Abstract
Members of the genus Chlamydia are obligate intracellular pathogens that have a unique biphasic developmental cycle and interactions with host cells. Many genes that dictate host infection tropism and, putatively, pathogenic manifestations of disease are clustered in a hypervariable region of the genome termed the plasticity zone (PZ). Comparative genomics studies have determined that an uncharacterized family of PZ genes encoding orthologs of eukaryotic and prokaryotic members of the phospholipase D (PLD) enzyme family varies among chlamydiae. Here, we show that the PZ PLD (pzPLD) of Chlamydia trachomatis are transcribed during both normal and persistent infection and that the corresponding PLD proteins are predominately localized in reticulate bodies on the inner leaflet of the inclusion membrane. Further, we show that strains of chlamydiae encoding the pzPLD, but not a strain lacking these genes, are inhibited by primary alcohols, potent PLD inhibitors, during growth in HeLa 229 cells. This inhibitory effect is amplified approximately 5,000-fold during recovery from persistent infection. These findings suggest that the chlamydial pzPLD may be important, strain-specific, pathogenesis factors in vivo.
Members of the genus Chlamydia are obligate intracellular bacteria that are ubiquitous pathogens of mammals. Despite a broad range of host species, disease manifestations, and tissue tropisms of these organisms in nature, the genome sequences of chlamydiae are remarkably similar (28-30). Chlamydia trachomatis strains that cause distinct diseases in humans, including trachoma and chlamydial sexually transmitted diseases, as well as the more distantly related mouse pathogen Chlamydia muridarum, exhibit near genomic synteny (28). Comparative genomics suggests that genes necessary for the intracellular survival of chlamydiae are conserved, while genetic shift is limited to host- and tissue-specific virulence genes. Diversity in infection tropism and disease potential correlates with genes located in a hypervariable portion of the chlamydial genome termed the plasticity zone (PZ). Although previous studies have suggested virulence-related functions for many PZ genes (2, 5, 7, 10, 25), a large group of open reading frames encoding proteins with homology to the phospholipase D (PLD) family enzymes remain uncharacterized (30).
PLD family enzymes of plants, animals, and bacteria retain a conserved HKD motif essential for enzymatic activity (14, 24). By definition, the primary reaction catalyzed by these enzymes is hydrolysis of phosphatidylcholine into phosphatidic acid and choline. However, more distant PLD family members include enzymes with varied functions, including cardiolipin synthases, phosphatidyl serine synthases (27), and nucleases, such as the Salmonella enterica serovar Typhimurium Nuc (26). PLD family enzymes are virulence factors of diverse viral and bacterial pathogens (9, 17, 23, 31, 32). The precise functions of many virulence-associated PLD family enzymes remain unclear; however, putative pathogenic mechanisms of these enzymes include disruption of host cell vesicular traffic (19), alteration of host cell signaling to promote pathogen uptake (9), direct toxic functions against host cells (17, 32), and disruption of host cell membranes (23). Interestingly, the PLD transphosphatidylation reaction can be blocked by primary alcohols, which substitute for water as a substrate to preferentially form phosphatidyl alcohols (27). Sensitivity to primary alcohols is used to elucidate the functions of diverse PLD family enzymes in vitro (9, 19).
Aspects of the chlamydial developmental cycle suggest roles for the PZ PLD family enzymes (pzPLD) in pathogenesis. First, chlamydiae parasitize (13) and modify host cell lipids (13, 15). However, the bacterial genes that direct this activity are unknown. Second, chlamydial inclusions resist fusion with the host endocytic pathway while simultaneously receiving sphingomyelin- and cholesterol-containing vesicles from the Golgi apparatus (6, 11). Described PLD functions include regulation of budding of vesicles from the Golgi (8) and host Golgi vesicle-viral fusion (19, 31). Collectively, the characterized roles of PLD in lipid metabolism and the intricate lipid exchange between chlamydiae and host cells suggest pzPLD may mediate these interactions.
We report here that only strains of chlamydiae that encode pzPLD are sensitive to primary alcohols. Further, these strains are unable to recover from persistence in the presence of primary alcohols. Finally, we show that pzPLD predominately localize to chlamydial reticulate bodies (RB) on the inner face of the inclusion membrane. The findings provide a functional description of the pzPLD and suggest that these enzymes may play a role in strain-specific chlamydial pathogenesis.
MATERIALS AND METHODS
Cell lines, chlamydial culture, and alcohol treatments.
Chlamydia muridarum strain Nigg (MoPn), Chlamydia trachomatis serovar D strain UW-3/CX, and the Chlamydia caviae guinea pig inclusion conjunctivitis strain (GPIC) were grown in HeLa 229 cells, and infectious elementary bodies (EB) were purified as previously described (4). Experimental infections were performed in MDMEM plus 10% fetal bovine serum (FBS) (normal infections) or in low-tryptophan MDMEM plus 10% dialyzed FBS (persistent infections) as previously described (1, 3). Recombinant human gamma interferon (IFN-γ) (R&D, Inc.) was used at a final concentration of 50 U/ml. Alcohols (Sigma) were added to the infection medium at 0.5% (wt/vol).
RNA isolation and Taqman qRT-PCR.
HeLa 229 cells were plated at 2.5 × 106 cells per well in six-well plates in either MDMEM plus 10% FBS (normal infections) or low-tryptophan MDMEM plus 10% dialyzed FBS and 50 U/ml IFN-γ (persistent infections) and then incubated for 24 h prior to infection. The cells were infected at a multiplicity of infection (MOI) of 1 or 10 with C. trachomatis EB by centrifugation as previously described (1, 4). Following infection, the cells were washed three times with Hanks' balanced salt solution and incubated in infection medium until RNA isolation was performed. EB or infected HeLa 229 cells were disrupted using Trizol reagent (1, 3, 8, 16, 24, and 42 h postinfection [p.i.]), and total RNA was extracted using standard procedures. Total RNA was resuspended in 100 μl of diethyl pyrocarbonate-treated water, treated with DNase I Free Turbo (Ambion) according to the manufacturer's instructions, and then diluted 1:3 for Taqman analysis. Taqman quantitative reverse transcription-PCR (qRT-PCR) was performed in an ABI 7500 thermocycler using Taqman one-step master mix (Applied Biosystems). The amplification cycle included 30 min at 48°C and 10 min at 95°C, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Taqman primer-probe sets were designed using Primer Express v. 1.5 software (Applied Biosystems). The sequences of all primers and probes are provided in Table 1. To determine absolute transcript copy numbers, signals were normalized to standard curves of purified C. trachomatis serovar D genomic DNA amplified in parallel with experimental samples. Gene expression was normalized to 16S rRNA levels.
TABLE 1.
Taqman primers and probes and RT-PCR primers
| Genea | Primer 1 | Primer 2 | Taqman probe |
|---|---|---|---|
| ct084 | ATCCATCCAAGTAGGTATGTTTGCT | TTGTGCGGCATTCAATTCA | CACTTTACCTCAGATTATb |
| ct154 | GTGCCCGTCATCACGAATC | AACATGATGTCTTGATGATGAGGA | CGCTCACATCGGCb |
| ct155 | AACACAGACTCCCGAAGACACTT | ACGACTTGGCTCGAAGATC | TTCTTCCTCCTCTAAACGAb |
| ct156 | TCCATCTCCCAGCAAATGC | TGTCTTCGGGAGTCTTGTGTTTT | CGCCGGATCCAAGAb |
| ct157 | GCCGTCGCGCTCGAT | TCTTTTGGAGAAGGAGTATTTTGAGAT | AACCCCTCCCCTCACAb |
| ct158 | CCTCCATTGCAAAGTCGGTTT | CCAGTTCGCAGAACCTGTG | ATCGATACCAACCTCCTTb |
| ct284 | GGCATTGATCCAATCAATCTTTC | AAAGGCTCGTCTCAATCATAGCA | AAAGCTAGATCCTCTTTCb |
| 16srRNA | GAGATTGGCCGCCAACACT | ACTGCAGCCTCCCGTAGGA | ACTGAGACACTGCCCb |
| ompA | GGTTTCGGCGGAGATCCT | AGTAACCAACACGCATGCTGAT | CCTTGCGCCACTTGGTGTGACGc |
| omcB | GAATATGTGATCTCCGTTTCCAATC | CCGGGAGAAAGAGTGTCTTCAA | GACGACATCTCGCAACACAAGATCTCCAc |
| euo | GGCTTTTATTCCGTGGGACA | AATGCGTGTAGCATAGTAAATCTTCTG | GGAACATCCAGCATGGACGCCAc |
Gene designation according to the sexually transmitted disease sequence database designation (http://www.stdgen.lanl.gov/) as provided by Los Alamos National Laboratory.
6-carboxyfluorescein (FAM)-minor groove binding group-labeled probe.
FAM-6-carboxytetramethyl-hodamine-labeled probe.
Cloning, expression, and purification of CT155.
CT155 was amplified from C. trachomatis serovar D genomic DNA by PCR using standard techniques. The PCR product was cloned into the Gateway Entry Vector SD/D/Topo (Invitrogen) and then recombined into the C-terminal His tag fusion expression vector pEXP-2 (Invitrogen). The final construct was confirmed by DNA sequencing. CT155 was expressed in Escherichia coli BL21 by treating log-phase cultures of bacteria with various concentrations of IPTG (isopropyl-β-d-thiogalactopyranoside), and was purified from cell pellets using a His Bind (Novagen) kit following the manufacturer's denaturing extraction protocol.
Production of polyclonal antibodies to CT155.
All animal experiments were approved by the National Institute of Allergy and Infectious Disease Animal Care and Use Committee and performed according to protocol. Mice were primed and boosted subcutaneously twice, at 2-week intervals, with 2 μg of purified recombinant CT155 in 200 μl Ribi adjuvant (Corixa) for each injection. Three weeks following the third boost, the mice were exsanguinated, and the sera were separated by centrifugation and finally stored in 10% glycerol supplemented with sodium azide. Polyclonal antibody specificity was confirmed by slot blotting of preimmune and immune sera against purified recombinant protein.
Western blotting of CT155, MOMP, and GAPDH.
HeLa 229 cells were plated in six-well plates and infected with C. trachomatis serovar D at an MOI of 1 as described above. At various intervals following infection or mock infection, the monolayers were washed twice with Hanks' balanced salt solution and then lysed in 300 μl of 2× Laemmli buffer. To isolate EB proteins, 111 μg of the purified EB preparation used for infections was suspended in 500 μl 2× Laemmli buffer. Protein samples were denatured at 100°C for 5 min, resolved on 10% Criterion Pre-cast sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels (Bio-Rad), and transferred to nitrocellulose membranes. The membranes were preblocked at room temperature for 2 h in phosphate-buffered saline (PBS) supplemented with 3% bovine serum albumin, 0.05% Tween 20, and 0.02% NaN3 and then incubated with mouse polyclonal anti-CT155 (1:500), mouse monoclonal anti-MOMP BB-5b (1:200), or mouse monoclonal anti-GAPDH (1:5,000) (Abcam) at room temperature overnight. The blots were washed extensively with PBS containing 3% bovine serum albumin and 0.05% Tween 20 and then incubated at room temperature for 2 h with goat anti-mouse horseradish peroxidase-conjugated secondary antibody (1:1,000) (MP Biomedicals Inc.). The blots were washed three times with PBS containing 0.05% Tween 20 and twice with PBS and then developed with 3,3′,5,5′-tetramethylbenzidine single solution (Invitrogen).
Confocal microscopy.
Monolayers of HeLa 229 cells in 24-well plates on glass coverslips were infected at an MOI of 0.2 for normal infection or at an MOI of 1.0 for persistent infection. The infected cells were incubated for various intervals postinfection in MDMEM (normal infections) or low-tryptophan MDMEM supplemented with 50 U/ml IFN-γ (persistent infections). The cells were fixed in 2% paraformaldehyde, treated with 0.2% saponin, and then incubated with rabbit anti-serovar D MOMP polyclonal antibodies and mouse anti-CT155 polyclonal antibodies prepared in this study. After being washed, the cells were incubated with secondary Alexa Fluor 647-conjugated goat anti-mouse and Alexa Fluor 488-conjugated anti-rabbit antibodies (Molecular Probes), and then the coverslips were washed, mounted, and visualized by confocal microscopy. The samples were examined with a Bio-Rad 1024 laser scanning confocal microscope using a Kr/Ar laser. Images were collected with Lasersharp 2000 imaging software (Bio-Rad). Data sets were processed by three-dimensional blind iterative deconvolution using Deblur software (AutoQuant Imaging), rendered with Imaris software (Bitplane Inc.), and processed with Adobe PhotoShop.
RESULTS
Genes located in the highly variable PZ of the chlamydial genome(s) were initially proposed by Read et al. to function as virulence factors (28, 29). Recent studies have shown that some PZ genes have specifically coevolved with chlamydiae and their respective hosts for the purpose of evading IFN-γ immunity (3, 5, 10, 25). Because of the critical nature of these characterized PZ genes, we were interested in understanding the roles of uncharacterized PZ genes in pathogenesis.
Schematics of the PZs of three mucosal chlamydial strains with rodent (C. muridarum and C. caviae) and human (C. trachomatis) host infection tropisms are shown in Fig. 1. Examples of PZ genes that have been described as possible virulence factors are the C. trachomatis tryptophan synthase operon (trpRBA) (5, 10) and C. muridarum cytotoxin genes (TC437, TC438, and TC439) (2, 25). The tryptophan synthase genes found in the human strain have been replaced in the mouse strain with a partial guanine nucleotide utilization operon (add-guaAB). C. trachomatis retains portions of a single copy of the MoPn cytotoxin gene (CT165-168). C. caviae, which is genetically diverse from the murine and human strains (29), has retained the add-guaAB operon, a single copy of an intact cytotoxin gene (tox), and an almost complete operon for tryptophan anabolism (trpRDCF, trpB-2, trpA, and kyuU) within its PZ. Interestingly, the pzPLD genes (Fig. 1), like trpRBA and the cytotoxin genes, are strain variable. All chlamydiae sequenced to date retain at least one ancestral PLD gene (chromosomal PLD) outside the PZ (7, 18, 20, 28, 29). However, C. trachomatis strains (7) and C. muridarum (28) encode multiple PLD orthologs within the PZ (CT154-58, TC432-436, TC440, and TC447) (Fig. 1) that are absent from C. caviae, C. pneumoniae, and parachlamydiae (18, 20, 29). We therefore undertook basic characterization studies of the pzPLD to better understand their potential role(s) in chlamydial pathogenesis.
FIG. 1.
Organization of PLD genes in the PZs of chlamydiae. Shown are the arrangements of genes in the PZ, and extra-PZ PLD, in C. trachomatis serovar D, C. muridarum, and C. caviae. The nomenclature is according to designations given by the National Center for Biotechnology Information for accession numbers NC_003361 (29), NC_002620 (28), and NC_000117 (30). PLD genes are shown in red, genes related to the C. muridarum large cytotoxin CT438 are in black, tryptophan biosynthesis genes are colored blue, and nucleotide biosynthesis- or acquisition-related genes are in yellow. PLD orthologs corresponding to pzPLD CT154 to CT158 of C. trachomatis and TC436 to TC432 in C. muridarum are absent in the PZ of C. caviae. C. muridarum encodes two additional pzPLD orthologs (TC440 and TC447) absent from the PZs of both C. trachomatis and C. caviae. CT084 and CT284 of C. trachomatis, TC357 of C. muridarum, and CCA357 and CCA457 of C. caviae encode PLDs that are located outside the PZ (indicated by double curved lines). The genes in white are intervening genes in the PZ which cannot be strictly sorted into any of the four aforementioned categories. The dual-colored C. caviae gene prsA functions in nucleotide and tryptophan biosynthesis. The arrows indicate the direction of gene transcription. Double curved lines indicate nonlinear breaks in the diagram between the extra-pzPLD and depicted portions of the PZ.
Chlamydial PLD genes exhibit distinct patterns of temporal expression during normal infection.
C. trachomatis gene expression profiles during normal and persistent infections, performed by microarray, has been described (1, 3). Although these studies determined that PLD genes (chromosomal and PZ) were expressed, the expression levels for all PLD genes were not corroborated by qRT-PCR throughout the growth cycle. We therefore undertook a comprehensive study of the temporal expression of PLD genes during C. trachomatis infection using qRT-PCR to characterize transcription of these genes during chlamydial development. Transcriptional profiles of the PLD genes were compared to those of three well-characterized developmentally regulated genes: early (euo), midcycle (ompA), and late (omcB) (3). The transcriptional profiles are shown in Fig. 2. As described previously (1), gradient-purified EB retained carryover transcripts for all of the control and experimental genes. However, the expression profiles of these transcripts following infection differed markedly. Expression of the early gene euo stabilized within 1 h p.i. and then increased exponentially throughout the developmental cycle. In contrast, transcripts for both the midcycle gene ompA and the late gene omcB decreased exponentially during the first 3 h p.i., indicating a lack of de novo transcription. The ompA transcripts significantly increased between 3 h and 8 h p.i., while omcB expression was unchanged, within the margin of error, during the same interval. Expression of the midcycle gene ompA increased exponentially between 8 h and 16 h p.i. and then remained relatively stable during the remainder of the developmental cycle. In contrast, omcB did not significantly increase until 16 h p.i., and expression of this gene then increased exponentially until completion of the developmental cycle at 42 h p.i. We compared expression of the PLD genes to these distinct transcriptional profiles. The PLD genes exhibited three patterns of temporal expression that did not correlate with their residence in the PZ (Fig. 2). Similar to euo, transcription of the chromosomal PLD CT284 decreased only slightly by 1 h p.i. and then increased exponentially throughout the remainder of the developmental cycle, categorizing it as an early gene. In contrast, expression of the second chromosomal PLD, CT084, and the five pzPLD genes resulted in profiles that were similar to mid- (CT156) and late-cycle (CT154, CT155, CT156, CT157, and CT158) genes. Expression of CT284 early in the developmental cycle suggests that this gene has a function distinct from those of the pzPLD genes. Interestingly, CT156 (predicted to encode a truncated pzPLD lacking the HKD motif) expression preceded that of all other pzPLD genes, possibly implying a regulatory rather than an enzymatic role for the gene. Collectively, the transcriptional data show that the PLD genes exhibit different temporal patterns of expression which do not necessarily correlate with their locations within or outside the PZ.
FIG. 2.
The pzPLD are expressed in the mid- to late developmental cycle. HeLa 229 cells were infected with C. trachomatis, and total RNA was harvested from EB or infected cells at 1, 3, 8, 16, 24, and 42 h p.i. The copy numbers of different transcripts (indicated in individual graphs) in each sample were determined by qRT-PCR. The experiment was repeated four times in triplicate, and data from a single representative experiment are depicted. The error bars indicate standard deviations of transcript copy numbers. Graph values marked with asterisks indicate transcripts that fell below the limit of reliable detection (approximately 1,000 total transcripts).
Chlamydial PLD genes are differentially expressed in normal and persistent infection.
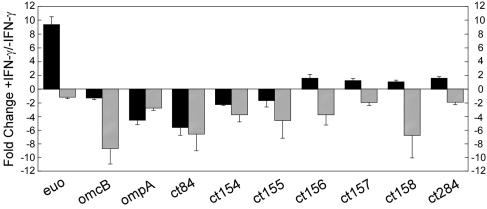
Previous studies have suggested that some, but not all, pzPLD genes are differentially expressed during IFN-γ-mediated persistent infections (1, 3). We compared the expressions of the pzPLD genes following normal and IFN-γ-induced persistent infections of HeLa 229 cells. We found that fewer chlamydial transcripts were detected during persistent versus normal growth. To compensate for this, we normalized the expression of genes in both sets to the respective 16S rRNA levels in each sample (Fig. 3). In agreement with a previous report, euo expression was markedly increased (9-fold) during persistent infection (1), while expression of the midcycle ompA and late-cycle omcB genes was decreased (5-fold and 1.5-fold, respectively). Interestingly, the PLD genes again exhibited differences in expression that did not strictly correlate with their chromosomal or PZ locations (Fig. 3). Expression of pzPLD CT156, CT157, and CT158, as well as chromosomal CT284, was slightly increased (1.5- to 2-fold) in persistent versus normal infections. In contrast, expression of chromosomal CT084 and pzPLD CT154 and CT155 decreased (two- to fivefold) during persistence. These results argue for functional diversity among chlamydial PLD genes and suggest that at least some of these genes might play roles in persistent infection.
FIG. 3.
pzPLD are differentially expressed during normal and persistent infections. HeLa 229 cells were normally or persistently infected (IFN-γ treated) with C. trachomatis at an MOI of 1, and total RNAs were harvested from infected cultures at 24 h p.i. The ratio of specific transcripts (indicated below the x axis) to 16S rRNA measured in normal-infection samples at 24 h was arbitrarily set to 1. Transcript-to-16S rRNA ratios in persistent infections are plotted as a change (n-fold) versus normal infection. The experiment was repeated four times in triplicate, and data from a single representative experiment are shown. The error bars indicate standard deviations of the change.
CT155 protein is present in EB and is expressed in the mid- to late developmental cycle.
Because our transcriptional data suggested that the pzPLD genes were transcribed during normal infection, we wanted to know if this transcription resulted in protein products. To test this, we performed kinetic Western blots on HeLa cells infected at a low MOI with C. trachomatis serovar D using antibodies to MOMP, the host-cell protein control GAPDH, and a representative pzPLD, CT155 (Fig. 4). Anti-CT155 polyclonal antibodies stained a single band of approximately 40 kDa in EB but failed to react with mock-infected HeLa 229 cells or with infected cells during the first 8 h p.i. However, between 16 and 24 h p.i., CT155 staining increased rapidly, and the protein was expressed throughout the remainder of the developmental cycle. As during transcriptional analysis, the pattern of MOMP protein expression was similar to that of CT155. MOMP was present in EB, but de novo expression of the protein did not increase substantially until the 24-h time point.
FIG. 4.
CT155 is present in EB and is expressed in the mid- to late developmental cycle. HeLa 229 cells were infected at an MOI of 1 with C. trachomatis serovar D EB, and whole-cell lysates were prepared at various intervals, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and blotted to nitrocellulose membranes. The nitrocellulose membranes were probed with antibodies to chlamydial CT155, MOMP, or the host cell control GAPDH. Both CT155 and MOMP were detected in EB preparations (EB protein equivalent to an MOI of ∼150), but not in mock-infected lysates (labeled M). Strong de novo expression of CT155 and MOMP was detected by 24 h p.i. and continued until the end of the developmental cycle. Equivalent amounts of lysates were loaded in each lane and blot.
PLDs (CT155) localize to chlamydial RB associated with the inclusion membrane.
Because our transcriptional and Western analyses identified developmental-cycle- and growth state-dependent regulation of PLD expression, we attempted to determine the localization of a representative PLD during infection. HeLa 229 cells, normally or persistently infected with C. trachomatis, were fixed and labeled with anti-CT155 antibodies and analyzed by confocal microscopy (Fig. 5). Similar to the results of transcriptional and Western analyses (Fig. 2 to 4), anti-CT155 antibody reacted with chlamydiae as early as 16 h p.i. (data not shown), with the strongest labeling occurring at 24 to 48 h p.i. (Fig. 5A). Anti-CT155 antibody reacted with large RB-like forms mainly located in close proximity to the inner leaflet of the inclusion membrane. In contrast to the preferential RB staining at the inclusion membrane seen with anti-CT155, MOMP staining was detectable on both RB and EB filling the inclusion lumen (Fig. 5B and C). CT155 labeling of chlamydiae in persistently infected cells was less intense but still clearly distinct from MOMP (data not shown). These results indicate that CT155 is primarily expressed in RB-like structures, which are in close proximity to, or in direct contact with, the inclusion membrane.
FIG. 5.
The pzPLD CT155 is present in RB that associate with the luminal face of the inclusion membrane. HeLa 229 cells were infected at an MOI of 0.2 and prepared for confocal microscopy at 40 h p.i. Paraformaldehyde-fixed, saponin-treated cells were dual labeled with rabbit anti-serovar D MOMP antibodies, mouse anti-serovar D CT155 antibodies, and Alexa-fluor-conjugated secondary antibodies. The micrographs show a single representative cross section from a normal inclusion at 40 h p.i. (A) Anti-CT155 channel in red. (B) Anti-MOMP channel in green. (C) Merged image of panels A and B. The areas of yellow coloration in panel C indicate pixels of CT155 and MOMP colocalization. Scale bars = 10 μm.
Primary alcohol inhibition of chlamydiae correlates with retention of pzPLD.
Primary alcohols, but not corresponding secondary alcohols, inhibit PLD activity (27). To determine if the pzPLD of chlamydiae are necessary for growth in vitro, we assessed the effects of primary alcohols on three chlamydial strains in a HeLa 229 cell model of infection (Fig. 6). C. trachomatis and the closely related MoPn strain retain nearly identical complements of pzPLD (Fig. 1). GPIC, a more distantly related pathogen, lacks pzPLD but retains two chromosomal loci with PLD homology (CCA357 and CCA453) (Fig. 1). 1-Butanol, but not 2-butanol, reduced the yield of infectious C. trachomatis and MoPn inclusion-forming units (IFU) to 5% and 8%, respectively, of untreated control cultures (Fig. 6). The alcohol effect was not restricted to 1-butanol; 1-propanol similarly inhibited both strains in HeLa 229 cells (data not shown). In contrast, GPIC resisted 1-butanol treatment in the one-step growth assay, with treated cells yielding 90% of the IFU of untreated controls (Fig. 6). These results demonstrate that the alcohol sensitivity of chlamydiae is strain specific and primary alcohol specific and correlates with pzPLD, but not extra-pzPLD, paralogs.
FIG. 6.
Alcohol sensitivity of chlamydiae is strain specific and primary alcohol specific and correlates with the complement of pzPLD. HeLa 229 cells were infected at an MOI of 1 with MoPn, C. trachomatis, or GPIC (indicated below the x axis). Following infection, the cultures received normal infection medium (black bars), infection medium supplemented with 1-butanol (open bars), or medium supplemented with 2-butanol (hatched bars). At 48 h p.i., the cultures were harvested, and recoverable IFU (y axis) were enumerated. The experiment was repeated twice in quadruplicate; representative data from a single experiment are depicted. The asterisks above the bars corresponding to 1-butanol treatment of C. trachomatis and MoPn indicate that the recoverable IFU of these cultures were decreased significantly (P < 0.05; 2-tailed unpaired t test) from those of 2-butanol-treated and untreated control cultures. The error bars depict standard deviations of recoverable IFU.
Primary alcohols inhibit C. trachomatis late in the developmental cycle.
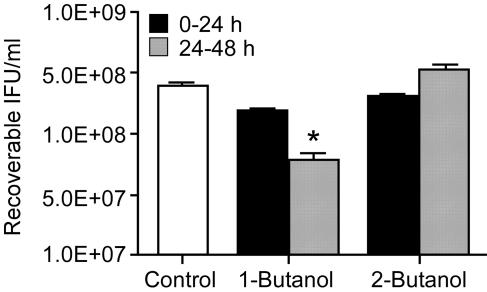
Chlamydiae share a characteristic developmental cycle in which they morphologically transition from infectious extracellular EB to intracellular replicative RB and then condense back into infectious EB. To determine if the effect of primary alcohols was associated with either the early (EB-to-RB transition), middle (RB replication), or late (RB-to-EB transition) portion of the developmental cycle, we varied the times of primary-alcohol addition in one-step growth experiments (Fig. 7). Consistent with the finding that the pzPLD are primarily expressed in the mid- to late developmental cycle, 1-butanol did not significantly inhibit C. trachomatis when it was present only during early stages of invasion and infection. Infected cells treated with 0.5% (vol/vol) 1-butanol for 24 h and then allowed 24 h of recovery in the absence of alcohol yielded 90% and 85% of the IFU of untreated and 2-butanol-treated control cells, respectively (Fig. 7). In contrast, addition of 1-butanol during the final 24 h of infection, a time of high pzPLD expression, resulted in an IFU yield similar to that of control cells treated with alcohol during the entire developmental cycle. Addition of 1-butanol during the final 24 h significantly decreased the IFU yield to 20% and 22% of that of untreated control or 2-butanol-treated cultures, respectively (Fig. 7). This result suggests that pzPLD are important for RB replication and/or maturation into infectious EB and are likely not required for chlamydial entry or the EB-to-RB transition.
FIG. 7.
C. trachomatis primary-alcohol sensitivity varies during the developmental cycle. HeLa 229 cells were infected with C. trachomatis at an MOI of 1. The infection medium was supplemented with 1-butanol or 2-butanol (indicated below the x axis) during the first 24 h (black bars) or second 24 h (hatched bars) of infection. Control cultures (open bar) received no alcohol during the entire course of infection. At 48 h p.i., the cultures were harvested, and recoverable IFU were enumerated (y axis). The experiment was repeated twice in quadruplicate; representative data from a single experiment are depicted. The asterisk indicates that 1-butanol treatment during the final 24 h of infection significantly decreased recoverable IFU (P < 0.05; two-tailed unpaired t test) from those of 2-butanol-treated and untreated control cultures. The error bars depict standard deviations of recoverable IFU.
Primary alcohols block rescue of C. trachomatis from IFN-γ-induced persistence.
Alcohol sensitivity experiments, transcriptional analysis, and microscopy each suggested that pzPLD mediate RB replication and/or maturation. During persistent infection, the chlamydial developmental cycle is apparently blocked between early RB development and RB division (3). Based on these prior results, we suspected that persistent chlamydiae might be sensitive to alcohol treatment. To test this, we assayed the sensitivity of C. trachomatis to alcohols during IFN-γ treatment and during tryptophan rescue from IFN-γ-induced persistence. HeLa 229 cells were grown in tryptophan-depleted medium containing IFN-γ for 24 h, infected with C. trachomatis, and then incubated for 24 h with or without alcohols. At 24 h p.i., the chlamydial cultures were rescued by replacing the medium with tryptophan-supplemented medium, with or without alcohols, until IFU harvest at 48 h p.i. Treatment with 1-butanol almost entirely blocked tryptophan-mediated rescue of chlamydiae from persistence (>5,000-fold) (Fig. 8). Chlamydiae in 2-butanol-treated cultures were rescued by tryptophan treatment at a level similar to that of untreated cultures (Fig. 8). Consistent with the negligible effect of 1-butanol during the first 24 h of normal development (Fig. 7), IFN-γ and 1-butanol treatments, prior to tryptophan rescue, failed to significantly decrease recoverable IFU (Fig. 8). These results suggest that pzPLD are necessary for tryptophan rescue from IFN-γ-mediated persistence.
FIG. 8.
Primary alcohols block tryptophan rescue of C. trachomatis from IFN-γ-mediated persistence. HeLa 229 cells were seeded in low-tryptophan MDMEM supplemented with 50 U/ml IFN-γ, incubated for 24 h, and infected with C. trachomatis at an MOI of 1. Following infection, the cultures were fed IFN-γ-supplemented MDMEM and incubated for an additional 24 h (IFN-γ treatment phase). The cultures were then washed and refed with MDMEM supplemented with 10× tryptophan and incubated for an additional 24 h (trytophan rescue phase) prior to recoverable-IFU harvest and enumeration (y axis). Experimental cultures were additionally treated with either 1-butanol or 2-butanol (indicated below the x axis) during IFN-γ treatment (black bars) or during tryptophan rescue (hatched bars). Control cultures (open bar) received no alcohol during either IFN-γ treatment or tryptophan rescue. The asterisk indicates that 1-butanol treatment during tryptophan rescue significantly decreased recoverable IFU (P < 0.05; two-tailed unpaired t test) from those of cultures treated with 1-butanol during IFN-γ treatment, cultures treated with 2-butanol during either IFN-γ treatment or tryptophan rescue, and untreated control cultures. The error bars depict standard deviations of recoverable IFU.
DISCUSSION
Functional genomics studies have determined that the chlamydial PZ is a hot spot for recombination in the relatively stable Chlamydia genomes. We show here that the pzPLD are primarily mid- to late-cycle genes that are expressed during both normal and persistent infections. At least one of the pzPLD, CT155, is located in RB-like structures associated with the inclusion membrane, consistent with the hypothesis that this protein might participate in direct interaction with host cells. Chlamydiae that retain pzPLD—but not GPIC, which lacks pzPLD—are sensitive to primary alcohols late in the developmental cycle, suggesting that these genes are enzymatically active in chlamydiae. Finally, we showed that primary alcohols block tryptophan rescue of C. trachomatis from IFN-γ-mediated persistence. These data suggest that the pzPLD might play a strain-specific role in chlamydial pathogenicity.
Assuming the pzPLD are classical PLDs, we propose a possible role for these proteins in acquisition and processing of host lipids. Chlamydiae incorporate a number of lipids from the host cell pool, including phosphatidylcholine, phosphatidyinositol, cardiolipin, and cholesterol (15, 33). Interestingly, metabolism of host phosphatidylcholine is accelerated in C. trachomatis-infected hepatocytes (16). PLDs are known to promote the fusion of lipid vesicles, suggesting another possible function for the pzPLD (21). Host-derived chlamydial lipids are primarily acquired via fusion with Golgi-derived vesicles (6, 13, 33), although the mechanisms underlying this traffic remain unclear. However, lipid traffic from the Golgi is at least partially dispensable, as Golgi disruption with brefeldin A has only a marginal inhibitory effect on chlamydial growth (33).
Another possibility is that pzPLD function in the acquisition of host ribonucleosides. Chlamydial pzPLD have homology to nucleases, such as Nuc (26); moreover, C. trachomatis is auxotrophic for three of four ribonucleosides and acquires all four from host cells (22). Three additional lines of correlative evidence support this speculation. First, the predicted isoelectric points of C. trachomatis pzPLD are highly basic (8.0 to 10.4) (30). The positive charge of these proteins, combined with the lack of obvious transmembrane domains, argues against the proteins interacting with lipids and for a nucleic acid binding function. Second, both C. trachomatis and C. muridarum inclusions are juxtaposed tightly with the nuclear envelope (12). The proximity of CT155 to the inclusion membrane, and presumably host nuclear pores, suggests these proteins might be positioned to receive and/or process host cell nuclear products late in the developmental cycle. Finally, primary-alcohol-insensitive C. caviae and pzPLD-negative C. pneumoniae, but not MoPn or C. trachomatis, retain pyrE, a critical gene in pyrimidine interconversion (20, 28-30). Collectively, these observations suggest that pzPLD may act in a specific manner to capture and utilize host nucleic acids.
In conclusion, our results suggest that the chlamydial pzPLD may be involved in strain-specific aspects of pathogenesis. The density of putative host-specific virulence genes in the PZ and the diversity of this region among chlamydiae suggest that the PZ may function as an adaptable pathogenicity island for this genus.
Acknowledgments
We thank Anita Mora and Gary Hettrick for graphics assistance and Kelly Matteson for manuscript formatting. We also thank John Carlson, Laszlo Kari, Jeff Shannon, William Whitmire, and Robert Heinzen for critical review of the manuscript.
Editor: D. L. Burns
REFERENCES
- 1.Belland, R. J., D. E. Nelson, D. Virok, D. D. Crane, D. Hogan, D. Sturdevant, W. L. Beatty, and H. D. Caldwell. 2003. Transcriptome analysis of chlamydial growth during IFN-gamma-mediated persistence and reactivation. Proc. Natl. Acad. Sci. USA 100:15971-15976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belland, R. J., M. A. Scidmore, D. D. Crane, D. M. Hogan, W. Whitmire, G. McClarty, and H. D. Caldwell. 2001. Chlamydia trachomatis cytotoxicity associated with complete and partial cytotoxin genes. Proc. Natl. Acad. Sci. USA 98:13984-13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belland, R. J., G. Zhong, D. D. Crane, D. Hogan, D. Sturdevant, J. Sharma, W. L. Beatty, and H. D. Caldwell. 2003. Genomic transcriptional profiling of the developmental cycle of Chlamydia trachomatis. Proc. Natl. Acad. Sci. USA 100:8478-8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caldwell, H. D., J. Kromhout, and J. Schachter. 1981. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect. Immun. 31:1161-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caldwell, H. D., H. Wood, D. Crane, R. Bailey, R. B. Jones, D. Mabey, I. Maclean, Z. Mohammed, R. Peeling, C. Roshick, J. Schachter, A. W. Solomon, W. E. Stamm, R. J. Suchland, L. Taylor, S. K. West, T. C. Quinn, R. J. Belland, and G. McClarty. 2003. Polymorphisms in Chlamydia trachomatis tryptophan synthase genes differentiate between genital and ocular isolates. J. Clin. Investig. 111:1757-1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carabeo, R. A., D. J. Mead, and T. Hackstadt. 2003. Golgi-dependent transport of cholesterol to the Chlamydia trachomatis inclusion. Proc. Natl. Acad. Sci. USA 100:6771-6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlson, J. H., S. F. Porcella, G. McClarty, and H. D. Caldwell. 2005. Comparative genomic analysis of Chlamydia trachomatis oculotropic and genitotropic strains. Infect. Immun 73:6407-6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, Y. G., A. Siddhanta, C. D. Austin, S. M. Hammond, T. C. Sung, M. A. Frohman, A. J. Morris, and D. Shields. 1997. Phospholipase D stimulates release of nascent secretory vesicles from the trans-Golgi network. J. Cell Biol. 138:495-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edwards, J. L., D. D. Entz, and M. A. Apicella. 2003. Gonococcal phospholipase D modulates the expression and function of complement receptor 3 in primary cervical epithelial cells. Infect. Immun. 71:6381-6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fehlner-Gardiner, C., C. Roshick, J. H. Carlson, S. Hughes, R. J. Belland, H. D. Caldwell, and G. McClarty. 2002. Molecular basis defining human Chlamydia trachomatis tissue tropism. A possible role for tryptophan synthase. J. Biol. Chem. 277:26893-26903. [DOI] [PubMed] [Google Scholar]
- 11.Fields, K. A., and T. Hackstadt. 2002. The chlamydial inclusion: escape from the endocytic pathway. Annu. Rev. Cell Dev. Biol. 14:14. [DOI] [PubMed] [Google Scholar]
- 12.Grieshaber, S., N. Grieshaber, and T. Hackstadt. 2003. Chlamydia trachomatis uses host cell dynein to traffic to the microtube organizing center in a p50 dynamitin independent process. J. Cell Biol. 116:3793-3802. [DOI] [PubMed] [Google Scholar]
- 13.Hackstadt, T., M. A. Scidmore, and D. D. Rockey. 1995. Lipid metabolism in Chlamydia trachomatis-infected cells: directed trafficking of Golgi-derived sphingolipids to the chlamydial inclusion. Proc. Natl. Acad. Sci. USA 92:4877-4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammond, S. M., Y. M. Altshulller, T. C. Sung, S. A. Rudge, K. Rose, J. Engebrecht, A. J. Morris, and M. A. Frohman. 1995. Molecular characterization of a human ARF-activated phosphatidylcholine specific phospholipase D defines a new and highly conserved family of genes. J. Biol. Chem. 270:29640-29643. [DOI] [PubMed] [Google Scholar]
- 15.Hatch, G. M., and G. McClarty. 1998. Cardiolipin remodeling in eukaryotic cells infected with Chlamydia trachomatis is linked to elevated mitochondrial metabolism. Biochem. Biophys. Res. Commun. 243:356-360. [DOI] [PubMed] [Google Scholar]
- 16.Hatch, T. P., and G. McClarty. 2004. C. trachomatis infection accelerates metabolism of phosphatidylcholine derived from low density lipoprotein but does not affect phosphatidylcholine secretion from hepatocytes. BMC Microbiol. 4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hinnebusch, B. J., A. E. Rudolph, P. Cherepanov, J. E. Dixon, T. G. Schwan, and A. Forsberg. 2002. Role of Yersinia murine toxin in survival of Yersinia pestis in the midgut of the flea vector. Science 296:733-735. [DOI] [PubMed] [Google Scholar]
- 18.Horn, M., A. Collingro, S. Schmitz-Esser, C. L. Beier, U. Purkhold, B. Fartmann, P. Brandt, G. J. Nyakatura, M. Droege, D. Frishman, T. Rattei, H. W. Mewes, and M. Wagner. 2004. Illuminating the evolutionary history of chlamydiae. Science 304:728-730. [DOI] [PubMed] [Google Scholar]
- 19.Husain, M., and B. Moss. 2002. Similarities in the induction of post-Golgi vesicles by the vaccinia virus F13L protein and phospholipase D. J. Virol. 76:7777-7789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalman, S., W. Mitchell, R. Marathe, C. Lammel, J. Fan, R. W. Hyman, L. Olinger, J. Grimwood, R. W. Davis, and R. S. Stephens. 1999. Comparative genomes of Chlamydia pneumoniae and C. trachomatis. Nat. Genet. 21:385-389. [DOI] [PubMed] [Google Scholar]
- 21.Liscovitch, M., M. Czarny, G. Fiucci, and X. Tang. 2000. Phospholipase D: molecular and cell biology of a novel gene family. Biochem. J. 345:401-415. [PMC free article] [PubMed] [Google Scholar]
- 22.McClarty, G., and B. Qin. 1993. Pyrimidine metabolism by intracellular Chlamydia psittaci. J. Bacteriol. 175:4652-4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNamara, P. J., W. A. Cuevas, and G. J. Songer. 1995. Toxic phospholipases D of Corynebacterium pseudotuberculosis, C. ulcerans and Arcanobacterium haemolyticum: cloning and sequence homology. Gene 156:113-118. [DOI] [PubMed] [Google Scholar]
- 24.Morris, A. J., J. Engebrecht, and M. A. Frohman. 1996. Structure and regulation of phospholipase D. Trends Pharmacol. Sci. 5:182-185. [DOI] [PubMed] [Google Scholar]
- 25.Nelson, D. E., D. P. Virok, H. Wood, C. Fehlner-Gardiner, R. M. Johnson, W. M. Whitmire, D. D. Crane, O. Steele-Mortimer, L. Kari, G. McClarty, and H. D. Caldwell. 2005. Chlamydial interferon gamma immune evasion is linked to host infection tropism. Proc. Natl. Acad. Sci. USA 102:10658-10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pohlman, R. F., F. Liu, L. Wang, M. I. More, and S. C. Winans. 1993. Genetic and biochemical analysis of an endonuclease encoded by the IncN plasmid pKM101. Nucleic Acids Res. 21:4867-4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ponting, C. P., and I. D. Kerr. 1996. A novel family of phospholipase D homologues that includes phospholipid synthases and putative endonucleases: identification of duplicated repeats and potential active site residues. Protein Sci. 5:914-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Read, T. D., R. C. Brunham, C. Shen, S. R. Gill, J. F. Heidelberg, O. White, E. K. Hickey, J. Peterson, T. Utterback, K. Berry, S. Bass, K. Linher, J. Weidman, H. Khouri, B. Craven, C. Bowman, R. Dodson, M. Gwinn, W. Nelson, R. DeBoy, J. Kolonay, G. McClarty, S. L. Salzberg, J. Eisen, and C. M. Fraser. 2000. Genome sequences of Chlamydia trachomatis MoPn and Chlamydia pneumoniae AR39. Nucleic Acids Res. 28:1397-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Read, T. D., G. S. Muers, R. C. Brunham, W. C. Nelson, I. T. Paulsen, J. F. Heidelberg, E. Holtzapple, H. Khouri, N. B. Federova, H. A. Carty, L. A. Umayam, D. H. Haft, J. Peterson, M. J. Beanan, O. White, S. L. Salzberg, R. Hsia, G. McClarty, R. G. Rank, P. M. Bavoil, and C. M. Fraser. 2003. Genome sequence of Chlamydiophilia caviae (Chlamydia psittaci GPIC): examining the role of niche-specific genes in the evolution of the Chlamydiaceae. Nucleic Acids Res. 31:2134-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stephens, R. S., S. Kalman, C. Lammel, J. Fan, R. Marathe, L. Aravind, W. Mitchell, L. Olinger, R. L. Tatusov, Q. Zhao, E. V. Koonin, and R. W. Davis. 1998. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science 282:754-759. [DOI] [PubMed] [Google Scholar]
- 31.Sung, T. C., R. L. Roper, Y. Zhang, S. A. Rudge, R. Temel, S. M. Hammond, A. J. Morris, B. Moss, J. Engebrecht, and M. A. Frohman. 1997. Mutagenesis of phospholipase D defines a superfamily including a trans-Golgi viral protein required for poxvirus pathogenicity. EMBO 16:4519-4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilderman, P. J., A. I. Vasil, Z. Johnson, and M. L. Vasil. 2001. Genetic and biochemical analyses of a eukaryotic-like phospholipase D of Pseudomonas aeruginosa suggest horizontal acquisition and a role for persistence in a chronic pulmonary infection model. Mol. Microbiol. 39:291-303. [DOI] [PubMed] [Google Scholar]
- 33.Wylie, J. L., G. M. Hatch, and G. McClarty. 1997. Host cell phospholipids are trafficked to and then modified by Chlamydia trachomatis. J. Bacteriol. 179:7233-7242. [DOI] [PMC free article] [PubMed] [Google Scholar]