dispensable for infections of J774 macrophage and mouse models (58). Work with purified B. suis VirB proteins has shown binding of the putative lytic transglycosylase VirB1 to VirB8, VirB9, and VirB11 (33). These interactions are believed to coordinate transmembrane assembly of the T4SS at the site of murein lysis by VirB1. Purified B. suis VirB5, which is similar to minor T-pilus components of other T4SSs (54, 64), interacts with VirB8 and VirB10, and these interactions are likely required for binding to VirB2, followed by pilus assembly (66). B. suis VirB4 fully complemented an A. tumefaciens virB4 mutant in a plant tumor assay (66), and B. suis VirB1 partly complemented virB1 gene defects in A. tumefaciens, showing that many protein-protein interactions are conserved (34).
Due to the pathogenicity of Brucella species and the requirement for biosafety level 3 containment, the options for biochemical studies on T4SS assembly in this organism are very limited. Based on our previous findings that some VirB components could be exchanged between the B. suis and the A. tumefaciens T4SS, we here expressed the entire B. suis virB operon in the heterologous host. Production of subsets of the B. suis VirB proteins increased the ability of A. tumefaciens to serve as recipient in T4SS-mediated plasmid conjugation experiments. Analyses of their membrane association and interactions further substantiated that the B. suis VirB proteins assembled into a T4SS with basic features similar to that of A. tumefaciens in the heterologous host.
MATERIALS AND METHODS
Cultivation of bacteria and yeast.
Overnight cultures of A. tumefaciens wild-type A348 and C58 (62) or strains carrying pTrc300 or virB operon constructs were grown in YEB medium (0.5% beef extract, 0.5% peptone, 0.1% yeast extract, 0.5% sucrose, 2 mM MgSO4) in the absence of antibiotics (wild-type strains) or with spectinomycin (300 μg/ml) and streptomycin (100 μg/ml) for plasmid propagation. The cells were then inoculated to an optical density at 600 nm (OD600) of 0.1 in liquid AB minimal medium (10 g of glucose/liter, 4 g of MES [morpholinoethanesulfonic acid]/liter, 0.3 g MgSO4 · 7 H2O/liter, 0.15 g of KCl/liter, 0.01 g of CaCl2/liter, 0.0025 g of FeSO4 · 7 H2O/liter, and 1 mM potassium phosphate [pH 5.5]) and grown for 5 h at 20°C, followed by plating of 1 ml on 15-cm-diameter AB agar plates with 0.5 mM IPTG (isopropyl-β-d-thiogalactopyranoside) for induction of the trc promoter or 200 μM acetosyringone (AS) for induction of the Agrobacterium virB promoter as indicated in individual experiments and further cultivation at 20°C for 3 days.
For the analysis of pLS1 recipient activity, donor A348 pLS1 (57) cells were cocultivated with UIA143 recipient cells (cured of Ti plasmid) carrying pTrc300 or virB operon plasmids in a 5:1 ratio for 3 days on AB minimal medium with 300 μM AS and 0.5 mM IPTG, followed by plating on YEB agar with antibiotics (carbenicillin at 150 μg/ml, streptomycin at 100 μg/ml, and spectinomycin at 300 μg/ml) for selection of donors, recipients, and transconjugants as described previously (34).
For the analysis of sodium dodecyl sulfate (SDS) sensitivity cells from overnight cultures grown in YEB medium were diluted to an OD600 of 0.1 in liquid AB minimal medium and cultivated for 2.5 to 3 h at 20°C, followed by aliquoting into wells of a 96-well microtiter plate in the presence or absence of 0.5 mM IPTG, the addition of SDS (0.025, 0.006, or 0.003%), and further cultivation and shaking for up to 60 h.
To study functional complementation of Agrobacterium virB defects by B. suis proteins in translocation of effector proteins we used the Cre reporter assay for translocation (CRAfT) (56). Here, we cocultivated Agrobacterium A348 containing plasmid pSDM3155, expressing a Cre-VirF fusion, with Saccharomyces cerevisiae strain LBY2 (56), in which Cre-mediated excision of a chromosomal URA3 gene was scored as colony growth on medium containing 5-fluoroorotic acid. The excision efficiency was calculated as number of 5-fluoroorotic acid-resistant colonies per output yeast.
B. suis strain 1330 was grown in tryptic soy broth (1.7% peptone from casein, 0.3% peptone from soy meal, 0.5% NaCl, 0.25% glucose, and 0.25% K2HPO4) or on tryptic soy agar.
Construction of B. suis virB manB mutant.
To construct B. suis 1330 (virB2::Tn5-manB), an internal fragment of the B. suis pgm gene was amplified by PCR (primers pgm5 [5′-TATGCGATGGGTGCGAAAGC-3′] and pgm3 [5′-GTTGGAGGTGACTGGCGTGA-3′]) and cloned into pGEM-T (Promega). Since ColE1-based vectors do not replicate in Brucella, this plasmid was introduced into B. suis 1330 virB2::Tn5 (27) by electroporation to inactivate the gene with insertional mutagenesis by homologous recombination. The rough phenotype of the resulting strain B. suis virB manB was checked by slide agglutination with O-antigen-specific sera and acriflavin.
Construction of B. suis virB operon constructs.
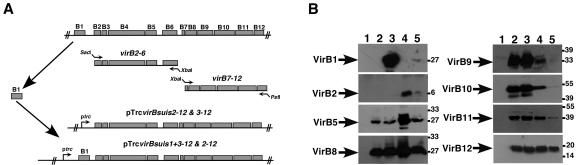
The trc promoter expression vector pTrc300 was constructed from pTrc200 (55), by cleavage at the NcoI site and removal of the overhanging 4-bp single-stranded DNA with mung bean nuclease, followed by blunt-end ligation. This modification permitted the expression of genes cloned into the polylinker without the need of directly fusing them to the NcoI site encoded ATG codon of pTrc200. For construction of virB operon vectors the following cloning strategy was used (Fig. 1A). First, virB2-6 (primers virBsuis2-5 [5′-GGCAGAGCTCGACATAAGGAATAAAGATCATGAAAAC-3′] and virBsuis6-3 [5′-GAGGTCTAGAAAGGCCCTAATCCCTGTTGAACTG-3′]) and virB7-12 (primers virBsuis7-5 [5′-GGCATCTAGAAGGAAATCATAATGAAAAAGGTAATCC-3′] and virBsuis12-3 [5′-GAGCCTGCAGGTTACTTGCGTAAAATTTCGATATC-3′]) operon fragments were PCR amplified from pUCvirB (49) by using the Expand Long Template PCR System (Roche). Next, virB2-6 and virB7-12 fragments were excised by SacI/XbaI and XbaI/PstI, respectively, and cloned into pTrc300 to give pTrcB2-6 and pTrcB7-12, respectively. The virB7-12 fragment was further excised from pTrcB7-12 with XbaI and PstI and cloned into the XbaI/PstI sites of pTrcB2-6, resulting in pTrcB2-12 and pTrcB3-12 (after detection of a missense mutation at the virB2 start codon). DNA manipulations such as DNA isolation cloning and sequencing were performed according to standard techniques (47). Next, the virB1 gene was PCR amplified from pUCvirB (primers virB1-5′ [5′-GCGCGAGCTCAGAAGGAGACGATCCTATGGTGCCA-3′] and virB1-3′ [5′-GCGCGAGCTCTTAGAAAACAACTACGCCGTCC-3′]), cloned into pCR2.1 by using the TOPO cloning system (Invitrogen), excised with SacI, and inserted into virB operon plasmids resulting in pTrcB1+3-12 and pTrcB1+2-12.
FIG. 1.
Cloning of the B. suis virB operon and production of VirB proteins in A. tumefaciens. (A) Construction of different virB operon-containing pTrc300 derivatives for IPTG-induced expression. Portions of the virB operon containing virB2-6 and virB7-12 were PCR amplified and cloned separately, followed by the construction of pTrcB2-12. pTrcB3-12 resulted from a spontaneous change at the virB2 start codon abolishing the expression of this gene. Cloning of the virB1 gene 5′ to the virB2-12 and virB3-12 operon resulted in pTrcB1+2-12 and pTrcB1+3-12. (B) Detection of VirB protein production in cells of UIA143 carrying pTrc300 (lane 1), pTrcB3-12 (lane 2), pTrcB1+3-12 (lane 3), pTrcB2-12 (lane 4), and pTrcB1+2-12 (lane 5). Cells were cultivated on AB minimal medium plates at 20°C for 3 days in the presence of IPTG for induction of the trc promoter, followed by cell lysis, SDS-PAGE, Western blotting, and analysis with B. suis VirB protein-specific antisera. Arrows indicate VirB proteins and molecular masses of reference proteins are shown on the right.
Generation of VirB protein-specific antisera.
For the generation of VirB12-specific antiserum, a 471-bp fragment of the gene corresponding to the processed periplasmic form of the protein (156 amino acids [16 to 172]) was PCR amplified from pUCvirB with oligonucleotides (VirB12-5 [5′-CAGGGTACCCTCCAGCCCGCCGAAGCC-3′] and virB12-3 [5′-GAGCTGCAGTTACTTGCGTAAAATTTCGATATCCAC-3′]), cleaved with Acc65I and PstI (restriction sites are underlined), and ligated with similarly cleaved vector pT7H6TrxFus (39). The hexahistidyl-TrxA fusion protein was overexpressed and purified by immobilized metal affinity chromatography as described previously (66), and 500 μg was used to immunize rabbits for the generation of an antiserum (BioGenes). A 15-amino-acid peptide (NGGLDKVNTSMQKVC) was used for the immunization of rabbits to generate a VirB2-specific antiserum (BioGenes). The generation of antisera for the detection of B. suis VirB1, VirB5, VirB8, VirB9, VirB10, and VirB11 was described previously (33, 66).
Isolation of T pili and subcellular fractions.
Cells were cultivated on AB minimal medium plates in the presence of AS or IPTG, followed by cell harvest and shearing for the isolation of T pili as described previously (54). Membrane fractions were separated from soluble fractions by cell lysis in a French press, followed by ultracentrifugation as described previously (66).
Analysis of protein-protein interactions by cross-linking.
Cells were cultivated in liquid AB minimal medium in the presence of 0.5 mM IPTG; the OD600 was adjusted to 1; and aliquots of 1 ml were sedimented, washed three times with phosphate-buffered saline pH 6 (PBS; 0.08% NaCl, 0.02% KCl, 0.14% Na2HPO4, and 0.024% KH2PO4 adjusted to pH 6), and suspended in 1 ml of the same buffer. The cross-linking agent bis(sulfosuccinimidyl)suberat (BS3; Pierce) was added at a concentration of 1 mM, followed by incubation for 30 min at room temperature and stopping of the reaction by the addition of 200 μl of Tris-HCl buffer (pH 6). The cells were then sedimented, washed once with PBS (pH 6), and subjected to SDS-polyacrylamide gel electrophoresis (PAGE) and Western blotting.
SDS-PAGE and Western blotting.
Agrobacterium cells and subcellular fractions were incubated in Laemmli sample buffer for 5 min at 100°C, followed by SDS-PAGE using the Laemmli (for proteins larger 20 kDa) (42) or the Schägger and Jagow (for proteins smaller 20 kDa) system (53). Western blotting and detection with a chemoluminescence system (Amersham Biosciences) was done according to standard protocols with A. tumefaciens and B. suis VirB protein-specific antisera (30).
Immunofluorescence analysis and image processing.
A. tumefaciens C58 carrying pTrcB3-B12 or pTrc300 grown on AB minimal medium plates was washed three times with PBS and fixed for 30 min in 4% paraformaldehyde, followed by three washes in PBS (0.08% NaCl, 0.02% KCl, 0.14% Na2HPO4, 0.024% KH2PO4 [pH 7]). Samples (30 μl) were applied to 0.1% polylysine-coated cover slides (Sigma) and dried. For permeabilization of the cell envelope, the cover slides were immersed in GET buffer (20 mM Tris-HCl [pH 7.5], 10 mM EDTA [pH 8], 50 mM glucose) containing 8 mg of lysozyme (Sigma)/ml, followed by a 10-min incubation at room temperature and three washes with PBS. Nonspecific binding sites were blocked by incubation with 1% bovine serum albumin (BSA) in PBS for 30 min, followed by treatment with primary antisera (1:200 dilution in PBS-1% BSA) at 4°C for 12 h. Next, the cover slides were washed three times in PBS and then incubated in Oregon green goat anti-rabbit immunoglobulin G (IgG)-coupled secondary antiserum (Molecular Probes) at a 1:200 dilution in PBS-1% BSA for 3 h in the dark. The samples were washed three times in PBS, treated with antifade solution (AF1; Citifluor), sealed with VALAP (vaseline-lanoline-paraffin [1:1:1]) on a microscope slide, and analyzed.
B. suis virB manB carrying pTrcB3-B12 or pTrc300 was grown as shaken tryptic soy broth culture at 37°C to an OD600 of 0.4, followed by induction of the trc promoter with 0.5 mM IPTG for 5 h. Cells were subsequently washed three times in PBS, fixed for 30 min in 3% paraformaldehyde, sedimented, and incubated in 1% Triton X-100 for 5 min for permeabilization of the cell envelope, followed by blocking with 1% BSA as described above and two washes in PBS. The cells were next treated with primary antiserum diluted in PBS (monoclonal anti-Omp31 [1:5] and polyclonal sera anti-VirB5 or anti-VirB8 [1:50 each]) for 2 h, followed by three washes with PBS. Cells were then treated with secondary antibodies (anti-rabbit IgG-fluorescein isothiocyanate conjugate [Sigma] and goat anti-mouse IgG-Texas red conjugate [Molecular Probes]) for 2 h, followed by three washes with PBS, treatment with antifade solution, sealing on a microscopy slide, and analysis.
Samples were analyzed by fluorescence microscopy with a Zeiss Axioplan microscope (filter set 9, BP 450 to 490, LP 515), the images were digitalized with a Spot-RT camera (Visitron Systems) and Spot 3.02 and IPLab 3.5 software, followed by processing with Adobe Photoshop 6 and Canvas 7 software.
RESULTS
Cloning and expression of the virB operon from B. suis in A. tumefaciens.
For the heterologous expression of the 11-kb B. suis virB operon, we followed a three-step procedure to assemble the operon from fragments. The reason for this procedure was to circumvent expression problems due to the intergenic regions between virB1/virB2 (contains conserved Brucella repeat sequence, BruRS1) and virB6/virB7 (49). In preliminary experiments we subcloned the entire virB operon including the intergenic regions, but this led to low-level constitutive expression of virB8-virB10 (data not shown). To avoid this complication, the regions encoding virB2-virB6 and virB7-virB12 were PCR amplified separately, cloned into the broad-host-range vector pTrc300, sequenced, and subsequently joined, resulting in pTrcB2-12 (Fig. 1A). We noticed a spontaneous mutation at the start codon of virB2 in one of our clones, and this vector was designated pTrcB3-12. The virB1 gene was subsequently PCR amplified and inserted 5′ to the operon, resulting in vectors pTrcB1+3-12 and pTrcB1+2-12. The plasmids were transformed into Ti plasmid-free A. tumefaciens strain UIA143. Western blot analysis with the available antisera was used to detect VirB protein production in IPTG-induced cells. We detected B. suis VirB1, VirB2, VirB5, and VirB8-VirB12 as expected by the composition of the operons (Fig. 1B). We noticed low levels of most VirB proteins in pTrcB1+2-12-carrying cells, and for this reason the strain was not analyzed further. To analyze whether the B. suis T4SS is functional in the heterologous host, we determined its ability to substitute for the A. tumefaciens virB operon using virulence, plasmid, and protein transfer assays.
Expression of B. suis virB operon stimulates pRSF1010 recipient competence.
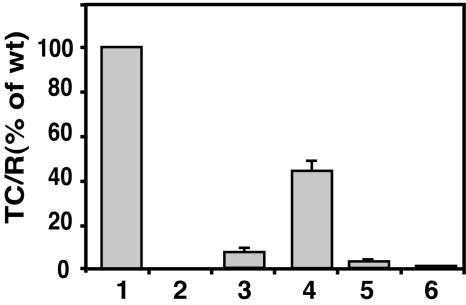
We have previously shown that B. suis VirB1 and VirB4 can partially or fully complement the corresponding Agrobacterium virB gene deletion mutant for virulence in plant infections (34). However, introduction of the B. suis virB operon constructs pTrcB1+2-12 and pTrcB1+3-12 did not restore the virulence of the virB operon deletion strain PC1000 of A. tumefaciens (26) in plant infection assays (data not shown). Similarly, expression of the B. suis virB genes in this strain did not permit conjugation of the IncQ plasmid pLS1 to other bacteria. Since Brucella does not encode a VirD4 homolog, we also assessed the transfer of the small mobilizable plasmid CloDF13, which encodes a coupling protein, into yeast recipients, but expression of the Brucella virB genes did not mediate its transfer (24). In line with these findings, using the CRAfT (63) we found that the B. suis VirB1 protein complemented an Agrobacterium virB1 mutant for translocation of a Cre recombinase-VirF fusion protein into yeast but saw no indication of complementation of the individual Agrobacterium virB gene deletions by pTrcB1+3-12 (data not shown). These results showed that the B. suis VirB proteins cannot fully substitute for all functions of the A. tumefaciens T4SS in transfer of virulence proteins or plasmid substrates. We next used an alternative plasmid conjugation assay based on the phenomenon that a subset of VirB proteins expressed in A. tumefaciens cells can increase recipient activity by 3 orders of magnitude (7, 46). Although the mechanism of this increase is not understood, it is dependent on the function of apparent subcomplexes of the VirB apparatus and therefore may serve as an assay for assembly of T4SS components. Using this assay, we found that conjugation of the IncQ plasmid pLS1 to Agrobacterium strain UIA143 expressing its native T4SS from pTiA6 was 3,330-fold more efficient than to the Ti plasmid-free strain UIA143 containing pTrc300 (Fig. 2 and Table 1). Expression of the Brucella VirB proteins also increased the recipient activity. The low-level expression from pTrcB1+2-12 increased the transfer efficiency nearly fourfold compared to UIA143 containing pTrc300. Higher levels of expression gave much greater increases in recipient activity, with a 265-fold increase of transfer to UIA143 pTrcB3-12 and a 1,460-fold increase to UIA143 pTrcB1+3-12, showing a clear role for VirB1. The increase in recipient activity was strictly dependent on trc promoter induction by IPTG in both cases. We need to emphasize that UIA143 does not carry a Ti plasmid, so that these results cannot be explained by the action of Agrobacterium VirB proteins in the recipient. These data suggest the assembly of at least a subset of Brucella VirB proteins in a T4SS-like complex in Agrobacterium. Interestingly, the presence of pTrcB2-12 only increased recipient activity by 55-fold, suggesting that the presence of VirB2 does not make the strains better recipients. In the next set of experiments, we characterized the molecular basis of this phenomenon.
FIG. 2.
Expression of the B. suis virB operon stimulates pLS1 transfer into A. tumefaciens. The recipient strain UIA143 carrying the Ti plasmid (bar 1), pTrc300 (bar 2), pTrcB3-12 (bar 3), pTrcB1+3-12 (bar 4), pTrcB2-12 (bar 5), and pTrcB1+2-12 (bar 6) were cultivated on AB minimal medium plates at 20°C for 3 days together with donor strain A348 pLS1 under virulence-inducing (+AS) conditions in the presence of IPTG. Exconjugants were identified by growth on selective agar media and the pLS1 transfer efficiency (transconjugants per recipient [TC/R]) into virulence gene-induced induced UIA143 pTiA6 (bar 1) was set to 100%. The standard deviation of results from three independent experiments is shown.
TABLE 1.
Conjugative transfer of pLS1 from A. tumefaciens donor A348 into recipient UIA143 carrying Ti plasmid pTiA6, pTrc300, pTrcB3-12, pTrcB1+3-12, pTrcB2-12, or pTrcB1+2-12a
| Donor | Recipient | No. of recipients (107) | TC | TC/recipient (frequency) | TC/recipient (%) | Fold increase relative to UIA143 pTrc300 | SDb |
|---|---|---|---|---|---|---|---|
| A348 pLS1 | UIA143 pTiA6 | 104 | 156,000 | 1.5 × 10−4 | 100 | 3,300 | 0 |
| A348 pLS1 | UIA143 pTrc300 | 62 | 28 | 4.52 × 10−8 | 0.03 | 1 | 0.01 |
| A348 pLS1 | UIA143 pTrcB3-12 | 150 | 18,000 | 1.2 × 10−5 | 8.0 | 265 | 0.51 |
| A348 pLS1 | UIA143 pTrcB1+3-12 | 108 | 71,000 | 6.57 × 10−5 | 43.8 | 1460 | 5.46 |
| A348 pLS1 | UIA143 pTrcB2-12 | 82 | 2,020 | 2.46 × 10−6 | 1.64 | 55 | 0.15 |
| A348 pLS1 | UIA143 pTrcB1+2-12 | 117 | 200 | 1.71 × 10−7 | 0.114 | 4 | 0.03 |
TC, transconjugants. The ratio of donors to recipients mixed for conjugation was 5 to 1.
Results are from three independent experiments.
The B. suis VirB proteins localize in the A. tumefaciens membranes.
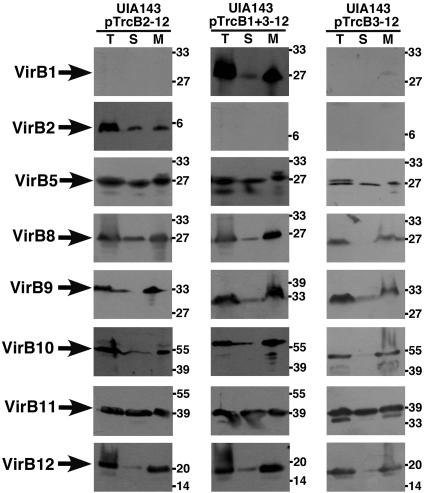
As a first step toward the characterization of B. suis T4SS assembly in A. tumefaciens, we studied the membrane association of VirB proteins. Strain UIA143 carrying pTrcB2-12, pTrcB1+3-12, and pTrcB3-12, respectively, was cultivated on AB minimal medium in the presence of IPTG and lysed, and the total cell lysate (Fig. 3, lanes T) was subjected to ultracentrifugation to separate the membranes (lanes M) from the soluble fraction (lanes S, cytoplasm and periplasm). Subsequent analysis of the subcellular fractions with specific antisera for VirB1, VirB2, VirB5, and VirB8-VirB12 showed that all VirB proteins were detected in the membranes but that most of them were also present to some extent in the soluble fraction (Fig. 3). We have not attempted to quantify the degree of membrane association, but it was evident that the B. suis VirB proteins did not associate as strongly with the membranes as their A. tumefaciens counterparts (38, 60). This indicates that assembly of the T4SS complex may not be as efficient as that of the A. tumefaciens system. We used different methods next to assess interactions between B. suis VirB proteins in the heterologous host.
FIG. 3.
Subcellular fractionation of B. suis VirB proteins produced in A. tumefaciens. Cells of UIA143 carrying pTrcB2-12, pTrcB1+3-12, and pTrcB3-12, respectively, were cultivated on AB minimal medium plates at 20°C for 3 days, followed cell lysis and membrane isolation. The protein content of subcellular fractions (total cell lysate [T], supernatant [S], and membrane fraction [M]) was analyzed by SDS-PAGE and Western blotting with B. suis VirB protein-specific antisera. Molecular masses of reference proteins are shown on the right.
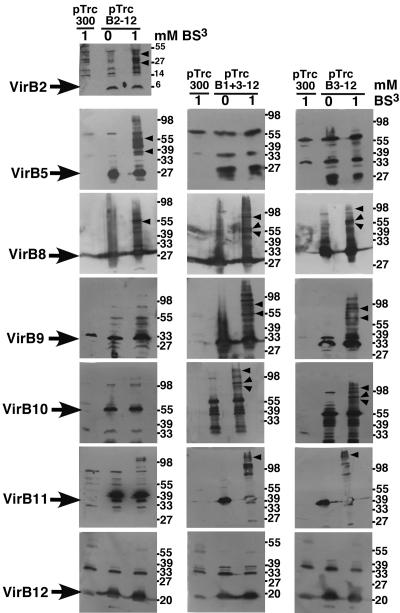
Cross-linking reveals differential interactions between B. suis VirB proteins in the cell envelope of A. tumefaciens.
Cross-linking agents have been used extensively to characterize interactions between A. tumefaciens VirB proteins and to determine the effects of virB gene deletions or amino acid changes in individual proteins on T4SS assembly (2, 3, 5, 10, 46). We here used the cross-linking agent BS3, which primarily cross-links proteins via Lys residues, to monitor VirB protein interactions in strain UIA143 carrying the different B. suis virB operon constructs. Cells carrying cloning vector pTrc300 and virB operon constructs pTrcB2-12, pTrcB1+3-12, and pTrcB3-12, respectively, cultivated on AB minimal medium were incubated with the cross-linking agent, followed by SDS-PAGE separation of cell lysates and Western blot detection with specific antisera. Similar to previous reports on the A. tumefaciens T4SS (46), multiple cross-linking products were detected with most antisera (Fig. 4). Due to the large number of putative interaction partners, it was not possible to unambiguously assign cross-linking products to pairwise interactions. However, we noted a striking correlation between the formation of cross-linking products and the ability to increase pLS1 transfer. As expected, VirB2 was only present in UIA143 pTrcB2-12, and its cross-linking products had molecular masses similar to those observed in the case of VirB5 in this strain. In contrast, cross-linking products of VirB5 were not observed in lysates from UIA143 carrying pTrcB3-12 and pTrcB1+3-12, indicating that VirB5 undergoes different interactions in the presence and in the absence of VirB2. Analysis with VirB core complex component-specific antisera (VirB8, VirB9, and VirB10) revealed substantial differences in the cross-linking patterns. The results indicate that the core components undergo certain interactions only in strains with strongly increased recipient competence, and similar observations were made with VirB11-specific antiserum (Fig. 4). No cross-linking products were detected with VirB12-specific antiserum, suggesting that it does not associate with the other B. suis T4SS components in A. tumefaciens. The results of the cross-linking experiments suggest that the T4SS core components form multiple interactions when they assemble into a complex competent to increase plasmid transfer. We used fluorescence microscopy next to localize this complex in A. tumefaciens and B. suis.
FIG. 4.
Cross-linking monitors protein-protein interactions between B. suis VirB proteins in A. tumefaciens. Cells of UIA143 carrying pTrc300, pTrcB2-12, pTrcB1+3-12, and pTrcB3-12, respectively, were cultivated on AB minimal medium plates at 20°C for 3 days, followed by cross-linking with BS3 (1 mM). Cell lysates from cross-linked and non-cross-linked samples were analyzed by SDS-PAGE and Western blotting with B. suis VirB protein-specific antisera. Arrows indicate monomeric proteins, and arrowheads indicate higher-molecular-mass cross-linking products differentiating interactions in UIA pTrcB2-12 from those in pTrcB1+3-12 and pTrcB3-12. Molecular masses of reference proteins are shown on the right.
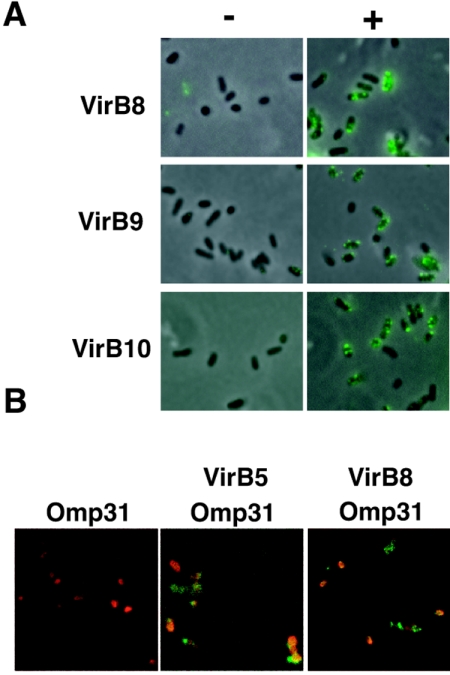
Immunofluorescence microscopy localizes B. suis VirB proteins in foci in the cell envelope.
Several VirB proteins have been shown to localize in foci on the surface of A. tumefaciens, which are believed to represent complexes of multiple T4SS components (35, 36, 40, 41). Although the functionality of these complexes has not been proven, it is likely that they constitute assembly sites of the T4SS in the cell envelope. To assess whether the B. suis T4SS components form similar foci when expressed in a heterologous host, we subjected A. tumefaciens strains carrying pTrcB3-12 and the control plasmid pTrc300 to immunofluorescence analysis with B. suis VirB8-, VirB9-, and VirB10-specific antisera. Analysis by fluorescence microscopy showed that, similar to their A. tumefaciens core protein homologs, they localized in the cell envelope and VirB8 was detected in the characteristic spot-like pattern (Fig. 5A). We next analyzed the localization of these proteins after expression from pTrcB3-12 in the natural host B. suis. Preliminary experiments with a B. suis 1330 virB2::Tn5 insertion mutant (27) (abolished expression of the native virB operon) gave very low levels of surface labeling with the anti-VirB sera. This suggested that the smooth lipopolysaccharide (LPS) blocked access of the antibodies as previously reported with monoclonal antibodies recognizing Brucella outer membrane proteins (16). To overcome this problem, we constructed a rough manB derivative (B. suis virB manB), followed by immunofluorescence analysis. Similar to the heterologous host, VirB8 and VirB5 were detected in the cell envelope in a spot-like pattern (Fig. 5B). The cell biological data show that the B. suis T4SS components localize in the cell envelope in complexes similar to those from A. tumefaciens. Taken together, the expression of the B. suis virB operon from an IPTG-inducible promoter on a broad-host-range plasmid leads to the production of B. suis VirB proteins, and they assemble in the cell envelope in defined regions, which is in accord with the results of the recipient assay and the cross-linking experiments. The following experiments were aimed at addressing the molecular basis for the ability of the B. suis T4SS to increase recipient activity.
FIG. 5.
Immunofluorescence analysis localizes B. suis VirB proteins in the cell envelope of A. tumefaciens. (A) A. tumefaciens strain C58 carrying pTrc300 (−) or pTrcB3-12 (+), respectively, was cultivated on AB minimal medium plates for 3 days in the presence of IPTG for trc promoter induction, followed by immunofluorescence analysis with primary VirB8-, VirB9-, or VirB10-specific antisera, and secondary Oregon green anti-rabbit antiserum. (B) Analysis of B. suis virB manB pTrcB3-12. Cells were cultivated in tryptic soy broth, and virB gene expression was induced with IPTG for 5 h. Immunofluorescence analysis was conducted with primary mouse Omp31-specific and rabbit VirB5- or VirB8-specific antisera, followed by the addition of secondary antibodies anti-rabbit IgG-fluorescein isothiocyanate conjugate and anti-mouse IgG-Texas red conjugate.
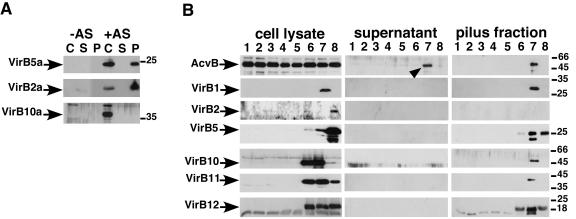
Assembly of B. suis T4SS components weakens the cell envelope of A. tumefaciens.
To analyze whether the B. suis T4SS assembles pilus-like structures in the heterologous host, we isolated extracellular high molecular mass structures by shearing of the cells, followed by ultracentrifugation. In samples from the A. tumefaciens wild-type control we detected the T-pilus major component VirB2a (VirBa indicating A. tumefaciens VirB protein) and the minor component VirB5a in the cells (lanes C) and in the sediment obtained after ultracentrifugation (lanes P), indicating pilus assembly as expected (Fig. 6A) (54). Other T4SS components such as VirB10a, VirE2a, and the periplasmic protein AcvB were only detected in the cells and not in the pilus fractions or in the supernatant after ultracentrifugation (lanes S) (Fig. 6A and B). The same fractionation procedure was applied to UIA143 carrying pTrc300 and the B. suis virB operon vectors, and the VirB proteins were detected in the subcellular fractions with specific antisera (Fig. 6B). The results were substantially different from observations made with A. tumefaciens VirB proteins, especially in case of UIA143 pTrcB1+3-12. Pilus fractions from this strain contained most VirB proteins and in addition the periplasmic AcvB. AcvB was also detected in the supernatant obtained after ultracentrifugation. The results indicated that AcvB was released from the periplasm during the shearing procedure. In pilus fractions isolated from UIA143 pTrcB3-12 only VirB12 was detected and AcvB was not present in the supernatant, indicating that the presence of VirB1 accounts for the major changes observed between the two strains. Pilus fractions from UIA143 pTrcB2-12 contained only VirB5 and VirB12. Since VirB5 is the minor pilus component in other T4SS, this suggested the formation of a pilus-like structure, but we did not detect VirB2 in these fractions. In addition, we did not detect pili by transmission electron microcopy in any of the B. suis virB operon-carrying strains (data not shown). Thus, in spite of the many similarities shown above, the B. suis T4SS expressed in the heterologous host did not share all of the structural features of the native Agrobacterium T4SS.
FIG. 6.
Expression of the B. suis virB1+3-12 operon induces periplasmic leakage in A. tumefaciens. Cells were cultivated on AB minimal medium plates at 20°C for 3 days, followed by shearing of the cells and analysis of protein content of subcellular fractions after SDS-PAGE and Western blotting: cell lysate (C), ultracentrifugation supernatant (S), and pellet (P [pilus fraction]). (A) A. tumefaciens wild-type C58 grown under noninducing (−AS) or virulence gene-inducing conditions (+AS). (B) A. tumefaciens wild type grown under noninducing (lanes 1) or virulence gene-inducing conditions (lanes 2) and Ti plasmid-free strain UIA143 grown without IPTG (lanes 3) or with IPTG (lanes 4). Strain UIA143 was grown with different virB operon plasmids in the presence of IPTG as follows: lanes 5, pTrc300; lanes 6, pTrcB3-12; lanes 7, pTrcB1+3-12; and lanes 8, pTrcB2-12. An arrowhead indicates periplasmic protein AcvB released into the supernatant by shearing. Antisera detected A. tumefaciens VirBa proteins or B. suis VirB proteins. The molecular masses of reference proteins are shown on the right.
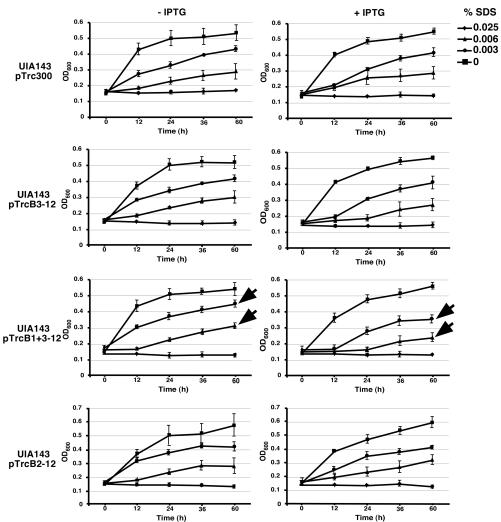
To monitor the weakening of the cell envelope permitting the leakage of periplasmic proteins, we tested the growth of cells carrying the different operon plasmids in the presence of various concentrations of the detergent SDS (at 0, 0.025, 0.006, or 0.003%) over a period of 3 days. This assay monitors the integrity of the cell envelope, and similar assays have previously been used to assess the effects of Rhizobium leguminosarum exo5 and Sinorhizobium meliloti bacA (25, 45). The addition of SDS to recipient strain UIA143 with or without cloning vectors at concentrations 0.003 and 0.006% successively inhibited growth, and cells did not grow at concentrations of 0.025% and higher (Fig. 7). The presence of pTrcB2-12 did not affect the sensitivity of strain UIA143 to the detergent. In contrast, the growth of UIA143 carrying pTrcB3-12 was slightly reduced in the presence of 0.003 and 0.006% SDS, and this effect was very obvious in the case of UIA143 pTrcB1+3-12 (Fig. 7). These results correlate with the release of periplasmic AcvB and suggest that production of the B. suis T4SS functional in the recipient assay reduces the integrity of the cell envelope. In the absence of ITPG induction of the trc promoter, we did not note any reduction of growth, showing that the sensitization of A. tumefaciens to SDS was strictly dependent on the expression of virB1+B3-12 and virB3-12 (Fig. 7).
FIG. 7.
Expression of B. suis virB operon constructs increases the sensitivity of A. tumefaciens to SDS. Cells of strain UIA143 carrying pTrc300 or different virB operon plasmids (pTrcB3-12, pTrcB1+3-12, or pTrcB2-12) were cultivated in liquid AB minimal medium at 20°C with or without IPTG for various times as indicated in the presence of various concentrations of SDS. The average of three independent experiments is given, and error bars show the standard deviations. Arrows indicate reduction of growth of UIA143 pTrcB1+3-12 upon induction of the trc promoter with IPTG.
DISCUSSION
The experiments reported here suggest that the B. suis VirB proteins assemble into a T4SS-like complex in the heterologous host A. tumefaciens. All VirB proteins were detected in the membranes, some of them localized in characteristic patterns in the cell envelope and the cross-linking patterns were reminiscent of those observed in case of the A. tumefaciens VirB homologs (2, 3, 5, 10, 46). Expression of subsets of B. suis VirB proteins in an Agrobacterium virB deletion mutant increased their competence as recipients in a conjugation assay, but the virB operon constructs did not fully complement T4SS functions. These results may indicate principal differences between the T4SSs of A. tumefaciens and B. suis or merely reflect the fact that some but not all VirB proteins can be exchanged. A principal difference between the two organisms is that B. suis does not encode a VirD4 homolog (50), suggesting that the coupling of substrate transport may follow a different mechanism and may be similar to that of the B. pertussis T4SS. VirD4 is required only on the donor but not on the recipient side in plasmid transfer experiments (7), and it is also dispensable for T-pilus formation (44), suggesting that it may not be required for functional assembly of the other T4SS components. We have previously shown that B. suis VirB4 and to some extent VirB1 could replace their Agrobacterium counterparts (34, 66), but others, such as VirB5, VirB6, and VirB11 could not (unpublished observations). This suggests that full T4SS functionality in A. tumefaciens requires interactions with specific sets of VirB and non-VirB assembly factors, DNA substrates, and/or coupling proteins, which cannot be conducted by the B. suis VirB proteins. Given that the sequence conservation between B. suis and A. tumefaciens VirB proteins is not high (amino acid identities of 18 to 32%, similarities of 46 to 65%) (49), it is not surprising that full complementation is not possible, but the results of the recipient assay suggest the correct assembly of a T4SS (7, 46). An alternative explanation is that overexpression of the B. suis VirB proteins in the heterologous host from the strong trc promoter and alteration of the operon structure may lead to protein production in a stoichiometry, which does not permit functional assembly. In the future, we will use alternative promoters, such as the A. tumefaciens virB and the arabinose-inducible pBAD promoter, to assess this possibility.
One of the interesting features of the different subsets of B. suis proteins is that strains carrying pTrcB1+2-12 and pTrcB2-12 were poorer recipients than those carrying pTrcB1+3-12 and pTrcB3-12. This suggests that the expression of Brucella VirB2 has a negative effect on the recipient assay. Similarly, the absence of VirB2 had an effect on the protein-protein interactions of VirB5, as well as of VirB8-VirB11 identified in the cross-linking experiments. This demonstrates that VirB proteins in recipient-competent (UIA143 pTrcB3-12 and pTrcB1+3-12) and less-competent (pTrcB1+2-12, pTrcB2-12) strains undergo different sets of interactions, which correlate with their assembly, and similar results were obtained in recipient assays with the A. tumefaciens T4SS (46). Also, marked variations of the levels of different VirB proteins were observed, which is in line with different overall structure and stability of the complexes. For example, the level of VirB5 was markedly increased in UIA143 pTrcB2-12 compared to the other strains, and the results of the cross-linking experiments indicated that this could rely on a direct interaction with and stabilization by VirB2. However, expression of virB2-virB12 did not lead to the assembly to T-pilus-like structures on the surface of A. tumefaciens, in spite of the fact that VirB5 was detected in high-molecular-mass extracellular fractions. One explanation of the negative effect of VirB2 on pLS1 recipient competence is that this protein may not undergo proper processing (signal peptide removal followed by cyclization [23]) due to the absence of the matching cofactors in A. tumefaciens. Incorrectly processed VirB2 may bind to VirB5 in nonproductive complexes and thereby negatively impact T4SS assembly. As an alternative explanation we hypothesize that production of VirB2 completes the assembly and “seals” the T4SS so that it cannot increase recipient competence. The molecular basis of the recipient stimulation phenomenon is currently unknown. It may be based on interactions between T4SS components in the donor and the recipient, and the exposed VirB2 pilus components may mediate this process. A. tumefaciens VirB2 is an important positive contributor in this assay, but if homologs from A. tumefaciens and B. suis did not interact it would explain why pTrcB2-12-carrying strains had a lower increase in recipient competence. In future, we will separately express VirB2 homologs from A. tumefaciens and B. suis and hybrid proteins in the recipient to directly test this hypothesis. Yet another interpretation becomes apparent if the levels of VirB9 and VirB10 in the different virB operon-carrying strains are compared. The levels of these proteins are most elevated in UIA143 carrying pTrcB3-12 and pTrcB1+3-12, respectively, suggesting that these T4SS core proteins may be principle factors for the increased recipient competence.
An interesting finding reported here is the weakening of the cell envelope, which is most pronounced in UIA143 pTrcB1+3-12. Cells carrying this plasmid had increased sensitivity to low concentrations of the detergent SDS, the periplasmic AcvB protein was released by shearing, and all VirB proteins were detected in extracellular high-molecular-mass fractions. Whereas the cell envelope was apparently more fragile, growth in YEB and AB minimal media was not reduced. This indicates that, in contrast to previous reports on cell lysis induced by overproduction of the plasmid R1 VirB1 homolog ORF169, the cells did not lyse (4). These results constitute an illustration of the cell envelope-permeabilizing potency of the lytic transglycosylase VirB1 (67), since the SDS sensitivity of UIA143 pTrcB3-12 was much less pronounced and release of VirB proteins and AcvB was not detected. We suggest that the release of high-molecular-mass VirB protein complexes from UIA143 pTrcB1+3-12 is a consequence of the absence of VirB2, which leads to an “open” recipient-competent complex, which is not stable and can thus be removed from the cells by shearing. It is intriguing to speculate that this “open” complex may reflect a natural status of the B. suis T4SS during the substrate translocation process. Thus far, there are few reports describing the actual channel properties of T4SSs, which may be due to the fact that this system is well sealed by VirB2 and other VirB proteins. The presence of the plasmid RP4 T4SS was shown to increase leakage of ATP from E. coli and to increase their permeability to certain lipophilic agents, but effects on growth of the cells have not been reported (18). We have analyzed the growth of 11 virB gene deletion variants of A348 (6) in the presence of various concentrations of SDS but did not determine any growth defects (not shown). Thus, the opening of the cell envelope in UIA143 pTrcB1+3-12 may either be due to the overexpression from the trc promoter or reflect a unique property of the B. suis T4SS.
Taken together, the findings presented here constitute the first comprehensive approach to study the Brucella T4SS with biochemical methods. The establishment of a heterologous system in a nonpathogenic host was an essential prerequisite for this strategy. The analysis of different virB operon constructs revealed novel features of this T4SS machinery, which may be generally applicable to those from other bacteria. We have not detected the translocation of substrates from A. tumefaciens cells carrying the B. suis T4SS, and this may reflect the fact that they do not undergo all protein-protein interactions necessary for translocation into the host. An alternative explanation is that the Agrobacterium VirD4 coupling protein, which is thought to be essential for recruitment of substrates, does not interact with the Brucella VirB proteins. However, a virB1 mutant could be complemented, suggesting that the Brucella transglycosylase activity is able to functionally complement for this deficit. As we have demonstrated assembly of the B. suis T4SS, it may be capable of translocation of Brucella substrates between cells. Translocated substrates have not been identified in Brucella, but the heterologous system may provide opportunities to study this process as well without the requirement for biosafety level 3 pathogen containment. We envisage that the expression of T4SSs from other organisms in heterologous hosts will permit similar insights into their specific features in the future.
Acknowledgments
We thank August Böck (Munich, Germany) for continued support and discussions.
This study was initiated with support from the EGIDE/DAAD French-German exchange program (PROCOPE) and the European Union (QLK2-CT-2001-01200). The Baron laboratory is supported by the Canadian Institutes of Health Research (CIHR grant MOP-64300), the Canada Foundation for Innovation, and the Ontario Innovation Trust. The O'Callaghan laboratory is supported by INSERM, the Université de Montpellier 1 (BQR), La Region Languedoc Roussillon, and La Ville de Nîmes.
Editor: D. L. Burns
REFERENCES
- 1.Azghani, A. O., S. Idell, M. Bains, and R. E. Hancock. 2002. Pseudomonas aeruginosa outer membrane protein F is an adhesin in bacterial binding to lung epithelial cells in culture. Microb. Pathog. 33:109-114. [DOI] [PubMed] [Google Scholar]
- 2.Baron, C., M. Llosa, S. Zhou, and P. C. Zambryski. 1997. C-terminal processing and cellular localization of VirB1, a component of the T-complex transfer machinery of Agrobacterium tumefaciens. J. Bacteriol. 179:1203-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baron, C., Y. R. Thorstenson, and P. C. Zambryski. 1997. Biochemical analysis of the complex between the lipoprotein VirB7 and VirB9 in the membranes of Agrobacterium tumefaciens. J. Bacteriol. 179:1211-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayer, M., R. Iberer, K. Bischof, E. Rassi, E. Stabentheiner, G. Zellnig, and G. Koraimann. 2001. Functional and mutational analysis of P19, a DNA transfer protein with muramidase activity. J. Bacteriol. 183:3176-3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beaupré, C. E., J. Bohne, E. M. Dale, and A. N. Binns. 1997. Interactions between VirB9 and VirB10 membrane proteins involved in movement of DNA from Agrobacterium tumefaciens into plant cells. J. Bacteriol. 179:78-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger, B. R., and P. J. Christie. 1994. Genetic complementation analysis of the Agrobacterium tumefaciens virB operon: virB2 through virB11 are essential virulence genes. J. Bacteriol. 176:3646-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohne, J., A. Yim, and A. N. Binns. 1998. The Ti plasmid increases the efficiency of Agrobacterium tumefaciens as a recipient in virB-mediated conjugal transfer of an IncQ plasmid. Proc. Natl. Acad. Sci. USA 95:7057-7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boschiroli, M. L., V. Foulongne, and D. O'Callaghan. 2001. Brucellosis: a worldwide zoonosis. Curr. Opin. Microbiol. 4:58-64. [DOI] [PubMed] [Google Scholar]
- 9.Boschiroli, M. L., S. Ouahrani-Bettache, V. Foulongne, S. Michaux-Charachon, B. G., A. Allardet-Servent, C. Cazevieille, J. P. Liautard, M. Ramuz, and D. O'Callaghan. 2002. The Brucella suis virB operon is induced intracellularly in macrophages. Proc. Natl. Acad. Sci. USA 99:1544-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cascales, E., and P. J. Christie. 2004. Definition of a bacterial type IV secretion pathway for a DNA substrate. Science 304:1170-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cascales, E., and P. J. Christie. 2003. The versatile bacterial type IV secretion systems. Nat. Rev. Microbiol. 1:137-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Celli, J., and J. P. Gorvel. 2004. Organelle robbery: Brucella interactions with the endoplasmic reticulum. Curr. Opin. Microbiol. 7:93-97. [DOI] [PubMed] [Google Scholar]
- 13.Celli, J., S. P. Salcedo, and J. P. Gorvel. 2005. Brucella coopts the small GTPase Sar1 for intracellular replication. Proc. Natl. Acad. Sci. USA 102:1673-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christie, P. J. 2004. Type IV secretion: the Agrobacterium VirB/D4 and related conjugation systems. Biochim. Biophys. Acta 1694:219-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christie, P. J., K. Atmakuri, V. Krishnamoorthy, S. Jakubowski, and E. Cascales. 2005. Biogenesis, architecture, and function of bacterial type IV secretion systems. Annu. Rev. Microbiol. 59:415-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cloeckaert, A., P. de Wergifosse, G. Dubray, and J. N. Limet. 1990. Identification of seven surface-exposed Brucella outer membrane proteins by use of monoclonal antibodies: immunogold labeling for electron microscopy and enzyme-linked immunosorbent assay. Infect. Immun. 58:3980-3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Comerci, D. J., M. J. Martinez-Lorenzo, R. Sieira, J. P. Gorvel, and R. A. Ugalde. 2001. Essential role of the VirB machinery in the maturation of the Brucella abortus-containing vacuole. Cell. Microbiol. 3:159-168. [DOI] [PubMed] [Google Scholar]
- 18.Daugelavicius, R., J. K. Bamford, A. M. Grahn, E. Lanka, and D. H. Bamford. 1997. The IncP plasmid-encoded cell envelope-associated DNA transfer complex increases cell permeability. J. Bacteriol. 179:5195-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delrue, R. M., C. Deschamps, S. Leonard, C. Nijskens, I. Danese, J. M. Schaus, S. Bonnot, J. Ferooz, A. Tibor, X. DeBolle, and J. J. Letesson. 2005. A quorum-sensing regulator controls expression of both the type IV secretion system and the flagellar apparatus of Brucella melitensis. Cell. Microbiol. 7:1151-1161. [DOI] [PubMed] [Google Scholar]
- 20.Delrue, R. M., M. Martinez-Lorenzo, P. Lestrate, I. Danese, V. Bielarz, P. Mertens, X. De Bolle, A. Tibor, J. P. Gorvel, and J. J. Letesson. 2001. Identification of Brucella spp. genes involved in intracellular trafficking. Cell. Microbiol. 3:487-497. [DOI] [PubMed] [Google Scholar]
- 21.DelVecchio, V. G., V. Kapatral, R. J. Redkar, G. Patra, C. Mujer, T. Los, N. Ivanova, I. Anderson, A. Bhattacharyya, A. Lykidis, G. Reznik, L. Jablonski, N. Larsen, M. D'Souza, A. Bernal, M. Mazur, E. Goltsman, E. Selkov, P. H. Elzer, S. Hagius, D. O'Callaghan, J. J. Letesson, R. Haselkorn, N. Kyrpides, and R. Overbeek. 2002. The genome sequence of the facultative intracellular pathogen Brucella melitensis. Proc. Natl. Acad. Sci. USA 99:443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.den Hartigh, A. B., Y. H. Sun, D. Sondervan, N. Heuvelmans, M. O. Reinders, T. A. Ficht, and R. M. Tsolis. 2004. Differential requirements for VirB1 and VirB2 during Brucella abortus infection. Infect. Immun. 72:5143-5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisenbrandt, R., M. Kalkum, E. M. Lai, R. Lurz, C. I. Kado, and E. Lanka. 1999. Conjugative pili of IncP plasmids, and the Ti plasmid T pilus are composed of cyclic subunits. J. Biol. Chem. 274:22548-22555. [DOI] [PubMed] [Google Scholar]
- 24.Escudero, J., A. Den Dulk-Ras, T. J. Regensburg-Tuink, and P. J. Hooykaas. 2003. VirD4-independent transformation by CloDF13 evidences an unknown factor required for the genetic colonization of plants via Agrobacterium. Mol. Microbiol. 47:891-901. [DOI] [PubMed] [Google Scholar]
- 25.Ferguson, G. P., R. M. Roop 2nd, and G. C. Walker. 2002. Deficiency of a Sinorhizobium meliloti BacA mutant in alfalfa symbiosis correlates with alteration of the cell envelope. J. Bacteriol. 184:5625-5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernandez, D., G. M. Spudich, X.-R. Zhou, and P. J. Christie. 1996. The Agrobacterium tumefaciens virB7 lipoprotein is required for stabilization of VirB proteins during assembly of the T-complex transport apparatus. J. Bacteriol. 178:3168-3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foulongne, V., G. Bourg, C. Cazevieille, S. Michaux-Charachon, and D. O'Callaghan. 2000. Identification of Brucella suis genes affecting intracellular survival in an in vitro human macrophage infection model by signature-tagged transposon mutagenesis. Infect. Immun. 68:1297-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Godfroid, J., A. Cloeckaert, J. P. Liautard, S. Kohler, D. Fretin, K. Walravens, B. Garin-Bastuji, and J. J. Letesson. 2005. From the discovery of the Malta fever's agent to the discovery of a marine mammal reservoir, brucellosis has continuously been a re-emerging zoonosis. Vet. Res. 36:313-326. [DOI] [PubMed] [Google Scholar]
- 29.Halling, S. M., B. D. Peterson-Burch, B. J. Bricker, R. L. Zuerner, Z. Qing, L. L. Li, V. Kapur, D. P. Alt, and S. C. Olsen. 2005. Completion of the genome sequence of Brucella abortus and comparison to the highly similar genomes of Brucella melitensis and Brucella suis. J. Bacteriol. 187:2715-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harlow, E., and D. Lane (ed.). 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 31.Hong, P. C., R. M. Tsolis, and T. A. Ficht. 2000. Identification of genes required for chronic persistence of Brucella abortus in mice. Infect. Immun. 68:4102-4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoover, D., and A. Friedlander. 1997. Brucellosis, p. 513-521. In F. Sidell, E. Takafuji, and D. Franz (ed.), Medical aspects of chemical and biological warfare. Office of the Surgeon General, Washington, D.C.
- 33.Höppner, C., A. Carle, D. Sivanesan, S. Hoeppner, and C. Baron. 2005. The putative lytic transglycosylase VirB1 from Brucella suis interacts with the type IV secretion system core components VirB8, VirB9, and VirB11. Microbiology. 151:3469-3482. [DOI] [PubMed] [Google Scholar]
- 34.Höppner, C., Z. Liu, N. Domke, A. N. Binns, and C. Baron. 2004. VirB1 orthologs from Brucella suis and pKM101 complement defects of the lytic transglycosylase required for efficient type IV secretion from Agrobacterium tumefaciens. J. Bacteriol. 186:1415-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Judd, P. K., R. B. Kumar, and A. Das. 2005. Spatial location and requirements for the assembly of the Agrobacterium tumefaciens type IV secretion apparatus. Proc. Natl. Acad. Sci. USA 102:11498-11503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Judd, P. K., R. B. Kumar, and A. Das. 2005. The type IV secretion apparatus protein VirB6 of Agrobacterium tumefaciens localizes to a cell pole. Mol. Microbiol. 55:115-124. [DOI] [PubMed] [Google Scholar]
- 37.Kohler, S., V. Foulongne, S. Ouahrani-Bettache, G. Bourg, J. Teyssier, M. Ramuz, and J. P. Liautard. 2002. The analysis of the intramacrophagic virulome of Brucella suis deciphers the environment encountered by the pathogen inside the macrophage host cell. Proc. Natl. Acad. Sci. USA 99:15711-15716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krall, L., U. Wiedemann, G. Unsin, S. Weiss, N. Domke, and C. Baron. 2002. Detergent extraction identifies different VirB protein subassemblies of the type IV secretion machinery in the membranes of Agrobacterium tumefaciens. Proc. Natl. Acad. Sci. USA 99:11405-11410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kromayer, M., R. Wilting, P. Tormay, and A. Böck. 1996. Domain structure of the prokaryotic selenocysteine-specific elongation factor SelB. J. Mol. Biol. 262:413-420. [DOI] [PubMed] [Google Scholar]
- 40.Kumar, R. B., and A. Das. 2002. Polar location and functional domains of the Agrobacterium tumefaciens DNA transfer protein VirD4. Mol. Microbiol. 43:1523-1532. [DOI] [PubMed] [Google Scholar]
- 41.Kumar, R. B., Y.-H. Xie, and A. Das. 2000. Subcellular localization of the Agrobacterium tumefaciens T-DNA transport pore proteins: VirB8 is essential for assembly of the transport pore. Mol. Microbiol. 36:608-617. [DOI] [PubMed] [Google Scholar]
- 42.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 43.Lai, E.-M., and C. I. Kado. 1998. Processed VirB2 is the major subunit of the promiscuous pilus of Agrobacterium tumefaciens. J. Bacteriol. 180:2711-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lai, E. M., O. Chesnokova, L. M. Banta, and C. I. Kado. 2000. Genetic and environmental factors affecting T-pilin export and T-pilus biogenesis in relation to flagellation of Agrobacterium tumefaciens. J. Bacteriol. 182:3705-3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laus, M. C., T. J. Logman, A. A. Van Brussel, R. W. Carlson, P. Azadi, M. Y. Gao, and J. W. Kijne. 2004. Involvement of exo5 in production of surface polysaccharides in Rhizobium leguminosarum and its role in nodulation of Vicia sativa subsp. nigra. J. Bacteriol. 186:6617-6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu, Z., and A. N. Binns. 2003. Functional subsets of the VirB type IV transport complex proteins involved in the capacity of Agrobacterium tumefaciens to serve as a recipient in virB-mediated conjugal transfer of plasmid RSF1010. J. Bacteriol. 185:3259-3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maniatis, T. A., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 48.Maria-Pilar, J. d. B., S. Dudal, J. Dornand, and A. Gross. 2005. Cellular bioterrorism: how Brucella corrupts macrophage physiology to promote invasion and proliferation. Clin. Immunol. 114:227-238. [DOI] [PubMed] [Google Scholar]
- 49.O'Callaghan, D., C. Cazevieille, A. Allardet-Servent, M. L. Boschiroli, G. Bourg, V. Foulongne, P. Frutus, Y. Kulakov, and M. Ramuz. 1999. A homologue of the Agrobacterium tumefaciens VirB and Bordetella pertussis Ptl type IV secretion systems is essential for intracellular survival of Brucella suis. Mol. Microbiol. 33:1210-1220. [DOI] [PubMed] [Google Scholar]
- 50.Paulsen, I. T., R. Seshadri, K. E. Nelson, J. A. Eisen, J. F. Heidelberg, T. D. Read, R. J. Dodson, L. Umayam, L. M. Brinkac, M. J. Beanan, S. C. Daugherty, R. T. Deboy, A. S. Durkin, J. F. Kolonay, R. Madupu, W. C. Nelson, B. Ayodeji, M. Kraul, J. Shetty, J. Malek, S. E. Van Aken, S. Riedmuller, H. Tettelin, S. R. Gill, O. White, S. L. Salzberg, D. L. Hoover, L. E. Lindler, S. M. Halling, S. M. Boyle, and C. M. Fraser. 2002. The Brucella suis genome reveals fundamental similarities between animal and plant pathogens and symbionts. Proc. Natl. Acad. Sci. USA 99:13148-13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roop, R. M., III, B. H. Bellaire, M. W. Valderas, and J. A. Cardelli. 2004. Adaptation of the brucellae to their intracellular niche. Mol. Microbiol. 52:621-630. [DOI] [PubMed] [Google Scholar]
- 52.Rouot, B., M.-T. Alvarez-Martinez, C. Marius, P. Mentanteau, L. Guilloteau, R.-A. Boigegrain, R. Zumbihl, D. O'Callaghan, N. Domke, and C. Baron. 2003. Production of the type IV secretion system differs among Brucella species as revealed with VirB5- and VirB8-specific antisera. Infect. Immun. 71:1075-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schägger, H., and G. von Jagow. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range of 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 54.Schmidt-Eisenlohr, H., N. Domke, C. Angerer, G. Wanner, P. C. Zambryski, and C. Baron. 1999. Vir proteins stabilize VirB5 and mediate its association with the T pilus of Agrobacterium tumefaciens. J. Bacteriol. 181:7485-7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmidt-Eisenlohr, H., N. Domke, and C. Baron. 1999. TraC of IncN plasmid pKM101 associates with membranes and extracellular high molecular weight structures in Escherichia coli. J. Bacteriol. 181:5563-5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schrammeijer, B., A. den Dulk-Ras, A. C. Vergunst, E. Jurado Jacome, and P. J. Hooykaas. 2003. Analysis of Vir protein translocation from Agrobacterium tumefaciens using Saccharomyces cerevisiae as a model: evidence for transport of a novel effector protein VirE3. Nucleic Acids Res. 31:860-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stahl, L. E., A. Jacobs, and A. N. Binns. 1998. The conjugal intermediate of plasmid RSF1010 inhibits Agrobacterium tumefaciens virulence and VirB-dependent export of VirE2. J. Bacteriol. 180:3933-3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun, Y. H., H. Garcia-Rolan, A. B. den Hartigh, D. Sondervan, and R. M. Tsolis. 2005. Brucella abortus VirB12 is expressed during infection, but it is not an essential component of the type IV secretion system. Infect. Immun. 73:6048-6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taminiau, B., M. Daykin, S. Swift, M. L. Boschiroli, A. Tibor, P. Lestrate, X. De Bolle, D. O'Callaghan, P. Williams, and J. Letesson. 2002. Identification of a quorum-sensing signal molecule in the facultative intracellular pathogen Brucella melitensis. Infect. Immun. 70:3004-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thorstenson, Y. R., G. A. Kuldau, and P. C. Zambryski. 1993. Subcellular localization of seven VirB proteins of Agrobacterium tumefaciens: implications for the formation of a T-DNA transport structure. J. Bacteriol. 175:5233-5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.U.S. Army Medical Research Institute of Infectious Diseases. 2001. Brucellosis, p. 36-43. In M. Kortepeter (ed.), Medical Management of Biological Casualties Handbook, Fourth Edition ed. U.S. Army Medical Research Institute of Infectious Diseases, Frederick, Md.
- 62.van Larebeke, N., G. Engler, M. Holsters, S. van den Elsacker, I. Zaenen, R. A. Schilperoort, and J. Schell. 1974. Large plasmids in Agrobacterium tumefaciens essential for crown gall-inducing ability. Nature 252:169-170. [DOI] [PubMed] [Google Scholar]
- 63.Vergunst, A. C., B. Schrammeijer, A. den Dulk-Ras, C. M. de Vlaam, T. J. Regensburg-Tuink, and P. J. Hooykaas. 2000. VirB/D4-dependent protein translocation from Agrobacterium into plant cells. Science 290:979-982. [DOI] [PubMed] [Google Scholar]
- 64.Yeo, H.-J., Q. Yuan, M. R. Beck, C. Baron, and G. Waksman. 2003. Structural and functional characterization of the VirB5 protein from the type IV secretion system encoded by the conjugative plasmid pKM101. Proc. Natl. Acad. Sci. USA 100:15947-15962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Young, E. J. 1995. An overview of human brucellosis. Clin. Infect. Dis. 21:283-289. [DOI] [PubMed] [Google Scholar]
- 66.Yuan, Q., A. Carle, C. Gao, D. Sivanesan, K. Aly, C. Höppner, L. Krall, N. Domke, and C. Baron. 2005. Identification of the VirB4-VirB8-VirB5-VirB2 pilus assembly sequence of type IV secretion systems. J. Biol. Chem. 280:26349-26359. [DOI] [PubMed] [Google Scholar]
- 67.Zahrl, D., M. Wagner, K. Bischof, M. Bayer, B. Zavecz, A. Beranek, C. Ruckenstuhl, G. Zarfel, and G. Koraimann. 2005. Peptidoglycan degradation by specialized lytic transglycosylases associated with type III and type IV secretion systems. Microbiology 151:3455-3467. [DOI] [PubMed] [Google Scholar]